Abstract
Kidney damage due to ischemia or rejection results in the accumulation of unfolded and misfolded proteins in the endoplasmic reticulum (ER) lumen, a condition known as “ER stress”. Inositol-requiring enzyme 1α (IRE1α), the first ER stress sensor found, is a type I transmembrane protein with kinase and endoribonuclease activity. Upon activation, IRE1α non-conventionally splices an intron from unspliced X-box binding protein 1 (XBP1u) mRNA to produce XBP1s mRNA that encodes the transcription factor, XBP1s, for the expression of genes encoding proteins that mediate the unfolded protein response (UPR). The UPR promotes the functional fidelity of ER and is required for secretory cells to sustain protein folding and secretory capability. Prolonged ER stress can lead to apoptosis, which may result in detrimental repercussions to organ health and has been implicated in the pathogenesis and progression of kidney diseases. The IRE1α-XBP1 signaling acts as a major arm of UPR and is involved in regulating autophagy, cell differentiation, and cell death. IRE1α also interacts with Activator Protein-1 (AP-1) and Nuclear Factor-κB (NF-κB) pathways to regulate inflammatory responses. Studies using transgenic mouse models highlight that the roles of IRE1α differ depending on cell type and disease setting. This review covers these cell-specific roles of IRE1α signaling and the potential for therapeutic targeting of this pathway in the context of ischemia and rejection affecting the kidneys.
Keywords: Endoplasmic reticulum, Acute kidney injury, Transplantation, Cell signaling, Cell survival
INTRODUCTION
Acute kidney injury (AKI) and chronic kidney diseases (CKD) continue to be major global healthcare burdens with poor clinical outcomes. CKD can progress over time and eventually lead to end-stage renal disease (ESRD), which requires dialysis or kidney transplantation as treatment. AKI is mainly resulted from ischemic kidney injury due to various causes such as sepsis, vascular lesions, multi-organ failure, or transplant surgery, and can lead to CKD. Despite ongoing research into the intricate cellular and molecular mechanisms involved, recent research has implicated the endoplasmic reticulum (ER) stress response network, known as the unfolded protein response (UPR) pathway, to be one of the cell malfunction mechanisms underlying various kidney ischemic diseases and allograft rejection post-transplantation.1
Ischemic insults can both directly and indirectly interfere with ER function via crosstalk among cell organelles (e.g. decreased mitochondrial adenosine triphosphate production), leading to the accumulation of unfolded and misfolded proteins in the ER lumen and the activation of downstream UPR.2 The UPR regulates several aspects of ER function, including protein synthesis, folding, and degradation, and determines cell fate by two instinct pathways ― adaptive pro-survival and pro-apoptotic pathways. During AKI, the pro-survival UPR pathway helps to restore ER homeostasis to promote cell survival and improves tissue repair. However, uncontrolled AKI leads to prolonged ER stress that can activate the pro-apoptotic pathway, leading to cell death and exacerbated inflammation.3 In eukaryotes, the UPR pathways are mediated by three stress sensors, including inositol-requiring enzyme 1 (IRE1), protein kinase RNA-like endoplasmic reticulum kinase (PERK), and activating transcription factor 6 (ATF6). Under basal conditions, these pathways are suppressed by chaperones, such as binding immunoglobulin protein (BiP, also known as glucose-regulated protein 78, GRP78); under ER stress, unfolded proteins cause BiP to dissociate from the transmembrane stress sensors and activate their distinctive, yet interconnected downstream pathways. Among the three ER stress sensors, IRE1 is the most conservative arm and has two isoforms — IRE1α (present in most cell types) and IRE1β (expressed by intestinal epithelial cells and airway mucous cells).3 Global IRE1α deletion causes embryonic lethality in mice, underscoring the importance of IRE1α in maintaining mammalian physiology.4 Recent research using conditional knockout or knockin mouse models has revealed that the activities and functions of IRE1α signaling vary among different cell types and are context-dependent (Table 1).5–19 This mini-review covers cell-specific roles of IRE1α and its downstream signaling, as well as the therapeutic potential of targeting this pathway in kidney ischemic injury and transplant rejection.
Table 1.
Mechanistic insights gained from mice with kidney and immune cell specific-deficiency or overexpression of IRE1α or XBP1.
| Mice | Key findings | References |
|---|---|---|
| Podocytes | ||
| PodocinCre IRE1αfl/fl | Male mice with podocyte-specific IRE1α KO show podocyte injury, albuminuria, disrupted glomerular capillary integrity, and impaired autophagy during aging. | 2017 Kaufman et al. 8 |
| PodocinCre IRE1αfl/fl | Mice with podocyte-specific IRE1α KO show exacerbated albuminuria and podocyte injury in adriamycin nephrosis, perturbated proteostasis, and ultrastructural and metabolic change in mitochondria. | 2020 Navarro-Betancourt et al.9 |
| PodocinCre XBP1fl/fl | Mice with podocyte-specific XBP1 KO show hyperglycemia-induced ER stress and aggravating diabetic nephropathy. | 2015 Madhusudhan etal.10 |
| PodocinCre XBP1fl/fl | Mice with podocyte-specific XBP1 KO show no histologic evidence of glomerular injury at baseline. | 2016 Hassan et al.11 |
| PodocinCre XBP1fl/fl Sec63fl/fl | Sec63 ablation induces ER stress genetically; mice with podocyte-specific XBP1 and Sec63 double KO show progressive albuminuria and glomerular apoptosis. | 2016 Hassan et al.11 |
| Tubular epithelial cells | ||
| Six2Cre XBP1fl/fl | Tubular cell-specific XBP1 KO mice have no phenotype at baseline but show decreased kidney injury and inflammation during LPS or sepsis-induced AKI. | 2019 Ferrè et al.5 |
| Ksp/rtTA TRE/XBP1s | Tubular cell-specific XBP1s overexpression causes tubule dilation and vacuolation, decline in kidney function, and 50% mortality in five days. | 2019 Ferrè et al.5 |
| Pkhd1Cre IRE1afl/fl | Collecting duct cell-specific IRE1α KO show no kidney injury or inflammatory phenotype at baseline. | 2019 Ishikawa et al.6 |
| Pkhd1Cre XBP1fl/fl | Collecting duct cell-specific XBP1 KO show no kidney injury or inflammatory phenotype at baseline. | 2019 Ishikawa et al.6 |
| Pkhd1Cre IRE1αfl/fl Sec63fl/fl | In the presence of ER stress, collecting duct cell-specific IRE1α KO show progressive interstitial inflammation, fibrosis, and decline in kidney function. | 2019 Ishikawa et al.6 |
| Pkhd1Cre XBP1fl/fl Sec63fl/fl | Similar to phenotypes in Pkhd1Cre IRE1αfl/fl Sec63fl/fl mice. | 2019 Ishikawa et al.6 |
| Pkhd1Cre IRE1afl/fl Xbp1fl/flSec63fl/fl | Similar to phenotypes in Pkhd1Cre IRE1αfl/fl Sec63fl/fl mice. | 2019 Ishikawa et al.6 |
| Slc5aCre-ERT2 XBP1fl/fl | Proximal tubular XBP1 KO exacerbates kidney fibrosis after IRI. | 2022 Chen et al.7 |
| T cells | ||
| CD4Cre XBP1fl/fl | CD4 T cells lacking XBP1 show normal T cell development and functions at baseline and improved influx of glutamine required for T cell mitochondrial respiration under glucose-deprived conditions. | 2018 Song et al.12 |
| CD4Cre IRE1αfl/fl | Similar findings in CD4cre XBP1fl/fl mice. | 2018 Song et al.12 |
| LckCre IRE1αfl/fl | T cell-specific IRE1α KO show normal T cell development and activation but reduced IL-4 mRNA stability and IL-4 protein production. | 2013 Kemp et al.13 |
| B cells | ||
| CD19Cre XBP1fl/fl | B cell-specific XBP1 KO limits plasma cell differentiation but antigen-specific memory B cell development is not affected. | 2009 Todd et al.14 |
| CD19Cre IRE1fl/fl | IRE1α is required for optimal antibody production but not isotype switching. | 2014 Benhammn et al.15 |
| Myeloid cells | ||
| LysMCre IRE1afl/fl | Myeloid IRE1α KO decreases pro-inflammatory cytokines induced by TLR activation. | 2013 Qiu et al.16 |
| LysMCre IRE1αfl/fl | Myeloid IRE1α KO promotes alternative activation of macrophages in the context of obesity. | 2017 Shan et al.17 |
| MRP8Cre IRE1αfl/fl | Neutrophil-specific IRE1α KO decreases mitoROS, IL-1β, and NETs production. | 2021 Abuaita et al.18 |
| CD11cCre XBP1fl/fl | DC-specific XBP1 deficiency improves antigen-presenting functions of DCs and inhibits tumor growth by promoting anti-tumor T cells. | 2015 Cubillos-Ruiz et al.19 |
Note: KO, knockout; TLR, Toll-like receptor; mitoROS, mitochondrial reactive oxygen species; NETs, neutrophil extracellular traps.
IRE1α SIGNALING NETWORK
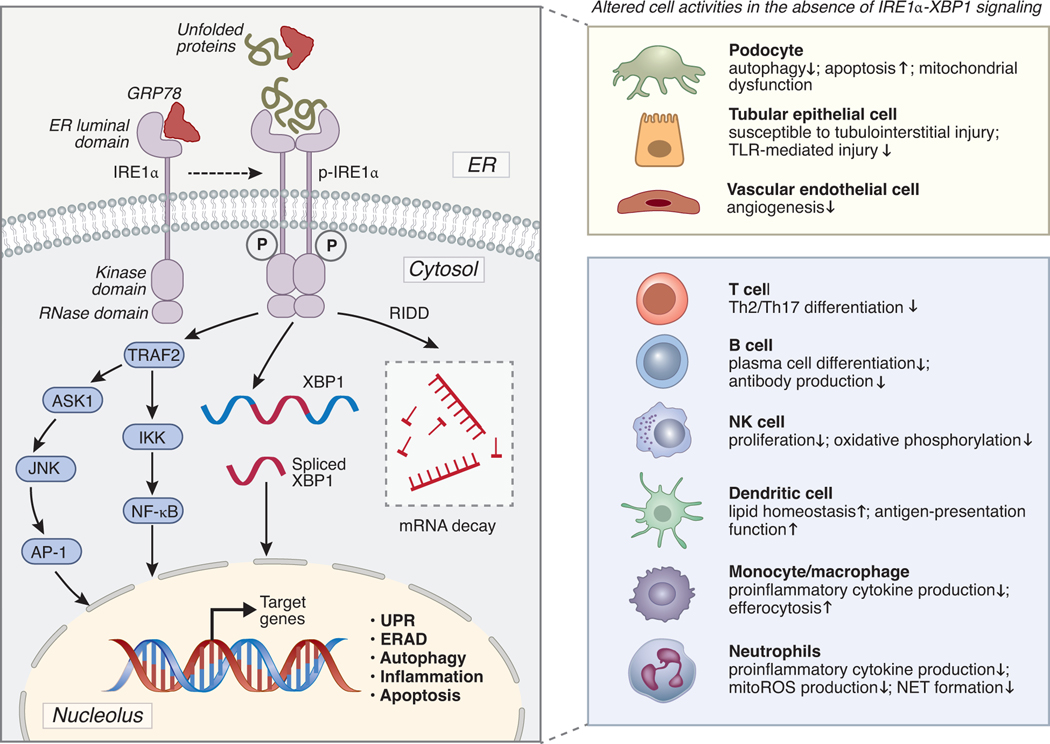
IRE1α is a type I transmembrane protein kinase with three domains: an ER luminal domain that detects protein-folding status, a cytosolic endoribonuclease (RNase) domain, and a cytosolic serine/threonine kinase domain. As reviewed by Karagöz et al.20, during ER stress and upon sensing unfolded proteins, IRE1α oligomerizes and undergoes trans-phosphorylation, thereby activating the RNase domain and downstream pathways. The activation of UPR is primarily distinguished by two mechanisms: (i) IRE1α initiates splicing of mRNA encoding X-box binding protein-1 (XBP1) that results in activation of its active XBP1s form, a widely encompassing transcription factor, and (ii) IRE1α executes a process known as regulated IRE1α-dependent decay (RIDD), which selectively degrades ER-bound mRNAs. When exposed to prolonged ER stress, IRE1α decreases its RNase activity in favor of a mechanism that controls apoptotic response pathways to determine cell fate. In addition to mediating the UPR, IRE1α also facilitates activation of other pathways. For instance, activated IRE1α combines with the adaptor protein tumor necrosis factor receptor-associated factor 2 (TRAF2) to form the complex IRE1α-TRAF2. This complex joins forces with the apoptosis signal-regulated kinase 1 (ASK1) and c-Jun N terminal kinase (JNK), which activates autophagy and inflammatory pathways involving the NF-κB transcription factor.21 The IRE1α signaling network and its cell-specific roles are summarized in Figure 1. The crosstalk between IRE1α and ATF6 or IRE1α and PERK pathways of the UPR has also been documented and well-reviewed elsewhere.18, 22
Figure 1. Schematic representation of IRE1α signaling network and its function in different kidney parenchymal cells and immune cells.
Under basal conditions, IRE1α is suppressed by ER-resident chaperones (e.g., GRP78). Under stressful conditions, unfolded proteins cause GRP78 to dissociate from the transmembrane stress sensor IRE1α, and the unfolded protein response leads to IRE1α oligomerization and phosphorylation, thereby IRE1α is activated and its RNase domain is stimulated. Induced RNase activity cleaves unspliced XBP1 mRNA, and the splicing of XBP1 leads to the production of XBP1s, which is a powerful transcription factor that can upregulate the expression of a broad variety of genes involved in stress adaption and cell survival. IRE1α RNase activity also contributes to mRNA and miRNA degradation through a mechanism known as regulated IRE1α-dependent decay (RIDD). Phosphorylated IRE1α interacts with TRAF2 to form the IRE1α-TRAF2 complex. This complex further interacts with ASK1 and JNK to activate AP-1 signaling. IRE1α-TRAF2 can also activate the NF-κB-mediated inflammatory pathway via interacting with IKK. The activation of different downstream pathways of IRE1α varies in different cell types and is context-dependent. The right panel of the figure summarized the functions of IRE1α in different cell activities. This figure was prepared using BioRender.com. GRP78, glucose-regulated protein 78; ER, endoplasmic reticulum; TRAF2, tumor necrosis factor receptor-associated factor; ASK1, apoptosis signal-regulated kinase 1; JNK, c-Jun N terminal kinase; AP-1, activator protein-1; IKK, the inhibitor of κB kinase; ERAD, ER-associated protein degradation; UPR, unfolded protein response; TLR, Toll-like receptor; mitoROS, mitochondrial reactive oxygen species; NET, neutrophil extracellular traps.
CELL-SPECIFIC ROLES OF IRE1α
IRE1α in podocytes
Podocytes are terminally differentiated cells essential for maintaining the permselectivity properties of the glomerular filter. IRE1α is required for podocyte homeostasis and maintenance.8, 9, 11 Studies have shown that aged male mice with podocyte-specific IRE1α deletion spontaneously develop age-dependent podocyte injury, autophagy impairment, mitochondrial ultrastructural and metabolic changes, and are susceptible to anti-glomerular basement membrane nephritis and adriamycin nephrosis.8, 9 Kaufman et al. further demonstrated that IRE1α is indispensable for the optimal formation of autophagosomes and that the mechanisms likely involve IRE1α-mediated upregulation of autophagy-related genes.8 IRE1α signaling in podocytes seems to involve both XBP1-dependent and independent pathways. XBP1 deletion showed no evidence of podocyte and glomerular abnormality at baseline,11 but caused increased podocyte apoptosis and glomerular injury in presence of ER stress induced either by hyperglycemia or removing Sec63, a heat shock protein-40 chaperone for proper protein folding.10, 11
IRE1α in tubular epithelial cells (TECs)
TECs do not require IRE1α or XBP1 for their maintenance, as mice with TEC-specific IRE1α or XBP1 deficiency do not exhibit renal injury at baseline.5, 6 However, IRE1α-XBP1 signaling is crucial for protein homeostasis (proteostasis) of TECs in pathological conditions.6, 7 In collecting duct cells, simultaneously deleting Sec63 with XBP1 and/or IRE1α results in chronic kidney interstitial inflammation and fibrosis that could be rescued by addition of XBP1s transgene in Sec63-IRE1α dual inactivation mice, indicating the critical role of XBP1s in modulating collecting duct cell proteostasis in response to ER stress.6 Similar observations were reported by Chen et al. showing that deletion of XBP1 in proximal TECs caused cell cycle arrest by downregulating Trap1, thereby enhancing kidney fibrosis in mice following kidney ischemia-reperfusion injury (IRI).7 However, another study by Ferrè et al. demonstrated that while the overexpression of XBP1s led to increased expression of UPR effector genes, such as Bip and C/EBP homologous protein (CHOP), and resulted in acute tubular necrosis (ATN), TEC-specific deletion of XBP1 was protective against lipopolysaccharide (LPS) or sepsis-induced AKI.5 Collectively, data from these studies support that IRE1α-XBP1 signaling in TECs can play opposing roles depending on different stages of injury.
IRE1α in vascular endothelial cells (EC)
Few studies have explored the roles of IRE1α in renal vascular endothelium; the activation status of endothelial IRE1α in kidney diseases remains unknown. Studies in cardiovascular disease models show that disturbed blood flow, angiotensin II activation, and oxidative stress could activate UPR signaling in ECs and persistent ER stress causes endothelial dysfunction.23 In ischemic tissues, knockdown of IRE1α or XBP1 inhibits the proliferation of ECs, and mice with EC-specific XBP1 deficiency show impaired angiogenesis.24 Sustained activation of XBP1, on the other hand, may cause EC apoptosis and activate the autophagy pathway via transcriptional control of Beclin-1.25 It is therefore reasonable to postulate that integrity and regeneration of kidney vascular endothelium require functional IRE1α-XBP1 signaling.
IRE1α in T and B lymphocytes
Adaptive immunity is mediated by both T cells and B cells. The impact of the IRE1α-XBP1 axis on T cells seems limited as this pathway is dispensable for the development and survival of T cells.12, 13 Studies on mice with T cell-specific IRE1α deficiency showed no changes in Th1, regulatory T cells (Tregs), and CD8 T cell populations in the thymus and spleen, but there was a reduction in Th2 differentiation. Mice lacking XBP1 in T cells showed a decrease in Th17 cell differentiation.13, 26 The significance of T cell IRE1α in kidney diseases remains to be explored.
Conversely, the IRE1α-XBP1 axis critically regulates B cell differentiation, and its activation is required for the terminal differentiation of B cells to plasma cells.27 Subsequent studies found both IRE1α kinase and RNase catalytic activities were required for XBP1 splicing and activation.28 Consequently, mice lacking IRE1α in B cells produce limited immunoglobulins upon B-cell receptor activation.14, 15, 27
IRE1α in myeloid cells
Myeloid cells, i.e. neutrophils, monocytes, macrophages, and dendritic cells (DCs), are major effectors in mediating innate immunity. Studies have shown that signaling through Toll-like receptor (TLR) pathways on myeloid cells activates IRE1α by catalyzing its ubiquitination, and the activation of IRE1α signaling facilitates the production of inflammatory cytokines such as IL-1β, tumor necrosis factor-α (TNF-α), and IL-6, which may exacerbate kidney injury.16, 18 Moreover, neutrophils secret reactive oxygen species (ROS) and extracellular traps (NETs) that promote tissue inflammation. Monocytes/macrophages also lead an extensive phagocytic system in clearing apoptotic cells (efferocytosis) and other cell debris to promote the resolution of kidney injury and inflammation. Recent studies suggest that IRE1α also regulates macrophage efferocytosis and polarization.17, 29, 30 Thus, it is worthwhile to investigate if IRE1α inhibition could improve macrophage-mediated tissue repair during ischemic kidney injury.
DCs are professional antigen-presenting cells critical for antigen recognition in both innate and adaptive immune responses. Studies show that DC-specific XBP1 deficiency improves lipid metabolism and antigen-presenting function in ovarian cancer models;19 however, a recent paper in preprint shows that in melanoma tumor models, IRE1α endonuclease was required for amplification of proinflammatory cytokine production and was necessary for efficient cross-presentation of melanoma-associated antigens. In addition, deficiency of IRE1α and XBP1 in DCs leads to decreased frequencies of effector T cells and accumulation of exhausted T-cell immunoglobulin and mucin domain 3 (TIM3)-positive CD8 T cells.31
POTENTIAL ROLE OF IRE1α IN KIDNEY ISCHEMIC INJURY AND TRANSPLANT REJECTION
IRE1α in kidney ischemic injury
Kidney ischemic injury is characterized by ATN, infiltration of inflammatory immune cells, and deterioration of renal function, and can lead to fibrosis if unresolved. Upregulation of IRE1α along with its downstream genes, such as XBP1s and CHOP, was observed in kidneys following ischemia in mouse AKI models.5, 7 With current understanding regarding cell-specific roles of IRE1α, one can speculate that IRE1α signaling in renal parenchymal cells versus immune cells can differentially contribute to the development of kidney ischemic injury. First, IRE1α-mediated UPR likely causes increased apoptosis of TECs and ATN.5 Second, while podocytes are less susceptible to ischemic injury compared to tubular cells and endothelial cells, extended ischemia causes podocyte effacement through the dissociation of slit diaphragm proteins, leading to fibrosis in the long term.32 In the acute phase, IRE1α signaling may play a cytoprotective role in maintaining podocyte integrity and proteostasis through crosstalk with the autophagy and mitochondrial pathways.9 Lastly, IRE1α activation likely promotes functions of neutrophils and monocytes/macrophages by 1) increasing the formation of ROS and NETs, 2) influencing macrophage differentiation via the production of proinflammatory cytokines such as IL-1β, IL-6, and TNF via NF-κB, and 3) inhibiting efferocytosis and regeneration of TECs, thus exacerbating AKI.
In the transplant setting, IRI occurs inevitably during transplant surgical procedures. Severe IRI can lead to delayed graft function (DGF), which is a well-recognized risk factor for acute and chronic kidney graft loss. During IRI, danger-associated molecular patterns (DAMPs) and pro-inflammatory cytokines released by injured tubular cells and endothelial cells activate the TLR signaling in kidney-resident macrophages/dendritic cells. Our group has previously reported that in mouse models of kidney transplantation with extended IRI, increased expressions of ER stress genes, such as IRE1α, XBP1s, and CHOP, were linked to early allograft injury, while donor kidney deficiency of Myd88-Trif signaling decreased ER stress genes and ameliorated kidney transplant IRI.33 Our data suggest that activation of IRE1α by innate immune receptors can result in upregulation of pro-apoptotic pathways in TECs and proinflammatory responses of myeloid cells, leading to exacerbated DGF. However, little is known regarding the role of podocyte-derived IRE1α in transplant IRI. Accelerated podocyte detachment has been observed in the early stage, which is linked to poor allograft outcomes.34 Given the aforementioned protective role of IRE1α in maintaining podocyte integrity, it is conceivable that dysregulation of podocyte IRE1α expression may contribute to progressive detachment of podocytes, driving long-term graft loss.
IRE1α in kidney transplant rejection
Kidney transplant rejection involves adaptive immune responses that occur days or weeks after transplantation and is primarily mediated by the host T and B cells in response to human leukocyte antigens (HLAs) in the donor kidney. With advances in immunomodulation therapies, T cell-mediated rejection has been well controlled. However, B cells and antibody-mediated rejection (AMR) remains a major barrier to long-term allograft survival. AMR can be more severe than cellular rejection and more difficult to treat, often not responding to typical immunosuppressive protocols.35 Additionally, monocytes/macrophages have been shown to be important modulators of the adaptive immune response, augmenting AMR. Our group found that IRE1α deficiency in B cells and myeloid cells also ameliorates antibody-mediated rejection and chronic allograft failure in mouse kidney transplant models (manuscript in preparation). Sun et al. have shown that XBP1 deletion in bone marrow-derived DCs results in immunosuppressive phenotypes, while treatment with these cells could prevent cardiac allograft rejection in mice, suggesting a regulatory role of DC-IRE1α in the transplant setting.36 With all considered, we propose that IRE1α influences kidney transplant rejection, AMR particularly through the following mechanisms: 1) sustaining donor-specific antibody production by promoting B cell differentiation to plasma cells via XBP1 activation; 2) augmenting macrophage activation/differentiation and cytokine production via NF-κB; 3) influencing antigen presentation by DCs; 4) adversely influencing TEC regeneration, and 5) contributing to progressive detachment of podocytes, driving long-term graft loss. The precise mechanisms of its action demand further investigations.
POTENTIAL THERAPEUTICS TARGETING IRE1α
Most IRE1α inhibitors (reviewed in Raymundo et al.37) have been developed towards the distinct cytosolic kinase and endoribonuclease enzymatic activity of IRE1α. RNase-specific IRE1α inhibitors, including Toyocamycin, STF-083010, 4μ8c, MKC-3946, OICR573, OICR464, and MKC-8866, were found to suppress cell proliferation and synergize with chemotherapy drugs. Blockade of XBP1 splicing by inhibition of IRE1α endoribonuclease domain38 or its kinase domain39 significantly inhibited growth of multiple myeloma cells and attenuated subcutaneous or orthometastatic growth of multiple myeloma in mice, respectively. Kinase-specific IRE1α inhibitors include Trierixin and Quino-trierixin. Particularly, ORIN1001 (a selective IRE1 RNase inhibitor) is now being tested in patients with idiopathic pulmonary fibrosis (Identifier: NCT04643769) and advanced solid tumors and relapsed refractory metastatic breast cancer (Identifier: NCT03950570). Targeting IRE1α using these inhibitors as therapeutic strategies holds promise for treatment of kidney ischemic injury and rejection and warrants further investigation.
However, targeting IRE1α could be a doubled-edged sword, as it may impair parenchymal cell regeneration while suppressing inflammation. Therefore, strategies need to be fine-tuned to minimize off-target effects. For instance, Feldman et al. reported that partial antagonists of IRE1α RNase (PAIRs) intermediately displace the helix αC in the IRE1α kinase domain, preserving XBP1 mRNA splicing while quelling destructive ER mRNA endonucleolytic decay, making it a promising drug candidate for fine-tuning the UPR pathway.40 Moreover, short-term application, local delivery, or site-specific delivery of IRE1α inhibitors may help avoid off-target impacts. In the transplant setting, suppressing IRE1α signaling in donor organs (e.g., pre-conditioning with perfusions) may be an effective approach. Other approaches such as chemical chaperones or autophagy modulators could be considered as an alternative.
CONCLUSION
The development and progression of kidney ischemic disease and kidney transplant rejection are largely driven by both UPR pathways and inflammatory responses. IRE1α is a key transducer that activates XBP1, JNK, and NF-κB, orchestrating the complex adaptive responses from UPR to inflammation. Targeting IRE1α holds promise for the treatment of kidney injury and allograft rejection. However, precautions should be taken when designing therapeutic strategy, considering that the roles of IRE1α differ depending on cell type and disease setting.
ACKNOWLEDGMENT
We thank Dr. Bima J. Hasjim, MD, Dr. Xinqiang Lai, MD PhD, Dr. Simon Cleary, PhD, and Charlie Lin, Jing Light Han, and Luke Andrew VanOsdol for careful review and edits for this manuscript. This work is supported by NIH 1P01AI112522–01A1, McCormick Foundation, and CTC Transplant Innovation Endowment Award.
Footnotes
CONFLICT OF INTEREST
All the authors declared no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cybulsky AV. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat Rev Nephrol 2017; 13: 681–696. [DOI] [PubMed] [Google Scholar]
- 2.Inoue T, Maekawa H, Inagi R. Organelle crosstalk in the kidney. Kidney Int 2019; 95: 1318–1325. [DOI] [PubMed] [Google Scholar]
- 3.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 2011; 334: 1081–1086. [DOI] [PubMed] [Google Scholar]
- 4.Iwawaki T, Akai R, Yamanaka S, et al. Function of IRE1 alpha in the placenta is essential for placental development and embryonic viability. Proc Natl Acad Sci U S A 2009; 106: 16657–16662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferre S, Deng Y, Huen SC, et al. Renal tubular cell spliced X-box binding protein 1 (Xbp1s) has a unique role in sepsis-induced acute kidney injury and inflammation. Kidney Int 2019; 96: 1359–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishikawa Y, Fedeles S, Marlier A, et al. Spliced XBP1 Rescues Renal Interstitial Inflammation Due to Loss of Sec63 in Collecting Ducts. J Am Soc Nephrol 2019; 30: 443459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen JH, Wu CH, Jheng JR, et al. The down-regulation of XBP1, an unfolded protein response effector, promotes acute kidney injury to chronic kidney disease transition. J Biomed Sci 2022; 29: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufman DR, Papillon J, Larose L, et al. Deletion of inositol-requiring enzyme-1alpha in podocytes disrupts glomerular capillary integrity and autophagy. Mol Biol Cell 2017; 28: 1636–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navarro-Betancourt JR, Papillon J, Guillemette J, et al. Role of IRE1alpha in podocyte proteostasis and mitochondrial health. Cell Death Discov 2020; 6: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madhusudhan T, Wang H, Dong W, et al. Defective podocyte insulin signalling through p85-XBP1 promotes ATF6-dependent maladaptive ER-stress response in diabetic nephropathy. Nat Commun 2015; 6: 6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan H, Tian X, Inoue K, et al. Essential Role of X-Box Binding Protein-1 during Endoplasmic Reticulum Stress in Podocytes. J Am Soc Nephrol 2016; 27: 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song M, Sandoval TA, Chae CS, et al. IRE1alpha-XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature 2018; 562: 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp KL, Lin Z, Zhao F, et al. The serine-threonine kinase inositol-requiring enzyme 1alpha (IRE1alpha) promotes IL-4 production in T helper cells. J Biol Chem 2013; 288: 33272–33282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Todd DJ, McHeyzer-Williams LJ, Kowal C, et al. XBP1 governs late events in plasma cell differentiation and is not required for antigen-specific memory B cell development. J Exp Med 2009; 206: 2151–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benhamron S, Hadar R, Iwawaky T, et al. Regulated IRE1-dependent decay participates in curtailing immunoglobulin secretion from plasma cells. Eur J Immunol 2014; 44: 867–876. [DOI] [PubMed] [Google Scholar]
- 16.Qiu Q, Zheng Z, Chang L, et al. Toll-like receptor-mediated IRE1alpha activation as a therapeutic target for inflammatory arthritis. EMBO J 2013; 32: 2477–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shan B, Wang X, Wu Y, et al. The metabolic ER stress sensor IRE1alpha suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat Immunol 2017; 18: 519–529. [DOI] [PubMed] [Google Scholar]
- 18.Abuaita BH, Sule GJ, Schultz TL, et al. The IRE1alpha Stress Signaling Axis Is a Key Regulator of Neutrophil Antimicrobial Effector Function. J Immunol 2021; 207: 210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, et al. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell 2015; 161: 1527–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karagoz GE, Acosta-Alvear D, Walter P. The Unfolded Protein Response: Detecting and Responding to Fluctuations in the Protein-Folding Capacity of the Endoplasmic Reticulum. Cold Spring Harb Perspect Biol 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishitoh H, Matsuzawa A, Tobiume K, et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev 2002; 16: 1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siwecka N, Rozpedek-Kaminska W, Wawrzynkiewicz A, et al. The Structure, Activation and Signaling of IRE1 and Its Role in Determining Cell Fate. Biomedicines 2021; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Battson ML, Lee DM, Gentile CL. Endoplasmic reticulum stress and the development of endothelial dysfunction. Am J Physiol Heart Circ Physiol 2017; 312: H355–H367. [DOI] [PubMed] [Google Scholar]
- 24.Zeng L, Xiao Q, Chen M, et al. Vascular endothelial cell growth-activated XBP1 splicing in endothelial cells is crucial for angiogenesis. Circulation 2013; 127: 1712–1722. [DOI] [PubMed] [Google Scholar]
- 25.Margariti A, Li H, Chen T, et al. XBP1 mRNA splicing triggers an autophagic response in endothelial cells through BECLIN-1 transcriptional activation. J Biol Chem 2013; 288: 859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brucklacher-Waldert V, Ferreira C, Stebegg M, et al. Cellular Stress in the Context of an Inflammatory Environment Supports TGF-beta-Independent T Helper-17 Differentiation. Cell Rep 2017; 19: 2357–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reimold AM, Iwakoshi NN, Manis J, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature 2001; 412: 300–307. [DOI] [PubMed] [Google Scholar]
- 28.Zhang K, Wong HN, Song B, et al. The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. J Clin Invest 2005; 115: 268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batista A, Rodvold JJ, Xian S, et al. IRE1alpha regulates macrophage polarization, PD-L1 expression, and tumor survival. PLoS Biol 2020; 18: e3000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yildirim Z, Baboo S, Hamid SM, et al. Intercepting IRE1 kinase-FMRP signaling prevents atherosclerosis progression. EMBO Mol Med 2022; 14: e15344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flores-Santibañez F, Rennen S, Fernandez D, et al. Nuanced role for dendritic cell intrinsic IRE1 RNase in the regulation of anti-tumor adaptive immunity. 2022: 2022.2007.2020.500838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner MC, Rhodes G, Wang E, et al. Ischemic injury to kidney induces glomerular podocyte effacement and dissociation of slit diaphragm proteins Neph1 and ZO-1. J Biol Chem 2008; 283: 35579–35589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu L, Lai X, Wang JJ, et al. Kidney-intrinsic factors determine the severity of ischemia/reperfusion injury in a mouse model of delayed graft function. Kidney Int 2020; 98: 1489–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naik AS, Afshinnia F, Aqeel J, et al. Accelerated podocyte detachment early after kidney transplantation is related to long-term allograft loss of function. Nephrol Dial Transplant 2019; 34: 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schinstock CA, Mannon RB, Budde K, et al. Recommended Treatment for Antibody-mediated Rejection After Kidney Transplantation: The 2019 Expert Consensus From the Transplantion Society Working Group. Transplantation 2020; 104: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun K, Fan C, Zhang J, et al. Prevention of alloimmune rejection using XBP1-deleted bone marrow-derived dendritic cells in heart transplantation. J Heart Lung Transplant 2022; 41: 1660–1671. [DOI] [PubMed] [Google Scholar]
- 37.Raymundo DP, Doultsinos D, Guillory X, et al. Pharmacological Targeting of IRE1 in Cancer. Trends Cancer 2020; 6: 1018–1030. [DOI] [PubMed] [Google Scholar]
- 38.Mimura N, Fulciniti M, Gorgun G, et al. Blockade of XBP1 splicing by inhibition of IRE1alpha is a promising therapeutic option in multiple myeloma. Blood 2012; 119: 5772–5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harnoss JM, Le Thomas A, Shemorry A, et al. Disruption of IRE1alpha through its kinase domain attenuates multiple myeloma. Proc Natl Acad Sci U S A 2019; 116: 16420–16429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldman HC, Ghosh R, Auyeung VC, et al. ATP-competitive partial antagonists of the IRE1alpha RNase segregate outputs of the UPR. Nat Chem Biol 2021; 17: 1148–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]