Abstract
Background
Telomere dysfunction can underly the development of idiopathic pulmonary fibrosis (IPF), and recent work suggests that patients with telomere syndromes might benefit from treatment with androgens, such as danazol.
Methods
This was a prospective observational cohort study. 50 patients with IPF received off-label treatment with danazol after they showed progressive disease under treatment with pirfenidone or nintedanib. The primary outcome was the difference in yearly decline in forced vital capacity (FVC) prior to (pre) and after (post) start of treatment with danazol.
Results
There was no significant difference in FVC-decline between 1 year pre and 1 year post start of danazol treatment (mean decline pre 395 mL (95% confidence interval (CI) 290–500) compared to post 461 mL (95% CI 259–712); p=0.46; paired t-test). 11 patients (22%) were still on danazol after 1 year, and 39 patients had stopped danazol, mainly because of side-effects (56%) or death (33%). In patients who were still using danazol after 1 year, FVC-decline significantly slowed down under danazol treatment (mean pre 512 mL (95% CI 308–716) versus post 198 mL (95% CI 16–380); p=0.04). Median survival post danazol was 14.9 months (95% CI 11.0–18.8).
Conclusion
Danazol as a treatment of last resort in patients with IPF did not lead to slowing of lung function decline and was associated with significant side-effects. It remains to be determined if earlier treatment or treatment of specific patient subgroups is beneficial.
Tweetable abstract
In this study, patients with advanced idiopathic pulmonary fibrosis (IPF) treated with danazol did not show slowing of lung function decline and side-effects were common. Standard treatment with danazol should not be offered to patients with advanced IPF. https://bit.ly/3shxssN
Introduction
Idiopathic pulmonary fibrosis (IPF) is a severe lung disease with a median survival of 3 years after diagnosis [1]. Progressive scarring of the lung leads to increasing breathlessness and eventually death. Treatment options include the antifibrotic drugs pirfenidone and nintedanib. Both drugs have been shown to slow disease progression, but do not cure the disease [2, 3]. So, even under antifibrotic treatment patients progress towards advanced fibrosis, which highlights the need for other medication.
Telomere dysfunction plays an important role in the pathogenesis of IPF [4]. Telomeres are repetitive nucleotide sequences at the ends of chromosomes. Telomeres shorten with every cell division, and thus telomeres become increasingly short with age. Critically short telomeres will eventually lead to cellular senescence or apoptosis [5]. Abnormally short telomeres lead to conditions of accelerated ageing, called short telomere syndromes. These conditions include dyskeratosis congenita and some forms of bone marrow failure, as well as IPF [6]. Telomere dysfunction in these patients is the result of a genetic mutation in a gene encoding a component of the telomere maintenance proteins [6].
IPF is the most common manifestation of a short telomere syndrome, [6] and mutations in telomere genes have been found in up to 35% of familial IPF cases and up to 11% of sporadic IPF cases [7, 8]. In addition, it has been found that a substantial proportion of IPF patients in whom no mutation was found has decreased telomere length compared to healthy controls and to patients with other chronic lung diseases [9–11]. In one study, over half of patients with IPF who had no known telomerase mutation did have short lung telomere length, in the same range as patients with a telomerase gene mutation [12].
Clinical studies in patients with aplastic anaemia or dyskeratosis congenita showed that patients with telomere-related gene mutations may benefit from androgen therapy [13–19]. The most extensive study to date reported on the use of danazol in patients with telomere syndromes [20]. Danazol is a synthetic hormone with light androgenic effects. The study included 27 patients, of whom 10 had overt pulmonary fibrosis, and two had a primary diagnosis of IPF. Most of the other patients had a primary diagnosis of aplastic anaemia with secondary pulmonary fibrosis. Patients were treated with oral danazol for 2 years. The main outcome parameter was peripheral blood telomere length, and this significantly increased during danazol treatment. Secondary outcome parameters included follow-up of chest computed tomography (CT) scans, which showed that the pulmonary fibrosis was radiologically stable in all but one patient. Also, the mean lung function was stable during the study period, whereas there was a mean lung function decline prior to the study period [20, 21].
Although these findings are promising, the efficacy of danazol for the treatment of patients with IPF is not yet well known. This study reports on the efficacy of danazol in patients with IPF to whom danazol was offered as an off-label treatment when there was progressive lung function decline despite treatment with pirfenidone or nintedanib.
Methods
Study subjects
Patients with IPF who had disease progression under the current standard-of-care, based on lung function (decline in forced vital capacity (FVC) >5% of predicted) and radiographic testing (worsening of fibrosis on high-resolution CT scan), and who started treatment with danazol at St. Antonius Hospital were included. Subjects were included starting in August 2018 up to July 2022. Per local protocol, patients were not eligible for treatment with danazol in case of severe cardiac, hepatic or renal disease, when they used statins metabolised by CYP3A4 (statins were switched if needed), when they had an androgen-dependent tumour (such as prostate cancer), when they had increased thromboembolic risk without adequate prophylactic measures taken (e.g. anticoagulation use), or when they were pregnant or of childbearing potential and unwilling to take adequate contraceptive measures during danazol treatment. Patients who had concomitant (severe) disease that was deemed likely to result in death within 30 days, or precluded the ability to participate in the study protocol, were also excluded.
All patients underwent clinical screening prior to starting treatment with danazol. This screening included the drawing of blood for laboratory workup (liver function tests, hormone profile, standard renal, electrolyte and other general labs, full blood count with differential count, lipid spectrum, prostate specific antigen (in men)), as well as ultrasound investigation of the liver and reproductive organs and a thrombophilia screening questionnaire. Repeat laboratory testing was done at 2, 4 and 8 weeks, as well as at 3, 6, 9 and 12 months. In the course of regular clinical care, follow-up of lung function was scheduled every 3 months, a chest radiograph was scheduled every 3 or 6 months and a CT scan was scheduled after 12 months.
The starting dose of danazol was 400 mg twice daily. Per local protocol, in case of suspected mild to moderate adverse effects, the dose was reduced in steps of 200 mg (i.e. to 300 mg twice daily, then to 200 mg twice daily, then to 100 mg twice daily). Step-wise dose reduction was continued until side-effects are acceptable. If a patient is unable to tolerate a dose of 100 mg twice daily, danazol was discontinued.
Study design
This was a prospective observational cohort study. Danazol was prescribed off-label to patients with IPF after shared decision making. The study was registered locally under protocol number NL 62034.100.17 and was approved by the local medical ethics committee (MEC-U; study number R17.047). The study was conducted in accordance with the principles of the Declaration of Helsinki. All patients provided written informed consent.
All subjects included in the study were followed up for at least 1 year, regardless of withdrawal from treatment, or until the subject's death or lung transplant. Patients still on treatment after 1 year of follow-up were followed further, while for patients that withdrew from treatment, follow-up ceased 1 year after start of treatment.
Laboratory investigations
Blood samples were taken around time of diagnosis. Details on leukocyte telomere length measurement and genetic analyses are provided in the supplementary material.
Statistical analysis
The main study parameter is difference in yearly decline in lung function between the period prior to danazol treatment and the first year after start of danazol treatment. Secondary outcome parameters are overall survival, radiological qualification of pulmonary fibrosis at baseline compared to during danazol treatment, and adverse events during danazol treatment.
For the primary analysis, yearly FVC-decline was compared between the period prior to therapy and the first year of the study period (i.e. the first year that a patient was receiving danazol) using a paired t-test. In case of missing data, including when patients had died, FVC-decline after 1 year was imputed using linear extrapolation of available lung function measurements after start of danazol treatment. When no pulmonary function testing after the start of danazol was available, FVC-decline after 1 year was imputed using linear extrapolation of the FVC-decline prior to start of danazol.
Secondary analyses included restricting included patients to those in whom lung function measurements were available at 12 months and restricting included patients to those who were still using danazol after 1 year. Secondary end points were compared between the period prior to therapy and the treatment period using paired t-tests, Chi-squared test or Fisher's exact test, where appropriate. For survival analyses, log-rank and Cox regression analyses were used, and patients were censored when follow-up ended, when they underwent lung transplantation, or when they were lost to follow-up. Data analyses were performed using IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, NY, USA). Linear mixed model analysis was done using R Statistics.
Results
The total cohort comprised 50 patients. Baseline characteristics at the time of diagnosis and when starting danazol treatment are provided in table 1. 44 (88%) of the patients were male, 16 (32%) had a first-degree family member with pulmonary fibrosis, 10 (20%) had a family history suggestive of a telomere syndrome and four (8%) had extrapulmonary disease suggestive of a telomere syndrome. All patients had received treatment with either pirfenidone or nintedanib prior to starting treatment with danazol, and 37 patients were still using pirfenidone or nintedanib when they started treatment with danazol. Patients started treatment with danazol a median of 2.44 years (interquartile range (IQR) 1.69–3.32) after the diagnosis of IPF.
TABLE 1.
Baseline characteristics of 50 patients with idiopathic pulmonary fibrosis who started treatment with danazol
| Male sex | 44 (88) |
| Age at diagnosis years | 67.6 (61.5–71.1) |
| Age at start of danazol years | 70.0 (66.2–73.8) |
| First-degree family member with pulmonary fibrosis | 16 (32) |
| Smoking status | |
| Current | 3 (6) |
| Former | 39 (78) |
| Never | 8 (16) |
| FVC % pred at diagnosis | 79 (68–87) |
| FVC % pred at start of danazol | 64 (56–80) |
| D LCO c % pred at diagnosis | 45 (37–51) |
| D LCO c % pred at start of danazol | 32 (25–37) |
| Treatment with antifibrotic agent prior to start of danazol | 50 (100) |
| Nintedanib | 44 (88) |
| Still using nintedanib at start of treatment with danazol | 28 (56) |
| Pirfenidone | 29 (58) |
| Still using pirfenidone at start of treatment with danazol | 9 (18) |
| Leukocyte telomere length# | 43 (86) |
| <10th percentile for age | 22 (51) |
| <1st percentile for age | 8 (19) |
| Whole exome sequencing | 36 (72) |
| Variant of unknown significance in telomere- or pulmonary fibrosis-related gene | 10 (28) |
| Likely pathogenic variant in telomere- or pulmonary fibrosis-related gene | 6 (17) |
| Any clinical suggestion of telomere syndrome | 18 (36) |
| Family history suggestive of telomere syndrome | 10 (20) |
| Extrapulmonary disease suggestive of telomere syndrome | 4 (8) |
| Haematological laboratory abnormalities | |
| Anaemia | 6 (12) |
| Macrocytosis | 9 (18) |
| Thrombocytopenia | 3 (6) |
| Leukopenia | 0 (0) |
Data are presented as n (%) or median (IQR). Percentages for subgroups calculated based upon the total number of patients for whom data were available. With whole exome sequencing, one patient was found to have two variants of unknown significance. Extrapulmonary disease suggestive of a telomere syndrome included liver cirrhosis in two patients, myelodysplastic syndrome in one patient, and otherwise unexplained cytopenia in two patients (one patient had cytopenia and liver cirrhosis). Anaemia, macrocytosis, thrombocytopenia and leukopenia were defined based on local laboratory lower-limit-of-normal levels. Any clinical suggestion of a telomere syndrome was defined as either a clinical history, family history or laboratory abnormality suggestive of a telomere syndrome [22]. FVC: forced vital capacity; DLCOc: diffusion capacity of the lung for carbon monoxide, corrected for haemoglobin level. #: this row indicates in how many patients leukocyte telomere length prior to starting treatment with danazol was available.
Leukocyte telomere length at diagnosis was available for 43 patients (86%) and was below the 10th percentile for age in 22 (51%). Whole exome sequencing, with results filtered for genes related to telomere syndromes or pulmonary fibrosis, was performed in 36 patients (72%) and revealed a likely pathogenic variant in six patients (17%) and a variant of unknown significance in nine patients (25%).
Danazol use
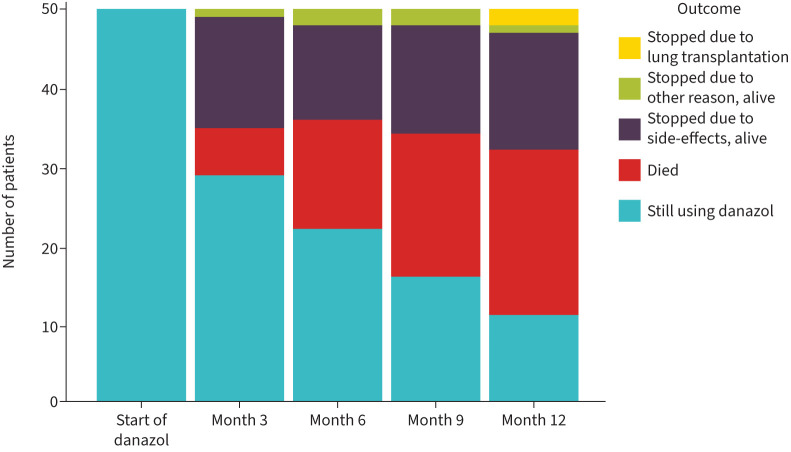
Patients received danazol for a median of 134 days (IQR 48–314 days). 39 patients (78%) stopped danazol prior to having received 1 year of treatment, mainly because of side-effects (56% of patients who stopped) or death (33% of patients who stopped) (figure 1; table 2). Presumed side-effects of danazol treatment were observed in 29 patients (58%) and included elevated liver enzymes, malaise, decreased renal function, myalgia and others (table 3). Dose adjustments were made in 14 patients (28%) and danazol was temporarily discontinued because of side-effects in 10 patients (20%). There was no significant difference in terms of side-effects between patients who had leukocyte telomere length below or above the 10th percentile for age (supplementary table S1), patients with or without any clinical suggestion of a telomere syndrome (supplementary table S2), or patients who were concurrently using antifibrotics or not (supplementary table S3).
FIGURE 1.
Outcomes of 50 patients with idiopathic pulmonary fibrosis treated with danazol, per 3 months. When a patient who had stopped using danazol died, this was reported as the outcome.
TABLE 2.
Duration of danazol use of 50 patients with idiopathic pulmonary fibrosis and reasons for stopping
| Duration of danazol use days, median (IQR) | 134 (48–314) |
| Stopped using danazol <1 year after start, n (%) | 39 (78) |
| Side-effects, n (%) | 22 (56) |
| Deceased, n (%) | 13 (33) |
| Progression of disease, n (%) | 3 (8) |
| Lung transplant, n (%) | 1 (3) |
| Still using danazol >1 year after start, n (%) | 11 (22) |
Percentages for subgroups calculated based upon the total number of patients for whom data were available.
TABLE 3.
Presumed side-effects of danazol in 50 patients with idiopathic pulmonary fibrosis
| Temporarily discontinued because of side-effects, n (%) | 10 (20) |
| Permanently discontinued because of side-effects, n (%) | 24 (48) |
| Dose adjustment made, n (%) | 14 (28) |
| Any side-effects, n (%) | 29 (58) |
| Elevated liver enzymes | 10 |
| Malaise | 6 |
| Decreased renal function | 4 |
| Myalgia | 4 |
| Oedema | 3 |
| Tiredness | 2 |
| Shivering | 2 |
| Stomach pain | 2 |
| Reduced appetite | 2 |
| Weight gain | 2 |
| Headache | 2 |
| Acne | 1 |
Lung function course
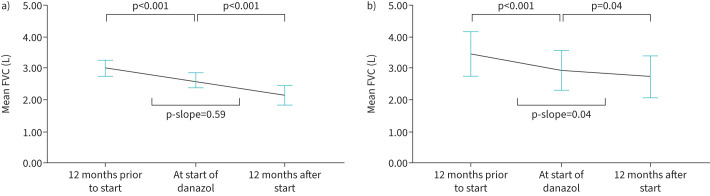
For all patients, the lung function declined significantly in the year prior to starting danazol, with a mean FVC-decline of 395 mL (95% confidence interval (CI) 290–500; p<0.001). In the year after starting danazol, the lung function also declined significantly, with a mean FVC-decline in the year after starting danazol of 485 mL (95% CI 259–712; p<0.0001; figure 2a). Lung function measurements after the start of danazol were available in 31 patients, and lung function decline was imputed from lung function decline prior to danazol in the remainder. There was no significant difference between FVC-decline in the year prior to starting danazol and in the year after starting danazol (p=0.46; paired t-test). In a linear mixed model, including age, sex and FVC at baseline as covariates, FVC-decline under danazol treatment did not significantly differ from FVC-decline prior to danazol treatment (estimated difference 90 mL (95% CI −150–330; p-value 0.462)).
FIGURE 2.
Forced vital capacity (FVC) in patients with IPF prior to, at start and 12 months after treatment with danazol. a) Mean FVC in 50 patients for the primary analysis at 12 months prior to starting danazol, at start of danazol and 12 months after starting danazol. b) Mean FVC in 11 patients who were still using danazol after 1 year. Whiskers represent 95% confidence intervals. p-values represent differences between FVC at two time points, and p-slope represents the difference between rates of decline (paired t-test).
FVC-decline in the year after starting danazol was not significantly different between patients who had leukocyte telomere length below or above the 10th percentile for age (supplementary table S1), or patients with or without any clinical suggestion of a telomere syndrome (supplementary table S2). When restricting the analysis to patients in whom lung function measurements were available at 12 months (n=13), there was no significant difference between FVC-decline in the year prior to starting danazol and in the year after starting danazol (mean pre 399 mL (95% CI 203–594) compared to post 265 mL (95% CI 74–457); p=0.32). For patients who were still using danazol after one year (n=11), the rate of FVC-decline was significantly higher in the year prior to starting danazol than under danazol (mean pre 512 mL (95% CI 308–716) versus post 198 mL (95% CI 16–380); p=0.04; figure 2b).
Radiographic imaging during follow-up was available for 34 patients, including CT scans in 18 patients (3–12 months after starting danazol). CT scans for 12 patients (67%) showed radiographic progression of fibrosis. In addition, radiographic progression of fibrosis was observed on chest radiograph in five patients for whom no CT scan was available.
Survival
1 year after starting treatment with danazol, 21 patients had died (eight of these patients had stopped danazol prior to dying), two patients underwent lung transplantation (one patient had stopped danazol prior to the transplantation) and 23 patients were still followed up (13 of these patients had stopped using danazol)). Patients who were still alive and using danazol after 12 months did not have significantly different baseline characteristics from the other patients (table 4). Median survival after start of treatment with danazol was 14.9 months (95% CI 11.0–18.8); figure 3).
TABLE 4.
Patient characteristics of 11 patients still alive and using danazol after 12 months compared to the rest of the cohort
| Still using danazol at 12 months | Not using danazol at 12 months | p-value | |
| Patients n | 11 | 39 | |
| Male sex, n (%) | 9 (82) | 35 (90) | 0.60 |
| Age at start of danazol years, median (IQR) | 69.7 (65.8–73.2) | 70.1 (66.2–74.0) | 0.55 |
| First-degree family member with pulmonary fibrosis, n (%) | 4 (36) | 12 (31) | 0.73 |
| Time after IPF diagnosis before start danazol years, median (IQR) | 3.6 (2.2–4.8) | 2.3 (1.3–3.0) | 0.06 |
| FVC % pred at start, median (IQR) | 66 (61–85) | 64 (50–79) | 0.29 |
| FVC-decline prior to start mL, mean (95% CI) | 470 (360–590) | 260 (80–600) | 0.14 |
| D LCO c % pred at start, median (IQR) | 37 (28–43) | 32 (24–36) | 0.11 |
| Still using antifibrotic agent, n (%) | 9 (82) | 28 (72) | 0.70 |
| Still using nintedanib at start of treatment with danazol, n (%) | 7 (78) | 21 (75) | 1.00 |
| Still using pirfenidone at start of treatment with danazol, n (%) | 2 (22) | 7 (25) | 1.00 |
| Leukocyte telomere length, n (%)# | 10 (91) | 33 (85) | 1.00 |
| <10th percentile for age, n (%) | 7 (70) | 15 (45) | 0.28 |
| <1st percentile for age, n (%) | 2 (20) | 6 (18) | 1.00 |
| Whole exome sequencing, n (%) | 10 (91) | 26 (67) | 0.15 |
| Variant of unknown significance in telomere- or pulmonary fibrosis-related gene, n (%) | 2 (20) | 8 (31) | 0.69 |
| Likely pathogenic variant in telomere- or pulmonary fibrosis-related gene, n (%) | 1 (10) | 5 (19) | 0.65 |
| Any clinical suggestion of telomere syndrome, n (%) | 3 (27) | 15 (38) | 2 |
| Family history suggestive of telomere syndrome, n (%) | 1 (9) | 9 (23) | 0.42 |
| Extrapulmonary disease suggestive of telomere syndrome, n (%) | 1 (9) | 3 (8) | 1.00 |
Patients not using danazol because they underwent lung transplantation or because they did not yet complete the first year of follow-up were excluded from this analysis. Percentages for subgroups calculated based upon the total number of patients for whom data were available. IPF: idiopathic pulmonary fibrosis; FVC: forced vital capacity; DLCOc: diffusion capacity of the lung for carbon monoxide, corrected for haemoglobin level. #: this row indicates in how many patients leukocyte telomere length prior to starting treatment with danazol was available.
FIGURE 3.
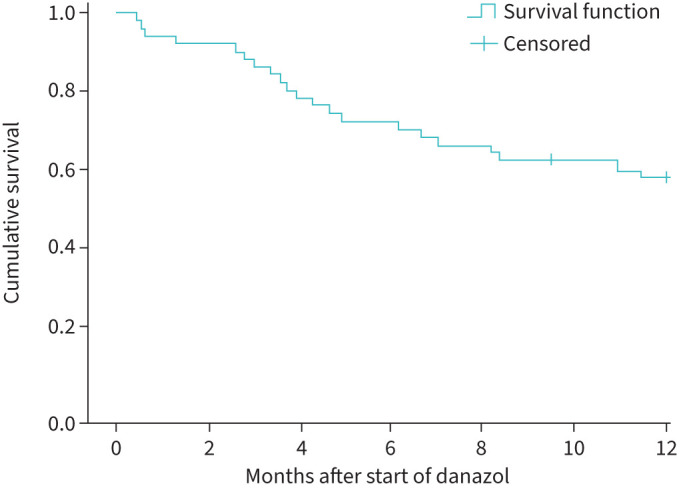
Survival in patients with IPF who were treated with danazol. Subjects were censored when lost to follow-up or when they underwent lung transplantation.
Discussion
In this prospective observational drug study, the use of danazol as a treatment of last resort for patients with IPF did not lead to slowing of lung function decline. Secondary outcomes, including radiographic progression and survival, also did not indicate a beneficial effect of danazol. Notably, the patients included in our study represent a subgroup of patients with an advanced stage of IPF. Prior to the start of danazol, patients showed a greater mean decline in FVC than observed in both the treatment and placebo arms of pirfenidone and nintedanib trials in patients with IPF [2, 3]. Median time after diagnosis and before starting danazol treatment was 2.44 years. After start of danazol, survival in the study cohort was low. It could be speculated that the patients included already had a too advanced stage of IPF, where the therapeutic potential for any treatment might have been limited. Therapeutic potential for telomere-targeting treatments may be higher in earlier stages of the disease. Notably, patients who remained on danazol for ≥1 year appeared to have a better DLCOc percentage of predicted at the start of danazol and showed a significant decrease in the rate of FVC-decline after 1 year of danazol therapy. This suggests that earlier start with danazol may have a positive effect.
Previously, danazol was shown to have a beneficial effect on patients with proven short telomere syndromes [20]. Other than to the advanced stage of IPF, the poor treatment effect might also be related to the fact that not all study participants had proven telomere dysfunction. Patients were not selected for low leukocyte telomere length or telomere-related gene mutations, although about half of the study participants did have leukocyte telomere length <10th percentile for age. When combining signs and symptoms suggestive for telomere-associated pathology, we found no evidence for their influence on outcome of danazol therapy. Albeit not statistically significant, when looking at patients who were still alive and using danazol after 12 months, a nominally higher percentage had leukocyte telomere length <10th percentile for age compared to other participants. Whether treatment with danazol is beneficial in such a specific subgroup will be addressed in the ongoing TELO-SCOPE study [23].
In recent years, the evidence for the role of androgens in the regulation of telomerase activity has increased. Telomere length in different cell lines can be modulated by sex hormones in vitro [24–27]. Oestrogen receptors act directly on the TERT (telomerase gene) promotor, whereas androgens influence expression of telomerase indirectly [26, 28]. Higher circulating levels of testosterone metabolites and oestrogen have been associated with longer leukocyte telomere length in healthy volunteers. Furthermore, a Chinese case–control study found decreased serum testosterone levels in patients with IPF, as well as an association between shorter leukocyte telomere length and lower serum testosterone levels in patients with IPF [29]. A recent UK Biobank study found that IPF was more common in females with earlier menopause and premature ovarian failure, and in men with lower bioavailable testosterone concentrations. Furthermore, lower concentrations of sex hormones were associated with faster disease progression. For both sexes, lower concentrations of sex hormones were associated with shorter leukocyte telomere length [30]. In addition, anecdotal reports on the beneficial effect of androgen treatment in patients with pulmonary fibrosis have been published after the study by Townsley and colleagues [19, 31, 32]. All in all, androgen treatment does still seem to be a theoretically attractive treatment option for patients with IPF.
Presumed side-effects of danazol were common in this study, as was the case in the study by Townsley and colleagues [20]. However, in our study a higher percentage of patients stopped treatment due to side-effects (42% in our study versus 19% in the previous study). Although the side-effect profile seemed similar, we hypothesise that in our study, because danazol was used as a treatment of last resort, side-effects might have been less acceptable to patients and treating physicians. Second, most patients used danazol as an add-on treatment and were already receiving treatment with antifibrotics. Some side-effects of antifibrotics overlap with the side-effects that we found, although concurrent antifibrotic use was not associated with a higher chance of side-effects in this study. We found no difference in lung function decline pre and post start of danazol suggesting that treatment did not progress disease or influence survival in the total group of patients.
Limitations other than those discussed above include the fact that this was not a randomised controlled trial and the absence of longitudinal data on leukocyte telomere length. Furthermore, there was missing data, which means that part of the data on the primary outcome had to be imputed, either based on FVC-decline prior to start of danazol, or based on FVC-decline after 3, 6 or 9 months under danazol. The missing data were partly related to the fact that patients with end-stage IPF did not come to the hospital as frequently and because a significant proportion of the study participants died prior to having completed 1 year follow-up and were therefore not missing at random.
In conclusion, in this study of 50 patients with IPF who showed evidence of disease progression despite treatment with pirfenidone or nintedanib, add-on treatment with danazol did not lead to slowing of lung function decline. Lung function decline pre- and post-treatment was worse than that in previous trials. Side-effects necessitated stopping treatment in 42% of patients. Whether earlier treatment or treatment of specific subgroups of patients with IPF would be beneficial remains to be determined.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00131-2023.SUPPLEMENT (445.4KB, pdf)
Acknowledgements
We thank the patients who participated in this study. We thank J.C. Kelder (St Antonius Hospital Academy) for his advice on statistical analysis.
Provenance: Submitted article, peer reviewed.
Support statement: This research was funded by the St Antonius Research Fund. The funder played no role in study design, data analysis, writing of the manuscript or the decision to submit. Funding information for this article has been deposited with the Crossref Funder Registry.
Author contributions: T.W. Hoffman, C.H.M. van Moorsel and J.C. Grutters planned the study. T.W. Hoffman collected data and performed data analysis. T.W. Hoffman, C.H.M. van Moorsel, J.J. van der Vis, D.H. Biesma and J.C. Grutters contributed to interpretation of the data. T.W. Hoffman wrote the first draft of the manuscript. T.W. Hoffman, C.H.M. van Moorsel, J.J. van der Vis, D.H. Biesma and J.C. Grutters critically reviewed and revised the manuscript. T.W. Hoffman is the guarantor of the overall content.
Data availability statement: The data that support the findings of this study are available from the corresponding author, T.W. Hoffman, upon reasonable request.
Conflict of interest: None declared.
References
- 1.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med 2018; 378: 1811–1823. doi: 10.1056/NEJMra1705751 [DOI] [PubMed] [Google Scholar]
- 2.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. doi: 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- 3.King TE, Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2083–2092. doi: 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- 4.Armanios M. Telomerase and idiopathic pulmonary fibrosis. Mutat Res 2012; 730: 52–58. doi: 10.1016/j.mrfmmm.2011.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Otin C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell 2013; 153: 1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev 2012; 13: 693–704. doi: 10.1038/nrg3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman TW, van Moorsel CHM, Borie R, et al. Pulmonary phenotypes associated with genetic variation in telomere-related genes. Curr Opin Pulm Med 2018; 24: 269–280. doi: 10.1097/MCP.0000000000000475 [DOI] [PubMed] [Google Scholar]
- 8.Borie R, Le Guen P, Ghanem M, et al. The genetics of interstitial lung diseases. Eur Respir Rev 2019; 28: 190053. doi: 10.1183/16000617.0053-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cronkhite JT, Xing C, Raghu G, et al. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med 2008; 178: 729–737. doi: 10.1164/rccm.200804-550OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snetselaar R, van Moorsel CH, Kazemier KM, et al. Telomere length in interstitial lung diseases. Chest 2015; 148: 1011–1018. doi: 10.1378/chest.14-3078 [DOI] [PubMed] [Google Scholar]
- 11.Dai J, Cai H, Li H, et al. Association between telomere length and survival in patients with idiopathic pulmonary fibrosis. Respirology 2015; 20: 947–952. doi: 10.1111/resp.12566 [DOI] [PubMed] [Google Scholar]
- 12.Van Batenburg AA, Kazemier KM, Van Oosterhout MFM, et al. From organ to cell: multi-level telomere length assessment in patients with idiopathic pulmonary fibrosis. PLoS One 2020; 15: e0226785. doi: 10.1371/JOURNAL.PONE.0226785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xin ZT, Beauchamp AD, Calado RT, et al. Functional characterization of natural telomerase mutations found in patients with hematologic disorders. Blood 2007; 109: 524–532. doi: 10.1182/blood-2006-07-035089 [DOI] [PubMed] [Google Scholar]
- 14.Ziegler P, Schrezenmeier H, Akkad J, et al. Telomere elongation and clinical response to androgen treatment in a patient with aplastic anemia and a heterozygous hTERT gene mutation. Ann Hematol 2012; 91: 1115–1120. doi: 10.1007/s00277-012-1454-x [DOI] [PubMed] [Google Scholar]
- 15.Islam A, Rafiq S, Kirwan M, et al. Haematological recovery in dyskeratosis congenita patients treated with danazol. Br J Haematol 2013; 162: 854–856. doi: 10.1111/bjh.12432 [DOI] [PubMed] [Google Scholar]
- 16.Khincha PP, Wentzensen IM, Giri N, et al. Response to androgen therapy in patients with dyskeratosis congenita. Br J Haematol 2014; 165: 349–357. doi: 10.1111/bjh.12748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zlateska B, Ciccolini A, Dror Y. Treatment of dyskeratosis congenita-associated pulmonary fibrosis with danazol. Pediatr Pulmonol 2015; 50: E48–E51. doi: 10.1002/ppul.23235 [DOI] [PubMed] [Google Scholar]
- 18.Kirschner M, Vieri M, Kricheldorf K, et al. Androgen derivatives improve blood counts and elongate telomere length in adult cryptic dyskeratosis congenita. Br J Haematol 2021; 193: 669–673. doi: 10.1111/BJH.16997 [DOI] [PubMed] [Google Scholar]
- 19.Clé DV, Catto LF, Darrigo LG, et al. Telomere elongation and clinical improvement in telomeropathy patients: a prospective clinical trial of nandrolone in telomeropathies. Blood 2019; 134: Suppl. 1, 2501–2501. doi: 10.1182/BLOOD-2019-130844 [DOI] [Google Scholar]
- 20.Townsley DM, Dumitriu B, Liu D, et al. Danazol treatment for telomere diseases. N Engl J Med 2016; 374: 1922–1931. doi: 10.1056/NEJMoa1515319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Townsley DM, Dumitriu B, Young NS. Danazol treatment for telomere diseases. N Engl J Med 2016; 375: 1095–1096. doi: 10.1056/NEJMc1607752 [DOI] [PubMed] [Google Scholar]
- 22.Hoffman TW, van der Vis JJ, Biesma DH, et al. Extrapulmonary manifestations of a telomere syndrome in patients with idiopathic pulmonary fibrosis are associated with decreased survival. Respirology 2022; 27: 959–965. doi: 10.1111/RESP.14264 [DOI] [PubMed] [Google Scholar]
- 23.MacKintosh JA, Pietsch M, Lutzky V, et al. TELO-SCOPE study: a randomised, double-blind, placebo-controlled, phase 2 trial of danazol for short telomere related pulmonary fibrosis. BMJ Open Respir Res 2021; 8: e001127. doi: 10.1136/BMJRESP-2021-001127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo C, Armbruster BN, Price DT, et al. In vivo regulation of hTERT expression and telomerase activity by androgen. J Urol 2003; 170: 615–618. doi: 10.1097/01.ju.0000074653.22766.c8 [DOI] [PubMed] [Google Scholar]
- 25.Bayne S, Liu JP. Hormones and growth factors regulate telomerase activity in ageing and cancer. Mol Cell Endocrinol 2005; 240: 11–22. doi:S0303-7207(05)00222-4 [DOI] [PubMed] [Google Scholar]
- 26.Calado RT, Yewdell WT, Wilkerson KL, et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood 2009; 114: 2236–2243. doi: 10.1182/blood-2008-09-178871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vieri M, Kirschner M, Tometten M, et al. Comparable effects of the androgen derivatives danazol, oxymetholone and nandrolone on telomerase activity in human primary hematopoietic cells from patients with dyskeratosis congenita. Int J Mol Sci 2020; 21: 1–12. doi: 10.3390/IJMS21197196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nanni S, Narducci M, Della Pietra L, et al. Signaling through estrogen receptors modulates telomerase activity in human prostate cancer. J Clin Invest 2002; 110: 219–227. doi: 10.1172/JCI15552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang C, Huang H, Zhang Q, et al. Relation between sex hormones and leucocyte telomere length in men with idiopathic pulmonary fibrosis. Respirology 2020; 25: 1265–1273. doi: 10.1111/RESP.13871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duckworth A, Ruth KS, Prague JK, et al. Study of the associations between short telomeres, sex hormones and pulmonary fibrosis. medRxiv 2022; preprint [ 10.1101/2022.09.29.22280270].doi 10.1101/2022.09.29.22280270]. [DOI] [Google Scholar]
- 31.Chambers DC, Lutzky VP, Apte SH, et al. Successful treatment of telomeropathy-related interstitial lung disease with immunosuppression and danazol. Respirol Case Reports 2020; 8: e00607. doi: 10.1002/RCR2.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dewald J, Attia S, Schriver E, et al. Progression of pulmonary fibrosis in a patient with telomere disease previously treated with danazol. Chest 2017; 152: A430. doi: 10.1016/j.chest.2017.08.457 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00131-2023.SUPPLEMENT (445.4KB, pdf)