Abstract
During the Coronavirus disease (COVID-19), the physical activity of older adults is at a lower level. The study aimed to examine the effectiveness of aerobic dancing on physical fitness and cognitive function in older adults. We conducted a randomized controlled trial with 34 older adults who were assigned into an aerobic dancing group and a control group. Three dance sessions weekly for 60 min were scheduled for the aerobic dancing group for a total of 12 weeks. Physical fitness, blood pressure, lipids, glucose, cognitive function were assessed before and after the intervention. Baseline adjusted Analysis of Covariance (ANCOVA) was used to determine whether outcome variables varied between groups at pre-test and post-test. Effect size (Cohen's d) was calculated to determine the differences between groups from baseline to post-test. After 12 weeks, we found that the aerobic dancing group showed significant improvement in memory (portrait memory: F = 10.45, p = 0.003, d = 1.18). The Limit of Stability (LOS) parameters in the aerobic dancing group displayed a significant increase after the intervention (right angle: F = 5.90, p = 0.022, d = 0.60; right-anterior angle: F = 4.23, p = 0.049, d = 0.12). Some beneficial effects were found on flexibility, grip strength, balance and subjective well-being (sit and reach: F = 0.25, p = 0.62, d = −0.40; grip strength: F = 3.38, p = 0.08, d = 0.89; one-legged standing with eyes closed: F = 1.26, p = 0.27, d = 0.50) in the aerobic dancing group. Aerobic dancing training was effective in improving memory and balance ability in older adults during the COVID-19 pandemic in China. In the future, aerobic dancing is a promising tool to encourage physical activity in older adults.
Keywords: Aerobic dancing, Older adults, Physical fitness, Cognitive function, Balance
Abbreviations:
- COVID-19
Coronavirus Disease 2019
- ANCOVA
Analysis of Covariance
- MMSE
The Mini-Mental State Examination
- SSJ
Soul Sexy Jazz
- PAR-Q
The Physical Activity Readiness Questionnaire
- PACES
The physical activity enjoyment scale
- mins
minutes
- cm
centimeters
- kg
kilograms
- s
seconds
- m
meters
- ml
milliliters
- L
Liters
- BMI
Body mass index
- LOS
Limit of stability
- PST
Postural Stability Test
- MCT
Motor Control Test
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- M
mean
- SD
Standard Deviation
Introduction
With the rapid development of China, the population of China is undergoing huge changes in terms of age structure. The results of the seventh national census showed that there were 264.02 million people in China over the age of 60 in 2021, and this is expected to grow to 300 million by 2025 (21% of the population).1 The enormous pressure of the aging population is a challenge for the government to maintain and improve the health and life quality for the older adults.2 Older adults have been reported to have increased risk of cardiovascular disease and unintentional injury due to increased sedentary time, descended physical activity, increased body fat, and decreased muscle mass.3 As the global population is aging, the number of people with dementia or Alzheimer's disease was expected to surge in the future, posing colossal challenges to social, healthcare, and economic system.4 Therefore, improving outcomes in these conditions are crucial to health as people age.
In 2020, the Coronavirus Disease 2019 (COVID-19, an infectious disease caused by the SARS-CoV-2 virus) has brought the fast-moving world to a standstill. In the context of COVID-19, the older adults are the most vulnerable group due to physical aging and weakened immunity and had been adversely affected by social distancing and physical activity restrictions. They are easily infected and at high risk of death from severe cases.5,6 The COVID-19 pandemic has forced governments in China to adopt lockdowns and home isolation measures as an epidemiological control strategy. The lack of physical activity and the increase in sedentary time among older adults could be associated with more severe COVID-19 outcomes.7 Some older adults are unable to engage in outdoor activities, and their daily life is seriously disrupted. They are prone to psychological problems such as feelings of loss, loneliness, and emptiness, which may further decrease their cognitive function and threaten their overall health.8 To avoid the negative effects of pandemic, exercise should be prescribed to older adults, which is of great importance for developing a healthy lifestyle and boosting their immunity.9,10 A recent meta-analysis suggested exercise benefits cognition in older adults, regardless of their cognitive status.11 Literature indicates that moderate intensity exercise in combination with cognitive, social, and emotional challenges can ameliorate a wide spectrum of age-related decline.12,13 Exercise is a safe way to improve health and promote healthy aging.14
Aerobic dancing, an enjoyable form of exercise, has the potential to improve physical and mental health. In recent years, besides its aesthetic and recreational values, the potentials of aerobic dancing for improving physical and mental well-being has been increasingly recognized.11,15,16 Two systematic reviews and meta-analyses of the effects of aerobic dancing on cognitive function in older adults with mild cognitive impairment showed aerobic dancing significantly improved global cognitive function and had a positive effect on improving executive function.17,18 An aerobic dancing intervention program (slow waltz) developed specifically for older adults improved short-term memory and executive function in the training group.19 Research also has shown home-based training is a low-cost, safe and effective exercise option to increase muscle strength and function in healthy and non-healthy older adults.20 During the COVID-19 pandemic, some older adults were unable to exercise in public places due to social isolation. Therefore, the combination of online and offline physical activity has gradually become an important form of exercise, such as dancing training at home. Maintaining a regular schedule of dancing in older adults could preserve cognitive, motor and perceptual abilities and prevent them from degradation.21 In an intervention trial conducted during the COVID-19 pandemic in Japan, 88 healthy older adults were divided into a Nordic walking group, an aerobic dancing group, or a control group for four-week (30 min three times a week) training, and the results showed that the aerobic dancing group significantly improved the general cognitive function and executive function.22 Innovative and enjoyable aerobic dancing interventions are needed to help mitigate the adverse effects of physical inactivity during COVID-19.23
To date, it has not been investigated if aerobic dancing could affect physical fitness, cognitive function, and balance ability in older Chinese adults during COVID-19 pandemic.22 This study aimed to investigate the effect of a 12-week aerobic dancing intervention in a group of older adults using a pre-post design. This study was a natural experiment with combination of online and offline aerobic dancing training, and the purpose was to examine the feasibility and acceptability and assess effects of aerobic dancing on physical fitness and cognitive function among older Chinese adults in the real world during periods like the COVID-19 pandemic. This study will help to develop long-term exercise habits and provide practical experience for the promotion of aerobic dancing in older adults. Findings will help future studies or programs to further promote physical activity in older adults in China or other countries.
Materials and methods
Study design
Before study enrollment, the older adults were informed about the general aims, the importance, relevance and detailed procedure of the study, and signed a written informed consent form. Participants were randomly assigned to 2 groups: aerobic dancing training or control group. The intervention lasted 12 weeks (exercise lasted 60 min three times a week). Changes in physical fitness and cognitive function were assessed at baseline and after the intervention. All participants were asked not to engage in any additional exercise activities during the 12 weeks intervention period. Unfortunately, eight weeks after the start of the aerobic dancing exercise, the outbreak of the epidemic in Xi'an and home quarantine policy in China led to only two months of in-person training completed. Therefore, the intervention was offered remotely via Tencent conferences as the online training platform in the last month. The study protocol was approved by the Shaanxi Normal University Ethics Committee (approval number: 202016001) and registered at Chinese Clinical Trial Registry (https://www.chictr.org.cn/).
Participants
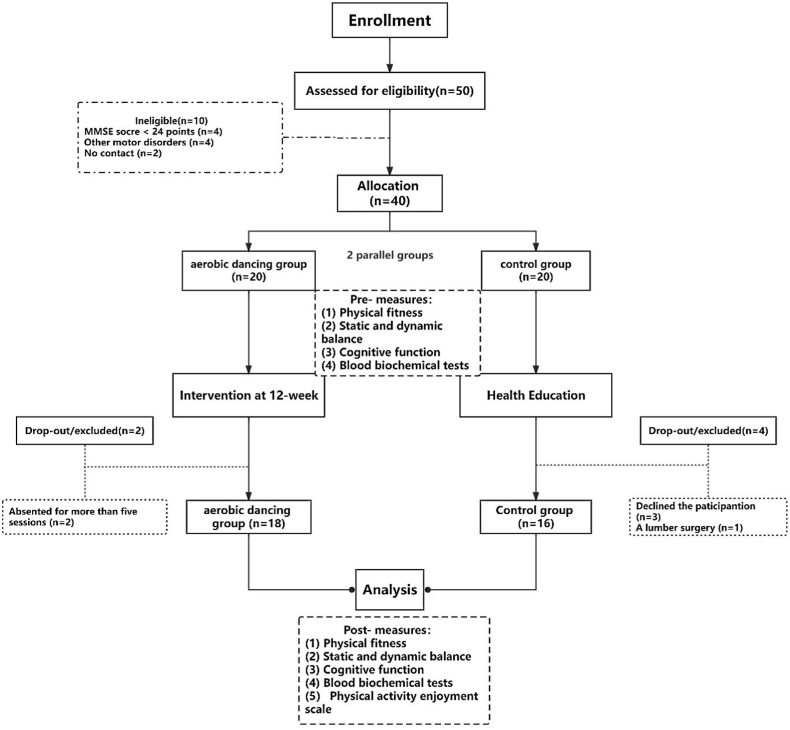
A total of 50 older adults were recruited from the surrounding community in Shaanxi Normal University. Inclusion criteria included: (1) aged between 60 and 80 years old, (2) willing to participate and to provide informed consent, (3) no physical disease, disability and had no contraindications to physical activity participation, (4) cognitive integrity, (5) possessed literacy and simple computer skills. Exclusion criteria for participants included: (1) neurological disorders, (2) history of cancer or psychiatric diseases, a previously implanted pacemaker, (3) The Mini-Mental State Examination (MMSE, which was used to assess the degree of cognitive functioning) score < 24 points. Two female participants in the aerobic group had to discontinue training due to being absent for more than five sessions. Four participants in the control group were dropped from the program due to time conflicts in the post-test (3 persons) and a lumbar surgery (1 person). Eligible participants were randomized to either an intervention group (to perform the Dance training program), or a control group (advised to follow their habitual daily routines). Thus, the final sample included 18 participants in the aerobic dancing group and 16 participants in the control group. Fig. 1 presented a flow diagram of the participants.
Fig. 1.
Flow chart of the study.
Interventions
Aerobic dancing intervention
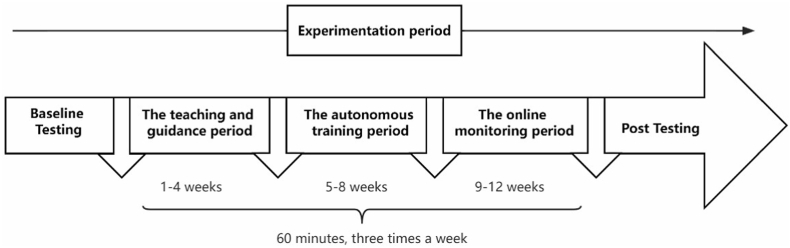
Participants were assigned to the 12-week aerobic dancing intervention at the gymnasium of Shaanxi Normal University for 60 min in three nonconsecutive days each week (Monday, Wednesday, and Friday evenings). Before the aerobic dancing training course, we conducted a pre-experiment in older adults to estimate the intensity of the dance. According to the results of the pre-experiment results, the moderate intensity aerobic dancing was selected. Each training session consisted of 60 min of dancing intervention that began with 10 min of warm-up included light dynamic movements focused on joint flexibility and ankle stability to prevent injury, followed by the main part (40 min) with 3 songs in different styles from Soul Sexy Jazz (SSJ, the prefabricated routine fitness course was led by the current popular jazz and presented with the basic elements of post-modern ballet), and 10 min for dynamic and static stretching after the aerobic dancing. The intensity of the whole dance intervention was moderate, with the heart rate at 108–125 beats per min.24 All participants wore the Fit Mao real-time heart rate monitor (Fit Mao, Shandong delay Cook Health Care Equipment Co. LTD, Dezhou, China) to track the heart rates during the training sessions. The study was completed between October 2021 and January 2022. In view of the current epidemic situation, this experiment was divided into three stages. The first month was the teaching and guidance period, which was led and guided by dance professionals. The second month was the autonomous training period. Subjects practiced freely according to their training conditions and proficiency. The third month was the online monitoring period, we leveraged pre-recorded video (Fig. 2.). Participants could follow along and work out during the video with professionals supervised online through the meeting link.
Fig. 2.
The intervention timeline.
Control condition
In order to ensure the accuracy of the experiment, the control group was instructed to maintain their usual routine as much as possible, and to refrain from any structured exercise or physical activity during the 12 weeks intervention period.
Measurements
Before the beginning of the experimental procedures and at the end of the three months of dancing intervention, all participants completed the following clinical evaluations. Questionnaires were measured by professionals in our team because some older adults had presbyopia issues.
The physical activity readiness questionnaire (PAR-Q)
PAR-Q was used to screen the physical activity readiness of recruited participants.25 Participants answered eleven yes/no questions (e.g. had previously experienced heart disease, asthma, high blood pressure, major surgery, etc.). Face-to-face structured questionnaires were collected at baseline and follow-up (after the intervention). The survey was taken before the other tests and intervention and lasted for 5 min.
Cognitive measurement
Before the intervention, the MMSE was used to assess the inclusion and exclusion criteria and screen participants (cut-off score of 23).26 The whole test lasted for about 20 min. The cognitive test adopted the “Basic cognitive ability test” prepared by Li Deming et al. The test included measures of general cognitive abilities such as memory, thinking and spatial imagery, as well as perceptual speed, cognitive efficiency and working memory. The test was applicable to older adults. The test was required to perform as quickly and correctly as possible. The higher the accuracy, the more scores, and higher scores indicated better cognitive functioning.
Intervention adherence
In measuring physical activity enjoyment, the Physical Activity Enjoyment Scale (PACES) was the most prominent instrument.27 To further explore adherence to the intervention, we used PACES from 0 (very negative) to 5 (very positive) after the intervention to measure the pleasure of the older adults during exercise. The measures took 5 min. The scale consisted of 16 items (9 positive and 7 negative poled items), with the highest score of 80 points and the lowest score of 16 points. Questionnaires with inconsistent answers to Question 5 and 13 would be invalid. The higher the score, the stronger the sense of happiness and pleasure, vice versa.
Physical fitness assessment
Weight and height were measured using an all-in-one machine (Guokang, GK 720, Zaozhuang, China) and an electronic weight scale and ultrasonic height sensor while standing barefoot in the designated position and looking straight ahead. Body mass index (BMI) was calculated using the standard formula (weight in kg divided by height in meters squared).
The body fat percentage was measured by bioelectrical impedance analysis (BiospaceCo. InBody 230, Seoul, South Korea). Waist Circumference was measured at the level of the umbilicus for the waist circumference using a non-elastic tape measure (Butterfly, China).
Grip strength (kilograms, kg) was measured using a digital dynamometer (Takei D T.K.K.5401, Takei Scientific Instruments, Tokyo, Japan).28 Participants were required to stand naturally with the palms of the hands to the inside of the body and both arms hanging naturally, with the dominant hand gripping the apparatus after the force was applied. No secondary force was allowed. During measurement, the grip meter should not touch the body and clothes. Two consecutive measurements were taken and the maximum value was recorded. Participants stood on the soles of their feet with electrodes in their hands.
One-legged standing with eyes closed test: the participants balanced on one leg on bare foot with their eyes closed. If they lost their balance and stood on both feet, the time of single-leg stance was recorded.
Vital capacity was measured by HK6800-FH Electronic Spirometer (Shenzhen Hengkangjiaye Technology Co., Ltd., Shenzhen, China). During the test, they were asked to breathe deeply until they could not breathe in anymore, then exhale slowly into the mouthpiece until the gas was exhaled. Two consecutive measurements were taken and the maximum value was recorded.
Reaction time, sit and reach were performed using anthropometrics testing stations (Taishan Sports Technology Co., LTD., TA106, Shenzhen, China). The participants sat on the mat with both feet in the designated position and legs straight. At the beginning of the test, the participants flexed and extended forward, keeping the leg straight, and slowly pushed the finger forward until it could no longer be pushed. Take two consecutive tests and record the best result. All test indicators were evaluated by two trials and the highest value was used. This machine provided automatic collection and storage of data.
Balance test
The dynamic balance ability experiment instrument used the Biodex dynamic balancing instrument (Biodex System SD with V4.x software, Biodex, America). The participant took off his shoes and stood on the test platform with his arms hanging down on both sides of his torso. The left and right heel coordinates of the participants were (F,8) and (F,14), with toes 30° apart (the line between the left and right heel and the third toe was parallel to the 15°line on the test platform). The stability level of the test platform was selected as level 7 and the test time was set to 20 s, repeated 3 times with rest for 20 s after each test. The dynamic balance test included three tests. The first test was the Limit of Stability (LOS). During each test trial, participants must shift their weight to move the cursor from the center target to a blinking target and back as quickly and with as little deviation as possible. The second test was the Postural Stability Test (PST). Participants focused their eyes at the screen so that the black dot on the screen was as close to the center of the cross axis as possible. The third test was the Motor Control Test (MCT). Participants needed to shift their center of gravity, moved the cursor from the central target to the blinking target and held for 0.25 s. Then they returned to the center cursor as soon as possible. The targets on the screen flashed in random order, repeating the same process for each target.
Cardiovascular risk factors
Blood pressure measurements were taken after the participants had rested in a chair in a quiet environment for 5 min. Measurements were taken using an electronic sphygmomanometer with the cuff wrapped around 80% of the upper arm and the lower end approximately 2 cm from the elbow joint. After measurement, the electronic sphygmomanometer was directly read and records.
Before and after the aerobic dancing intervention, fasting blood samples (following a fast of at least 10 h overnight) were collected from each participant (control group and experimental group, n = 34). The hospital used an AU480 Automatic Biochemical Analyzer (Beckman Coulter Co., Ltd., Brea, CA, USA). to perform venous blood collection from each participant. The levels of total cholesterol, triglyceride, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and insulin were measured. All assays were processed in a single batch at an accredited testing laboratory.
Statistical analyses
The sample size was calculated by the G∗Power 3.1 software (Franz Faul-University of Kiel, Kiel, Germany). In a previous pilot study on the effect of aerobic dancing on physical fitness and cognitive function,29 the effect size d was determined to be 0.4, with α = 0.05, power = 0.8 in Analysis of Covariance (ANCOVA) in G∗Power. Thus, the initial sample size was calculated to be 52. Due to the limited condition in the study and the withdrawal of participants during the intervention, the final sample size was 34. The final power was 0.61 in the ANCOVA statistical test according to calculation in the G∗Power.
Statistical calculation and management of all data were processed by SPSS 23.0. Descriptive statistics for all variables were reported as means ± standard deviations. The normal distribution of baseline characteristics of participants was verified by the Kolmogorov-Smirnov test. For quantitative outcomes, normality and homogeneity of variance have been tested (p > 0.05). Baseline comparisons among groups were performed using independent t-tests or Chi-square tests. Due to the small sample size of this study, there was significant differences in some variables at baseline among older adults. Also, those covariates may have potential confounding effect, which was not statistically significant. Thus, the ANCOVA was performed for the dependent variables to determine whether the interventions produced significant effect, with age, sex, education level and moderate to high activity levels as covariables. Results were considered statistically significant if p < 0.05. Cohen's d was calculated to further compare the differences between groups from baseline to post-test.30 Magnitude of effect sizes were categorized as none (0 ≤ Cohen's d < 0.20), small (0.20 ≤ Cohen's d < 0.50), medium (0.50 ≤ Cohen's d < 0.80), and large (Cohen's d ≥ 0.80).
Results
Recruitment and participants’ characteristics
All participants completed the whole study without injuries or adverse responses to the dancing occurring. Participant characteristics were detailed in Table 1. There was no significant difference between the two groups regarding the measures during the pre-intervention test.
Table 1.
Participants’ characteristics at baseline.
| Aerobic dancing Group n = 18 |
Control Group n = 16 |
t/χ2 | p | |
|---|---|---|---|---|
| M(SD) or n (%) | M(SD) or n (%) | |||
| Age | 65.22(4.32) | 65.75(3.24) | −0.40 | 0.69 |
| Female | 16(88.9) | 10(62.5) | 3.28 | 0.07 |
| Urban household registration | 18(100) | 13(81.3) | 3.70 | 0.05 |
| Degree in | 1.25 | 0.87 | ||
| Primary school | 1(5.6) | 1(6.3) | ||
| Junior high school | 2(11.1) | 2(12.5) | ||
| High school | 6(33.3) | 7(43.8) | ||
| junior college | 5(27.8) | 2(12.5) | ||
| Bachelor or above | 4(22.2) | 4(25.0) | ||
| Smoking | 0(0.0) | 1(6.3) | 1.16 | 0.28 |
| Sport injury | 3.03 | 0.55 | ||
| Without | 7(38.9) | 7(43.8) | ||
| Knee | 5(27.8) | 7(43.8) | ||
| Vertebral | 4(22.2) | 1(6.3) | ||
| Diverse | 1(5.6) | 0(0.0) | ||
| Other | 1(5.6) | 1(6.3) | ||
| Chronic disease | 1.15 | 0.77 | ||
| Without | 9(50.0) | 7(18.8) | ||
| Hypertension/hyperlipidemia/hyperglycemia | 2(11.1) | 4(25.0) | ||
| Other diseases | 7(38.9) | 5(31.3) |
Note: M = mean, SD = Standard Deviation. p for between-group differences.
Physical fitness and cardiovascular risk factors
The descriptive statistics of physical fitness and cardiovascular risk factors were shown in Table 2. Differences in baseline measures were tested between groups. Significant differences were found in grip strength and diastolic blood pressure between the two groups (p < 0.05). No significance was found in other variables.
Table 2.
Comparison of the changes in physical fitness and cardiovascular risk factors between the two groups following the 12-week intervention.
| Aerobic dancing group |
Control group |
F | p | d | Effect size | 1-β | |||
|---|---|---|---|---|---|---|---|---|---|
| Pre M(SD) | Post M(SD) | Pre M(SD) | Post M(SD) | ||||||
| Physical fitness | |||||||||
| Waist circumference (cm) | 85.1(7.3) | 83.4(8.1) | 87.7(9.9) | 86.7(10.2) | 0.02 | 0.89 | −0.11 | – | |
| Body mass index (kg/m2) | 23.1(3.0) | 23.3(2.7) | 23.6(3.7) | 23.9(3.5) | 0.02 | 0.88 | −0.11 | – | |
| Body fat percentage (%) | 28.0(5.3) | 29.2(4.2) | 25.1(6.7) | 26.1(6.8) | 0.06 | 0.81 | 0.04 | – | |
| Vital capacity (ml) | 2 455.9(512.0) | 2 332.6(726.2) | 2 646.6(736.4) | 2 556.7(844.9) | 0.01 | 0.91 | −0.08 | – | |
| Grip strength (kg)# | 24.8(3.8) | 25.0(3.7) | 30.3(9.1) | 28.7(9.0) | 3.38 | 0.08 | 0.89 | L | 0.71 |
| One-legged standing with eyes closed (s) | 4.4(2.0) | 11.3(12.1) | 4.1(3.0) | 6.2(5.2) | 1.26 | 0.27 | 0.50 | M | 0.29 |
| Sit and reach (cm) | 10.6(7.9) | 10.7(7.3) | 5.2(11.0) | 6.9(10.5) | 0.25 | 0.62 | −0.40 | S | |
| cardiovascular risk factors | |||||||||
| Systolic blood pressure (mmHg) | 126.8(16.2) | 129.2(17.3) | 122.3(12.9) | 132.1(8.9) | 3.00 | 0.09 | −0.65 | M | 0.45 |
| Diastolic blood pressure (mmHg)# | 78.3(9.4) | 79.3(6.2) | 70.8(10.4) | 77.3(8.2) | 0.47 | 0.50 | −0.73 | M | 0.54 |
| Total cholesterol (mmol/L) | 5.5(0.9) | 5.5(1.1) | 5.3(1.2) | 5.0(0.9) | 0.53 | 0.47 | 0.31 | S | |
| Triglyceride (mmol/L) | 1.7(1.2) | 1.5(1.0) | 1.4(0.7) | 1.6(0.8) | 0.39 | 0.54 | −0.32 | S | |
| High-density lipoprotein (mmol/L) | 1.9(0.7) | 1.6(0.3) | 1.6(0.4) | 1.5(0.5) | 0.58 | 0.45 | −0.54 | M | 0.33 |
| Low-density lipoprotein (mmol/L) | 3.3(0.9) | 3.1(0.9) | 3.1(0.8) | 2.8(0.5) | 0.55 | 0.46 | 0.23 | S | |
| Insulin (μU/ml) | 7.4(3.5) | 7.3(3.2) | 6.5(2.4) | 5.9(2.5) | 2.08 | 0.16 | 0.33 | S | |
Note: M = mean, SD = Standard Deviation, cm = centimeters, kg = kilograms, m = meters, ml = milliliters, s = seconds, L = liters. p for between-group differences. Effect size: None (−) 0–0.20, Small (S) 0.20–0.50, Medium (M) 0.05–0.80, Large (L) > 0.80.
#:Baseline test values were significantly different between the aerobic dancing group and the control group(p < 0.05).
According to Table 2, grip strength, one-legged standing with eyes closed of the older adults in the aerobic dancing group were improved to some extent after the intervention and their changes were larger than those in the control group, respectively. In addition, grip strength had a large effect size, and one-legged standing with eyes closed had medium effect sizes. Concerning waistline circumference, BMI, body fat mass and vital capacity, there were no significant between the two groups at the post-intervention (p > 0.05).
Compared with the control group, there were no differences in cardiovascular risk factors between the groups. Systolic blood pressure, diastolic blood pressure and HDL had medium effect sizes.
Cognitive function
Table 3 showed the changes in cognitive function. Compared with the control group, the portrait memory of the older adults in the aerobic dancing group was significantly enhanced, where the dance group had a significantly larger mean score change than the control (p < 0.01). Besides, there were no significant improvements between the two groups in any of the other cognitive functions including symbol search, operation span, spatial cognition and similar tests.
Table 3.
Comparison of the changes in cognitive function between the two groups following the 12-week intervention.
| Cognitive function | Aerobic dancing group |
Control group |
F | p | d | Effect size | 1-β | ||
|---|---|---|---|---|---|---|---|---|---|
| Pre |
Post |
Pre |
Post |
||||||
| M(SD) | M(SD) | M(SD) | M(SD) | ||||||
| Symbol search | 31.6(5.9) | 32.3(5.5) | 30.1(7.3) | 30.0(7.2) | 0.78 | 0.38 | 0.17 | – | |
| Operation span | 4.7(2.6) | 4.5(3.1) | 5.1(3.2) | 5.1(3.1) | 0.02 | 0.90 | −0.10 | – | |
| Portrait memory | 20.0(5.2) | 24.8(7.9) | 20.7(9.2) | 18.2(8.8) | 10.45 | 0.003∗∗ | 1.18 | L | 0.91 |
| Spatial cognition | 5.7(3.0) | 4.9(1.8) | 4.9(2.3) | 3.9(2.9) | 0.90 | 0.35 | 0.09 | – | |
| Similar tests | 33.1(14.0) | 32.8(12.3) | 31.4(14.5) | 36.0(11.7) | 1.01 | 0.33 | −0.43 | S | |
Note: M = mean, SD = Standard Deviation.
p for between-group differences, ∗∗p < 0.01.
Effect size: None (−) 0–0.20, Small (S) 0.20–0.50, Medium (M) 0.05–0.80, Large (L) > 0.80.
Balance
The descriptive statistics of balance were shown in Table 4. With respect to the LOS, the right angle (p = 0.022) and right-anterior angle (p = 0.049) in the aerobic dancing group were significantly higher than those in the control group after the program completion. On the other hand, the motion control and stable position, either in the aerobic dancing group or control group, did not show a significant difference between pre- and post-intervention.
Table 4.
Comparison of the changes in Limit of Stability (LOS), Motion control Test (MCT), Postural Stability Test (PST) between the two groups following the 12-week intervention.
| Balance index | Aerobic dancing group |
Control group |
F | p | d | Effect size | 1-β | ||
|---|---|---|---|---|---|---|---|---|---|
| Pre M(SD) | Post M(SD) | Pre M(SD) | Pos M(SD) | ||||||
| LOS | |||||||||
| The average angle | 5.9(0.6) | 6.3(0.6) | 6(0.6) | 6(0.6) | 2.46 | 0.13 | 0.49 | S | |
| Front angle | 6.9(1.2) | 7.5(0.9) | 7(0.8) | 7.1(1.0) | 4.14 | 0.05 | 0.33 | S | |
| Back angle | 3.8(0.6) | 3.7(0.4) | 3.8(0.6) | 3.4(0.9) | 1.32 | 0.26 | 0.42 | S | |
| Left angle | 6.3(0.5) | 6.5(1.0) | 6.5(1.2) | 6.6(0.8) | 1.37 | 0.25 | 0.05 | – | |
| Right angle | 6.3(0.7) | 6.4(1.1) | 6.4(0.6) | 5.9(1.2) | 5.90 | 0.022∗ | 0.60 | M | 0.40 |
| Left-anterior angle | 7.6(1.0) | 8.3(0.8) | 7.7(1.0) | 8(1.0) | 1.65 | 0.21 | 0.21 | S | |
| Right-anterior angle | 7.7(0.7) | 8.0(1.0) | 7.4(0.8) | 7.5(1.2) | 4.23 | 0.049∗ | 0.12 | – | |
| Left-rear angle | 4.7(0.8) | 4.8(0.8) | 4.8(0.9) | 4.9(1.0) | 1.38 | 0.25 | 0.07 | – | |
| Right-rear angle | 4.5(0.8) | 4.9(0.9) | 4.7(0.8) | 4.7(0.8) | 0.31 | 0.58 | 0.28 | S | |
| MCT | |||||||||
| Total completion time (s) | 58.0(8.6) | 141.4(31.7) | 60.4(14.7) | 134.1(18.4) | 1.28 | 0.27 | 0.35 | S | |
| Total efficiency score | 0.4(0.1) | 0.4(0.1) | 0.4(0.1) | 0.4(0.1) | 0.10 | 0.75 | 0.45 | S | |
| PST | |||||||||
| Overall stability index | 0.7(0.2) | 0.8(0.2) | 0.7(0.2) | 0.8(0.2) | 0.24 | 0.63 | −0.06 | – | |
| Overall shaking index | 0.4(0.6) | 0.2(0.2) | 0.2(0.4) | 0.2(0.2) | 0.11 | 0.74 | −0.25 | S | |
Note: M = mean, SD = Standard Deviation, s = seconds, LOS = Limit of stability, MCT = Motor Control Test, PST = Postural Stability Test.
p for between-group differences, ∗p < 0.05.
Effect size: None (−) 0–0.20, Small (S) 0.20–0.50, Medium (M) 0.05–0.80, Large (L) > 0.80.
Intervention adherence
In Table 5, Cronbach's alpha for the PACES in the aerobic dancing group was 0.80. It was indicated that the questionnaire was highly reliable. Similarly, it could be seen from the table that most older adults had a high level of pleasure in the process of aerobic dancing exercise and could get happiness and satisfaction in aerobic dancing intervention.
Table 5.
The Physical Activity Enjoyment Scale (PACES) in aerobic dancing group.
| n | M(SD) | Minimum score | Maximum score | α | |
|---|---|---|---|---|---|
| Aerobic dancing Group | 18 | 75.89(3.61) | 68 | 80 | 0.80 |
Note: M = mean, SD = Standard Deviation. α: Cronbach's alpha (> 0.80 Excellent confidence level, > 0.70 Acceptable confidence level, > 0.65 Need a big rework but acceptable, < 0.65 Given up).
Discussion
The primary aim of the present study was to determine the effect of aerobic dancing training on physical fitness, balance ability and cognitive function in older adults after a 12-week intervention during the COVID-19 pandemic. The primary findings of this study were that the portrait memory and LOS (right angle, right-anterior angle) were significantly improved in the older adults after three months (two months of offline teaching and one month of online practice) of aerobic dancing training. In addition, in terms of physical fitness, the grip strength, the lower-body balance ability (one-legged standing with eyes closed) and flexibility (sit and reach) were also enhanced in the aerobic dancing group after the intervention compared to the pre-intervention period. This study provides an appropriate intensity and type exercise to improve muscle strength, maintain body posture and short-term memory in Chinese older adults during the COVID-19 pandemic. The program was not effective for other variables of physical fitness or cognitive function, and longer period of intervention with further follow-up measures are recommended for future studies in this population.
The effect of aerobic dancing on physical fitness and cardiovascular risk factors
Some older adults who stayed in the aerobic dancing intervention for 12-week showed improvements in grip strength, sit and reach, and one-legged standing with eyes closed. The results were consistent with some previous studies. For example, a study with 24-week creative dance in older adults (65–80 years old) found that older adults can significantly improve their strength, aerobic endurance, flexibility, balance and motor agility through intervention.31 Moreover, a research had shown that 2 months of dance therapy significantly increased the single-leg balance and improved cardiorespiratory fitness in postmenopausal women.32 Kinematic analysis showed older adults have greater hip movement and over-reliance on hip muscles to maintain upright posture.33 Therefore, the longer one-legged standing with eyes closed in the aerobic dancing group may partly be attributed to the improved ankle muscle strength caused by aerobic dancing training. In a recent study, 88 healthy older adults were randomly assigned to dance group, walking group or control group, and the results showed muscle quality improved the most in the dance group.22 In addition, the leg flexibility significantly increased in the sedentary individuals with a 16-week aerobic dancing intervention than the same counterparts without the intervention of aerobic dancing.34 For the body fat mass and BMI, there were no significant improvements in the aerobic dancing group after the intervention. This is different from a study who reported a significant reduce in reduced body fat and abdominal thickness after dance intervention.15 It was possible that the intervention did not involve strict dietary control, resulting in an increase in body fat percentage in some participants.
No improvements have been observed in blood biochemical testing results after the intervention, which was consistent with a study displaying no significant difference in blood profiles after the intervention of the aerobic dancing.35 However, numerous studies had shown that adherence to the aerobic exercise program was key for long-term maintenance of cardiovascular health.36 Physical activity have been shown to improve blood pressure in people with and without hypertension.36,37 Strong evidence demonstrated physical activity reduced the risk of cardiovascular disease progression.38 A result suggested that regular aerobic training was beneficial for blood pressure control and cardiovascular disease risk reduction.39 A reduction in total cholesterol was considered the gold standard in preventative cardiovascular medicine. A Report of the American Heart Association Task Force on Clinical Practice Guidelines indicated that it was recommended to continue regular moderate-intensity physical activity, in patients with increased diabetes mellitus risk.40 A study suggested that aerobic dancing training had favorable effects on LDL, triglyceride, and systolic and diastolic blood pressure in individuals with hypertension on a thiazide.41 More future studies are need to better understand the effect of aerobic dancing on those cardiovascular risk factors.
In this study, although grip strength, one-legged standing with eyes closed and blood pressure were not significantly improved after intervention, their effect size levels were Large and Medium. Moreover, we have computed the achieved power and found t the 1-β were less than 0.8, indicating that the sample size of this study was small, and this may limit our ability to detect improvements in physical fitness and cardiovascular risk factors associated with aerobic dancing. We tried our best to recruit more participants, but due to the impact of the epidemic, the number of recruited participants were still limited.
The effect of aerobic dancing on cognitive function
The research indicated a decline in cognitive function with aging could be attenuated by the aerobic dancing intervention.42,43 With respect to cognitive function, the portrait memory in the dance program improved significantly, compared to non-dance intervention group. Furthermore, aerobic dancing intervention also improved symbol search of the older adults to a certain extent. This positive result was similar to the studies which have shown aerobic dancing can significantly improve processing speed and episodic memory.44 These results suggested that motor imagery stimulated the activation of motion-related basal ganglia loops and frontal lobe regions, affecting short-term memory and the selection and execution of actions.45 Learning aerobic dancing requires repetition, concentration and cognitive processing, especially memory. This may account for the improved portrait memory and symbol search ability in the aerobic dancing group compared with the control group. In another study comparing aerobic dancing and Nordic walking, the aerobic dancing group showed significant improvement in visuospatial skills, which was inconsistent with our results.22 This may be due to the lack of spatial cognitive training in our aerobic dancing training.
The reasons for the change of cognitive function have been discussed in previous studies. Lin Tw et al. demonstrated that exercise can improve the expression of synaptic plasticity genes, as well as the benign effects on hippocampal volume and density and complexity of dendritic spines, and promote the regeneration of hippocampal dentate gyrus neurons.46 A systematic review found that aerobic exercise, especially moderate to high-intensity aerobic exercise, was more effective to slow down the cognitive decline.47 Aerobic dancing training can elevate social engagement and interaction, which benefitted cognitive function in the older adults.12,48,49 A recent study showed that a contextual preventive effect of community-level sports group participation on developing cognitive impairment among older individuals.10,13,49 It was important to note that aerobic dancing, as a concurrent dual-task training, improved dual-task performance in older adults with variability in gait and cognitive tasks. This training was memorized, planned, and cognitively processed while performing aerobic exercise.22 In summary, aerobic dancing may improve certain aspects of cognition, and that could even reduce the likelihood of developing cognitive impairments.
On the contrary, some previous studies have reported no improvement in cognitive function with aerobic dancing interventions.29 Possible reasons for the difference may be that the music speed and movement rhythm in our study are faster than some easier aerobic dance. Angevaren et al. investigated the relationship between intensity of exercise and cognition. The results suggest that cognitive function is associated with exercise intensity, and can significantly improve brain processing speed, memory and mental agility.50 Another reason may be that, in the context of the epidemic, the old adults in our study have reduced daily physical activity at baseline, which may have a lot of room for progress.
The effect of aerobic dancing on balance ability
We also observed a partial significant effect on the factors affecting the balance changes induced by the aerobic dancing intervention, as indicated by less LOS in the right and right-anterior directions compared with the control group. The reason may be that the aerobic dancing training performed in this study all started movements from the right direction. Filar-Mierzwa et al. compared aerobic dancing and general exercises, they confirmed that dance significantly improved the participants' LOS.51 Older adults’ who participated in aerobic dancing had a significant improvement in dynamic balance increasing the amplitude of upper trunk oscillations during periodic sway after the intervention.52 With the increase of age, the strength of ankle joint muscle decreases, leading to the decrease of LOS. Aerobic dancing focused on ankle movement and shifting the center of gravity between the feet to constantly rotate the torso and maintain balance. Therefore, the increase in the limits of stability may be attributed to increased range of ankle motion and ankle muscle strength.53
Although many previous studies had reported improvements in MCT and PST after aerobic dancing intervention,16,54 our results did not show such changes occurred after dance training. There was some research to support that lower limb muscle strength was strongly correlated with balance.55 It was the basis of balance ability, and it would directly affect the related indicators of balance. To some extent, better balance means better muscle strength.56 Aerobic dancing intervention studies had demonstrated that dance could actively control body posture and improve core stability in some degree.57 We suspected that SSJ training may not improve core stability in the older adults, and thus PST indicators did not improve and remain unchanged. Studies have shown that balance ability was closely related to visuospatial perception.58, 59, 60 Spatial cognition is impaired in some older adults, which may affect balance.61 The older adults who often participate in aerobic dancing exercise can enhance the stimulation of visual and vestibular organs and improve the dynamic balance ability of the human body.62 A number of studies suggested that aerobic dancing exercise is an ideal form of exercise for improving dynamic balance in older adults.52,63 However, no improvement effect was observed in our study, which may be due to the lack of enough intervention time and non-standard movements of the older adults during the third stage of home-based exercise. More efforts are necessary to find out the influence of aerobic dancing on dynamic balance in older adults.
Strengths and limitations
There were several strengths to this study. First, aerobic dancing, as an art form, helps the older adults to enjoy life and work out at the same time. It is not only an appropriate aerobic exercise for improving physical fitness and cognitive function, but also increases physical activity and stimulates the older adults’ exercise interest, which are principal considerations for participants.64 Second, as far as we are aware, this is one of the first studies to provide a comprehensive synthesis of the effect of aerobic dancing intervention on physical fitness and cognitive function for the Chinese older adults during the COVID-19 pandemic. Third, this experiment was completed under strict scientific supervision to ensure the quality and safety of the intervention. These results are crucial for researchers and medical care personnel to provide insight that may have potential implications to physical and cognitive health of older adults.
The study had several limitations. First, the number of participants was small and had an attrition rate due to difficulties in recruiting during the COVID-19 pandemic. Second, during the intervention, we did not control the participants' diet properly, which may also have contributed to the lack of improvement in blood biochemical testing and body fat percentage. Third, more women than men were recruited in this study. In the future, it is indispensable to conducted longer and more intensive interventions and add regular protein supplementation to produce stronger experimental effects. Finally, due to the limited conditions, we did not add another exercise group as an active control group, to observe whether aerobic dancing had a significant effect beyond other regular exercise on the physical fitness and cognitive function in older adults.
Conclusions
Aerobic dancing exercise has significant positive effect on some aspects of physical fitness and cognitive function in older adults during the COVID-19 pandemic. The portrait memory and LOS were significantly improved in the older adults after three months of aerobic dancing training. In addition, the grip strength, the lower-body balance ability and flexibility were also enhanced in the aerobic dancing group after the intervention compared to the pre-intervention period. This study provides a simple and feasible exercise program to improve physical fitness and cognitive function for the older adults during COVID-19 pandemic. It may serve as a tool to create physical activity that improves physical fitness and cognitive functioning to some extent. For more significant effect of aerobic dancing on physical fitness and cognitive function, longer period of intervention with further follow-up measures are recommended for future studies in this population.
Submission statement
We confirm that this article has not been published before and is not under consideration for publication elsewhere. All authors have seen the manuscript and approved to submit to your journal.
Ethical approval statement
Informed consent was obtained from each participate, and the study was approved by the Shaanxi Normal University Ethics Committee (approval number: 202016001) and registered at Chinese Clinical Trial Registry (https://www.chictr.org.cn/).
Authors’ contributions
LW: conceptualization, writing original draft preparation and writing—review and editing. CZ, LZ: methodology. MZ, CZ and LZ: investigation. JG: writing-review and editing. ZL and FG: project administration. WZ: conceptualization, supervision and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the MOE (Ministry of Education in China) Project of Humanities and Social Sciences (20YJC890053), and Shaanxi Province Social Science Foundation Program (2020Q009).
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all the funds for their support and the valuable contributions of all investigators and participants.
References
- 1.The Seventh Census. 2022. http://www.stats.gov.cn/ztjc/zdtjgz/zgrkpc/dqcrkpc/ggl/202105/t20210519_1817701.html [Google Scholar]
- 2.Zhong X., Yan X., Liang H., Xia R., Chen B., Zhao H.J. Evaluation of eight-style Tai chi on cognitive function in patients with cognitive impairment of cerebral small vessel disease: study protocol for a randomised controlled trial. BMJ Open. 2021;11(2) doi: 10.1136/bmjopen-2020-042177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donath L., Roth R., Hohn Y., Zahner L., Faude O. The effects of Zumba training on cardiovascular and neuromuscular function in female college students. Eur J Sport Sci. 2014;14(6):569–577. doi: 10.1080/17461391.2013.866168. [DOI] [PubMed] [Google Scholar]
- 4.Lutz W., Sanderson W., Scherbov S. The coming acceleration of global population ageing. Nature. 2008;451(7179):716–719. doi: 10.1038/nature06516. [DOI] [PubMed] [Google Scholar]
- 5.Jowell A., Carstensen L.L., Barry M. A life-course model for healthier ageing: lessons learned during the COVID-19 pandemic. The Lancet. Healthy Longevity. 2020;1(1):e9–e10. doi: 10.1016/S2666-7568(20)30008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghram A., Briki W., Mansoor H., Al-Mohannadi A.S., Lavie C.J., Chamari K. Home-based exercise can be beneficial for counteracting sedentary behavior and physical inactivity during the COVID-19 pandemic in older adults. Postgrad Med. 2021;133(5):469–480. doi: 10.1080/00325481.2020.1860394. [DOI] [PubMed] [Google Scholar]
- 7.Chaabene H., Prieske O., Herz M., et al. Home-based exercise programmes improve physical fitness of healthy older adults: a PRISMA-compliant systematic review and meta-analysis with relevance for COVID-19. Ageing Res Rev. 2021;67 doi: 10.1016/j.arr.2021.101265. [DOI] [PubMed] [Google Scholar]
- 8.Ceban F., Ling S., Lui L.M.W., et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langhammer B., Bergland A., Rydwik E. The Importance of physical activity exercise among older people. BioMed Res Int. 2018;2018 doi: 10.1155/2018/7856823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawes P., Cruickshanks K.J., Fischer M.E., Klein B.E.K., Klein R., Nondahl D.M. Hearing aid use and long-term health outcomes: hearing handicap, mental health, social engagement, cognitive function, physical health and mortality. Int J Audiol. 2015;54(11):838–844. doi: 10.3109/14992027.2015.1059503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan J.S.Y., Wu J., Deng K., Yan J.H. The effectiveness of dance interventions on cognition in patients with mild cognitive impairment: a meta-analysis of randomized controlled trials. Neurosci Biobehav Rev. 2020;118:80–88. doi: 10.1016/j.neubiorev.2020.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Brustio P.R., Liubicich M.E., Chiabrero M., Rabaglietti E. Dancing in the golden age: a study on physical function, quality of life, and social engagement. Geriatr Nurs. 2018;39(6):635–639. doi: 10.1016/j.gerinurse.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Penninkilampi R., Casey A.N., Singh M.F., Brodaty H. The association between social engagement, loneliness, and risk of dementia: a systematic review and meta-analysis. JAD. 2018;66(4):1619–1633. doi: 10.3233/JAD-180439. [DOI] [PubMed] [Google Scholar]
- 14.Woods J.A., Hutchinson N.T., Powers S.K., et al. The COVID-19 pandemic and physical activity. Sports Med Health Sci. 2020;2(2):55–64. doi: 10.1016/j.smhs.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barone Gibbs B., Hivert M.F., Jerome G.J., et al. Physical activity as a critical component of first-line treatment for elevated blood pressure or cholesterol: who, what, and how?: a scientific statement from the American heart association. Hypertension. 2021;78(2) doi: 10.1161/HYP.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 16.Su Z., Zhao J. Comparative study of the effects of Tai Chi and Square Dance on immune function, physical health, and life satisfaction in urban empty-nest older adults. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.721758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Y., Zhong Q., Ji J., et al. Effects of aerobic dance on cognition in older adults with mild cognitive impairment: a systematic review and meta-analysis. J Alzheimers Dis. 2020;74(2):679–690. doi: 10.3233/JAD-190681. [DOI] [PubMed] [Google Scholar]
- 18.Murillo-Garcia A., Villafaina S., Collado-Mateo D., Leon-Llamas J.L., Gusi N. Effect of dance therapies on motor-cognitive dual-task performance in middle-aged and older adults: a systematic review and meta-analysis. Disabil Rehabil. 2021;43(22):3147–3158. doi: 10.1080/09638288.2020.1735537. [DOI] [PubMed] [Google Scholar]
- 19.Kosmat H., Vranic A. The efficacy of a dance intervention as cognitive training for the old-old. J Aging Phys Activ. 2017;25(1):32–40. doi: 10.1123/japa.2015-0264. [DOI] [PubMed] [Google Scholar]
- 20.Kis O., Buch A., Stern N., Moran D.S. Minimally supervised home-based resistance training and muscle function in older adults: a meta-analysis. Arch Gerontol Geriatr. 2019;84 doi: 10.1016/j.archger.2019.103909. [DOI] [PubMed] [Google Scholar]
- 21.Kattenstroth J.C., Kolankowska I., Kalisch T., Dinse H.R. Superior sensory, motor, and cognitive performance in elderly individuals with multi-year dancing activities. Front Aging Neurosci. 2010;2:31. doi: 10.3389/fnagi.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyazaki A., Okuyama T., Mori H., Sato K., Kumamoto K., Hiyama A. Effects of two short-term aerobic exercises on cognitive function in healthy older adults during COVID-19 confinement in Japan: a pilot randomized controlled trial. Int J Environ Res Publ Health. 2022;19(10):6202. doi: 10.3390/ijerph19106202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster P.P. How does dancing promote brain reconditioning in the elderly? Front Aging Neurosci. 2013;5:4. doi: 10.3389/fnagi.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livesey G., Taylor R., Livesey H.F., et al. Dietary glycemic index and load and the risk of type 2 diabetes: a systematic review and updated meta-analyses of prospective cohort studies. Nutrients. 2019;11(6):1280. doi: 10.3390/nu11061280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas S., Reading J., Shephard R.J. Revision of the physical activity readiness questionnaire (PAR-Q) Can J Sport Sci. 1992;17(4):338–345. [PubMed] [Google Scholar]
- 26.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Chen C., Weyland S., Fritsch J., et al. A short version of the Physical Activity Enjoyment Scale: development and psychometric properties. IJERPH. 2021;18(21) doi: 10.3390/ijerph182111035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts H.C., Denison H.J., Martin H.J., et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 29.Merom D., Grunseit A., Eramudugolla R., Jefferis B., Mcneill J., Anstey K.J. Cognitive benefits of social dancing and walking in old age: the dancing mind randomized controlled trial. Front Aging Neurosci. 2016;8:26. doi: 10.3389/fnagi.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen J. second ed. Routledge; 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 31.Cruz-Ferreira A., Marmeleira J., Formigo A., Gomes D., Fernandes J. Creative dance improves physical fitness and life satisfaction in older women. Res Aging. 2015;37(8):837–855. doi: 10.1177/0164027514568103. [DOI] [PubMed] [Google Scholar]
- 32.Serrano-Guzmán M., Aguilar-Ferrándiz M.E., Valenza C.M., Ocaña-Peinado F.M., Valenza-Demet G., Villaverde-Gutiérrez C. Effectiveness of a flamenco and sevillanas program to enhance mobility, balance, physical activity, blood pressure, body mass, and quality of life in postmenopausal women living in the community in Spain: a randomized clinical trial. Menopause. 2016;23(9):965–973. doi: 10.1097/GME.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 33.Nagai K., Okita Y., Ogaya S., Tsuboyama T. Effect of higher muscle coactivation on standing postural response to perturbation in older adults. Aging Clin Exp Res. 2017;29(2):231–237. doi: 10.1007/s40520-016-0554-1. [DOI] [PubMed] [Google Scholar]
- 34.Barranco-Ruiz Y., Paz-Viteri S., Villa-González E. Dance fitness classes improve the health-related quality of life in sedentary women. Int J Environ Res Publ Health. 2020;17(11):3771. doi: 10.3390/ijerph17113771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mann S., Beedie C., Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. 2014;44(2):211–221. doi: 10.1007/s40279-013-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Raimondo D., Buscemi S., Musiari G., et al. Ketogenic diet, physical activity, and hypertension—a narrative review. Nutrients. 2021;13(8):2567. doi: 10.3390/nu13082567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopes D.P.S., Ribeiro I.S., Santos D.C., et al. Regular physical activity reduces the proinflammatory response in older women with diabetes and hypertension in the postmenopausal phase. Exp Gerontol. 2021;152 doi: 10.1016/j.exger.2021.111449. [DOI] [PubMed] [Google Scholar]
- 38.Pescatello L.S., Buchner D.M., Jakicic J.M., et al. Physical activity to prevent and treat hypertension: a systematic review. Med Sci Sports Exerc. 2019;51(6):1314–1323. doi: 10.1249/MSS.0000000000001943. [DOI] [PubMed] [Google Scholar]
- 39.Boeno F.P., Ramis T.R., Munhoz S.V., et al. Effect of aerobic and resistance exercise training on inflammation, endothelial function and ambulatory blood pressure in middle-aged hypertensive patients. J Hypertens. 2020;38(12):2501–2509. doi: 10.1097/HJH.0000000000002581. [DOI] [PubMed] [Google Scholar]
- 40.Grundy S.M., Stone N.J., Bailey A.L., et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2018;139(25):e1046–e1081. doi: 10.1161/CIR.0000000000000624. 2019. [DOI] [PubMed] [Google Scholar]
- 41.Maruf F.A., Akinpelu A.O., Salako B.L. A randomized controlled trial of the effects of aerobic dance training on blood lipids among individuals with hypertension on a thiazide. High Blood Pres Cardiovasc Prev. 2014;21(4):275–283. doi: 10.1007/s40292-014-0063-2. [DOI] [PubMed] [Google Scholar]
- 42.Flodin P., Jonasson L.S., Riklund K., Nyberg L., Boraxbekk C.J. Does aerobic exercise influence intrinsic brain activity? an aerobic exercise intervention among healthy old adults. Front Aging Neurosci. 2017;9:267. doi: 10.3389/fnagi.2017.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jonasson L.S., Nyberg L., Kramer A.F., Lundquist A., Riklund K., Boraxbekk C.J. Aerobic exercise intervention, cognitive performance, and brain structure: results from the physical influences on brain in aging (PHIBRA) study. Front Aging Neurosci. 2017;8:336. doi: 10.3389/fnagi.2016.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Y., Wu H., Qi M., et al. Effects of a specially designed aerobic dance routine on mild cognitive impairment. Clin Interv Aging. 2018;13:1691–1700. doi: 10.2147/CIA.S163067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng X., Li G., Jia Y., et al. Effects of dance intervention on global cognition, executive function and memory of older adults: a meta-analysis and systematic review. Aging Clin Exp Res. 2020;32(1):7–19. doi: 10.1007/s40520-019-01159-w. [DOI] [PubMed] [Google Scholar]
- 46.Lin T.W., Chen S.J., Huang T.Y., et al. Different types of exercise induce differential effects on neuronal adaptations and memory performance. Neurobiol Learn Mem. 2012;97(1):140–147. doi: 10.1016/j.nlm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Law C.K., Lam F.M., Chung R.C., Pang M.Y. Physical exercise attenuates cognitive decline and reduces behavioural problems in people with mild cognitive impairment and dementia: a systematic review. J Physiother. 2020;66(1):9–18. doi: 10.1016/j.jphys.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 48.Taylor-Piliae R.E., Newell K.A., Cherin R., Lee M.J., King A.C., Haskell W.L. Effects of Tai Chi and western exercise on physical and cognitive functioning in healthy community-dwelling older adults. J Aging Phys Activ. 2010;18(3):261–279. doi: 10.1123/japa.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsuji T., Kanamori S., Miyaguni Y., Hanazato M., Kondo K. Community-level sports group participation and the risk of cognitive impairment. Med Sci Sports Exerc. 2019;51(11):2217–2223. doi: 10.1249/MSS.0000000000002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Angevaren M., Vanhees L., Wendel-Vos W., et al. Intensity, but not duration, of physical activities is related to cognitive function. Eur J Cardiovasc Prev Rehabil. 2007;14(6):825–830. doi: 10.1097/HJR.0b013e3282ef995b. [DOI] [PubMed] [Google Scholar]
- 51.Filar-Mierzwa K., Długosz-Boś M., Marchewka A., Aleksander-Szymanowicz P. Effect of different forms of physical activity on balance in older women. J Women Aging. 2021;33(5):487–502. doi: 10.1080/08952841.2020.1718579. [DOI] [PubMed] [Google Scholar]
- 52.Sofianidis G., Hatzitaki V., Douka S., Grouios G. Effect of a 10-week traditional dance program on static and dynamic balance control in elderly adults. J Aging Phys Activ. 2009;17(2):167–180. doi: 10.1123/japa.17.2.167. [DOI] [PubMed] [Google Scholar]
- 53.Filar-Mierzwa K., Długosz M., Marchewka A., Dąbrowski Z., Poznańska A. The effect of dance therapy on the balance of women over 60 years of age: the influence of dance therapy for the elderly. J Women Aging. 2017;29(4):348–355. doi: 10.1080/08952841.2016.1194689. [DOI] [PubMed] [Google Scholar]
- 54.Lu T., Song Q.H., Xu R.M., et al. Dance combined with magnetic pulse stimulates the ability of walk and balance in elder people. Int J Clin Exp Med. 2015;8(3):4381–4386. [PMC free article] [PubMed] [Google Scholar]
- 55.Song Q.H., Zhang Q.H., Xu R.M., et al. Effect of Tai-chi exercise on lower limb muscle strength, bone mineral density and balance function of elderly women. Int J Clin Exp Med. 2014;7(6):1569–1576. [PMC free article] [PubMed] [Google Scholar]
- 56.Liu K., Wang H., Xiao J., Taha Z. Analysis of human standing balance by largest lyapunov exponent. Comput Intell Neurosci. 2015;2015 doi: 10.1155/2015/158478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hofgaard J., Ermidis G., Mohr M. Effects of a 6-week Faroese chain dance programme on postural balance, physical function, and health profile in elderly subjects: a pilot study. BioMed Res Int. 2019;2019 doi: 10.1155/2019/5392970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lally H., Hart A.R., Bay A.A., Kim C., Wolf S.L., Hackney M.E. Association between motor subtype and visuospatial and executive function in mild-moderate Parkinson disease. Arch Phys Med Rehabil. 2020;101(9):1580–1589. doi: 10.1016/j.apmr.2020.05.018. [DOI] [PubMed] [Google Scholar]
- 59.Oki M., Matsumoto M., Yoshikawa Y., et al. Risk factors for falls in patients with Alzheimer disease: a retrospective study of balance, cognition, and visuospatial ability. Dement Geriatr Cogn Dis Extra. 2021;11(1):58–63. doi: 10.1159/000514285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu S., Sui Y., Shen Y., et al. Effects of virtual reality intervention on cognition and motor function in older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. Front Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.586999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oki M., Matsumoto M., Yoshikawa Y., et al. Risk factors for falls in patients with Alzheimer disease: a retrospective study of balance, cognition, and visuospatial ability. Dement Geriatr Cogn Dis Extra. 2021;11(1):58–63. doi: 10.1159/000514285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu Z., Zhou L., Gong W., et al. The Effect of 6-week combined balance and plyometric training on dynamic balance and quickness performance of elite badminton players. Int J Environ Res Publ Health. 2022;19(3):1605. doi: 10.3390/ijerph19031605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Granacher U., Muehlbauer T., Bridenbaugh S.A., et al. Effects of a salsa dance training on balance and strength performance in older adults. Gerontology. 2012;58(4):305–312. doi: 10.1159/000334814. [DOI] [PubMed] [Google Scholar]
- 64.Guzman J., Aguiñaga S., Balbim G.M., Lamar M., Marques I.G., Marquez D.X. The effects of the BAILAMOS dance program on hippocampal volume in older Latinos: a randomized controlled pilot study. Transl Behav Med. 2021;11(10):1857–1862. doi: 10.1093/tbm/ibab009. [DOI] [PMC free article] [PubMed] [Google Scholar]