Abstract
In the present study the dynamics of antigenemia and coproantigens were studied in patients with Fasciola hepatica infection during an outbreak occurring in La Palma, Pinar del Río, in the West Province of Cuba. Stool and serum samples were collected from 67 patients and 40 healthy subjects. Stool samples were studied by a simple gravity sedimentation technique and an ES78 sandwich enzyme-linked immunosorbent assay (ELISA) for observation of eggs and detection of parasite coproantigens, respectively. Serum samples were also studied by the ES78 sandwich ELISA and an indirect ELISA to detect circulating antigens and antibodies, respectively. At the beginning of the study, 8 of 67 patients had patent infections and 59 had prepatent infections, which was determined by the recent consumption of lettuce contaminated with metacercariae of F. hepatica, the presence of clinical symptoms, and the absence of Fasciola eggs in their stools. Patients with prepatent infections were monitored by all techniques until patency. Circulating antigens were not detected in patients with patent infections. However, coproantigens were clearly detected in all patients with patent infections. On the other hand, 28.8% of patients with prepatent infections tested positive for circulating antigens and 81.4% tested positive for coproantigens in the first stool sample studied. Only two other coproantigen determinations were necessary to diagnose 93.2% of the patients. While circulating antigen levels diminished in all patients during the infection, coproantigen levels increased. The present study demonstrates that the ES78 sandwich ELISA is a better tool than parasitological examination for diagnosis of active early infection, since by the combination of the circulating-antigen detection assay and the coproantigen detection assay 91% of patients were able to be diagnosed at the beginning of the study. In contrast, a coprologic analysis repeated over several weeks was necessary to diagnose 100% of the patients.
Fasciolosis is a disease caused by digenetic trematodes of the genus Fasciola, of which Fasciola hepatica is the most common. It is a cause of economic losses in the animal husbandry industry (11, 17, 18). In comparison with animal infections, human infections are uncommon. However, clinical cases have been reported in more than 40 countries and from all continents. Humans are usually infected by the ingestion of aquatic plants that harbor the infectious metacercariae. The diagnosis of fasciolosis is complicated because parasite eggs are not found during the prepatent period (2, 15) when juvenile worms migrate through the intestinal wall to the peritoneal cavity (at 1 week), penetrate the liver parenchyma (at 5 to 7 weeks), and pass into the biliary tract where they ultimately reach maturity (at 2 months and more). Once the worms have matured, diagnosis still remains difficult, since eggs are frequently excreted at irregular intervals.
We have previously reported the determination of circulating antigens and coproantigens by means of an ES78 sandwich enzyme-linked immunosorbent assay (ELISA) as a good alternative for the diagnosis of chronic F. hepatica infection (6, 9) and the assessment of cure after specific chemotherapy (8, 9). In the present communication, we report the dynamics of both circulating antigens and coproantigens in comparison with the parasitological examination during the study of a human fasciolosis outbreak. The diagnostic values of the antigen detection assays and a conventional antibody assay were compared to that of the simple gravity sedimentation technique as the “gold standard.”
MATERIALS AND METHODS
Patients.
Patients in this study came from La Palma, Pinar del Río, West Cuba. In January 1995 an outbreak of fasciolosis occurred in this community of 8,721 inhabitants, of whom 81 developed clinical symptoms. The epidemiological study performed during the outbreak demonstrated that the individuals were infected by consuming lettuce contaminated with metacercariae of F. hepatica during only a 1-week period, so the actual time of infection could be determined. In addition, as all patients were symptomatic, the incubation period, which ranged from 2 to 13 weeks postinfection (mean, 6 weeks), could also be determined.
After interviews, a total of 67 patients from this outbreak were included in the present study. Healthy subjects from the same community as the patients were also interviewed, and 40 who denied the recent ingestion of water plants and had no history of F. hepatica or other parasite infection were also included in the study. Informed consent was obtained from all participants in this study. Two consecutive stool samples were collected from individuals and processed by microscopical examination using a simple gravity sedimentation technique. Individuals for whom these samples tested negative for F. hepatica eggs were monitored for 3 months at biweekly intervals. Stool suspensions were prepared from stool samples collected and studied by sandwich ELISA to detect coproantigens. A serum sample was also taken from each individual at the time of stool collection and was studied by indirect and sandwich ELISAs to detect antibodies to F. hepatica and circulating antigens, respectively.
For this study, the time between the onset of infection and collection of a specimen was designated the postinfection time (IT). Thus, at the beginning of the study, stool and serum samples were available from patients with ITs ranging from 40 to 240 days postinfection (for 5 patients, 40 to 60 days; for 17 patients, 61 to 80 days; for 37 patients, 81 to 120 days; and for 8 patients, 121 to 240 days).
Simple gravity sedimentation technique.
The simple gravity sedimentation technique performed in the present study was similar to that described by Ash and Orihel (1). Briefly, 10 to 20 g of feces of each patient was thoroughly mixed in 200 ml of tap water. After suspension through two layers of wet gauze, the mixture was transferred to a conical sedimentation flask and allowed to stand for 2 h. The supernatant was then carefully decanted, and the sediment was resuspended by adding more tap water and allowing it to stand for 1 h. This wash procedure was repeated twice until a clarified supernatant was achieved. The sediment was finally transferred to slides and examined for parasite eggs.
Antibody detection ELISA.
The ELISA for the detection of antibodies to F. hepatica ES antigens was performed according to a preestablished protocol (5). Briefly, polystyrene microtiter plates (Maxisorp; Nunc) were coated with ES antigens (40 μg/ml) and, after being washed, were blocked with 5% nonfat dry milk. Serum samples were tested at a dilution of 1:800. After the plates were washed, peroxidase–anti-human immunoglobulin G (IgG) conjugate (Sigma) diluted 1:5,000 was added to the wells. Following incubation, the plates were washed and incubated with the substrate solution. Absorbance was read at 492 nm with a Multiskan Organon ELISA reader. A previously determined cutoff value of 0.38 was used in the present study (5). Serum samples with absorbances higher than this value were considered positive.
Sandwich ELISA.
A sandwich ELISA for detection of circulating ES antigens and coproantigens was performed as previously described, with the monoclonal antibody (MAb) ES78 as the capture antibody and a polyclonal antibody-peroxidase conjugate as the secondary antibody. The production of both MAbs (mouse IgG2a) and polyclonal antibodies (rabbit IgG) directed against ES antigens has been described previously (6). Briefly, polystyrene microtiter plates (Maxisorp; Nunc) were coated with MAb ES78 (5 μg/ml) and, after being washed, were blocked with nonfat dry milk. Serum samples or stool suspensions (prepared by mixing 1 g of stool with 2 ml of tap water) were added undiluted. After the plates were washed, IgG anti-ES antigen–peroxidase conjugate, diluted 1:1,000, was added to the wells. Following incubation, the plates were washed again and incubated with the substrate solution. Absorbance was read as described above. A previously determined cutoff value of 0.24 was used for both circulating antigen and coproantigen determination (6, 9). Sera or stool suspensions with absorbances higher than this value were considered positive.
Statistical methods.
Each antibody or antigen determination was performed in duplicate, and the results were expressed as the mean absorbance for each determination. The coprological technique results served as the “gold standard.” We defined a true-positive result as the presence of F. hepatica eggs in stool samples and a true-negative result as the absence of eggs in stool during the whole study and the lack of clinical symptoms consistent with infection. Antigen and antibody detection assays were evaluated in terms of their sensitivity (percentage of positive results among the total number of coprologically positive individuals) and specificity (percentage of negative results among total number of coprologically negative individuals). To investigate if any association exists between the IT of patients and the positivity of circulating-antigen and coproantigen detection assays, a nonparametric Mann-Whitney U test was used. Wilcoxon’s matched-pair signed-rank test was used to compare the results before and after egg excretion. All statistical procedures were performed by using the STATGRAF System (version 2.1).
RESULTS
When the first stool samples from 67 patients with clinical symptoms of fasciolosis were examined, F. hepatica eggs were found in 8 patients (11.94%), with an IT range of 121 to 240 days (136 ± 45 days) (unless otherwise stated, results are given as means ± standard deviations). The samples from the other 59 patients (88.06%), with ITs of 40 to 120 days (63 ± 26.6 days), tested negative for parasite eggs. Therefore, the latter patients were considered to be in the prepatent period of infection.
Patients with patent infections all tested negative for circulating antigens. However, all tested highly positive for coproantigens in a range of absorbance between 0.54 and 2.9, with a mean value of 1.45 ± 0.84. On the other hand, 53 of 59 patients (89.8%) with prepatent infections tested positive for circulating antigen and/or coproantigen. Only five patients (8.5%) tested positive for circulating antigens, with an absorbance range of 0.33 to 0.72 (0.45 ± 0.13). The ITs for these patients ranged from 40 to 60 days (45 ± 5.6 days). The 12 other patients (20.3%), with ITs of 61 to 80 days (67 ± 4.5 days), tested positive for both circulating antigens and coproantigens, with ranges of absorbance of 0.25 to 0.33 (0.28 ± 0.11) and 0.28 to 1.8 (0.84 ± 0.43), respectively. The 36 other patients (61.0%), with ITs of 81 to 120 (88 ± 18.8), tested positive only for coproantigens in a range of absorbance of 0.3 to 2.9 (1.09 ± 0.47). Six patients with prepatent infections (10.2%) tested negative for circulating antigens and coproantigens in the first samples studied. When serum samples from patients with prepatent and patent infections were studied by an indirect ELISA to detect specific antibodies to F. hepatica ES antigens, all tested positive, with absorbances ranging from 0.66 to 1.88 (1.08 ± 0.38) and 0.51 to 177 (1.38 ± 0.48), respectively.
When the antigen determination results were correlated with the ITs, a highly significant difference was observed between the mean IT of patients with patent infection and the mean IT of those with prepatent infection (Z = 5.5; P < 0.0001). We also found a significant difference between the mean IT of patients who tested positive for circulating antigens only and the mean IT of patients who tested positive for coproantigens only (Z = 5.4; P < 0.0001). The mean IT of patients who at the same time tested positive for circulating antigens and coproantigens was also statistically different from that of patients who tested positive for coproantigens only (Z = 3.57; P < 0.01). In addition, a highly significant difference was found between the mean ITs of patients with prepatent and patent infection who tested positive for coproantigens only (Z = 5.45; P < 0.0001). No healthy subjects tested positive for either circulating antigens or coproantigens. However, when healthy subjects were studied by the antibody assay, 12 (30%) were recorded as positive while the remaining 28 individuals (70%) were recorded as negative, with absorbance values ranging from 0.4 to 0.85 (0.65 ± 0.33) and 0.06 to 0.36 (0.19 ± 0.08), respectively.
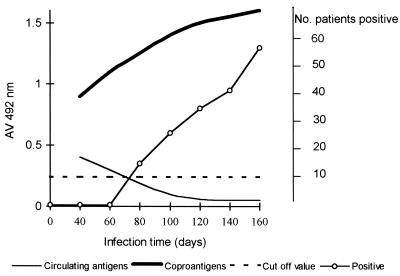
Table 1 shows the follow-up study performed with samples from patients with prepatent infections by means of parasitological examination and the ES78 antigen detection assays. As shown, most patients who initially tested positive for circulating antigens tested negative a few weeks later and remained negative until the end of the study. Parasite eggs were found in all patients, but repeated stool examinations over several weeks were necessary to diagnose 100% of the patients. In contrast, coproantigens were detected in the first stool sample studied for 48 of 59 patients (81.4%) and only two other coproantigen determinations were necessary to diagnose 93.2% of patients. Coproantigen levels increased in each patient, reaching the highest absorbance value when the parasitological examination was positive. After patency, coproantigen levels ranged from 0.48 to 2.9, with a mean value of 1.59 ± 0.72 (Fig. 1). A highly significant difference was found between mean coproantigen levels before and after egg excretion (Z = 5.399; P < 0.0001). Four of six patients who at the beginning of the study tested negative for both circulating antigens and coproantigens remained negative after patency.
TABLE 1.
Follow-up study performed on 59 patients with prepatent F. hepatica infection
| Mean IT ± SD (days) | No. (%) of patients positive:
|
||
|---|---|---|---|
| For circulating antigens | For coproantigens | By stool examination | |
| 63 ± 26.6 | 17 (28.8) | 48 (81.4) | 0 (0) |
| 81 ± 28.5 | 2 (3.4) | 54 (91.5) | 19 (32.2) |
| 96 ± 34.0 | 0 (0) | 55 (93.2) | 28 (47.5) |
| 111 ± 44.4 | 0 (0) | 55 (93.2) | 37 (62.7) |
| 126 ± 52.1 | 0 (0) | 55 (93.2) | 41 (69.5) |
| 141 ± 65.6 | 0 (0) | 55 (93.2) | 59 (100.0) |
FIG. 1.
Dynamics of antigenemia, coproantigens, and parasite eggs in patients with F. hepatica infection. Positive, positive for eggs.
Compared with the results of repeated parasitological examination as the “gold standard,” the sensitivity of a single parasitological examination at the beginning of the study was 11.94% (8 of 67 patients tested positive), with a false-negative rate of 88.06%. At the same time, use of the circulating antigen detection assay combined with the coproantigen assay was able to detect 91% of infected patients (61 of 67 patients, including those with patent and prepatent infection, tested positive). At the end of the study, sensitivity of the antigen detection assays (ascribed to the coproantigen determination) was 94%, with a false-negative rate of 6.0%. Specificity was 100%. When the antibody assay was compared with parasitological examination, a sensitivity of 100% and a specificity of 70% were obtained (Table 2).
TABLE 2.
Sensitivity and specificity of the antigen detection assay (ES78 sandwich ELISA) and the antibody assay (ELISA) at the end of the follow-up study
| Result by assay | Result by parasitological examinationa
|
|
|---|---|---|
| Positive | Negative | |
| Antigen detectionb | ||
| Positive | 63 | 0 |
| Negative | 4 | 40 |
| Antibody detection | ||
| Positive | 67 | 12 |
| Negative | 0 | 28 |
Negative and positive cases are presented as percentages based on the results of a sedimentation technique repeated over 3 months.
Results obtained at the end of the study attributable to the coproantigen assay.
DISCUSSION
Previous studies have estimated that a period of at least 3 to 4 months is necessary for F. hepatica flukes to attain sexual maturity in humans (2, 19). Thus, patients that at the beginning of the present study had ITs of 40 to 120 days and tested negative by parasitological examinations could have been experiencing prepatent infection. This view was supported by the observation that most of these patients tested positive for circulating antigens, coproantigens, or both. In addition, all tested highly positive for antibodies against F. hepatica ES antigens. The finding of parasite eggs in stools of all these patients after repeated parasitological examinations over several weeks verified this conclusion.
It has been demonstrated that evaluation of a single stool specimen is an insensitive means of detecting most gastrointestinal helminths (3, 13). In the present study we demonstrated that this is particularly true for F. hepatica, since it was necessary to perform repeated coprologic analyses over several weeks to diagnose 100% of the patients. However, although a simple sedimentation technique allows the diagnosis of 100% of patients with minimal glassware and reagent requirements, it is time-consuming and, therefore, inappropriate when the study of a large amount of samples is required. In contrast, by a single circulating-antigen determination combined with a coproantigen determination, 91% of patients were able to be diagnosed from the beginning of the study, even when most of them were in the prepatent period of infection. Confirmation that these patients were true positives was obtained during the follow-up study, when parasite eggs were sequentially found in stool samples from all patients. These results demonstrated that the ES78 sandwich ELISA is a better tool than parasitological examination for diagnosis of active early infection and could potentially replace the parasitological examination in acute fasciolosis outbreak studies.
There are only a few studies in which the incubation period of F. hepatica in humans has been accurately determined (12, 16, 17). Fortunately, a determination of the incubation period was possible during the outbreak in the present study, since the exact period of infection could be determined and all patients were symptomatic. This permitted us to calculate the approximate ITs of patients at each time interval studied and consequently to monitor the dynamics of antigenemia and coproantigens during the prepatent infection. Our experimental study with rats (10) and those with mice previously reported by Langley and Hillyer (14) demonstrated that during a primary prepatent infection, antigenemia developed during weeks 1 to 3 postinfection, a time when immature flukes are actively migrating through the liver parenchyma and excreting large amounts of antigens into the bloodstream. After this period, antigenemia decreases and becomes undetectable over the course of infection. In the present study, 17 patients with ITs of 40 to 80 days tested positive for circulating antigens, which reflects a prolonged antigenemia compared to those in the above-mentioned studies. However, the dynamics observed in both studies were similar, since the levels of antigen detected in patient serum diminished and became undetectable early during the infection. Note that five patients with ITs of 60 to 80 days consistently tested negative for circulating antigens. It is possible that the periods of antigenemia experienced by these patients were shorter than those experienced by others. Unfortunately, no patients with ITs of <40 days were included in the study, and consequently the complete antigenemia curve could not be studied and the earliest time when mean circulating antigens become negative in humans was not able to be determined. On the other hand, the low levels of circulating antigens detected in patients suggest that they were included in the study at the end of the antigenemia period (Fig. 1). As clinical symptoms of fasciolosis are nonpathognomonic or absent (2), patients seldom seek medical attention early during the prepatent period of infection. For this reason, the probability of detecting infected patients by means of circulating-antigen determination is extremely low, which could be a typical behavior for a primary fasciolosis in humans.
The results for the detection of circulating antigens obtained in the present study differ from those previously reported in humans with chronic infection (6). In the former study (6) all the patients had a history of eating contaminated raw vegetables over the course of several months before diagnosis. A possible explanation for this discrepancy is that these patients were reinfected and the antigenemia was a consequence of the overlapping of subsequent prepatent phase onto the primary patent infection.
In contrast to antigenemia, most patients with ITs of >60 days showed detectable levels of coproantigens which increased during the infection. The dynamics observed for coproantigens are also consistent with those previously observed in our experimental study with rats (10). The observed increase in the coproantigen levels during the course of infection may be proportional to the sexual maturity process of the parasite in the bile ducts. The observation that coproantigens were detected in most patients several weeks before patency is consistent with our own previous studies (9) and those of Youssef et al. (20), in which coproantigens were detected in patients with acute fasciolosis several weeks before Fasciola eggs could be observed. Taking into account that levels of coproantigens are less affected by immunocomplex formation than circulating antigens and that coproantigens are detectable during both phases of infection, its detection could be the most feasible procedure for diagnosing active acute and chronic infection.
Another observation in the present study is that four patients with repeated coproantigen-negative results tested positive for parasite eggs in the last stool examination. Only one Fasciola egg was observed in the stools of each of these patients, while more eggs were observed in stools of the coproantigen-positive patients. As parasite eggs were not counted in the present study, the possible relationship between coproantigens and the egg count was not investigated. However, other studies have demonstrated that coproantigens are correlated with the Fasciola egg count (9) and the parasite burden (4). Therefore, the absence of coproantigens in these patients may be due to a very light infection and the consequently undetectable levels of antigens in stools.
We also found that antibody levels to F. hepatica ES antigens remain uniformly high in all infected individuals during prepatent and patent phases. In addition, other authors have observed that antibodies to parasites are still high for many years (13). Although the demonstration of antibodies in sera provides indirect proof of infection, the results must be interpreted with caution, since our study demonstrated that 30% of healthy subjects were antibody positive. It is unlikely that cross-reactivity with parasites other than F. hepatica influenced these results, because specificity to the F. hepatica ES antigens used has been extensively proven in previous studies (5, 7). As all patients and healthy subjects in the present study live in the same area of endemicity, the probability of their eating contaminated vegetables or drinking contaminated water may be high. Therefore, the antibody levels detected in the healthy persons may reflect a past infection.
In conclusion, this is the first comprehensive study using ES78 sandwich ELISA to describe the dynamics of circulating antigens and coproantigens during the prepatent phase of F. hepatica infection in humans. The results reported herein suggest that antigenemia and coproantigen levels follow dynamics similar to those previously described for a primary infection in rats. Circulating antigens become detectable early during the acute phase, while coproantigens are detectable several weeks before egg excretion and during patency. The ES78 sandwich ELISA for detection of coproantigens permitted us finally to diagnose 94% of the patients, while a repeated stool examination over several weeks was necessary to diagnose 100% of the patients. In addition, both the circulating-antigen assay and the coproantigen detection assay have an important advantage over antibody detection for diagnosis, in that the presence of antigens implies active infection. Thus, the circulating-antigen and coproantigen assays could be a simple, fast, and accurate diagnosis tool for human fasciolosis.
ACKNOWLEDGMENTS
We thank M. Celestino from the local Hospital of La Palma, Pinar del Río, Cuba, for his help in collecting stool and serum samples from patients included in the present study. We are also grateful to Oscar Otero and Domingo Galvez for technical assistance and to Ana Valdes-Portela for reviewing the manuscript.
REFERENCES
- 1.Ash L R, Orihel T C. Parasites: a guide to laboratory procedures and identification. Chicago, Ill: Society of Clinical Pathologists Press; 1987. pp. 30–31. [Google Scholar]
- 2.Chen M G, Mott K E. Progress in assessment of morbidity due to Fasciola hepatica infection. A review of recent literature. Trop Dis Bull. 1990;8:2–38. [Google Scholar]
- 3.De Vlas S, Gryseels B. Underestimation of Schistosoma mansoni prevalences. Parasitol Today. 1992;8:274–277. doi: 10.1016/0169-4758(92)90144-q. [DOI] [PubMed] [Google Scholar]
- 4.Duménigo B E, Espino A M, Finlay C M. Detection of Fasciola hepatica antigens in cattle faeces by a monoclonal antibody-based sandwich immnunoassay. Res Vet Sci. 1996;60:278–279. doi: 10.1016/s0034-5288(96)90055-7. [DOI] [PubMed] [Google Scholar]
- 5.Espino A M, Duménigo B E, Fernández R, Finlay C M. Immunodiagnosis of human fascioliasis by enzyme-linked immunosorbent assay using excretory secretory products. Am J Trop Med Hyg. 1987;37:605–608. doi: 10.4269/ajtmh.1987.37.605. [DOI] [PubMed] [Google Scholar]
- 6.Espino A M, Marcet R, Finlay C M. Detection of circulating excretory secretory antigens in human fascioliasis by sandwich enzyme-linked immunosorbent assay. J Clin Microbiol. 1990;28:2637–2640. doi: 10.1128/jcm.28.12.2637-2640.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espino A M, Seuret N, Finlay C M, Duménlgo B E. Antigenos de Fasciola hepatica. Su utilidad en el diagnóstico de la fascioliasis humana. Rev Cub Med Trop. 1991;43:151–155. [PubMed] [Google Scholar]
- 8.Espino A M, Millan J C, Finlay C M. Detection of antibodies and circulating excretory secretory antigens for assessing cure of patients with fascioliasis. Trans R Soc Trop Med Hyg. 1992;86:649. doi: 10.1016/0035-9203(92)90174-b. [DOI] [PubMed] [Google Scholar]
- 9.Espino A M, Finlay C M. Sandwich enzyme-linked immunosorbent assay for detection of excretory secretory antigens in humans with fascioliasis. J Clin Microbiol. 1994;32:190–193. doi: 10.1128/jcm.32.1.190-193.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espino A M, Marcet R, Finlay C M. Fasciola hepatica: detection of antigenemia and coproantigens in experimentally infected rats. Exp Parasitol. 1997;85:117–120. doi: 10.1006/expr.1996.4112. [DOI] [PubMed] [Google Scholar]
- 11.Froyd G. Liver fluke in Great Britain: a survey of affected livers. Vet Rec. 1975;97:492–495. [PubMed] [Google Scholar]
- 12.Hardman E W, Jones R L H, Davies A H. Fascioliasis. A large outbreak. Br Med J. 1970;3:502–505. doi: 10.1136/bmj.3.5721.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hillyer G V, Soler de Galanes J, Rodríguez J, Bjorland J, Silva de Lagrava M, Ramírez Guzman S, Bryan R T. Use of the Falcon assay screening test-enzyme-linked immunosorbent assay (FAST ELISA) and the enzyme-linked immunotransfer blot (EITB) to determine the prevalence of human fascioliasis in the Bolivian Altiplano. Am J Trop Med Hyg. 1992;46:603–609. doi: 10.4269/ajtmh.1992.46.603. [DOI] [PubMed] [Google Scholar]
- 14.Langley R J, Hillyer G V. Detection of circulating parasite antigen in murine fascioliasis by two-site enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1989;41:472–478. doi: 10.4269/ajtmh.1989.41.472. [DOI] [PubMed] [Google Scholar]
- 15.Levine D M, Hillyer G V, Flores S I. Comparison of counterelectrophoresis, the enzyme-linked immunosorbent assay and kato faecal examination for the diagnosis of fascioliasis in infected mice and rabbits. Am J Trop Med Hyg. 1980;29:602–608. doi: 10.4269/ajtmh.1980.29.602. [DOI] [PubMed] [Google Scholar]
- 16.Ragab M, Farag H F. On human fascioliasis in Egypt. J Egypt Med Assoc. 1978;61:773–780. [PubMed] [Google Scholar]
- 17.Rimbault C. Une épidémie de distomatose dans une communauté rurale de Haute-Loire. Thèse de doctorat en médecine. Clermont-Ferrand, France: Faculté de Médecine, Université de Clermont-Ferrand I; 1981. [Google Scholar]
- 18.Sexton J L, Miner A R, Campbell N J. Fasciola hepatica: immunoprecipitation analysis of biosynthetically labelled antigen using sera from infected sheep. Parasite Immunol. 1991;13:105–108. doi: 10.1111/j.1365-3024.1991.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 19.Wei D X. Fasciola and fascioliasis. Chin J Vet Med. 1984;9:16–18. [Google Scholar]
- 20.Youssef F G, Mansour N S, Azis A E. Early diagnosis of human fascioliasis by the detection of coproantigens using counterimmunoelectrophoresis. Trans R Soc Trop Med Hyg. 1991;85:383–384. doi: 10.1016/0035-9203(91)90300-n. [DOI] [PubMed] [Google Scholar]