Abstract
Children with autism spectrum disorders (ASD) or autism are more prone to gastrointestinal (GI) disorders than the general population. These disorders can significantly affect their health, learning, and development due to various factors such as genetics, environment, and behavior. The causes of GI disorders in children with ASD can include gut dysbiosis, immune dysfunction, food sensitivities, digestive enzyme deficiencies, and sensory processing differences. Many studies suggest that numerous children with ASD experience GI problems, and effective management is crucial. Diagnosing autism is typically done through genetic, neurological, functional, and behavioral assessments and observations, while GI tests are not consistently reliable. Some GI tests may increase the risk of developing ASD or exacerbating symptoms. Addressing GI issues in individuals with ASD can improve their overall well-being, leading to better behavior, cognitive function, and educational abilities. Proper management can improve digestion, nutrient absorption, and appetite by relieving physical discomfort and pain. Alleviating GI symptoms can improve sleep patterns, increase energy levels, and contribute to a general sense of well-being, ultimately leading to a better quality of life for the individual and improved family dynamics. The primary goal of GI interventions is to improve nutritional status, reduce symptom severity, promote a balanced mood, and increase patient independence.
Keywords: Gastrointestinal disorders; Autism spectrum disorders, Children; Gut microbiota; Ketogenic diet; Gluten-free casein-free diet, Dietary management
Core Tip: Children with autism spectrum disorder often experience gastrointestinal (GI) disorders that can significantly impact their health, learning, and development. Various factors, including genetics, environment, and behavior, can cause these disorders. Common causes include gut dysbiosis, immune dysfunction, food sensitivity, digestive enzyme deficiencies, and sensory processing differences. Proper management can improve well-being, cognitive function, behavior, and educational abilities. GI interventions enhance nutrition, reduce symptoms, promote balanced moods, and increase independence.
INTRODUCTION
The prevalence of autism spectrum disorders (ASD) has increased since Leo Kanner was first illustrated in 1943 and continues to rise[1]. ASD is a neurodevelopmental disorder, which includes, in addition to autism, Asperger’s disorder, and pervasive developmental disorder-not otherwise specified. Pervasive developmental disorders, which include Rett’s disorder, childhood disintegrative disorder, and overactive disorder, are also related. It may overlap with attention deficit hyperactivity disorder[2]. The prevalence of ASD varies from country to country, with an average of 1% worldwide. In the United States, the incidence of ASD can be as high as 1/59 in 2014, according to the centres for disease control and prevention[3], while it can reach 1/64 in the United Kingdom[4]. The prevalence of ASD may be underestimated in some parts of the world, such as Bahrain, where it is estimated to be 1/1000, due to missed diagnoses and lack of official recordings[1]. ASD is more common in boys than girls, with a higher prevalence in non-Hispanic white children and a lower prevalence in Hispanic and African American/black children, with variability in Asian/Pacific residents[5]. Although the exact cause of ASD remains unclear, it is widely accepted that the development of this condition is influenced by a complex interplay of various factors, including genetic predisposition, biological determinants such as advanced parental age, and environmental, immunological, and psychosocial factors[6].
ASD presents in various ways and is characterized by a combination of social, cognitive, sensory, motor, and perceptual symptoms that typically emerge before age three. Children with ASD exhibit behaviors, communication patterns, social interactions, and learning styles that differ from typically developing children. They may struggle with social communication and interaction, including difficulty giving eye contact, displaying limited expressions of emotion, and showing little interest in others or playing with them. They may also have restricted interests, such as playing with the same toys in the same way repeatedly, becoming agitated over minor changes in routine, and developing an obsessive interest in specific parts of objects, the environment, or the body. Additionally, they may engage in repetitive or stereotyped behaviors, such as repeating words, phrases, or sections of videos, flapping their hands, rocking their body, or spinning in circles. Children with ASD may also experience delays in language development, movement, sensory, and cognitive or learning skills[7,8].
It has been observed that children with ASD are at a higher risk of developing medical comorbidities than the general population. These medical conditions can adversely affect their overall health, hinder their learning abilities, and worsen their autistic symptoms. Among the common medical conditions observed in patients with ASD, gastrointestinal (GI) disorders are particularly prevalent[9]. In this review, we highlight the connection between GI issues and ASD, emphasizing the importance of understanding the role of GI health in managing ASD.
Prevalence of GI disorders in children with ASD
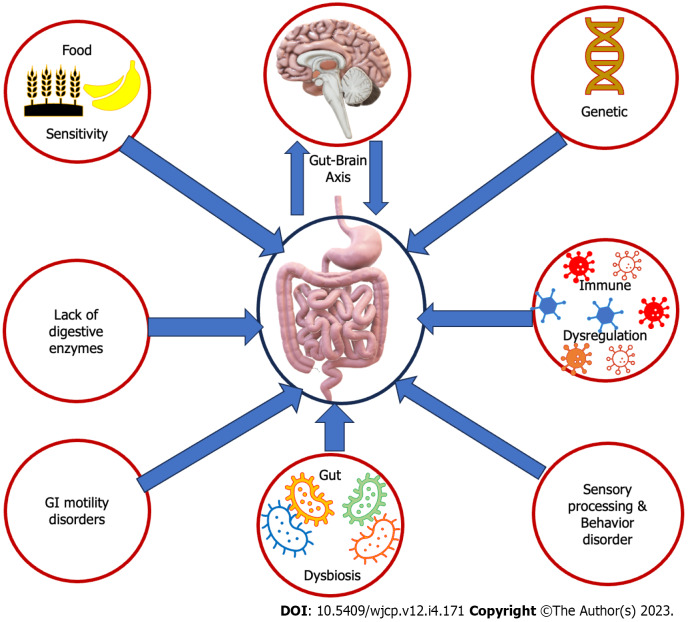
It has been observed that patients with ASD commonly experience GI problems, with a prevalence rate between 46% and 84% in children with ASD. These problems manifest in numerous ways, including food intolerance and/or sensitivities, nausea and/or vomiting, chronic constipation, chronic diarrhea, gastroesophageal reflux and/or disease, chronic flatulence, abdominal discomfort, ulcers, inflammatory bowel disease, colitis, and/or failure to thrive. Additionally, food allergies are more prevalent in children with ASD, with a rate of 20%-25% compared to only 5%-8% in children without ASD[10]. Several factors contribute to this higher prevalence (Figure 1). It is established that genetic factors participate in both ASD and GI disorders. Specific genetic mutations and variations are detected in individuals with ASD, which may impact the development and operation of the GI system[11]. One of the primary reasons is an imbalance and microbiota and dysregulation in the gut microbiota. Children with ASD often exhibit dysbiosis, resulting in GI issues[12]. Another factor is immune system dysfunction, which some children with ASD experience. This can lead to gut inflammation and further contribute to GI problems. Food sensitivities and allergies, particularly to gluten and casein, are also more common in children with ASD. These sensitivities can trigger symptoms like abdominal pain, diarrhea, and constipation. In addition, some children with ASD may have deficiencies in digestive enzymes, which can impact the breakdown of food components. This inadequate enzyme activity can lead to malabsorption and GI disturbances[13]. It’s not uncommon for children with ASD to experience GI motility issues, which can lead to problems like constipation or diarrhea. These issues can arise from various factors, including abnormal serotonin levels linked to ASD and GI dysfunction[14]. Children with ASD frequently display selective and limited eating habits, leading to unbalanced diets that lack essential nutrients. These dietary restrictions may contribute to GI issues like constipation or diarrhea[15]. Additionally, individuals with ASD often exhibit sensory processing differences, making them hypersensitive or hyposensitive to sensory stimuli. These differences may affect their perception of GI sensations, resulting in discomfort or altered responses to normal digestive processes[16]. Unfortunately, the general public, some parents, and professionals may misinterpret sudden improper behaviors due to GI discomfort from children with ASD as lousy behavior that came up from nowhere or just to avoid activity. For instance, during an activity, the child might have severe abdominal or GI pain that forces him/her to scream and move from the place. Meanwhile, the adults may interpret the child’s behavior as improper and escape trial. Children with ASD are more liable to have dysautonomia and abnormal dietary metabolites. Furthermore, gut and brain communications are bidirectional through the gut-brain axis. Children with ASD often have altered gut-brain communication, leading to an impaired gut-brain axis. This can contribute to digestive issues and affect GI function[17]. Children with ASD frequently display selective and limited eating habits, leading to unbalanced diets that lack essential nutrients. Their insistence on sticking to stereotypical diets can result in inadequate fiber and fluid intake, causing GI symptoms. These dietary restrictions may also contribute to GI issues like constipation or diarrhea[15]. Certain medications can also affect bowel function, such as stimulants causing abdominal pain and βeta-blockers causing constipation, diarrhea, and stomach irritation[18].
Figure 1.
Increase the prevalence of gastrointestinal disorders in children with autism spectrum disorders. These factors may include genetic variations, abnormal gut-brain axis, dysbiosis, immune dysfunction, food sensitivities, digestive enzyme deficiencies, sensory processing and integration differences, dysautonomia, and abnormal behaviors. GI: Gastrointestinal.
Children with ASD may experience pain and discomfort due to GI disorders, which can interfere with their learning. Children with ASD who are nonverbal may exhibit behavioral problems like posturing, self-injury, or outbursts without apparent causes due to unrecognized GI disorders, specifically reflux esophagitis and disaccharide malabsorption. However, these manifestations may be mistakenly overlooked as a behavioral problem instead of a medical condition since many children with ASD have difficulty expressing their symptoms or discomfort to their parents or physicians. Lactase deficiency, common in children with ASD and not associated with intestinal inflammation or injury, may also contribute to abdominal discomfort, pain, and obvious behavioral problems[19]. It can be challenging to diagnose GI symptoms in children with ASD due to the lack of established clinical guidelines that prioritize routine assessment of potential medical conditions or GI issues in this population. This is particularly challenging because many children with ASD are nonverbal and cannot convey pain or discomfort through language. Even those who can communicate verbally may have trouble describing subjective experiences or symptoms compared to their typically developing children. As a result, guidelines that specifically address this issue are crucial[20]. Healthcare professionals should know that children with ASD may experience GI dysfunction, mainly if they exhibit unusual postures or movements, have trouble sleeping, are intolerant to certain foods, or display aggressive or self-harming behaviors. To properly assess these issues, clinicians should gather a comprehensive GI and nutritional history covering the patient's eating habits, allergies or food sensitivities, and bowel movements in children with ASD[21].
It’s essential to consider a child’s sleep history when diagnosing underlying GI disorders, as they may manifest as disturbed sleeping patterns[22]. Healthcare providers should thoroughly review the child’s medication, growth history, and sleep habits. Additionally, they should be able to identify vocal, sensory, or motor behaviors that may indicate the presence of pain related to GI disorders. Vocal behaviors associated with GI disorders may include throat clearing, guttural sounds, spitting up in infants, ear rubbing, habitual coughing, or difficulty swallowing. Motor behaviors related to GI disorders include seeking belly pressure, pointing behaviors, certain repetitive behaviors, abnormal neck or body posture, and aggressive or self-injurious behaviors. Studies have shown a strong correlation between aggressive behavior and underlying GI disorders[23], and more severe autistic features tend to be linked to severe GI symptoms. GI disorder symptoms are more likely associated with sleep disruptions and food intolerances. Therefore, clinicians should consider these associations when assessing and treating comorbidities and screening for constipation, diarrhea, or soiling of underwear in children with ASD with prominent rigid-compulsive symptoms[24].
If a child with ASD experiences eczema, vocal, sensory, or motor signs, aggressive or self-injurious behaviors, chronic constipation or diarrhea, or chronic spitting or vomiting, pediatricians should consider referring them for GI evaluation. It is common for children with ASD who exhibit GI symptoms to have increased intestinal permeability. One way to evaluate this is by measuring plasma zonulin level, a valuable blood marker[25]. Endoscopy can also reveal signs of allergic esophagitis, acid reflux damage, allergic changes, or evidence of inflammatory bowel disease in patients with ASD and abdominal manifestations[26]. Effective medical treatment of GI disorders may lead to improvements in behavioral problems. If abdominal pain or discomfort is present, psychotropic medications may not be effective and may even worsen the problem due to GI adverse effects. The microbiota-gut-brain axis is an emerging concept that suggests modulation of the gut microbiota could lead to new therapeutic modalities for different complex central nervous system disorders[27].
While studies have not found a higher prevalence of celiac disease in ASD, it’s important to note that one child out of 68 with celiac disease may develop ASD, and one child out of 130 with ASD may develop celiac disease. Even without GI symptoms, those with celiac disease have been found to have a strong association with epilepsy, cerebral calcifications, and positive responses to dietary changes. Investigating and treating celiac disease, non-celiac gluten sensitivity, and epilepsy could potentially lead to positive outcomes for those with ASD, even without typical GI symptoms or overt seizures[28]. It’s crucial to consider non-celiac gluten or wheat sensitivity in children with ASD, especially if they have irritable bowel symptoms and a history of atopy and allergies[29]. Medical professionals should also be aware of the possibility of non-celiac gluten sensitivity in patients with ASD who present with atopic disease, migraine, mood, and anxiety disorders. While many children with ASD have experienced positive results from a gluten-free, soy-free, and dairy-free diet, it’s crucial to have a celiac test performed before attempting any dietary changes. The gluten-free diet remains the only effective treatment for those with gluten sensitivity, regardless of the manifestations. It’s essential to prioritize the overall health and well-being of patients with ASD, and understanding the potential impact of diet and gluten sensitivity can lead to better outcomes[30].
Understanding the gut-brain connection in children with ASD
The idea of the “second brain” pertains to the enteric nervous system, an intricate network of neurons in the GI tract’s walls. The enteric nervous system is also known as the “gut brain“ or “second brain“ because it can function autonomously from the central nervous system. It has its own reflexes and sensory abilities[31]. The enteric nervous system consists of millions of neurons communicating with each other and the central nervous system via the vagus nerve and other nerve pathways. It plays a crucial role in regulating different digestion aspects, such as food movement through the digestive tract, secretion of digestive enzymes, and absorption of nutrients[32]. The connection between the brain and gut is a complex interconnection known as the gut-brain axis. This pathway allows for two-way communication between the central nervous and GI systems. It comprises various components, including the enteric nervous system, gut microbiota, immune system, and autonomic nervous system[33]. The gut-brain axis regulates physiological processes like digestion and metabolism, influencing cognitive function, behavior, and other brain functions. Communication between the gut and brain is facilitated through various mechanisms, such as neurocrine and endocrine pathways[34]. Within the digestive system are trillions of microorganisms collectively known as the gut microbiota. These microorganisms are vital in maintaining gut health, digestion, nutrient absorption, and immune function and significantly impact human health, including their influence on brain development and function[35]. The gut microbiota is a crucial part of the gut-brain axis and is involved in signaling from the gut microbiota to the brain. The brain, in turn, can influence the composition of gut microbiota and control it through neurotransmitters such as serotonin and dopamine, neuromuscular control of peristalsis, stress-induced cortisol, and stimulation of mucus secretion. On the other hand, the gut affects the brain through vagus nerve activation, neuropeptides, and neurotransmitters like leptin and serotonin, immune signaling through secretory immunoglobulin A, and mucous membrane barrier integrity signaling through zonulin protein and short chain fatty acids (SCFAs) like butyrate[36,37]. Additionally, the microbiota can impact the brain through various mechanisms, like the production of neurotransmitters such as gamma-aminobutyric acid, serotonin, and dopamine, the production of SCFAs like butyric acid, propionic acid, and acetic acid, stimulation of the sympathetic nervous system, and induction of mucosal serotonin release, subsequently affecting memory and learning processes in the brain[38].
Children with ASD experience numerous changes that disrupt their gut-brain axis. One significant change is an imbalance in their gut microbiota composition, known as dysbiosis. This imbalance can disrupt the gut’s normal functioning, potentially contributing to the development of ASD symptoms[39]. Research has shown that children with ASD have reduced microbial diversity, overgrowth of certain harmful bacteria, and imbalances in the beneficial to pathogenic bacteria ratio compared to neurotypical individuals. These changes can impact the production of essential metabolites, neurotransmitters, neuronal sensory processing and integration, and immune signaling molecules, affecting brain development and behavior. The gut microbiota also can produce and modulate neurotransmitters, including serotonin, dopamine, and gamma-aminobutyric acid[12]. These neurotransmitters are critical in regulating mood, sensory processing and integration, behavior, and cognition. Disruptions in the gut microbiota can lead to imbalances in neurotransmitter production, potentially impacting the neurodevelopmental processes associated with ASD. Serotonin, a hormone linked to social behavior and communication, has been found to have altered levels in individuals with ASD. In addition, imbalances in gamma-aminobutyric acid levels have been linked to anxiety and behavioral issues commonly seen in children with ASD[40]. Gut dysbiosis may contribute to GI symptoms commonly observed in children with ASD, such as abdominal pain, constipation, and diarrhea[41].
There is a link between the gut and brain in individuals with ASD that goes beyond microbial composition and neuroactive compounds. Children with ASD have been observed to experience GI inflammation and immune dysregulation, and chronic inflammation. When the immune system in the gut is activated, it can release pro-inflammatory cytokines that can communicate with the brain, causing changes in neural function[42]. Furthermore, immune dysregulation may lead to a compromised blood-brain barrier, which can allow immune cells and inflammatory molecules to enter the brain, resulting in additional effects on neurological processes[43]. Chronic inflammation in the gut can lead to a "leaky gut", which is an increased intestinal permeability. Increased gut permeability allows bacteria and their byproducts, such as lipopolysaccharides, to pass into the bloodstream, causing systemic inflammation and immune activation[44]. Such inflammation can affect the blood-brain barrier and contribute to neuroinflammation, impacting brain function and behavior and potentially contributing to the cognitive and behavioral symptoms observed in children with ASD[45]. The gut-brain connection is also influenced by metabolic and nutritional factors, which are critical components. Children with ASD often have altered metabolic profiles and frequent nutrient deficiencies. Deficiencies in vitamins, minerals, and essential fatty acids affect brain development and function. Additionally, the composition and diversity of the gut microbiota can be influenced by dietary factors, which can impact the gut-brain axis[46].
Research on the gut-brain connection in children with ASD presents new opportunities for diagnosis and treatment. By studying the composition and function of gut microbiota and examining gut permeability and immune markers, potential biomarkers for ASD diagnosis may be uncovered. Furthermore, interventions that target the gut microbiota, such as probiotics, prebiotics, and dietary adjustments, have shown promise in mitigating ASD-related symptoms and improving overall well-being[47]. To further understand the gut-brain connection in children with ASD, more comprehensive research is necessary. Longitudinal studies are required to determine whether gut dysbiosis precedes the onset of ASD symptoms or is a consequence of the disorder. Additionally, investigating the impact of early-life interventions, like breastfeeding and antibiotic exposure, on the gut microbiota and subsequent ASD risk is crucial. Technological advancements, such as metagenomics and metabolomics, will provide more comprehensive insights into the mechanisms underlying the gut-brain connection in ASD[48].
Common symptoms and common mechanisms for GI and ASD spectrum disorders
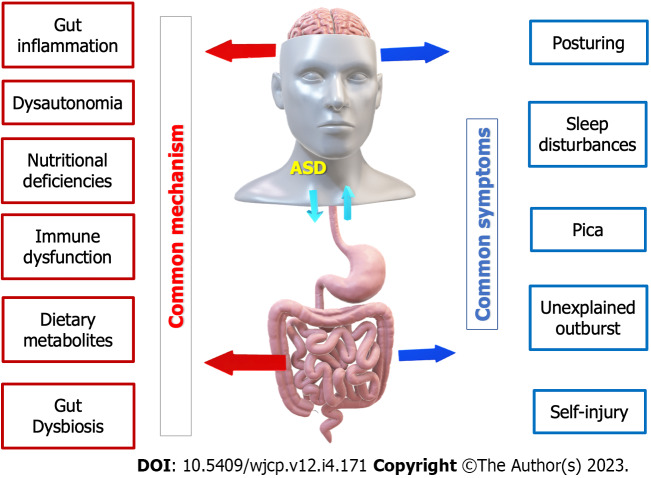
While GI disorders and ASD are distinct conditions, some overlapping symptoms and potential shared mechanisms exist (Figure 2). It’s important to note that researchers are still studying these associations, and the exact nature of the relationship between GI disorders and ASD is not fully understood[20]. Some common GI symptoms include abdominal pain, diarrhea, constipation, bloating, and gastroesophageal reflux, observed in individuals with ASD and may contribute to their behavioral and sensory issues. Both GI disorders and ASD also involve sensory sensitivities, such as heightened responses to certain textures, physical pressure, movement, sounds, tastes, and smells. Additionally, many individuals with both conditions exhibit food selectivity, preferring specific foods while avoiding others, and may limit their diet due to food intolerances and/or sensitivities[49].
Figure 2.
The mutual interaction between autism spectrum disorders and gastrointestinal disorders. ASD: Autism spectrum disorders.
Common mechanisms between the two conditions include gut-brain axis disruptions, gut and systemic inflammation, altered serotonin levels, and genetic factors. For example, alterations in gut microbiota composition, intestinal permeability, and abnormal immune responses have been implicated in GI disorders and ASD[44]. Chronic low-grade inflammation in the gut may lead to systemic inflammation, which can affect brain function and contribute to cognitive, sensory processing and integration, and behavioral symptoms seen in ASD. Additionally, altered serotonin levels have been found in both conditions, suggesting a potential link between them[50]. Furthermore, GI disorders and ASD have a genetic component, and certain genetic variations may make individuals more susceptible to developing one condition when the other is present[51].
Gastroesophageal reflux
Gastroesophageal reflux is a common GI issue where stomach acid and contents flow back into the esophagus. It can affect individuals with or without ASD, but studies suggest that those with ASD may have a higher prevalence of gastroesophageal reflux[52]. The exact reasons for this are not fully understood, but possible factors include delayed gastric emptying, abnormal esophageal motility, and differences in sensory processing[20].
Individuals with ASD who suffer from gastroesophageal reflux may exhibit abnormal behaviors due to various factors. Although the link between gastroesophageal reflux and abnormal behaviors in ASD is not entirely understood, sensory sensitivities are believed to play a significant role[53]. This is because discomfort related to gastroesophageal reflux can trigger physical discomfort and pain, leading to irritability, restlessness, or agitation, which may manifest as abnormal behaviors[54]. Communication challenges may also contribute to the problem, as patients with ASD may have difficulty expressing their discomfort or pain verbally. Instead, they may use unconventional ways of communicating their distress, such as self-stimulating or challenging behaviors[55]. Individuals with ASD thrive on routines and predictability, making gastroesophageal reflux significantly disrupt their daily routines. For instance, mealtimes or feeding schedules may be affected, leading to frustration or anxiety, which can trigger changes in behavior or increased rigidity in following established patterns. Heightened anxiety and stress levels can trigger or exacerbate abnormal behaviors to cope with physical and emotional discomfort[21].
Diagnosing gastroesophageal reflux in individuals with ASD can be difficult as they may exhibit atypical or subtle symptoms. These could include irritability, behavioral changes, eating refusal, gagging, choking, or discomfort during feeding. Communication difficulties may further hinder their ability to accurately express or describe their symptoms[52]. As such, it is crucial to identify the signs of gastroesophageal reflux and seek appropriate medical attention. A healthcare professional specializing in ASD can determine the underlying cause of gastroesophageal reflux and suggest treatments like dietary adjustments, medications to reduce acid reflux, or other interventions as required[56]. Managing gastroesophageal reflux in individuals with ASD involves following standard guidelines for gastroesophageal reflux treatment. These may include making lifestyle changes such as adjusting their diet, elevating their head during sleep, managing their weight, and refraining from lying down right after eating. Depending on the severity of the condition, medications like antacids, proton pump inhibitors, or histamine-2 receptor blockers may be prescribed to reduce acid production or neutralize stomach acid[57].
Helping individuals with ASD communicate their discomfort and pain can be achieved through various methods, such as alternative communication, visual aids, or assistive technology[58]. Additionally, sensory therapy plans and integration strategies can be implemented at home to manage discomfort related to gastroesophageal reflux. This can involve activities that provide sensory input, offering comfort items like weighted blankets or fidget toys, or creating a sensory-friendly eating environment[59]. Consulting certified occupational therapists with a sensory integration specialty can be beneficial in creating specific plans to address abnormal sensory processes that contribute to behaviors associated with gastroesophageal reflux. These plans may include using sensory tools, training alternative coping skills, providing visual schedules, or employing positive reinforcement techniques[60]. Establishing routines and maintaining predictability can also help individuals with ASD manage disruptions caused by gastroesophageal reflux. Precise visual schedules and social stories can assist them in understanding and preparing for changes in their daily routine[61]. Consistent monitoring, follow-up, and open communication between caregivers, healthcare professionals, and educators are essential for effectively managing gastroesophageal reflux in individuals with ASD[62].
Abdominal pain
Individuals with ASD commonly report experiencing abdominal pain, which can have varying severities and causes, including GI and non-GI factors. To determine the underlying cause, conducting a comprehensive differential diagnosis is significantly essential. Several GI causes may affect individuals with ASD. These include gastroesophageal reflux disease, constipation, irritable bowel syndrome, food intolerances or sensitivities, and inflammatory bowel disease[5]. Gastroesophageal reflux disease is caused by stomach acid flowing back into the esophagus and can cause discomfort and irritation. Constipation can be more prevalent in individuals with ASD and may lead to abdominal pain. Irritable bowel syndrome is a functional GI disorder characterized by changes in bowel habits, bloating, and abdominal pain. Some individuals with ASD may have specific intolerances or sensitivities to certain foods, such as gluten or lactose. Inflammatory bowel disease, including crohn’s disease and ulcerative colitis, involves chronic digestive tract inflammation and can cause abdominal pain[63].
Aside from GI causes, other factors can lead to abdominal pain, such as sensory sensitivities, urinary tract infections, musculoskeletal issues, anxiety and stress, and medication side effects. Individuals with ASD may experience heightened sensory responses, leading to discomfort from certain sensations like pressure on the abdomen, clothing textures, or internal sensations. Urinary tract infections, including bladder infections, can also cause abdominal pain, especially for those with difficulty expressing or communicating their symptoms[64]. Muscle strain, spasms, or other musculoskeletal problems can result in abdominal pain. Emotional factors like anxiety and stress can also have physical manifestations like abdominal pain. Furthermore, some medications used to treat ASD symptoms or other co-occurring conditions may list abdominal pain as a potential side effect[65].
Diagnosing abdominal pain in children with ASD can be challenging due to various factors. Communication and behavioral differences associated with ASD can make it difficult to assess the severity and identify the potential underlying causes of the pain. Children with ASD may have difficulty expressing their symptoms verbally or have limited communication skills[66]. Describing the location, intensity, or nature of their abdominal pain can be challenging, making it difficult for healthcare providers to assess the severity of the pain and identify potential underlying causes. They may also have heightened sensory sensitivities that can affect their perception and response to pain[67]. They may exhibit atypical reactions or engage in self-injury as a response to pain, which can complicate diagnosis. In addition, abdominal pain can present with various underlying causes, some of which may overlap with common behavioral or GI symptoms seen in ASD. Differentiating between primary GI issues and pain-related behaviors associated with ASD can be complex[68].
Diagnostic overshadowing is another problem involving correctly identifying abdominal pain in children with ASD. Healthcare providers may attribute symptoms solely to a child’s ASD rather than considering other potential underlying medical conditions[69]. Abdominal pain may be overlooked or dismissed as a behavioral issue related to ASD, leading to delayed diagnosis and appropriate treatment. Furthermore, children with ASD often experience high anxiety levels and have difficulty coping with medical procedures or assessments, making it harder to conduct thorough physical examinations, laboratory tests, or imaging studies to identify the cause of abdominal pain[70]. It can be challenging for some patients with ASD to access appropriate healthcare, as they may encounter various barriers. These barriers can range from difficulties in healthcare settings to sensory overload or difficulty adapting to new environments. Unfortunately, limited access to healthcare can lead to delays in diagnosis and appropriate treatment, especially when addressing abdominal pain[71].
When children with ASD experience abdominal pain, they may express their discomfort differently. They may resort to aggression or self-injurious behaviors if they cannot effectively communicate their pain or frustration. Additionally, they may become more agitated or irritable, exhibit social withdrawal or isolation, and display changes in eating habits or disruptive behaviors during mealtimes. Chronic abdominal pain can also increase anxiety and emotional dysregulation, leading to outbursts, meltdowns, or heightened anxiety levels. Furthermore, sleep disturbances can occur, which can further exacerbate behavioral challenges and affect overall well-being and functioning[66,72].
To address these challenges, healthcare professionals may employ various strategies. One method uses visual aids, such as visual pain scales or communication boards, to help children express their pain levels and location[73]. Another approach is closely observing behavioral changes or non-verbal cues that may indicate discomfort or pain. Involving parents, caregivers, or teachers familiar with the child’s behavior patterns can also provide valuable insights into changes in behavior or routines. A multidisciplinary team of pediatricians, gastroenterologists, psychologists, and occupational therapists can provide a comprehensive assessment and accurate diagnosis in complex cases[56]. It is crucial to acknowledge that every person with ASD is unique, and their medical background, sensory processing profiles, and behavioral patterns differ. Individual situations must be considered when assessing and addressing abdominal discomfort[74].
Diarrhea
Diarrhea can occur in individuals with ASD, just as it can affect anyone else. While diarrhea is not directly related to ASD, individuals with ASD may be more prone to specific GI issues, including diarrhea. Diarrhea can affect individuals with ASD for various reasons, and certain factors may increase these patients’ diarrhea risk. These factors include sensory sensitivities and dietary preferences, dietary changes and restrictions, food intolerances and sensitivities, GI disorders, medications and supplements, and anxiety and stress. Pica is also a significant risk factor for diarrhea in children with ASD[49]. Pica, which is eating non-food items, is a behavior sometimes exhibited by individuals with ASD. While pica itself may not directly cause diarrhea, there are several ways by which ingesting non-food substances can increase the risk of developing GI issues, including diarrhea. These include irritation of the digestive system, blockages in the digestive tract, bacterial or parasitic infections, and nutritional imbalances. It’s important to understand that pica is a complex behavior with various underlying causes and can occur in individuals with or without ASD[75]. Chronic diarrhea could result from immune dysfunction, irritable bowel syndrome, inflammatory bowel disease, intestinal infection, food allergies, celiac disease, lactose intolerance, or excessive intake of sugary liquids such as fruit juices. In some cases, fecal encopresis due to severe chronic constipation can be misdiagnosed as chronic diarrhea[76]. However, it is essential to remember that not all individuals with ASD will experience diarrhea or have an increased risk compared to the general population. Each person is unique, and other co-occurring conditions or individual factors may also play a role[8].
Sometimes, children with ASD may display behaviors that resemble or are mistaken for diarrhea. These behaviors can be categorized into four groups: Sensory-seeking behaviors, repetitive or ritualistic behaviors, communication difficulties, and sensory sensitivities[77]. Sensory-seeking behaviors involve playing with materials that may resemble the appearance or consistency of diarrhea, such as enjoying squishing or smearing substances like playdough, mud, or other malleable materials, which may appear similar to diarrhea but are not actual fecal matter[78]. Repetitive behaviors may involve movements or actions around the diaper area that can be mistaken for diarrhea. Still, it is crucial to differentiate between purposeful behaviors and physical neuro-sensory symptoms. Children with ASD may have difficulties expressing themselves verbally, leading to unconventional means of communication that may be misinterpreted as indicating diarrhea. Lastly, sensory sensitivities can cause discomfort or aversion to certain physical sensations around the diaper area, which may be misinterpreted as diarrhea[79].
Managing diarrhea in patients with ASD requires a personalized approach that considers both general strategies for diarrhea management and specific considerations for the individual’s unique needs and challenges associated with ASD. Hydration is crucial to keep the individual hydrated, and a balanced diet that includes easy-to-digest and gentle foods is key. Probiotics can help restore the natural balance of gut flora and aid digestion; medications or supplements may be necessary in some cases. Visual and social support, sensory accommodation, communication support, and personalized strategies are crucial for managing diarrhea effectively. It’s essential to collaborate with healthcare professionals, clinical occupational therapists specialized and certified in sensory integration, and caregivers familiar with the individual's abilities to provide tailored strategies for managing diarrhea[80,81].
Constipation
Constipation is a common GI problem that individuals with ASD may be more susceptible to. While there is no direct link between ASD and constipation, some studies suggest that individuals with ASD are prone to experiencing constipation 3.5 times more than the general population[82]. The reasons for this are unclear, but several factors could contribute. Many individuals with ASD have sensory sensitivities, including sensitivities to certain textures or tastes of food. This may result in a limited diet lacking fiber-rich foods, leading to constipation. Some individuals with ASD may have limited food preferences or engage in selective eating patterns that exclude fruits, vegetables, and other high-fiber foods necessary for regular bowel movements[83]. Individuals with ASD may have difficulty recognizing and responding to bodily cues, such as the urge to have a bowel movement. This can lead to a delay in seeking a restroom or actively avoiding using the bathroom due to sensory sensitivities or rigid routines[84]. Some medications commonly prescribed for individuals with ASD, such as antipsychotics or certain antiepileptic drugs, can have constipation as a side effect, which can exacerbate the problem. Differences in the gut microbiome of individuals with ASD have been found in some studies, and changes in gut microbiota can influence bowel function and potentially contribute to constipation[85].
For individuals with ASD, constipation can sometimes lead to or be interpreted as abnormal behaviors. Although the precise connection between constipation and such behaviors is not yet fully understood, several factors could contribute to this association. One factor is sensory sensitivities, as many individuals with ASD are sensitive to certain sensations, and this discomfort can extend to constipation[86]. The physical discomfort and pain caused by constipation may lead to increased irritability, restlessness, or agitation, which could manifest as abnormal behaviors[87]. Communication challenges can also be a factor, as some individuals with ASD may have difficulty verbally expressing their discomfort or pain. They may instead resort to unconventional methods of communicating their distress[66]. Furthermore, individuals with ASD rely heavily on routines and predictability, and constipation can interfere with their daily routines, causing frustration or anxiety, leading to changes in behavior or increased rigidity in following established patterns[88]. Finally, constipation can cause discomfort and pain, leading to increased anxiety and stress levels in individuals with ASD, which can trigger or worsen abnormal behaviors to cope with physical and emotional distress[89].
When evaluating constipation in children with ASD, a comprehensive investigation is necessary to identify root causes and appropriate interventions. It’s crucial to obtain a detailed medical history to understand the child's bowel habits, diet, fluid intake, and any previous diagnoses or treatments related to constipation. A physical examination is necessary to identify signs of constipation and any physical abnormalities that could contribute to it. A bowel movement diary can provide valuable information about bowel patterns, triggers, and dietary habits[90]. A dietary assessment is also essential to identify potential dietary factors contributing to constipation and assess the intake of fiber-rich foods, fruits, vegetables, and fluids[91]. Toileting skills should be evaluated to determine any challenges or difficulties related to recognizing the need to use the bathroom or any routines that may interfere with regular bowel movements[92]. Additional medical tests may be necessary to determine the severity of constipation or rule out underlying conditions. Collaboration with specialists may be beneficial in complex cases or if there are concerns about underlying medical conditions. An individualized management plan can be developed to address sensory sensitivities, communication challenges, or behavioral factors that may impact the child’s ability to manage constipation effectively[93].
When dealing with constipation-related behaviors in individuals with ASD, it’s essential to consider several approaches. Firstly, recognizing the signs of constipation and seeking medical intervention is vital. It’s advisable to consult a healthcare professional experienced in ASD, as they can help identify the underlying cause of constipation and recommend appropriate treatments such as dietary modifications, fiber supplements, stool softeners, or laxatives[88]. Secondly, encouraging and facilitating effective communication can help individuals with ASD express their discomfort and pain. Alternative communication methods, such as visual aids or assistive technology, can enhance communication and reduce frustration[58]. Thirdly, sensory strategies can help manage discomfort related to constipation. This may include providing sensory input through appropriate sensory activities, offering comfort items like weighted blankets or fidget toys, or creating a sensory-friendly bathroom environment to make bowel movements more comfortable[94]. Fourthly, collaborating with behavioral therapists or professionals experienced in working with individuals with ASD can be helpful. They can develop behavior support plans that address the specific abnormal behaviors associated with constipation. Strategies may include teaching alternative coping skills, providing visual schedules, or implementing positive reinforcement techniques[95]. Lastly, maintaining established routines and predictability as much as possible can help individuals with ASD manage the disruptions caused by constipation. Clear visual schedules and social stories can help them understand and prepare for changes in their daily routine[96].
All the previous strategies should work hand in hand with strategies aimed at relieving constipation. One effective strategy is adjusting one's diet to include fiber, fruits, vegetables, and whole grains. Individuals with sensory sensitivities may need gradual food diversification and the introduction of new textures and tastes[97]. Staying hydrated by drinking plenty of fluids, especially water, can help soften the stool and ease bowel movements[98]. Establishing a regular bathroom routine, preferably after meals, can also help develop a consistent routine for bowel movements. Regular exercise and physical activity can stimulate bowel movements and promote healthy digestion. If constipation persists and is associated with medication side effects, consulting with a healthcare professional is essential to explore potential adjustments in medication or additional treatments[99]. Regular follow-up and monitoring are crucial to assess the effectiveness of interventions and make necessary adjustments.
Coeliac disease
Coeliac disease and ASD are two distinct medical conditions that can sometimes exist simultaneously in an individual. Coeliac disease is an autoimmune condition that is triggered by consuming foods that contain gluten. It affects the small intestine, leading to inflammation and damage to the lining, which may cause various GI symptoms and nutritional deficiencies[100]. Although some studies and case reports suggest a possible link between coeliac disease and ASD, the exact nature of this connection remains unclear. Some scientists suggest that shared genetic or immune system abnormalities might contribute to some individuals’ co-occurrence of these conditions[101]. However, further research is necessary to understand this association’s underlying mechanisms and prevalence. Despite studies finding no higher prevalence of coeliac disease in patients with ASD, one child per 68 children with coeliac disease will develop ASD, and one child per 130 children with ASD will develop coeliac disease[9]. There is a strong association between coeliac disease, even in the absence of GI symptoms, epilepsy, and cerebral calcifications, and positive responses to dietary changes in these patients. Investigation and treatment of coeliac disease, non-coeliac gluten sensitivity (NCGS), and epilepsy, even without typical GI symptoms or overt seizures, could yield positive outcomes for patients with ASD[28].
It is essential to consider the possibility of non-coeliac gluten or wheat sensitivity in children with ASD, who are more likely to have atopy and allergies, especially if they show symptoms of irritable bowel[29]. When neurological manifestations with probable autoimmune etiology are unclear, it is recommended to determine the transglutaminase-2 autoantibody titer to consider the possibility of gluten sensitivity[102]. The relationship between coeliac disease and abnormal behavior in children with ASD is complex and not yet fully understood. Both coeliac disease and ASD share some symptoms, such as GI issues, irritability, anxiety, and sleep disturbances[103]. Consuming gluten can lead to inflammation and other immune responses that may affect behavior and cognition. Coeliac disease can also cause nutrient deficiencies, such as deficiencies in vitamins and minerals, which might contribute to abnormal behavior or exacerbate existing behavioral challenges in children with ASD[104]. While implementing a gluten-free diet in children with ASD who have coeliac disease or gluten sensitivity may improve behavior, communication, and social interaction, the evidence in this area is limited. Not all studies have shown consistent results. It's important to note that the impact of coeliac disease on behavior can vary among individuals[102].
It’s worth noting that having coeliac disease doesn’t necessarily mean an individual has ASD, and vice versa. These conditions can occur separately. However, if a child with ASD experiences GI symptoms or other signs that suggest coeliac disease, it's crucial to seek advice from a healthcare professional. Diagnosing coeliac disease in children with ASD follows a similar process to diagnosing coeliac disease in the general population, such as measuring tissue transglutaminase antibody test, determining HLA typing, and performing intestinal biopsies[105]. The gluten-free diet is currently the only effective treatment available and should be recommended for all patients with gluten sensitivity, regardless of the type of manifestations. Medical professionals should be aware of the possibility of NCGS in some patients with ASD, particularly those with atopic disease, migraine, mood, and anxiety disorders. Although many children with ASD improve on a gluten-free, soy-free, and dairy-free diet, it is essential to perform a celiac test before attempting this diet[106].
Impact of GI disorders on children with ASD
GI disorders can significantly impact children with ASD. GI disorders can affect all the child’s life aspects; behavior, quality of life and well-being, social interaction: Communication abilities, education capabilities, and sleep patterns, and increase the risk of epilepsy. There is a strong correlation between GI symptoms and the severity of ASD, indicating that children more severely affected by ASD are likely to have severe GI symptoms. Health professionals should consider the possibility of GI dysfunction in patients with ASD, especially those presenting with strange posturing or movements, sleep disorders, food intolerances, and aggressive or self-injurious behaviors[80]. Table 1 summarizes the impact of GI disorders on the various life aspects of patients with ASD.
Table 1.
Impact of gastrointestinal disorders on children with autism spectrum disorders
|
Domain
|
The impact
|
| Patient’s behavior | Abnormal posturing, self-injury, sudden outbursts, social withdrawal or isolation, and changes in eating habits |
| Self-stimulatory behaviors | |
| Toe walking, increased irritability, restlessness, and agitation | |
| Poor attention, reduced food intake, or avoidance of certain foods | |
| Poor response to psychotropic medications | |
| Patient’s social interaction | Difficult engagement in social interactions |
| Anxiety, irritability, and withdrawal from social situations with social avoidance | |
| Decrease the ability to engage in social play, follow social cues, or maintain relationships with peers and family members | |
| Poor development of social skills | |
| Patient’s sleep | Disrupted sleep patterns |
| Disruption of sleep-wake cycles regulation | |
| Patient’s epilepsy | Altered brain electrical activity |
| Increased risk of epileptic tendencies | |
| Decrease epilepsy thresholds | |
| Interactions with anti-epileptic medications | |
| Patient’s education | Increase absenteeism and poor attendance |
| Poor academic performance | |
| Poor participation | |
| Decreased ability to concentrate | |
| Poor engagement in learning activities | |
| Reduced motivation and participation in classroom activities | |
| Poor participation in group activities | |
| Poor participation in food-related activities | |
| Poor cognitive functioning | |
| Patient’s quality of life | Poor eating, sleeping, and social interactions |
| Reduced appetite, causing poor nutrition and weight loss |
Impact on the patient’s behavior
GI disorders can cause pain-induced agitation and irritability in children, leading to abnormal behaviors in individuals with ASD. For instance, GI pain may be mistaken for behavioral issues like abnormal posturing, self-injury, sudden outbursts, social withdrawal or isolation, and changes in eating habits. Individuals with ASD and oesophageal ulcerations may exhibit self-stimulatory behaviors in response to the pain or discomfort they experience[79]. Additionally, gastroesophageal reflux can lead to frequent nighttime awakenings. If the patient is experiencing constipation, it can lead to a range of behavioral changes such as toe walking, increased irritability, restlessness, and agitation. The patient may also experience abnormal sleep patterns, such as difficulties falling asleep, frequent awakenings, or restless sleep, resulting in daytime irritability, fatigue, and behavioral challenges[107]. Constipation can cause poor attention, reduced food intake, or avoidance of certain foods. Additionally, toilet training problems could be caused by chronic diarrhea or constipation. If the patient with ASD frequently digs into the rectal area, it could indicate anal itching, which parasitic infestations like enterobiasis may cause. However, if the GI disorder is identified and treated with medical intervention, the behavioral problem may decrease. It's important to note that psychotropic medications may not be effective and could worsen the issue if they have adverse GI effects, especially when abdominal pain or discomfort is present[108]. Table 2 summarizes the different behavior changes associated with GI disorders in individuals with ASD.
Table 2.
The different behavior changes that result from various gastrointestinal disorders
|
GI disorder
|
Behavior changes
|
| Gastroesophageal reflux with/without ulcerations | Self-stimulatory behaviors (commonly called stimming) |
| Constant eating/drinking/swallowing (grazing behavior) | |
| Frequent nighttime awakenings, abnormal posturing, pushing out the jaw, straining the neck, and tapping the throat, increased self-injury, and other challenging behaviors | |
| Abdominal pain | Increased anxiety levels and emotional dysregulation, leading to outbursts and meltdowns |
| Repetitive rocking and other repetitive behaviors. | |
| Blinking, sudden screaming, spinning, and fixed look | |
| Agitation: Pacing, jumping up and down | |
| Sleep disturbances, exacerbating the behavioral challenges and affecting overall well-being and functioning | |
| Constipation | Tip-toe walking |
| Increased irritability, restlessness, and agitation | |
| Abnormal sleep patterns, such as difficulties falling asleep, frequent awakenings, or restless sleep | |
| Daytime irritability, poor attention, fatigue, and behavioral challenges | |
| Reduced food intake, or avoidance of certain foods | |
| Toilet training problems | |
| Diarrhea | Social withdrawal if the child is experiencing discomfort or embarrassment due to diarrhea |
| Increased self-stimulatory behaviors like engagement in repetitive or self-stimulatory behaviors to self-soothe, especially during the time of discomfort | |
| Toilet training problems | |
| Change appetite with changing eating patterns or food preferences |
Impact on the patient’s social interaction
For individuals with ASD, GI disorders can significantly affect their social interactions. The GI disorders-related discomfort can make it difficult for individuals with ASD to engage in social interactions, leading to anxiety, irritability, or a desire to withdraw from social situations[109]. The sensory discomfort associated with GI issues, like nausea or GI distress, can be overwhelming and make it challenging for individuals to focus on social interactions or be present in social environments. The discomfort or pain caused by GI disorders may also interfere with verbal communication or affect the individual's ability to engage in reciprocal conversation, leading to limited social interactions and potential misunderstandings[67]. In addition, GI disorders-related behavioral changes can impact social interactions by affecting their ability to engage in social play, follow social cues, or maintain relationships with peers and family members. Meanwhile, GI disorders can increase anxiety in individuals with ASD, leading to social avoidance. They may develop fear or anxiety related to social situations due to concerns about experiencing GI symptoms or being unable to manage them in public. This social avoidance can limit opportunities for social interaction and hinder the development of social skills[110].
Impact on the patient’s sleep
Individuals with ASD spectrum disorder often experience disrupted sleep patterns due to GI disorders. Studies have found a higher prevalence of GI issues among those with ASD, although the exact relationship between the two is still being researched. Symptoms such as constipation, diarrhea, abdominal pain, and acid reflux can cause significant discomfort and pain, making it challenging for people with ASD to fall asleep and stay asleep[111]. Moreover, sensory sensitivities among people with ASD can exacerbate the discomfort caused by such symptoms, making relaxation and sleep difficult. As individuals with ASD rely on routines and predictability to feel secure, any disruptions caused by GI disorders can lead to increased anxiety and difficulty in transitioning to sleep[112]. Changes in diet, medication, or medical procedures can also contribute to sleep disturbances. Additionally, GI disorders can disrupt the regulation of sleep-wake cycles, affecting the production and regulation of hormones and neurotransmitters essential for sleep regulation[113]. Therefore, it is crucial to address GI issues in people with ASD to manage physical symptoms and improve overall well-being. Effective management strategies may involve dietary modifications, medication, behavioral interventions, and addressing sensory sensitivities. Pediatric gastroenterologists and ASD-specializing dietitians can collaborate to develop these strategies[114].
Impact on the patient’s brain activities and epileptic tendencies
Individuals with ASD who experience GI disorders may be at risk of epileptic tendencies due to the potential influence on brain electrical activities. The gut-brain axis facilitates bidirectional communication between the gut and brain, meaning disruptions in the gut, such as inflammation, altered gut microbiota, or intestinal permeability, can impact brain function and neural electrical activities[115]. Therefore, GI disorders in individuals with ASD may contribute to dysregulation in brain electrical activities through this gut-brain connection[116].
GI disorders have the potential to cause inflammation in the gut, which in turn releases pro-inflammatory molecules that can affect the brain and lead to neuroinflammation. This neuroinflammation has been linked to various neurological conditions, including epilepsy, and can result in altered brain electrical activity and an increased likelihood of seizures[40]. Chronic inflammation in the gut may also contribute to developing or worsening epileptic tendencies. Interestingly, some individuals with ASD, epilepsy, and GI disorders may share genetic factors contributing to these conditions. Specifically, genetic variations and mutations related to GI function and neuronal excitability can increase the risk of epilepsy[117]. These shared genetic factors may influence brain electrical activities and contribute to the co-occurrence of GI disorders and epileptic tendencies in individuals with ASD[118].
Individuals with ASD who are prone to epilepsy may experience seizures triggered by GI disorders, especially when combined with sensory sensitivities. Specific triggers, including certain foods, GI pain, or changes in gut microbiota, can increase the likelihood of seizures in susceptible individuals[119]. GI discomfort or inflammation can lower the seizure threshold and cause abnormal brain electrical activities. It is crucial to consider potential interactions between medications used to treat GI disorders and those prescribed for epilepsy[120]. Some medications, such as antacids, proton pump inhibitors, or antibiotics, may affect the efficacy of antiepileptic drugs or increase the risk of adverse effects. Careful management and medication monitoring are essential to minimize potential interactions and ensure optimal treatment for both conditions[121].
It is crucial to understand how GI disorders affect brain electrical activities and epileptic tendencies in individuals with ASD to provide comprehensive care[44]. A multidisciplinary approach involving gastroenterologists, neurologists, and other healthcare professionals must address GI and neurological aspects. Treatment strategies may include dietary modifications, targeted therapies for GI disorders, antiepileptic medications, and lifestyle adjustments to manage both conditions effectively. It is important to note that while there is an association between ASD, GI disorders, and epilepsy, the exact mechanisms underlying these relationships are still being investigated[122]. The impact of GI disorders on brain electrical activities and epileptic tendencies can vary among individuals with ASD, making personalized care plans tailored to each person's unique needs and challenges essential for optimal outcomes[123].
Impact on the child’s learning
For individuals with ASD, GI disorders can significantly impact their learning and education. These disorders can affect various aspects of a student’s educational experience, including academic performance, attendance, participation, and overall well-being[124]. GI disorders can impact education for individuals with ASD in different ways. GI disorders often cause physical discomfort and pain, making it challenging for students to concentrate and engage in learning activities. Persistent abdominal pain, bloating, or other symptoms can distract and affect students’ ability to focus on their studies[125]. GI disorders can cause fatigue and a lack of energy in individuals with ASD, reducing motivation and participation in classroom activities. This makes it difficult for students to actively engage in lessons, complete assignments, or participate in group activities. Many individuals with ASD have sensitivities/over-responsivity that extend to food textures, pressure, tastes, smells, and GI movement[126]. In the case of GI disorders, certain foods or dietary restrictions may be necessary, which can further limit food choices and cause additional stress or anxiety for the student. This can make it difficult for them to navigate school environments where food-related activities are common[127]. Severe GI symptoms may lead to increased absenteeism from school. Students with ASD who experience frequent GI distress may need to miss school days or leave early due to discomfort or medical appointments. This can result in missed instruction, reduced participation in classroom activities, and disruptions in educational progress[128]. GI disorders can contribute to behavioral changes and emotional distress in individuals with ASD. The discomfort and pain associated with GI issues can lead to increased irritability, anxiety, or even meltdowns. These behavioral and emotional challenges can disrupt the learning environment and entirely hinder the student’s ability to engage in educational activities[129]. Research suggests that there may be a bidirectional relationship between GI health and cognitive functioning. GI inflammation and imbalances in gut bacteria may affect mental processes, including attention, concentration, memory, and executive functioning. Consequently, students with ASD and GI disorders may experience difficulties in sensory and information processing and integration, problem-solving, and academic achievement[130]. Some individuals with autism and GI disorders may require specific dietary modifications to manage their symptoms. These dietary restrictions may limit their food choices and require accommodations in school settings. Collaborating with school staff may be necessary to ensure meeting the student’s nutritional needs while maintaining a safe and inclusive environment[131].
Collaboration is key in addressing the educational impact of GI disorders on individuals with ASD. Educators, school staff, and parents can work together to support these students by implementing various strategies. This includes maintaining open communication between all parties to comprehensively understand the student's needs and challenges[132]. It also involves creating a supportive and understanding school environment that considers the unique needs and challenges of students with ASD and GI disorders. Sensory-friendly strategies, like providing quiet spaces for breaks and accommodating sensory sensitivities related to food, can also be implemented. Developing individualized learning plans or 504 plans in collaboration with healthcare professionals and families is crucial to address the student’s specific needs[133]. Providing access to appropriate healthcare interventions, such as medication management, dietary adjustments, or therapies to manage GI symptoms, is also essential. Communication and collaboration between healthcare providers and school personnel help ensure consistency in managing the student’s GI issues[134].
Academic accommodations should be offered. These accommodations may include flexible schedules, extra time for assignments or tests, and access to support services like occupational therapy and clinical psychology counseling. Creating a supportive and inclusive classroom environment that promotes understanding, empathy, and tolerance for individual differences is also crucial[135]. Sensory needs can be addressed through sensory environments, breaks, or by utilizing personalized/customized sensory tools by occupational therapists-sensory integration specialists to alleviate discomfort or anxiety related to GI issues. By addressing the impact of GI disorders on education and providing appropriate support, individuals with ASD can have a better chance of achieving their learning goals and maximizing their potential[67].
Impact on the patient’s quality of life
Individuals with ASD may experience a significant decline in their quality of life due to GI disorders. These disorders, including constipation, diarrhea, acid reflux, and abdominal pain, can cause persistent physical discomfort and pain. The discomfort can be distressing and affect daily activities, such as eating, sleeping, and social interactions[136]. It can also reduce appetite, cause poor nutrition, and lead to weight loss, further affecting overall health and well-being. Many individuals with ASD have sensory sensitivities, and GI symptoms can exacerbate these sensitivities[137]. The discomfort caused by GI issues, such as bloating or stomach pain, can be overwhelming for individuals with heightened sensory sensitivity, leading to increased anxiety and challenges in daily activities. Individuals with ASD often rely on routines and predictability to feel secure and comfortable. GI disorders can disrupt daily routines, increasing stress and anxiety[67]. Changes in diet, medication regimens, or medical procedures related to managing GI issues may require adjustments to established routines, which can be challenging for individuals with ASD and impact their overall quality of life. GI symptoms can affect social interactions and participation in activities[138]. Individuals with ASD may experience embarrassment, anxiety, or discomfort due to GI issues, leading to social withdrawal or avoidance of certain situations. This can impact their ability to form and maintain relationships, participate in social events, and engage in educational or vocational settings. Living with GI disorders can affect the emotional well-being of individuals with ASD[139]. Chronic pain, discomfort, and the challenges of managing GI symptoms can increase stress, anxiety, and depression. These emotional factors can further impact the overall quality of life and make engaging in activities and enjoying daily life difficult[140].
Improving the quality of life for individuals with ASD requires addressing their GI disorders. Seeking medical evaluation and management from healthcare professionals, such as pediatric gastroenterologists, occupational therapists specializing in sensory integration, and dietitians specializing in ASD, can effectively treat GI symptoms. Treatment options may include dietary modifications, medications, behavioral interventions, and occupational therapy based on sensory integration[56]. Equally important is providing support and understanding within the individual’s social environment, including family, friends, and educators. Creating a supportive and inclusive environment that accommodates the individual’s needs and ensures access to appropriate healthcare and educational resources can significantly enhance their overall quality of life[141]. It is essential to recognize that each person with ASD and GI disorders is unique, and the impact on their quality of life may vary. Therefore, a personalized approach that considers the individual’s specific needs, challenges, and strengths is essential for promoting their well-being and enhancing their overall quality of life[142].
GI tract as a key for the diagnosis and evaluation of ASD
There is no definitive way to diagnose ASD based on examining the GI tract. Healthcare professionals, like psychiatrists, psychologists, and developmental pediatricians, primarily rely on behavioral assessments and observations to diagnose ASD. These assessments evaluate a person’s social interactions, communication skills, and repetitive or restricted behaviors[143]. Although some have claimed that specific tests, such as stool analysis or measurements of specific gut bacteria, could help diagnose ASD, no consistent scientific evidence supports these claims[144]. Therefore, these tests are not regarded as reliable diagnostic tools for ASD. However, specific GI tests may lead to a higher likelihood of developing ASD or experiencing more severe symptoms of ASD.
Gut microbiota print
Research suggests that changes to a child’s intestinal microbiota during their early years can impact their emotional and cognitive development later on. Specifically, certain species of bacteria found in the gut microbiota (or the absence of others) may play a significant role in the development of ASD[145,146]. Jendraszak et al[147] showed that children with ASD have a distinct fecal microbiota pattern different from the neurotypically developed children with lower Bifidobacterium spp. Kang et al[148] conducted a study comparing the bacterial composition in fecal samples from 19 children with ASD who had varying GI symptoms and 20 neurotypical controls with minimal GI symptoms. They used high-throughput sequencing of the 16S rDNA gene. The study found that the presence of autistic symptoms, rather than the severity of GI symptoms, was associated with lower levels of the bacterial genera Prevotella, Coprococcus, and unclassified Veillonellaceae. Furthermore, in their research, Adam et al[149] discovered a significant correlation between the severity of ASD and GI symptoms. This suggests that children with more severe ASD are more prone to experiencing intense GI symptoms and vice versa. It is also possible that underlying GI issues could contribute to the severity of ASD symptoms. However, it is regrettable that two recent meta-analyses have revealed inconsistent outcomes in investigating the intestinal microbiota of children with ASD through cohort studies[150,151].
A study by Wu et al[152] aimed to confirm previous research on the gut microbiome’s association with ASD. They utilized machine learning techniques to identify potential biomarkers for ASD through feature selection and classification evaluation in training, validation, and independent diagnosis cohorts. The results revealed that Prevotella, Roseburia, Ruminococcus, Megasphaera, and Streptococcus could be potential biomarkers for ASD. Prevotella showed significant differences between patients with ASD and typical neuro-developers. Qureshi et al[153] investigated the differences in gut microbial metabolites between children with ASD and GI disorders and typically developing children without GI disorders. They also examined the effects of gut microbiota transfer therapy (MTT) on the fecal metabolites of the group with ASD. Using machine learning, they created 5-metabolite fecal models for classification, which showed significant changes before and after gut MTT. The developed multivariate metabolite models can potentially categorize children with ASD from typically developed children effectively. These machine-learning models can also diagnose children with ASD by comparing their gut microbiome data with subjects with and without ASD. However, these findings did not align with the prediction model established by Zhai et al[154]. Various factors, such as environmental conditions and calculation methods, may influence intestinal microbiota composition. Additionally, the quality control of sequencing data may also affect the prediction model’s accuracy[155]. Further studies are necessary to explore the gut microbiome’s characteristics in ASD, particularly regarding interventions.
Alternations in gut permeability
Research has found that patients with ASD often experience changes in the integrity of their intestinal barrier. In one study, 75% of patients with ASD showed a reduction in the expression of "tight junction" components that form the barrier in the intestine[156]. Zonulin is a protein that regulates the tight junctions between enterocytes and controls how permeable the intestines are. In patients with ASD, the zonulin levels were higher than in healthy controls, which was associated with the severity of ASD symptoms[157]. This suggests that zonulin could be a valuable biomarker for a subgroup of children with ASD who have GI issues related to changes in intestinal integrity. Still, not all studies have confirmed this[158].
GI Tract as a key component in the management of ASD
Effectively managing GI disorders in patients with ASD can significantly benefit their overall well-being, improving their quality of life, better behavior, cognitive function, and educational abilities[159]. Addressing underlying GI issues such as chronic constipation, diarrhea, or gastroesophageal reflux can help alleviate physical discomfort and pain experienced by individuals with ASD. This can, in turn, lead to better digestion, enhanced nutrient absorption, and improved appetite. Properly addressing GI problems can also positively impact mood, attention, irritability, and hyperactivity, potentially reducing challenging behaviors[20]. As individuals with ASD often face communication and social interaction challenges, relieving GI symptoms can improve comfort, reduce distress, and better regulate their sensory systems, creating a more favorable environment for communication and social engagement[21]. Promoting a balanced gut microbiota may also positively affect brain function and cognition in individuals with ASD[160]. Relief from GI symptoms can improve sleep patterns, increase energy levels, and contribute to a general sense of well-being, ultimately leading to a better quality of life for the individual and improved family dynamics[161]. Therefore, the main aim of GI interventions is not to treat ASD but to improve nutritional status, reduce concomitant symptoms, ensure a balanced mood with decreasing anxiety, impulse control, aggression, and stereotypy, and increase the patient’s independence.
Dietary intervention: Treating ASD through dietary interventions has garnered attention and research, although there is limited and contentious scientific evidence to support their effectiveness. It is crucial to recognize that ASD is a multifaceted neurological condition with diverse symptoms and underlying causes, and there is no universally applicable treatment. Nonetheless, specific dietary interventions have been studied in the context of ASD. Many individuals with ASD turn to nutritional interventions, with or without clinical supervision, to help alleviate GI and behavioral symptoms[162]. Different types of dietary interventions can be used for ASD, grouped into four main categories: elimination dietary therapy [e.g., gluten-/casein-free diet, oligoantigenic diet, and specific carbohydrate diet (SCD)], modification dietary therapy (e.g., modified ketogenic diet), supplementation dietary therapy (e.g., minerals, vitamins like Vitamin B6, high dose Vitamin B12, and Vitamin D, antioxidants/polyphenolic compounds, omega 3, omega 6, and camel milk), and exclusion dietary therapy (e.g., excluding food additives)[163]. Although there is significant interest in nutritional interventions for individuals with ASD, there is currently no consensus on the optimal dietary approach to pursue[164]. A meta-analysis conducted by Yu et al[165] has revealed that implementing specific dietary therapies can effectively improve the core symptoms associated with ASD. Adopting a gluten-free diet has been shown to impact social behaviors positively. Despite the promising outcomes, it is essential to note that the small sample size of randomized controlled trials currently limits the effectiveness of dietary therapy for ASD. Therefore, further well-designed and high-quality clinical trials are required to validate these conclusions.
Gluten-free casein-free (GFCF) diet
Eliminating gluten and casein from the diet is a commonly discussed approach for managing ASD. These proteins are found in wheat, other grains, and dairy products. The belief is that they may exacerbate ASD symptoms in certain individuals. It has been observed that children with ASD may have a sensitivity or intolerance to gluten and casein and may exhibit elevated levels of antibodies against certain substances, namely anti-gliadin, anti-casein, and dipeptidyl peptidase 4-a digestive enzyme that plays a crucial role in the breakdown of gliadin into various peptides including gliadinomorphin-7 which has “opioid activity”, able to increase gut membrane permeability, stimulate opioid receptors, and decrease social interaction observed in children with ASD. Gluten also induces a state of systemic inflammation, including neuroinflammation[166,167]. It should be noted that among individuals with ASD, some may also have celiac disease or non-celiac gluten sensitivity. A double-blind, randomized controlled study by Hyman et al[168] involved 14 children between the ages of 3 and 5 with ASD. These children were put on a GFCF diet, and after 6 wk, they were given “dietary challenges” in the form of weekly snacks containing either gluten, casein, gluten plus casein, or neither. The children were monitored for an additional three months after three months of challenges. The study’s findings indicate that the dietary challenges did not significantly affect the children’s sleep quality, hyperactivity, or ASD behaviors. According to a systematic review conducted by Piwowarczyk et al[169], there is little evidence to support the effectiveness of a GFCF diet in alleviating symptoms of ASD in children. A recent meta-analysis by Quan et al[170] suggests that a GFCF diet may effectively reduce stereotypical behaviors and improve cognition in children with ASD. Despite most studies being single-blind, the potential benefits of a GFCF diet are encouraging. There is a need for further studies on a larger scale to confirm these findings.
SCD
A SCD is a dietary approach that limits the intake of complex carbohydrates and specific sugars such as grains, starchy vegetables, lactose, and most processed sugars that are difficult to digest and may negatively affect gut health. On the other side, it allows the intake of specific types of carbohydrates that are believed to be easier to digest, such as monosaccharides and certain disaccharides. It is commonly used to manage conditions such as inflammatory bowel disease and celiac disease. This diet is believed to improve gut health, reduce inflammation, and potentially alleviate some ASD symptoms associated with gut-related issues[171,172]. Although some anecdotes exist on individuals with ASD experiencing better behavior, digestion, and overall well-being through the SCD, scientific research on its effectiveness for ASD is limited. Most studies have been hindered by limitations such as small sample sizes, absence of control groups, and variations in study design, making it difficult to arrive at definite conclusions regarding its efficacy. Therefore, it is crucial to approach SCD for ASD with caution and under the guidance of healthcare professionals[173,174].
Ketogenic diet
The ketogenic diet is a high-fat, low-carbohydrate, and adequate-protein diet that has gained attention for its potential therapeutic effects in various neurological conditions, including epilepsy. The ketogenic diet aims to shift the body’s metabolism into ketosis, which primarily relies on fat for energy instead of carbohydrates. This metabolic state is likely to affect brain function and neurotransmitter activity, which may have implications for individuals with neurological conditions[175]. There are some physicians and families who have looked into using the ketogenic diet as a way to improve symptoms of ASD. Research suggests that the ketogenic diet can positively affect children with ASD by improving energy metabolism, reducing oxidative stress, controlling neurotransmitters, inhibiting the mammalian target of the rapamycin signaling pathway, and modulating the gut microbiota. These neuroprotective benefits demonstrate the ketogenic diet’s potential as a helpful intervention for children with ASD[176]. The effectiveness of the ketogenic diet in addressing ASD symptoms is highly individualized and varies greatly depending on the child and their family’s unique situation[177].
Although there have been some reports of anecdotal evidence showing improvement in behavior, communication, and social interaction, there has not been much research done on the effects of this diet on ASD. It’s worth mentioning that while the ketogenic diet has shown some promise as a treatment for ASD, the scientific evidence is limited and inconclusive due to small sample sizes and a lack of control groups in most studies[178]. This makes it difficult to draw definitive conclusions about its effectiveness. Additionally, the ketogenic diet is highly specialized and restrictive, requiring close monitoring and supervision from healthcare professionals such as registered dietitians or physicians specializing in ketogenic diets. It can be challenging to implement and maintain, and there are potential risks and nutritional concerns, particularly for growing children[179]. When considering using the ketogenic diet for managing ASD, monitoring and providing proper guidance is essential. This includes assessing the need for dietary modifications, monitoring nutritional adequacy, and ensuring overall health and well-being[180].
Camel milk therapy
Camel milk is a nutritious and healthy alternative to cow’s milk. It contains essential vitamins, minerals, and immunoglobulins, providing hypoallergenic, antioxidant, antibacterial, and antiviral properties. Moreover, it is easier to digest than milk from other ruminants making it more appealing to a broader range of consumers[181]. Recently, there has been growing interest in using camel milk as a possible aid for ASD. Camel milk advocates believe that it possesses distinct properties that could benefit individuals with ASD. Both research and personal accounts have suggested that camel milk may contain certain properties that could benefit those with ASD. These include lower lactose levels, unique protein structures, higher levels of vitamins and minerals, possible immune-regulating effects, and the ability to reduce oxidant stress[182,183]. Many people with ASD and their families have reported experiencing better behavior, digestion, and overall health after incorporating camel milk into their diet. According to the research conducted by Al-Ayadhi et al[184], the consumption of camel milk for a period of two weeks has been found to enhance the Childhood Autism Rating Scale (CARS), Social Responsiveness Scale (SRS), and ASD treatment evaluation checklist in children with ASD, as compared to those who consumed a placebo. According to a meta-analysis conducted by Kandeel and El-Deeb[185], the use of raw and boiled camel milk in treating ASD led to significantly lower CARS scores compared to the use of a placebo. The use of camel milk may be limited due to its comparatively high cost and short shelf-life. When it comes to discussing the potential benefits of camel milk for ASD symptoms, it’s crucial to be cautious. The current research in this field is insufficient and frequently relies on small-scale studies with methodological constraints[186]. To ascertain the actual impact of camel milk on ASD symptoms, we require more rigorous and controlled studies.
High-dose Methylcobalamin (Vitamin B12) therapy
Methylcobalamin, or Vitamin B12, is a crucial nutrient that occurs naturally in the body. It is vital in various biochemical processes, including DNA synthesis and maintaining the nervous system[187,188]. Recent studies have examined the potential use of high-dose Methylcobalamin therapy to help individuals with ASD. Supporters of this therapy claim that it can enhance language and communication skills, behavior, attention, and overall well-being. They suggest it may have neuroprotective and neurotrophic effects, influencing brain function and behavior in people with ASD[189-191]. Research has shown that methylcobalamin may affect the redox status and potentially improve the clinical symptoms associated with ASD[192]. Some individuals with ASD may also have metabolic or genetic issues that could impact their vitamin B12 levels or metabolism[193]. Therefore, they can get benefit from methylcobalamin therapy.
A study by Čorejová et al[194] found that a 200-d treatment with 500 μg methylcobalamin orally daily gradually improved the clinical and psychological condition of children and adults with ASD. The social domain showed the most significant improvement, followed by cognitive, behavioral, and communication characteristics. The study also established a strong correlation between the changes in the clinical and psychological status and the level of reduced glutathione and reduced/oxidized glutathione ratio. According to a meta-analysis conducted by Rossignol and Frye[195], administering subcutaneous injections of methylcobalamin Vitamin B12 (64.5-75 µg/kg/dose two-to-three times weekly) can effectively improve metabolic abnormalities and clinical symptoms of ASD. These symptoms include expressive communication, personal and domestic daily living skills, interpersonal, play-leisure, and coping social skills, as well as sleep disorders, GI symptoms, hyperactivity, tantrums, nonverbal intellectual quotient, vision, eye contact, echolalia, stereotypy, anemia, and nocturnal enuresis. However, it should be noted that some patients may experience mild non-serious side effects such as hyperactivity, irritability, trouble sleeping, aggression, and worsening behaviors[195]. However, there are limitations to these studies, such as small sample sizes, lack of control groups, and variations in study design, making it difficult to determine the effectiveness of Methylcobalamin therapy for ASD.
Omega-3 supplement
Omega-3 fatty acids are vital polyunsaturated fats crucial to brain function and development. They are commonly found in fatty fish like salmon, mackerel, and sardines, as well as in some plant-based sources such as flaxseeds and walnuts[196]. Studies suggest that omega-3 fatty acids have anti-inflammatory and neuroprotective properties, which may support cognitive and behavioral processes, brain health, and development[197]. Although researchers have shown interest in exploring the potential benefits of omega-3 fatty acids, specifically eicosapentaenoic acid and docosahexaenoic acid , as a dietary intervention for ASD, the scientific evidence in this area is still limited and inconclusive[198]. Some studies indicate that omega-3 fatty acid supplementation may help address deficiencies associated with certain omega-3 fatty acids in the blood of some individuals with ASD, potentially improving ASD symptoms[199]. According to a meta-analysis conducted by Cheng et al[200], it has been found that omega-3 fatty acid supplementation may have positive effects on hyperactivity, lethargy, and stereotypy in individuals with ASD. However, the results of studies investigating the effects of omega-3 fatty acid supplementation in individuals with ASD have been mixed, and more extensive, well-controlled studies are needed to establish the efficacy and optimal dosing of omega-3 fatty acids for ASD[201]. Although omega-3 fatty acids may have potential benefits, they should be considered as part of a comprehensive treatment plan for ASD that includes a range of evidence-based interventions, therapies, educational strategies, and support services tailored to the specific needs of each person with ASD[199].
Other nutritional supplements
Studies have been conducted to investigate the effects of dietary supplements like vitamin B6, selenium, and magnesium on patients with ASD. Vitamin B6, a water-soluble vitamin, is crucial in various physiological processes, such as synthesizing brain function compounds and metabolizing neurotransmitters and amino acids[202]. Vitamin B6 supplementation has been explored as a potential treatment for ASD, but the scientific evidence supporting its effectiveness is limited and inconclusive. The interest in vitamin B6 for ASD stems from its involvement in the metabolism of neurotransmitters like dopamine and serotonin, which play a crucial role in brain function and behavior[203,204]. Some studies have examined the effects of vitamin B6 and magnesium supplementation, known as the “B6-Mg protocol”, on individuals with ASD. While some studies have reported improvements in certain behaviors like social interaction, communication, and sensory issues, others have shown no significant effects. Study results may be due to differences in study design, participant characteristics, and outcome measures[205]. When discussing dietary interventions for ASD, it is crucial to exercise prudence and consider the unique needs, preferences, and circumstances of each individual with ASD. A holistic treatment approach for ASD typically comprises a blend of therapies, educational interventions, support services, and customized strategies tailored to the specific requirements of the individual[206].
Oral antifungal gut decontamination: There has been research into the possibility of fungi overgrowth in the GI tract contributing to ASD. Some scientists and medical professionals believe that excessive growth of fungi in the gut (gut de-fungemia hypothesis) may affect the behaviors and symptoms associated with ASD. The toxins released by an overgrowth of Candida species, a type of fungi, can enter the bloodstream and impact the brain, potentially leading to ASD symptoms. However, it is essential to remember that there is currently limited and inconclusive scientific evidence to support this theory[207]. Baker and Shaw[208] tested this theory by treating a child with symptomatic ASD. They found heavy GI fungal colonization with Aspergillus. They used the antifungal probiotic Saccharomyces boulardii, followed by increasing doses of itraconazole for strong antifungal therapy. The child experienced complete recovery from all ASD symptoms and even developed excellent academic, athletic, and musical skills. The recovery was accompanied by a significant reduction in urine markers of Aspergillus colonization, supporting the theory[208]. In a systematic review conducted by Herman and Herman[209], it was found that there were no significant differences between patients with and without antifungal medication. Therefore, it cannot be confirmed that Candida overgrowth is linked to ASD in children or causes ASD. Additionally, their findings do not entirely support the theory that Candida overgrowth is associated with GI issues and impacts ASD-related behavioral symptoms. Although there have been reports of changes in gut fungal composition among individuals with ASD, such as higher levels of Candida species, the link between increased gut candidiasis and ASD has not been definitively established. Small sample sizes, methodological issues, and inconsistent results often limit these studies.
Modification of gut microbiota: Researchers interested in managing ASD have given a growing interest in microbiota-based interventions, which involve modifying the gut microbiota. The gut microbiota is a diverse community of microorganisms that reside in the GI tract and play a crucial role in various aspects of health, including immune function, metabolism, and brain development[210]. Research studies have revealed that people with ASD may have different gut microbiota compared to those without the condition. This has prompted researchers to explore whether gut microbiota plays a role in developing and expressing ASD symptoms. The goal is to improve ASD-related symptoms by adjusting the gut microbiota to a healthier composition[47]. There are different approaches to modifying the gut microbiota, including: Antibiotics and dietary interventions, probiotic and prebiotic interventions, and fecal microbiota transplant (FMT) therapy.
Antibiotics and dietary interventions
There is a conflicting opinion regarding the beneficial use of antibiotics in restoring gut dysbiosis and reducing GI and behavioral symptoms in children with ASD. However, there are still ongoing debates surrounding the use of antibiotics. Antibiotics eliminate harmful and beneficial bacteria in patients with ASD, which may increase the likelihood of GI illnesses in affected children. As a result, antibiotic therapy may not be the most effective approach for restoring gut microbiota balance[211]. Studies have demonstrated that a Mediterranean diet can improve GI health, cardiovascular diseases, and neurobehavioral health outcomes[212]. The effectiveness of a ketogenic diet has also been explored, with positive results in mitigating neurobehavioral symptoms[213]. The gluten-free and GFCF diet is another popular approach, with some studies showing favorable results in improving symptoms such as communication, social interaction, and GI issues[214]. However, recent studies have shown that restrictive diets can potentially have harmful effects, resulting in nutrient deficiencies and high costs for families. The standard of dietary intervention is also strict and may not apply to all ASD patients[215].
Probiotic and prebiotic interventions
Probiotics are microorganisms that can provide health benefits when taken in sufficient quantities. There have been studies on using specific probiotic strains to balance gut bacteria and potentially improve symptoms in people with ASD[216]. However, the evidence for the effectiveness of probiotics in treating ASD is limited and inconsistent[217]. Prebiotics are non-digestible fibers that can promote the growth of healthy gut bacteria. By consuming prebiotics, the gut microbiota can become more balanced[218]. Some studies have looked into the effects of prebiotic supplements on individuals with ASD, but further research is required to determine the impact on ASD symptoms[219]. As the gut-brain axis has two-way communication, probiotic intervention may decrease the prevalence of ASD by affecting related gene expression in infants. However, the specificity of the gut microbiota in different patients suggests the need for precision medicine tailored to specific subpopulations of patients[220].
According to Sanctuary et al[221] pilot study, the intake of Bifidobacterium infantis probiotic and a bovine colostrum product for five weeks was well-tolerated and effectively lowered the occurrence of specific GI symptoms. Additionally, it reduced the occurrence of certain abnormal behaviors. Furthermore, a study by Kong et al[222] found that using a combination of Lactobacillus plantarum PS128 probiotic for 28 wk and oxytocin for 12 wk resulted in significant improvement in the SRS, aberrant behavior checklist, and clinical global impression scale. The combination also led to the growth of favorable gut microbiome network hubs. The study also revealed that the effectiveness of the combination treatment in enhancing social cognition is highly linked to the abundance of the Eubacterium hallii group.
FMT therapy
A potential solution for gut dysbiosis in children with ASD is a FMT. This procedure transfers fecal microbiota from healthy donors into an individual’s GI tract to balance their gut microbiota. FMT has shown promising results in treating certain GI conditions and can improve GI and neurobehavioral symptoms in younger children with ASD[223]. However, it may only work for some older patients. Despite its benefits, FMT can cause adverse reactions in some patients, so a modified protocol called MTT was developed. MTT involves oral vancomycin treatment, bowel cleansing, and oral or rectal administration of standardized human gut microbiota[224]. Studies have shown that MTT can improve behavioral symptoms and increase the diversity of gut bacteria in children with ASD. The effects of MTT can last for at least two years after treatment. These results suggest that overall gut microbe rebalancing may be a promising approach to treating gut microbiota dysbiosis in ASD[47]. However, FMT use in ASD is still in its early stages and requires further research to establish its safety and efficacy.
When seeking interventions for individuals with ASD, it is essential to remember that while microbiota-based treatments show promise, they are still a developing field. Further research is necessary to determine their effectiveness, optimal protocols, and long-term effects on those with ASD[225]. Healthcare professionals with expertise in this field are recommended to oversee these interventions. Additionally, a comprehensive approach that includes evidence-based therapies, educational interventions, support services, and individualized strategies tailored to the specific needs of each person with ASD should be considered. Modifying the gut microbiota should be viewed as a component of a more extensive treatment plan, and healthcare professionals should be consulted when deciding on specific interventions[226].
Limitations of the study
It’s important to remember that there are some limitations to the article’s findings regarding the association between GI health and ASD symptoms. While the article suggests a potential link, it cannot definitively establish a causal relationship. It’s worth noting that many of the studies included in the review relied on observational data or retrospective analysis, which may introduce biases and confounding factors that can impact the results. Additionally, some of the studies were limited by small sample sizes or specific populations, making it difficult to generalize the findings. To better understand the relationship between these factors, we need more large-scale studies with strong controls to minimize potential biases and confounding factors.
CONCLUSION
This review article delves into the extensive research on the connection between GI health and ASD. We have summarized the key findings from the literature, highlighted areas of agreement and disagreement, and examined the implications of these outcomes for future research. Children with ASD are more susceptible to GI disorders than the general population, which can significantly impact their health, learning, and development due to genetic, environmental, and behavioral factors. GI disorders can arise from gut dysbiosis, immune dysfunction, food sensitivities, digestive enzyme deficiencies, and sensory processing and integration differences. Current research suggests that numerous children with ASD experience GI disorders, and effective management is critical. Behavioral assessments and observations are the preferred methods for diagnosing ASD, and GI tests are not consistently supported by scientific evidence for reliable diagnostic tools. However, certain GI tests may increase the risk of developing ASD or experiencing more severe symptoms. Addressing GI problems in individuals with ASD can improve their overall well-being, leading to better behavior, cognitive function, and learning abilities. Properly addressing GI issues can relieve physical discomfort and pain, promoting better digestion, enhanced nutrient absorption, and improved appetite. Alleviating GI symptoms can improve sleep patterns, increase energy levels, and contribute to a general sense of well-being, ultimately leading to a better quality of life for the individual and improved family dynamics. The primary objective of GI interventions is to improve nutritional status, reduce symptom severity, promote a balanced mood, and increase patient independence.
Recommendation
It is essential to support research on GI disorders’ causes, mechanisms, and treatments in children with ASD. Healthcare professionals should receive training on diagnosing and treating such conditions and implementing evidence-based interventions. Effective management of GI problems can be achieved through medical team intervention and management, occupational therapy based on sensory integration in all settings the child uses, communication, and behavior support plans. Early identification and treatment of these disorders can improve overall well-being and prevent long-term complications. A multi-transdisciplinary approach is often necessary to provide comprehensive care.
ACKNOWLEDGEMENTS
We thank the anonymous referees for their valuable suggestions.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Faculty of Medicine, Tanta University, Egypt; University Medical Center, King Abdulla Medical City. Arabian Gulf University.
Peer-review started: July 10, 2023
First decision: August 8, 2023
Article in press: August 17, 2023
Specialty type: Pediatrics
Country/Territory of origin: Bahrain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chew KS, Malaysia; Morais MB, Brazil S-Editor: Qu XL L-Editor: A P-Editor: Zhang XD
Contributor Information
Mohammed Al-Beltagi, Pediatric Department, Faculty of Medicine, Tanta University, Algharbia, Tanta 31511, Egypt; Pediatrics, Univeristy Medical Center, King Abdulla Medical City, Arabian Gulf University, Dr. Sulaiman Al Habib Medical Group, Manama, Manama 26671, Bahrain. mbelrem@hotmail.com.
Nermin Kamal Saeed, Medical Microbiology Section, Pathology Department, Salmaniya Medical Complex, Ministry of Health, Manama, Manama 12, Bahrain; Medical Microbiology Section, Pathology Department, Irish Royal College of Surgeon, Bahrain, Muharraq, Busaiteen 15503, Bahrain.
Adel Salah Bediwy, Pulmonology Department, Faculty of Medicine, Tanta University, Algharbia, Tanta 31527, Egypt; Pulmonology Department, University Medical Center, King Abdulla Medical City, Arabian Gulf University, Dr. Sulaiman Al Habib Medical Group, Manama, Manama 26671, Bahrain.
Reem Elbeltagi, Medicine, The Royal College of Surgeons in Ireland-Bahrain, Muharraq, Busiateen 15503, Bahrain.
Rawan Alhawamdeh, Pediatrics Research, and Development Department, Genomics Creativity and Play Center, Manama, Manama 0000, Bahrain; Pediatrics Research, and Development Department, SENSORYME Dubai 999041, United Arab Emirates.
References
- 1.Ratajczak HV. Theoretical aspects of autism: causes--a review. J Immunotoxicol. 2011;8:68–79. doi: 10.3109/1547691X.2010.545086. [DOI] [PubMed] [Google Scholar]
- 2.McBain RK, Kareddy V, Cantor JH, Stein BD, Yu H. Systematic Review: United States Workforce for Autism-Related Child Healthcare Services. J Am Acad Child Adolesc Psychiatry. 2020;59:113–139. doi: 10.1016/j.jaac.2019.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McPartland J, Volkmar FR. Autism and related disorders. Handb Clin Neurol. 2012;106:407–418. doi: 10.1016/B978-0-444-52002-9.00023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baird G, Simonoff E, Pickles A, Chandler S, Loucas T, Meldrum D, Charman T. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP) Lancet. 2006;368:210–215. doi: 10.1016/S0140-6736(06)69041-7. [DOI] [PubMed] [Google Scholar]
- 5.Al-Biltagi M, Saeed NK, Qaraghuli S. Gastrointestinal disorders in children with autism: Could artificial intelligence help? Artif Intell Gastroenterol. 2022;3 [Google Scholar]
- 6.Becerra TA, von Ehrenstein OS, Heck JE, Olsen J, Arah OA, Jeste SS, Rodriguez M, Ritz B. Autism spectrum disorders and race, ethnicity, and nativity: a population-based study. Pediatrics. 2014;134:e63–e71. doi: 10.1542/peds.2013-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahmani M, Sarrafchi A, Shirzad H, Rafieian-Kopaei M. Autism: Pathophysiology and Promising Herbal Remedies. Curr Pharm Des. 2016;22:277–285. doi: 10.2174/1381612822666151112151529. [DOI] [PubMed] [Google Scholar]
- 8.Hyman SL, Levy SE, Myers SM COUNCIL ON CHILDREN WITH DISABILITIES, SECTION ON DEVELOPMENTAL AND BEHAVIORAL PEDIATRICS. Identification, Evaluation, and Management of Children With Autism Spectrum Disorder. Pediatrics. 2020;145 doi: 10.1542/peds.2019-3447. [DOI] [PubMed] [Google Scholar]
- 9.Al-Beltagi M. Autism medical comorbidities. World J Clin Pediatr. 2021;10:15–28. doi: 10.5409/wjcp.v10.i3.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu G, Snetselaar LG, Jing J, Liu B, Strathearn L, Bao W. Association of Food Allergy and Other Allergic Conditions With Autism Spectrum Disorder in Children. JAMA Netw Open. 2018;1:e180279. doi: 10.1001/jamanetworkopen.2018.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rylaarsdam L, Guemez-Gamboa A. Genetic Causes and Modifiers of Autism Spectrum Disorder. Front Cell Neurosci. 2019;13:385. doi: 10.3389/fncel.2019.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fattorusso A, Di Genova L, Dell'Isola GB, Mencaroni E, Esposito S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients. 2019;11 doi: 10.3390/nu11030521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bresnahan M, Hornig M, Schultz AF, Gunnes N, Hirtz D, Lie KK, Magnus P, Reichborn-Kjennerud T, Roth C, Schjølberg S, Stoltenberg C, Surén P, Susser E, Lipkin WI. Association of maternal report of infant and toddler gastrointestinal symptoms with autism: evidence from a prospective birth cohort. JAMA Psychiatry. 2015;72:466–474. doi: 10.1001/jamapsychiatry.2014.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gabriele S, Sacco R, Altieri L, Neri C, Urbani A, Bravaccio C, Riccio MP, Iovene MR, Bombace F, De Magistris L, Persico AM. Slow intestinal transit contributes to elevate urinary p-cresol level in Italian autistic children. Autism Res. 2016;9:752–759. doi: 10.1002/aur.1571. [DOI] [PubMed] [Google Scholar]
- 15.Schröder SS, Danner UN, Spek AA, van Elburg AA. Problematic eating behaviours of autistic women-A scoping review. Eur Eat Disord Rev. 2022;30:510–537. doi: 10.1002/erv.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marco EJ, Hinkley LB, Hill SS, Nagarajan SS. Sensory processing in autism: a review of neurophysiologic findings. Pediatr Res. 2011;69:48R–54R. doi: 10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu Y, Zhao F. Microbiota-gut-brain axis in autism spectrum disorder. J Genet Genomics. 2021;48:755–762. doi: 10.1016/j.jgg.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Harris C, Card B. A pilot study to evaluate nutritional influences on gastrointestinal symptoms and behavior patterns in children with Autism Spectrum Disorder. Complement Ther Med. 2012;20:437–440. doi: 10.1016/j.ctim.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Fulceri F, Morelli M, Santocchi E, Cena H, Del Bianco T, Narzisi A, Calderoni S, Muratori F. Gastrointestinal symptoms and behavioral problems in preschoolers with Autism Spectrum Disorder. Dig Liver Dis. 2016;48:248–254. doi: 10.1016/j.dld.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Wasilewska J, Klukowski M. Gastrointestinal symptoms and autism spectrum disorder: links and risks - a possible new overlap syndrome. Pediatric Health Med Ther. 2015;6:153–166. doi: 10.2147/PHMT.S85717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madra M, Ringel R, Margolis KG. Gastrointestinal Issues and Autism Spectrum Disorder. Psychiatr Clin North Am. 2021;44:69–81. doi: 10.1016/j.psc.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang XL, Liang S, Zou MY, Sun CH, Han PP, Jiang XT, Xia W, Wu LJ. Are gastrointestinal and sleep problems associated with behavioral symptoms of autism spectrum disorder? Psychiatry Res. 2018;259:229–235. doi: 10.1016/j.psychres.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 23.Prosperi M, Santocchi E, Muratori F, Narducci C, Calderoni S, Tancredi R, Morales MA, Guiducci L. Vocal and motor behaviors as a possible expression of gastrointestinal problems in preschoolers with Autism Spectrum Disorder. BMC Pediatr. 2019;19:466. doi: 10.1186/s12887-019-1841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holingue C, Newill C, Lee LC, Pasricha PJ, Daniele Fallin M. Gastrointestinal symptoms in autism spectrum disorder: A review of the literature on ascertainment and prevalence. Autism Res. 2018;11:24–36. doi: 10.1002/aur.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ajamian M, Steer D, Rosella G, Gibson PR. Serum zonulin as a marker of intestinal mucosal barrier function: May not be what it seems. PLoS One. 2019;14:e0210728. doi: 10.1371/journal.pone.0210728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coury DL, Ashwood P, Fasano A, Fuchs G, Geraghty M, Kaul A, Mawe G, Patterson P, Jones NE. Gastrointestinal conditions in children with autism spectrum disorder: developing a research agenda. Pediatrics. 2012;130 Suppl 2:S160–S168. doi: 10.1542/peds.2012-0900N. [DOI] [PubMed] [Google Scholar]
- 27.Mukhtar K, Nawaz H, Abid S. Functional gastrointestinal disorders and gut-brain axis: What does the future hold? World J Gastroenterol. 2019;25:552–566. doi: 10.3748/wjg.v25.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genuis SJ, Bouchard TP. Celiac disease presenting as autism. J Child Neurol. 2010;25:114–119. doi: 10.1177/0883073809336127. [DOI] [PubMed] [Google Scholar]
- 29.Rubenstein E, Schieve L, Bradley C, DiGuiseppi C, Moody E, Thomas K, Daniels J. The prevalence of gluten free diet use among preschool children with autism spectrum disorder. Autism Res. 2018;11:185–193. doi: 10.1002/aur.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niewinski MM. Advances in celiac disease and gluten-free diet. J Am Diet Assoc. 2008;108:661–672. doi: 10.1016/j.jada.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol. 2016;13:517–528. doi: 10.1038/nrgastro.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 33.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 34.Saeed NK, Al-Beltagi M, Bediwy AS, El-Sawaf Y, Toema O. Gut microbiota in various childhood disorders: Implication and indications. World J Gastroenterol. 2022;28:1875–1901. doi: 10.3748/wjg.v28.i18.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bull MJ, Plummer NT. Part 1: The Human Gut Microbiome in Health and Disease. Integr Med (Encinitas) 2014;13:17–22. [PMC free article] [PubMed] [Google Scholar]
- 36.Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128–133. doi: 10.1016/j.brainres.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osadchiy V, Martin CR, Mayer EA. The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin Gastroenterol Hepatol. 2019;17:322–332. doi: 10.1016/j.cgh.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125:926–938. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srikantha P, Mohajeri MH. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20092115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Xu J, Chen Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients. 2021;13 doi: 10.3390/nu13062099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martínez-González AE, Andreo-Martínez P. The Role of Gut Microbiota in Gastrointestinal Symptoms of Children with ASD. Medicina (Kaunas) 2019;55 doi: 10.3390/medicina55080408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siniscalco D, Schultz S, Brigida AL, Antonucci N. Inflammation and Neuro-Immune Dysregulations in Autism Spectrum Disorders. Pharmaceuticals (Basel) 2018;11 doi: 10.3390/ph11020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson-Agramonte MLA, Noris García E, Fraga Guerra J, Vega Hurtado Y, Antonucci N, Semprún-Hernández N, Schultz S, Siniscalco D. Immune Dysregulation in Autism Spectrum Disorder: What Do We Know about It? Int J Mol Sci. 2022;23 doi: 10.3390/ijms23063033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dargenio VN, Dargenio C, Castellaneta S, De Giacomo A, Laguardia M, Schettini F, Francavilla R, Cristofori F. Intestinal Barrier Dysfunction and Microbiota-Gut-Brain Axis: Possible Implications in the Pathogenesis and Treatment of Autism Spectrum Disorder. Nutrients. 2023;15 doi: 10.3390/nu15071620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madore C, Leyrolle Q, Lacabanne C, Benmamar-Badel A, Joffre C, Nadjar A, Layé S. Neuroinflammation in Autism: Plausible Role of Maternal Inflammation, Dietary Omega 3, and Microbiota. Neural Plast. 2016;2016:3597209. doi: 10.1155/2016/3597209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van De Sande MM, van Buul VJ, Brouns FJ. Autism and nutrition: the role of the gut-brain axis. Nutr Res Rev. 2014;27:199–214. doi: 10.1017/S0954422414000110. [DOI] [PubMed] [Google Scholar]
- 47.Taniya MA, Chung HJ, Al Mamun A, Alam S, Aziz MA, Emon NU, Islam MM, Hong SS, Podder BR, Ara Mimi A, Aktar Suchi S, Xiao J. Role of Gut Microbiome in Autism Spectrum Disorder and Its Therapeutic Regulation. Front Cell Infect Microbiol. 2022;12:915701. doi: 10.3389/fcimb.2022.915701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brett BE, de Weerth C. The microbiota-gut-brain axis: A promising avenue to foster healthy developmental outcomes. Dev Psychobiol. 2019;61:772–782. doi: 10.1002/dev.21824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leader G, Abberton C, Cunningham S, Gilmartin K, Grudzien M, Higgins E, Joshi L, Whelan S, Mannion A. Gastrointestinal Symptoms in Autism Spectrum Disorder: A Systematic Review. Nutrients. 2022;14 doi: 10.3390/nu14071471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Israelyan N, Margolis KG. Reprint of: Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacol Res. 2019;140:115–120. doi: 10.1016/j.phrs.2018.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoon SH, Choi J, Lee WJ, Do JT. Genetic and Epigenetic Etiology Underlying Autism Spectrum Disorder. J Clin Med. 2020;9 doi: 10.3390/jcm9040966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamionkowski S, Shibli F, Ganocy S, Fass R. The relationship between gastroesophageal reflux disease and autism spectrum disorder in adult patients in the United States. Neurogastroenterol Motil. 2022;34:e14295. doi: 10.1111/nmo.14295. [DOI] [PubMed] [Google Scholar]
- 53.Dominick KC, Davis NO, Lainhart J, Tager-Flusberg H, Folstein S. Atypical behaviors in children with autism and children with a history of language impairment. Res Dev Disabil. 2007;28:145–162. doi: 10.1016/j.ridd.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Yasuda Y, Hashimoto R, Nakae A, Kang H, Ohi K, Yamamori H, Fujimoto M, Hagihira S, Takeda M. Sensory cognitive abnormalities of pain in autism spectrum disorder: a case-control study. Ann Gen Psychiatry. 2016;15:8. doi: 10.1186/s12991-016-0095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edelson SM. Understanding Challenging Behaviors in Autism Spectrum Disorder: A Multi-Component, Interdisciplinary Model. J Pers Med. 2022;12 doi: 10.3390/jpm12071127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buie T, Campbell DB, Fuchs GJ 3rd, Furuta GT, Levy J, Vandewater J, Whitaker AH, Atkins D, Bauman ML, Beaudet AL, Carr EG, Gershon MD, Hyman SL, Jirapinyo P, Jyonouchi H, Kooros K, Kushak R, Levitt P, Levy SE, Lewis JD, Murray KF, Natowicz MR, Sabra A, Wershil BK, Weston SC, Zeltzer L, Winter H. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125 Suppl 1:S1–18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- 57.Linday LA, Tsiouris JA, Cohen IL, Shindledecker R, DeCresce R. Famotidine treatment of children with autistic spectrum disorders: pilot research using single subject research design. J Neural Transm (Vienna) 2001;108:593–611. doi: 10.1007/s007020170059. [DOI] [PubMed] [Google Scholar]
- 58.Brignell A, Chenausky KV, Song H, Zhu J, Suo C, Morgan AT. Communication interventions for autism spectrum disorder in minimally verbal children. Cochrane Database Syst Rev. 2018;11:CD012324. doi: 10.1002/14651858.CD012324.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Addison LR, Piazza CC, Patel MR, Bachmeyer MH, Rivas KM, Milnes SM, Oddo J. A comparison of sensory integrative and behavioral therapies as treatment for pediatric feeding disorders. J Appl Behav Anal. 2012;45:455–471. doi: 10.1901/jaba.2012.45-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cooper K, Loades ME, Russell AJ. Adapting Psychological Therapies for Autism - Therapist Experience, Skills and Confidence. Res Autism Spectr Disord. 2018;45:43–50. doi: 10.1016/j.rasd.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lima Antão JYF, Oliveira ASB, Almeida Barbosa RT, Crocetta TB, Guarnieri R, Arab C, Massetti T, Antunes TPC, Silva APD, Bezerra ĹMP, Mello Monteiro CB, Abreu LC. Instruments for augmentative and alternative communication for children with autism spectrum disorder: a systematic review. Clinics (Sao Paulo) 2018;73:e497. doi: 10.6061/clinics/2017/e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodriguez L, Morley-Fletcher A, Winter H, Timothy B. Evaluation of Gastroesophageal Reflux Disease in Children on the Autism Spectrum: A Study Evaluating the Tolerance and Utility of the BRAVO Wireless pH Monitoring. J Pediatr Gastroenterol Nutr. 2022;75:450–454. doi: 10.1097/MPG.0000000000003561. [DOI] [PubMed] [Google Scholar]
- 63.Lanyi J, Flynn C, Mannion A, Maher L, Naughton K, Leade G. Abdominal Pain in Children and Adolescents with Autism Spectrum Disorder: a Systematic Review. Rev J Autism Dev Disord. 9:280–289. [Google Scholar]
- 64.Neuhaus E, Bernier RA, Tham SW, Webb SJ. Gastrointestinal and Psychiatric Symptoms Among Children and Adolescents With Autism Spectrum Disorder. Front Psychiatry. 2018;9:515. doi: 10.3389/fpsyt.2018.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cvejic RC, Arnold SRC, Foley KR, Trollor JN. Neuropsychiatric profile and psychotropic medication use in adults with autism spectrum disorder: results from the Australian Longitudinal Study of Adults with Autism. BJPsych Open. 2018;4:461–466. doi: 10.1192/bjo.2018.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fitzpatrick R, McGuire BE, Lydon HK. Improving pain-related communication in children with autism spectrum disorder and intellectual disability. Paediatr Neonatal Pain. 2022;4:23–33. doi: 10.1002/pne2.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mazurek MO, Vasa RA, Kalb LG, Kanne SM, Rosenberg D, Keefer A, Murray DS, Freedman B, Lowery LA. Anxiety, sensory over-responsivity, and gastrointestinal problems in children with autism spectrum disorders. J Abnorm Child Psychol. 2013;41:165–176. doi: 10.1007/s10802-012-9668-x. [DOI] [PubMed] [Google Scholar]
- 68.Allely CS. Pain sensitivity and observer perception of pain in individuals with autistic spectrum disorder. ScientificWorldJournal. 2013;2013:916178. doi: 10.1155/2013/916178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Solomon O, Heritage J, Yin L, Maynard DW, Bauman ML. ‘What Brings Him Here Today?’: Medical Problem Presentation Involving Children with Autism Spectrum Disorders and Typically Developing Children. J Autism Dev Disord. 2016;46:378–393. doi: 10.1007/s10803-015-2550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mingins JE, Tarver J, Waite J, Jones C, Surtees AD. Anxiety and intellectual functioning in autistic children: A systematic review and meta-analysis. Autism. 2021;25:18–32. doi: 10.1177/1362361320953253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Strömberg M, Liman L, Bang P, Igelström K. Experiences of Sensory Overload and Communication Barriers by Autistic Adults in Health Care Settings. Autism Adulthood. 2022;4:66–75. doi: 10.1089/aut.2020.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Minshawi NF, Hurwitz S, Fodstad JC, Biebl S, Morriss DH, McDougle CJ. The association between self-injurious behaviors and autism spectrum disorders. Psychol Res Behav Manag. 2014;7:125–136. doi: 10.2147/PRBM.S44635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McTee HM, Mood D, Fredrickson T, Thrasher A, Bonino AY. Using Visual Supports to Facilitate Audiological Testing for Children With Autism Spectrum Disorder. Am J Audiol. 2019;28:823–833. doi: 10.1044/2019_AJA-19-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Young S, Hollingdale J, Absoud M, Bolton P, Branney P, Colley W, Craze E, Dave M, Deeley Q, Farrag E, Gudjonsson G, Hill P, Liang HL, Murphy C, Mackintosh P, Murin M, O'Regan F, Ougrin D, Rios P, Stover N, Taylor E, Woodhouse E. Guidance for identification and treatment of individuals with attention deficit/hyperactivity disorder and autism spectrum disorder based upon expert consensus. BMC Med. 2020;18:146. doi: 10.1186/s12916-020-01585-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fields VL, Soke GN, Reynolds A, Tian LH, Wiggins L, Maenner M, DiGuiseppi C, Kral TVE, Hightshoe K, Ladd-Acosta C, Schieve LA. Association between pica and gastrointestinal symptoms in preschoolers with and without autism spectrum disorder: Study to Explore Early Development. Disabil Health J. 2021;14:101052. doi: 10.1016/j.dhjo.2020.101052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shankar S, Rosenbaum J. Chronic diarrhoea in children: A practical algorithm-based approach. J Paediatr Child Health. 2020;56:1029–1038. doi: 10.1111/jpc.14986. [DOI] [PubMed] [Google Scholar]
- 77.Kirby AV, Boyd BA, Williams KL, Faldowski RA, Baranek GT. Sensory and repetitive behaviors among children with autism spectrum disorder at home. Autism. 2017;21:142–154. doi: 10.1177/1362361316632710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Call CR, Williams ME, Lau D, Nyp SS. The Complexity of Making a Diagnosis. J Dev Behav Pediatr. 2020;41:242–244. doi: 10.1097/DBP.0000000000000792. [DOI] [PubMed] [Google Scholar]
- 79.Chakraborty P, Carpenter KLH, Major S, Deaver M, Vermeer S, Herold B, Franz L, Howard J, Dawson G. Gastrointestinal problems are associated with increased repetitive behaviors but not social communication difficulties in young children with autism spectrum disorders. Autism. 2021;25:405–415. doi: 10.1177/1362361320959503. [DOI] [PubMed] [Google Scholar]
- 80.Holingue C, Poku O, Pfeiffer D, Murray S, Fallin MD. Gastrointestinal concerns in children with autism spectrum disorder: A qualitative study of family experiences. Autism. 2022;26:1698–1711. doi: 10.1177/13623613211062667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holingue C, Kalb LG, Musci R, Lukens C, Lee LC, Kaczaniuk J, Landrum M, Buie T, Fallin MD. Characteristics of the autism spectrum disorder gastrointestinal and related behaviors inventory in children. Autism Res. 2022;15:1142–1155. doi: 10.1002/aur.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ofei SY, Fuchs GJ 3rd. Constipation Burden in Children with Autism Spectrum Disorder: Emergency Department and Healthcare Use. J Pediatr. 2018;202:12–13. doi: 10.1016/j.jpeds.2018.06.057. [DOI] [PubMed] [Google Scholar]
- 83.Harris HA, Micali N, Moll HA, van Berckelaer-Onnes I, Hillegers M, Jansen PW. The role of food selectivity in the association between child autistic traits and constipation. Int J Eat Disord. 2021;54:981–985. doi: 10.1002/eat.23485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wiggins LD, Nadler C, Hepburn S, Rosenberg S, Reynolds A, Zubler J. Toileting Resistance Among Preschool-Age Children with and Without Autism Spectrum Disorder. J Dev Behav Pediatr. 2022;43:216–223. doi: 10.1097/DBP.0000000000001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fu SC, Lee CH, Wang H. Exploring the Association of Autism Spectrum Disorders and Constipation through Analysis of the Gut Microbiome. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18020667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marler S, Ferguson BJ, Lee EB, Peters B, Williams KC, McDonnell E, Macklin EA, Levitt P, Margolis KG, Beversdorf DQ, Veenstra-VanderWeele J. Association of Rigid-Compulsive Behavior with Functional Constipation in Autism Spectrum Disorder. J Autism Dev Disord. 2017;47:1673–1681. doi: 10.1007/s10803-017-3084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sparks B, Cooper J, Hayes C, Williams K. Constipation in Children with Autism Spectrum Disorder Associated with Increased Emergency Department Visits and Inpatient Admissions. J Pediatr. 2018;202:194–198. doi: 10.1016/j.jpeds.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 88.Maslen C, Hodge R, Tie K, Laugharne R, Lamb K, Shankar R. Constipation in autistic people and people with learning disabilities. Br J Gen Pract. 2022;72:348–351. doi: 10.3399/bjgp22X720077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Failla MD, Gerdes MB, Williams ZJ, Moore DJ, Cascio CJ. Increased pain sensitivity and pain-related anxiety in individuals with autism. Pain Rep. 2020;5:e861. doi: 10.1097/PR9.0000000000000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Coe A, Ciricillo J, Mansi S, El-Chammas K, Santucci N, Bali N, Lu PL, Damrongmanee A, Fei L, Liu C, Kaul A, Williams KC. Evaluation of Chronic Constipation in Children With Autism Spectrum Disorder. J Pediatr Gastroenterol Nutr. 2023;76:154–159. doi: 10.1097/MPG.0000000000003662. [DOI] [PubMed] [Google Scholar]
- 91.Sanctuary MR, Kain JN, Angkustsiri K, German JB. Dietary Considerations in Autism Spectrum Disorders: The Potential Role of Protein Digestion and Microbial Putrefaction in the Gut-Brain Axis. Front Nutr. 2018;5:40. doi: 10.3389/fnut.2018.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mannion K, Leader A, Geraldine The Assessment and Treatment of Toileting Difficulties in Individuals with Autism Spectrum Disorder and Other Developmental Disabilities. Rev J Autism Dev Disord. 2017;4:1–15. [Google Scholar]
- 93.Peeters B, Noens I, Philips EM, Kuppens S, Benninga MA. Autism spectrum disorders in children with functional defecation disorders. J Pediatr. 2013;163:873–878. doi: 10.1016/j.jpeds.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 94.Piccin S, Crippa A, Nobile M, Hardan AY, Brambilla P. Video modeling for the development of personal hygiene skills in youth with autism spectrum disorder. Epidemiol Psychiatr Sci. 2018;27:127–132. doi: 10.1017/S2045796017000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reche-Olmedo L, Torres-Collado L, Compañ-Gabucio LM, Garcia-de-la-Hera M. The Role of Occupational Therapy in Managing Food Selectivity of Children with Autism Spectrum Disorder: A Scoping Review. Children (Basel) 2021;8 doi: 10.3390/children8111024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alhuzimi T. Stress and emotional wellbeing of parents due to change in routine for children with Autism Spectrum Disorder (ASD) at home during COVID-19 pandemic in Saudi Arabia. Res Dev Disabil. 2021;108:103822. doi: 10.1016/j.ridd.2020.103822. [DOI] [PubMed] [Google Scholar]
- 97.Inoue R, Sakaue Y, Kawada Y, Tamaki R, Yasukawa Z, Ozeki M, Ueba S, Sawai C, Nonomura K, Tsukahara T, Naito Y. Dietary supplementation with partially hydrolyzed guar gum helps improve constipation and gut dysbiosis symptoms and behavioral irritability in children with autism spectrum disorder. J Clin Biochem Nutr. 2019;64:217–223. doi: 10.3164/jcbn.18-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mulay KV, Karthik SV. Managing constipation in children with ASD - A challenge worth tackling. Pediatr Neonatol. 2022;63:211–219. doi: 10.1016/j.pedneo.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 99.Furuta GT, Williams K, Kooros K, Kaul A, Panzer R, Coury DL, Fuchs G. Management of constipation in children and adolescents with autism spectrum disorders. Pediatrics. 2012;130 Suppl 2:S98–105. doi: 10.1542/peds.2012-0900H. [DOI] [PubMed] [Google Scholar]
- 100.Giuffrè M, Gazzin S, Zoratti C, Llido JP, Lanza G, Tiribelli C, Moretti R. Celiac Disease and Neurological Manifestations: From Gluten to Neuroinflammation. Int J Mol Sci. 2022;23 doi: 10.3390/ijms232415564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Croall ID, Hoggard N, Hadjivassiliou M. Gluten and Autism Spectrum Disorder. Nutrients. 2021;13 doi: 10.3390/nu13020572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu XB, Uhde M, Green PH, Alaedini A. Autoantibodies in the Extraintestinal Manifestations of Celiac Disease. Nutrients. 2018;10 doi: 10.3390/nu10081123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ahmed E, Mansour A, Amer A, Barakat T, Baz H. Screening of gastrointestinal symptoms and celiac disease in children with autism spectrum disorder. The Egyptian Journal of Otolaryngology. 2022;38 [Google Scholar]
- 104.Aljada B, Zohni A, El-Matary W. The Gluten-Free Diet for Celiac Disease and Beyond. Nutrients. 2021;13 doi: 10.3390/nu13113993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abdel-Maksoud M, Aly El-Gabry D, Al Kayoumi T, Alketbi J, Mohamednour D, Elhassan Elamin M, Subhash Reddy M, Al Yafei ZA, Stip E, Abdel Aziz K, Arnone D. Measures of gluten-related reactivity in children with autism spectrum disorders in the absence of overt gastrointestinal symptoms: a pilot study from the United Arab Emirates. J Int Med Res. 2020;48:300060520952655. doi: 10.1177/0300060520952655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Radzikowski A, Wojnar M, Kulus M, Zalewski T. [Evaluation of the effect of gluten-free diet on nutritional status of children with florid celiac disease] Pediatr Pol. 1989;64:150–154. [PubMed] [Google Scholar]
- 107.Khanijow V, Prakash P, Emsellem HA, Borum ML, Doman DB. Sleep Dysfunction and Gastrointestinal Diseases. Gastroenterol Hepatol (N Y) 2015;11:817–825. [PMC free article] [PubMed] [Google Scholar]
- 108.Koch E, Demontis D. Drug repurposing candidates to treat core symptoms in autism spectrum disorder. Front Pharmacol. 2022;13:995439. doi: 10.3389/fphar.2022.995439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vellingiri B, Aishwarya SY, Benita Jancy S, Sriram Abhishek G, Winster Suresh Babu H, Vijayakumar P, Narayanasamy A, Mariappan S, Sangeetha R, Valsala Gopalakrishnan A, Parthasarathi R, Iyer M. An anxious relationship between Autism Spectrum Disorder and Gut Microbiota: A tangled chemistry? J Clin Neurosci. 2022;99:169–189. doi: 10.1016/j.jocn.2022.03.003. [DOI] [PubMed] [Google Scholar]
- 110.Mazefsky CA, Schreiber DR, Olino TM, Minshew NJ. The association between emotional and behavioral problems and gastrointestinal symptoms among children with high-functioning autism. Autism. 2014;18:493–501. doi: 10.1177/1362361313485164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bresciani G, Da Lozzo P, Lega S, Bramuzzo M, Di Leo G, Dissegna A, Colonna V, Barbi E, Carrozzi M, Devescovi R. Gastrointestinal Disorders and Food Selectivity: Relationship with Sleep and Challenging Behavior in Children with Autism Spectrum Disorder. Children (Basel) 2023;10 doi: 10.3390/children10020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parmar KR, Porter CS, Dickinson CM, Pelham J, Baimbridge P, Gowen E. Visual Sensory Experiences From the Viewpoint of Autistic Adults. Front Psychol. 2021;12:633037. doi: 10.3389/fpsyg.2021.633037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao M, Tuo H, Wang S, Zhao L. The Effects of Dietary Nutrition on Sleep and Sleep Disorders. Mediators Inflamm. 2020;2020:3142874. doi: 10.1155/2020/3142874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Berry RC, Novak P, Withrow N, Schmidt B, Rarback S, Feucht S, Criado KK, Sharp WG. Nutrition Management of Gastrointestinal Symptoms in Children with Autism Spectrum Disorder: Guideline from an Expert Panel. J Acad Nutr Diet. 2015;115:1919–1927. doi: 10.1016/j.jand.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 115.Ding M, Lang Y, Shu H, Shao J, Cui L. Microbiota-Gut-Brain Axis and Epilepsy: A Review on Mechanisms and Potential Therapeutics. Front Immunol. 2021;12:742449. doi: 10.3389/fimmu.2021.742449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saurman V, Margolis KG, Luna RA. Autism Spectrum Disorder as a Brain-Gut-Microbiome Axis Disorder. Dig Dis Sci. 2020;65:818–828. doi: 10.1007/s10620-020-06133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zahra A, Wang Y, Wang Q, Wu J. Shared Etiology in Autism Spectrum Disorder and Epilepsy with Functional Disability. Behav Neurol. 2022;2022:5893519. doi: 10.1155/2022/5893519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pan PY, Taylor MJ, Larsson H, Almqvist C, Lichtenstein P, Lundström S, Bölte S. Genetic and environmental contributions to co-occurring physical health conditions in autism spectrum condition and attention-deficit/hyperactivity disorder. Mol Autism. 2023;14:17. doi: 10.1186/s13229-023-00548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Darch H, McCafferty CP. Gut microbiome effects on neuronal excitability & activity: Implications for epilepsy. Neurobiol Dis. 2022;165:105629. doi: 10.1016/j.nbd.2022.105629. [DOI] [PubMed] [Google Scholar]
- 120.Al-Beltagi M, Saeed NK. Epilepsy and the gut: Perpetrator or victim? World J Gastrointest Pathophysiol. 2022;13:143–156. doi: 10.4291/wjgp.v13.i5.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yibirin M, De Oliveira D, Valera R, Plitt AE, Lutgen S. Adverse Effects Associated with Proton Pump Inhibitor Use. Cureus. 2021;13:e12759. doi: 10.7759/cureus.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kerr M Guidelines Working Group, Scheepers M, Arvio M, Beavis J, Brandt C, Brown S, Huber B, Iivanainen M, Louisse AC, Martin P, Marson AG, Prasher V, Singh BK, Veendrick M, Wallace RA. Consensus guidelines into the management of epilepsy in adults with an intellectual disability. J Intellect Disabil Res. 2009;53:687–694. doi: 10.1111/j.1365-2788.2009.01182.x. [DOI] [PubMed] [Google Scholar]
- 123.Jiang CC, Lin LS, Long S, Ke XY, Fukunaga K, Lu YM, Han F. Signalling pathways in autism spectrum disorder: mechanisms and therapeutic implications. Signal Transduct Target Ther. 2022;7:229. doi: 10.1038/s41392-022-01081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mallory C, Keehn B. Implications of Sensory Processing and Attentional Differences Associated With Autism in Academic Settings: An Integrative Review. Front Psychiatry. 2021;12:695825. doi: 10.3389/fpsyt.2021.695825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nikolov RN, Bearss KE, Lettinga J, Erickson C, Rodowski M, Aman MG, McCracken JT, McDougle CJ, Tierney E, Vitiello B, Arnold LE, Shah B, Posey DJ, Ritz L, Scahill L. Gastrointestinal symptoms in a sample of children with pervasive developmental disorders. J Autism Dev Disord. 2009;39:405–413. doi: 10.1007/s10803-008-0637-8. [DOI] [PubMed] [Google Scholar]
- 126.Santhanam SP. An Interactive and Neurodiversity-Affirming Approach to Communication Supports for Autistic Students Through Videogaming. Lang Speech Hear Serv Sch. 2023;54:120–139. doi: 10.1044/2022_LSHSS-22-00027. [DOI] [PubMed] [Google Scholar]
- 127.Doreswamy S, Bashir A, Guarecuco JE, Lahori S, Baig A, Narra LR, Patel P, Heindl SE. Effects of Diet, Nutrition, and Exercise in Children With Autism and Autism Spectrum Disorder: A Literature Review. Cureus. 2020;12:e12222. doi: 10.7759/cureus.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hillier A, Goldstein J, Murphy D, Trietsch R, Keeves J, Mendes E, Queenan A. Supporting university students with autism spectrum disorder. Autism. 2018;22:20–28. doi: 10.1177/1362361317699584. [DOI] [PubMed] [Google Scholar]
- 129.Ferguson BJ, Dovgan K, Takahashi N, Beversdorf DQ. The Relationship Among Gastrointestinal Symptoms, Problem Behaviors, and Internalizing Symptoms in Children and Adolescents With Autism Spectrum Disorder. Front Psychiatry. 2019;10:194. doi: 10.3389/fpsyt.2019.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mitrea L, Nemeş SA, Szabo K, Teleky BE, Vodnar DC. Guts Imbalance Imbalances the Brain: A Review of Gut Microbiota Association With Neurological and Psychiatric Disorders. Front Med (Lausanne) 2022;9:813204. doi: 10.3389/fmed.2022.813204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cermak SA, Curtin C, Bandini LG. Food selectivity and sensory sensitivity in children with autism spectrum disorders. J Am Diet Assoc. 2010;110:238–246. doi: 10.1016/j.jada.2009.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bowman KS, Suarez VD, Weiss MJ. Standards for Interprofessional Collaboration in the Treatment of Individuals With Autism. Behav Anal Pract. 2021;14:1191–1208. doi: 10.1007/s40617-021-00560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Carlier S, Vorlet P, Sá Dos Reis C, Malamateniou C. Strategies, challenges and enabling factors when imaging autistic individuals in Swiss medical imaging departments. J Med Imaging Radiat Sci. 2022 doi: 10.1016/j.jmir.2022.11.002. [DOI] [PubMed] [Google Scholar]
- 134.Dineen-Griffin S, Garcia-Cardenas V, Williams K, Benrimoj SI. Helping patients help themselves: A systematic review of self-management support strategies in primary health care practice. PLoS One. 2019;14:e0220116. doi: 10.1371/journal.pone.0220116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bailey AJ. The neuroscience of autism education. Autism Res. 2008;1:207. doi: 10.1002/aur.36. [DOI] [PubMed] [Google Scholar]
- 136.Leader G, O'Reilly M, Gilroy SP, Chen JL, Ferrari C, Mannion A. Comorbid Feeding and Gastrointestinal Symptoms, Challenging Behavior, Sensory Issues, Adaptive Functioning and Quality of Life in Children and Adolescents with Autism Spectrum Disorder. Dev Neurorehabil. 2021;24:35–44. doi: 10.1080/17518423.2020.1770354. [DOI] [PubMed] [Google Scholar]
- 137.Brede J, Babb C, Jones C, Elliott M, Zanker C, Tchanturia K, Serpell L, Fox J, Mandy W. "For Me, the Anorexia is Just a Symptom, and the Cause is the Autism": Investigating Restrictive Eating Disorders in Autistic Women. J Autism Dev Disord. 2020;50:4280–4296. doi: 10.1007/s10803-020-04479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Babinska K, Celusakova H, Belica I, Szapuova Z, Waczulikova I, Nemcsicsova D, Tomova A, Ostatnikova D. Gastrointestinal Symptoms and Feeding Problems and Their Associations with Dietary Interventions, Food Supplement Use, and Behavioral Characteristics in a Sample of Children and Adolescents with Autism Spectrum Disorders. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17176372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dovgan K, Gynegrowski K, Ferguson BJ. Bidirectional relationship between internalizing symptoms and gastrointestinal problems in youth with Autism Spectrum Disorder. J Autism Dev Disord. 2022 doi: 10.1007/s10803-022-05539-6. [DOI] [PubMed] [Google Scholar]
- 140.Salleh MR. Life event, stress and illness. Malays J Med Sci. 2008;15:9–18. [PMC free article] [PubMed] [Google Scholar]
- 141.Andrulis DP, Brach C. Integrating literacy, culture, and language to improve health care quality for diverse populations. Am J Health Behav. 2007:31 Suppl 1: S122–S133. doi: 10.5555/ajhb.2007.31.supp.S122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Oakley BF, Tillmann J, Ahmad J, Crawley D, San José Cáceres A, Holt R, Charman T, Banaschewski T, Buitelaar J, Simonoff E, Murphy D, Loth E. How do core autism traits and associated symptoms relate to quality of life? Findings from the Longitudinal European Autism Project. Autism. 2021;25:389–404. doi: 10.1177/1362361320959959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hodges H, Fealko C, Soares N. Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation. Transl Pediatr. 2020;9:S55–S65. doi: 10.21037/tp.2019.09.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Garcia-Gutierrez E, Narbad A, Rodríguez JM. Autism Spectrum Disorder Associated With Gut Microbiota at Immune, Metabolomic, and Neuroactive Level. Front Neurosci. 2020;14:578666. doi: 10.3389/fnins.2020.578666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017;595:489–503. doi: 10.1113/JP273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hughes HK, Rose D, Ashwood P. The Gut Microbiota and Dysbiosis in Autism Spectrum Disorders. Curr Neurol Neurosci Rep. 2018;18:81. doi: 10.1007/s11910-018-0887-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jendraszak M, Gałęcka M, Kotwicka M, Regdos A, Pazgrat-Patan M, Andrusiewicz M. Commercial microbiota test revealed differences in the composition of intestinal microorganisms between children with autism spectrum disorders and neurotypical peers. Sci Rep. 2021;11:24274. doi: 10.1038/s41598-021-03794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, Krajmalnik-Brown R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8:e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11:22. doi: 10.1186/1471-230X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu M, Xu X, Li J, Li F. Association Between Gut Microbiota and Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Front Psychiatry. 2019;10:473. doi: 10.3389/fpsyt.2019.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Iglesias-Vázquez L, Van Ginkel Riba G, Arija V, Canals J. Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Nutrients. 2020;12 doi: 10.3390/nu12030792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu T, Wang H, Lu W, Zhai Q, Zhang Q, Yuan W, Gu Z, Zhao J, Zhang H, Chen W. Potential of gut microbiome for detection of autism spectrum disorder. Microb Pathog. 2020;149:104568. doi: 10.1016/j.micpath.2020.104568. [DOI] [PubMed] [Google Scholar]
- 153.Qureshi F, Adams J, Hanagan K, Kang DW, Krajmalnik-Brown R, Hahn J. Multivariate Analysis of Fecal Metabolites from Children with Autism Spectrum Disorder and Gastrointestinal Symptoms before and after Microbiota Transfer Therapy. J Pers Med. 2020;10 doi: 10.3390/jpm10040152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhai Q, Cen S, Jiang J, Zhao J, Zhang H, Chen W. Disturbance of trace element and gut microbiota profiles as indicators of autism spectrum disorder: A pilot study of Chinese children. Environ Res. 2019;171:501–509. doi: 10.1016/j.envres.2019.01.060. [DOI] [PubMed] [Google Scholar]
- 155.Galloway-Peña J, Hanson B. Tools for Analysis of the Microbiome. Dig Dis Sci. 2020;65:674–685. doi: 10.1007/s10620-020-06091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fiorentino M, Sapone A, Senger S, Camhi SS, Kadzielski SM, Buie TM, Kelly DL, Cascella N, Fasano A. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism. 2016;7:49. doi: 10.1186/s13229-016-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Esnafoglu E, Cırrık S, Ayyıldız SN, Erdil A, Ertürk EY, Daglı A, Noyan T. Increased Serum Zonulin Levels as an Intestinal Permeability Marker in Autistic Subjects. J Pediatr. 2017;188:240–244. doi: 10.1016/j.jpeds.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 158.Dalton N, Chandler S, Turner C, Charman T, Pickles A, Loucas T, Simonoff E, Sullivan P, Baird G. Gut permeability in autism spectrum disorders. Autism Res. 2014;7:305–313. doi: 10.1002/aur.1350. [DOI] [PubMed] [Google Scholar]
- 159.Patusco R, Ziegler J. Role of Probiotics in Managing Gastrointestinal Dysfunction in Children with Autism Spectrum Disorder: An Update for Practitioners. Adv Nutr. 2018;9:637–650. doi: 10.1093/advances/nmy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mangiola F, Ianiro G, Franceschi F, Fagiuoli S, Gasbarrini G, Gasbarrini A. Gut microbiota in autism and mood disorders. World J Gastroenterol. 2016;22:361–368. doi: 10.3748/wjg.v22.i1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ali T, Choe J, Awab A, Wagener TL, Orr WC. Sleep, immunity and inflammation in gastrointestinal disorders. World J Gastroenterol. 2013;19:9231–9239. doi: 10.3748/wjg.v19.i48.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Karhu E, Zukerman R, Eshraghi RS, Mittal J, Deth RC, Castejon AM, Trivedi M, Mittal R, Eshraghi AA. Nutritional interventions for autism spectrum disorder. Nutr Rev. 2020;78:515–531. doi: 10.1093/nutrit/nuz092. [DOI] [PubMed] [Google Scholar]
- 163.Taha Z, Abdalhai KA. A Review of the Efficacy of the Dietary Intervention in Autism Spectrum Disorder. Open Access Maced J Med Sci. 2021;18 [Google Scholar]
- 164.Amadi CN, Orish CN, Frazzoli C, Orisakwe OE. Dietary interventions for autism spectrum disorder: An updated systematic review of human studies. Psychiatriki. 2022;33:228–242. doi: 10.22365/jpsych.2022.073. [DOI] [PubMed] [Google Scholar]
- 165.Yu Y, Huang J, Chen X, Fu J, Wang X, Pu L, Gu C, Cai C. Efficacy and Safety of Diet Therapies in Children With Autism Spectrum Disorder: A Systematic Literature Review and Meta-Analysis. Front Neurol. 2022;13:844117. doi: 10.3389/fneur.2022.844117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pruimboom L, de Punder K. The opioid effects of gluten exorphins: asymptomatic celiac disease. J Health Popul Nutr. 2015;33:24. doi: 10.1186/s41043-015-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Trivedi MS, Shah JS, Al-Mughairy S, Hodgson NW, Simms B, Trooskens GA, Van Criekinge W, Deth RC. Food-derived opioid peptides inhibit cysteine uptake with redox and epigenetic consequences. J Nutr Biochem. 2014;25:1011–1018. doi: 10.1016/j.jnutbio.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stewart PA, Hyman SL, Schmidt BL, Macklin EA, Reynolds A, Johnson CR, James SJ, Manning-Courtney P. Dietary Supplementation in Children with Autism Spectrum Disorders: Common, Insufficient, and Excessive. J Acad Nutr Diet. 2015;115:1237–1248. doi: 10.1016/j.jand.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 169.Piwowarczyk A, Horvath A, Łukasik J, Pisula E, Szajewska H. Gluten- and casein-free diet and autism spectrum disorders in children: a systematic review. Eur J Nutr. 2018;57:433–440. doi: 10.1007/s00394-017-1483-2. [DOI] [PubMed] [Google Scholar]
- 170.Quan L, Xu X, Cui Y, Han H, Hendren RL, Zhao L, You X. A systematic review and meta-analysis of the benefits of a gluten-free diet and/or casein-free diet for children with autism spectrum disorder. Nutr Rev. 2022;80:1237–1246. doi: 10.1093/nutrit/nuab073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Obih C, Wahbeh G, Lee D, Braly K, Giefer M, Shaffer ML, Nielson H, Suskind DL. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition. 2016;32:418–425. doi: 10.1016/j.nut.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 172.Barnhill K, Devlin M, Moreno HT, Potts A, Richardson W, Schutte C, Hewitson L. Brief Report: Implementation of a Specific Carbohydrate Diet for a Child with Autism Spectrum Disorder and Fragile X Syndrome. J Autism Dev Disord. 2020;50:1800–1808. doi: 10.1007/s10803-018-3704-9. [DOI] [PubMed] [Google Scholar]
- 173.Żarnowska I, Chrapko B, Gwizda G, Nocuń A, Mitosek-Szewczyk K, Gasior M. Therapeutic use of carbohydrate-restricted diets in an autistic child; a case report of clinical and 18FDG PET findings. Metab Brain Dis. 2018;33:1187–1192. doi: 10.1007/s11011-018-0219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ābele, Silvija, Meija, Laila, Folkmanis, Valdis and Tzivian, Lilian “Specific Carbohydrate Diet (SCD/GAPS) and Dietary Supplements for Children with Autistic Spectrum Disorder” Proceedings of the Latvian Academy of Sciences. Section B. Natural, Exact, and Applied Sciences. 2021;75:417–425. [Google Scholar]
- 175.Shaaban S, Al-Beltagi M, El Rashidy O, Nassar M, El Gendy Y. Ketogenic diet in childhood epilepsy: clinical algorithm in a tertiary care center. Front Pediatr. 2023;11:1221781. doi: 10.3389/fped.2023.1221781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li Q, Liang J, Fu N, Han Y, Qin J. A Ketogenic Diet and the Treatment of Autism Spectrum Disorder. Front Pediatr. 2021;9:650624. doi: 10.3389/fped.2021.650624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Albers J, Kraja G, Eller D, Eck K, McBrian D, Bain JM. Assessing the feasibility of using the ketogenic diet in autism spectrum disorder. J Hum Nutr Diet. 2023;36:1303–1315. doi: 10.1111/jhn.13115. [DOI] [PubMed] [Google Scholar]
- 178.Díaz Vargas D, Leonario Rodríguez M. Effectiveness of nutritional interventions on behavioral symptomatology of autism spectrum disorder: a systematic review. Nutr Hosp. 2022;39:1378–1388. doi: 10.20960/nh.04155. [DOI] [PubMed] [Google Scholar]
- 179.Varesio C, Grumi S, Zanaboni MP, Mensi MM, Chiappedi M, Pasca L, Ferraris C, Tagliabue A, Borgatti R, De Giorgis V. Ketogenic Dietary Therapies in Patients with Autism Spectrum Disorder: Facts or Fads? A Scoping Review and a Proposal for a Shared Protocol. Nutrients. 2021;13 doi: 10.3390/nu13062057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vallamchetla SK. Exploring the Therapeutic Potential of the Ketogenic Diet on Neurological Disorders: A Comprehensive Review. J Neurol Neuromedicine. 2023;7:1–8. [Google Scholar]
- 181.Oselu S, Ebere R, Arimi JM. Camels, Camel Milk, and Camel Milk Product Situation in Kenya in Relation to the World. Int J Food Sci. 2022;2022:1237423. doi: 10.1155/2022/1237423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Al-Ayadhi LY, Elamin NE. Camel Milk as a Potential Therapy as an Antioxidant in Autism Spectrum Disorder (ASD) Evid Based Complement Alternat Med. 2013;2013:602834. doi: 10.1155/2013/602834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mihic T, Rainkie D, Wilby KJ, Pawluk SA. The Therapeutic Effects of Camel Milk: A Systematic Review of Animal and Human Trials. J Evid Based Complementary Altern Med. 2016;21:NP110–NP126. doi: 10.1177/2156587216658846. [DOI] [PubMed] [Google Scholar]
- 184.Al-Ayadhi LY, Halepoto DM, Al-Dress AM, Mitwali Y, Zainah R. Behavioral Benefits of Camel Milk in Subjects with Autism Spectrum Disorder. J Coll Physicians Surg Pak. 2015;25:819–823. [PubMed] [Google Scholar]
- 185.Kandeel M, El-Deeb W. The Application of Natural Camel Milk Products to Treat Autism-Spectrum Disorders: Risk Assessment and Meta-Analysis of Randomized Clinical Trials. Bioinorg Chem Appl. 2022;2022:6422208. doi: 10.1155/2022/6422208. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 186.Shabo, Yosef and Yagil, Reuven “Etiology of autism and camel milk as therapy”. International Journal on Disability and Human Development. 2005;4:67–70. [Google Scholar]
- 187.O'Leary F, Samman S. Vitamin B12 in health and disease. Nutrients. 2010;2:299–316. doi: 10.3390/nu2030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fakhroo A, Al-Hammadi M, Fakhroo L, Al-Ali F, Snobar R, Al-Beltagi M, Kamal A. The Effect of High-Dose Methyl Vitamin B12 Therapy on Epileptogenesis in Rats: An In Vivo Study. Cureus. 2023;15:e35929. doi: 10.7759/cureus.35929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bertoglio K, Jill James S, Deprey L, Brule N, Hendren RL. Pilot study of the effect of methyl B12 treatment on behavioral and biomarker measures in children with autism. J Altern Complement Med. 2010;16:555–560. doi: 10.1089/acm.2009.0177. [DOI] [PubMed] [Google Scholar]
- 190.Hendren RL, James SJ, Widjaja F, Lawton B, Rosenblatt A, Bent S. Randomized, Placebo-Controlled Trial of Methyl B12 for Children with Autism. J Child Adolesc Psychopharmacol. 2016;26:774–783. doi: 10.1089/cap.2015.0159. [DOI] [PubMed] [Google Scholar]
- 191.James SJ, Melnyk S, Fuchs G, Reid T, Jernigan S, Pavliv O, Hubanks A, Gaylor DW. Efficacy of methylcobalamin and folinic acid treatment on glutathione redox status in children with autism. Am J Clin Nutr. 2009;89:425–430. doi: 10.3945/ajcn.2008.26615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Frye RE, Melnyk S, Fuchs G, Reid T, Jernigan S, Pavliv O, Hubanks A, Gaylor DW, Walters L, James SJ. Effectiveness of methylcobalamin and folinic Acid treatment on adaptive behavior in children with autistic disorder is related to glutathione redox status. Autism Res Treat. 2013;2013:609705. doi: 10.1155/2013/609705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Žigman T, Petković Ramadža D, Šimić G, Barić I. Inborn Errors of Metabolism Associated With Autism Spectrum Disorders: Approaches to Intervention. Front Neurosci. 2021;15:673600. doi: 10.3389/fnins.2021.673600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Čorejová A, Fazekaš T, Jánošíková D, Repiský J, Pospíšilová V, Miková M, Rauová D, Ostatníková D, Kyselovič J, Hrabovská A. Improvement of the Clinical and Psychological Profile of Patients with Autism after Methylcobalamin Syrup Administration. Nutrients. 2022;14 doi: 10.3390/nu14102035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rossignol DA, Frye RE. The Effectiveness of Cobalamin (B12) Treatment for Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. J Pers Med. 2021;11 doi: 10.3390/jpm11080784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dighriri IM, Alsubaie AM, Hakami FM, Hamithi DM, Alshekh MM, Khobrani FA, Dalak FE, Hakami AA, Alsueaadi EH, Alsaawi LS, Alshammari SF, Alqahtani AS, Alawi IA, Aljuaid AA, Tawhari MQ. Effects of Omega-3 Polyunsaturated Fatty Acids on Brain Functions: A Systematic Review. Cureus. 2022;14:e30091. doi: 10.7759/cureus.30091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52. doi: 10.3389/fnagi.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Agostoni C, Nobile M, Ciappolino V, Delvecchio G, Tesei A, Turolo S, Crippa A, Mazzocchi A, Altamura CA, Brambilla P. The Role of Omega-3 Fatty Acids in Developmental Psychopathology: A Systematic Review on Early Psychosis, Autism, and ADHD. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18122608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bent S, Bertoglio K, Hendren RL. Omega-3 fatty acids for autistic spectrum disorder: a systematic review. J Autism Dev Disord. 2009;39:1145–1154. doi: 10.1007/s10803-009-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cheng YS, Tseng PT, Chen YW, Stubbs B, Yang WC, Chen TY, Wu CK, Lin PY. Supplementation of omega 3 fatty acids may improve hyperactivity, lethargy, and stereotypy in children with autism spectrum disorders: a meta-analysis of randomized controlled trials. Neuropsychiatr Dis Treat. 2017;13:2531–2543. doi: 10.2147/NDT.S147305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mankad D, Dupuis A, Smile S, Roberts W, Brian J, Lui T, Genore L, Zaghloul D, Iaboni A, Marcon PM, Anagnostou E. A randomized, placebo controlled trial of omega-3 fatty acids in the treatment of young children with autism. Mol Autism. 2015;6:18. doi: 10.1186/s13229-015-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Parra M, Stahl S, Hellmann H. Vitamin B₆ and Its Role in Cell Metabolism and Physiology. Cells. 2018;7 doi: 10.3390/cells7070084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Obara T, Ishikuro M, Tamiya G, Ueki M, Yamanaka C, Mizuno S, Kikuya M, Metoki H, Matsubara H, Nagai M, Kobayashi T, Kamiyama M, Watanabe M, Kakuta K, Ouchi M, Kurihara A, Fukuchi N, Yasuhara A, Inagaki M, Kaga M, Kure S, Kuriyama S. Potential identification of vitamin B6 responsiveness in autism spectrum disorder utilizing phenotype variables and machine learning methods. Sci Rep. 2018;8:14840. doi: 10.1038/s41598-018-33110-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Khan F, Rahman MS, Akhter S, Momen ABI, Raihan SG. Vitamin B6 and Magnesium on Neurobehavioral Status of Autism Spectrum Disorder: A Randomized, Double-Blind, Placebo Controlled Study. Bangladesh Journal of Medicine. 2021;32:12–18 . [Google Scholar]
- 205.Nye C, Brice A. Combined vitamin B6-magnesium treatment in autism spectrum disorder. Cochrane Database Syst Rev. 2005;2005:CD003497. doi: 10.1002/14651858.CD003497.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Srinivasan P. A review of dietary interventions in autism. Ann Clin Psychiatry. 2009;21:237–247. [PubMed] [Google Scholar]
- 207.Burrus CJ. A biochemical rationale for the interaction between gastrointestinal yeast and autism. Med Hypotheses. 2012;79:784–785. doi: 10.1016/j.mehy.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 208.Baker S, Shaw W. Case Study: Rapid Complete Recovery From An Autism Spectrum Disorder After Treatment of Aspergillus With The Antifungal Drugs Itraconazole And Sporanox. Integr Med (Encinitas) 2020;19:20–27. [PMC free article] [PubMed] [Google Scholar]
- 209.Herman A, Herman AP. Could Candida Overgrowth Be Involved in the Pathophysiology of Autism? J Clin Med. 2022;11 doi: 10.3390/jcm11020442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bibbò S, Ianiro G, Giorgio V, Scaldaferri F, Masucci L, Gasbarrini A, Cammarota G. The role of diet on gut microbiota composition. Eur Rev Med Pharmacol Sci. 2016;20:4742–4749. [PubMed] [Google Scholar]
- 211.Vargason T, McGuinness DL, Hahn J. Gastrointestinal Symptoms and Oral Antibiotic Use in Children with Autism Spectrum Disorder: Retrospective Analysis of a Privately Insured U.S. Population. J Autism Dev Disord. 2019;49:647–659. doi: 10.1007/s10803-018-3743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Marí-Bauset S, Llopis-González A, Zazpe I, Marí-Sanchis A, Suárez-Varela MM. Fat intake in children with autism spectrum disorder in the Mediterranean region (Valencia, Spain) Nutr Neurosci. 2016;19:377–386. doi: 10.1179/1476830515Y.0000000029. [DOI] [PubMed] [Google Scholar]
- 213.Kaviyarasan S, Chung Sia EL, Retinasamy T, Arulsamy A, Shaikh MF. Regulation of gut microbiome by ketogenic diet in neurodegenerative diseases: A molecular crosstalk. Front Aging Neurosci. 2022;14:1015837. doi: 10.3389/fnagi.2022.1015837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Baspinar B, Yardimci H. Gluten-Free Casein-Free Diet for Autism Spectrum Disorders: Can It Be Effective in Solving Behavioural and Gastrointestinal Problems? Eurasian J Med. 2020;52:292–297. doi: 10.5152/eurasianjmed.2020.19230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Białek-Dratwa A, Szymańska D, Grajek M, Krupa-Kotara K, Szczepańska E, Kowalski O. ARFID-Strategies for Dietary Management in Children. Nutrients. 2022;14 doi: 10.3390/nu14091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yang H, Liu Y, Cai R, Li Y, Gu B. A narrative review of relationship between gut microbiota and neuropsychiatric disorders: mechanisms and clinical application of probiotics and prebiotics. Ann Palliat Med. 2021;10:2304–2313. doi: 10.21037/apm-20-1365. [DOI] [PubMed] [Google Scholar]
- 217.Abdellatif B, McVeigh C, Bendriss G, Chaari A. The Promising Role of Probiotics in Managing the Altered Gut in Autism Spectrum Disorders. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21114159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, Berenjian A, Ghasemi Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods. 2019;8 doi: 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Grimaldi R, Gibson GR, Vulevic J, Giallourou N, Castro-Mejía JL, Hansen LH, Leigh Gibson E, Nielsen DS, Costabile A. A prebiotic intervention study in children with autism spectrum disorders (ASDs) Microbiome. 2018;6:133. doi: 10.1186/s40168-018-0523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lu C, Rong J, Fu C, Wang W, Xu J, Ju XD. Overall Rebalancing of Gut Microbiota Is Key to Autism Intervention. Front Psychol. 2022;13:862719. doi: 10.3389/fpsyg.2022.862719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sanctuary MR, Kain JN, Chen SY, Kalanetra K, Lemay DG, Rose DR, Yang HT, Tancredi DJ, German JB, Slupsky CM, Ashwood P, Mills DA, Smilowitz JT, Angkustsiri K. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS One. 2019;14:e0210064. doi: 10.1371/journal.pone.0210064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kong XJ, Liu J, Liu K, Koh M, Sherman H, Liu S, Tian R, Sukijthamapan P, Wang J, Fong M, Xu L, Clairmont C, Jeong MS, Li A, Lopes M, Hagan V, Dutton T, Chan SP, Lee H, Kendall A, Kwong K, Song Y. Probiotic and Oxytocin Combination Therapy in Patients with Autism Spectrum Disorder: A Randomized, Double-Blinded, Placebo-Controlled Pilot Trial. Nutrients. 2021;13 doi: 10.3390/nu13051552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li N, Chen H, Cheng Y, Xu F, Ruan G, Ying S, Tang W, Chen L, Chen M, Lv L, Ping Y, Chen D, Wei Y. Fecal Microbiota Transplantation Relieves Gastrointestinal and Autism Symptoms by Improving the Gut Microbiota in an Open-Label Study. Front Cell Infect Microbiol. 2021;11:759435. doi: 10.3389/fcimb.2021.759435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S, Pollard EL, Roux S, Sadowsky MJ, Lipson KS, Sullivan MB, Caporaso JG, Krajmalnik-Brown R. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5:10. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hadrich D. Microbiome Research Is Becoming the Key to Better Understanding Health and Nutrition. Front Genet. 2018;9:212. doi: 10.3389/fgene.2018.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Johnson D, Letchumanan V, Thurairajasingam S, Lee LH. A Revolutionizing Approach to Autism Spectrum Disorder Using the Microbiome. Nutrients. 2020;12 doi: 10.3390/nu12071983. [DOI] [PMC free article] [PubMed] [Google Scholar]