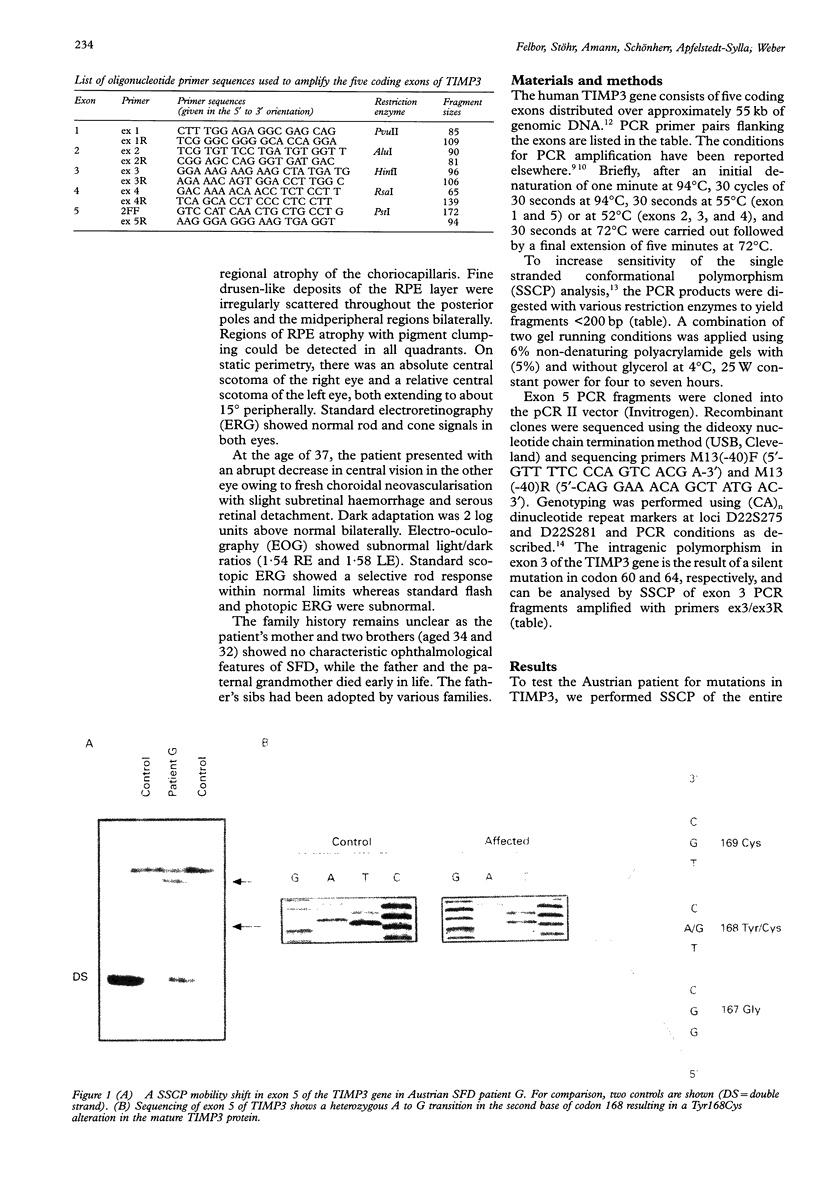
Abstract
Sorsby's fundus dystrophy (SFD) is a rare autosomal dominant macular disorder with age of onset usually in the fourth decade. It is characterised by loss of central vision owing to subretinal neovascularisation and disciform macular degeneration. In an effort to identify the SFD gene, the disease locus was first mapped to chromosome 22q13-qter by genetic linkage analysis, the same chromosomal region as the gene encoding the tissue inhibitor of metalloproteinases-3 (TIMP3). Subsequently, two separate mutations in TIMP3 were found in affected members of two unrelated SFD pedigrees (Tyr168Cys and Ser181Cys). More recently, two additional SFD related mutations, Ser156Cys and Gly167Cys, have provided further confirmation that heterozygous mutations in TIMP3 are causally responsible for the SFD phenotype. We now report the occurrence of the Tyr168Cys mutation in an SFD patient of Austrian descent and show that this mutation found earlier in an American SFD family arose independently. The new findings add to an emerging pattern of SFD mutations which all seem to affect the C-terminal region of the mature TIMP3 protein. In addition, all known mutations cause a change of an amino acid to a cysteine residue. This suggests a critical role for the additional C-terminal free thiol group in SFD pathogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apte S. S., Mattei M. G., Olsen B. R. Cloning of the cDNA encoding human tissue inhibitor of metalloproteinases-3 (TIMP-3) and mapping of the TIMP3 gene to chromosome 22. Genomics. 1994 Jan 1;19(1):86–90. doi: 10.1006/geno.1994.1016. [DOI] [PubMed] [Google Scholar]
- Capon M. R., Marshall J., Krafft J. I., Alexander R. A., Hiscott P. S., Bird A. C. Sorsby's fundus dystrophy. A light and electron microscopic study. Ophthalmology. 1989 Dec;96(12):1769–1777. doi: 10.1016/s0161-6420(89)32664-9. [DOI] [PubMed] [Google Scholar]
- Felbor U., Stöhr H., Amann T., Schönherr U., Weber B. H. A novel Ser156Cys mutation in the tissue inhibitor of metalloproteinases-3 (TIMP3) in Sorsby's fundus dystrophy with unusual clinical features. Hum Mol Genet. 1995 Dec;4(12):2415–2416. doi: 10.1093/hmg/4.12.2415. [DOI] [PubMed] [Google Scholar]
- Jacobson S. G., Cideciyan A. V., Regunath G., Rodriguez F. J., Vandenburgh K., Sheffield V. C., Stone E. M. Night blindness in Sorsby's fundus dystrophy reversed by vitamin A. Nat Genet. 1995 Sep;11(1):27–32. doi: 10.1038/ng0995-27. [DOI] [PubMed] [Google Scholar]
- Leco K. J., Khokha R., Pavloff N., Hawkes S. P., Edwards D. R. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is an extracellular matrix-associated protein with a distinctive pattern of expression in mouse cells and tissues. J Biol Chem. 1994 Mar 25;269(12):9352–9360. [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Murphy G., Houbrechts A., Cockett M. I., Williamson R. A., O'Shea M., Docherty A. J. The N-terminal domain of tissue inhibitor of metalloproteinases retains metalloproteinase inhibitory activity. Biochemistry. 1991 Aug 20;30(33):8097–8102. doi: 10.1021/bi00247a001. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Pauleikhoff D., Chen J. C., Chisholm I. H., Bird A. C. Choroidal perfusion abnormality with age-related Bruch's membrane change. Am J Ophthalmol. 1990 Feb 15;109(2):211–217. doi: 10.1016/s0002-9394(14)75989-6. [DOI] [PubMed] [Google Scholar]
- Pavloff N., Staskus P. W., Kishnani N. S., Hawkes S. P. A new inhibitor of metalloproteinases from chicken: ChIMP-3. A third member of the TIMP family. J Biol Chem. 1992 Aug 25;267(24):17321–17326. [PubMed] [Google Scholar]
- Polkinghorne P. J., Capon M. R., Berninger T., Lyness A. L., Sehmi K., Bird A. C. Sorsby's fundus dystrophy. A clinical study. Ophthalmology. 1989 Dec;96(12):1763–1768. doi: 10.1016/s0161-6420(89)32654-6. [DOI] [PubMed] [Google Scholar]
- SORSBY A., MASON M. E. J. A fundus dystrophy with unusual features. Br J Ophthalmol. 1949 Feb;33(2):67–97. doi: 10.1136/bjo.33.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B. H., Vogt G., Pruett R. C., Stöhr H., Felbor U. Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby's fundus dystrophy. Nat Genet. 1994 Dec;8(4):352–356. doi: 10.1038/ng1294-352. [DOI] [PubMed] [Google Scholar]
- Weber B. H., Vogt G., Wolz W., Ives E. J., Ewing C. C. Sorsby's fundus dystrophy is genetically linked to chromosome 22q13-qter. Nat Genet. 1994 Jun;7(2):158–161. doi: 10.1038/ng0694-158. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- Willenbrock F., Crabbe T., Slocombe P. M., Sutton C. W., Docherty A. J., Cockett M. I., O'Shea M., Brocklehurst K., Phillips I. R., Murphy G. The activity of the tissue inhibitors of metalloproteinases is regulated by C-terminal domain interactions: a kinetic analysis of the inhibition of gelatinase A. Biochemistry. 1993 Apr 27;32(16):4330–4337. doi: 10.1021/bi00067a023. [DOI] [PubMed] [Google Scholar]