Abstract
Characterization of 49 rotavirus-positive stool specimens from children with diarrhea in the state of Rio de Janeiro, Brazil, in 1996 and 1997 revealed a great diversity of rotavirus G types. Conventional types G1 and G3 accounted for 27 and 12% of the infections, respectively, whereas 60% of the infections were caused by unconventional types G5 (25%), G10 (16%), and G8 (4%) and mixed G types (16%).
Rotavirus is the major cause of acute diarrhea in children worldwide and an important cause of infantile death in the developing world (12). The virus belongs to the Reoviridae family and possesses a genome of 11 double-stranded RNA segments. Group A rotavirus is classified into G and P types on the basis of the specificity of the VP7 and VP4 proteins, respectively, presented on the outer shell of the virus. Among the 14 rotavirus G serotypes already described, 10 have been recovered from humans. Serotypes G1 to G4 have been the most widespread types and are consequently the targets for development of vaccines; serotypes G5, G6, G8 to G10, and G12 have been less commonly found (11). Serotype G5 has been recovered mostly from pigs and less frequently from horses. However, the G5 serotype was recently described as an important pathogen of humans in Brazil (7, 9). Serotypes G6, G8, and G10 are major pathogens in cattle and have been sporadically recovered from humans (1, 4, 14, 15, 17). The present study is a follow-up of an epidemiological survey performed in Brazil (9, 16). We report here the results of a survey in two cities of the state of Rio de Janeiro, Brazil, in which rotavirus strains bearing type G8 and G10 specificities were detected. To our knowledge, this is the first report of such rotavirus types in South America among either humans or animals.
Forty-nine rotavirus-positive stool specimens from children under 5 years of age with acute diarrhea were collected between March 1996 and December 1997 from three laboratories in the city of Rio de Janeiro and from one laboratory in the neighboring city of Niterói. The four centers attend patients with different educational and socioeconomical backgrounds who live in neighborhoods with distinct levels of sanitation. The samples were initially analyzed by polyacrylamide gel electrophoresis and/or latex agglutination for the presence of rotavirus, and the rotavirus-positive specimens were further characterized by a PCR-based typing assay for identification of the G types (5, 6).
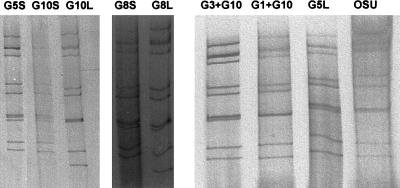
The distribution of the rotavirus G types detected in this study is shown in Table 1. As in previous studies in Brazil, no marked seasonality was observed, with rotavirus diarrhea cases being detected year round (9, 16). Rotavirus type G1, which is the most common serotype recovered from humans worldwide, was also the most prevalent in this survey, while type G3 was detected at a lower frequency. The other common human rotavirus types, G2 and G4, were not found. Type G5 was the second most prevalent of the serotypes found. Interestingly, we found one G5 isolate that exhibited a short electropherotype, different from the electropherotypes of other human serotype G5 isolates described thus far (Fig. 1) (7, 9). Even though mixed infections were detected in 16% of the specimens by reverse transcription-PCR assay, none of those samples exhibited a number of RNA bands in the electrophoresis profile that would suggest mixed infection (i.e., more than 11), as shown for two specimens in Fig. 1.
TABLE 1.
Distribution of rotavirus G types in the cities of Rio de Janeiro and Niterói, RJ, Brazil, in 1996 and 1997
| Year | No. of samples belonging to type:
|
Total | |||||
|---|---|---|---|---|---|---|---|
| G1 | G3 | G5 | G8 | G10 | Mixed G | ||
| 1996 | 4 | 4 | 9 | 0 | 0 | 4a | 21 |
| 1997 | 9 | 2 | 3 | 2 | 8 | 4b | 28 |
| Total (%) | 13 (27) | 6 (12) | 12 (25) | 2 (4) | 8 (16) | 8 (16) | 49 (100) |
One each G1 plus G5 and G5 plus G8; two G1 plus G3 plus G5.
One each of the following: G1 plus G5, G1 plus G10, G3 plus G10, and G8 plus G10.
FIG. 1.
Polyacrylamide gel electrophoresis of eight stool specimens, with their respective G serotypes indicated, and the OSU strain. S, short electropherotype; L, long electropherotype.
Surprisingly, 15 rotavirus isolates (30.0%) from 14 specimens were identified as type G8 or G10. Those types have not been described previously in Brazil among either humans or animals. This finding added two new types to the already complex pool of rotavirus strains that had been found to circulate in Brazil (9). Rotavirus serotype G8 was first described as an agent of human diarrhea in Indonesia (10). However, the frequency of detection of this serotype as a human pathogen is still very low. Since the first description of three rotavirus strains in Indonesia with a super-short electropherotype bearing G8 specificity (10), very few reports of such a serotype have been published. One European survey detected six G8 isolates in Finland and one in Italy, all of them exhibiting long electropherotypes (4). Another study, in Nigeria, detected one type G8 isolate with a short electropherotype (1). Among animals, rotavirus type G8 has been detected in calves and, on at least one occasion, in pigs (6, 15). The animal isolates showed long electropherotypes. In Brazil, rotavirus type G8 was first recognized in a mixed infection with a G5 strain in 1996. In 1997 it was detected in two single infections and in one mixed infection with a G10 strain. One of the single isolates exhibited a short, but not super-short, electropherotype, while all of the others exhibited long electropherotypes (Fig. 1). Those specimens were provided by three different centers in the city of Rio de Janeiro.
Rotavirus serotype G10 has been detected frequently among cattle and occasionally among horses, pigs, and lambs (11, 14, 15). It has also been detected among humans on a few occasions. A survey in India detected rotavirus type G10 in asymptomatical infected children (3). In Thailand, type G10 rotavirus was detected in a single child with diarrhea (17). Furthermore, in a seroepidemiological survey in Ecuador, 8% of sera from children who underwent natural rotavirus exposure neutralized a serotype G10 strain (2). We report herein the emergence of rotavirus G10 in Brazil. Contrary to the previous studies, rotavirus serotype G10 was detected in a high percentage (39%; eight single infections and three mixed infections) of the Brazilian specimens obtained between April and September 1997, all of which were from outpatients with moderate to severe diarrhea. The diarrheal cases were apparently sporadic. One of the isolates exhibited a short electropherotype, while the others possessed long electropherotypes (Fig. 1), which reinforces the hypothesis that the infections were caused by different variants of type G10 rotavirus. The G10 isolates were detected among specimens collected from three different centers in two cities, Rio de Janeiro and Niterói. One of those centers is located in a wealthy area of the city of Rio de Janeiro, whereas the other two centers are located in middle-class areas of Rio de Janeiro and Niterói. No serotype G10 isolate was detected in the fourth center, which attends a poor population of a slum in Rio de Janeiro. During the same period, infections with rotavirus serotypes G1, G3, and G5 were also detected in those centers. These findings seem to indicate that rotavirus type G10 is already widespread among the populations of the two largest cities in the state of Rio de Janeiro. Further studies will show whether rotavirus type G10 will become a prevalent serotype among Brazilians, as happened with type G5 rotaviruses (9).
Evidence for animal-human rotavirus transmission has been reported on several occasions (3, 16). In Brazil, previous studies have revealed an enormous number of mixed infections with rotaviruses bearing a wide range of G serotype specificities which are commonly found in humans or animals (9, 16). Together, these events may facilitate the emergence of rotavirus reassortants (8). In this survey, all five different rotavirus G types detected (G1, G3, G5, G8, and G10) were found in mixed infections. One of the serotype G10 isolates exhibited a unique short electropherotype. It is possible that this isolate is the product of a reassortment between a type G10 animal strain and a human strain, since short electropherotypes have been almost exclusively found in human strains. However, further studies are needed to verify this hypothesis.
This report emphasizes some important aspects of the diversity of rotavirus strains in Brazil: it reports the presence of rotavirus types G8 and G10 in the country and their association with diarrhea in children; it describes the dissemination of those rotavirus types in the populations of two cities in the state of Rio de Janeiro; and it confirms the previous observation of a high frequency of mixed infections, a fact that should facilitate the occurrence of natural reassortment and, therefore, the amplification of rotavirus diversity (8).
The rotavirus typing studies performed in Brazil have demonstrated an extremely high diversity and complexity of G serotypes (9, 16). Nevertheless, a number of isolates have been left untyped, suggesting the possibility that they belong to unconventional serotypes. Indeed, isolates with such unusual serotypes were found in the present study. These findings may explain, in part, the low level of protection achieved in a vaccine trial in Brazil (13). Because the rotavirus vaccine candidates currently being tested possess antigens for only types G1 to G4, these data raise a critical concern about the efficacy of such vaccines in situations similar to that in Brazil. They may not offer protection against type G5, which has been shown to be an epidemiologically important serotype in Brazil (9, 16), or against type G10, which appears to be emerging in Rio de Janeiro and may become an established serotype in the Brazilian population. Furthermore, these data reinforce the need for a continuous laboratory surveillance of rotavirus types in order to identify the most common ones circulating in different populations and to detect the introduction of new rotavirus types.
Acknowledgments
We thank Luíz Otoni, Giovani C. V. Costa, and Maria Odete O. Carvalho for supplying the stool samples used in this study.
This work was partially supported by CNPq, FINEP, FUJB, and FAPERJ, Brazil, and the TWAS, Italy.
REFERENCES
- 1.Adah M I, Rohwedder A, Olaleyly O D, Werchau H. Nigerian rotavirus serotype G8 could not be typed by PCR due to nucleotide mutation at the 3′ end of the primer binding site. Arch Virol. 1997;142:1881–1887. doi: 10.1007/s007050050206. [DOI] [PubMed] [Google Scholar]
- 2.Brüssow H, Offit P A, Sidoti J. Neutralizing antibodies to heterologous animal rotavirus serotypes 5, 6, 7, and 10 in sera from Ecuadorian children. J Clin Microbiol. 1991;29:869–873. doi: 10.1128/jcm.29.5.869-873.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunn S J, Greenberg H B, Ward R L, Nakagomi O, Burns J W, Vo P T, Pax K A, Das M, Gowda K, Rao C D. Serotypic and genotypic characterization of human serotype 10 rotavirus from asymptomatic neonates. J Clin Microbiol. 1993;31:165–169. doi: 10.1128/jcm.31.1.165-169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerna G, Sarasini A, Zentilin L, DiMatteo A, Miranda P, Parea A, Battaglia M, Milanese G. Isolation in Europe of 69M-like (serotype 8) human rotavirus strains with either subgroup I or II specificity and a long RNA electropherotype. Arch Virol. 1990;112:27–40. doi: 10.1007/BF01348983. [DOI] [PubMed] [Google Scholar]
- 5.Gouvea V, Glass R I, Woods P, Taniguchi K, Clark H F, Forrester B, Fang Z-Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gouvea V, Santos N, Timenetsky M D C. Identification of bovine and porcine rotavirus G types by PCR. J Clin Microbiol. 1994;32:1338–1340. doi: 10.1128/jcm.32.5.1338-1340.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gouvea V, de Castro L, Timenetsky M D C, Greenberg H, Santos N. Rotavirus serotype G5 associated with diarrhea in Brazilian children. J Clin Microbiol. 1994;32:1408–1409. doi: 10.1128/jcm.32.5.1408-1409.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gouvea V, Brantly M. Is rotavirus a population of reassortants? Trend Microbiol. 1995;3:159–162. doi: 10.1016/s0966-842x(00)88908-8. [DOI] [PubMed] [Google Scholar]
- 9.Gouvea V, Santos N. Molecular epidemiology of rotavirus in Brazil: a model for the tropics? Virus Res Rev. 1997;2:15–24. [Google Scholar]
- 10.Hasegawa A, Inouye S, Matsuno S, Yamaoka K, Eko R, Suharyono W. Isolation of human rotavirus with a distinct RNA electrophoretic pattern from Indonesia. Microbiol Immunol. 1984;6:719–722. doi: 10.1111/j.1348-0421.1984.tb00726.x. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino, Y., and A. Z. Kapikian. 1996. Classification of rotavirus VP4 and VP7 serotypes. Arch. Virol. 12(Suppl.):99–111. [DOI] [PubMed]
- 12.Kapikian A Z, Chanock R M. Rotaviruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1657–1708. [Google Scholar]
- 13.Linhares A C, Gabbay Y B, Mascarenhas J D P, de Freitas R B, Oliveira C S, Bellesi N, Monteiro T A F, Lainson Z L, Ramos F L P, Valente S A. Immunogenicity, safety and efficacy of tetravalent rhesus-human, reassortant rotavirus vaccine in Bélem, Brazil. Bull W H O. 1996;74:491–500. [PMC free article] [PubMed] [Google Scholar]
- 14.Pongsuwanna Y, Taniguchi K, Chiwakul M, Urasawa T, Wakasugi F, Jayavasu C, Urasawa S. Serological and genomic characterization of porcine rotaviruses in Thailand: detection of a G10 porcine rotavirus. J Clin Microbiol. 1996;34:1050–1057. doi: 10.1128/jcm.34.5.1050-1057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taniguchi K, Urasawa T, Pongsuwanna Y, Choonthanom M, Jayavasu C, Urasawa S. Molecular and antigenic analyses of serotypes 8 and 10 of bovine rotavirus in Thailand. J Gen Virol. 1991;72:2929–2937. doi: 10.1099/0022-1317-72-12-2929. [DOI] [PubMed] [Google Scholar]
- 16.Timenetsky M D C S T, Santos N, Gouvea V. Survey of rotavirus G and P types associated with human gastroenteritis in São Paulo, Brazil, from 1986 to 1992. J Clin Microbiol. 1994;32:2622–2624. doi: 10.1128/jcm.32.10.2622-2624.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urasawa S, Hasegawa A, Urasawa T, Taniguchi K, Wakasugi F, Suzuki H, Inouye I, Pongprot B, Supawadee J, Suprasert S, Rangsiyanons P, Tonusin S, Yamazi Y. Antigenic and genetic analyses of human rotavirus in Chiang Mai, Thailand: evidence for a close relationship between human and animal rotavirus. J Infect Dis. 1992;166:227–234. doi: 10.1093/infdis/166.2.227. [DOI] [PubMed] [Google Scholar]