Abstract
Childhood trauma may confer risk for poorer adult health through changes in systemic inflammation. Emotion regulation may plausibly moderate associations between childhood trauma and adult psychological well-being, but it remains unclear whether moderation effects extend to differences in systemic inflammation. To examine whether childhood trauma and emotion regulation separately and interactively predict prospective changes in C-reactive protein (CRP) and interleukin-6 (IL-6) and whether biopsychosocial factors account for observed associations. Healthy midlife adults (N = 331) retrospectively reported on childhood trauma, current trait-level cognitive reappraisal and expressive suppression, and had their blood drawn. At baseline and then a median of 2.85 years later, 279 of the 331 participants had their blood drawn, body mass index calculated, and reported on health behaviours (smoking, sleep), psychological distress (perceived stress, depressive symptoms), and years of education. Childhood trauma predicted prospective increases in CRP (B= 0.004, p= 0.049), which were partially accounted for by differences in adiposity, psychological distress, and health behaviours. In contrast, cognitive reappraisal predicted prospective decreases in IL-6 (B = −0.007, p = 0.006), which were independent of biopsychosocial influences. Cognitive reappraisal further moderated the association between childhood trauma and prospective changes in IL-6 (B = −0.001, p = 0.012) such that childhood trauma predicted greater IL-6 increases but only among adults lower in cognitive reappraisal (B = 0.006, p = 0.007). There were no main or moderation effects of expressive suppression (ps > 0.05). Cognitive reappraisal may attenuate IL-6 changes over time and may moderate the prospective association between childhood trauma and systemic inflammation in midlife.
Keywords: childhood trauma, cognitive reappraisal, expressive suppression, systemic inflammation
In the United States and other high-income countries, one in 10 youth experience physical, sexual, or psychological abuse or neglect each year, which can have lasting effects on psychological and physiological well-being into adulthood (Gilbert et al., 2009). Adults who experience childhood trauma are at heightened risk for a host of health conditions, including depression (Humphreys et al., 2020), cancer (Holman et al., 2016), cardiovascular diseases and type II diabetes (Basu et al., 2017). Childhood trauma may confer risk for poorer health through various behavioural, psychological, and physiological pathways, including increased levels of peripheral, low-grade systemic inflammation (Kerr et al., 2021).
Nonetheless, individual differences in psychosocial risk and protective characteristics may influence the extent to which childhood trauma contributes to poorer health and changes in systemic inflammation (Fritz et al., 2018). Emotion regulation refers to a set of strategies that individuals use to modulate their emotions (Gross et al., 2006). Although emotion regulation may moderate psychological well-being outcomes among childhood trauma survivors (England-Mason et al., 2017), what is still not clear is whether the influence of emotion regulation strategies on psychological well-being among adults exposed to childhood trauma extends to indicators of their physical health, such as systemic inflammation. This study will therefore examine whether emotion regulation strategies moderate the extent to which retrospective reports of childhood trauma are associated with prospective, multi-year changes in markers of systemic inflammation among a sample of midlife adults.
Cognitive reappraisal and expressive suppression are two emotion regulation strategies that have received attention for their physical health relevance. Cognitive reappraisal involves reframing one’s interpretation of an event to change an emotional experience, such as viewing a setback as a learning opportunity. In contrast, expressive suppression involves inhibiting the outward expression of an emotion despite feeling the emotion internally (e.g., maintaining a neutral expression despite feeling anger or frustration when receiving negative feedback).
Although each of these emotion regulation strategies can be socially adaptive depending on the context, trait-level cognitive reappraisal is generally considered health-protective, whereas trait-level expressive suppression is generally viewed as detrimental for health. For example, among midlife adults, Appleton et al. (2013) found that greater use of cognitive reappraisal was cross-sectionally associated with lower levels of peripheral C-reactive protein (CRP) and that greater expressive suppression was associated with higher levels of CRP. Extending these findings, Ellis et al. (2019) found that crosssectional associations between cognitive reappraisal, expressive suppression, and systemic inflammation were partly accounted for by differences in concurrent perceived stress and sleep quality. Although these studies add to our understanding of how emotion regulation strategies associate with concurrent levels of systemic inflammation, it is unknown whether these emotion regulation strategies relate to changes in immune parameters and interact with childhood trauma to contribute to changes in systemic inflammation over time.
Indeed, little attention has been given to whether emotion regulation strategies in adulthood moderate the link between childhood trauma and prospective changes in systemic inflammation. Yet, there is reason to believe that cognitive reappraisal and expressive suppression would act as moderators. Specifically, trait-level emotion regulation strategies are often cultivated in childhood and adolescence such that youth who are facing abuse and neglect may gravitate towards emotion regulation strategies that are adaptive within their home environment (Wadsworth, 2015). These strategies may include engaging in cognitive reappraisal to reduce psychological distress (Boyes et al., 2016) or using expressive suppression to preserve the parent-child relationship (Gross & Cassidy, 2019). The habitual use of these emotion regulation strategies over time could in turn exacerbate or mitigate the effects of childhood trauma on changes in systemic inflammation. For example, Jones et al. (2018) found that under conditions of chronic family stress, youth higher in cognitive reappraisal had indicators of better cardiometabolic health (e.g., lower resting blood pressure) compared to their peers who were lower in reappraisal. In contrast, youth who were higher in expressive suppression evidenced decreased sensitivity of immune cells to the anti-inflammatory effects of glucocorticoids under conditions of chronic family stress.
It is unclear whether the moderation effects of emotion regulation that Jones et al. (2018) observed would extend into adulthood, years after childhood trauma has occurred. Some support for this possibility comes from research examining emotion regulation strategies among bereaved older adults. Although the authors did not examine moderation effects specifically, Lopez et al. (2020) found an independent effect of expressive suppression on a measure of inflammatory response to challenge (i.e., greater stimulated pro-inflammatory cytokine production) among a sample of bereaved adults, and this effect held after adjusting for biopsychosocial factors including health behaviours and depressive symptoms. Despite being very different experiences, both the loss of a spouse and childhood trauma are types of psychosocial stressors that have been associated with greater systemic inflammation (e.g., Kerr et al., 2021; Knowleset al., 2019), and the strength of this association may vary depending on whether a person is higher or lower in cognitive reappraisal or expressive suppression.
The present study aims to (1) examine whether childhood trauma, cognitive reappraisal, and expressive suppression are associated with prospective, multi-year changes in peripheral levels of CRP and interleukin-6 (IL-6), two markers of systemic inflammation, and (2) whether cognitive reappraisal and expressive suppression moderate associations between retrospective reports of childhood trauma and prospective changes in systemic inflammation in adulthood. Because childhood trauma and emotion regulation strategies may influence systemic inflammation through various psychosocial and physiological pathways, we further consider whether any significant findings are accounted for by individual differences in adiposity (body mass index [BMI]), health behaviours (self-reported sleep, smoking status), psychological distress (depressive symptoms, perceived stress) and socioeconomic position in adulthood (years of education) as these could point to potential areas of intervention.
In ancillary analyses, we will test for sex differences given that men and women may differ in their use of emotion regulation strategies (McRae et al., 2008) and in their exposure to childhood trauma (Tolin & Foa, 2006). Because prior research suggests that associations between childhood trauma and systemic inflammation may be stronger, or only observed among, women (Ehrlich et al., 2021; Osborn & Widom, 2020), we hypothesize that childhood trauma may be associated with greater prospective increases among women compared to men in the sample. We further consider whether associations between childhood trauma, emotion regulation strategies, and systemic inflammation differ depending on whether childhood trauma is characterized by threat or deprivation (McLaughlin & Sheridan, 2016). This analysis is exploratory and we do not have a directional hypothesis given that much of the research on the differential effects of neglect and abuse on health outcomes have focussed on emotional well-being or brain processes and not changes in systemic inflammation over time.
1 |. METHODS
1.1 |. Participants
A community sample of 331 midlife adults (ages 30–51 years, 167 self-reported as female, 69.5% identifying as white, non-Hispanic) were recruited from Allegheny county, Pennsylvania, to participate in the Pittsburgh Imaging Project. Participants completed a series of baseline visits and a follow-up visit approximately 3 years later (median = 2.85 years, interquartile range = 3.47 years). To be included, adults needed to be free of common chronic physical health conditions (e.g., clinical cardiovascular diseases, cancer, diabetes, pulmonary and respiratory diseases) and not currently diagnosed with a substance or mood disorder at baseline. Adults were also ineligible if they were regularly taking lipid-lowering, weight loss, insulin, cardiovascular, hypoglycemic, glucocorticoid, or psychotropic medications or were pregnant at baseline. Additional eligibility criteria for the study that are not specific to the analyses presented here were as follows: being colourblind, claustrophobic, having a neurological condition, history of cerebrovascular trauma, and having ferromagnetic implants of any kind. All participants provided informed consent and the University of Pittsburgh approved the study. See Table 1 for sample characteristics. For additional details on recruitment methods, study design, and sample characteristics, please see Gianaros et al. (2017, 2022).
TABLE 1.
Sample descriptives
| n (%) | M (SD) | |
|---|---|---|
|
| ||
| Race | ||
| Asian or Asian American | 15 (4.5) | |
| Black or African American | 80 (24.2) | |
| Indigenous or Native American | 0(0) | |
| White or Caucasian American | 230 (69.5) | |
| Bi- or multi-racial American | 3 (0.9) | |
| Other race | 3 (0.9) | |
| Female sex assigned at birth | 167 (50.5) | |
| Male sex assigned at birth | 164 (49.5) | |
| Age (baseline) | 40.24 (6.24) | |
| Childhood trauma (total) | 36.21 (11.35) | |
| Neglect | 16.35 (6.29) | |
| Abuse | 19.86 (6.77) | |
| Reappraisal | 30.84 (5.83) | |
| Suppression | 14.30 (4.71) | |
| Psychosocial and physiological pathways (follow-up) | ||
| BMI | 27.51 (5.23) | |
| Sleep score (total) | 5.03 (2.99) | |
| Smoking status | ||
| Former or current smoker | 103 (37.1) | |
| Never smoked | 228 (68.9) | |
| Perceived stress | 12.68 (6.61) | |
| Depressive symptoms | 4.47 (4.84) | |
| Education (years) | 16.88 (3.31) | |
| Systemic inflammation | ||
| IL-6 (baseline, pg/mL, raw) | 1.50 (1.22) | |
| IL-6 (follow-up, pg/mL, raw) | 1.88 (1.24) | |
| CRP (baseline, mg/L, raw) | 0.24 (0.38) | |
| CRP (follow-up, mg/L, raw) | 0.29 (0.46) | |
Note: Of the 331 participants who completed the baseline visit, 279 were retained for the follow-up visit. The participants who were lost to follow-up were younger than the 279 who were retained, but did not differ with respect to race, sex assigned at birth, childhood trauma, cognitive reappraisal, expressive suppression, CRP, or IL-6 as baseline. Abbreviations: BMI, body mass index; CRP, C-reactive protein; IL-6, interleukin-6; M, mean; SD, standard deviation.
1.2 |. Procedure
As part of baseline and follow-up visits, participants provided written consent, had their blood drawn and anthropometric measurements taken, and completed a set of questionnaires and a semi-structured interview. Other study procedures not included in the analyses described here included psychophysiological assessments and an fMRI scanning session. Data collection occurred between 2008 and 2017.
1.3 |. Measures
1.3.1 |. Childhood Trauma Questionnaire
As part of the baseline visit, participants retrospectively reported on experiences of childhood trauma by completing the 28-item Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 2003). On a five-point Likert scale from 1 (‘never true’) to 5 (‘very often true’), participants indicated to what extent statements in the following five domains were reflective of their own experiences ‘growing up as a child and a teenager’: physical abuse (‘People in my family hit me so hard that it left me with bruises or marks’), emotional abuse (‘People in my called me things like stupid, lazy, or ugly’), sexual abuse (‘Someone tried to touch me in a sexual way, or tried to make me touch them’), physical neglect (‘My parents were too drunk or high to take care of the family’), and emotional neglect (‘There was someone in my family who helped me feel that I was important or special’). Positively phrased items were reverse scored. We then summed across the five subscales to create a total CTQ score, with higher summary scores indicating greater childhood trauma. A childhood abuse variable was also calculated by summing across the physical, emotional, and sexual abuse subscales and a childhood neglect variable was created by summing scores on the physical and emotional neglect subscales. In the sample, the CTQ had strong internal reliability when considering the scale in its entirety (α = 0.90) and when considering abuse (α = 0.87) and neglect separately (α = 0.88).
1.3.2 |. Emotion Regulation Questionnaire
At baseline, participants reported on their habitual use of cognitive reappraisal and expressive suppression by completing the 10-item Emotion Regulation Questionnaire (ERQ) (Gross & John, 2003). On a scale of 1 (‘strongly disagree’) to 7 (‘strongly agree’), participants indicated to what extent they agreed with statements about modulating their emotions. Six items assessed cognitive reappraisal (‘When I want to feel less negative emotion, I change the way I’m thinking about the situation’) and four items focussed on expressive suppression (‘I keep my emotions to myself’). Individual items for each subscale were summed to create a total score for cognitive reappraisal and expressive suppression, respectively. Internal consistency was adequate for each subscale (reappraisal: α= 0.80, suppression: α = 0.75).
1.3.3 |. Systemic inflammation
Participants were instructed to fast from food, drink (except water), exercise, and tobacco products for 8 h prior to their baseline and follow-up visit. All blood draws were completed between 7:00 and 11:00 AM and participants were rescheduled if they experienced symptoms of acute infection, were taking antibiotics or antivirals, or were vaccinated or received a tattoo in the prior 2 weeks. Participants’ blood was drawn by antecubital venipuncture into sodium citrate and serum separator tubes that were centrifuged within 1 h of the blood draw. Plasma and serum samples were aliquoted and frozen at −80°C until batch processing.
The University of Pittsburgh’s Behavioural Immunology Laboratory measured IL-6 in plasma samples using a high-sensitivity enzyme-linked immunoassay kit (Human IL-6 Quantikine HS ELISA, R&D system; detection range 0.2–10 pg/ml; intra-assay coefficient of variation (CV) = 4.24%). Serum samples were sent to the university’s Clinical Services Laboratory where CRP was measured using a high-sensitivity CRP assay with CRPH reagent and the SYNCHRON LX system (Beckman Coulter, Inc.; intra-assay CV = 5.0%). All samples were run in duplicate for both analytes.
1.3.4 |. Covariates
At baseline, participants reported on their age, sex assigned at birth, and racial and ethnic identity. Based on the sample distribution, participants were categorized as male (n = 164; 49.5%) or female (n = 167; 50.5%) and either belonging to a racial or ethnic minority group (n = 101; 30.5%) or white, non-Hispanic (n = 230; 69.5%). We also calculated the time in years between the baseline and follow-up visit (median = 2.85 years, interquartile range = 3.47 months).
1.3.5 |. Physiological, psychosocial, and health behaviour pathways
Individual differences in health behaviours and physiological and psychosocial characteristics could account for associations between childhood trauma, emotion regulation strategies, and prospective changes in systemic inflammation. At the follow-up visit, we estimated participants’ level of adiposity by taking their height and weight, which were used to calculate body mass index (BMI; kg/m2). To assess health behaviours, participants reported on their sleep by completing the Pittsburgh Sleep Questionnaire Index (PSQI; Buysse et al., 1989), and indicated whether they currently or previously smoked cigarettes or consumed tobacco products. For sleep, we used the Pittsburgh Sleep Quality Index (PSQI) total sleep score, which estimates global sleep quality and has a range of 0–21. The mean sleep score in the sample was 5.03, which is suggestive of sleep disturbance or poorer quality sleep, on average (Buysse et al., 1989). Given the distribution of smoking behaviours, participants were categorized as current or former smokers (n = 103; 37.1%) versus non-smokers (n = 228; 68.9%). To assess psychological distress, participants completed the 21-item Beck Depression Inventory (BDI; Beck et al., 1996) and 10-item Perceived Stress Scale (PSS; Cohen et al., 1994), both of which had acceptable internal consistency within the sample (BDI: α = 0.84, PSS: α = 0.89). Participants also indicated how many years of education they completed, which was used as a proxy of adult socioeconomic position.
1.4 |. Statistical analyses
Research questions and analyses were pre-registered on Open Science Framework (OSF). In transparency, the analyses presented here deviate from those initially proposed. Instead of averaging IL-6 and CRP values across time points as pre-registered, we assessed changes in inflammatory markers over time. This decision was made because inflammatory markers did not meet the pre-registered effect size threshold for the correlations across baseline and follow-up time points, particularly for IL-6. Accordingly, the prospective study design was leveraged to examine changes over time. For completeness of reporting, results based on average CRP and IL-6 levels across time points (pre-registered) can be found in the supplemental file.
Prior to fitting regression analyses, we examined variable distributions and correlations between study variables. Multiple regression analyses examined the separate main effects of childhood trauma and emotion regulation strategies on prospective levels of peripheral inflammation by predicting CRP and IL-6 levels at follow-up, adjusting for baseline levels of CRP and IL-6, respectively. Using Hayes’ PROCESS macro moderation models were fit with childhood trauma as the predictor and either cognitive reappraisal or expressive suppression as the moderator. Significant interaction effects were probed at ±1 standard deviation (SD) above and below the sample mean for cognitive reappraisal and expressive suppression and to assess the conditional effects of childhood trauma on changes in peripheral levels of systemic inflammation. For interaction models with significant conditional effects falling outside of ±1 SD, we ran a Johnson-Neyman region of significance test, which includes the score at which conditional effects became significant (p < 0.05) and the percentage of participants with scores in this range. For significant main and moderation effects only, we then added adiposity (i.e., BMI), health behaviours (PSQI, smoking status), psychological distress (PSS, BDI), and years of education one at a time to the models to see whether significant effects were accounted for by individual differences in these psychosocial and physiological characteristics and health behaviours. Baseline levels of systemic inflammation, age, sex, race, and the number of years between baseline and follow-up visits were included as covariates in all analyses.
In ancillary analyses, we considered potential sex differences and whether associations between childhood trauma, emotion regulation strategies, and prospective changes in systemic inflammation differed depending on whether childhood trauma was characterized by threat (i.e., sum of physical, emotional, and sexual abuse subscales) or deprivation (i.e., sum of physical and emotional neglect subscales). For sex differences, we fit moderation models using PROCESS with either childhood trauma, cognitive reappraisal, or expressive suppression as the predictor variable and sex assigned at birth variable (0 = male, 1 = female) as the moderator.
In post-hoc sensitivity analyses, we excluded participants who developed and were receiving treatment for new chronic health conditions that emerged in between their baseline and follow-up visits as these conditions and the medications to treat these conditions could contribute to changes in systemic inflammation. At the follow-up visit, participants reported on whether they had experienced a range of health conditions including a cardiac event (myocardial infarction; n = 1), traumatic ischaemic attack (n = 1) or cancer (adenocarcinoma; n = 1). They also reported whether they were told by a doctor on two separate occasions that they had certain chronic health conditions that needed to be managed with medications, including high blood pressure (n = 5; one of whom had both high blood pressure and myocardial infarction) and chronic obstructive pulmonary disease (n = 1). We also learnt at the time of follow-up that one participant included at baseline had been taking blood pressure lowering medications for decades. There was also another participant who was involved in a multi-vehicle car accident shortly before their follow-up visit and endorsed high levels of posttraumatic stress, anxiety, and depressive symptoms at follow-up. Consequently, we ran post-hoc sensitivity analyses excluding this participant and the nine other participants diagnosed with and taking medication for one or more of the above listed chronic health conditions. Results remained substantively the same as when including these participants, with one exception detailed below. As such, the results presented here are based on data that includes these 10 participants. All statistical analyses were performed in R (R Core Team).
2 |. RESULTS
2.1 |. Preliminary descriptives
When examining variable distributions, one outlying IL-6 value at baseline (15.223 pg/ml) was excluded from analyses for being greater than 9 standard deviations above the mean, which is unlikely to be biological plausible in a slightly overweight (BMI = 26.4%), healthy adult in the absence of infection. IL-6 and CRP values at baseline and follow-up were not normally distributed and subsequently log-transformed. Of the 331 participants who completed baseline, 279 participants were retained at follow-up. Participants who completed only the baseline assessments were slightly younger (38.37 years) than the participants who completed both baseline and follow-up visits (40.58 years; t[329] = −2.372, p = 0.018), but did not differ with respect to race, self-reported sex assigned at birth, childhood trauma, cognitive reappraisal, expressive suppression, CRP, or IL-6 at baseline (ps > 0.40).
Cognitive reappraisal and expressive suppression were not reliably correlated with one another (r = 0.037, p = 0.500) and they variably related to childhood trauma, both when considering total childhood trauma (cognitive reappraisal: r = 0.055, p = 0.323; expressive suppression: r = 0.047, p = 0.393) and neglect (cognitive reappraisal: r = −0.056, p = 0.312, expressive suppression: r = 0.129, p = 0.019) and abuse separately (cognitive reappraisal: r = 0.143, p = 0.009, expressive suppression: r = −0.041, p = 0.460). Peripheral levels of IL-6 at baseline and follow-up were moderately correlated (r = 0.381, p < 0.001), with stronger correlations between CRP levels at baseline and follow-up (r = 0.679, p < 0.001). See Table 2 for a summary of all bivariate correlations.
TABLE 2.
Correlation matrix
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| 1. Cognitive reappraisal | 1 | |||||||||||||||
| 2. Expressive suppression | 0.037 | 1 | ||||||||||||||
| 3. Childhood trauma (total) | 0.055 | 0.047 | 1 | |||||||||||||
| 4. Neglect | −0.056 | 0.129 | 0.858 | 1 | ||||||||||||
| 5. Abuse | 0.143 | −0.041 | 0.879 | 0.510 | 1 | |||||||||||
| 6. IL-6 (baseline, log) | −0.089 | 0.144 | 0.115 | 0.098 | 0.100 | 1 | ||||||||||
| 7. CRP (baseline, log) | 0.015 | 0.025 | 0.022 | 0.030 | 0.009 | 0.394 | 1 | |||||||||
| 8. IL-6 (follow-up, log) | −0.127 | 0.121 | 0.086 | 0.053 | 0.101 | 0.381 | 0.334 | 1 | ||||||||
| 9. CRP (follow-up, log) | −0.023 | −0.036 | 0.156 | 0.093 | 0.180 | 0.395 | 0.679 | 0.512 | 1 | |||||||
| 10. Age (baseline) | 0.026 | 0.076 | −0.025 | 0.003 | −0.044 | 0.113 | −0.007 | 0.099 | 0.029 | 1 | ||||||
| 11. Years between visits | 0.061 | −0.006 | 0.036 | 0.043 | 0.021 | 0.011 | 0.016 | 0.057 | 0.081 | −0.023 | 1 | |||||
| 12. Body mass index (follow-up) | −0.059 | −0.047 | 0.071 | 0.046 | 0.080 | 0.335 | 0.391 | 0.405 | 0.511 | 0.090 | 0.028 | 1 | ||||
| 13. Perceived stress (follow-up) | −0.088 | 0.055 | 0.244 | 0.288 | 0.146 | 0.062 | −0.020 | 0.122 | 0.070 | −0.033 | 0.058 | 0.055 | 1 | |||
| 14. Depressive symptoms (follow-up) | −0.028 | 0.019 | 0.192 | 0.238 | 0.103 | 0.101 | 0.048 | 0.113 | 0.139 | 0.021 | 0.019 | 0.030 | 0.612 | 1 | ||
| 15. Sleep score (total; follow-up) | −0.035 | 0.027 | 0.169 | 0.175 | 0.126 | 0.140 | 0.096 | 0.148 | 0.120 | 0.004 | 0.011 | 0.059 | 0.399 | 0.384 | 1 | |
| 16. Education (years) | 0.023 | −0.011 | −0.088 | −0.080 | −0.073 | −0.231 | −0.157 | −0.342 | −0.163 | −0.094 | −0.120 | −0.204 | −0.083 | −0.115 | −0.2171 | 1 |
Note: Bold values denote correlations significant at the p < 0.01 level; Italics values indicate correlations significant at the p < 0.05 level.
Abbreviations: CRP, C-reactive protein; IL-6, interleukin-6.
2.2 |. Primary analyses
Do childhood trauma, cognitive reappraisal, and expressive suppression separately contribute to prospective changes in peripheral levels of systemic inflammation?
Please see Table 3 for results of main effects models. For significant moderation effects, see Figure 1 for interactions between childhood trauma and cognitive reappraisal and Figure 2 for interactions between childhood trauma and expressive suppression. Greater childhood trauma was associated with prospective, multiyear increases in CRP (B = 0.004, SE = 0.002, p = 0.049) but not IL-6 (B = 0.001, SE = 0.001, p = 0.603). This association was independent of years of education (B = 0.005, SE = 0.002, p = 0.044), but became non-significant when accounting for BMI (B = 0.004, SE = 0.002, p = 0.070), smoking and sleep behaviour (B = 0.004, SE = 0.002, p = 0.060), and depressive symptoms and perceived stress (B = 0.004, SE = 0.002, p = 0.064).
TABLE 3.
Multiple regression models examining the separate main effects of childhood trauma, cognitive reappraisal, and expressive suppression on prospective changes in markers of systemic inflammation among a sample of young and midlife adults
| Total childhood trauma |
Cognitive reappraisal |
Expressive suppression |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | Std. B | P | B | SE | Std. B | P | B | SE | Std. B | P | |
| IL-6 at follow-up (pg/ml; log) | ||||||||||||
| Intercept | 0.192 | 0.140 | 0.170 | 0.405 | 0.150 | 0.007 | 0.179 | 0.138 | 0.198 | |||
| Race/ethnic minority | 0.126 | 0.032 | 0.224 | <0.001 | 0.140 | 0.032 | 0.248 | <0.001 | 0.126 | 0.032 | 0.222 | <0.001 |
| Female sex assigned at birth | −0.003 | 0.029 | −0.006 | 0.914 | 0.007 | 0.028 | 0.013 | 0.812 | 0.008 | 0.030 | 0.015 | 0.800 |
| Age at baseline | 0.001 | 0.002 | 0.035 | 0.533 | 0.002 | 0.002 | 0.044 | 0.434 | 0.001 | 0.002 | 0.029 | 0.613 |
| Years between visits | −0.051 | 0.028 | −0.102 | 0.071 | −0.050 | 0.028 | −0.100 | 0.073 | −0.052 | 0.028 | −0.104 | 0.066 |
| II-6 at baseline (log) | 0.350 | 0.057 | 0.352 | <0.001 | 0.346 | 0.056 | 0.348 | <0.001 | 0.345 | 0.057 | 0.347 | <0.001 |
| Total childhood trauma | 0.001 | 0.001 | 0.030 | 0.603 | ||||||||
| Cognitive reappraisal | −0.007 | 0.003 | −0.155 | 0.006* | ||||||||
| Expressive suppression | 0.003 | 0.003 | 0.062 | 0.294 | ||||||||
| Model fit | R2 = 0.210, adjusted R2 = 0.191 | R2 = 0.232, adjusted R2 = 0.214 | R2 = 0.212, adjusted R2 = 0.194 | |||||||||
| CRP at follow-up (mg/L; log) | ||||||||||||
| Intercept | −0.393 | 0.230 | 0.089 | −0.104 | 0.250 | 0.678 | −0.193 | 0.225 | 0.394 | |||
| Race/ethnic minority | 0.089 | 0.054 | 0.082 | 0.096 | 0.114 | 0.054 | 0.104 | 0.034 | 0.111 | 0.053 | 0.101 | 0.039 |
| Female sex assigned at birth | 0.005 | 0.049 | 0.005 | 0.924 | 0.020 | 0.049 | 0.020 | 0.679 | 0.003 | 0.050 | 0.003 | 0.958 |
| Age at baseline | <0.001 | 0.004 | 0.003 | 0.950 | <0.001 | 0.004 | 0.005 | 0.918 | 0.001 | 0.004 | 0.011 | 0.824 |
| Years between visits | −0.016 | 0.039 | −0.019 | 0.689 | −0.015 | 0.040 | −0.018 | 0.706 | −0.016 | 0.040 | −0.019 | 0.693 |
| CRP at baseline (log) | 0.675 | 0.050 | 0.657 | <0.001 | 0.675 | 0.051 | 0.657 | <0.001 | 0.680 | 0.051 | 0.662 | <0.001 |
| Total childhood trauma | 0.004 | 0.002 | 0.096 | 0.049* | ||||||||
| Cognitive reappraisal | −0.005 | 0.004 | −0.056 | 0.253 | ||||||||
| Expressive suppression | −0.005 | 0.005 | −0.046 | 0.362 | ||||||||
| Model fit | R2 = 0.480, adjusted R2 = 0.467 | R2 = 0.474, adjusted R2 = 0.460 | R2 = 0.473, adjusted R2 = 0.459 | |||||||||
Note:
p < 0.05.
Participants retrospectively reported on exposure to childhood trauma by completing the Childhood Trauma Questionnaire, which assesses experiences of abuse (physical, emotional, sexual) and neglect (physical, emotional) in childhood. Cognitive reappraisal and expressive suppression were measured using the Emotion Regulation Questionnaire, which includes six items to assess cognitive reappraisal and four items to assess expressive suppression. CRP and IL-6 were log-transformed prior to fitting regression models.
Abbreviations: B, unstandardized beta coefficient; CRP, C-reactive protein; IL-6, interleukin-6; SE, standard error; Std. B, standardized beta coefficient.
FIGURE 1.
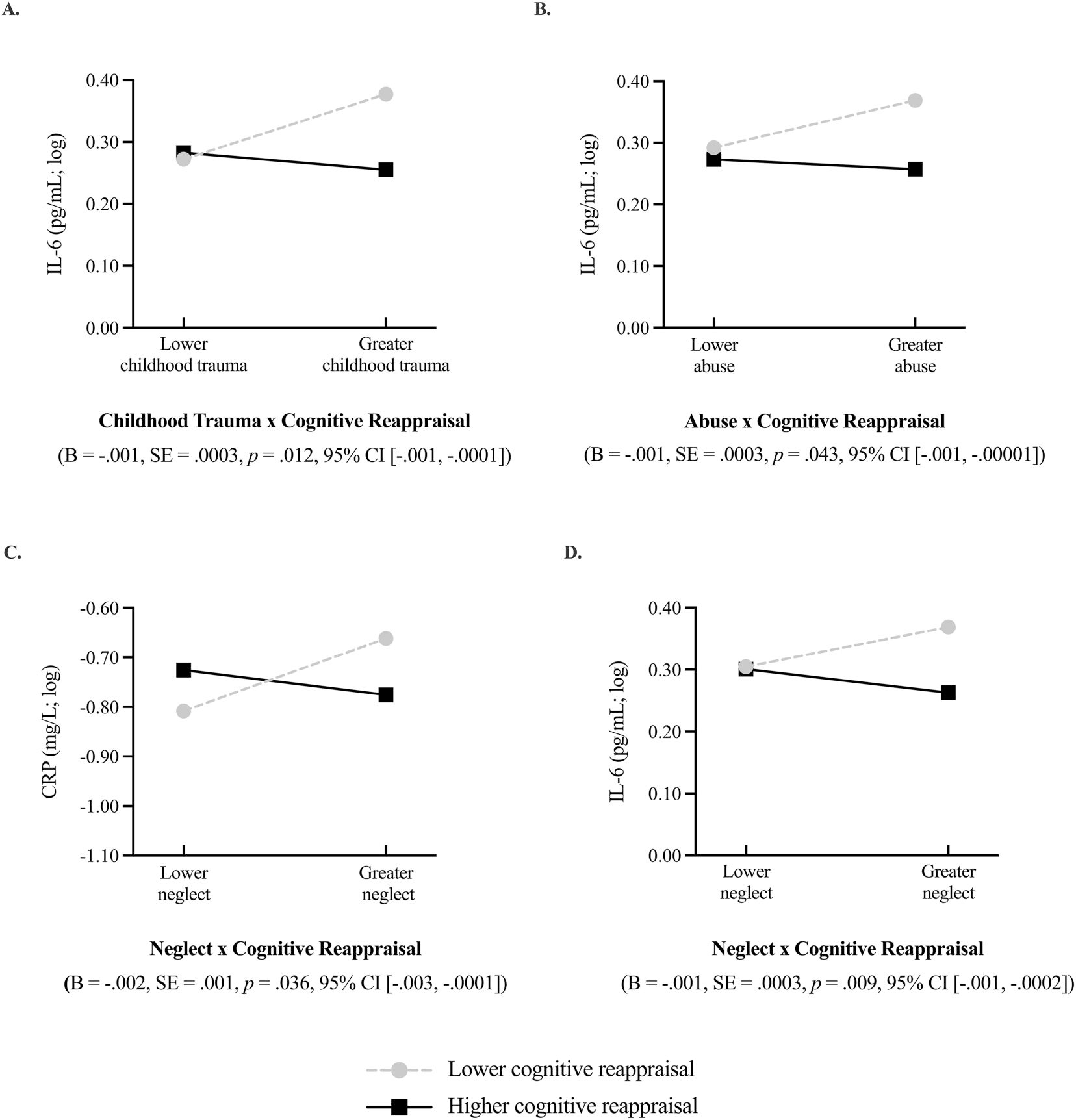
Trait-level cognitive reappraisal moderated the association between retrospective reports of childhood trauma (total, neglect, abuse) and prospective changes in C-reactive protein (CRP) and interleukin-6 (IL-6). Greater childhood trauma was associated with prospective increases in systemic inflammation, but only among adults who lower in cognitive reappraisal. Moderation effects are graphed at one standard deviation (SD) above and below the mean level of childhood trauma and cognitive reappraisal reported in the sample. The black solid line represents associations for higher levels of cognitive reappraisal (+1 SD) and the light grey, dashed line represents associations for lower levels of cognitive reappraisal (−1 SD). IL-6 and CRP were log-transformed prior to fitting interaction models, hence the negative values. Figure 1A depicts the interaction between total childhood trauma and cognitive reappraisal on prospective changes in IL-6. Figure 1B depicts the interaction between childhood abuse and cognitive reappraisal on prospective changes in IL-6. Figure 1C shows the interaction between childhood neglect and cognitive reappraisal on propsective changes in CRP, and Figure 1D shows the between childhood neglect and cognitive reappraisal on prospective changes in IL-6.
FIGURE 2.
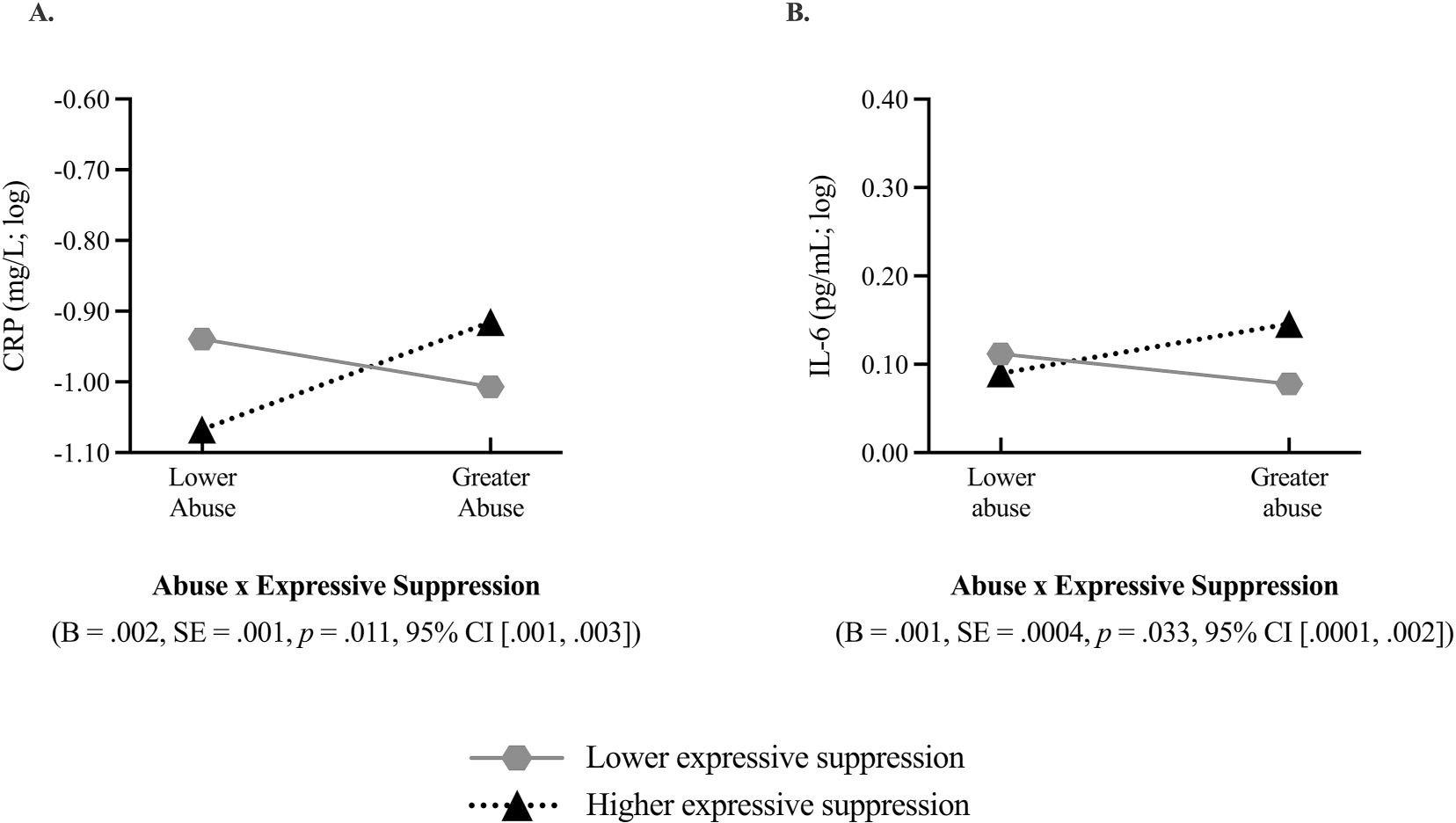
Trait-level expressive suppression moderated the association between retrospective reports of childhood abuse and prospective changes in C-reactive protein (CRP) and interleukin-6 (IL-6). Greater childhood trauma was associated with prospective increases in systemic inflammation, but only among adults higher in expressive suppression. Moderation effects are graphed at one standard deviation (SD) above and below the mean level of childhood trauma and expressive suppression reported in the sample. The dotted black line represents associations at higher levels of expressive suppression (+1 SD) and the solid, dark grey line reflects associations at lower levels of expressive suppression (−1 SD). IL-6 and CRP were log-transformed prior to fitting interaction models, hence the negative values. Figure 2A depicts the interaction between childhood abuse and expressive suppression on prospective changes in CRP and Figure 2B depicts the interaction between childhood abuse and expressive suppression on prospective changes in IL-6.
Greater cognitive reappraisal was associated with a prospective, multi-year decrease in IL-6 (B = −0.007, SE = 0.003, p = 0.006), but not CRP (B = −0.005, SE = 0.004, p = 0.253). The association between cognitive reappraisal and prospective change in IL-6 was independent of BMI (B =−0.006, SE = 0.002, p = 0.014), sleep and smoking behaviour (B = −0.007, SE = 0.003, p = 0.005), depressive symptoms and perceived stress (B = −0.007, SE = 0.003, p = 0.009), and years of education (B = −0.006, SE = 0.002, p = 0.011) when included separately and then in a saturated model (B = −0.005, SE = 0.002, p = 0.037). When running sensitivity analyses, these results remained substantively the same except that the association between cognitive reappraisal and IL-6 became marginal when included in the saturated model of all psychosocial and physiological pathways (B = −0.005, SE = 0.003, p = 0.063). There were no main effects of suppression on prospective change in IL-6 (B = 0.003, SE = 0.003, p = 0.294) or CRP (B = −0.005, SE = 0.005, p = 0.362).
Do cognitive reappraisal and expressive suppression moderate associations between childhood trauma and prospective changes in peripheral levels of systemic inflammation?
Cognitive reappraisal moderated the association between childhood trauma and prospective change in IL-6 (B = −0.001, SE = 0.0003, p = 0.012; Figure 1a) but not CRP (B = −0.001, SE = 0.001, p = 0.115). Participants who reported exposure to greater childhood trauma had greater prospective increases in IL-6, but only if they were lower in cognitive reappraisal (B = 0.006, SE = 0.002, p = 0.007). Among participants relatively higher in cognitive reappraisal, there was no association between childhood trauma and prospective levels of IL-6. This moderation effect was independent of BMI (B = −0.001, SE = 0.0002, p = 0.020), smoking and sleep behaviour (B = −0.001, SE = 0.0003, p = 0.006), perceived stress and depressive symptoms (B = −0.001, SE = 0.0003, p = 0.007) and years of education (B = −0.001, SE = 0.0003, p = 0.008) when considered separately and then in saturated models (B = −0.001, SE = 0.0002, p = 0.007).
Expressive suppression did not moderate associations between childhood trauma and CRP (B = 0.001, SE = 0.001, p = 0.117) or IL-6 (B = 0.001, SE = 0.0003, p = 0.074).
2.3 |. Ancillary analyses
Do associations between childhood trauma, emotion regulation, and prospective changes in peripheral levels of systemic inflammation differ by self-reported sex assigned at birth?
There were no sex differences (all ps > 0.10; please see Table S1).
Do associations between childhood trauma, emotion regulation, and prospective changes in peripheral levels of systemic inflammation differ depending on whether trauma is abusive or neglectful?
Childhood abuse was associated with greater prospective increases in CRP (B = 0.009, SE = 0.004, p = 0.023), but not IL-6 (B = 0.001, SE = 0.002, p = 0.579). There was no main effect of childhood neglect on prospective changes in CRP (B = 0.004, SE = 0.003, p = 0.246) or IL-6 (B = 0.001, SE = 0.002, p = 0.716).
Both cognitive reappraisal and expressive suppression moderated the association between childhood abuse and markers of systemic inflammation in ways consistent with prior research. Cognitive reappraisal moderated the association between childhood abuse and prospective change in IL-6 (B = −0.001, SE = 0.0003, p = 0.043; Figure 1b) such that greater childhood abuse was associated with greater prospective increases in IL-6, but only among participants lower in cognitive reappraisal (−1 SD; B = 0.006, SE = 0.003, p = 0.040). There were no moderation effects for CRP (B = 0.001, SE = 0.001, p = 0.518). When considering expressive suppression, there was a moderation effect of expressive suppression on the association between childhood abuse and prospective change in CRP (B = 0.002, SE = 0.0008, p = 0.011; Figure 2a) and IL-6 (B = 0.001, SE = 0.0003, p = 0.033; Figure 2b), such that greater childhood abuse was associated with greater prospective increases in systemic inflammation but only among participants higher in expressive suppression (CRP: B = 0.013, SE = 0.005, p = 0.020; IL-6: B = 0.005, SE = 0.003, p = 0.079). However, the conditional effects of childhood abuse on prospective changes in IL-6 were only significant for suppression scores greater than 6.712 units (9.7% of participants) as indicated by the Johnson-Neyman region of significance test.
When considering childhood neglect, moderation effects were only observed for cognitive reappraisal and not expressive suppression (CRP: B = 0.0003, SE = 0.001, p = 0.636; IL-6: B = −0.0002, SE = 0.0003, p = 0.472). Cognitive reappraisal moderated the association between childhood neglect and prospective change in CRP (B = −0.002, SE = 0.0007, p = 0.036; Figure 1c) and IL-6 (B = −0.001, SE = 0.0003, p = 0.009; Figure 1d) such that greater childhood neglect was associated with greater prospective increases in systemic inflammation but only among participants lower in cognitive reappraisal (CRP: B = 0.012, SE = 0.006, p = 0.050; IL-6: B = 0.005, SE = 0.002, p = 0.042).
3 |. DISCUSSION
We examined whether retrospective reports of childhood trauma and trait-level cognitive reappraisal and expressive suppression in adulthood separately and interactively predicted prospective changes in peripheral levels of systemic inflammation. Importantly, we found that childhood trauma was associated with prospective increases in peripheral levels of IL-6, but only among participants who were relatively lower in cognitive reappraisal. Similar patterns were observed when considering abuse and neglect separately. Greater abuse and neglect were each associated with increases in prospective levels of IL-6 (and to a lesser extent CRP), but only among participants lower in cognitive reappraisal. This fairly consistent pattern supports a growing body of research focussed on the stress-buffering effects of cognitive reappraisal (Shahane et al., 2019), particularly when considering uncontrollable stressors, such as childhood trauma (Troy et al., 2013). For example, Kalia and Knauft (2020) and Boyes et al. (2016) found that cognitive reappraisal buffered individuals from the effects of adverse childhood experiences on perceived stress in adulthood and adolescence, respectively. Stress-buffering effects of cognitive reappraisal could reflect a decrease in negative emotions and perceived stress in response to concurrent stressors. Cognitive reappraisal has been further tied to social functioning and feelings of life satisfaction more broadly (Cutuli, 2014), which appear health-protective (e.g., Diener & Chan, 2011), even among individuals who have experienced childhood trauma (Logan-Greene et al., 2014). An important next step will be to consider how trait-level cognitive reappraisal and expressive suppression may jointly relate to associations between childhood trauma and inflammation, as the combination of being lower in suppression and higher in cognitive reappraisal has been linked to better physical and mental health outcomes in other samples (Eftekhari et al., 2009; Raymond et al., 2019).
Expressive suppression did not moderate the association between total childhood trauma and changes in systemic inflammation. However, suppression did exacerbate the extent to which childhood abuse was associated with changes in systemic inflammation. Adults higher in expressive suppression had greater prospective increases in both CRP and IL-6. Because expressive suppression requires the inhibition of external behaviour despite the internal experience of an emotion, this strategy requires effortful control that may be physiologically and psychologically taxing, especially for individuals who habitually use this strategy (Cutuli, 2014). Thus, for youth who regularly experience abuse, expressive suppression (e.g., not expressing anger or sadness when being hit or berated) may mitigate the escalation of abuse but come at a cost to psychological and physiological functioning. Moreover, the habitual use of expressive suppression has been linked to greater negative affect and less social support (Cutuli, 2014), which are also associated with systemic inflammation (Marsland et al., 2008) and may exacerbate the effect of childhood trauma on systemic inflammation (Runsten et al., 2013).
We did not find moderation effects of expressive suppression on changes in systemic inflammation when considering childhood neglect, however. It is not clear why this might be the case. It is possible that people who experience childhood neglect are more likely to use different emotion regulation strategies, such as avoidance or disengagement, which we did not consider here. Childhood neglect has also been associated with difficulties identifying different emotional states (Aust et al., 2013; Pollak et al., 2000), which may make it more challenging to employ a desired emotion regulation strategy. For example, someone who has experienced neglect may not know whether they are experiencing sadness, anger, or both, which could make it more difficult to suppress their outward expression of these emotions.
When considering how childhood trauma and emotion regulation strategies separately related to changes in systemic inflammation, results were generally consistent with prior literature. Childhood trauma was associated with prospective increases in CRP but not IL-6, and this was partly accounted for by individual differences in adiposity, health behaviours, and psychological distress. This aligns with prior work linking greater childhood trauma exposure to higher BMIs, riskier health behaviours, and greater perceived stress (e.g., Brindle et al., 2018; Min et al., 2013; Nelson et al., 2017; Ruiz & Font, 2020), all of which may increase systemic inflammation over time (McDade et al., 2006; Park et al., 2005; Raison et al., 2006). Nonetheless, the size of the effect was modest and at the upper end of the conventional threshold for determining statistical significance.
Similarly, abuse (but not neglect) was associated with prospective increases in CRP. This pattern partially supports the dimensional model of adversity, which posits that neglect and abuse have differential effects on health and well-being (McLaughlin & Sheridan, 2016). Characterized by high deprivation and low threat, neglect may reflect the absence of sufficient social and cognitive stimulation, whereas abuse (i.e., high threat and low deprivation) may contribute to poorer social-emotional processing, especially to threatening or ambiguous stimuli (McLaughlin & Sheridan, 2016). Childhood abuse has also been associated with lower resting connectivity and stress-related activity patterns in brain networks that may be involved in both affective processing and immune regulation (Banihashemi et al., 2015, 2022). Thus, abuse could contribute to increasing inflammation through central changes in visceral control networks (Kraynak et al., 2018, 2019), or via emotional processing and threat appraisal. Nonetheless, reappraisal moderated associations between abuse, neglect, and changes in inflammation. This suggests that for adults reporting childhood trauma, reappraisal and suppression may differentially relate to brain networks jointly involved in affective regulation and inflammatory control (Gianaros et al., 2014; Koban et al., 2021; Kraynak et al., 2018).
As hypothesized, cognitive reappraisal was associated with a decrease in IL-6 from baseline to follow-up, building upon prior cross-sectional research linking greater cognitive reappraisal to lower systemic inflammation (Appleton et al., 2013). However, unlike Ellis et al. (2019), associations were independent of biopsychosocial factors and health behaviours. This suggests that cognitive reappraisal may uniquely influence changes in systemic inflammation above and beyond biopsychosocial factors and possibly via other pathways not studied here (e.g., physical activity, diet).
Counter to prior cross-sectional work (Appleton et al., 2013), expressive suppression did not predict prospective changes in IL-6 or CRP. The absence of main effects could suggest that suppressing emotions may matter more for concurrent versus prospective levels of systemic inflammation or that expressive suppression indirectly influences changes in systemic inflammation through health behaviours and sleep (Ellis et al., 2019). The ERQ also estimates general, trait-level expressive suppression so we cannot infer whether suppressing negative emotions specific to childhood trauma may have differential effects on inflammation compared to suppressing emotions more generally. ERQ scores also do not tell us how well or how frequently a person implements these strategies and relies on participants’ perceptions of the emotion regulations strategies they employ. To assess whether expressive suppression relates to prospective changes in systemic inflammation over time and for whom, experimental tasks or daily diary methods that assess both general emotion regulation strategies and in the context of childhood trauma are needed.
Interestingly, there were differences in findings when considering changes in CRP versus IL-6. Specifically, when considering changes in CRP, there were two main effects of trauma (total, abuse) and two interaction effects (neglect by reappraisal, abuse by suppression). For IL-6, there was one main effect of reappraisal and four interaction effects (total trauma by reappraisal, neglect by reappraisal, abuse by reappraisal, abuse by suppression). As such, it is difficult to discern whether the overall pattern in results for IL-6 versus CRP is meaningful or not. One possibility is that CRP may be more strongly influenced by longer-lasting events (e.g., childhood trauma happening over years of childhood) whereas IL-6 levels may be more sensitive to temporal experiences, including dynamic affective processes in the past days, weeks, or years. This is partially supported by meta-analytic evidence finding reliable increases in IL-6, but not CRP in response to acute laboratory stressors (Marsland et al., 2017). Nonetheless, we are hesitant to overinterpret these differences given that there were significant effects for CRP when considering interactions between suppression and abuse and reappraisal and neglect. Thus, in addition to replication studies, more research is needed to better understand how emotion regulation and childhood trauma come to be associated with changes in systemic inflammation and whether these pathways are distinct for CRP versus IL-6.
This study must be considered in view of several limitations. Foremost, this was a community sample of adults who, at baseline, were not currently diagnosed with chronic physical or mental health conditions. As such, we may have underestimated the effects of childhood trauma and emotion regulation strategies on prospective changes in systemic inflammation. Future studies should include clinical samples of adults with chronic physical or psychiatric conditions for whom the negative consequences of childhood trauma on emotion regulation and physical and mental health may be more severe. Yet, we still found associations between mild-to-moderate childhood trauma and prospective increases in systemic inflammation among adults who were higher in expressive suppression or lower in cognitive reappraisal, although again the effect sizes were modest. The study included retrospective reports of childhood trauma and self-reports of emotion regulation strategies and health behaviours. An important next step will be to leverage data from prospective, longitudinal studies beginning in childhood to better understand how emotion regulation strategies unfold in the context of childhood trauma and whether associations between childhood trauma, emotion regulation strategies, and systemic inflammation change over time. Moreover, it is unclear whether associations change depending on whether individuals receive therapeutic interventions that focus on developing emotion regulation strategies and addressing cognitive distortions, such as trauma-focussed Cognitive Behaviour Therapy. Future studies could also consider other types of emotion regulation strategies, such as denial and acceptance.
There are also additional methodological limitations to consider. We used BMI as an approximation of adiposity; however, body fat percentage or visceral fat may better estimate adiposity. Similarly, we used years of education as a rough proxy of socioeconomic status, which could be more adequately measured using an index that considers multiple measures of prestige, wealth, and subjective social status in comparison to others. We also did not have information on participants’ gender identity, which may differ from their sex assigned at birth and differentially relate to study findings (Juster et al., 2019). We were limited to a single blood draw at each timepoint and there is the possibility of batch and freezer effects. Although CRP levels are generally stable over time, peripheral levels of IL-6 are more dynamic and multiple assessments of IL-6 levels at a single time point may better estimate stable inter-individual differences in circulating IL-6. Nonetheless, potential confounds were mitigated by rescheduling individuals who reported current or recent infections or vaccinations, and having participants complete fasting, morning blood draws and refraining from eating, exercising, smoking, and taking anti-inflammatory medications prior to appointments. Results also held in post-hoc sensitivity analyses excluding participants who developed chronic physical health conditions in between their two visits, with only one attenuated effect.
Despite these limitations, this study has several strengths. Although the strict inclusion criteria may limit generalizability of study findings, these criteria enable the identification of associations between childhood trauma, emotion regulation, and changes in systemic inflammation that are not attributed to extraneous factors, such as pre-existing chronic conditions and medications. We also included two markers of systemic inflammation and examined multiyear changes from baseline to follow-up. Adiposity, psychological distress, and health behaviours were also considered as potential biopsychosocial pathways underlying observed associations. In ancillary analyses, we also considered potential sex differences and whether results differed depending on the nature of the childhood trauma. Although there were no observed sex differences, we did find that cognitive reappraisal and expressive suppression moderated associations between childhood abuse and neglect and prospective changes in markers of systemic inflammation, but in different ways.
Adding to prior cross-sectional research, we found that cognitive reappraisal attenuates increases in IL-6 and may offset the extent to which childhood trauma contributes to greater increases in systemic inflammation in adulthood. Findings further suggest that greater habitual use of expressive suppression may exacerbate associations between childhood abuse and increases in peripheral levels of CRP and IL-6 over time. Preliminary study findings support the notion of cultivating cognitive reappraisal, particularly among adults who may have experienced childhood trauma, which may have benefits for physiological functioning, as indicated by slower changes in systemic inflammation over time.
Supplementary Material
ACKNOWLEDGEMENTS
Research reported in this manuscript was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health under award numbers R01-HL089850 and P01-HL040962 (PI: Peter Gianaros) and 5T32HL007560-37 (PIs: Rebecca Thurston & Peter Gianaros). The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
The authors report no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
De-identified data and an Rmarkdown file for the analyses presented in the manuscript can be accessed on Open Science Framework (OSF): https://osf.io/t324s/.
REFERENCES
- Appleton AA, Buka SL, Loucks EB, Gilman SE, & Kubzansky LD (2013). Divergent associations of adaptive and maladaptive emotion regulation strategies with inflammation. Health Psychology, 32(7), 748–756. 10.1037/a0030068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aust S, Alkan Härtwig E, Heuser I, & Bajbouj M (2013). The role of early emotional neglect in alexithymia. Psychological Trauma Theory Research Practice and Policy, 5(3), 225–232. 10.1037/a0027314 [DOI] [Google Scholar]
- Banihashemi L, Peng CW, Rangarajan A, Karim HT, Wallace ML, Sibbach BM, Singh J, Stinley MM, Germain A, & Aizenstein HJ (2022). Childhood threat is associated with lower resting-state connectivity within a central visceral network. Frontiers in Psychology, 13, 805049. 10.3389/fpsyg.2022.805049. https://europepmc.org/articles/PMC8927539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banihashemi L, Sheu LK, Midei AJ, & Gianaros PJ (2015). Childhood physical abuse predicts stressor-evoked activity within central visceral control regions. Social Cognitive and Affective Neuroscience, 10(4), 474–485. 10.1093/scan/nsu073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, McLaughlin KA, Misra S, & Koenen KC (2017). Childhood maltreatment and health impact: The examples of cardiovascular disease and type 2 diabetes mellitus in adults. Clinical Psychology: Science and Practice, 24(2), 125–139. 10.1111/cpsp.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Steer R, & Brown G (1996). Beck depression inventory: Manual (2nd ed.). The Psychological Corporation. [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, & Zule W (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect, 27(2), 169–190. 10.1016/s0145-2134(02)00541-0 [DOI] [PubMed] [Google Scholar]
- Boyes ME, Hasking PA, & Martin G (2016). Adverse life experience and psychological distress in adolescence: Moderating and mediating effects of emotion regulation and rumination. Stress and Health, 32(4), 402–410. 10.1002/smi.2635 [DOI] [PubMed] [Google Scholar]
- Brindle RC, Cribbet MR, Samuelsson LB, Gao C, Frank E, Krafty RT, Thayer JF, Buysse DJ, & Hall MH (2018). The relationship between childhood trauma and poor sleep health in adulthood. Psychosomatic Medicine, 80(2), 200–207. 10.1097/PSY.0000000000000542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF III, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1994). Perceived stress scale. Measuring Stress: A Guide for Health and Social Scientists, 10(2), 1–2. [Google Scholar]
- Cutuli D (2014). Cognitive reappraisal and expressive suppression strategies role in the emotion regulation: An overview on their modulatory effects and neural correlates. Frontiers in systems neuroscience, 8(175). 10.3389/fnsys.2014.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E, & Chan MY (2011). Happy people live longer: Subjective well-being contributes to health and longevity. Applied Psychology: Health and Well-Being, 3(1), 1–43. 10.1111/j.1758-0854.2010.01045.x [DOI] [Google Scholar]
- Eftekhari A, Zoellner LA, & Vigil SA (2009). Patterns of emotion regulation and psychopathology. Anxiety, Stress & Coping, 22(5), 571–586. 10.1080/10615800802179860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich KB, Miller GE, Rogosch FA, & Cicchetti D (2021). Maltreatment exposure across childhood and low-grade inflammation: Considerations of exposure type, timing, and sex differences. Developmental Psychobiology, 63(3), 529–537. 10.1002/dev.22031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis EM, Prather AA, Grenen EG, & Ferrer RA (2019). Direct and indirect associations of cognitive reappraisal and suppression with disease biomarkers. Psychology & Health, 34(3), 336–354. 10.1080/08870446.2018.1529313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- England-Mason G, Kimber M, Khoury J, Atkinson L, MacMillan H, & Gonzalez A (2017). Difficulties with emotion regulation moderate the association between childhood history of maltreatment and cortisol reactivity to psychosocial challenge in postpartum women. Hormones and Behavior, 95, 44–56. 10.1016/j.yhbeh.2017.07.007 [DOI] [PubMed] [Google Scholar]
- Fritz J, de Graaff AM, Caisley H, van Harmelen A-L, & Wilkinson PO (2018). A systematic review of amenable resilience factors that moderate and/or mediate the relationship between childhood adversity and mental health in young people. Frontiers in Psychiatry, 9, 230. 10.3389/fpsyt.2018.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Kuan DC-H, Schirda BL, Jennings JR, Sheu LK, Hariri AR, Gross JJ, & Manuck SB (2014). An inflammatory pathway links atherosclerotic cardiovascular disease risk to neural activity evoked by the cognitive regulation of emotion. Biological Psychiatry, 75(9), 738–745. 10.1016/j.biopsych.2013.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Rasero J, DuPont CM, Kraynak TE, Gross JJ, McRae K, Wright AGC, Verstynen TD, & Barinas-Mitchell E (2022). Multivariate brain activity while viewing and reappraising affective scenes does not predict the multiyear progression of preclinical atherosclerosis in otherwise healthy midlife adults. Affective Science, 3(2), 406–424. 10.1007/s42761-021-00098-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Uyar F, Koushik J, Jennings JR, Wager TD, Singh A, & Verstynen TD (2017). A brain phenotype for stressor-evoked blood pressure reactivity. Journal of American Heart Association, 6(9), e006053. 10.1161/JAHA.117.006053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, & Janson S (2009). Burden and consequences of child maltreatment in high-income countries. The Lancet, 373(9657), 68–81. 10.1016/s0140-6736(08)61706-7 [DOI] [PubMed] [Google Scholar]
- Gross JJ, & John OP (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85(2), 348–362. 10.1037/0022-3514.85.2.348 [DOI] [PubMed] [Google Scholar]
- Gross JJ, Richards JM, & John OP (2006). Emotion regulation in everyday life. In Snyder DK, Simpson J, & Hughes JN (Eds.), Emotion regulation in couples and families: Pathways to dysfunction and health (pp. 13–35). American Psychological Association. 10.1037/11468-001 [DOI] [Google Scholar]
- Gross J, & Cassidy J (2019). Expressive suppression of negative emotions in children and adolescents: Theory, data, and a guide for future research. Developmental Psychology, 55(9), 1938–1950. 10.1037/dev0000722 [DOI] [PubMed] [Google Scholar]
- Holman DM, Ports KA, Buchanan ND, Hawkins NA, Merrick MT, Metzler M, & Trivers KF (2016). The association between adverse childhood experiences and risk of cancer in adulthood: A systematic review of the literature. Pediatrics, 138(Supplement 1), S81–S91. 10.1542/peds.2015-4268l [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, LeMoult J, Wear JG, Piersiak HA, Lee A, & Gotlib IH (2020). Child maltreatment and depression: A meta-analysis of studies using the Childhood Trauma Questionnaire. Child Abuse & Neglect, 102, 104361. 10.1016/j.chiabu.2020.104361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EJ, Lam PH, Hoffer LC, Chen E, & Schreier HM (2018). Chronic family stress and adolescent health: The moderating role of emotion regulation. Psychosomatic Medicine, 80(8), 764–773. 10.1097/PSY.0000000000000624 [DOI] [PubMed] [Google Scholar]
- Juster R-P, de Torre MB, Kerr P, Kheloui S, Rossi M, & Bourdon O (2019). Sex differences and gender diversity in stress responses and allostatic load among workers and LGBT people. Current Psychiatry Reports, 21(11), 110. 10.1007/s11920-019-1104-2 [DOI] [PubMed] [Google Scholar]
- Kalia V, & Knauft K (2020). Emotion regulation strategies modulate the effect of adverse childhood experiences on perceived chronic stress with implications for cognitive flexibility. PLoS One, 15(6), e0235412. 10.1371/journal.pone.0235412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DM, McDonald J, & Minnis H (2021). The association of child maltreatment and systemic inflammation in adulthood: A systematic review. PLoS One, 16(4), e0243685. 10.1371/journal.pone.0243685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles LM, Ruiz JM, & O’Connor M-F (2019). A systematic review of the association between bereavement and biomarkers of immune function. Psychosomatic Medicine, 81(5), 415–433. 10.1097/PSY.0000000000000693 [DOI] [PubMed] [Google Scholar]
- Koban L, Gianaros PJ, Kober H, & Wager TD (2021). The self in context: Brain systems linking mental and physical health. Nature Reviews Neuroscience, 22(5), 309–322. 10.1038/s41583-021-00446-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraynak TE, Marsland AL, Hanson JL, & Gianaros PJ (2019). Retrospectively reported childhood physical abuse, systemic inflammation, and resting corticolimbic connectivity in midlife adults. Brain, Behavior, and Immunity, 82, 203–213. 10.1016/j.bbi.2019.08.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraynak TE, Marsland AL, Wager TD, & Gianaros PJ (2018). Functional neuroanatomy of peripheral inflammatory physiology: A meta-analysis of human neuroimaging studies. Neuroscience & Biobehavioral Reviews, 94, 76–92. 10.1016/j.neubiorev.2018.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan-Greene P, Green S, Nurius PS, & Longhi D (2014). Distinct contributions of adverse childhood experiences and resilience resources: A cohort analysis of adult physical and mental health. Social Work in Health Care, 53(8), 776–797. 10.1080/00981389.2014.944251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez RB, Brown RL, Wu E-LL, Murdock KW, Denny BT, Heijnen C, & Fagundes C (2020). Emotion regulation and immune functioning during grief: Testing the role of expressive suppression and cognitive reappraisal in inflammation among recently bereaved spouses. Psychosomatic Medicine, 82(1), 2–9. 10.1097/psy.0000000000000755 [DOI] [PubMed] [Google Scholar]
- Marsland AL, Prather AA, Petersen KL, Cohen S, & Manuck SB (2008). Antagonistic characteristics are positively associated with inflammatory markers independently of trait negative emotionality. Brain, Behavior, and Immunity, 22(5), 753–761. 10.1016/j.bbi.2007.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Walsh C, Lockwood K, & John-Henderson NA (2017). The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain, Behavior, and Immunity, 64, 208–219. 10.1016/j.bbi.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Hawkley LC, & Cacioppo JT (2006). Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: The Chicago health, aging, and social relations study. Psychosomatic Medicine, 68(3), 376–381. 10.1097/01.psy.0000221371.43607.64 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, & Sheridan MA (2016). Beyond cumulative risk: A dimensional approach to childhood adversity. Current Directions in Psychological Science, 25(4), 239–245. 10.1177/0963721416655883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gabrieli JJD, & Gross JJ (2008). Gender differences in emotion regulation: An fMRI study of cognitive reappraisal. Group Processes & Intergroup Relations, 11(2), 143–162. 10.1177/1368430207088035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min MO, Minnes S, Kim H, & Singer LT (2013). Pathways linking childhood maltreatment and adult physical health. Child Abuse & Neglect, 37(6), 361–373. 10.1016/j.chiabu.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J, Klumparendt A, Doebler P, & Ehring T (2017). Childhood maltreatment and characteristics of adult depression: Meta-analysis. British Journal of Psychiatry, 210(2), 96–104. 10.1192/bjp.bp.115.180752 [DOI] [PubMed] [Google Scholar]
- Osborn M, & Widom CS (2020). Do documented records and retrospective reports of childhood maltreatment similarly predict chronic inflammation? Psychological Medicine, 50(14), 2406–2415. 10.1017/S0033291719002575 [DOI] [PubMed] [Google Scholar]
- Park HS, Park JY, & Yu R (2005). Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6. Diabetes Research and Clinical Practice, 69(1), 29–35. 10.1016/j.diabres.2004.11.007 [DOI] [PubMed] [Google Scholar]
- Pollak S, Cicchetti D, Hornung K, & Reed A (2000). Recognizing emotion infaces: Developmental effects of child abuse and neglect. Developmental Psychology, 36(5), 679–688. 10.1037/0012-1649.36.5.679 [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, & Miller AH (2006). Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends in Immunology, 27(1), 24–31. 10.1016/j.it.2005.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond C, Marin M-F, Juster R-P, & Lupien SJ (2019). Should we suppress or reappraise our stress?: The moderating role of reappraisal on cortisol reactivity and recovery in healthy adults. Anxiety, Stress & Coping, 32(3), 286–297. 10.1080/10615806.2019.1596676 [DOI] [PubMed] [Google Scholar]
- Ruiz AL, & Font SA (2020). Role of childhood maltreatment on weight and weight-related behaviors in adulthood. Health Psychology, 39(11), 986–996. 10.1037/hea0001027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runsten S, Kauniskangas K, Koskenvuo M, Rautava P, Vainio O, & Korkeila J (2013). Can social support alleviate inflammation associated with childhood adversities? Nordic Journal of Psychiatry, 68(2), 137–144. 10.3109/08039488.2013.786133 [DOI] [PubMed] [Google Scholar]
- Shahane AD, Lopez RB, & Denny BT (2019). Implicit reappraisal as an emotional buffer: Reappraisal-related neural activity moderates the relationship between inattention and perceived stress during exposure to negative stimuli. Cognitive, Affective, & Behavioral Neuroscience, 19(2), 355–365. 10.3758/s13415-018-00676-x [DOI] [PubMed] [Google Scholar]
- Tolin DF, & Foa EB (2006). Sex differences in trauma and posttraumatic stress disorder: A quantitative review of 25 years of research. Psychological Bulletin, 132(6), 959–992. 10.1037/0033-2909.132.6.959 [DOI] [PubMed] [Google Scholar]
- Troy AS, Shallcross AJ, & Mauss IB (2013). A person-by-situation approach to emotion regulation: Cognitive reappraisal can either help or hurt, depending on the context. Psychological Science, 24(12), 2505–2514. 10.1177/0956797613496434 [DOI] [PubMed] [Google Scholar]
- Wadsworth ME (2015). Development of maladaptive coping: A functional adaptation to chronic, uncontrollable stress. Child Development Perspectives, 9(2), 96–100. 10.1111/cdep.12112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data and an Rmarkdown file for the analyses presented in the manuscript can be accessed on Open Science Framework (OSF): https://osf.io/t324s/.


