Abstract
The intersection of big data and artificial intelligence (AI) has resulted in advances in numerous areas, including machine learning, computer vision, and natural language processing. Although there are many potentially transformative applications of AI in health care, including precision medicine, this industry has been slow to adopt these technologies. At the same time, the operations of health care have historically been system-directed and physician-directed rather than patient-centered. The application of AI to patient-reported outcome measures (PROMs), which provide insight into patient-centered health outcomes, could steer research and healthcare delivery toward decisions that optimize outcomes important to patients. Historically, PROMs have only been collected within research registries. However, the increasing availability of PROMs within electronic health records has led to their inclusion in big data ecosystems, where they can inform or be informed by other data elements. The use of big data to analyze PROMs can help establish norms, evaluate data distribution, and determine proportions of patients achieving change or threshold standards. This information can be used for benchmarking, risk adjustment, predictive modeling, and ultimately improving the health of individuals and populations.
The terms “big data,” “machine learning,” and “artificial intelligence (AI)” have become catchphrases, at times promoting the concept that if we simply combine large enough databases with the right AI, we can solve any problem and gain hither-to-now unknown, profound insights that will solve the world's most challenging problems. This oversimplification belies the necessary contribution of human experts in both data science and the disciplines to which these advanced analytics are applied. Such partnerships have resulted in numerous advances that affect our daily lives with applications, such as facial recognition (eg, iPhone and US Customs and Border Protection), recommender systems (eg, Amazon and Spotify), autonomous driving (eg, Tesla), and natural language processing (eg, Google Translate and ChatGPT). These partnerships are especially important in health care, where the stakes are higher and the consequences of errors can be more severe than in other industries.1 Although there have been some successful routine applications of AI to health care, including transcription of medical notes and the reading of electrocardiograms, the development and adoption of AI applications has lagged relative to other industries. One obstacle is fragmentation across the health system and the data within it. In addition, there are concerns about the reliability and interpretability of AI algorithms in health care, as well as potential ethical issues around using AI to make decisions that could have life-or-death consequences for patients.1
The healthcare industry has similarly been slow to adopt patient-centric care for what care is delivered and how it is delivered and in determining how successful care is defined. Patient-reported outcome measures (PROMs) provide insight into patient-centered health outcomes, including pain, function, and quality of life. Historically collected within research registries, these data are increasingly collected as a standard of care and now comprise a growing segment of data within electronic health records. Increasing availability and density of PROMs within big data affords us the opportunity to understand relationships between care processes and patient-centered outcomes, establish PROM-based care standards, and predict care outcomes.
Big Data
Big data has been described by the SAS Institute as “data that is so large, fast, or complex that it’s difficult or impossible to process using traditional methods.” More importantly, the big data revolution is about the advanced analytics that can be applied to large data sets and the insights we can glean.
Big data can be characterized by the "four V's": volume, velocity, variety, and veracity. The volume of data within health care is immense and rapidly expanding. In 2013, the global health data volume was estimated to be 153 exabytes (1 exabyte = 1018 bytes). Seven years later, in 2020, the estimated volume was 2,314 exabytes; for comparison, the total global data volume in 2000 was three exabytes.2 These health data reside in a variety of databases including paper and electronic medical records (EMRs), administrative data sets that are used to track utilization of health services and facilitate payment, and increasingly in wearable devices. For variety, health data come in three main forms: structured, such as the fields in registries or administrative databases; unstructured, such as the information included in a medical note or image; and semistructured, such as an EMR or picture archiving and communication system, which maintain structured information about the unstructured notes or images. Veracity, the trustworthiness of the data, has particular relevance to health care, where decisions based on inaccurate or incomplete data can have serious consequences.1 Veracity encompasses both accuracy and completeness. The veracity of research registry data is generally high because notable resources are invested to ensure both the accuracy (eg, cancer registries may access pathology reports to verify the cancer type) and completeness (eg, multiple channels may be used to contact participants to ensure high-response rates) of collected data elements. By contrast, EMR data may have lower veracity regarding completeness for certain data elements across patients (eg, different clinical data elements are collected for different patients), whereas administrative data may suffer from inaccuracy.
PROMs data, which are structured, have historically been collected within research registries along with other associated structured data, such as disease severity or stage, treatment, and device characteristics. Increasingly, PROMs are being collected within care delivery to better understand care outcomes and inform care decisions3 and may be stored within electronic health records or other clinical databases in association with a variety of other structured and unstructured data elements. As such, PROM data have become components of “big data” ecosystems, where they can inform or be informed by many other data elements. For example, PROM data add important patient-centric context to other data elements in device registries, which historically have focused on the mechanical performance and longevity of a device. Conversely, other data elements in an EMR or registry could be used to risk adjust PROM scores.
Registries as a Source of Big Data in Orthopaedics
Patient registries are organized systems that use observational study methods to collect uniform data to evaluate specified outcomes for a population (defined by a particular disease, condition, or exposure) to serve one or more predetermined scientific, clinical, or policy purposes.4 In orthopaedics, patient registries may be used by individual surgeons or institutions to track a variety of conditions and procedures. By combining data collected by the many surgeons and institutions within a country, national arthroplasty registries have been able to amass enough information to be considered “big data.” First among these are the Swedish Knee Arthroplasty Register, which started in 19755 and the Swedish Hip Arthroplasty Register, initiated in 1979.6 Since then, numerous countries have developed national arthroplasty registries, including Australia, Belgium, Canada, Croatia, Denmark, Netherlands, Egypt, Finland, France, Germany, Hungary, New Zealand, Norway, Pakistan, Portugal, Romania, Slovenia, Slovakia, Switzerland, the United Kingdom (minus Scotland), and the United States.7,8
In the United States, the Mayo Clinic pioneered the development of institutional arthroplasty registries, establishing its joint replacement registry in 1969.9 Other regional and institutional registries in the United States include the Massachusetts General Hospital–led Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement, the Michigan Arthroplasty Registry Collaborative Quality Initiative, and the Kaiser Permanente National Total Joint Replacement Registry.8 In 2009, the American Joint Replacement Registry was created, and since 2017, it has been managed by the American Academy of Orthopaedic Surgeons. With data from hospitals, ambulatory surgery centers, and private practice groups in all 50 states and the District of Columbia, the American Joint Replacement Registry has become the largest hip and knee arthroplasty registry in the world by annual procedure count. Nevertheless, the UK National Mayo Clinic Joint Replacement database is still the largest in total number of procedures.
The use of big data from orthopaedic registries has several benefits for clinical practice, research, and quality improvement. For example, it can be used to identify risk factors that are associated with adverse outcomes after surgical procedures. This information can then be used to inform patient care, making it safer and more effective. In addition, big data can be used to monitor the performance of surgical devices and implants and to identify areas for improvement. This information can be shared with manufacturers, who can make changes to improve the safety and effectiveness of their products.
Although rates of revision surgery and implant longevity remain the focus of many existing arthroplasty registries, they are gradually incorporating data on PROMs.10,11 One review reported that approximately 18 arthroplasty registries collect PROMs on all or a sample of hip and knee arthroplasty patients.12 We have updated those findings and included the cumulative volume of procedures (where available), which totals more than 10 million (Table 1).
Table 1.
Characteristics and Cumulative Volume of Selected Hip and Knee Arthroplasty Registries by Volume
| Registry | Primary Hip Arthroplasty Volume | Primary Knee Arthroplasty Volume | General Health PROM | Condition-specific PROM | Data Collection Time Points |
| UK National Joint Registry Established in 2003 |
1,344,357 (all) | 1,442,051 (all) | EQ-5D | OHS/OKS | Preoperative, 6 mo |
| AJRR Established in 2012 |
821,640 (THA) | 1,306,719 (TKA) | VR-12, PROMIS-10 global | HOOS-JR/KOOS-JR | Preoperative |
| Australian Orthopaedic Association National Joint Replacement Registry Established in 2003 |
599,656 (THA) | 829,272 (TKA) | EQ-5D | HOOS-12/KOOS-12 | Preoperative |
| Swedish Arthroplasty Register Established in 1975 (knee); 1979 (hip) |
515,703 (all) | 333,693 (all) | EQ-5D | HOOS-12/KOOS-12 | Preoperative, 1, 6, and 10 yr (hip) Preoperative, 1 yr (knee) |
| Dutch Arthroplasty Register Established in 2007 |
386,956 (THA) | 309,340 (TKA) | EQ-5D | OHS/OKS | Preoperative, 3 mo, 6 mo, 1 yr |
| Canadian Joint Replacement Registry Established in 2016 |
295,285 (all) | 343,374 (all) | EQ-5D | OHS/OKS | Preoperative, 1 yr |
| MARCQI Established in 2012 |
128,938 (THA) | 206,860 (TKA) | PROMIS-10 global | HOOS-JR/KOOS-JR | Preoperative, 5-13 wk, 5-13 mo, 2, 5, and 10 yr |
| Norwegian Arthroplasty Register Established in 1994 |
218,445 (THA) | 102,649 (all) | EQ-5D | HOOS/KOOS | 1 and 2 yr |
| Swiss National Joint Registry (PROMs collected by the Geneva arthroplasty registry) Established in 2012 |
177,710 (THA) | 134,923 (TKA) | SF-12 | WOMAC, UCLA, HHS | Preoperative, 1, 5, 10, and 15 yr |
All = includes partial arthroplasties, AJRR = American Joint Replacement Registry, EQ-5D = EuroQol 5-Dimension Health Outcome Survey, HHS = Harris Hip Score, HOOS = Hip Disability and Osteoarthritis Outcome Score, HOOS-JR = Hip Disability and Osteoarthritis Outcome Score for Joint Replacement, KOOS = Knee Injury and Osteoarthritis Outcome Score, KOOS-JR = Knee Injury and Osteoarthritis Outcome Score for Joint Replacement, MARCQI = Michigan Arthroplasty Registry Collaborative Quality Initiative, OHS = Oxford Hip Score, OKS = Oxford Knee Score, PROM = patient-reported outcome measure, PROMIS = Patient-Reported Outcome Measurement Information System, SF-36 = 36-Item Short Form Health Survey, THA = total hip arthroplasty, TKA = total knee arthroplasty, UCLA = University of California at Los Angeles Activity Score, VAS = visual analog scale, VR-12 = Veterans RAND 12-item survey, WOMAC = Western Ontario and McMaster Universities Arthritis Index
Adapted from Wilson et al.12
There are many advantages of including PROMs into these registries. First, it has the potential to improve patient care because it allows healthcare providers to understand the patient's experience and make informed decisions about their care. Second, it may enhance surgeon performance analysis through institutional and/or external benchmarking. Third, it leads to improved patient satisfaction because patients appreciate the opportunity to provide input on their outcomes and experience. Finally, it increases providers’ understanding of procedure effectiveness by adding a patient-centered measurement to their assessment tools.
Using Big Data to Gain Insights About/From PROMs
Big data are used across many industries to inform our understanding about the characteristics of populations and how they have historically behaved and increasingly to predict future characteristics and behavior. PROMs can be applied for benchmarking, risk adjustment, predictive modeling, and population health management.
A first application of big PROMs data would be to deepen our understanding of PROMs. PROMs data along with other healthcare data in a big data ecosystem can be used to establish norms, evaluate data distribution, and determine proportions of patients achieving change or threshold standards. These norms can be established in different subpopulations, where previously, PROMs data and patient-generated health data to identify subpopulations were limited. This information increases the usefulness of PROMs in two ways. First, by identifying subgroups that may have relatively poor outcomes, which allows for targeted intervention for population health management. Second, these present important benchmarks that allow providers to identify values outside of the norm, given patients' demographics and clinical characteristics.
Risk identification, risk adjustment, and prediction are closely related tasks in population health management. Currently, these tasks use patient demographics, medical conditions, and comorbidities as predictors of risk. These predictors are easily extracted from the EMR or through claims data. Patient self-reported data in the form of PROMs present a new element for segmenting and defining population risk on factors that were previously not captured systematically, such as patient behaviors, mental health, stress, functional status, and level of physical activity.13 For example, the patient health questionnaire, which measures symptoms of depression, has been used to stratify mortality risk among patients with heart failure14 and diabetes.15 Preoperative PROM scores for physical and mental health have been used to stratify patients at risk of poor outcomes after total joint arthroplasty16 and to identify patients at high risk of death after hip fracture.17 Risk identification can provide useful information for primary prevention by detecting early signs and symptoms and provide an opportunity to mitigate more severe symptoms, permanent damage, and added complications related to disease progression.
Risk adjustment is used to account for factors outside of health care that may influence measures of cost, quality, and outcomes. This is particularly important when comparing quality across providers because variations may be due to factors inherent to the population being measured and outside of provider control. Providers may serve populations with different risk profiles, and appropriate risk adjustment allows for fair comparison of performance. This is also beneficial in comparing performance across time to account for a provider's patient mix at a given point in time. The use of PROMs as risk adjustors is currently limited, likely because they are not readily available for large populations. In an evaluation of the risk models used to adjust payments to Medicare Advantage (MA) plans, the General Accounting Office18 found that the models underestimated spending for those with functional limitations and overestimated spending for those without such limitations, concluding that the models would be improved if they accounted for beneficiaries' ability to do daily tasks. Were they routinely collected and included in the data sets used to develop MA risk models, PROMs could improve the accuracy of these models.
Like all other outcomes, patient-reported outcomes are subject to influences unrelated to health care. However, the factors that may affect PROMs are not well-delineated, and the methods to risk adjust are not well-described. Consequently, few PROMs are currently risk-adjusted. One exception is the US Centers for Medicare & Medicaid Services (CMS) total hip arthroplasty (THA)/total knee arthroplasty measure, which assesses the proportion of patients undergoing these procedures that achieve a substantial clinical benefit.19 This measure uses age, sex, patient comorbidities, health literacy, and a single item from the Oswestry Disability Index for risk adjustment. Data on age, sex, and comorbidities will be obtained from Medicare claims files, and hospitals will be required to report data on health literacy and the Oswestry Disability Index along with Hip Disability and Osteoarthritis Outcome Score for Joint Replacement/Knee Injury and Osteoarthritis Outcome Score for Joint Replacement scores to CMS starting in 2024 to support mandatory reporting in 2027.20,21
Starting in 2023, hospitals are required to report social determinants of health for Medicare beneficiaries to CMS, including food insecurity, housing instability, transportation needs, utility difficulties, and interpersonal safety. The availability of these data within CMS data sets will provide an opportunity to study their effects on PROMs also housed within CMS data.
Predictive modeling uses risk factors and statistical techniques to predict which patients and populations are at high risk of increased utilization and waste, increased cost, and poor outcomes. PROMs may be used as predictors or as outcomes to be predicted. Different methods can be used to predict outcomes. Conventional statistical models have long been used for prediction in clinical research and in the development of scoring algorithms using risk factors as predictors of outcomes such as the Framingham Risk Score and Charlson Comorbidity Index.22,23 Similarly, PROMs have been used within conventional statistical models to predict the likelihood of a variety of outcomes, including revision surgery after THA,24 return to work after cardiac rehabilitation,25 and hospital readmissions.25 With the widespread implementation of the EMR and increased computing power, machine learning techniques are increasingly being used to predict outcomes as well. These techniques have been used to predict patient-reported outcomes after procedures, including total joint arthroplasty26 and the treatment of various musculoskeletal conditions, including lower back pain27 and end-stage ankle arthritis.28
Tools for Risk Adjustment and Prediction
Statistical models are used in prediction to understand the relationship between risk factors and outcomes and determine factors for risk adjustment. Statistical models can provide information on multiple factors simultaneously or factors without simple cut points where simple thresholds and decision support tools can quickly become cumbersome or fail. Regression models are commonly used to estimate the effect of risk factors on the likelihood of an outcome. These models are a simplified mathematical representation of how you think independent variables (also called risk factors or predictors) and dependent variables (also called outcomes) will relate to each other. Examples of predictors are patient characteristics, baseline measurements, and chronic conditions. Regression models can be used to determine which predictors meaningfully affect an outcome and produce coefficients that quantify the effect of that factor. Coefficients can then be applied to the factors for individual patients to predict their likelihood of an outcome.13
Unlike conventional statistical methods for which models of parameters and assumptions are set from the start, machine learning often imposes fewer assumptions. Machine learning is focused on creating systems or programs that learn from data to make predictions or decisions. To do this, machine learning requires more data than traditional statistical modeling. The increasing availability of big data has made applications of machine learning more feasible. Examples include deep learning, neural networks, and random forests.29-31 As data increase in scale and complexity, machine learning can distill information from data without having to impose many assumptions. Hence, the utility of machine learning increases with the amount of data available and may be no better than conventional methods in smaller data sets.32
Both statistical modeling and machine learning can be used to answer the same types of questions of prediction, classification, and decision making (Table 2). In fact, many methods and techniques of machine learning and statistical models rely on the same underlying mathematical concepts, and there are hybrid forms of machine learning and statistical models. Choosing between machine learning and statistical models lies with the goals of prediction, data available, and the interest in the interpretation of specific factors. Interpretability of results can vary with machine learning algorithms. This is because machine learning may choose the optimal model using an algorithm, but this does not always produce plausible or intuitive results. Although these models may produce accurate predictions, they can be a black box for understanding of the relationship between predictors and outcomes. If the goal is pure prediction, this may be less important. Machine learning allows for developing complex models because more combinations of factors and model structures can be tested to optimize the prediction model. This increases the number of tests conducted to find an optimal model, and more data are needed to do this. Data constraints should be considered when choosing between statistical models and machine learning approaches.33
Table 2.
Advantages and Disadvantages of Statistical Modeling and Machine Learning for Developing Predictive Models
| Advantages | Disadvantages | Example | |
| Statistical modeling | Transparency in predictors in the model Allows for interpretation of predictors Can be modeled with less data |
Strong assumptions associated with modeling Need human input and technical expertise to guide predictor selection Need to select an appropriate model to ensure accuracy More up-front time in conceptualizing the model |
Determining whether patient stress predicts revision surgery 1 yr after surgery above and beyond other known clinical risk factors |
| Machine learning | Fewer assumption Less human input needed in determining predictors Focus on developing the best prediction May develop more complex models May uncover unexpected predictors |
Requires more data Predictors are not guided by subject matter expertise and may not be interpretable Complex models may not be interpretable Predictor results may not be available or interpretable depending on the method |
Developing an accurate risk or prediction score for revision surgery 1 yr after surgery |
PROMs as Quality Measures
There is increasing interest among policy makers to promote patient-centered care and use patient-reported outcomes as measures of healthcare quality. For example, the US CMS,34 Canadian pros National Steering Committee,35 and European Union36 Organisation for Economic Co-operation and Development37 have each identified PROMs as fundamental measures of patient-centered care. To advance the development of quality measures based on PROMs, CMS contracted the National Quality Forum to develop technical guidance for measure developers for PROM-based performance measures.38 Similarly, the OECD's Patient-Reported Indicator Surveys initiative is working to standardize PROM-based performance measures.39 The International Consortium of Outcome Measures, a US 501c corporation inspired by Michael Porter's healthcare value framework,21 has made a standardized methodology publicly available to develop patient-centered outcome measure sets including PROMs.40 To date, measure sets have been developed for 40 conditions, including hip and knee osteoarthritis, rheumatoid arthritis, hand and wrist conditions, and lower back pain.40
The implementation of PROMs as quality measures for public reporting and/or payment is currently limited but expected to grow. The National Health Service publicly reports PROM-based health gains among patients undergoing hip arthroplasty, knee arthroplasty, and, up to September 2017, varicose vein and groin hernia surgery in England.41 CMS currently uses two PROMs from the Health Outcomes Survey for public reporting and value-based payments for MA plans and 17 from a variety of measure developers in the Merit-Based Incentive Payment System for clinicians, including 11 that are applicable to orthopaedics. In 2027, CMS will start reporting on Hospital Compare, the proportion of total knee arthroplasty/THA patients who achieve a substantial clinical benefit as part of the Hospital Inpatient Quality Reporting program. In advance of the mandatory reporting in 2027, there are two voluntary reporting periods in 2025 and 2026 during which CMS will not publicly report the performance of participating hospitals but rather share with those hospitals their performance relative to other participating hospitals across the nation. Data collection for the voluntary 2025 reporting started in October 2022. Data collection for the voluntary 2026 reporting started in April 2023.20
Pulling It All Together
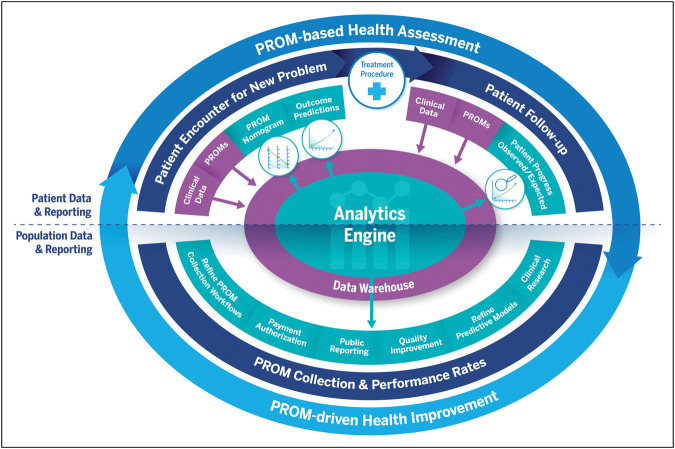
The virtuous cycle for PROMs (Figure 1) refers to a continuous process that involves the collection, analysis, and use of patient-generated data to improve patient outcomes.3 It starts with the collection of patient-generated data through various means, such as surveys, interviews, and other forms of self-reported information. These data provide valuable information regarding patient perspectives on their health, including symptoms, functional status, and overall quality of life. These data are ideally housed in a central data warehouse.
Figure 1.
Virtuous cycle of PROMs for health assessment and improvement. Reproduced with permission from NEJM Catalyst.2 PROM = patient-reported outcome measure.
The next step in the virtuous cycle is the analysis of the collected data through an analytics engine. This involves using statistical and analytical methods to identify trends, patterns, and relationships in the data. The goal is to uncover meaningful insights that can inform the development of new and more effective treatments, develop predictive outcomes models, and identify areas for improvement in the current clinical care delivery system. Ideally, the data warehouse and analytical engine can be leveraged in real time for clinical research, quality improvement, public reporting, and payment authorization.
Finally, the virtuous cycle is completed by using the insights generated from the analysis to improve patient outcomes. This may involve developing new treatments, improving current treatments, or making changes to the healthcare delivery system to better meet the needs of patients. By continuously collecting and analyzing patient-generated data, healthcare providers and researchers can identify areas for improvement, which can then be acted on to improve patient outcomes. This creates a self-reinforcing cycle of improvement.
Critical to the execution of this virtual cycle is embedding PROMs collection into clinical practice. The importance of PROMs collection should be emphasized for both clinicians and patients. Successful implementation depends on (1) integration of PROMs collection in the EMR, (2) recognition by all members of the care team and patients that PROMs have clinical meaning and importance, (3) patient engagement in the collection and review of PROMs, (4) routine PROMs collection at each clinical encounter, and (5) regular sharing and analysis of PROM data with clinicians.
Challenges That Remain
Moving from patient-level data to transform PROMs into “big data” is subject to many challenges. They start with the decision on how to collect PROMs. Although most PROMs have originally been developed for paper and pencil administration, electronic collection has multiple advantages, including easier delivery of surveys (eg, e-mail and Short Message Service [SMS]), automatic score calculations, and algorithms that reduce the number of required questions using item response theory.3,42
The next challenge is to implement the electronic administration of PROMs, which includes both technological and operational complexities. First, institutions need to weigh between collecting PROMs through external vendors or building them directly into their EMR. The first option usually comes with off-the-shelf solutions, which may be relatively easy to implement, but often require a very labor-intensive process if integration with EMR is desired. In most cases, true integration, with seamless bidirectional data exchange, may never be accomplished.3 On the other hand, building PROMs directly into the EMR has multiple benefits, such as eliminating the need for an extra vendor, viewing PROMs scores directly in the chart, and incorporating these results into clinical notes, which can greatly improve the shared decision-making process.43 Still, several EMR systems do not possess strong customization options for questionnaires and patient portals and may not be as adaptable as solutions provided by specialized vendors regarding customization and calculating intricate scores. Operational hurdles include reluctance on the part of staff to adopt new workflows and low patient completion rates, which may hinder generalizability of results.
If regional, national, and/or international data sharing is warranted, the lack of health records interoperability is frequently the most obvious barrier, not to mention legal requirements for data sharing agreements. Interoperability refers to the ability of different EMR systems to share and exchange patient data seamlessly, including PROMs. This is a critical aspect of healthcare information technology because it enables healthcare providers to access complete and accurate patient information, regardless of where the information was originally recorded. The goal of interoperability is to improve the quality of patient care, reduce medical errors, and enhance the coordination of care between healthcare providers. In the United States, the Office of the National Coordinator for Health Information Technology has developed standards and guidelines for EMRs to ensure that patient data can be exchanged securely and accurately between different EMR systems.44 The Office of the National Coordinator for Health Information Technology has also established the Nationwide Health Information Network to facilitate the exchange of health information between different healthcare organizations. Moreover, the use of a common terminology to identify each PROM data elements, such as the Logical Observation Identifiers Names and Codes, could simplify integration and interoperability. The Logical Observation Identifiers Names and Codes was first developed for laboratory and clinical observations and now includes more than 600 PROM questionnaires.45
Finally, as presented in Table 1, the regional differences in PROMs collected for just two of the most common orthopaedic procedures highlight the need for the development of common data sets and crosswalks between various PROMs. The Patient-Reported Outcome (PRO) Rosetta Stone (PROsetta Stone) initiative has developed and applied methods to link Patient-Reported Outcomes Measurement Information System instruments with other related instruments (eg, 36-Item Short Form Health Survey, Veterans RAND 12-item Survey, and Brief Pain Inventory) to increase the range of PRO assessment options within a consistent and uniform metric. It provides equivalent scores for different scales that measure the same health outcome.46
Conclusion
The integration of PROMs within big data ecosystems has the potential to revolutionize health care. The combination of PROMs and other healthcare data provides a wealth of information that can be used to establish norms, evaluate data distributions, predict outcomes, and improve shared decision making and ultimately patient-centered care. The use of big data from orthopaedic registries has already demonstrated numerous benefits for clinical practice, research, and quality improvement. As the healthcare industry continues to adopt patient-centric care and the collection of PROMs data becomes more widespread, the potential for big data to inform our understanding of healthcare outcomes and inform decision making will only continue to grow. The future of health care is intertwined with the integration of big data and PROMs. However, many structural challenges must be overcome before the full potential of AI in health care can be realized.
Footnotes
None of the following authors or any immediate family member has received anything of value from or has stock or stock options held in a commercial company or institution related directly or indirectly to the subject of this article: MacLean, Antao, Chin, and McLawhorn.
Contributor Information
Vinicius C. Antao, Email: antaov@hss.edu.
Amy S. Chin, Email: china@hss.edu.
Alexander S. McLawhorn, Email: mclawhorna@hss.edu.
References
- 1.ChatGPT: Conversation with Catherine MacLean. Available at: https://chat.openai.com/. Accessed April 13, 2023. [Google Scholar]
- 2.Schwendicke F, Krois J: Data dentistry: How data are changing clinical care and research. J Dent Res 2022;101:21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacLean CH, Antao VC, Fontana MA, Sandhu HS, McLawhorn AS: PROMs: Opportunities, challenges, and unfinished business. NEJM Catalyst 2021;2. [Google Scholar]
- 4.Gliklich RE, Dreyer NA, Leavy MB: Registries for Evaluating Patient Outcomes: A User's Guide. Rockville, MD: Agency for Healthcare Research and Quality (US), 2014. Available at: https://www.ncbi.nlm.nih.gov/books/NBK208626/. Accessed February 1, 2023. [PubMed] [Google Scholar]
- 5.Knutson K, Lewold S, Robertsson O, Lidgren L: The Swedish knee arthroplasty register. A nation-wide study of 30,003 knees 1976-1992. Acta Orthop Scand 1994;65:375-386. [DOI] [PubMed] [Google Scholar]
- 6.Malchau H, Herberts P, Eisler T, Garellick G, Söderman P: The Swedish total hip replacement register. J Bone Joint Surg Am 2002;84(suppl 2):2-20. [DOI] [PubMed] [Google Scholar]
- 7.Delaunay C: Registries in orthopaedics. Orthop Traumatol Surg Res 2015;101:S69-S75. [DOI] [PubMed] [Google Scholar]
- 8.Hughes RE, Batra A, Hallstrom BR: Arthroplasty registries around the world: Valuable sources of hip implant revision risk data. Curr Rev Musculoskelet Med 2017;10:240-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry DJ, Kessler M, Morrey BF: Maintaining a hip registry for 25 years. Mayo Clinic experience. Clin Orthop Relat Res 1997;344:61-68. [DOI] [PubMed] [Google Scholar]
- 10.Rolfson O, Eresian Chenok K, Bohm E, et al. : Patient-reported outcome measures in arthroplasty registries. Acta Orthop 2016;87(suppl 1):3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rolfson O, Bohm E, Franklin P, et al. : Patient-reported outcome measures in arthroplasty registries report of the patient-reported outcome measures working group of the international society of arthroplasty registries Part II. Recommendations for selection, administration, and analysis. Acta Orthop 2016;87(suppl 1):9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson I, Bohm E, Lübbeke A, et al. : Orthopaedic registries with patient-reported outcome measures. EFORT Open Rev 2019;4:357-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duncan IG: Healthcare Risk Adjustment and Predictive Modeling. New Hartford, CT: ACTEX Publications, 2011. [Google Scholar]
- 14.Deveney TK, Belnap BH, Mazumdar S, Rollman BL: The prognostic impact and optimal timing of the Patient Health Questionnaire depression screen on 4-year mortality among hospitalized patients with systolic heart failure. Gen Hosp Psychiatry 2016;42:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann M, Köhler B, Leichsenring F, Kruse J: Depression as a risk factor for mortality in individuals with diabetes: A meta-analysis of prospective studies. PLoS One 2013;8:e79809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berliner JL, Brodke DJ, Chan V, SooHoo NF, Bozic KJ: Can preoperative patient-reported outcome measures Be used to predict meaningful improvement in function after TKA? Clin Orthop Relat Res 2017;475:149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Risk Factors for Poor Outcomes After Hip Fracture Patients in the Robust Elderly: Are Patient Reported Outcomes Important? ACR Meeting Abstracts. Available at: https://acrabstracts.org/abstract/risk-factors-for-poor-outcomes-after-hip-fracture-patients-in-the-robust-elderly-are-patient-reported-outcomes-important/. Accessed February 1, 2023. [Google Scholar]
- 18.Office USGA: Medicare Advantage: Benefits and Challenges of Payment Adjustments Based on Beneficiaries' Ability to Perform Daily Tasks. Available at: https://www.gao.gov/products/gao-18-588. Accessed February 1, 2023. [Google Scholar]
- 19.CMS: Methodology. Available at: https://qualitynet.cms.gov/inpatient/measures/THA_TKA/methodology. Accessed February 1, 2023. [Google Scholar]
- 20.CMS: THA/TKA PRO-PM Overview. Available at: https://qualitynet.cms.gov/inpatient/measures/THA_TKA. Accessed February 1, 2023. [Google Scholar]
- 21.Porter ME: What is value in health care? N Engl J Med 2010;363:2477-2481. [DOI] [PubMed] [Google Scholar]
- 22.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB: Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837-1847. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373-383. [DOI] [PubMed] [Google Scholar]
- 24.Singh JA, Schleck C, Harmsen S, Lewallen D: Clinically important improvement thresholds for Harris Hip Score and its ability to predict revision risk after primary total hip arthroplasty. BMC Musculoskelet Disord 2016;17:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salzwedel A, Koran I, Langheim E, et al. : Patient-reported outcomes predict return to work and health-related quality of life six months after cardiac rehabilitation: Results from a German multi-centre registry (OutCaRe). PLoS One 2020;15:e0232752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontana MA, Lyman S, Sarker GK, Padgett DE, MacLean CH: Can machine learning algorithms predict which patients will achieve minimally clinically important differences from total joint arthroplasty? Clin Orthop Relat Res 2019;477:1267-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verma D, Jansen D, Bach K, Poel M, Mork PJ, d'Hollosy WON: Exploratory application of machine learning methods on patient reported data in the development of supervised models for predicting outcomes. BMC Med Inform Decis Mak 2022;22:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waly FJ, Yeo EMN, Wing KJ, Penner MJ, Veljkovic A, Younger ASE: Relationship of preoperative patient-reported outcome measures (PROMs) to postoperative success in end-stage ankle arthritis. Foot Ankle Int 2020;41:253-258. [DOI] [PubMed] [Google Scholar]
- 29.Brownlee J: What is machine learning? Machine Learning Mastery, 2013. Available at: https://machinelearningmastery.com/what-is-machine-learning/. Accessed February 1, 2023. [Google Scholar]
- 30.SHARP SIGHT: What's the Difference Between Machine Learning, statiStics, and Data Mining?, 2016. Available at: https://www.sharpsightlabs.com/blog/difference-machine-learning-statistics-data-mining/. Accessed February 1, 2023. [Google Scholar]
- 31.Beam AL, Kohane IS: Big data and machine learning in health care. JAMA 2018;319:1317-1318. [DOI] [PubMed] [Google Scholar]
- 32.Oosterhoff JHF, Gravesteijn BY, Karhade AV, et al. : Feasibility of machine learning and logistic regression algorithms to predict outcome in orthopaedic trauma surgery. J Bone Joint Surg Am 2022;104:544-551. [DOI] [PubMed] [Google Scholar]
- 33.Harrell F: Statistical Thinking - Road Map for Choosing Between Statistical Modeling and Machine Learning, 2018. Available at: https://www.fharrell.com/post/stat-ml/. Accessed February 1, 2023. [Google Scholar]
- 34.CMS: Person-Centered Innovation – An Update on the Implementation of the CMS Innovation Center's Strategy, 2022. Available at: https://cmmicoordinator.survey.fm/was-this-helpful?iframe=https%3A%2F%2Finnovation.cms.gov%2Fstrategic-direction&ft=1. Accessed February 1, 2023. [Google Scholar]
- 35.Ahmed S, Barbera L, Bartlett SJ, et al. : A catalyst for transforming health systems and person-centred care: Canadian national position statement on patient-reported outcomes. Curr Oncol 2020;27:90-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enhancing Value in European Health Systems: The Role of Outcomes Measurement. Available at: https://www.eu-patient.eu/globalassets/policy/patientssafety/value-of-health-consensus-document.pdf. [Google Scholar]
- 37.Measuring What Matters: The Patient-Reported Indicator Surveys, 2019. Available at: https://www.oecd.org/health/health-systems/Measuring-what-matters-the-Patient-Reported-Indicator-Surveys.pdf. [Google Scholar]
- 38.NQF: Patient-Reported Outcome Measures to Patient-Reported Outcome Performance Measures - Technical Guidance Final Report. Available at: https://www.qualityforum.org/Publications/2022/11/Patient-Reported_Outcome_Measures_to_Patient-Reported_Outcome_Performance_Measures_-_Technical_Guidance_Final_Report.aspx. Accessed February 1, 2023. [Google Scholar]
- 39.Patient-Reported Indicator Surveys (PaRIS) - OECD. Available at: https://www.oecd.org/health/paris/. Accessed February 1, 2023. [Google Scholar]
- 40.ICHOM: Patient-Centered Outcome Measures. Available at: https://www.ichom.org/patient-centered-outcome-measures/. Accessed February 3, 2023. [Google Scholar]
- 41.NHS Digital. Patient Reported Outcome Measures (PROMs). Available at: https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/patient-reported-outcome-measures-proms. Accessed February 1, 2023. [Google Scholar]
- 42.Cella D, Gershon R, Lai JS, Choi S: The future of outcomes measurement: Item banking, tailored short-forms, and computerized adaptive assessment. Qual Life Res 2007;16:133-141. [DOI] [PubMed] [Google Scholar]
- 43.Kotronoulas G, Kearney N, Maguire R, et al. : What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol 2014;32:1480-1501. [DOI] [PubMed] [Google Scholar]
- 44.Adler-Milstein J, Garg A, Zhao W, Patel V: A survey of health information exchange organizations in advance of A Nationwide connectivity framework. Health Aff 2021;40:736-744. [DOI] [PubMed] [Google Scholar]
- 45.Huff SM, Rocha RA, McDonald CJ, et al. : Development of the logical observation identifier Names and Codes (LOINC) vocabulary. J Am Med Inform Assoc 1998;5:276-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi SW, Podrabsky T, Mckinney N, Schalet BD, Cook KF, Cella D: Prosetta Stone® Methodology a Rosetta Stone for Patient Reported Outcomes.