Highlights
-
•
The flaviviruses encoded NS1 protein is a multifunctional secreted glycoprotein.
-
•
NS1 crystal structure has been determined and unraveled broader and deeper functions.
-
•
NS1 is involved in the escaping of host signal immune pathway mediated by PRRs.
-
•
The flavivirus NS1 can directly induce specific tissues damage in the infected host.
Keywords: Flavivirus, Nonstructural protein 1, Crystal structure, Innate immune evasion, Tissue-specific damage
Abstract
Flaviviruses include medically important mosquito-borne pathogens, such as Zika virus (ZIKV), Japanese encephalitis virus (JEV), dengue virus (DENV) and West Nile virus (WNV), that cause hundreds of millions of infections each year. Currently, there are no approved effect therapies against mosquito-borne flaviviruses. The flaviviruses encoded nonstructural protein 1 (NS1) is a secreted glycoprotein widely involved in viral replication, immune evasion, and directly causing tissue-specific damage during flaviviruses infection. Upon viral infection of host cell, NS1 can be found in multiple oligomeric forms and include a dimer on the cell surface, and a soluble secreted hexameric lipoparticle. In the recent decade, the detailed crystal structure of several flaviviruses NS1 have been determined and unraveled its broader and deeper functions. Consistent with the potential immune function revealed by its structure, NS1 is involved in the escaping of host signal immune pathway mediated by pattern recognition receptors (PRRs), including RIG-I-like receptors (RLRS) and Toll-like receptors (TLRs). Moreover, the flavivirus NS1 is efficiently secreted by infected cells and circulates in the blood of the host to directly induce specific tissues damage. The NS1 of ZIKV, JEV and WNV changes the permeability of brain microvascular endothelial cell to cause endothelial cell dysfunction and promote virus pathogenesis. DENV NS1 can induce systemic tissues damage in humans through multiple strategies. Mutations of several key amino acids in NS1 can reduce the neurovirulence of the flavivirus. In this article, we provide an overview of the latest research on this fascinating protein in these disparate areas.
1. Introduction
The flavivirus genus contains enveloped, positive-sense RNA viruses that are transmitted by mosquito and tick vectors (Calisher and Gould, 2003). Flaviviruses comprises many important human pathogens, including Zika virus (ZIKV), Japanese encephalitis virus (JEV), dengue virus (DENV), West Nile virus (WNV), yellow fever virus (YFV), and tick-borne encephalitis virus (TBEV) (Kuno et al., 1998; Suthar et al., 2013; Wilder-Smith et al., 2017). It is estimated that nearly half of the world's population is at risk of being infected with these arboviruses (Alcala et al., 2018). In humans, flaviviruses cause systemic or neurotropic-infection and lead to a series of outcomes, from asymptomatic infections to severe, even fatal encephalitis. Flaviviruses infection causes clinical symptoms mainly characterized by viral encephalitis (JEV and WNV), hemorrhagic and vascularleakage manifestations (DENV and YFV), and congenital microcephaly and Guillain-Barre´ syndrome associated with ZIKV infection (Heymann et al., 2016; Kuno et al., 1998).
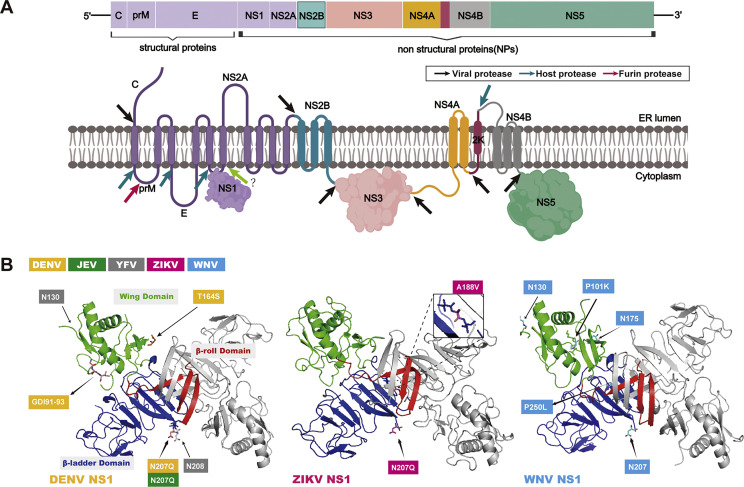
The flavivirus genome comprises approximately 11 kb nucleotides and the viral genomic RNA consists of a single open reading frame (ORF) that encodes three structural proteins (capsid, membrane, and envelope) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) (Suthar et al., 2013). The single ORF is translated to a polyprotein and then cleaved by viral and host proteases (Fig. 1). The structural proteins of flaviviruses play crucial roles in virus entry, fusion, and assembly. The nonstructural proteins are the main components of viral replication complexes (RC), and play an essential role in viral RNA replication and the evasion of the host immune response (Xia et al., 2018). A large number of studies have showed that flaviviruses encoded NS1, NS4B, and NS5 play a crucial role in inhibiting the expression of interferon (IFN) and other antiviral cytokines (Dalrymple et al., 2015; Shimojima et al., 2014; Wu et al., 2017).
Fig. 1.
Model of flavivirus genome and encoded NS1 three-dimensional structure. (A) The genome and encoded proteins of flaviviruses. (B) NS1 dimer: Represented by the 3D structure of the DENV NS1 dimer, one of the monomers is colored gray, and the other monomer is divided into three colors according to the domain: β-roll (red), wing (green), and β-ladder (blue). Those amino acid sites thought to be involved in innate immune evasion and induction of tissue-specific damage are annotated with labels representing the colors of the different virus strains. Structure factor files have been deposited in the RCSB Protein Data Bank (PDB) database under the accession codes 4O6B for DENV-2 NS1, 5GS6 for ZIKV NS1, and 4O6C for WNV NS1. Molecular graphics were performed using the PyMOL Molecular Graphics system.
Flavivirus NS1 is perhaps the most important protein among all the encoded proteins and this fascinating protein has reported more than 50 years. It was first recognized as a viral antigen in 1970 in the serum and brain of DENV infected mice (Brandt et al., 1970). Current knowledge reveals that NS1 plays numerous roles in viral replication and disease. NS1 is highly immunogenic and both the protein and the elicited antibodies have been showed the paradoxical roles of protection and pathogenesis in infected hosts (Muller and Young, 2013). In recent years, accompanied by the report of the detailed crystal structure of flavivirus NS1 protein, the broader and deeper protein functions have been unraveled. This review aims to summary the research progress of NS1, especially its structure, and its role in evading host innate immune and directly causing tissue-specific damage.
2. Flavivirus NS1: a multifunctional protein
NS1 is a unique non-structural protein, with a molecular weight between 48 and 55kD according to the degree of glycosylation, and is a conserved glycoprotein in flaviviruses. As a multifunctional glycoprotein, NS1 can be divided into three forms: (1) a monomer, (2) a dimer (membrane-bound protein, mNS1), and (3) a hexamer (secreted protein, sNS1). NS1 protein is found both in and out of cells, and its glycosylation status can directly affect viral replication, virulence intensity and immune escape (Somnuke et al., 2011). The dimer NS1, which is required for viral RNA synthesis, is formed in the virus-induced vesicle envelope and binds to the luminal side of the ER membrane. It is generally believed that in the early stage of infection, intracellular NS1 is translated into a dimer structure in the ER cavity and participates in RNA replication, and then secreted to the extracellular in the form of hexamer lipoprotein particles.
NS1′ is a unique protein derived from NS1, which has been found in the extracellular environment of JEV, WNV, and DENV infections (Firth and Atkins, 2009; Melian et al., 2010). It is generally believed that NS1’ is produced by programmed ribosomal frameshiftthat causes 52 amino acids added to the C-terminus of NS1. NS1′ is commonly associated with flavivirus replication complexes and plays a role in viral neuroinvasiveness (Melian et al., 2010; Xie et al., 2022; Young et al., 2013). In addition, multiple reports have showed that JEV NS1′ is involved in regulating the host antiviral response (Li et al., 2021).
2.1. Structure of NS1 and its corresponding function
Understanding the structure of NS1 can help to analyze its function and provide a basis for the development the vaccine of NS1. NS1 three-dimensional structure and an overview of the marked key functional sites are shown in Fig. 1. Akey et al. (2014) have produced full-length, glycosylated NS1 of WNV and DENV-2 using a baculovirus expression system and successfully resolved the dimer and hexamer structures of NS1. The resolved crystal structure of flavivirus NS1 showed that the NS1 monomer contained three domains: β-roll (amino acid 1-29), wing (amino acid 30-180) and central β-ladder (amino acid 181-352). "β-roll" domain is the smallest of the three domains. The "wing" domain contains two glycosylation sites (Asn130, Asn175), an internal disulfide bond (Cys55-Cys143) and two discrete subdomains. It is connected to the "β-roll" by a discontinuous subdomain and also to the central "β-ladder" domain by a disulfide bond, which like a wing projecting from the central β-ladder domain. The "β-ladder" is the main structural domain of NS1, and each monomer has nine β-chains arranged like ladders (Akey et al., 2014). Recent report found that ZIKV NS1 crystal structure was similar to the NS1 structure of other flaviviruses, including DENV and WNV (Xu et al., 2016). In addition, it has been found that a dipeptide (Arg10-Gln11) of WNV NS1 that interacts with the transmembrane protein NS4B is localized in a loop of the β-roll (Youn et al., 2012). This suggests that hydrophobic protrusions are strong candidates for the interaction of dimer NS1 with the ER membrane and with the replication complex via the transmembrane protein NS4A/B (Lindenbach and Rice, 1999).
Secreted NS1 (sNS1) is a soluble hexamer proteolipid particle. Three NS1 dimers are juxtaposed on the lipid bilayer, host lipids are breaked and trapped within the central channel of the hexamer, and eventually lipoprotein particles are released outside the cell via the secretory pathway (Gutsche et al., 2011). Its crystal structure consists of three β-roll facing the interior of the hexamer, with a central lipid-rich core linked together by weak hydrophobic interactions, while the outer surface contains “spaghetti loop”, the glycosylation site, and the “wing” domain disordered loop. By mapping the linear epitope of the NS1 protein onto the hexamer structure of the NS1 protein (Vita et al., 2010), it can be found that the frequently recognized epitopes are mainly concentrated in the easily accessible “wing” domain disordered loop, the C-terminal top of “β-ladder”, and the hydrophobic protrusion region (Akey et al., 2014). These together indicate that NS1 can be recognized by the host immune system before or after hexamer formation.
Although flaviviruses NS1 is highly similar in structure, different flaviviruses NS1 exhibit functional differences, and variations in NS1 and host protein interactions among viruses may lead to differences in pathogenesis. It has been proposed that there is a strong association between the peptide motif centered at position 10 of the NS1 protein encoded by flaviviruses and the localization of NS1 to the plasma membrane surface or secretion of NS1 in the cell supernatant. This peptide motif of WNV NS1 facilitates the interaction with the endoplasmic reticulum membrane, thereby facilitating the retention of NS1 at the plasma membrane. In contrast, the DENV NS1 peptide motif at this corresponding position weakly associates with this moiety, thus facilitating secretion into the cell supernatant (Youn et al., 2010), which further explains the higher levels of sNS1 in DENV infected individuals. A recent report compared the characteristics of the C-terminal β-ladder domain of NS1 among flaviviruses, suggesting that NS1-C of JEV was similar to WNV and ZIKV, but different from DENV (Poonsiri et al., 2018a). JEV NS1-C does not bind to liposomes, and the C terminus is not responsible for binding to the cell membrane via glycosaminoglycans (GAGs) (Poonsiri et al., 2018b).
2.2. NS1 involved in complement system
NS1 can activate the complement system. The complement system is an important component of the innate immune system and a bridge connecting the innate and adaptive immune responses. Both DENV soluble and membrane-associated forms NS1 can activate the complement system. This leads to the deposition of C5b-C9 membrane attack complex on the cell surface, which increases the expression and secretion of vasoactive cytokines, resulting in increased vascular permeability leading to more severe clinical symptoms (Avirutnan et al., 2006). The C4 protein promotes inflammatory responses, and sNS1 has been reported to bind C4 and C1s, prompting the cleavage of C4 to C4b to stimulate the canonical/lectin pathway (Fuchs et al., 2010).
NS1 can also be involved in complement escape mechanisms. C1q-NS1 binding prevents assembly of antibody-related C1q complexes, thereby helping to prevent activation of the classical pathway of complement, this interaction way plays a role in ADE in DENV infection (Mehlhop et al., 2007). The NS1 of WNV regulates C3b cleavage and inactivation of the alternative pathway C3 convertase by interacting with the negative regulator factor H, thereby exerting immune evasion and resisting complement activation (Zipfel et al., 2002). These results suggest that secreted NS1 and membrane surface NS1 can inhibit the activation of complement and reduce the immune response to virus.
3. Evasion of host innate immune by NS1
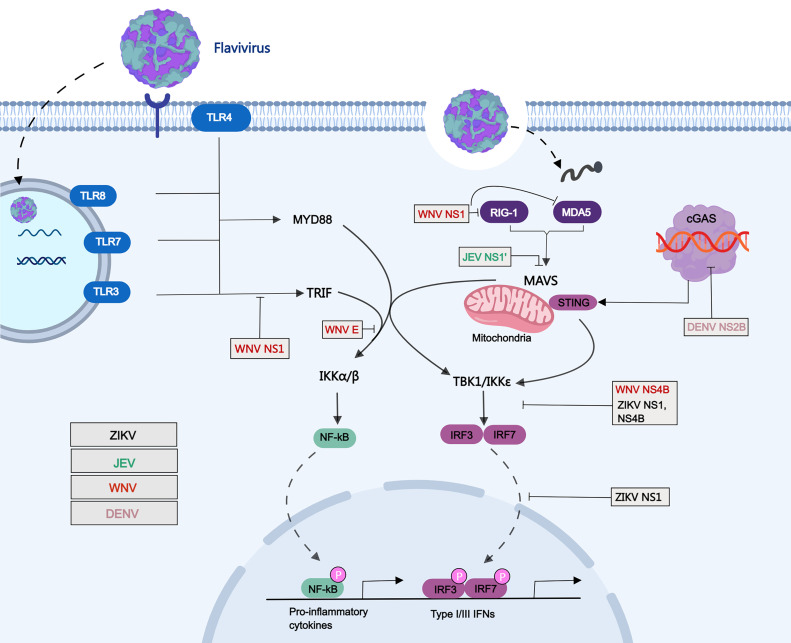
The innate immune system is the first line of defense against the invasion of pathogens and plays an essential role in the stability of the environment in humans and animals. Flaviviruses can be recognized by host pattern recognition receptors (PRRs), which leads to the activation of type I interferon (IFN) immune signaling pathways (Nazmi et al., 2014). The innate and adaptive immune responses of the body can develop corresponding strategies against flavivirus invasion. During flavivirus infection, PRRs recognize viral components, known as pathogen-associated molecular patterns (PAMPS), and then trigger the antiviral responses by producing type I IFNs (Zhang et al., 2017). Currently, there are three main types of PRRS, namely Toll-like receptors (TLRs), RIG-I-like receptors (RLRS), and NOD-like receptors. However, to antagonize the antiviral response to IFN, many viruses have evolved different strategies to combat IFN production. It has been shown that some non-structural proteins, especially NS1 of flaviviruses can antagonize the antiviral response induced by the TLRs or RLRS pathways, thereby helping flaviviruses evade the host immune response (Fig. 2).
Fig. 2.
Schematic of evasion of the host immune response by flavivirus NS1. Schematic representation of the mechanisms utilized by flaviviruses NS1 to inhibit the immune response. Viral components of flaviviruses were sensed via RIG-I and TLR3/4/7/8. TLR3 and TLR4/7/8 recruit TRIF and MyD88 adaptor respectively, while RIG-I interacts with MAVS adaptor. These adaptors were then activate downstream TBK1 and IKKε kinases, which then leads to phosphorylation of IRF3. The phosphorylated IRF3 translocates to the nucleus, thus inducing the production of type I IFNs. NS1 of ZIKV inhibits IFN-β production by preventing TBK1 phosphorylation, and promotes the clevage of cGAS by inhibiting the proteasomal degradation of caspase-1. JEV NS1′ inhibits the production of IFN-β by reducing MAVS expression. NS1 of WNV interact with RIG-I and MDA5 receptors and degrade their expression to inhibit IFN-β production, and simultaneously to inhibit TLR3-mediated IFN-β expression. DENV NS2B directly target cGAS and causing its degradation.
3.1. TLRs pathways evasion
The TLRs are important for recognizing virus infection and play a crucial role in the initiation of innate immunity. TLRs are comprised of leucine-rich repeats (LRRs), a transmembrane domain, and a cytoplasmic domain designated the Toll/IL-1 receptor homology (TIR) domain (Takeuchi and Akira, 2009). So far, at least 13 TLRs have been identified, of which TLR1 to TLR9 are shared by humans and mice, TLR10 functions only in humans, and TLR11 to TLR13 are specific to mice. The PAMPs recognized by TLRs are different due to their localization characteristics and structure.
TLRs act as intermediates that interact with viral replication products and transmit signals to a range of adapters and kinases that ultimately lead to transcriptional activation of cytokines and type I interferon genes. TLR pathway plays a crucial role in the process of sensing and clearing pathogens, and is divided into two pathways according to whether it contains myeloid differentiation factor 88 (MyD88) as previous reported (Kawai and Akira, 2010). TLR3 recruits TRIF proteins, which transmit signals to downstream molecules that in turn induce type I interferon production (Han et al., 2014).
Previous studies have shown that TLRS play an essential role in the detection and response to flavivirus infection. TLR3(-/-) mice are highly susceptible to JEV and exhibit severe neuroinflammatory responses as well as increased blood-brain barrier (BBB) permeability, while TLR4(-/-) mice have significantly increased immunity to JEV and effectively antagonize virus replication and inflammation by inducing innate immune responses to JEV (Han et al., 2014). Similarly, TLR3 plays a role in promoting host immune responses during DENV infection in humans and animals (Guo et al., 2018). However, TLR3 may mediate WNV invading brain tissue causing lethal encephalitis and promote the replication of WNV in the brain (Wang et al., 2004). A number of studies have showed that the nonstructural proteins, especially NS1 protein, of flaviviruses have engaged in host immune antagonism and pathogenesis after virus infection. Recent report showed that ZIKV NS1, NS2B-NS3 and NS4B proteins antagonize cellular antiviral immune responses in a variety of ways (Wu et al., 2017).
The NS1 protein of WNV, including the secreted form, blocks the nuclear translocation of IRF3 via TLR3 signaling, thereby inhibiting TLR3-mediated IFN-β and cytokine production (Crook et al., 2014). Secreted NS1 can significantly inhibit WNV replicon mediated cytokine transcription via TLR3-mediated signaling. In addition, immunizing TLR3-deficient mice with NS1 protein partially reduces the invasion of brain inflammatory cells induced by WNV infection, but the specific molecular mechanism remains unclear (Patel et al., 2019). A previous report showed that WNV NS1 inhibits TLR3-induced transcriptional activation of the IFN-β and NF-κB promoters (Wilson et al., 2008). Controversially, it also has been reported that NS1, encoded by WNV and DENV-2, does not inhibit TLR3-induced IFN-β production (Baronti et al., 2010). NS1 is an important protein that activates TLR2 and TLR6 in response to DENV infection, which can exacerbate the production of proinflammatory cytokines. The production of proinflammatory cytokines during DENV infection may lead to increased vascular permeability and leakage, which also explains the underlying mechanisms leading to more severe manifestations of DENV infection (Chen et al., 2015).
3.2. RLRs pathways evasion
RLRs receptors include RIG-I, MDA5 and laboratory of genetics and physiology 2 (LGP2). RIG-I and MDA5 are viral sensors in the host's innate immune system, which can recognize RNA molecules of viruses invading cells and trigger immune responses. RIG-I or MDA5 then interacts with mitochondrial antiviral signal proteins (MAVS; also known as VISA, IPS1 or Cardif) to activate downstream inhibitors of κB kinase ε (IKKε), which then leads to phosphorylation of key transcription factor IRF3. The phosphorylated IRF3 translocates to the nucleus, thus inducing the production of type I IFN and the inflammatory reaction (Takeuchi and Akira, 2009). Flavivirus can inhibit the production of interferon through RLRs pathway, such as ZIKV, JEV and WNV (Wu et al., 2017; Zhang et al., 2017; Zhou et al., 2020). According to previous studies, the encoded non structural proteins in these viruses can inhibit RLRs-induced type I IFN production through different strategies (Wu et al., 2017; Xia et al., 2018; Zhang et al., 2017).
Previous studies have proved that RIG-I is the main sensor for the induction of type I IFN and interferon stimulated genes (ISGs) by ZIKV infection in A549 cells. Conversely, the deletion of RIG-I will promote virus replication (Schilling et al., 2020). Recent report showed that ZIKV NS1 and NS4B can inhibit the production of type I IFN by targeting TBK1 through involving RIG-I signaling pathways (Wu et al., 2017). Interestingly, a single A188V amino acid substitution of NS1 in ZIKV prevalent strain promotes virus escape from host interferon induction compare to pre-epidemic strains. This mutation enables ZIKV NS1 interacting with TBK1 and reduces the phosphorylation of TBK1, leading to inhibit IFN-β induction (Xia et al., 2018). In order to inhibit the sensing of DENV by mitochondrial DNA in host cells during virus infection, NS2B protein encoded by DENV directly target cGAS and causing its degradation (Aguirre et al., 2017).
Both of TLR3 and RIG-I signaling pathways play the essential roles in JEV induced microglial inflammation (Jiang et al., 2014). JEV can infect swine testicular (ST) cell and increase the expression of RIG-I and activates the downstream transcription factor NF-κB. Meanwhile, the inflammatory cytokines expression was obviously inhibited in JEV infected ST cells after knockout of RIG-I or treatment with the NF-κB specific inhibitor (Zheng et al., 2019). Previous studies have showed that JEV NS1′ can inhibit the production of IFN-β and ISGs by targeting MAVS and promote virus replication (Zhou et al., 2018, 2020). At the same time, it was found that NS1′ could up-regulate the expression of microRNA-22 (miR-22), which a key regulator for MAVS expression during JEV infection, by increasing the combination of CREB and c-Rel with the miR-22 promoter region (Zhou et al., 2020). In addition, JEV NS1′ can interact with host cyclin-dependent kinase 1 (CDK1) protein, interrupting CDK1 dephosphorylation, and lead to inhibit MAVS-mediated IFN-β production (Li et al., 2021).
RIG-I and MDA5 also play an essential role in inducing innate immune response in vivo and in vitro to control WNV infection. In addition, the susceptibility of mice lacking these two PRRs to WNV infection reproduces the MAVS phenotype (Errett et al., 2013). Similarly, DENV can promote the expression of RIG-I and MDA5, activate the phosphorylation of IRF3, and then induce IFN-β production (Urcuqui-Inchima et al., 2017). Our recent studies have shown that NS1 of WNV can inhibit IFN-β production by targeting RIG-I and MDA5. Mechanically, NS1 interact with RIG-I and MDA5 receptors and degrade their expression. In addition, WNV NS1 inhibits K63 linked ubiquitination of RIG-I, thus inhibiting the activation of downstream signal molecules in RLR signal pathway (Zhang et al., 2017).
In addition, ZIKV NS2A and NS4A proteins inhibited the RIG-I-mediated signaling pathway by inhibiting the activity of the NF-κB promoter, and both NS2A and NS4A down-regulated MDA5 in a dose-dependent manner (Lee et al., 2020a). At the same time, NS1 interacts with NS4A and NS4B in the ER lumen and plays an important role in the formation of replication complexes (Lindenbach and Rice, 1999). Based on the above analysis, we speculate that when the NS1 protein is involved in innate immunity to exert its function, other viral proteins may also be involved to help the virus complete immune escape.
4. Role of NS1 in inducing tissue-specific damage
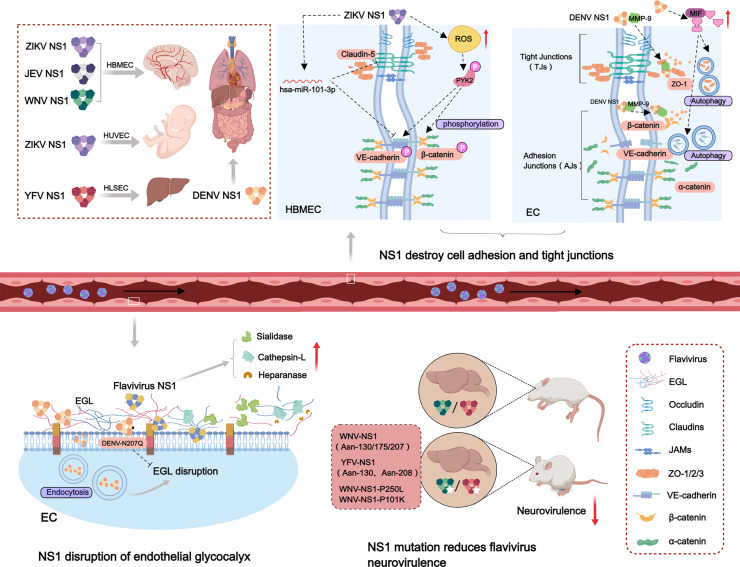
The flavivirus NS1 is efficiently secreted by infected cells and circulates in the blood of the host, and blood levels of NS1 have been found to correlate with disease severity in flaviviruses caused diseases, especially in dengue patients (Muller and Young, 2013). An overview of NS1 inducing tissue-specific damage is shown in Fig. 3. The NS1 of flaviviruses, including ZIKV, JEV, WNV, DENV and YFV, selectively changes the permeability of endothelial cells, incuding human brain microvascular endothelial cell (HBMEC), human umbilical vein endothelial cell (HUVEC) and human liver sinusoidal microvascular endothelial cell (HLSEC), leading to endothelial cell dysfunction and inducing vascular leakage to promote the pathogenesis of flaviviruses (Puerta-Guardo et al., 2019). ZIKV NS1 mainly induces hyperpermeability of umbilical vein and brain endothelial cells. In both JEV and WNV, which cause neurological damage in the brain, NS1 interacts only with glycocalyx on brain vascular endothelial cells. NS1 of DENV enhanced endothelial cells in the lung, vascular endothelium, brain, umbilical vein and liver permeability. These results suggest that the NS1 of flaviviruses influences virus tissue tropism and disease manifestation, suggesting a complex and important significance of NS1 in the mechanism of flavivirus induced pathogenesis.
Fig. 3.
Model of flavivirus NS1 to induce tissue-specific damage and its contribution to virus pathogenesis. Flavivirus infections can induce specific tissues damage, including brain, lung, liver, vascular endothelium, and the placenta during pregnancy, to cause neurotropic-encephalitic or systemic diseases. ZIKV NS1 causes hyperpermeability of HBMEC and HUVEC. NS1 of JEV and WNV mainly induces the permeability change of HBMEC. YFV NS1 mainly causes the dysfuntion of HLSEC. NS1 of DENV can induce systemic tissues damage in humans. ZIKV NS1 phosphorylate β-catenin and VE-cadherin via increasing ROS expression, and simultaneously to inhibit VE-cadherin and claudin-5 expression to destroy the adhesion junctions of HBMEC. DENV NS1 recruits MMP-9 to interact with β-catenin and ZO-1 to disrupt cells tight junctions (TJs) and adhesion junctions (AJs). DENV NS1 also promotes MIF and induces cell autophagy, leading to intercellular connections disruption. NS1 of ZIKV, JEV, and DENV down-regulates the sialic acid on the surface of endothelial cells, and can up-regulate the expression of cathepsin L and heparanase to destroy EGL and lead to endothelial cell damage. DENV NS1 N207Q mutant abolish cell endocytosis and EGL disruption. The N-linked glycosylation sites mutation of WNV and YFV NS1 reduces the neurovirulence of the mutant virus. NS1-P250L and NS1-P101K mutation of WNV reduce the virus neuroinvasiveness.
Previous studies have demonstrated that the flavivirus NS1 protein is endocytosed into endothelial cells in a dynamin- and clathrin-dependent manner (Wang et al., 2019). NS1 endocytosis is necessary for its biological function of disrupting endothelial barrier and inducing vascular hyperpermeability. Recent studies have identified SRB1 in hepatic cells and SRB1-like receptors in mosquito cells as cellular receptors for DENV and ZIKV NS1 binding, and only cells expressing the SRB1 are able to internalize NS1 (Alcala et al., 2022). In addition, the interaction between NS1 and vascular endothelial cells is tissue specific. A recent study identified the flavivirus NS1 wing and β-ladder domains are important factors contributing to tissue-specific endothelial dysfunction. Residues 91 to 93 (GDI) in wing domain are the key molecules that determine the tissue-specific binding of NS1 to endothelial cells. The investigators hypothesized that residues 91 to 93 could directly interact with specific factors on the surface of host endothelial cells, facilitating association with the plasma membrane and mediating tissue-specific interactions. Another speculation is that residues 91 to 93 regulate the flexibility of the flexible loop of the NS1 residue W115-W118-G119 (WWG) motif, which predicts sites of interaction with endothelial cells. The ability of flavivirus NS1 to interact specifically with tissues should also consider the factor of host endothelial cells. Depending on the tissue of endothelial cell origin, the composition and proportions of the components that make up the endothelial cell surface glycocalyx may vary considerably, which in turn may lead to different binding of viral proteins to distinct tissues (Lo et al., 2022).
4.1. NS1 induces brain tissue damage
ZIKV infection primarily causes brain disease, characterized by congenital microcephaly in newborns and Guillain-Barre´ syndrome in adults. So currently studies have mainly focused on the role of ZIKV NS1 in brain tissue. Previous studies have found that flavivirus NS1 dependent on glycogen synthase beta (GSK-3β) induces mislocalization of intercellular junction proteins β-catenin and VE-cadherin in endothelial cells of human umbilical vein and brain tissue in a tissue-specific manner, thereby damaging the endothelial barrier and triggering vascular leakage (Puerta-Guardo et al., 2022). Cell junctions include tight junctions (TJs) and adhesion junctions (AJs). Recent report showed that ZIKV NS1 can stimulate phosphorylation of AJ proteins on human brain microvascular endothelial cells and secretes NS1 in a by stander manner to inhibit TJs. The intrinsic mechanism is that ZIKV NS1 stimulates NADPH-dependent increase in the production of reactive oxygen species (ROS) and the redox-sensitive tyrosine kinase, further phosphorylating β-catenin and VE-cadherin (Rastogi and Singh, 2020). In addition, ZIKV NS1 has been shown to inhibit VE-cadherin and claudin-5 expression via hsa-miR-101-3P, which disrupt intercellular junctions, compromise the integrity of human brain microvascular endothelial cells and cross the BBB causing brain disease (Bhardwaj and Singh, 2021). ZIKV NS1 may affect fetal brain development through the placenta, and has been shown to induce increased permeability of the human placenta by destroying glycosaminoglycans (GAGs) on trophoblastic cells (Puerta-Guardo et al., 2020).
Endothelial glycocalyx-like layer (EGL) is a multi-glycoprotein complex that covers the surface of vascular endothelial cells, providing a buffer to the cell membrane and maintaining vascular function (Reitsma et al., 2007). Sialic acid and heparan sulfate proteoglycan are the main components of EGL, a recent study has demonstrated that JEV NS1 down-regulates the sialic acid modification of human brain endothelial cells, and can up-regulate the expression of cathepsin L to degrade heparan sulfate proteoglycan, destroy EGL and lead to brain endothelial cell damage (Pan et al., 2022). Interestingly, its mutant protein NS1 N207Q significantly weakened the endothelial damage. These results indicated that JEV NS1 play an important role in the damage of brain endothelial cell. The molecular mechanism of JEV NS1 destroying the blood-brain barrier and causing brain tissue damage deserves further study.
The main symptoms of WNV infection are neurological diseases such as encephalitis and meningitis. WNV NS1 mainly acts on endothelial cells in the central nervous system and has an important correlation with virus neuroinvasiveness. Among several nonstructural protein region mutants (NS2A (A30P), NS3(P249H), NS4B (P38G, C102S, E249G)) obtained, only the NS1-P250L mutant exhibited 0 % lethality in mammalian in vitro and in vivo models, and significantly reduced the neuroinvasive power of the virus (Szentpali-Gavaller et al., 2016). Recently report showed that circulating levels of WNV NS1 were associated with virus transmission into the mouse brain. The generated WNV NS1-P101K mutant can alleviate virus infection in the brain without changing virus replication, NS1 binding to endothelial cells, and NS1 interaction with complement proteins. In addition, the WNV infectious ability in mice brain can be rescued after injection of exogenous NS1 protein to improve its circulating level. This suggests that circulating NS1 contributes to the spread of the virus to the central nervous system and promotes the occurrence of the disease (Wessel et al., 2021). All these findings indicate that the flaviviruses NS1 plays an important role in the role of virus in causing tissue damage.
4.2. NS1 causes peripheral tissue damage
Patients with DENV infection manifestas asymptomatic, classic dengue and more severe dengue fever, including dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). Severe dengue patients are usually associated with increased vascular fragility and plasma leakage resulting in hypovolemic, organ failure and shock. DENV NS1 is the key protein to induce the endothelial hyperpermeability and vascular leakage of virus infection. Previous report has showed that NS1 of DENV disrupts endothelial cell integrity by releasing proinflammatory cytokines and chemokines via TLR4 activation in murine macrophages and human peripheral blood monocytes (PBMC), leading to vascular fragility (Modhiran et al., 2015). In addition, NS1 can bind to TLR4 on platelets, causing platelets aggregate and leading to thrombocytopenia and bleeding (Chao et al., 2019).
There are four serotypes of dengue virus, and secondary infection with heterologous dengue serotypes is one of the factors that causes severe dengue fever, which is thought to be caused by an immune response triggered by serotype cross-reactive antibodies (Guzman et al., 2013). DENV NS1 polyclonal mouse serum and NS1 monoclonal antibody could specifically block vascular leakage in mice in vivo and increased permeability in human endothelial cells in vitro. DENV1, DENV3 and DENV4 NS1 immunization could mitigate the lethal risk of vascular leakage induced by DENV2 infection in mice (Beatty et al., 2015). However, it was later illustrated that NS1 immunization or passive transfer of NS1 antibodies did not provide protection in the A129 (lacking type I IFN) and AG129 (lacking type I and II IFN) mouse models inoculated with two highly virulent strains of DENV2, including the non-mouse-adapted strain D2Y98P and the clinical isolate strain from Singapore (Lee et al., 2020b). The contradiction between the two studies is related to DENV strains, suggesting that the pathogenic role of sNS1 is likely to be DENV strain dependent. In conclusion, NS1 protein has a complex role in the pathogenesis of DENV infection.
DENV NS1 leads to endothelial barrier dysfunction and vascular endothelial hyperpermeability mainly through disruption of intercellular junctions and the EGL degradation. In dengue patients, vascular endothelial hyperpermeability was found to be restored in patients treated with fluid resuscitation, suggesting that vascular leakage is not caused by structural disruption of endothelial cells but may be due to disruption of cell junctions caused by vasoactive cytokines (Forster, 2008). Tight junction proteins and adhesion junction proteins have functions such as stabilizing the cytoskeleton, controlling paracellular permeability and transmitting intercellular signals to maintain the stability of the endothelial barrier (Mehta and Malik, 2006). Macrophage migration inhibitor factor (MIF) can disrupt endothelial connexins zona occludens protein-1 (ZO-1) and VE-cadherin to increase vascular permeability. DENV NS1 stimulated dermal cells to produce MIF and induced autophagy in human endothelial cells, thereby destroying intercellular connections and resulting in endothelial hyperpermeability (Chen et al., 2016). Matrix metalloproteinases (MMPs) are a class of enzymes that degrade most of the protein components of the extracellular matrix and maintain the dynamic homeostasis of the extracellular matrix. Recent study showed that DENV NS1 and MMP-9 synergistically induce hyperpermeability and vascular leakage in human endothelial cells and mouse tissues. Mechanically, NS1 recruit MMP-9 to interact with the cell adhesion protein β-catenin and the tight junction factor ZO-1 and ZO-2 to digest important adhesion and tight junction proteins, causing vascular leakage and endothelial hyperpermeability (Pan et al., 2021).
In addition to intercellular junctions, the EGL is also an important factor in determining endothelial barrier function, and these two factors together control vascular homeostasis. DENV NS1 can up-regulate sialidase expression, resulting in sialic acid lysis on the surface of endothelial cells. DENV NS1 and JEV NS1 share common features in the molecular mechanism of EGL destruction. In addition, NS1 also promote cathepsin L activity and increase the expression of heparanase-1 (HPA-1) and cleave transmembrane heparan sulfate proteoglycan (CD138) on the surface of endothelial cells, leading to an increase in blood CD138 levels (Puerta-Guardo et al., 2016). However, the exact mechanism by which NS1 activates these enzymes remains unclear. In generally, DENV NS1 disrupts the endothelial EGL structure of the endothelium and lead to endothelial barrier dysfunction, resulting in vascular hyperpermeability and systemic vascular leakage. In addition, it has been shown that NS1-induced secretion of HPA-1 and MMP-9 is MIF-dependent, which suggests a complex mechanism for DENV NS1-induced vascular endothelial hyperpermeability (Chen et al., 2018).
4.3. NS1 mutations reduce flavivirus neurovirulence
Flavivirus NS1 first forms a dimer, then a tetramer and a hexamer, which are secreted by infected cells (Lee et al., 2020b). The N-linked glycosylation sites of NS1 stabilize the morphology of its hexamer and promote the secretion of NS1. The two N-linked glycosylation sites (Asn-130 and Asn-208) mutation of YFV NS1 significantly reduces the neurovirulence of the mutant virus in mice (Muylaert et al., 1996). WNV NS1 has three N-linked glycosylation sites at asparagine residues (NS1130, NS1175, NS1207) , eliminating these glycosylation sites by mutating asparagine to alanine that attenuated the neuroinvasiveness of the virus in mice. The researchers then replaced NS1130 aspartate with serine and glutamate to produce a mutant that was more stable and greatly reduced neurovirulence in mice (Whiteman et al., 2011).
The Asn-207 site is highly conserved in a variety of flaviviruses, such as DENV, ZIKV and WNV. A glycosylated residue N207Q mutation in NS1 was identified, which can abolish the role in NS1 disrupting the EGL and inducing endothelial hyperpermeability compared to wild-type NS1. Wild-type NS1 can be rapidly internalized depending on clathrin and dynein, which activates EGL degradation and causes endothelial dysfunction, while the NS1 N207Q mutant is retained on the cell surface and does not undergo endocytosis (Wang et al., 2019). In addition, the T164S mutation in NS1 of DENV cause the increase of sNS1 production in human PBMC, and lead to more severe disease manifestations in infected mice characterized by tissue inflammation and faster death. Molecular dynamics simulations predicted NS1 hexamer instability in this mutant strain, which also illustrated the importance of secretory NS1 in inducing disease (Chan et al., 2019).
Flavivirus NS1 protein has many functions, such as participating in virus replication, involving in host innate immunity, as vaccine target (Choy et al., 2020; Muller and Young, 2013; Rastogi et al., 2016). We speculate that NS1 mainly play an important role in escaping host innate immune response and causing tissue damage. Flaviviruses NS1, including JEV and WNV, can antagonize the host's innate immune response through TLRs or RLRs pathway. In contrast, the NS1 protein encoded by DENV and ZIKV mainly causes tissue damage and directly aggravate the dieases symptoms. In view of the increasing evidence suggested that NS1 plays a key role in regulating host innate immune response and directly inducing tissue damage, we should focus on the research in this area in the future, and further analyze the specific role of NS1, and whether there is a direct relationship between these two aspects. At the same time, these studies will support the development of new attenuated vaccines based on NS1 modification.
5. Conclusions
Flaviviruses include important mosquito-borne pathogens and cause systemic or neurotropic encephalitic pathology in humans with no specific treatment. In this article, we have tried to provide a comprehensive introduction of the functional characteristics of NS1, its three-dimensional structures of different forms, its role in viral replication and as a vaccine candidate and diagnostic biomarker, especially involved in escaping of host innate immune and inducing tissue-specific damage. This key enigmatic protein has showed its somewhat contradictory role in the induction of both protection and pathogenesis in the infected host. Given the extensive role of NS1 in the disease progression of flaviviruses, a combination treatment approach targeting its multiple role of NS1 may provide a magical effect to treat clinical cases of flaviviruses in the future.
CRediT authorship contribution statement
Quan Zeng: Writing – original draft. Jiaqi Liu: Writing – original draft. Chenlin Hao: Writing – original draft. Bo Zhang: Writing – review & editing. Honglei Zhang: Writing – original draft.
Declaration of Competing Interest
We would like to submit our manuscript entitled “Making sense of flavivirus non-strctural protein 1 in innate immune evasion and inducing tissue-specific damage” for the consideration as a review article in Virus Research. We declare that we have no conflict of interest. Moreover, we have no financial and personal relationships with other people or organizations that can inappropriately influence our work.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (32002297), and the Outstanding Talents of Henan Agricultural University (30500632).
Data availability
No data was used for the research described in the article.
References
- Aguirre S., Luthra P., Sanchez-Aparicio M.T., Maestre A.M., Patel J., Lamothe F., Fredericks A.C., Tripathi S., Zhu T., Pintado-Silva J., Webb L.G., Bernal-Rubio D., Solovyov A., Greenbaum B., Simon V., Basler C.F., Mulder L.C., Garcia-Sastre A., Fernandez-Sesma A. Dengue virus NS2B protein targets cGAS for degradation and prevents mitochondrial DNA sensing during infection. Nat. Microbiol. 2017;2:17037. doi: 10.1038/nmicrobiol.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akey D.L., Brown W.C., Dutta S., Konwerski J., Jose J., Jurkiw T.J., DelProposto J., Ogata C.M., Skiniotis G., Kuhn R.J., Smith J.L. Flavivirus NS1 structures reveal surfaces for associations with membranes and the immune system. Science. 2014;343(6173):881–885. doi: 10.1126/science.1247749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcala A.C., Maravillas J.L., Meza D., Ramirez O.T., Ludert J.E., Palomares L.A. Dengue virus NS1 uses scavenger receptor B1 as a cell receptor in cultured cells. J. Virol. 2022;96(5) doi: 10.1128/jvi.01664-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcala A.C., Palomares L.A., Ludert J.E. Secretion of nonstructural protein 1 of dengue virus from infected mosquito cells: facts and speculations. J. Virol. 2018;92(14) doi: 10.1128/JVI.00275-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avirutnan P., Punyadee N., Noisakran S., Komoltri C., Thiemmeca S., Auethavornanan K., Jairungsri A., Kanlaya R., Tangthawornchaikul N., Puttikhunt C., Pattanakitsakul S.N., Yenchitsomanus P.T., Mongkolsapaya J., Kasinrerk W., Sittisombut N., Husmann M., Blettner M., Vasanawathana S., Bhakdi S., Malasit P. Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J. Infect. Dis. 2006;193(8):1078–1088. doi: 10.1086/500949. [DOI] [PubMed] [Google Scholar]
- Baronti C., Sire J., de Lamballerie X., Querat G. Nonstructural NS1 proteins of several mosquito-borne flavivirus do not inhibit TLR3 signaling. Virology. 2010;404(2):319–330. doi: 10.1016/j.virol.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Beatty P.R., Puerta-Guardo H., Killingbeck S.S., Glasner D.R., Hopkins K., Harris E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci. Transl. Med. 2015;7(304):304ra141. doi: 10.1126/scitranslmed.aaa3787. [DOI] [PubMed] [Google Scholar]
- Bhardwaj U., Singh S.K. Zika virus NS1 suppresses VE-cadherin and claudin-5 via hsa-miR-101-3p in human brain microvascular endothelial cells. Mol. Neurobiol. 2021;58(12):6290–6303. doi: 10.1007/s12035-021-02548-x. [DOI] [PubMed] [Google Scholar]
- Brandt W.E., Cardiff R.D., Russell P.K. Dengue virions and antigens in brain and serum of infected mice. J. Virol. 1970;6(4):500–506. doi: 10.1128/jvi.6.4.500-506.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher C.H., Gould E.A. Taxonomy of the virus family flaviviridae. Adv. Virus Res. 2003;59:1–19. doi: 10.1016/s0065-3527(03)59001-7. [DOI] [PubMed] [Google Scholar]
- Chan K.W.K., Watanabe S., Jin J.Y., Pompon J., Teng D., Alonso S., Vijaykrishna D., Halstead S.B., Marzinek J.K., Bond P.J., Burla B., Torta F., Wenk M.R., Ooi E.E., Vasudevan S.G. A T164S mutation in the dengue virus NS1 protein is associated with greater disease severity in mice. Sci. Transl. Med. 2019;11(498) doi: 10.1126/scitranslmed.aat7726. [DOI] [PubMed] [Google Scholar]
- Chao C.H., Wu W.C., Lai Y.C., Tsai P.J., Perng G.C., Lin Y.S., Yeh T.M. Dengue virus nonstructural protein 1 activates platelets via Toll-like receptor 4, leading to thrombocytopenia and hemorrhage. PLoS Pathog. 2019;15(4) doi: 10.1371/journal.ppat.1007625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.R., Chao C.H., Liu C.C., Ho T.S., Tsai H.P., Perng G.C., Lin Y.S., Wang J.R., Yeh T.M. Macrophage migration inhibitory factor is critical for dengue NS1-induced endothelial glycocalyx degradation and hyperpermeability. PLoS Pathog. 2018;14(4) doi: 10.1371/journal.ppat.1007033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.R., Chuang Y.C., Lin Y.S., Liu H.S., Liu C.C., Perng G.C., Yeh T.M. Dengue virus nonstructural protein 1 induces vascular leakage through macrophage migration inhibitory factor and autophagy. PLoS Negl. Trop. Dis. 2016;10(7) doi: 10.1371/journal.pntd.0004828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Ng M.M., Chu J.J. Activation of TLR2 and TLR6 by dengue NS1 protein and its implications in the immunopathogenesis of dengue virus infection. PLoS Pathog. 2015;11(7) doi: 10.1371/journal.ppat.1005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy M.M., Ng D.H.L., Siriphanitchakorn T., Ng W.C., Sundstrom K.B., Tan H.C., Zhang S.L., Chan K.W.K., Manuel M., Kini R.M., Chan K.R., Vasudevan S.G., Ooi E.E. A non-structural 1 protein G53D substitution attenuates a clinically tested live dengue vaccine. Cell Rep. 2020;31(6) doi: 10.1016/j.celrep.2020.107617. [DOI] [PubMed] [Google Scholar]
- Crook K.R., Miller-Kittrell M., Morrison C.R., Scholle F. Modulation of innate immune signaling by the secreted form of the West Nile virus NS1 glycoprotein. Virology. 2014;458-459:172–182. doi: 10.1016/j.virol.2014.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple N.A., Cimica V., Mackow E.R. Dengue virus NS proteins inhibit RIG-I/MAVS signaling by blocking TBK1/IRF3 phosphorylation: dengue virus serotype 1 NS4A is a unique interferon-regulating virulence determinant. mBio. 2015;6(3):e00553. doi: 10.1128/mBio.00553-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errett J.S., Suthar M.S., McMillan A., Diamond M.S., Gale M., Jr. The essential, nonredundant roles of RIG-I and MDA5 in detecting and controlling West Nile virus infection. J. Virol. 2013;87(21):11416–11425. doi: 10.1128/JVI.01488-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth A.E., Atkins J.F. A conserved predicted pseudoknot in the NS2A-encoding sequence of West Nile and Japanese encephalitis flaviviruses suggests NS1′ may derive from ribosomal frameshifting. Virol. J. 2009;6:14. doi: 10.1186/1743-422X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster C. Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 2008;130(1):55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A., Lin T.Y., Beasley D.W., Stover C.M., Schwaeble W.J., Pierson T.C., Diamond M.S. Direct complement restriction of flavivirus infection requires glycan recognition by mannose-binding lectin. Cell Host Microbe. 2010;8(2):186–195. doi: 10.1016/j.chom.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H.Y., Zhang X.C., Jia R.Y. Toll-like receptors and RIG-I-like receptors play important roles in resisting flavivirus. J. Immunol. Res. 2018;2018 doi: 10.1155/2018/6106582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutsche I., Coulibaly F., Voss J.E., Salmon J., d'Alayer J., Ermonval M., Larquet E., Charneau P., Krey T., Megret F., Guittet E., Rey F.A., Flamand M. Secreted dengue virus nonstructural protein NS1 is an atypical barrel-shaped high-density lipoprotein. Proc. Natl. Acad. Sci. U. S. A. 2011;108(19):8003–8008. doi: 10.1073/pnas.1017338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman M.G., Alvarez M., Halstead S.B. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch. Virol. 2013;158(7):1445–1459. doi: 10.1007/s00705-013-1645-3. [DOI] [PubMed] [Google Scholar]
- Han Y.W., Choi J.Y., Uyangaa E., Kim S.B., Kim J.H., Kim B.S., Kim K., Eo S.K. Distinct dictation of Japanese encephalitis virus-induced neuroinflammation and lethality via triggering TLR3 and TLR4 signal pathways. PLoS Pathog. 2014;10(9) doi: 10.1371/journal.ppat.1004319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann D.L., Hodgson A., Sall A.A., Freedman D.O., Staples J.E., Althabe F., Baruah K., Mahmud G., Kandun N., Vasconcelos P.F., Bino S., Menon K.U. Zika virus and microcephaly: why is this situation a PHEIC? Lancet. 2016;387(10020):719–721. doi: 10.1016/S0140-6736(16)00320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R., Ye J., Zhu B., Song Y., Chen H., Cao S. Roles of TLR3 and RIG-I in mediating the inflammatory response in mouse microglia following Japanese encephalitis virus infection. J. Immunol. Res. 2014;2014 doi: 10.1155/2014/787023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kuno G., Chang G.J., Tsuchiya K.R., Karabatsos N., Cropp C.B. Phylogeny of the genus flavivirus. J. Virol. 1998;72(1):73–83. doi: 10.1128/jvi.72.1.73-83.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.Y., Nguyen T.T.N., Myoung J. Zika Virus-encoded NS2A and NS4A strongly downregulate NF-kappaB promoter activity. J. Microbiol. Biotechnol. 2020;30(11):1651–1658. doi: 10.4014/jmb.2011.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.X., Ting D.H.R., Boey C.P.H., Tan E.T.X., Chia J.Z.H., Idris F., Oo Y., Ong L.C., Chua Y.L., Hapuarachchi C., Ng L.C., Alonso S. Relative contribution of nonstructural protein 1 in dengue pathogenesis. J. Exp. Med. 2020;217(9) doi: 10.1084/jem.20191548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Zhou D., Jia F., Zhang L., Ashraf U., Li Y., Duan H., Song Y., Chen H., Cao S., Ye J. Japanese encephalitis virus NS1′ protein interacts with host CDK1 protein to regulate antiviral response. Microbiol. Spectr. 2021;9(3) doi: 10.1128/Spectrum.01661-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach B.D., Rice C.M. Genetic interaction of flavivirus nonstructural proteins NS1 and NS4A as a determinant of replicase function. J. Virol. 1999;73(6):4611–4621. doi: 10.1128/jvi.73.6.4611-4621.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo N.T.N., Roodsari S.Z., Tin N.L., Wong M.P., Biering S.B., Harris E. Molecular determinants of tissue specificity of flavivirus nonstructural protein 1 interaction with endothelial cells. J. Virol. 2022;96(19) doi: 10.1128/jvi.00661-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhop E., Ansarah-Sobrinho C., Johnson S., Engle M., Fremont D.H., Pierson T.C., Diamond M.S. Complement protein C1q inhibits antibody-dependent enhancement of flavivirus infection in an IgG subclass-specific manner. Cell Host Microbe. 2007;2(6):417–426. doi: 10.1016/j.chom.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D., Malik A.B. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Melian E.B., Hinzman E., Nagasaki T., Firth A.E., Wills N.M., Nouwens A.S., Blitvich B.J., Leung J., Funk A., Atkins J.F., Hall R., Khromykh A.A. NS1′ of flaviviruses in the Japanese encephalitis virus serogroup is a product of ribosomal frameshifting and plays a role in viral neuroinvasiveness. J. Virol. 2010;84(3):1641–1647. doi: 10.1128/JVI.01979-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modhiran N., Watterson D., Muller D.A., Panetta A.K., Sester D.P., Liu L., Hume D.A., Stacey K.J., Young P.R. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci. Transl. Med. 2015;7(304):304ra142. doi: 10.1126/scitranslmed.aaa3863. [DOI] [PubMed] [Google Scholar]
- Muller D.A., Young P.R. The flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antivir. Res. 2013;98(2):192–208. doi: 10.1016/j.antiviral.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Muylaert I.R., Chambers T.J., Galler R., Rice C.M. Mutagenesis of the N-linked glycosylation sites of the yellow fever virus NS1 protein: effects on virus replication and mouse neurovirulence. Virology. 1996;222(1):159–168. doi: 10.1006/viro.1996.0406. [DOI] [PubMed] [Google Scholar]
- Nazmi A., Dutta K., Hazra B., Basu A. Role of pattern recognition receptors in flavivirus infections. Virus Res. 2014;185:32–40. doi: 10.1016/j.virusres.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Pan Junhui L.Y., Zhenjie Liang, Xingmiao Yang, Shengda Xie, Ruibing Cao. Preliminary study on injury of brain endothelium by Japanese encephalitis virus NS1 protein. J. Nanjing Agric. Univ. 2022;8(31) [Google Scholar]
- Pan P., Li G., Shen M., Yu Z., Ge W., Lao Z., Fan Y., Chen K., Ding Z., Wang W., Wan P., Shereen M.A., Luo Z., Chen X., Zhang Q., Lin L., Wu J. DENV NS1 and MMP-9 cooperate to induce vascular leakage by altering endothelial cell adhesion and tight junction. PLoS Pathog. 2021;17(7) doi: 10.1371/journal.ppat.1008603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Sinigaglia A., Barzon L., Fassan M., Sparber F., LeibundGut-Landmann S., Ackermann M. Role of NS1 and TLR3 in pathogenesis and immunity of WNV. Viruses. 2019;11(7):603. doi: 10.3390/v11070603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poonsiri T., Wright G.S.A., Diamond M.S., Turtle L., Solomon T., Antonyuk S.V. Structural study of the C-terminal domain of nonstructural protein 1 from Japanese encephalitis virus. J. Virol. 2018;92(7) doi: 10.1128/JVI.01868-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poonsiri T., Wright G.S.A., Diamond M.S., Turtle L., Solomon T., Antonyuk S.V., Pfeiffer J.K. Structural study of the C-terminal domain of nonstructural protein 1 from Japanese encephalitis virus. J. Virol. 2018;92(7) doi: 10.1128/JVI.01868-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puerta-Guardo H., Biering S.B., de Sousa F.T.G., Shu J., Glasner D.R., Li J., Blanc S.F., Beatty P.R., Harris E. Flavivirus NS1 triggers tissue-specific disassembly of intercellular junctions leading to barrier dysfunction and vascular leak in a GSK-3beta-dependent manner. Pathogens. 2022;11(6):615. doi: 10.3390/pathogens11060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puerta-Guardo H., Glasner D.R., Espinosa D.A., Biering S.B., Patana M., Ratnasiri K., Wang C., Beatty P.R., Harris E. Flavivirus NS1 triggers tissue-specific vascular endothelial dysfunction reflecting disease tropism. Cell Rep. 2019;26(6):1598–1613. doi: 10.1016/j.celrep.2019.01.036. e1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puerta-Guardo H., Glasner D.R., Harris E. Dengue virus NS1 disrupts the endothelial glycocalyx, leading to hyperpermeability. PLoS Pathog. 2016;12(7) doi: 10.1371/journal.ppat.1005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puerta-Guardo H., Tabata T., Petitt M., Dimitrova M., Glasner D.R., Pereira L., Harris E. Zika Virus nonstructural protein 1 disrupts glycosaminoglycans and causes permeability in developing human placentas. J. Infect. Dis. 2020;221(2):313–324. doi: 10.1093/infdis/jiz331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi M., Sharma N., Singh S.K. Flavivirus NS1: a multifaceted enigmatic viral protein. Virol. J. 2016;13:131. doi: 10.1186/s12985-016-0590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi M., Singh S.K. Zika virus NS1 affects the junctional integrity of human brain microvascular endothelial cells. Biochimie. 2020;176:52–61. doi: 10.1016/j.biochi.2020.06.011. [DOI] [PubMed] [Google Scholar]
- Reitsma S., Slaaf D.W., Vink H., van Zandvoort M.A., oude Egbrink M.G. The endothelial glycocalyx: composition, functions, and visualization. Pflug. Arch. 2007;454(3):345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling M., Bridgeman A., Gray N., Hertzog J., Hublitz P., Kohl A., Rehwinkel J. RIG-I Plays a dominant role in the induction of transcriptional changes in Zika Virus-infected cells, which protect from virus-induced cell death. Cells. 2020;9(6):1476. doi: 10.3390/cells9061476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojima M., Takenouchi A., Shimoda H., Kimura N., Maeda K. Distinct usage of three C-type lectins by Japanese encephalitis virus: DC-SIGN, DC-SIGNR, and LSECtin. Arch. Virol. 2014;159(8):2023–2031. doi: 10.1007/s00705-014-2042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somnuke P., Hauhart R.E., Atkinson J.P., Diamond M.S., Avirutnan P. N-linked glycosylation of dengue virus NS1 protein modulates secretion, cell-surface expression, hexamer stability, and interactions with human complement. Virology. 2011;413(2):253–264. doi: 10.1016/j.virol.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar M.S., Aguirre S., Fernandez-Sesma A. Innate immune sensing of flaviviruses. PLoS Pathog. 2013;9(9) doi: 10.1371/journal.ppat.1003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentpali-Gavaller K., Lim S.M., Dencso L., Banyai K., Koraka P., Osterhaus A.D., Martina B.E., Bakonyi T., Balint A. In vitro and in vivo evaluation of mutations in the NS region of lineage 2 West Nile Virus associated with neuroinvasiveness in a mammalian model. Viruses. 2016;8(2):49. doi: 10.3390/v8020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Innate immunity to virus infection. Immunol. Rev. 2009;227(1):75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuqui-Inchima S., Cabrera J., Haenni A.L. Interplay between dengue virus and Toll-like receptors, RIG-I/MDA5 and microRNAs: implications for pathogenesis. Antivir. Res. 2017;147:47–57. doi: 10.1016/j.antiviral.2017.09.017. [DOI] [PubMed] [Google Scholar]
- Vita R., Zarebski L., Greenbaum J.A., Emami H., Hoof I., Salimi N., Damle R., Sette A., Peters B. The immune epitope database 2.0. Nucleic Acids Res. 2010;38(Database issue):D854–D862. doi: 10.1093/nar/gkp1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Puerta-Guardo H., Biering S.B., Glasner D.R., Tran E.B., Patana M., Gomberg T.A., Malvar C., Lo N.T.N., Espinosa D.A., Harris E. Endocytosis of flavivirus NS1 is required for NS1-mediated endothelial hyperpermeability and is abolished by a single N-glycosylation site mutation. PLoS Pathog. 2019;15(7) doi: 10.1371/journal.ppat.1007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Town T., Alexopoulou L., Anderson J.F., Fikrig E., Flavell R.A. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 2004;10(12):1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- Wessel A.W., Dowd K.A., Biering S.B., Zhang P., Edeling M.A., Nelson C.A., Funk K.E., DeMaso C.R., Klein R.S., Smith J.L., Cao T.M., Kuhn R.J., Fremont D.H., Harris E., Pierson T.C., Diamond M.S. Levels of circulating NS1 impact west Nile Virus spread to the brain. J. Virol. 2021;95(20) doi: 10.1128/JVI.00844-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman M.C., Wicker J.A., Kinney R.M., Huang C.Y., Solomon T., Barrett A.D. Multiple amino acid changes at the first glycosylation motif in NS1 protein of West Nile virus are necessary for complete attenuation for mouse neuroinvasiveness. Vaccine. 2011;29(52):9702–9710. doi: 10.1016/j.vaccine.2011.09.036. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith A., Gubler D.J., Weaver S.C., Monath T.P., Heymann D.L., Scott T.W. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect. Dis. 2017;17(3):e101–e106. doi: 10.1016/S1473-3099(16)30518-7. [DOI] [PubMed] [Google Scholar]
- Wilson J.R., de Sessions P.F., Leon M.A., Scholle F. West Nile virus nonstructural protein 1 inhibits TLR3 signal transduction. J. Virol. 2008;82(17):8262–8271. doi: 10.1128/JVI.00226-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Liu Q., Zhou J., Xie W., Chen C., Wang Z., Yang H., Cui J. Zika virus evades interferon-mediated antiviral response through the co-operation of multiple nonstructural proteins in vitro. Cell Discov. 2017;3:17006. doi: 10.1038/celldisc.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H., Luo H., Shan C., Muruato A.E., Nunes B.T.D., Medeiros D.B.A., Zou J., Xie X., Giraldo M.I., Vasconcelos P.F.C., Weaver S.C., Wang T., Rajsbaum R., Shi P.Y. An evolutionary NS1 mutation enhances Zika virus evasion of host interferon induction. Nat. Commun. 2018;9(1):414. doi: 10.1038/s41467-017-02816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S., Pan J., Zhang Q., Guan R., Yang X., Yang X., Liang Z., Cao R. Japanese encephalitis virus (JEV) NS1′ enhances the viral infection of dendritic cells (DCs) and macrophages in pig tonsils. Microbiol. Spectr. 2022;10(4) doi: 10.1128/spectrum.01147-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Song H., Qi J., Liu Y., Wang H., Su C., Shi Y., Gao G.F. Contribution of intertwined loop to membrane association revealed by Zika virus full-length NS1 structure. EMBO J. 2016;35(20):2170–2178. doi: 10.15252/embj.201695290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn S., Cho H., Fremont D.H., Diamond M.S. A short N-terminal peptide motif on flavivirus nonstructural protein NS1 modulates cellular targeting and immune recognition. J. Virol. 2010;84(18):9516–9532. doi: 10.1128/JVI.00775-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn S., Li T., McCune B.T., Edeling M.A., Fremont D.H., Cristea I.M., Diamond M.S. Evidence for a genetic and physical interaction between nonstructural proteins NS1 and NS4B that modulates replication of West Nile virus. J. Virol. 2012;86(13):7360–7371. doi: 10.1128/JVI.00157-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L.B., Melian E.B., Khromykh A.A. NS1′ colocalizes with NS1 and can substitute for NS1 in West Nile virus replication. J. Virol. 2013;87(16):9384–9390. doi: 10.1128/JVI.01101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.L., Ye H.Q., Liu S.Q., Deng C.L., Li X.D., Shi P.Y., Zhang B. West Nile virus NS1 antagonizes interferon beta production by targeting RIG-I and MDA5. J. Virol. 2017;91(18) doi: 10.1128/JVI.02396-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., Wang X., Liu Y., Li Y., Long S., Gu C., Ye J., Xie S., Cao S. Japanese Encephalitis Virus infection induces inflammation of swine testis through RIG-I-NF-kB signaling pathway. Vet. Microbiol. 2019;238 doi: 10.1016/j.vetmic.2019.108430. [DOI] [PubMed] [Google Scholar]
- Zhou D., Jia F., Li Q., Zhang L., Chen Z., Zhao Z., Cui M., Song Y., Chen H., Cao S., Ye J. Japanese encephalitis virus NS1′ protein antagonizes interferon beta production. Virol. Sin. 2018;33(6):515–523. doi: 10.1007/s12250-018-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Li Q., Jia F., Zhang L., Wan S., Li Y., Song Y., Chen H., Cao S., Ye J. The Japanese encephalitis virus NS1′ protein inhibits Type I IFN production by targeting MAVS. J. Immunol. 2020;204(5):1287–1298. doi: 10.4049/jimmunol.1900946. [DOI] [PubMed] [Google Scholar]
- Zipfel P.F., Skerka C., Hellwage J., Jokiranta S.T., Meri S., Brade V., Kraiczy P., Noris M., Remuzzi G. Factor H family proteins: on complement, microbes and human diseases. Biochem. Soc. Trans. 2002;30(Pt 6):971–978. doi: 10.1042/bst0300971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.