Summary
The long-term clinical outcomes of severe obesity due to leptin signaling deficiency are unknown. We carry out a retrospective cross-sectional investigation of a large cohort of children with leptin (LEP), LEP receptor (LEPR), or melanocortin 4 receptor (MC4R) deficiency (n = 145) to evaluate the progression of the disease. The affected individuals undergo physical, clinical, and metabolic evaluations. We report a very high mortality in children with LEP (26%) or LEPR deficiency (9%), mainly due to severe pulmonary and gastrointestinal infections. In addition, 40% of surviving children with LEP or LEPR deficiency experience life-threatening episodes of lung or gastrointestinal infections. Although precision drugs are currently available for LEP and LEPR deficiencies, as yet, they are not accessible in Pakistan. An appreciation of the severe impact of LEP or LEPR deficiency on morbidity and early mortality, educational attainment, and the attendant stigmatization should spur efforts to deliver the available life-saving drugs to these children as a matter of urgency.
Keywords: monogenic obesity, leptin-signaling deficiency, age-related changes, mortality, morbidity, body growth, metabolism, oxidative stress, consanguinity
Graphical abstract
Highlights
-
•
Remarkably high morbidity and mortality in children with LEP or LEPR deficiency
-
•
High incidence of pulmonary and GI infections is main cause of deaths
-
•
Oxidative stress levels notably higher in LEP than in LEPR or MC4R deficiency
-
•
Emphasis on delivery of precision medication for affected children of this region
In this report, Saeed et al. investigate the natural history of leptin-signaling deficiency in a large cohort of affected children. The study reveals high morbidity and mortality in these patients at an early age. Lack of available treatment facilities for these patients calls for immediate action.
Introduction
Biallelic, (likely) pathogenic variants in LEP encoding leptin and LEPR encoding its receptor, leptin receptor (LEPR), that follow highly penetrant Mendelian inheritance lead to a congenital LEP-signaling-deficient state, causing very-early-onset severe obesity due to persistent hyperphagia. The first evidence of the (likely) pathogenic variants in the LEP gene in the homozygous state resulting in LEP deficiency was reported in two obese cousins of Pakistani origin,1 whereas the first inactivating homozygous mutation in LEPR was found in three French sisters from Algeria.2 Since then, 22 pathogenic mutations in LEP and 55 in LEPR have been reported, most of them in highly consanguineous populations.3,4
The physiological role of LEP as a key initiator of hypothalamic neural circuitry that drives the central melanocortin pathway in the regulation of appetite is well established.3,5 LEP’s binding to its receptor results in the production of α- and β-melanocyte-stimulating hormones (α-MSH and β-MSH) from pro-opiomelanocortin (POMC), peptides that act as key satiety signals by activating the melanocortin 4 receptor (MC4R) on second-order neurons. Thus, LEP-driven central melanocortin signaling is the main central regulator of energy balance, food intake, and body weight.
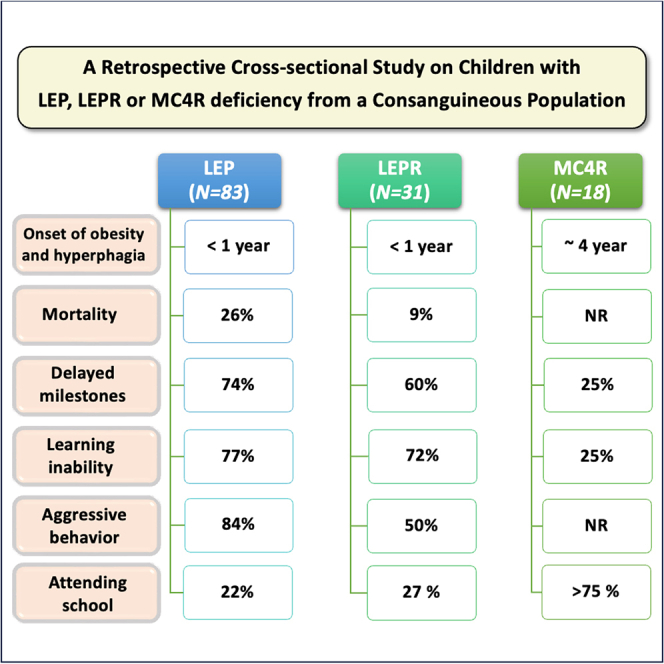
Clinically, the initial descriptions of young patients with obesity consequent to LEP or LEPR deficiency were rather similar.6,7,8 Born with normal weight, the recessive mutation carriers gain excessive weight during infancy.9,10 In rodents and humans, LEP and LEPR deficiencies are associated with hyperinsulinemia, hypothyroidism, and hypogonadotropic hypogonadism resulting in delay or absence of pubertal development.7,11,12 Furthermore, there are some data to suggest that immune response of individuals with LEP-signaling deficiency may be seriously compromised with reduced numbers and proliferative function of T cells and vulnerability to infections during childhood.13,14,15 However, it is noteworthy that most of the existing clinical knowledge on LEP deficiency is based on individual case reports or on small pooled data from different ethnicities.3 Prospective studies of genetically homogeneous individuals with LEP-signaling deficiency have not been carried out so far. Thus, the long-term consequences of LEP deficiency are still unknown. Since 2012, we have constituted a cohort of children with severe obesity from consanguineous families for the study of genetic etiology of obesity in the Pakistani population (Severe Obesity in Pakistani Population [SOPP] study). We have previously reported an exceptionally high prevalence of monogenic obesity (49%) due to (likely) pathogenic variants in known obesity-associated genes, which included 33% of homozygous point mutations or copy-number variations (CNVs) in LEP, LEPR, and MC4R.16
Here, we present a retrospective clinical history of 92 children suffering from severe obesity due to LEP and 32 cases with LEPR deficiency, all from a single geographical region of Punjab, Pakistan. In addition, 21 cases of patients with severe obesity carrying (likely) pathogenic homozygous variants in MC4R, from the same population, have been included for comparison.
Results
Identification of mutations in SOPP cohort
The genetic screening of 454 families from our cohort, with severe obesity, identified 132 probands (29%) and 13 family members with homozygous or compound heterozygous (likely) pathogenic variants in LEP, LEPR, or MC4R (Table 1). Eighty-three children (53 males; 30 females) and nine family members (5 males; 4 females) carried 11 different homozygous (likely) pathogenic variants in LEP. These included five loss-of-function mutations (three frameshift and two splice site), one insertion or deletion (indel), and five missense mutations. With the exception of the two missense mutations, where cases presented high levels of circulating bioinactive LEP, the rest of the children presented with undetectable levels of LEP. The frameshift c.398del (p.G133Vfs∗15) was shown to be the most predominant LEP mutation identified in 62 patients (76%; 39 males and 23 females). This mutation was first reported in 1997 in a UK family of Pakistani origin.1 Interestingly, 54 probands carrying this mutation belonged to the Pakistani subethnicity Arain, suggesting a founder effect. For the remaining LEP mutations, no notable association with a particular region of origin or subethnicity was noticeable in this cohort (Table 1). Thirty-one probands (13 males; 18 females) and one family member were identified with 21 different (likely) pathogenic homozygous or compound heterozygous variants in LEPR. Among these, 17 were loss-of-function mutations including 5 splice site (n = 14), four nonsense (n = 5), one start loss (n = 1), three frameshift (n = 3), one compound heterozygous (n = 1), and three CNVs with homozygous deletions of various lengths (44–52 kb). The four missense (n = 4) mutations were identified in single cases, in SOPP cohort and were not identified in the other 1,328 children with severe obesity of the same ancestry recruited to the Genetics of Obesity Study (https://www.goos.org.uk) and, therefore, are likely to be private mutations. Eighteen probands (9 males; 9 females) and 3 family members (1 male; 2 females) were found to carry different homozygous mutations in MC4R. These included five missense, two nonsense, one frameshift, and one indel variants (Table 1).
Table 1.
Pathogenic LEP, LEPR, and MC4R mutations identified in probands from SOPP cohort in relation to subethnic groups
| Mutation | Type of mutation | 1st reported/novel | REVEL score | No. probands | Subethnicity | |
|---|---|---|---|---|---|---|
| LEP | c.398del (p.G133Valfs∗15) | frameshift | Montague et al.1 | – | 62 | Arain (n = 54), Rajput, Mughal, Gazar, Jutt (n = 2), Tamboo, N/Aa (n = 2) |
| LEP | c.313C>T (p.R105W) | missense | Strobel et al.17 | 0.686 (Dam) | 1 | Sahotra |
| LEP | c.-28-16_-3del | splice site | Saeed et al.8 | – | 2 | Mashki, Rajput |
| LEP | c.-29 + 1G>C | splice site | Saeed et al.8 | – | 4 | Sheikh (n = 2), Sahotra, Lotay |
| LEP | c.298G>A (p.D100N) | missense | Saeed et al.8 | 0.610 (Dam) | 1 | Sheikh |
| LEP | c.309C>A (p.N103K) | missense | Mazen et al.18 | 0.523 (Dam) | 2 | Arain, Rehmani |
| LEP | c.314G>A (p.R105Q) | missense | Saeed et al.16 | 0.467 (Uncer)b | 1 | Ansari |
| LEP | c.350G>A (p.C117Y) | missense | Saeed et al.8 | 0.33 (Uncer)b | 2 | Warraich (n = 2) |
| LEP | c.104_106del (p.I35del) | in-frame deletion | Fatima et al.19 | – | 5 | Rajput (n = 2), Jutt (n = 2), Bhatti |
| LEP | c.417delC (p.Y140Tfs∗8) | frameshift | Saeed et al.16 | – | 2 | Syed Bukhari, Jutt |
| LEP | c.481_482del (p.L161Gfs∗10) | frameshift | Fatima et al.19 | – | 1 | Jutt |
| LEPR | c.2396-1G>T | splice site | Saeed et al.10 | – | 7 | Jutt, Rajput (n = 4), Ansari (n = 2) |
| LEPR | c.2396-2A>G | splice site | Saeed et al.16 | – | 2 | Rajput, Bayi |
| LEPR | c.704-1G>A | splice site | Saeed et al.16 | – | 3 | Ansari, Bhatti, Khansani |
| LEPR | c.2213-3C>G | splice site | Saeed et al.16 | – | 1 | Kakazai |
| LEPR | c.994 + 2T>C | splice site | – | – | 1 | Mughal |
| LEPR | c.2T>C (p.?) | start loss | Saeed et al.16 | – | 1 | Awan |
| LEPR | c.1674G>A (p.W558∗) | nonsense | Saeed et al.10 | – | 2 | Siddiqui, Sheikh |
| LEPR | c.133_136dup p.(Tyr46∗) | nonsense | novel | – | 1 | Lalikher |
| LEPR | c.2114G>A (p.W705∗) | nonsense | Saeed et al.16 | – | 1 | Dogar |
| LEPR | c.489T>A (p.Y163∗) | nonsense | novel | – | 1 | Barfizai |
| LEPR | c.1810T>A (p.C604S) | missense | Saeed et al.16 | 0.609 (Dam) | 1 | Gujar |
| LEPR | c.2627C>T (p.P876L) | missense | Saeed et al.16 | 0.647 (Dam) | 1 | Mughal |
| LEPR | c.40G>A (p.E14K) | missense | Saeed et al.16 | 0.116 (Ben)c | 1 | Rehmani |
| LEPR | c.2153A>G (p.N718S) | missense | Saeed et al.16 | 0.665 (Dam) | 1 | Jutt |
| LEPR | c.2899_2900insAT(p.A967Dfs∗7) | frameshift | Saeed et al.16 | – | 1 | Jutt |
| LEPR | c.3268_3269del (p.S1090Wfs∗6) | frameshift | Bhatt et al.20 | – | 1 | Arain |
| LEPR | c.1738delG(p.E580Kfs∗37) | frameshift | Saeed et al.16 | – | 1 | Arain |
| LEPR | c.2875T>A (p.R959∗) and c.2872G>A (p.E958K) | compound heterozygous | Saeed et al.16 | – | 1 | Gujar |
| LEPR | ∼44 kb homozygous deletion including exons 3–20 of LEPR | CNV | Saeed et al.16 | – | 1 | Gujar |
| LEPR | ∼61 kb homozygous deletion including exons 3–6 of LEPR | CNV | Saeed et al.8 | – | 1 | Rajput |
| LEPR | 52 kb homozygous deletion | CNV | Saeed et al.16 | – | 1 | Rajput |
| MC4R | c.633_636delCTAT (p.Y212Sfs∗5) | frameshift | Saeed et al.16 | – | 1 | Khokhar |
| MC4R | c.47G>A (p.W16∗) | nonsense | Marti et al.21 | – | 3 | Jutt, Rajput (n = 2) |
| MC4R | c.63_64del (p.Y21∗) | nonsense | Saeed et al.16 | – | 2 | Arain (n = 2) |
| MC4R | c.601_612 del TTCTTCACCATG (p.F201_M204del) | in-frame deletion | Saeed et al.16 | – | 1 | Rehmani |
| MC4R | c.206T>C (p.I69T) | missense | Tan et al.22 | 0.592 (Dam) | 1 | Jutt |
| MC4R | c.482 T>C (p.M161T) | missense | Tan et al.22 | 0.488 (Uncer) | 3 | Pathan (n = 2), Memon |
| MC4R | c.493C>T (p.R165W) | missense | Hinney et al.23 | 0.501 (Dam) | 4 | Arain (n = 2), Jutt Sayal, Lashari |
| MC4R | c.811T>C (p.C271R) | missense | Farooqi et al.24 | 0.844 (Dam) | 1 | Bhatti |
| MC4R | c.947 T>C (p.I316S) | missense | Yeo et al.25 | 0.442 (Uncer) | 2 | Rajput, Sheikh |
LEP: NM_000230.3; LEPR: NM_002303.6; MC4R: NM_005912.3. Dam, damaging; Uncer, uncertain; Ben, benign.
Not available.
Undetectable levels of leptin hormone.
Located at essential splice site.
Clinical phenotypes
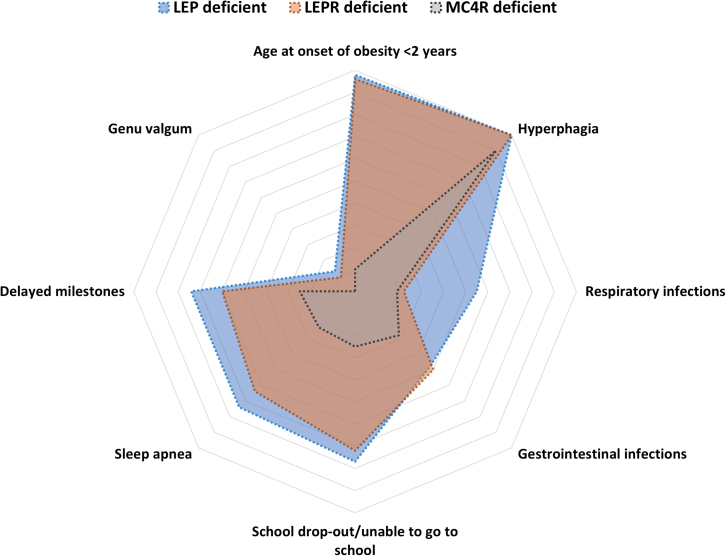
The most prominent and ubiquitous characteristic of children identified with biallelic (likely) pathogenic variants in LEP, LEPR, and MC4R genes was the onset of severe obesity and hyperphagia at a very young age—they lacked satiety and were constantly in demand of food. If denied, they demanded more food by crying and turning aggressive. The majority of the children with LEP or LEPR deficiency displayed excessive weight gain and hyperphagia that were noticed at a far earlier age in postnatal life (before 1 year of age; mean ages in children with LEP deficiency: 0.3 ± 0.04 years [n = 83] and in children with LEPR deficiency: 0.9 ± 0.1 years [n = 27]) as compared with those in children with MC4R deficiency (mean age: 4 ± 3.3 years [n = 20]).
Children deficient for LEP or LEPR presented a more precocious and profound hyperphagia than those with MC4R deficiency, with frequent aggressive demands for food during the day and when waking up at night. The craving for food was reported relatively less intense and frequent in children with MC4R deficiency. At the time of clinical examination, 84% of children with LEP, 79% with LEPR, and 61% with MC4R deficiency suffered from severe hyperphagia, whereas in the remaining affected subjects, the craving for food, though frequent, was reported as less severe or as moderate (Figure 1). It is noteworthy that with advancing age, no exacerbation of severity of hyperphagia was observed. On the contrary, a considerable variation in demand for food with time was observed in children with LEP or LEPR deficiency. This could partly be due to parents’ care and efforts to divert the attention of the child’s reward system to other healthy activities of choice.
Figure 1.
Radar chart representing the severity of the obesity-associated risks in children with LEP or LEPR deficiency compared with those with MC4R deficiency
No dysmorphic features or congenital malformations were observed in any of the obese probands. However, acanthosis nigricans was often present in the three mutant groups and was most predominant as dark velvety patches of skin around the neck (Figure S1A). Furthermore, prominent skin folds bracing the subcutaneous fat were invariably present on the forearms and trunk regions of children deficient for LEP or LEPR at an early stage of life but were less conspicuous in MC4R-deficient age-matched individuals (Figure S1B).
Morbidity
The majority of the children deficient for LEP or LEPR (55% and 38%, respectively) suffered from recurrent episodes of serious respiratory afflictions such as pneumonia and upper respiratory tract infections. Also, the majority of the children deficient for LEP or LEPR (74% and 64%, respectively) were assessed as hypoxic. The second most common complication observed is related to gastrointestinal infections often leading to severe diarrhea. About 40% of the living children with LEP or LEPR deficiency had one or more hospital admissions for intensive care management of these complications. In addition, genu valgum was identified in 13% of children deficient for LEP and 9% of children with LEPR deficiency but was not reported in the group with MC4R deficiency (Figure 1). Vision refractive errors were reported in 16% of probands with LEP deficiency but not in the other two groups.
Mortality
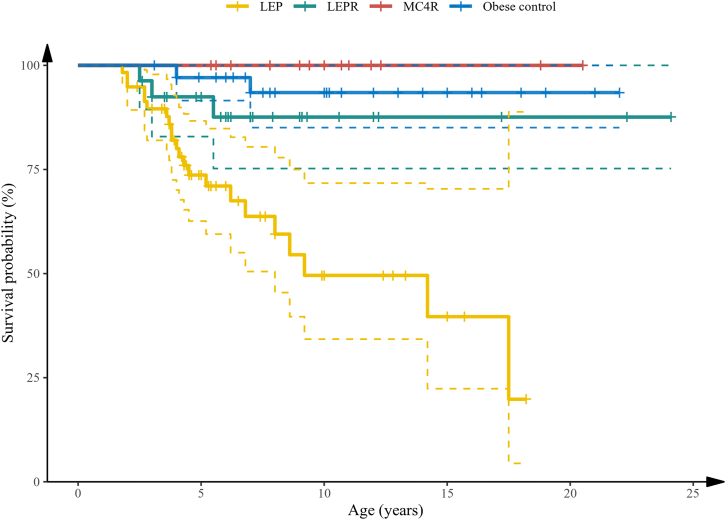
Deaths were reported in 26% of children with LEP deficiency and 9% of children with LEPR deficiency (Figure 1; Table S1). In the majority of these cases, the cause of death was diagnosed as respiratory failure due to pneumonia or other respiratory infections, and the second most common cause of death was severe diarrheal episodes. No deaths have so far been reported in children deficient for MC4R from this cohort. The Kaplan-Meier survival estimates demonstrate a remarkably low survival rate in children with LEP-signaling deficiency (p < 0.05; Figure 2).
Figure 2.
Survival curve of children with LEP, LEPR, and MC4R deficiencies and severely obese children negative for mutations in known obesity genes (obese controls)
Kaplan-Meier group log-rank p < 0.05.
Physical activity and social behavior
Gross motor development milestones were noticeably delayed in 74% of children with LEP, 60% with LEPR, and 25% with MC4R deficiencies, presumably and partly due to excessive adiposity. Thus, the ability to independently sit and walk was acquired by these children at a much later stage compared with their non-obese siblings or with age-matched healthy subjects. Aggressive behavior in the majority of children with LEP or LEPR deficiency (84% and 50%, respectively) was reported toward their siblings and peers but not toward their parents. The relative inability to learn and remember new things was variable in affected children but was more evident in children with LEP or LEPR deficiency (77% and 72%, respectively) as compared with those of children with MC4R deficiency (25%). Overall, only a small percentage of the children with LEP deficiency (22%) or with LEPR deficiency (27%) over the age of 5 years were reported to be attending school compared with 75% of children with MC4R deficiency. Furthermore, those children who initially attended school very often dropped out prematurely mainly due to their inability to keep in step with their peers in studies, a lack of integration and acceptance by their peers, frequent health problems, and socio-psychological pressures (Figure 1). A lag in learning abilities as evidenced by difficulty in learning/remembering new things, delayed milestones, aggressive behavior, and high dropout rates from school in children with LEP and LEPR mutations probably indicate an age-related delay in cognitive ability. However, no attempt was made to quantitatively assess cognitive limitations in these patients.
Body growth
Longitudinal growth data in relation to age in the three mutant groups and in control subjects with healthy body weight from age 1–15 years are presented in Figure S2 and S2 and Tables S3A. The mean height was significantly greater (p < 0.05) in children with MC4R deficiency than in the other two study groups, whereas no significant differences in growth were found between children deficient for LEP or LEPR (Table S3A). The estimated linear growth in children with MC4R deficiency was also significantly increased compared with the age- and gender-matched controls (8.7 ± 2.3; p = 0.0001). We observed a similar trend (but statistically non-significant) in individuals with LEP or LEPR deficiency (1.7 ± 1.6 and 2.3 ± 1.9, respectively) (Table S4; Figure S2). No remarkable differences were found in longitudinal growth in individuals carrying mutations and the control subjects during the first 10 years of life (Table S3A). However, we noticed a trend toward an increased growth in individuals with MC4R deficiency in children ≥10 years old compared with age-matched children with LEP or LEPR deficiency and also compared with the control subjects. Age-related body weight and body mass data are summarized in Figure S3 and Tables S2, S3B, and S3C.
Metabolic characteristics
The overall metabolic characteristics of children with mutations in the three genes and those with healthy body weight are presented in Table 2. As expected, insulin levels were significantly higher in the three mutant groups compared with control values and increased progressively with advancing age (Table S5A). At the age of 10–15 years, hyperinsulinemia appeared milder in the group with LEP deficiency as compared with the patients with LEPR and MC4R deficiencies (mean 49 versus 104 and 66 μIU/mL in children with LEP, LEPR, or MC4R deficiency, respectively) (range of insulin assay: 0–300 μIU/mL) (Table S5A).
Table 2.
Endocrine and glycemic characteristics of children with LEP, LEPR, and MC4R mutations and lean controls
| Characteristic | LEP deficient | LEPR deficient | MC4R deficient | Controls |
|---|---|---|---|---|
| Leptin, ng/mL (n) | 0.1 ± 0.0a,b (108) | 31.9 ± 2.7c,d (49) | 26.7 ± 3.5c,d (26) | 3.1 ± 0.1a,b (68) |
| Insulin, μIU/mL (n) | 24.6 ± 2.3a,b,d (111) | 41.8 ± 5.9c,d (49) | 49.0 ± 10.3c,d (26) | 6.9 ± 0.5a,b,c (61) |
| Cortisol, μg/dL (n) | 16.2 ± 0.8a,b,d (111) | 13.3 ± 0.7c,d (49) | 10.9 ± 0.8c (26) | 9.4 ± 0.3a,c (61) |
| TSH, μIU/mL (n) | 2.2 ± 0.1 (112) | 2.3 ± 0.2 (49) | 2.1 ± 0.2 (26) | 2.2 ± 0.1 (61) |
| HbA1C (n) | 5.2 ± 0.1d (29) | 5.0 ± 0.1b,d (19) | 5.9 ± 0.5a,d (7) | 4.2 ± 0.1a,b,c (40) |
| RBG∗, mg/dL (n) | 95.7 ± 3.2 (30) | 99.7 ± 3.7 (23) | 109.1 ± 7.8 (15) | 102.8 ± 2.6 (40) |
| T4, μg/dL (n) | 8.4 ± 0.6 (23) | 7.0 ± 0.5 (11) | 7.6 ± 0.9 (15) | 8.1 ± 0.3 (27) |
| T3, ng/mL (n) | 1.8 ± 0.1 (23) | 1.9 ± 0.2 (11) | 1.6 ± 0.1 (15) | 1.5 ± 0.1 (27) |
Data represent mean ± SEM.
∗Random blood glucose
Statistically significant p <0.05 (Scheffe’s multiple test) vs. LEPR deficient.
Statistically significant p <0.05 (Scheffe’s multiple test) vs. MC4R deficient.
Statistically significant p <0.05 (Scheffe’s multiple test) vs. LEP deficient.
Statistically significant p <0.05 (Scheffe’s multiple test) vs. controls.
LEP concentrations in LEPR deficiency tended to be higher in the first 5 years of life than those of the children with MC4R deficiency (p < 0.05) (Table S5B). As anticipated, serum LEP concentrations were below the sensitivity level of the assay in children with LEP deficiency with the exception of two individuals (0.7 and 1.5 years of age) with the p.N103K mutation and one individual (2 years of age) with the p.D100N mutation in LEP. These cases present high levels of circulation of immunoreactive, but bioinactive, LEP protein (mean: 49 ng/mL) as also reported previously.26
Mean cortisol levels (morning) were significantly higher (p < 0.05) in LEP deficiency than those in the other two groups and tended to decline with age (Table S5C) but were indistinguishable from the lean controls in patients with MC4R deficiency (Table 2). Remarkably, mean serum thyroid-stimulating hormone (TSH), T4, and T3 concentrations were within the healthy range in children with LEP, LEPR, or MC4R deficiency and were not different from those of healthy subjects (Tables 2 and S5D). Mean HbA1c ranged between 5 and 5.9 in the three mutant groups compared to a mean of 4.2 in the control group (Table 2). Mean random blood glucose (RBG) levels were within the healthy range in the three mutant groups and were comparable with lean control values (Table 2), except in one individual with MC4R deficiency (RBG = 199 mg/dL).
Oxidative stress
Peripheral levels of malondialdehyde (MDA) and 8-OHdG, critical biomarkers of oxidative stress that occurs as a consequence of an imbalance between the formation of free oxygen radicals and inactivation of these species by the antioxidant defense system, and of glutathione (GSH), an antioxidant biomarker, were measured in a restricted number of probands. Serum levels of MDA and 8-OHdG were significantly higher and of GSH lower in individuals with LEP or LEPR deficiency compared with those with MC4R deficiency (p < 0.05) (Table S5E). Circulating levels of MDA and 8-OHdG were maximal in children with LEP deficiency.
Discussion
Our study demonstrates a high mortality rate in obese children with LEP deficiency and to a lesser extent in children with LEPR deficiency. While increased susceptibility to infection and mortality have been reported in case reports of these conditions previously,7,27 this large clinical series of children definitively establishes the severe clinical impact of these conditions. In contrast, no severely obese child with MC4R deficiency of same ethnic origin died during the study. Indeed, all children with severe obesity in this study belong to the same geographical region and share similar socioeconomic status (low middle economy). Previous studies indicate that the country’s disease burden may be overtly influenced by the socio-economic index.28 In LEP-signaling deficiency, the mortality rate may even be underestimated, as ∼30% of the families could not be contacted following the initial presentation. Health records indicated that deaths of the children were mostly due to respiratory and gastrointestinal infections. These effects may be due to lowered immunity and possibly a lack of a permissive effect of LEP on immune cells.13,29
Previously, clinical investigation of three patients with LEP deficiency demonstrated an increased frequency of infections predominantly of the respiratory tract when compared with their wild-type siblings. T cell number and function were impaired in the subjects with a reduced number of CD4+ T cells, reduced T cell proliferation, and cytokine response, but no gross abnormalities related to thermoregulation were reported.13 These findings of T cell responsiveness were consistent with observations in ob/ob mice.30 Therapeutic doses of recombinant LEP normalized the T cell function.13 These findings suggest that LEP is a crucial metabolic signal that allows activation and proliferation of T cells, thus linking nutritional status, cellular metabolism, and immunity.31
Another outcome of this investigation is the observation that as many as 40% of living young patients with LEP or LEPR deficiency had experienced serious health problems posing a life-threatening risk and necessitating hospitalization for intensive care management. According to a recent UNICEF (The United Nations Children’s Fund) report, mortality in children under 5 years of age (excluding neonatal mortality, which constitutes more than half of the under-5 deaths in the country) is estimated at 2.5% in Pakistan for the year 2020 (Monitoring the situation of children and women. Pakistan, Key demographic indicators 2020: https://data.unicef.org/country/pak/). Our data unequivocally demonstrate that LEP deficiency leads to a remarkably higher mortality rate, presumably due to lowered immunity leading to high risk of infections in these children. Also, according to the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019, neonatal disorders, congenital defects, diarrheal diseases, and respiratory infections have been shown to be among the top ten main causes of premature mortality and years of life lost in Pakistan.28
Although severe obesity has been considered a long-term risk factor of early morbidity, disability, and premature death in adulthood, here we present a well-defined non-syndromic form of genetic obesity in childhood shown to be so dramatically life threatening. Indeed, more common forms of severe early-onset obesity are associated with metabolic and cardiovascular disease, as well as with some cancers later in life, but not with high infection rates (with the recent exception of COVID19 in relatively young adults). Earlier research on LEP-signaling deficiency in human and animals suggested impaired immunity that may contribute to the severity of common respiratory and digestive infections.13 Although we were not able to carry out direct evaluation of humoral and cellular immunity in our children with obesity, we found a high systemic oxidative stress level and a marked depletion of the antioxidant GSH in obese carriers with homozygous loss-of-function mutations in LEP or LEPR genes. In comparison, the oxidative stress level was found to be lower in patients with MC4R deficiency. Earlier studies suggested a positive correlation between body mass index and levels of oxidative stress,32,33 but here we demonstrate that the level of oxidative stress related to obesity may differ even among well-defined conditions of genetic obesity. In spite of a close phenotypic identity between LEP and LEPR mutation carriers, morbidity in children with LEP deficiency is surprisingly much higher than that of patients with LEPR deficiency. We also found a higher index of oxidative stress and DNA damage in children deficient for LEP compared with those with LEPR deficiency, suggesting a more severe impairment in cellular or humoral immunity of unknown nature.
The very early onset of weight gain due to severe hyperphagia in children with LEP or LEPR deficiency is well documented,6 and in this respect, we confirm that children with LEP or LEPR deficiency are indistinguishable.7,16,29 In addition, our ethnically homogeneous study unambiguously shows that the onset of severe obesity is ∼3 years earlier in both LEP and LEPR deficiencies compared with the children deficient for MC4R.
Here, we also document the high incidence of delay in learning ability, possibly due to intellectual impairment, and aggressive behavior in children with LEP or LEPR deficiency that appear to be milder or absent in children with MC4R deficiency. This is also evidenced by the observation that only ∼25% of children with LEP or LEPR deficiency could attend school after the age of 5 years compared with 75% of children with MC4R deficiency. Also, long-term schooling was nearly impossible for children deficient for LEP or LEPR. This other dramatic outcome is further supported by our recent study on a group of severely obese teenagers from the same geographical region and with the same level of consanguinity (mean BMI = 37 kg/m2; mean age = 18 years) who were regularly attending school. Remarkably, this cohort did not include a single subject carrying a mutation in the LEP gene.34
LEP-deficient ob/ob and LEPR-deficient db/db mice have decreased linear body growth.35 In human, previous reports on linear growth due to defective LEP or LEPR signaling have been controversial,2 with reports of both accelerated36,37 and diminished2,38 linear growth. Here, our data in a much larger cohort demonstrate no remarkable differences in linear growth in children with LEP or LEPR deficiency compared with age-matched lean controls up to the age of 15 years. In the present investigation, we observed a significantly increased mean linear growth in children with MC4R deficiency as compared with the other two groups of mutation carriers. Previously, MC4R deficiency has also been associated with increased linear growth due to enhanced osteogenesis.24
Hyperinsulinemia, though significantly more pronounced in individuals with LEPR or MC4R deficiency compared with those with LEP deficiency, increased with age in all three mutant groups. Raised HbA1c levels (but still within the healthy range) were found in children with MC4R deficiency but to a lesser extent in children deficient for LEP or LEPR. Consequently, none of these children had thus far developed a risk of diabetes. The euglycemic condition in affected children in this study is in concordance with previous observations.24
Hypercortisolemia was more prominent in children with LEP deficiency compared with that in patients with LEPR deficiency, whereas cortisol levels were in the healthy range in patients with MC4R deficiency and were indistinguishable from those of the lean controls, as previously reported.8 This corresponds to the high cortisol levels seen in ob/ob and db/db mice.39,40 Observations on thyroid function in subjects with these mutations were again conflicting.13,41 Notably, in this large study, serum TSH, T4, and T3 levels were within the healthy range in all the three mutant groups, thus excluding thyroid abnormalities in children in this study. However, here no attempt was made to estimate free T4 levels.
Conclusions
In summary, comparative data from this retrospective cross-sectional study indicate a distinctly higher level of morbidity in children with LEP or LEPR deficiency compared with those with homozygous loss-of-function mutations in the MC4R gene. Current or novel medications against monogenic forms of obesity, though available in many developed countries, are unfortunately lacking in Pakistan—a country with the world’s highest recorded prevalence of LEP-signaling deficiency. The treatments include hormone replacement therapy with recombinant leptin for subjects with LEP deficiency, the MC4R agonist setmelanotide for LEPR deficiency,42 and glucagon-like peptide-1 receptor agonists for subjects with MC4R deficiency.43,44 The fact that a sizable population of children are failing to achieve normal educational development and are becoming seriously ill and dying prematurely as the result of a deficiency in hormonal signaling for which relatively simple peptide treatments are readily available highlights serious flaws in the global system through which drugs are developed and made available to those who most need them.
Limitations of the study
This investigation has certain limitations. It is necessarily a cross-sectional study and lacks the advantage of an organized follow-up regimen. A regular follow-up was not possible because of logistic problems, as a sizable proportion of affected families, especially those residing in rural and remote areas of the province/country, did not respond to a follow-up call or could not be contacted the second time.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Critical commercial assays | ||
| Leptin | Monobind | Cat# 10825-300A |
| Cortisol | Monobind | Cat# 3625-300A |
| Insulin | Monobind | Cat# 2425-300A |
| Thyroid-stimulating hormone (TSH) | Monobind | Cat# 6025-300A |
| 8-Hydroxy-2′-deoxyguanosine (8-OHdG) | Elabsciences | Cat# E-EL-0028 |
| Glucose | Certeza Medical | Cat# GL-110 |
| HbA1c | Biohermes HbA1c | Cat# HbA1c EZ 2.0 |
| Software and algorithms | ||
| SPSS 25 | IBM | https://www.ibm.com/products/spss-statistics |
| R 4.0.2 | The R Foundation | https://cran.r-project.org/bin/windows/base/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to the lead contact, Dr. Sadia Saeed (s.saeed08@imperial.ac.uk)
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
Human subjects
This retrospective cohort study is based on 145 cases (132 probands and 13 siblings) of monogenic obesity due to homozygous loss-of-function mutations in the LEP, LEPR and MC4R genes (Table S1). These mutations were identified through genetic screening of 454 unrelated children with severe early-onset obesity recruited to the SOPP [Severe obesity in Pakistani Population] study, from consanguineous population of Pakistan. The inclusion criteria were based on a body mass index standard deviation score (BMI SDS) of ≥3.5 and age of obesity onset ≤5 years. Where possible, other affected family members were also included in the study. In addition, a group of age-matched children negative for these mutations and of normal body weight have been included as lean controls.
Ethics statement
The study was approved by the relevant institutional ethical committees, and all participants or their parents/guardians provided written informed consent. The study was conducted according to the principles outlined in the Declaration of Helsinki. A detailed interview with the patient and/or guardian, was carried out and medical history was recorded at the time of first recruitment as well as at the time of any follow-up examination. Anthropomorphic measurements were carried out and blood samples obtained (between 10 a.m.–12 noon), for subsequent genetic and hormonal analysis.
Method details
Genetic analysis
Initially, all the 454 probands with severe obesity were genetically screened for mutations in LEP and MC4R genes through Sanger sequencing. DNA of the probands found negative for the mutations in these two genes, was further analyzed through conventional or augmented whole exome sequencing (WES). The pathogenicity of the mutations was determined by following the American College of Medical Genetics and Genomics (ACMG) criteria. The screening methods have been described in detail elsewhere.34,45 Sixty-three of these affected children (56 probands and 7 siblings) were also clinically screened at a more advanced age but at variable intervals. Necessarily, we have included data obtained from these patients at their subsequent visit (or follow-up) as independent values in our age-related cross-sectional observations.
Biochemical determinations
Metabolic and oxidative stress biomarkers were measured in serum. Leptin, insulin, cortisol, thyroid-stimulating hormone (TSH), and 8-hydroxy-2′-deoxyguanosine (8-OHdG), were determined using commercially available ELISA kits (leptin, insulin, cortisol, TSH: Monobind, Lake Forest, USA; 8OHdG: Elabscience, Houston, USA). Serum levels of malondialdehyde (MDA) and glutathione (GSH) were determined spectrophotometrically. Samples were analyzed in duplicate. The intra- and inter-assay variations were less than 11% for each assay. Random blood glucose and glycated hemoglobin A1c (HbA1c) were determined at presentation.
Quantification and statistical analyses
Statistical analysis
Comparisons between the traits were made using Scheffe’s test. For association analysis between trait and study groups, we applied a linear regression model on different traits to assess the effect of the LEP, LEPR and MC4R mutant groups compared to the lean control group adjusted by gender and age. p-values <0.05 were considered statistically significant. Since the number of children in the three mutant groups, was not equally distributed across age (or in relation to age), the present data have also been analyzed in three consecutive windows of 5 years for comparative purposes. The results were analyzed using the SPSS data analysis program.
The families of the three mutant groups as well as obese children with yet undiagnosed causes were approached for reporting any deaths among the affected individuals. Survival in the three mutant groups and obese controls was estimated in cases of death, and at the age of the last reported visit or contact by phone. Survival curves were estimated and plotted among the three mutant groups and obese controls, using the Kaplan-Meier method with R.
Acknowledgments
We thank the patients and their families for their participation in the study. We thank Frédéric Allegaert, Timothée Beke, and Stefan Gaget for technical assistance. This work was supported by funding from the Medical Research Council (MRC) MR/S026193/1 (P.F.) and the Pakistan Academy of Sciences (M.A.). S.S. is supported by a project grant from the Medical Research Council (MR/S026193/1). Further support was provided by the National Center for Precision Diabetic Medicine – PreciDIAB, which is jointly supported by the French National Agency for Research (ANR-18-IBHU-0001), the European Union (FEDER), the Hauts-de-France Regional Council, and the European Metropolis of Lille (MEL).
Author contributions
S.S., S.O’R., M.A., and P.F. conceptualized the study. S.S., M.A., and P.F. wrote the first draft of the manuscript. J.M., I.S.F., G.S.H.Y., S.O’R., and A.B. contributed to amending the first draft. S.S., R.K., Q.M.J., and M.A. collected samples and performed biochemical analysis. S.S., A.B., M.A., and P.F. performed analysis of the genetic data. S.S., R.K., Q.M.J., L.N., S.H., M.C., R.K., A.B., and M.A. performed statistical analysis. J.M., M.A., H.A., W.I.K., and T.A.B. identified and recruited families with obesity. S.O’R., S.S., M.A., and P.F. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the revision process of the manuscript and approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: September 1, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.101187.
Contributor Information
Sadia Saeed, Email: s.saeed08@imperial.ac.uk.
Muhammad Arslan, Email: muhammadarslan@fccollege.edu.pk.
Philippe Froguel, Email: p.froguel@imperial.ac.uk.
Supplemental information
Data and code availability
All data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon reasonable request.
References
- 1.Montague C.T., Farooqi I.S., Whitehead J.P., Soos M.A., Rau H., Wareham N.J., Sewter C.P., Digby J.E., Mohammed S.N., Hurst J.A., et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 2.Clément K., Vaisse C., Lahlou N., Cabrol S., Pelloux V., Cassuto D., Gourmelen M., Dina C., Chambaz J., Lacorte J.-M., et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 3.Saeed S., Arslan M., Froguel P. Genetics of obesity in consanguineous populations: toward precision medicine and the discovery of novel obesity genes. Obesity. 2018;26:474–484. doi: 10.1002/oby.22064. [DOI] [PubMed] [Google Scholar]
- 4.Stenson P.D., Ball E.V., Mort M., Phillips A.D., Shiel J.A., Thomas N.S.T., Abeysinghe S., Krawczak M., Cooper D.N. Human gene mutation database (HGMD®): 2003 update. Hum. Mutat. 2003;21:577–581. doi: 10.1002/humu.10212. [DOI] [PubMed] [Google Scholar]
- 5.Loos R.J.F., Yeo G.S.H. The genetics of obesity: from discovery to biology. Nat. Rev. Genet. 2022;23:120–133. doi: 10.1038/s41576-021-00414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farooqi I.S., O'Rahilly S. 20 years of leptin: human disorders of leptin action. J. Endocrinol. 2014;223:T63–T70. doi: 10.1530/JOE-14-0480. [DOI] [PubMed] [Google Scholar]
- 7.Ozata M., Ozdemir I.C., Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J. Clin. Endocrinol. Metab. 1999;84:3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 8.Saeed S., Bonnefond A., Manzoor J., Shabbir F., Ayesha H., Philippe J., Durand E., Crouch H., Sand O., Ali M., et al. Genetic variants in LEP, LEPR, and MC4R explain 30% of severe obesity in children from a consanguineous population. Obesity. 2015;23:1687–1695. doi: 10.1002/oby.21142. [DOI] [PubMed] [Google Scholar]
- 9.Saeed S., Butt T.A., Anwer M., Arslan M., Froguel P. High prevalence of leptin and melanocortin-4 receptor gene mutations in children with severe obesity from Pakistani consanguineous families. Mol. Genet. Metabol. 2012;106:121–126. doi: 10.1016/j.ymgme.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Saeed S., Bonnefond A., Manzoor J., Philippe J., Durand E., Arshad M., Sand O., Butt T.A., Falchi M., Arslan M., Froguel P. Novel LEPR mutations in obese Pakistani children identified by PCR-based enrichment and next generation sequencing. Obesity. 2014;22:1112–1117. doi: 10.1002/oby.20667. [DOI] [PubMed] [Google Scholar]
- 11.York D.A., Otto W., Taylor T.G. Thyroid status of obese (ob/ob) mice and its relationship to adipose tissue metabolism. Comp. Biochem. Physiol. B. 1978;59:59–65. doi: 10.1016/0305-0491(78)90271-7. [DOI] [PubMed] [Google Scholar]
- 12.Batt R.A., Everard D.M., Gillies G., Wilkinson M., Wilson C.A., Yeo T.A. Investigation into the hypogonadism of the obese mouse (genotype ob/ob) Reproduction. 1982;64:363–371. doi: 10.1530/jrf.0.0640363. [DOI] [PubMed] [Google Scholar]
- 13.Farooqi I.S., Matarese G., Lord G.M., Keogh J.M., Lawrence E., Agwu C., Sanna V., Jebb S.A., Perna F., Fontana S., et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tschöp J., Nogueiras R., Haas-Lockie S., Kasten K.R., Castañeda T.R., Huber N., Guanciale K., Perez-Tilve D., Habegger K., Ottaway N., et al. CNS leptin action modulates immune response and survival in sepsis. J. Neurosci. 2010;30:6036–6047. doi: 10.1523/JNEUROSCI.4875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alti D., Sambamurthy C., Kalangi S.K. Emergence of leptin in infection and immunity: scope and challenges in vaccines formulation. Front. Cell. Infect. Microbiol. 2018;8:147. doi: 10.3389/fcimb.2018.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saeed S., Arslan M., Manzoor J., Din S.M., Janjua Q.M., Ayesha H., Ain Q.-t., Inam L., Lobbens S., Vaillant E., et al. Genetic causes of severe childhood obesity: a remarkably high prevalence in an inbred population of Pakistan. Diabetes. 2020;69:1424–1438. doi: 10.2337/db19-1238. [DOI] [PubMed] [Google Scholar]
- 17.Strobel A., Issad T., Camoin L., Ozata M., Strosberg A.D. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat. Genet. 1998;18:213–215. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- 18.Mazen I., El-Gammal M., Abdel-Hamid M., Amr K. A novel homozygous missense mutation of the leptin gene (N103K) in an obese Egyptian patient. Mol. Genet. Metabol. 2009;97:305–308. doi: 10.1016/j.ymgme.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Fatima W., Shahid A., Imran M., Manzoor J., Hasnain S., Rana S., Mahmood S. Leptin deficiency and leptin gene mutations in obese children from Pakistan. Int. J. Pediatr. Obes. 2011;6:419–427. doi: 10.3109/17477166.2011.608431. [DOI] [PubMed] [Google Scholar]
- 20.Bhatt A., Purani C., Bhargava P., Patel K., Agarbattiwala T., Puvar A., Shah K., Joshi C.G., Dhamecha N., Prabhakar M., Joshi M. Whole exome sequencing reveals novel LEPR frameshift mutation in severely obese children from Western India. Mol. Genet. Genomic Med. 2019;7:e00692. doi: 10.1002/mgg3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marti A., Corbalán M.S., Forga L., Martinez J.A., Hinney A., Hebebrand J. A novel nonsense mutation in the melanocortin-4 receptor associated with obesity in a Spanish population. Int. J. Obes. Relat. Metab. Disord. 2003;27:385–388. doi: 10.1038/sj.ijo.0802244. [DOI] [PubMed] [Google Scholar]
- 22.Tan K., Pogozheva I.D., Yeo G.S.H., Hadaschik D., Keogh J.M., Haskell-Leuvano C., O'Rahilly S., Mosberg H.I., Farooqi I.S. Functional characterization and structural modeling of obesity associated mutations in the melanocortin 4 receptor. Endocrinology. 2009;150:114–125. doi: 10.1210/en.2008-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinney A., Schmidt A., Nottebom K., Heibült O., Becker I., Ziegler A., Gerber G., Sina M., Görg T., Mayer H., et al. Several mutations in the melanocortin-4 receptor gene including a nonsense and a frameshift mutation associated with dominantly inherited obesity in humans. J. Clin. Endocrinol. Metab. 1999;84:1483–1486. doi: 10.1210/jcem.84.4.5728. [DOI] [PubMed] [Google Scholar]
- 24.Farooqi I.S., Keogh J.M., Yeo G.S.H., Lank E.J., Cheetham T., O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 25.Yeo G.S.H., Lank E.J., Farooqi I.S., Keogh J., Challis B.G., O'Rahilly S. Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum. Mol. Genet. 2003;12:561–574. doi: 10.1093/hmg/ddg057. [DOI] [PubMed] [Google Scholar]
- 26.Fischer-Posovszky P., Funcke J.-B., Wabitsch M., Kuhnle-Krahl U., Lahr G., Debatin K.-M., Vatter P., Gierschik P., Moepps B., Fischer-Posovszky P. Biologically inactive leptin and early-onset extreme obesity. N. Engl. J. Med. 2015;372:1266–1267. doi: 10.1056/NEJMoa1406653. [DOI] [PubMed] [Google Scholar]
- 27.Farooqi I.S., O'rahilly S. Mutations in ligands and receptors of the leptin–melanocortin pathway that lead to obesity. Nat. Clin. Pract. Endocrinol. Metabol. 2008;4:569–577. doi: 10.1038/ncpendmet0966. [DOI] [PubMed] [Google Scholar]
- 28.GBD 2019 Pakistan Collaborators. Dangel W.J., Ostroff S.M., Kiani A.G., Glenn S.D., Abbas J., Afzal M.S., Afzal S., Ahmad S., Ahmed A. The state of health in Pakistan and its provinces and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Global Health. 2023;11:e229–e243. doi: 10.1016/S2214-109X(22)00497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farooqi I.S., Wangensteen T., Collins S., Kimber W., Matarese G., Keogh J.M., Lank E., Bottomley B., Lopez-Fernandez J., Ferraz-Amaro I., et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N. Engl. J. Med. 2007;356:237–247. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lord G.M., Matarese G., Howard J.K., Baker R.J., Bloom S.R., Lechler R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 31.Saucillo D.C., Gerriets V.A., Sheng J., Rathmell J.C., MacIver N.J. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J. Immunol. 2014;192:136–144. doi: 10.4049/jimmunol.1301158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vincent H.K., Taylor A.G. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int. J. Obes. 2006;30:400–418. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 33.Marseglia L., Manti S., D’Angelo G., Nicotera A., Parisi E., Di Rosa G., Gitto E., Arrigo T. Oxidative stress in obesity: a critical component in human diseases. Int. J. Mol. Sci. 2014;16:378–400. doi: 10.3390/ijms16010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saeed S., Janjua Q.M., Haseeb A., Khanam R., Durand E., Vaillant E., Ning L., Badreddine A., Berberian L., Boissel M., et al. Rare Variant Analysis of Obesity-Associated Genes in Young Adults With Severe Obesity From a Consanguineous Population of Pakistan. Diabetes. 2022;71:694–705. doi: 10.2337/db21-0373. [DOI] [PubMed] [Google Scholar]
- 35.Campfield L.A., Smith F.J., Guisez Y., Devos R., Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 36.Mazen I., El-Gammal M., Abdel-Hamid M., Farooqi I.S., Amr K. Homozygosity for a novel missense mutation in the leptin receptor gene (P316T) in two Egyptian cousins with severe early onset obesity. Mol. Genet. Metabol. 2011;102:461–464. doi: 10.1016/j.ymgme.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Andiran N., Çelik N., Andiran F. 2011. Homozygosity for two missense mutations in the leptin receptor gene (P316T; W646C) in a Turkmenian girl with severe early-onset obesity. [PubMed] [Google Scholar]
- 38.Gill R., Cheung Y.H., Shen Y., Lanzano P., Mirza N.M., Ten S., Maclaren N.K., Motaghedi R., Han J.C., Yanovski J.A., et al. Whole-exome sequencing identifies novel LEPR mutations in individuals with severe early onset obesity. Obesity. 2014;22:576–584. doi: 10.1002/oby.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahima R.S., Prabakaran D., Mantzoros C., Qu D., Lowell B., Maratos-Flier E., Flier J.S. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 40.O'Rahilly S. Life without leptin. Nature. 1998;392:330–331. doi: 10.1038/32769. [DOI] [PubMed] [Google Scholar]
- 41.Paz-Filho G., Delibasi T., Erol H.K., Wong M.-L., Licinio J. Congenital leptin deficiency and thyroid function. Thyroid Res. 2009;2:11–14. doi: 10.1186/1756-6614-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clément K., van den Akker E., Argente J., Bahm A., Chung W.K., Connors H., De Waele K., Farooqi I.S., Gonneau-Lejeune J., Gordon G., et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020;8:960–970. doi: 10.1016/S2213-8587(20)30364-8. [DOI] [PubMed] [Google Scholar]
- 43.Iepsen E.W., Have C.T., Veedfald S., Madsbad S., Holst J.J., Grarup N., Pedersen O., Brandslund I., Holm J.-C., Hansen T., Torekov S.S. GLP-1 receptor agonist treatment in morbid obesity and type 2 diabetes due to pathogenic homozygous melanocortin-4 receptor mutation: a case report. Cell Rep. Med. 2020;1:100006. doi: 10.1016/j.xcrm.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iepsen E.W., Zhang J., Thomsen H.S., Hansen E.L., Hollensted M., Madsbad S., Hansen T., Holst J.J., Holm J.-C., Torekov S.S. Patients with obesity caused by melanocortin-4 receptor mutations can be treated with a glucagon-like peptide-1 receptor agonist. Cell Metabol. 2018;28:23–32.e3. doi: 10.1016/j.cmet.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Montagne L., Derhourhi M., Piton A., Toussaint B., Durand E., Vaillant E., Thuillier D., Gaget S., De Graeve F., Rabearivelo I., et al. CoDE-seq, an augmented whole-exome sequencing, enables the accurate detection of CNVs and mutations in Mendelian obesity and intellectual disability. Mol. Metabol. 2018;13:1–9. doi: 10.1016/j.molmet.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon reasonable request.