Abstract
Background:
Epidemiological evidence for gestational polycyclic aromatic hydrocarbon (PAH) exposure and adverse child cognitive outcomes is mixed; little is known about critical windows of exposure.
Objective:
We investigated associations between prenatal PAH exposure and child cognition in a large, multi-site study.
Methods:
We included mother–child dyads from two pooled prospective pregnancy cohorts (CANDLE and TIDES, N = 1,223) in the ECHO-PATHWAYS Consortium. Seven urinary mono-hydroxylated PAH metabolites were measured in mid-pregnancy in both cohorts as well as early and late pregnancy in TIDES. Child intelligence quotient (IQ) was assessed between ages 4–6. Associations between individual PAH metabolites and IQ were estimated with multivariable linear regression. Interaction terms were used to examine effect modification by child sex and maternal obesity. We explored associations of PAH metabolite mixtures with IQ using weighted quantile sum regression. In TIDES, we averaged PAH metabolites over three periods of pregnancy and by pregnancy period to investigate associations between PAH metabolites and IQ.
Results:
In the combined sample, PAH metabolites were not associated with IQ after full adjustment, nor did we observe associations with PAH mixtures. Tests of effect modification were null except for the association between 2-hydroxynaphthalene and IQ, which was negative in males (βmales = −0.67 [95%CI:−1.47,0.13]) and positive in females (βfemales = 0.31 [95%CI:−0.52,1.13])(pinteraction = 0.04). In analyses across pregnancy (TIDES-only), inverse associations with IQ were observed for 2-hydroxyphenanthrene averaged across pregnancy (β = −1.28 [95%CI:−2.53,−0.03]) and in early pregnancy (β = −1.14 [95%CI:−2.00,−0.28]).
Significance:
In this multi-cohort analysis, we observed limited evidence of adverse associations of early pregnancy PAHs with child IQ. Analyses in the pooled cohorts were null. However, results also indicated that utilizing more than one exposure measures across pregnancy could improve the ability to detect associations by identifying sensitive windows and improving the reliability of exposure measurement. More research with multiple timepoints of PAH assessment is warranted.
Keywords: Polycyclic aromatic hydrocarbons, Neurodevelopment, Prenatal
1. Introduction
Accumulating epidemiological evidence suggests that prenatal exposure to polycyclic aromatic hydrocarbons (PAHs), a prevalent environmental toxicant resulting from the incomplete combustion of fossil fuels and other organic materials, is associated with adverse neurocognitive development. Predominant sources of PAH exposure include inhalation of ambient air pollution, smoking, and from diet through consuming grilled or barbequed meats(Agency for Toxic Substances and Disease Registry (ATSDR), 1995). Neurodevelopmental deficits in children associated with prenatal PAH exposure have included differences in attention, behavior, and cognition(Jedrychowski et al., 2015; Perera et al., 2014). Hypothesized mechanisms for neurotoxic effects include disruption of pathways that regulate neuronal differentiation, synapse formation, and synapse plasticity(McCallister et al., 2008; Deanna D Wormley et al., 2004), epigenetic modifications (Herbstman et al., 2012), endocrine disruption(Takeda et al., 2004), oxidative stress and neuroinflammation(Saunders et al., 2006), and impairment of long-term potentiation of hippocampus, critical to learning and memory (D. D. Wormley et al., 2004).
Several previous studies have specifically investigated associations between prenatal PAH exposure and childhood IQ(Edwards et al., 2010; Perera et al., 2012, 2009). Two of these studies, conducted in New York City, and Krakow, Poland, demonstrated lower childhood IQ (Perera et al., 2009) or nonverbal reasoning ability (Edwards et al., 2010) associated with prenatal PAH exposure measured in persona. In contrast a third study in Chongqing, China of prenatal PAHs measured in cord blood showed no association with child IQ (Perera et al., 2012). Measures of prenatal PAH exposure via personal air monitoring have the benefit of capturing longer periods of exposure than PAHs measured in urine, are limited to airborne sources, and do not capture exposure by routes such as dietary intake. This is important, as diet is estimated to contribute up to 70–90% of total PAH exposure, but with substantial geographic and temporal variability(Agency for Toxic Substances and Disease Registry (ATSDR), 1995; Skupinska et al., 2004; Suzuki and ´ Yoshinaga, 2007). Furthermore, previous work largely aggregates measures of various PAH compounds into a single exposure assessment, whether by constructing a composite of PAH concentrations in air, or by measuring PAHs in cord blood at birth. This does not allow for evaluating associations of individual PAH metabolites, or co-exposure to multiple PAH metabolites, which may have different neurodevelopmental toxicities that are not apparent in single-pollutant models. Further, the use of a single exposure metric may be prone to misclassification error as it does not account for intraindividual variability and may not reflect patterns of exposure over pregnancy; nor does it allow for the exploration of windows of vulnerability to prenatal PAH exposure.
Another gap in the current literature is the limited research on child or pregnancy-related factors that might modify adverse associations between PAH and child cognition or other neurodevelopmental endpoints. Studies of prenatal exposures to similar toxicants such PM2.5 and child IQ show differences in outcomes by child sex, with inverse associations more prominent in boys than girls (Chiu et al., 2016; Lertxundi et al., 2019). Brain development differs between males and females and sex hormones are believed to interact with the neuroendocrine and immune systems in modulating the fetus’ response to stressors (McCarthy et al., 2017). We also hypothesized that maternal body composition could interact with PAH exposure on fetal brain development. PAHs are lipophilic and accumulate in fatty tissue(Pastor-Belda et al., 2019), and studies of other lipophilic environmental contaminants demonstrate that they are transferred from adipose tissue to placenta (Kapraun et al., 2022), potentially exposing fetuses of women with higher body mass to higher exposure to PAHs even given the same level of environmental exposure.
Our study adds to the existing literature by estimating the associations between prenatal exposure to PAH and childhood cognition at age 4–6 years in a large, diverse, multi-site U.S. sample, with robust adjustment for potential confounders. We measured PAHs in mid-pregnancy urine, which captures all routes of PAH exposure. We hypothesized that higher prenatal PAH exposure would be associated with lower childhood IQ. We also use contemporary mixtures methods to estimate associations between mixtures of PAH metabolites and early childhood cognition. We assessed whether sex or pre-pregnancy body mass index modified associations between prenatal PAH and childhood IQ. Finally, in a subset of participants, we evaluated multiple time points of prenatal exposure to PAH in relation to childhood IQ to better estimate associations across pregnancy and to explore periods during which the fetus may be more susceptible to the impacts of PAH exposure.
2. Subjects and methods
2.1. Design and participants
The current investigation is part of the National Institutes of Health Environmental influences on Child Health Outcomes (ECHO) PATHWAYS Consortium, which pooled data from three pre-existing cohorts (LeWinn et al., 2022). Two cohorts of the ECHO PATHWAYS Consortium had mid-pregnancy PAH data and were included in this analysis: the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study, in Memphis, TN; and The Infant Development and Environment Study (TIDES) in San Francisco, CA, Minneapolis, MN, Rochester, NY, and Seattle, WA. Design, recruitment, and data collection for CANDLE and TIDES have been previously described(LeWinn et al., 2022). In brief, CANDLE enrollment of pregnant mothers occurred between 2006 and 2011. Participant follow-up included two prenatal study visits in mid and late pregnancy, biospecimen collection, and ongoing postnatal follow up of mothers and offspring. Mother-child pairs were followed prenatally and at regular intervals with clinic and home visits and telephone surveys, including clinic visits at approximately 4 years of age. The TIDES study recruited mothers between 2010 and 2012, with three prenatal study visits occurring in early, mid, and late pregnancy that included biospecimen collection and maternal surveys. Mother-child pairs were followed with questionnaires and a clinic visit at approximately 6 years of age. For the current study, CANDLE and TIDES participants were included if they were singleton births with a mid-pregnancy prenatal urinary PAH measure and a measure of IQ. We excluded children born less than 34 weeks of pregnancy, as well as children of mothers who smoked during pregnancy, as the relationship between children born prematurely and of mothers who smoke and child IQ is well-established in the literature (Herrmann et al., 2008; Soleimani et al., 2014). Maternal prenatal smokers were defined using self-reported smoking at any point in pregnancy, or had urinary cotinine levels of greater than 200 ng/ml measured in mid or late pregnancy(Schick et al., 2017). The CANDLE study was approved by the institutional review board (IRB) of the University of Tennessee Health Sciences Center. TIDES was approved by the IRBs of the University of California, San Francisco, University of Minnesota, University of Rochester Medical Center, and University of Washington. The current analysis was conducted by ECHO PATHWAYS and was approved by the University of Washington IRB.
2.2. Exposure assessment
For CANDLE participants, urine collection occurred in mid-pregnancy, at a mean of 23.0 weeks (SD = 3.0). In TIDES, collection occurred once each in early-, mid-, and late-pregnancy, with mid-pregnancy samples used in the main analyses. Early pregnancy urinary measures were taken, on average, at 10.9 weeks’ gestation (SD = 2.4); mid-pregnancy, at a mean of 20.8 weeks (SD = 3.8); and late-pregnancy, at a mean of 32.7 weeks (SD = 3.1). The methods for the extraction and processing of urinary hydroxy-PAHs (OH-PAHs) has been published previously(Guo et al., 2013). Briefly, extraction of OH-PAHs from urine was performed by liquid–liquid extraction followed by LC-MS/MS analysis (Guo et al., 2013). A Waters Acquity I-Class UPLC system (Waters; Milford, MA, USA) was used for chromatographic separation of PAHs, connected with an Acquity UPLC BEH C18 column (50 × 2.1 mm, 1.7 μm, Waters; Milford, MA, USA). The identification and quantification of PAH metabolites was performed on an ABSCIEX 5500 triple quadrupole mass spectrometer (Applied Biosystems; Foster City, CA, USA). The laboratory participated in several external quality assurance schemes to validate OH-PAHs assay successfully(Kannan et al., 2021). Individual urinary OH-PAH metabolites were included in this analysis if they were detected in at least 70% of the pooled TIDES and CANDLE study sample. These include 1-hydroxypyrene, 1- and 2-hydroxynaphthalene, 1/9-, 2-, and 3-hydroxyphenanthrene, and 2/3/9-hydroxyfluorene. In addition, we analyzed the molar sums of the hydroxynaphthalene and hydroxyphenanthrene metabolites for separate analyses. For OH-PAH measured below the limit of detection (LOD), we substituted the value of .
2.3. Outcome
Child cognition was assessed using a validated measure of IQ in each cohort. In CANDLE, IQ was measured at approximately age 4 years using the Stanford-Binet Intelligence Scales, Fifth Edition (SB-5). The SB-5 included 10 subtests addressing five cognitive factors, including knowledge, fluid reasoning, quantitative reasoning, visual-spatial processing, and working memory. The IQ score in the SB-5 has excellent internal consistency and test–retest reliability (0.98 and 0.92, respectively)(Sattler, 2018). In TIDES, the Wechsler Intelligence Scale for Children, Fifth Edition (WISC-V), was administered at approximately 6 years(Raiford, 2018). For the WISC-V, IQ was derived from five subtests and included Vocabulary (Verbal Comprehension), Block Design (Visual Spatial), Matrix Reasoning (Fluid Reasoning), Digit Span (Working Memory), and Coding (Processing Speed). This combination represents one primary subtest from each index scale. IQ was calculated using the Tellegen and Briggs formula(Wechsler, 2003). Reliability and validity coefficients for this five-subtest version are 0.945 and 0.934 respectively (Sattler, 2018). This approach is comparable to prior studies(Mollon et al., 2018) which have utilized an abbreviated four-subtest version of the WISC-IV to assess child cognition. IQ scores from both the SB-5 and WISC-V were standardized to age in 3-month intervals to a mean score of 100 and a standard deviation of 15. While the specific tests used to capture the different cognitive domains may vary between SB-5 and WISC-V, performance on the instruments is highly correlated in typically developing children and they provide a standardized metric of overall cognitive performance (Roid, 2003). This approach is similar to prior studies which sought to examine child IQ across different instruments (Kullar et al., 2019; Lanphear et al., 2005; Ni et al., 2022). All examiners were thoroughly trained on the administration and scoring of the SB-5 or WISC-V by licensed psychologists. They participated in didactic instruction and guided practice, inter-rater reliability exercises, as well as weekly supervision by psychologists post-training. Overall reliability equal to or greater than 90% was required for the examiners to administer the measure independently. To identify potential data entry and scoring errors, we used logic checks (e.g., comparing raw scores to expected standardized scores) and reviewed outliers (i.e., scores significantly above or below the normative mean).
2.4. Other variables
Mothers reported on their own and their child’s characteristics at prenatal and postnatal study visits, including child race, marital status, pre-pregnancy body mass index (BMI), maternal education attainment, household size, smoking and tobacco use during pregnancy and in the postnatal period, length of breastfeeding and family income. Family income was adjusted for regional price differences and inflation using established econometric methods from the U.S. Bureau of Economic Analysis (BEA)(Bureau of Economic Analysis, 2021). Medical record abstraction was used to collect information on infant sex, gestational age, and birthweight. Child secondhand tobacco smoke exposure was reported at the child’s age 4–6 visit by maternal survey. Mothers were asked if they, the child’s father, child’s caregiver or care provider, or any other person living in the home with the child smoked (Yes/No). Child opportunity was measured using the Education and Social and Economic domains of the Child Opportunity Index, linked to maternal address at enrollment(Acevedo-Garcia et al., 2014). Maternal IQ was assessed at the age 4–6 visit using the Wechsler Abbreviated Scale of Intelligence (WASI) short form in both cohorts(Axelrod, 2002).
2.5. Statistical analysis
We described the demographics of the study sample overall as well as for the component CANDLE and TIDES cohorts. For descriptive summaries of OH-PAH metabolites, we standardized raw PAH values to the median study sample urinary specific gravity (SG) to account for urinary dilution, as follows:
Where represents the standardized PAH value, represents the raw PAH value, represents the median specific gravity for the PAH metabolites, and represents the specific gravity for the PAH metabolite(Levine and Fahy, 1945). To assess the relationship between OH-PAH and IQ, we used multivariable linear regression, with separate models for each individual urinary metabolite (and the hydroxynaphthalene and hydroxyphenanthrene sums). Unless specified, all analyses were restricted to participants without missing covariate data. Consistent with prior studies of urinary measures of prenatal PAH exposure and health outcomes(Ferguson et al., 2017) (Abid et al., 2014; Ferguson et al., 2017), we used raw, log-transformed OH-PAH metabolite concentrations in the multivariable linear regressions and an adjustment term for specific gravity was included to account for urinary dilution. Estimates and 95% CI were multiplied by ln (2) to calculate change in IQ per 2-fold increase in OH-PAH metabolite or parent compound sum. We took a staged approach to covariate adjustment to examine the influence of increasing adjustment on effect estimates. Potential confounders were determined a priori and defined as factors either directly or indirectly associated with prenatal PAH exposure and child IQ. We also identified selected precision variables with well-established relationships with child IQ although not likely to be associated with prenatal PAH exposure(Lewinn et al., 2020). Supplemental Fig. 1 shows a Directed Acyclic Graph for covariate selection and regression model development. A “minimal” model adjusted for study site (five categories), child sex (binary), child age (continuous), analysis batch (four categories), specific gravity (continuous), and an interaction between specific gravity and cohort to account for urinary dilution in each cohort’s OH-PAH measures. A “full” model, considered the main analysis, additionally adjusted for maternal education (four categories), maternal IQ (continuous), maternal age (continuous), marital status (binary, married or living as married vs. not), birth order (binary, first born vs. not), prenatal urinary cotinine (continuous), secondhand smoke exposure (binary, any vs. none), breastfeeding practice (binary, none vs. any), an interaction between household income and household count, each of the education and economics domains of the Child Opportunity Index (continuous), and child race (three categories). Race and ethnicity are conceptualized as important social constructs that, in this analysis, may capture unmeasured confounding due to exposures inequitably distributed by race and associated with both exposure to air pollution and EF (e.g., other toxic exposures like lead). Finally, an “extended” model additionally included gestational age (continuous) and birthweight (continuous), potential confounders that were not included in the full model because they may also lie on the causal pathway.
2.6. Sensitivity analyses
We conducted a number of sensitivity analyses. To evaluate alternate methods of accounting for urinary dilution, we standardized individual OH-PAHs as well as urinary cotinine to SG via the above formula rather than adjusting for SG as a covariate. We substituted below LOD OH-PAH values by censored likelihood multiple imputation (CLMIClick or tap here to enter text.)(Boss et al., 2019), using fixed effects meta-analysis rather than our primary pooling approach since differences in OH-PAH distributions across cohorts precluded fitting pooled CLMI models. To compare with our primary analysis’s exclusion of participants with missing covariates (i.e. complete case analysis), we imputed missing covariates using the method of multiple imputation by chained equations. To assess whether our results might be driven by heterogeneous associations across study sites or cohort, we performed a series of “full” regressions leaving one study site out in turn as well as repeating the main analyses by study cohort. To exclude the influence of participants with intellectual disability, who may have underlying neurodevelopmental differences linked to their level of cognition, we repeated our analyses after restricting our sample to participants with IQ above 70. As a final sensitivity analyses, we repeated analyses using more parsimonious adjustment models with covariate adjustment comparable to prior cohort studies of prenatal PAH exposure and IQ in New York and Krakow (Edwards et al., 2010; Perera et al., 2009).
2.7. Secondary analyses
As a secondary analysis, we examined the associations between mixtures of OH-PAH metabolites and early childhood IQ by weighted quantile sum (WQS) regression(Carrico et al., 2015; Czarnota et al., 2015). OH-PAH levels were standardized to specific gravity via the formula above and transformed into deciles. We estimated weighted sums of individual OH-PAH metabolites to comprise the WQS score. To improve the sensitivity to detect the associations between the WQS score and IQ in multivariable linear regression models, we used bootstrap resampling methods to estimate index weights, using 1000 bootstrap samples for each analysis. We separately tested for positive or negative associations between the OH-PAH mixture and IQ. Estimates were adjusted for all covariates in the “full” model. To correct for potential Type 1 error, for any full sample WQS analysis that resulted in a 95% CI that did not include the null, we estimated a permutation test p-value (Day et al., 2022).
To investigate possible effect modification by child sex and maternal pre-pregnancy BMI, we used multivariable linear regression and added an interaction term between each modifier and OH-PAH metabolite. We estimated stratum-specific associations and corresponding 95% CI in each group and evaluated evidence of effect modification using p-values for interaction with a significance threshold of 0.05. Pre-pregnancy BMI was dichotomized as overweight/obese (≥25 kg/m2) vs. not (less than25 kg/m2). All estimates were adjusted for covariates in the “full” model.
2.8. Analyses across pregnancy
In the study sample from the TIDES cohort that had OH-PAH measures from all three periods of pregnancy, we examined associations of pregnancy-averaged OH-PAH exposures and IQ as well as estimating period-specific associations to explore the possibility of differing susceptibility to PAH exposure at different points in pregnancy. 1-Hydroxypyrene and 2/3/9-hydroxyfluorene were excluded from these TIDES-only analyses due to the high proportion of samples below LOD in the participant sample. We calculated pregnancy-average OH-PAH metabolite concentrations over the three periods of pregnancy and examined associations with IQ. In addition, for each of the three periods in pregnancy, we separately performed multivariable linear regression. We then examined OH-PAHs during each period in pregnancy with and without mutual adjustment for the other two periods to assess the independent association of OH-PAH at a particular window in pregnancy on childhood IQ. All regressions were performed with the “full” model described above.
All analyses were completed in R version 3.6.5 (R Development Core Team). P-values less than 0.05 were deemed statistically significant.
3. Results
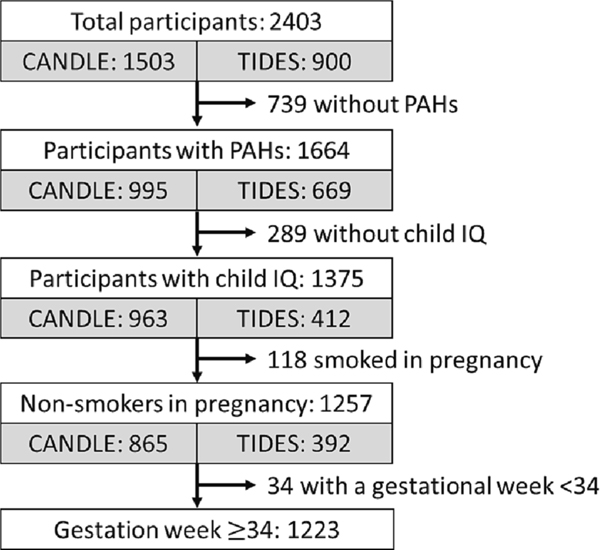
The inclusion of enrolled CANDLE and TIDES participants in the analytic sample is illustrated in Fig. 1. Of the 2,403 participants enrolled in CANDLE and TIDES, 1,375 had both prenatal PAH measures and child IQ. After excluding 118 prenatal smokers and 34 children born prior to 34 weeks, the study sample comprised 838 CANDLE participants and 385 TIDES participants, for a total of 1,223. Participants in the analytic sample were similar across sociodemographic and maternal characteristics to the study population at enrollment (Supplemental Table 1). Descriptive characteristics of the analytic sample are presented in Table 1. Compared to child participants in CANDLE, those in TIDES were more often reported to be White (69% TIDES, 29% CANDLE), more likely to ever breastfeed (93% TIDES, 68% CANDLE), and less likely to be exposed to second hand smoke (2% TIDES, 25% CANDLE); they had higher income households on average ($105 k ± 58 k TIDES, $42 k ± 29 k CANDLE) with fewer household members. Mothers in TIDES were more educated (80% with some college or beyond, 46% in CANDLE), more likely to be married (87%, 59% CANDLE), and less often overweight or obese (44%, 61% CANDLE) (Table 1).
Fig. 1.
Study sample construction from constituent cohorts.
Table 1.
Demographic characteristics of the study sample.
| Cohort |
|||
|---|---|---|---|
| Characteristic | Total (N = 1223) | CANDLE (N = 838) | TIDES (N = 385) |
| Child age | |||
| Mean (SD) | 4.96 (±1.04) | 4.32 (±0.48) | 6.33 (±0.34) |
| Child sex | |||
| Female | 641 (52 %) | 430 (51 %) | 211 (55 %) |
| Male | 582 (48 %) | 408 (49 %) | 174 (45 %) |
| Child race | |||
| White | 500 (42 %) | 242 (29 %) | 258 (69 %) |
| Black | 572 (48 %) | 527 (64 %) | 45 (12 %) |
| Other | 128 (11 %) | 58 (7 %) | 70 (19 %) |
| Birth order | |||
| First born | 541 (45 %) | 339 (40 %) | 202 (54 %) |
| Gestational week | |||
| Mean (SD) | 39.2 (±1.42) | 39.1 (±1.30) | 39.4 (±1.63) |
| Breastfeeding | |||
| Ever breastfed | 929 (76 %) | 574 (68 %) | 355 (93 %) |
| Postnatal secondhand smoking exposure | |||
| Yes | 214 (18 %) | 206 (25 %) | 8 (2 %) |
| Maternal age at delivery (year) | |||
| Mean (SD) | 28.5 (±5.95) | 27.0 (±5.60) | 31.7 (±5.33) |
| Region-, inflation-adjusted household income, $ | |||
| Mean (SD) | 62,200 (±50,100) | 42,400 (±29,300) | 105,000 (±58,300) |
| Household counts | |||
| 2–3 | 271 (23 %) | 186 (22 %) | 85 (23 %) |
| 4 | 497 (41 %) | 313 (38 %) | 184 (50 %) |
| 5 | 257 (21 %) | 193 (23 %) | 64 (17 %) |
| >=6 | 173 (14 %) | 140 (17 %) | 33 (9 %) |
| Maternal education | |||
| Less than high school | 91 (7 %) | 73 (9 %) | 18 (5 %) |
| High school/GED | 438 (36 %) | 378 (45 %) | 60 (16 %) |
| College/technical school | 408 (33 %) | 272 (32 %) | 136 (36 %) |
| Graduate or Professional degree | 281 (23 %) | 114 (14 %) | 167 (44 %) |
| Marital status | |||
| Married/living as married | 829 (68 %) | 495 (59 %) | 334 (87 %) |
| Single/living as single | 393 (32 %) | 342 (41 %) | 51 (13 %) |
| Pre-pregnancy weight status | |||
| Under/normal weight | 535 (44 %) | 326 (39 %) | 209 (56 %) |
| Overweight/obese Maternal IQ | 675 (56 %) | 509 (61 %) | 166 (44 %) |
| Mean (SD) | 101 (±17.4) | 96.6 (±16.4) | 111 (±15.5) |
| 2nd trimester urinary cotinine (ng/mL) | |||
| Mean (SD) | 2.85 (±12.2) | 3.35 (±13.0) | 1.77 (±10.2) |
| 2nd trimester urinary specific gravity | |||
| Mean (SD) | 1.02 (±0.00749) | 1.02 (±0.00706) | 1.01 (±0.00795) |
| Child Opportunity Educational Index (Prenatal) | |||
| Mean (SD) | −0.0242 (±0.0740) | −0.0442 (±0.0660) | 0.0190 (±0.0718) |
| Child Opportunity Economics Index (Prenatal) | |||
| Mean (SD) | −0.0513 (±0.265) | −0.106 (±0.264) | 0.0660 (±0.227) |
| Recruitment site | |||
| Memphis | 838 (69 %) | 838 (100 %) | – |
| San Francisco | 96 (8 %) | – | 96 (25 %) |
| Minneapolis | 110 (9 %) | – | 110 (29 %) |
| Rochester | 91 (7 %) | – | 91 (24 %) |
| Seattle | 88 (7 %) | – | 88 (23 %) |
| Child IQ at age 4–6 visit | |||
| Mean (SD) | 102.9 (±15.6) | 101.2 (15.1) | 106.6 (16.2) |
Missing: child race (n = 23), birth order (n = 10), breastfeeding (n = 3), postnatal secondhand smoke exposure (n = 36), maternal age at delivery (n = 18), household income (n = 62), household count (n = 25), maternal education (n = 5), maternal IQ (n = 9), specific gravity (n = 3), Child Opportunity Educational Index (n = 24), Child Opportunity Economics Index (n = 24).
3.1. Distribution of prenatal urinary OH-PAHs and IQ
The distribution of OH-PAH values in the analytic sample and by cohort, standardized to SG, are described in Supplemental Table 2. The proportion of sample below LOD was highest for 1-hydroxypyrene and 2/3/9-hydroxyfluroene and lowest for 2-hydroxynapthalene (Supplemental Table 2). Urinary OH-PAH concentrations were higher in CANDLE than TIDES participants for all metabolites with the exception of 3-hydroxyphenanthrene and 1-hydroxypyrene (Supplemental Table 2). OH-PAHs exhibited moderate-to-strong pairwise correlations (Supplemental Table 3). Child IQ averaged 102.9 (standard deviation 15.6) in the pooled study sample.
3.2. Associations between urinary OH-PAHs and IQ
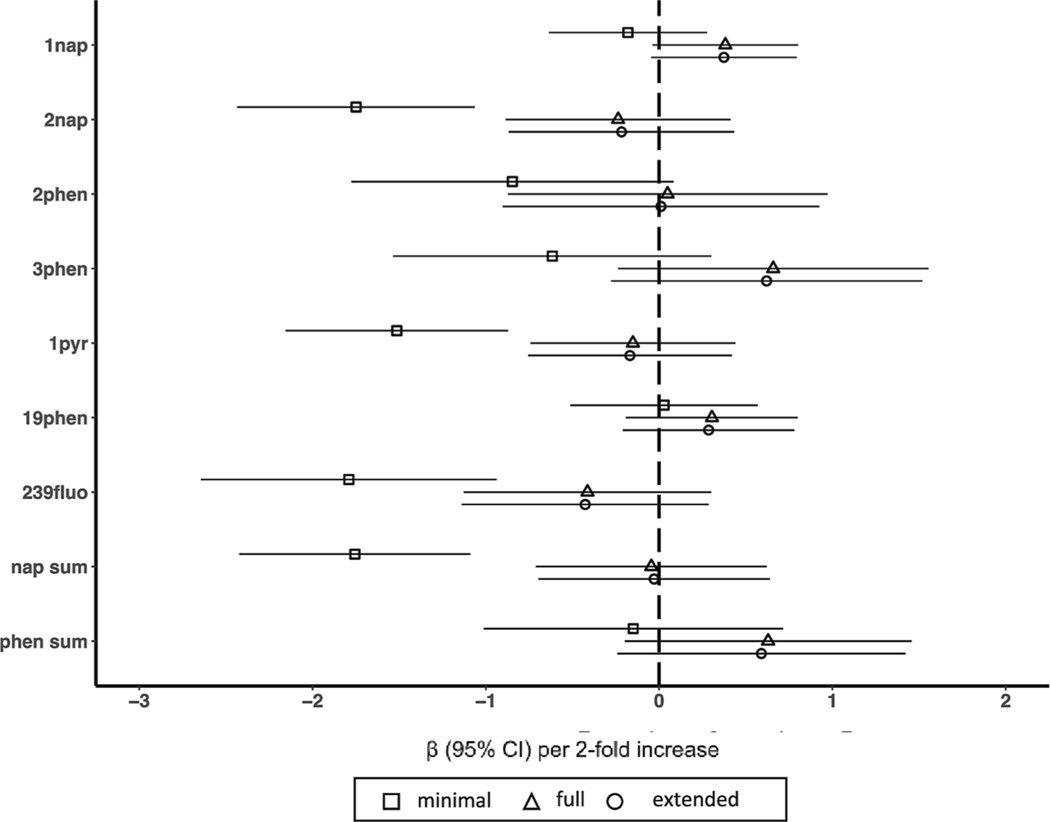
In minimally adjusted models, we observed significant inverse associations between several individual OH-PAH metabolites and IQ, most notably for 2-hydroxynapthalene, 1-hydroxypyrene, and 2/3/9-hydroxyfluorene. None of these associations persisted after full or extended adjustment for covariates and there were no meaningful differences between the results of the full and expanded adjustment models (Fig. 2).
Fig. 2.
Associations between individual polycyclic aromatic hydrocarbons and early childhood IQ. Models represent effect estimates (symbols) and 95% confidence intervals (bars) from linear regressions. The minimal adjustment model includes study site (categorical, 5 categories), child sex (binary), child age (continuous), batch (categorical), specific gravity (continuous), and an interaction between specific gravity and cohort to account for urinary dilution in each cohort’s OH-PAH measures. The fully adjustment model includes terms in the minimal adjustment model plus maternal education (categorical, 4 categories), maternal IQ (continuous), maternal age (continuous), marital status (binary, married or living as married vs. not), birth order (binary, first born vs. not), prenatal urinary cotinine (continuous), secondhand smoke exposure (binary, any vs. none), breastfeeding practice (binary, none vs. any), an interaction between household income and household count, child race, and each of the education and economics domains of the Child Opportunity Index (continuous). The extended adjustment model includes terms in the full adjustment model plus gestational age (continuous) and birthweight (continuous). All estimates represent effect per 2-fold increase in log OH-PAH. Abbreviations: 1nap = 1-hydroxynaphthalene, 2nap = 2-hydroxynaphthalene, 2phen = 2-hydroxyphenanthrene, 3phen = 3-hydroxyphenanthrene, 19-phen = 1/9-hydroxyphenanthrene, 239fluo = 2/3/9-hydroxyfluorene, 1pyr = 1-hydroxypyrene, nap sum = sum of hydroxynaphthalenes, phen sum = sum of hydroxyphenanthrenes, 95% CI = 95% Confidence Interval.
3.3. Sensitivity analyses
Estimates were similar when OH-PAH values were standardized to specific gravity to account for urinary dilution rather than by including specific gravity as a covariate (Supplemental Fig. 2); 1-hydroxynaphthalene was positively associated with IQ (β = 0.61, 95% CI: 0.02, 1.22; p = 0.04). Other sensitivity analyses, including using CLMI to impute OH-PAH values below LOD, restricting the study sample to those with IQ over 70, imputation of missing covariates, leave-one-out analyses (exclusion of individual study sites one at a time) and by study cohort, and using more parsimonious adjustment were also similar to the main analyses (Supplemental Figs. 3–7).
3.4. Secondary analyses
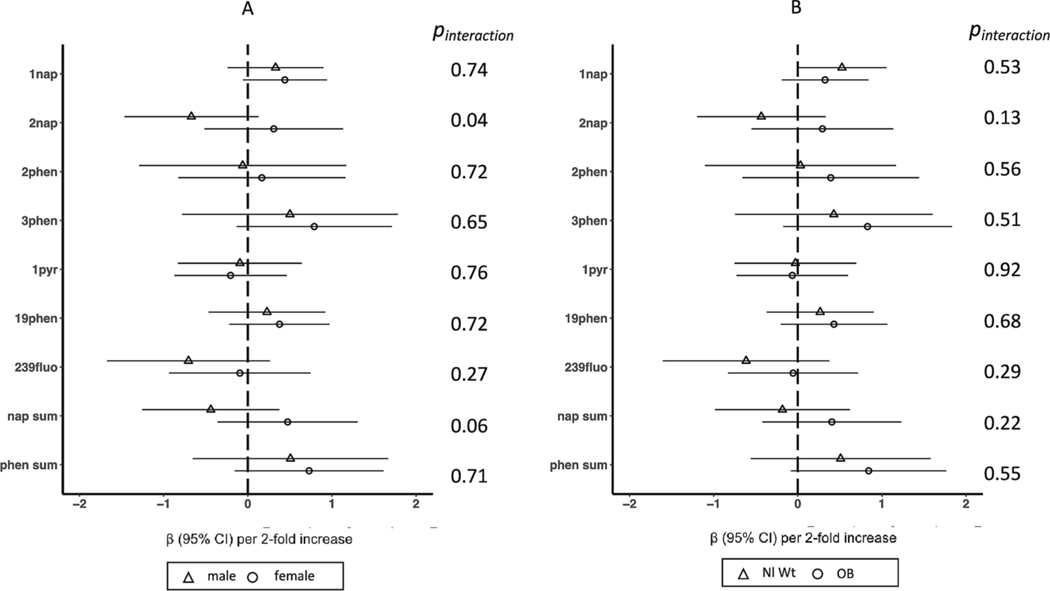
In secondary analyses of OH-PAH mixtures using WQS, we did not observe any association, either positive or negative, between a mixture of OH-PAHs and IQ (Table 2). Estimates of effect modification by child sex are provided in Fig. 3. There was no evidence of association between OH-PAHs and IQ in either males or females and there was no statistical evidence for effect modification by sex with the exception of 2-hydroxynapthalene, which was associated with lower IQ in males (βmales = −0.67, 95% CI: −1.5, 0.13), but not associated with IQ in females (βfemales = 0.31, 95% CI: −0.52, 1.1) (p-value for interaction = 0.04) (Fig. 3). We observed no evidence for effect modification by maternal pre-pregnancy BMI (Fig. 3).
Table 2.
Estimated effects and metabolite weights of OH-PAH matrix and early childhood IQ from WQS regression, by direction.
| Direction | Estimate | SE | p-value | 95% CI | Permutation p-value | |
|---|---|---|---|---|---|---|
| Positive | 0.39 | 0.20 | 0.05 | −0.01 | 0.79 | 0.15 |
| Negative | −0.18 | 0.24 | 0.45 | −0.64 | 0.28 | |
| Weight | Direction | |||||
|
|
|
|||||
| Positive | Negative | |||||
|
|
|
|
|
|||
| 3phen | 0.459 | 2nap | 0.467 | |||
| 19phen | 0.282 | 1pyr | 0.358 | |||
| 1nap | 0.227 | 239fluo | 0.119 | |||
| 239fluo | 0.018 | 2phen | 0.045 | |||
| 1pyr | 0.008 | 1nap | 0.010 | |||
| 2nap | 0.006 | 19phen | 0.002 | |||
| 2phen | 0.001 | 3phen | <0.001 |
Models represent effect estimates and 95% confidence intervals from Weighted Quantile Sum Regression, adjusted for study site (categorical, 5 categories), child sex (binary), child age (continuous), batch (categorical), specific gravity (continuous), and an interaction between specific gravity and cohort to account for urinary dilution in each cohort’s PAH measures, maternal education (categorical, 4 categories), maternal IQ (continuous), maternal age (continuous), marital status (binary, married or living as married vs. not), birth order (binary, first born vs. not), prenatal urinary cotinine (continuous), secondhand smoke exposure (binary, any vs. none), breastfeeding practice (binary, none vs. any), an interaction between household income and household count, child race, and each of the education and economics domains of the Child Opportunity Index (continuous).
Abbreviations: 1nap = 1-hydroxynaphthalene, 2nap = 2-hydroxynaphthalene, 2phen = 2-hydroxyphenanthrene, 3phen = 3-hydroxyphenanthrene, 19-phen = 1/9-hydroxyphenanthrene, 239fluo = 2/3/9-hydroxyfluorene, 1pyr = 1-hydroxypyrene, OH-PAH = Hydroxy-Polycyclic Aromatic Hydrocarbon, WQS = Weighted Quantile Sum.
Fig. 3.
Associations between individual polycyclic aromatic hydrocarbons and early childhood IQ, stratified by A) child sex and B) pre-pregnancy body mass index. Models represent effect estimates (symbols) and 95% confidence intervals (bars) from linear regressions. The full adjustment model includes study site (categorical, 5 categories), child sex (binary), child age (continuous), batch (categorical), specific gravity (continuous), and an interaction between specific gravity and cohort, maternal education (categorical, 4 categories), maternal IQ (continuous), maternal age (continuous), marital status (binary, married or living as married vs. not), birth order (binary, first born vs. not), prenatal urinary cotinine (continuous), secondhand smoke exposure (binary, any vs. none), breastfeeding practice (binary, none vs. any), an interaction between household income and household count, child race, and each of the education and economics domains of the Child Opportunity Index (continuous). All estimates represent effect per 2-fold increase in log OH-PAH. Abbreviations: 1nap = 1-hydroxynaphthalene, 2nap = 2-hydroxynaphthalene, 2phen = 2-hydroxyphenanthrene, 3phen = 3-hydroxyphenanthrene, 19-phen = 1/9-hydroxyphenanthrene, 239fluo = 2/3/9-hydroxyfluorene, 1pyr = 1-hydroxypyrene, nap sum = sum of hydroxynaphthalenes, phen sum = sum of hydroxyphenanthrenes, 95% CI = 95% Confidence Interval, Nl Wt = Normal weight, OB = Obese.
3.5. Associations between urinary PAHs and IQ across pregnancy
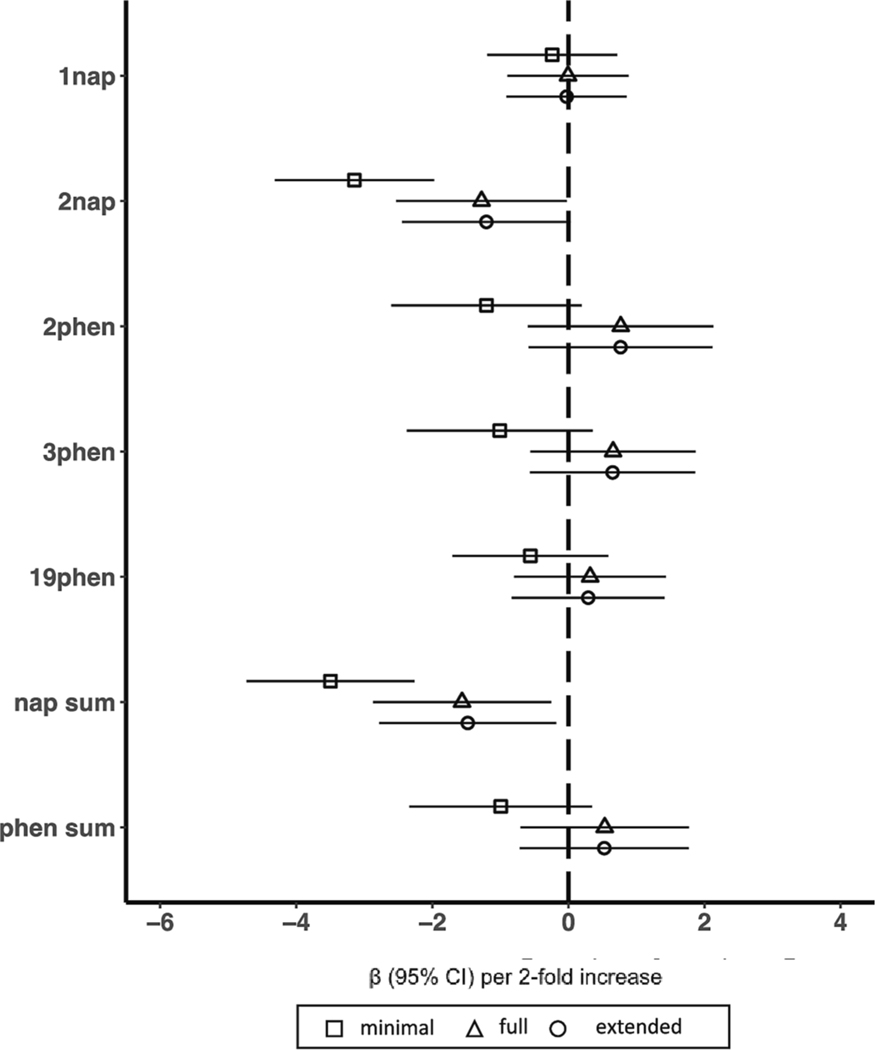
There were 356 TIDES participants who had prenatal OH-PAHs measured in all three periods of pregnancy and IQ data in childhood. The distribution of prenatal PAH metabolite concentrations averaged over all of pregnancy and by period of pregnancy are provided in Supplemental Table 4. Intraclass correlation coefficients for PAH metabolites across pregnancy ranged from 0.29 to 0.47 (Supplemental Table 5). Associations between prenatal OH-PAH and IQ averaged across three periods of pregnancy are shown in Fig. 4. 2-hydroxynapthalene (β = −1.28 [95%CI: −2.53, −0.03]) and sum of hydroxynapthalenes (β = −1.56 [95%CI: −2.87, −0.26]) were associated with lower IQ in fully adjusted models (Fig. 4). Associations of prenatal OH-PAH exposure and IQ stratified by pregnancy period with and without mutual adjustment for exposure in the other periods are shown in Supplemental Figures 6 and 7, respectively. In period-specific analyses, we observed an association between early pregnancy 2-hydroxynaphthalene and lower IQ that persisted with full (β = −1.14 [95%CI: −2.00, −0.28]) and extended (β = −1.15 [95%CI: −2.01, −0.29]) adjustment for covariates (Supplemental Figure 6) but was not statistically significant after adjustment for OH-PAHs in the other two pregnancy periods (Supplemental Figure 7).
Fig. 4.
Associations of polycyclic aromatic hydrocarbons averaged across trimesters of pregnancy and early childhood IQ in the TIDES cohort. Models represent effect estimates (symbols) and 95% confidence intervals (bars) from linear regressions. The minimal adjustment model includes study site (categorical, 5 categories), child sex (binary), child age (continuous), batch (categorical), specific gravity (continuous), and an interaction between specific gravity and cohort to account for urinary dilution in each cohort’s OH-PAH measures. The fully adjustment model includes terms in the minimal adjustment model plus maternal education (categorical, 4 categories), maternal IQ (continuous), maternal age (continuous), marital status (binary, married or living as married vs. not), birth order (binary, first born vs. not), prenatal urinary cotinine (continuous), secondhand smoke exposure (binary, any vs. none), breastfeeding practice (binary, none vs. any), an interaction between household income and household count, child race, and each of the education and economics domains of the Child Opportunity Index (continuous). The extended adjustment model includes terms in the full adjustment model plus gestational age (continuous) and birthweight (continuous). All estimates represent effect per 2-fold increase in log OH-PAH. Abbreviations: 1nap = 1-hydroxynaphthalene, 2nap = 2-hydroxynaphthalene, 2phen = 2-hydroxyphenanthrene, 3phen = 3-hydroxyphenanthrene, 19-phen = 1/9-hydroxyphenanthrene, nap sum = sum of hydroxynaphthalenes, phen sum = sum of hydroxyphenanthrenes, 95% CI = 95% Confidence Interval.
In additional secondary analyses we explored effect modification by child sex and pre-pregnancy BMI on pregnancy-averaged and early pregnancy associations of 2-hydroxynapthalene and IQ. While none of the tests for interaction were significant, patterns suggest interesting areas for future research in larger samples. We observed inverse associations between pregnancy-averaged 2-hydroxynapthalene and IQ in males (βmales = −1.59 [95% CI: −3.11, −0.06]) but not females (βfemales = −0.97 [95% CI: −2.84,0.89] (pinteraction = 0.60). The pattern was similar for early pregnancy 2-hydroxynapthalene exposure, with lower IQ in males (βmales = −1.50 [95% CI: −2.55,−0.45]), but no association in females (βfemales = −0.68 [95% CI:−1.85,0.48]) and no significant interaction (pinteraction = 0.24). Analyses of effect modification by pre-pregnancy BMI showed inverse associations between pregnancy-averaged 2-hydroxynapthalene and IQ in normal weight and underweight women (βnormal= −2.06 [95% CI:−3.73,−0.38]), with no evidence of associations in overweight or obese women (βobese = −0.30 [95% CI: −1.98, 1.37]) and without evidence of interaction (pinteraction = 0.12). Associations were similar for early pregnancy 2-hydroxynapthalene and IQ (βnormal = −1.67, [95% CI: −2.98, −0.35]; (βobese = −0.75, [95% CI: −1.71, 0.21]) pinteraction = 0.21).
4. Discussion
To the best of our knowledge, this is the largest prospective study to date of prenatal PAH exposure and preschool and early school-aged cognition and the first to examine associations using exposure measures across three windows of pregnancy. For our primary analyses, we examined associations of mid-pregnancy OH-PAH and IQ using a multi-center pooled cohort, taking advantage of a relatively large and diverse sample spanning several regions of the U.S. In this pooled sample, we did not observe evidence of associations between individual OH-PAH metabolites nor OH-PAH mixtures with child IQ. Moreover, we did not observe evidence of effect modification by child sex or maternal obesity, with the exception of potential sex-specific associations of 2-hydroxynapthalene, which was associated with lower IQ in boys but not girls.
In secondary analyses, we leveraged the availability of multiple OH-PAH measures across pregnancy in the smaller cohort, TIDES, to address two specific gaps in the existing literature: 1) potential measurement error resulting from single time point OH-PAH assessment, and 2) examination of sensitive periods during pregnancy. OH-PAH metabolites in a spot urine sample reflect exposure only in the hours prior to urine collection, due to the short half-lives of OH-PAHs(Li et al., 2012). While it is standard for epidemiological studies to include a single measure of prenatal PAH exposure (Edwards et al., 2010; Perera et al., 2009), this approach is likely vulnerable to measurement error and may bias measured associations toward the null(Cathey et al., 2018). To address this limitation, we calculated pregnancy-average exposures using measures in early, mid and late pregnancy in a subset of our study sample with multiple OH-PAH measures available. These pregnancy-average OH-PAH metabolites should better approximate exposure across pregnancy. We observed that pregnancy-average 2-hydroxynapthalene as well as the sum of naphthalene mono-hydroxylated metabolites were both associated with lower IQ. If these reflect true adverse impacts of PAHs upon fetal neurodevelopment, it may be that such associations are more easily detectable by averaging multiple measures of PAHs across pregnancy than using a single pregnancy assessment. In addition, we used the multiple measures of OH-PAHs available in TIDES to explore evidence for sensitive windows of exposure. These analyses indicated that adverse associations between IQ and OH-PAH metabolites were most evident for early pregnancy exposures, in particular for 2-hydroxynapthalene. This suggests that early pregnancy may be a period of greater sensitivity, which is consistent with evidence for some other chemical exposures(Zhu et al., 2020). However, our study was not specifically designed to test sensitive periods of PAH exposure during pregnancy, which would require a larger sample size sufficiently powered to enable formal testing of differences across timing of exposure (Sánchez et al., 2011); thus, these preliminary findings need to be replicated in other study populations.
Epidemiological evidence of adverse associations between PAHs and child cognitive development has been somewhat inconsistent. In a New York City cohort of non-smoking Black and Dominican-American mothers, prenatal PAH exposure measured in personal air in the third trimester of pregnancy was associated with developmental delay in their children at age 3(Perera et al., 2006) and reduced IQ at age 5(Perera et al., 2009). In a second cohort of mother–child dyads in Krakow, prenatal PAH exposure measured by personal air in the second or third trimester of pregnancy was associated with lower nonverbal reasoning skills at age 5(Edwards et al., 2010). In contrast, a third prospective cohort of mother–child dyads in Chongqing, China showed no association between prenatal PAHs measured in DNA adducts and IQ at age 5, and associations of PAH with IQ only in the presence of interaction with environmental tobacco smoke(Perera et al., 2012). Likewise, a cross-sectional study using data from the National Health and Nutrition Examination Survey found no evidence of associations between urinary PAH metabolites and the need for educational assistance in middle childhood, though their PAH measures were contemporaneous with their outcome rather than a reflection of the in utero environment(Abid et al., 2014).
There are several possible explanations for heterogeneity in the literature, including variability in magnitude of exposure between populations. For example, the ambient air PAH concentrations measured in the Krakow cohort were very high relative to measurements from other urban settings in Europe(Binková et al., 1995) and Canada (Nethery et al., 2012), and more than five times that observed in the New York cohort(Jedrychowski et al., 2005). Differences in the method used to measure PAHs (e.g., urine, cord blood, personal air), furthermore, preclude direct comparison of PAH concentrations across cohorts. The PAH metabolites captured in our urinary measures were limited to low molecular weight PAHs, which reflect both dietary and air pollution sources (Agency for Toxic Substances and Disease Registry (ATSDR), 1995). High molecular weight PAHs are largely excreted in feces (Ramesh et al., 2004), not urine, and nearly all of the high molecular weight PAHs measured in our study were excluded from analyses due to a large proportion of samples being below the limit of detection. The urinary OH-PAH metabolites concentrations measured in this study were similar to other studies of pregnant women and women participating in NHANES(Woodruff et al., 2011). The largely null findings of mid-pregnancy PAH in our full cohort could be explained by relatively low PAH exposures. The composition of prenatal PAH mixtures also varies across study regions and may lead to inconsistency across studies. For example, in the Chongqing cohort, the major source of PAH was a coal-fired power plant that operated the winter before child participants were born, which may have led to a substantially different profile of PAH exposure(Perera et al., 2012). In comparison, a major source of airborne PAHs in New York City is thought to be vehicular traffic, which may be more similar to the sources and thus the composition of PAH mixtures encountered in our cohort(Perera et al., 2009).
Between-study heterogeneity in results could also stem from differences in methodological approach. We observed that several individual OH-PAH metabolites were negatively associated with IQ in the full cohort analysis but only in the model with minimal covariate adjustment, and our associations were greatly attenuated with full covariate adjustment. The New York and Krakow studies(Edwards et al., 2010; Perera et al., 2009) utilized less robust adjustment models, including using maternal education alone as a proxy for socioeconomic status, while we controlled for multiple domains and levels of socioeconomic status. We considered whether the results of prior studies were affected by residual confounding; to explore this, we conducted additional sensitivity analyses utilizing similar covariate adjustment as in the New York and Krakow studies. Even with this less robust adjustment, we saw no evidence of associations between prenatal OH-PAH metabolites and IQ in our dataset, which suggests that covariate adjustment cannot entirely explain the discrepancies between studies.
Effect modification may also contribute to the inconsistency in findings across studies. Based on possible mechanisms for neurotoxicity of prenatal PAH, we hypothesized that associations may vary by participant characteristics, including infant sex and maternal obesity. In both our full cohort and TIDES-only analyses, 2-hydroxynapthalene was associated with lower IQ in boys but not girls. This is notable as studies in animals and humans suggest that the neurodevelopmental consequences from environmental toxicant exposure differ between males and females(Gade et al., 2021). PAHs in particular are linked to neuroinflammation, (Saunders et al., 2006; Deanna D Wormley et al., 2004) which trigger sex-specific responses in the fetus,(McCarthy et al., 2017) and are theorized to underlie differences in the frequency and severity of neurodevelopmental disorders between sexes(McCarthy et al., 2017). PAHs also accumulate in fatty tissue (Pastor-Belda et al., 2019), potentially exposing fetuses of overweight or obese women to a greater burden of PAH exposure even at similar environmental exposures (Vizcaino et al., 2014). We saw no evidence for differences in associations by maternal obesity in the full cohort analyses. In our TIDES-only analyses we observed inverse associations with IQ for 2-hydroxynapthalene in normal and underweight women and not overweight or obese women, but without statistical evidence for effect modification. Given the mixed results and exploratory nature of some of these analyses, future studies that are well-powered to detect effect modification and with larger variation in exposure are needed to characterize the populations most vulnerable to PAH exposure.
Despite primarily null findings in this analysis and others, a growing body of toxicological evidence provides biological plausibility for neurotoxic effects of prenatal PAH exposure. A number of pathophysiological mechanisms have been proposed for how PAHs harm the developing brain. PAHs are transferred across the placenta, easily cross the blood–brain barrier, and are accumulated and metabolized in the brain(Rouet et al., 1981). PAH exposure is associated with endocrine disruption(Takeda et al., 2004) and alterations to global methylation, implying epigenetic modification (Herbstman et al., 2012), with subsequent effects on neurodevelopment. Toxicological studies also point to oxidative stress, suggesting that PAH metabolism increases intracellular reactive oxygen species and inhibits the brain antioxidant scavenging system(Saunders et al., 2006), as well as initiating p38MAP kinase pathways and activation of inflammatory microglial cells and bystander neuronal death(Dutta et al., 2010). Other suggested mechanisms include downregulation of developmental ionotropic glutamate receptor sub-unit expression, which is critical for synaptic formation and the maintenance of synaptic plasticity mechanisms during cortical and hippocampal development(D. D. Wormley et al., 2004; Deanna D Wormley et al., 2004).
There are important limitations to our study. Our primary analyses leveraged the large sample size of a multi-cohort sample but relied on a single measure of prenatal PAH exposure. As discussed above, this is an important limitation because urinary OH-PAH metabolites reflect exposure in approximately the prior 12 h(Jongeneelen et al., 1990) and have exhibited moderate to high intraindividual variability in some (Cathey et al., 2018) but not all(Dobraca et al., 2018) prior analyses of urinary PAHs measured repeatedly. Notably, we were able to reduce the measurement error associated with using a single timepoint in our analysis using our subsample of TIDES participants with three measures across pregnancy. However these three spot urinary PAH measures reflect exposure over a relatively short window of time and as a result limited our ability to further investigate window-specific effects. Second, we were interested in both the associations of individual PAH compounds as well as their potential effects as a mixture; we therefore had a large number of prospectively defined analyses, increasing the opportunity for chance findings. A third possible limitation in our study sample is the pooling of two cohorts that have different demographics, average age at outcome assessment, and measures of IQ. Our “leave one site out” sensitivity analyses supported robustness of the study results to cohort and site inclusion or exclusion. Although the SB-5 and WISC-V IQ measures were standardized to age and estimates were adjusted for age at assessment, the children administered the WISC-V were older on average than those given the SB-5, which may have contributed to the differences in IQ scores, and we cannot exclude the possibility of residual confounding by child age. Fourth, all of our PAH measures were subject to left-censoring due to batch-specific LOD, which could be a source of bias in measured associations.(Hewett and Ganser, 2007). Our method of imputing PAH measures using the value of is a limitation as it centers all imputed values on a single value without variability as opposed to a true distribution of low exposures. It is reassuring that our results were not sensitive to using likelihood-based CLMI in place of the primary approach of substituting the value of , but this is still a limitation since CLMI assumes a common distribution of PAHs across batches, which was not observed in our data (Supplemental Table 2). Finally, because we excluded children of mothers who smoked during pregnancy, our results are only generalizable to a non-smoking population.
In summary, this study represents the largest and most robustly adjusted epidemiological analysis of prenatal PAH exposure and childhood cognition, including examination of multiple measurements of PAH across time in a subsample. In our primary analysis of mid-pregnancy PAH exposure in the overall, multi-cohort sample, we observed no associations. However, in secondary analyses in the TIDES cohort, we found that average exposure across pregnancy and early pregnancy exposure to 2-hydroxynapthalene were each associated with lower IQ. Better-powered studies assessing multiple prenatal PAH measures as well as early pregnancy exposures to PAH are needed to confirm these findings and advance our understanding of PAH impacts on child neurodevelopment.
Supplementary Material
Acknowledgments
The authors thank the participating families for their time and commitment to the CANDLE and TIDES cohorts. We also appreciate the dedication of the study research staff and investigators who have worked diligently independently and collaboratively to generate the ECHO PATHWAYS Consortium.
Funding
The ECHO PATHWAYS Consortium is funded by NIH (UG3/UH3OD023271, P30ES007033). The Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study was funded by the Urban Child Institute and NIH R01HL109977. The Infant Development and Environment Study (TIDES) study was funded by NIH R01ES016863, NIH R01ES25169, and National Institute of Environmental Health Sciences (NIEHS) Intramural Funding (ZIA103313): Reproductive outcomes and oxidative stress in TIDES (ROOST). Dr. Kannan analyzed OH-PAH metabolites in TIDES with support from the New York University ECHO Cohort Center (UG3/UH3OD023305 (PI: Leonardo Trasande)). Dr. Barrett is supported in part by the NIEHS sponsored Rutgers Center for Environmental Exposure and Disease (CEED) grant: P30ES005022. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This manuscript has been reviewed by PATHWAYS for scientific content and consistency of data interpretation with previous PATHWAYS publications.
Abbreviations:
- 1nap
1-hydroxynaphthalene
- 2nap
2-hydroxynaphthalene
- 2phen
2-hydroxyphenanthrene
- 3phen
3-hydroxyphenanthrene
- 19-phen
1/9-hydroxyphenanthrene
- 239fluo
2/3/9-hydroxyfluorene
- 1pyr
1-hydroxypyrene
- nap sum
sum of hydroxynaphthalenes
- phen sum
sum of hydroxyphenanthrenes
- 95% CI
95% Confidence Interval
- BMI
Body mass index
- CLMI
Censored Likelihood Multiple Imputation
- LOD
Limit of Detection
- IQ
Intellectual Quotient
- OH-PAH
Hydroxy-Polycyclic Aromatic Hydrocarbon
- PAH
Polycyclic Aromatic Hydrocarbon
- WISC-V
Wechsler Intelligence Scale for Children Fifth Edition
- WQS
Weighted Quantile Sum Regression
Footnotes
Ethical Approval
ECHO PATHWAYS research activities were approved by the University of Washington Human Subjects Division (#STUDY00000638).
CRediT authorship contribution statement
Bob Sun: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review and editing. Erin R Wallace: Conceptualization, Methodology, Formal analysis, Visualization, Writing – original draft, Writing – review and editing. Yu Ni: Formal analysis, Writing – review and editing. Christine T. Loftus: Conceptualization, Methodology, Writing – review and editing. Adam Szpiro: Methodology, Writing – review and editing. Drew Day: Methodology, Writing – review and editing. Emily S. Barrett: Writing – review and editing. Ruby HN Nguyen: Writing – review and editing. Kurunthachalam Kannan: Resources, Formal analysis. Morgan Robinson: Resources, Formal analysis. Nicole R. Bush: Funding acquisition, Writing – review and editing. Sheela Sathyanarayana: Funding acquisition, Writing – review and editing. Alex Mason: Funding acquisition, Writing – review and editing. Shanna H. Swan: Funding acquisition, Writing – review and editing. Leonardo Trasande: Funding acquisition, Writing – review and editing. Catherine J. Karr: Conceptualization, Methodology, Funding acquisition, Supervision, Writing – review and editing. Kaja Z. LeWinn: Conceptualization, Methodology, Funding acquisition, Supervision, Writing – review and editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2023.108009.
Data Availability
The data utilized for this study are not publicly available but de-identified data may be available on request, subject to approval by the internal review board and under a formal data use agreement. Contact the corresponding author for more information.
References
- Abid Z, Roy A, Herbstman JB, Ettinger AS, 2014. Urinary polycyclic aromatic hydrocarbon metabolites and attention/deficit hyperactivity disorder, learning disability, and special education in U.S. children aged 6 to 15. J. Environ. Public Health 2014, 628508. 10.1155/2014/628508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo-Garcia D, McArdle N, Hardy EF, Crisan UI, Romano B, Norris D, Baek M, Reece J, 2014. The child opportunity index: improving collaboration between community development and public health. Health Aff (Millwood) 33, 1948–1957. 10.1377/hlthaff.2014.0679. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR), 1995. Toxicological profile for polycyclic aromatic hydrocarbons, U.S. Department of Health and Human Services. [PubMed] [Google Scholar]
- Axelrod BN, 2002. Validity of the Wechsler abbreviated scale of intelligence and other very short forms of estimating intellectual functioning. Assessment 9, 17–23. 10.1177/1073191102009001003. [DOI] [PubMed] [Google Scholar]
- Binková B, Lewtas J, Míšková I, Leníšcek J, Šrám R, 1995. DNA adducts and personal air monitoring of carcinogenic polycyclic aromatic hydrocarbons in an environmentally exposed population. Carcinogenesis 16, 1037–1046. 10.1093/CARCIN/16.5.1037. [DOI] [PubMed] [Google Scholar]
- Boss J, Mukherjee B, Ferguson KK, Aker A, Alshawabkeh AN, Cordero JF, Meeker JD, Kim S, 2019. Estimating Outcome-Exposure Associations when Exposure Biomarker Detection Limits vary Across Batches. Epidemiology 30, 746–755. 10.1097/EDE.0000000000001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Economic Analysis, 2021. Real Personal Income & Regional Price Parities Methodology [WWW Document]. U.S. Department of Commerce; https://www.bea.gov/data/prices-inflation/regional-price-parities-state-and-metro-area (accessed 4.17.22. [Google Scholar]
- Carrico C, Gennings C, Wheeler DC, Factor-Litvak P, 2015. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J Agric Biol Environ Stat 20, 100–120. 10.1007/s13253-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathey A, Ferguson KK, McElrath TF, Cantonwine DE, Pace G, Alshawabkeh A, Cordero JF, Meeker JD, 2018. Distribution and predictors of urinary polycyclic aromatic hydrocarbon metabolites in two pregnancy cohort studies. Environ Pollut 232, 556–562. 10.1016/j.envpol.2017.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YHM, Hsu HHL, Coull BA, Bellinger DC, Kloog I, Schwartz J, Wright RO, Wright RJ, 2016. Prenatal particulate air pollution and neurodevelopment in urban children: Examining sensitive windows and sex-specific associations. Environ Int 87, 56–65. 10.1016/J.ENVINT.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnota J, Gennings C, Wheeler DC, 2015. Assessment of weighted quantile sum regression for modeling chemical mixtures and cancer risk. Cancer Inform 14, 159–171. 10.4137/CIN.S17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DB, Sathyanarayana S, Lewinn KZ, Karr CJ, Mason WA, Szpiro AA, 2022. A Permutation Test-Based Approach to Strengthening Inference on the Effects of Environmental Mixtures: Comparison between Single-Index Analytic Methods. Environ Health Perspect 130. 10.1289/EHP10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobraca D, Lum R, Sjödin A, Calafat AM, Laurent CA, Kushi LH, Windham GC, 2018. Urinary biomarkers of polycyclic aromatic hydrocarbons in pre- and peripubertal girls in Northern California: Predictors of exposure and temporal variability. Environ Res 165, 46–54. 10.1016/J.ENVRES.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta K, Ghosh D, Nazmi A, Kumawat KL, Basu A, 2010. A common carcinogen benzo[a]pyrene causes neuronal death in mouse via microglial activation. PLoS One 5, e9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SC, Jedrychowski W, Butscher M, Camann D, Kieltyka A, Mroz E, Flak E, Li Z, Wang S, Rauh V, Perera F, 2010. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children’s intelligence at 5 years of age in a prospective cohort study in Poland. Environ Health Perspect 118, 1326–1331. 10.1289/ehp.0901070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Mcelrath TF, Pace GG, Weller D, Zeng L, Pennathur S, Cantonwine DE, Meeker JD, Sci E, Author T, 2017. Urinary polycyclic aromatic hydrocarbon metabolite associations with biomarkers of inflammation, angiogenesis, and oxidative stress in pregnant women HHS Public Access Author manuscript. Environ Sci Technol 51, 4652–4660. 10.1021/acs.est.7b01252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gade M, Comfort N, Re DB, 2021. Sex-specific neurotoxic effects of heavy metal pollutants: Epidemiological, experimental evidence and candidate mechanisms. Environ Res 201. 10.1016/j.envres.2021.111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Senthilkumar K, Alomirah H, Moon H-B, Minh TB, Mohd MA, Nakata H, Kannan K, 2013. Concentrations and profiles of urinary polycyclic aromatic hydrocarbon metabolites (OH-PAHs) in several Asian countries. Environ Sci Technol 47, 2932–2938. 10.1021/es3052262. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Tang D, Zhu D, Qu L, Sjödin A, Li Z, Camann D, Perera FP, 2012. Prenatal exposure to polycyclic aromatic hydrocarbons, benzo[a]pyrene-DNA adducts, and genomic DNA methylation in cord blood. Environ Health Perspect 120, 733–738. 10.1289/EHP.1104056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M, King K, Weitzman M, 2008. Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment. Curr Opin Pediatr 20, 184–190. 10.1097/MOP.0B013E3282F56165. [DOI] [PubMed] [Google Scholar]
- Hewett P, Ganser GH, 2007. A comparison of several methods for analyzing censored data. Ann Occup Hyg 51, 611–632. 10.1093/ANNHYG/MEM045. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Galas A, Pac A, Flak E, Camman D, Rauh V, Perera F, 2005. Prenatal ambient air exposure to polycyclic aromatic hydrocarbons and the occurrence of respiratory symptoms over the first year of life. Eur J Epidemiol 20, 775–782. 10.1007/S10654-005-1048-1. [DOI] [PubMed] [Google Scholar]
- Jedrychowski WA, Perera FP, Camann D, Spengler J, Butscher M, Mroz E, Majewska R, Flak E, Jacek R, Sowa A, 2015. Prenatal exposure to polycyclic aromatic hydrocarbons and cognitive dysfunction in children. Environ Sci Pollut Res Int 22, 3631–3639. 10.1007/s11356-014-3627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongeneelen FJ, van Leeuwen FE, Oosterink S, Anzion RB, van der Loop F, Bos RP, van Veen HG, 1990. Ambient and biological monitoring of cokeoven workers: determinants of the internal dose of polycyclic aromatic hydrocarbons. Br J Ind Med 47, 454–461. 10.1136/oem.47.7.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan K, Stathis A, Mazzella MJ, Andra SS, Barr DB, Hecht SS, Merrill LS, Galusha AL, Parsons PJ, 2021. Quality assurance and harmonization for targeted biomonitoring measurements of environmental organic chemicals across the Children’s Health Exposure Analysis Resource laboratory network. Int J Hyg Environ Health 234. 10.1016/J.IJHEH.2021.113741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapraun DF, Zurlinden TJ, Verner MA, Chiang C, Dzierlenga MW, Carlson LM, Schlosser PM, Lehmann GM, 2022. A Generic Pharmacokinetic Model for Quantifying Mother-to-Offspring Transfer of Lipophilic Persistent Environmental Chemicals. Toxicol. Sci 189, 155–174. 10.1093/TOXSCI/KFAC084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullar SS, Shao K, Surette C, Foucher D, Mergler D, Cormier P, Bellinger DC, Barbeau B, Sauvé S, Bouchard MF, 2019. A benchmark concentration analysis for manganese in drinking water and IQ deficits in children. Environ Int 130. 10.1016/J.ENVINT.2019.05.083. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R, 2005. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect 113, 894–899. 10.1289/EHP.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lertxundi A, Andiarena A, Martínez MD, Ayerdi M, Murcia M, Estarlich M, Guxens M, Sunyer J, Julvez J, Ibarluzea J, 2019. Prenatal exposure to PM2.5 and NO2 and sex-dependent infant cognitive and motor development. Environ Res 174, 114–121. 10.1016/j.envres.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Levine L, Fahy J, 1945. Evaluation of urinary lead concentrations. I. The significance of the specific gravity. J Ind Hyg Toxicol 27, 217–223. [Google Scholar]
- Lewinn KZ, Bush NR, Batra A, Tylavsky F, Rehkopf D, 2020. Identification of Modifiable Social and Behavioral Factors Associated with Childhood Cognitive Performance. JAMA Pediatr 174, 1063–1072. 10.1001/JAMAPEDIATRICS.2020.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeWinn KZ, Karr CJ, Hazlehurst M, Carroll K, Loftus C, Nguyen R, Barrett E, Swan SH, Szpiro AA, Paquette A, Moore P, Spalt E, Younglove L, Sullivan A, Colburn T, Byington N, Sims Taylor L, Moe S, Wang S, Cordeiro A, Mattias A, Powell J, Johnson T, Norona-Zhou A, Mason A, Bush NR, Sathyanarayana S, 2022. Cohort profile: the ECHO prenatal and early childhood pathways to health consortium (ECHO-PATHWAYS). BMJ Open 12, e064288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Romanoff L, Bartell S, Pittman EN, Trinidad DA, McClean M, Webster TF, Sjödin A, 2012. Excretion profiles and half-lives of ten urinary polycyclic aromatic hydrocarbon metabolites after dietary exposure. Chem Res Toxicol 25, 1452–1461. 10.1021/TX300108E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallister MM, Maguire M, Ramesh A, Aimin Q, Liu S, Khoshbouei H, Aschner M, Ebner FF, Hood DB, 2008. Prenatal exposure to benzo(a)pyrene impairs later-life cortical neuronal function. Neurotoxicology 29, 846–854. 10.1016/j.neuro.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Nugent BM, Lenz KM, 2017. Neuroimmunology and neuroepigenetics in the establishment of sex differences in the brain. Nat Rev Neurosci 18, 471–484. 10.1038/NRN.2017.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollon J, David AS, Zammit S, Lewis G, Reichenberg A, 2018. Course of Cognitive Development From Infancy to Early Adulthood in the Psychosis Spectrum. JAMA Psychiat. 75, 270–279. 10.1001/JAMAPSYCHIATRY.2017.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nethery E, Wheeler AJ, Fisher M, Sjödin A, Li Z, Romanoff LC, Foster W, Arbuckle TE, 2012. Urinary polycyclic aromatic hydrocarbons as a biomarker of exposure to PAHs in air: A pilot study among pregnant women. J Expo Sci Environ Epidemiol. 10.1038/jes.2011.32. [DOI] [PubMed]
- Ni Y, Loftus CT, Szpiro AA, Young MT, Hazlehurst MF, Murphy LE, Tylavsky FA, Mason WA, Lewinn KZ, Sathyanarayana S, Barrett ES, Bush NR, Karr CJ, 2022. Associations of Pre- and Postnatal Air Pollution Exposures with Child Behavioral Problems and Cognitive Performance: A U.S. Multi-Cohort Study. Environ. Health Perspect. 130. 10.1289/EHP10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Belda M, Campillo N, Arroyo-Manzanares N, Torres C, Pérez-Cárceles MD, Hernández-Córdoba M, Viñas P, 2019. Bioaccumulation of Polycyclic Aromatic Hydrocarbons for Forensic Assessment Using Gas Chromatography-Mass Spectrometry. Chem Res Toxicol 32, 1680–1688. 10.1021/ACS.CHEMRESTOX.9B00213. [DOI] [PubMed] [Google Scholar]
- Perera FP, Li Z, Whyatt R, Hoepner L, Wang S, Camann D, Rauh V, 2009. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics 124, e195–e202. 10.1542/peds.2008-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Chang HW, Tang D, Roen EL, Herbstman J, Margolis A, Huang TJ, Miller RL, Wang S, Rauh V, 2014. Early-life exposure to polycyclic aromatic hydrocarbons and ADHD behavior problems. PLoS One 9. 10.1371/journal.pone.0111670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F, Li TY, Lin C, Tang D, 2012. Effects of prenatal polycyclic aromatic hydrocarbon exposure and environmental tobacco smoke on child IQ in a Chinese cohort. Environ Res 114, 40–46. 10.1016/j.envres.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai W-Y, Tang D, Diaz D, Hoepner L, Barr D, Tu Y-H, Camann D, Kinney P, 2006. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect 114, 1287–1292. 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiford SE, 2018. The Wechsler Intelligence Scale for Children—Fifth Edition Integrated., in: Contemporary Intellectual Assessment: Theories, Tests, and Issues, 4th Ed. The Guilford Press, New York, NY, US, pp. 303–332. [Google Scholar]
- Ramesh A, Walker SA, Hood DB, Guillén MD, Schneider K, Weyand EH, 2004. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int J Toxicol 23, 301–333. 10.1080/10915810490517063. [DOI] [PubMed] [Google Scholar]
- Roid GH, 2003. Stanford-Binet Intelligence Scales, Fifth Edition (SB: V). Riverside Publishing, Itasca, IL. [Google Scholar]
- Rouet P, Alexandrov K, Markovits P, Frayssinet C, Dansette PM, 1981. Metabolism of benzo[a]pyrene by brain microsomes of fetal and adult rats and mice. Induction by 5,6 benzoflavone, comparison with liver and lung microsomal activities. Carcinogenesis 2, 919–926. 10.1093/CARCIN/2.9.919. [DOI] [PubMed] [Google Scholar]
- Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM, 2011. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect 119, 409–415. 10.1289/EHP.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler JM, 2018. Assessment of children : cognitive foundations and applications, 6th ed. Jerome M Sattler, Publisher Inc., La Mesa, CA. [Google Scholar]
- Saunders CR, Das SK, Ramesh A, Shockley DC, Mukherjee S, 2006. Benzo(a) pyrene-induced acute neurotoxicity in the F-344 rat: Role of oxidative stress. J. Appl. Toxicol 26, 427–438. 10.1002/JAT.1157. [DOI] [PubMed] [Google Scholar]
- Schick SF, Blount BC, Jacob PR, Saliba NA, Bernert JT, el Hellani A, Jatlow P, Pappas RS, Wang L, Foulds J, Ghosh A, Hecht SS, Gomez JC, Martin JR, Mesaros C, Srivastava S, St Helen G, Tarran R, Lorkiewicz PK, Blair IA, Kimmel HL, Doerschuk CM, Benowitz NL, Bhatnagar A, 2017. Biomarkers of exposure to new and emerging tobacco delivery products. Am J Physiol Lung Cell Mol Physiol 313, L425–L452. 10.1152/ajplung.00343.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skupińska K, Misiewicz I, Kasprzycka-Guttman T, 2004. Polycyclic aromatic hydrocarbons: Physicochemical properties, environmental appearance and impact on living organisms. Acta Poloniae Pharmaceutica - Drug Research 61, 233–240. [PubMed] [Google Scholar]
- Soleimani F, Zaheri F, Abdi F, 2014. Long-term neurodevelopmental outcomes after preterm birth. Iran Red Crescent Med J 16, e17965–e. 10.5812/ircmj.17965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Yoshinaga J, 2007. Inhalation and dietary exposure to polycyclic aromatic hydrocarbons and urinary 1-hydroxypyrene in non-smoking university students. Int Arch Occup Environ Health 81, 115–121. 10.1007/S00420-007-0188-X. [DOI] [PubMed] [Google Scholar]
- Takeda K, Tsukue N, Yoshida S, 2004. Endocrine-disrupting activity of chemicals in diesel exhaust and diesel exhaust particles. Environ Sci 11, 33–45. [PubMed] [Google Scholar]
- Vizcaino E, Grimalt JO, Glomstad B, Fernández-Somoano A, Tardón A, 2014. Gestational weight gain and exposure of newborns to persistent organic pollutants. Environ Health Perspect 122, 873–879. 10.1289/EHP.1306758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 2003. Wechsler Intelligence Scale for Children—WISC-IV. The Psychological Corporation, New York. [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM, 2011. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect 119, 878–885. 10.1289/EHP.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormley DD, Chirwa S, Nayyar T, Wu J, Johnson S, Brown LA, Harris E, Hood DB, 2004a. Inhaled benzo(a)pyrene impairs long-term potentiation in the F1 generation rat dentate gyrus. Cell Mol Biol (Noisy-le-grand) 50, 715–721. [PubMed] [Google Scholar]
- Wormley DD, Ramesh A, Hood DB, 2004b. Environmental contaminant-mixture effects on CNS development, plasticity, and behavior. Toxicol Appl Pharmacol 197, 49–65. 10.1016/j.taap.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Zhu Y. Duo, Wu XY, Yan S. Qin, Huang K, Tong J, Gao H, Xie Y, Tao S. Man, Ding P, Zhu P, Tao F. Biao, 2020. Domain- and trimester-specific effect of prenatal phthalate exposure on preschooler cognitive development in the Ma’anshan Birth Cohort (MABC) study. Environ Int 142. 10.1016/J.ENVINT.2020.105882. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data utilized for this study are not publicly available but de-identified data may be available on request, subject to approval by the internal review board and under a formal data use agreement. Contact the corresponding author for more information.