Abstract
Background:
Only a limited number of patients with major depressive disorder (MDD) respond to a first course of antidepressant medication (ADM). We investigated the feasibility of creating a baseline model to determine which these would be among patients beginning ADM treatment in the US Veterans Health Administration (VHA).
Methods:
A 2018–2020 national sample of n=660 VHA patients receiving ADM treatment for MDD completed an extensive baseline self-report assessment near the beginning of treatment and a 3-month self-report follow-up assessment. Using baseline self-report data along with administrative and geospatial data, an ensemble machine learning method was used to develop a model for 3-month treatment response defined by the Quick Inventory of Depression Symptomatology Self-Report and a modified Sheehan Disability Scale. The model was developed in a 70% training sample and tested in the remaining 30% test sample.
Results:
35.7% of patients responded to treatment. The prediction model had an area under the ROC curve (SE) of 0.66 (0.04) in the test sample. A strong gradient in probability (SE) of treatment response was found across three subsamples of the test sample using training sample thresholds for high [45.6% (5.5)], intermediate [34.5% (7.6)], and low [11.1% (4.9)] probabilities of response. Baseline symptom severity, comorbidity, treatment characteristics (expectations, history, and aspects of current treatment), and protective/resilience factors were the most important predictors.
Conclusions:
Although these results are promising, parallel models to predict response to alternative treatments based on data collected before initiating treatment would be needed for such models to help guide treatment selection.
Keywords: Antidepressant medication, Clinical decision support, Depression, Machine learning, Treatment response, Veterans Health Administration
INTRODUCTION
Major depressive disorder (MDD) has high prevalence and high impairment (GBD 2019 Diseases and Injuries Collaborators, 2020). The two primary first-line MDD treatments are psychotherapy and antidepressant medication (ADM; Qaseem, Barry, & Kansagara, 2016). ADM is the more common treatment despite most patients preferring psychotherapy (McHugh, Whitton, Peckham, Welge, & Otto, 2013) due to lower cost and wider availability (Hockenberry, Joski, Yarbrough, & Druss, 2019). But some MDD patients do not respond to ADMs (Cipriani et al., 2018; Kazdin et al., 2021; Little, 2009) but do to psychotherapy or an ADM-psychotherapy combination. However, the latter treatments often are provided only after months of unsuccessful ADM treatment (Day et al., 2021). A meaningful proportion of patients drop out before receiving other treatments (Larson et al., 2021). A strategy to predict likelihood of responding before initiating ADM treatment could be of value.
Many multivariable models have been developed, typically using machine learning (ML) methods (Chekroud et al., 2021; Ermers, Hagoort, & Scheepers, 2020; Lee et al., 2018), to predict depression treatment response. Most such models can be faulted, though, for (i) low external validity because of restriction to clinical trial samples; (ii) focus on biomarkers infeasible to use in routine clinical practice; (iii) including many fewer predictors than documented in the literature; or (iv) suboptimal analytic methods.
The current report presents results of a study designed to address these problems by analyzing an observational sample of patients recruited near beginning ADM treatment and administered an extensive baseline battery of self-report questions to assess predictors of ADM treatment response found in previous studies. The patients were followed for 3 months to assess treatment response. The data were analyzed using a state-of-the-art stacked generalization ML method.
MATERIALS AND METHODS
Sample
As detailed elsewhere (Puac-Polanco et al., 2021), a probability sample of patients beginning MDD outpatient treatment was selected from Veterans Health Administration (VHA) EHRs December 2018-June 2020. Inclusion criteria were: (i) beginning first outpatient MDD treatment in the past year; and (ii) receiving ADM prescription and/or psychotherapy referral. Exclusions were: (i) 12-month suicide attempt; (ii) lifetime diagnoses of bipolar disorder, nonaffective psychosis, dementia, intellectual disability, autism, Tourette’s disorder, stereotyped movement disorder, or borderline intellectual functioning; (iii) lifetime prescriptions of mood stabilizers or antipsychotic medications (Supplementary Table 1). The exclusion of 12-month suicide attempts was made because such patients in VHA are placed on a high-risk list that leads to intensive case management, making the experiences of these patients unrepresentative of the more general patient population.
Recruitment letters were sent to 55,106 provisionally eligible patients the day after their first outpatient visit. The letter described the study purposes and the requirements of self-report web- or phone-based baseline assessment taking 45 minutes and at 3-months follow-up taking 20 minutes, with compensation of $50 and $25, respectively, for the two assessments. A team member then attempted to contact each patient over the next week (3 call attempts). Of the 17,000 reached, 6,298 agreed to participate and 4,164 completed the baseline assessment (Supplementary Figure 1). At baseline, 809 respondents had received an ADM prescription without referral to psychotherapy and were otherwise eligible. The 660 of these 809 who completed the 3-month assessment are the focus of this report. The protocol was approved by the Institutional Review Board of Syracuse VA Medical Center, Syracuse, New York.
Measures
Treatment response:
Two-week depressive symptoms were assessed with the 16-item Quick Inventory of Depression Symptomatology Self-Report (QIDS-SR; Rush et al., 2003). A modified version of the Sheehan Disability Scale (SDS; Leon, Olfson, Portera, Farber, & Sheehan, 1997) was used to assess role impairment by asking patients how much depression interfered with the ability to work, participate in family and home life, or participate in social activities in the past 2 weeks on a 0–10 visual analog scale with response options of not at all (0), mildly (1–3), moderately (4–6), markedly (7–9), and extremely (10) (Cronbach’s α=0.85).
Treatment response was defined as either (i) a 3-month QIDS-SR score no more than half its baseline value or (ii) a baseline SDS score of 4–10 in any role impairment domain along with a 3-month SDS score of 0–3 in all such domains. A similar composite definition of ADM treatment response was used in previous research (Huang et al., 2018; Wang et al., 2018; Zilcha-Mano et al., 2021).
Predictors:
Numerous recent reports have carried out reviews or meta-analyses of research on baseline predictors of response to individual types of depression treatment (e.g., Furukawa et al., 2021; Noma et al., 2019) or treatment in general pooled across multiple treatment types (e.g., Buckman et al., 2021a; Buckman et al., 2022; Buckman et al., 2021b; Buckman et al., 2021c), which are referred to collectively as prognostic predictors. Other reviews have examined baseline variables that interact significantly with treatment type to predict outcomes (Maj et al., 2020; Perlman et al., 2019; Perna, Alciati, Daccò, Grassi, & Caldirola, 2020), which are referred to as prescriptive predictors. Predictors from all important domains of either prescriptive or prognostic predictors were included in our baseline questionnaire or abstracted from EHRs or government small-area geospatial databases linked to patient residential addresses. Included here were six domains involving the episodes (symptom frequency, severity, subtypes, clinical staging, psychiatric comorbidities, functioning and quality of life), two others involving stressors (early environmental exposures, recent environmental stressors), and three involving personality/cognition (personality scales, neurocognition, dysfunctional cognitive schemas). A separate domain of “protective/resilience factors” assessed patient psychological characteristics (e.g., coping styles, self-reported psychological resilience) and environmental resources (e.g., access to supportive social relationships; access to material resources). Two other domains included information about comorbid physical disorders and family history of psychopathology.
We also included information about socio-demographics and treatment characteristics associated in previous research with differential depression treatment response (Constantino, Vîslă, Coyne, & Boswell, 2018; Kraus, Kadriu, Lanzenberger, Zarate Jr, & Kasper, 2019). Treatment characteristics included self-reports about current expectations, which were assessed as of the time of baseline rather than asking patients to recall their expectations prior to making their initial treatment contact. This time frame is relevant because, as noted above, the baseline assessment was carried out only after treatment started. Treatment characteristics also included patient self-reports about past treatment experiences and EHR data on treatment histories and ADM types prescribed in the current treatment. The latter were classified as norepinephrine-dopamine reuptake inhibitors (NDRI), serotonin antagonist reuptake inhibitors (SARI), serotonin modulator and stimulators (SMS), serotonin-norepinephrine reuptake inhibitors (SNRI), selective serotonin reuptake inhibitors (SSRI), tricyclic antidepressants (TCA), and tetracyclic antidepressants (TeCA). We also included a dummy variable for ADMs suggested as most effective in controlled trials (i.e., escitalopram, mirtazapine, paroxetine, sertraline, venlafaxine) (Cipriani et al., 2018; Kazdin et al., 2021; Little, 2009). Other dummy variables were included for typical combinations of ADMs with baseline symptoms (e.g., trazodone with sleep disturbance, duloxetine with severe physical pain). We also recorded whether the treatment provider was the patient’s regular primary care physician, someone else in the same primary care office, or someone at a mental health specialty clinic.
Categorical variables were coded as dummy indicators. Quantitative variables were standardized to a mean of 0 and variance of 1 and discretized into quintiles to create stabilized predictors and nested dichotomies. These transformations resulted in 2,768 potential predictors (Supplementary Tables 2–4). Item-level missingness was handled by single imputation carried out in the total sample before defining separate training and test samples, with missing values imputed to the mode for dichotomous and categorical variables and to the mean for ordinal and interval variables.
Analysis methods
The R program sbw (Zubizarreta, Li, Allouah, & Greifer, 2021) was used to make weighting adjustments for: (i) discrepancies in baseline EHR variables between eligible VHA patients and the 809 baseline respondents and (ii) discrepancies in baseline survey variables between the 660 3-month follow-up respondents and nonrespondents (Zubizarreta, 2015).
The Super Learner (SL) stacked generalization ML method (Polley, LeDell, Kennedy, Lendle, & van der Laan, 2021) was used to develop a prediction model in the weighted sample of 3-month respondents. SL generates predictions from a weighted combination of conventional and flexible ML algorithms in an ensemble. Our SL specification used 10-fold cross-validation (10F-CV) to generate a weighted composite that performs at least as well in expectation as the best algorithm in the ensemble (Polley, Rose, & van der Laan, 2011). The appeal of stacked generalization over single algorithms is improved predictive accuracy by virtue of combining results across algorithms that include a wide range of functional forms (Polley et al., 2011). Consistent with recommendations (LeDell, van der Laan, & Petersen, 2016), a diverse set of algorithms was included in the SL ensemble (Supplementary Table 5). Some prior computational psychiatric studies have used similar stacked generalization procedures (Karrer et al., 2019; Ziobrowski et al., 2021a).
We estimated the SL model in a stratified (by the outcome variable) random 70% training sample n=462) and validated it in the remaining 30% test sample (n=198). Prediction strength, defined as area under the receiver operating characteristic curve [AUC (ROC)], was compared across a wide range of hyperparameter settings for each algorithm in the 10F-CV sample (Supplementary Table 5). Predictors were selected independently in each 10F-CV fold with a range of constraints on predictor number using lasso regression (Park & Casella, 2008) for linear models and Bayesian additive regression trees (BART) (Chipman, George, & McCulloch, 2010) for nonadditive models. Comparisons of AUC (ROC) estimated in the full training sample and 10F-CV allowed determination of how much each learner (i.e., combination of number of allowed predictors and hyperparameter values for a given algorithm) was overfitting and CV prediction strength. A subset of learners with balance between these two criteria was selected for the final SL ensemble. Once the final SL model was estimated, 10F-CV was used for model calibration in the 10F-CV sample based on isotonic regression (Lindhiem, Petersen, Mentch, & Youngstrom, 2020).
Models were assessed in the test sample by how well predicted probability of treatment response ranked patients on observed response (i.e., discrimination). The AUC (ROC) and the AUC of the precision recall curve [AUC (PRC)] were compared for the SL and a simpler benchmark lasso regression model whose penalty parameter was selected via internal cross-validation, both estimated in the training sample and applied to the test sample. Operating characteristics in the test sample were then inspected across quantiles of predicted probability of response. Operating characteristics included conditional and cumulative sensitivity (SN; the proportion of all patients responding to treatment who were in the quantile) and positive predictive value (PPV; the prevalence of treatment response in the decile). A locally estimated scatterplot smoothed calibration curve (Austin & Steyerberg, 2014) with .75 bandwidth was used to quantify model calibration in the test sample using the integrated calibration index (ICI) and expected calibration error (ECE) (Austin & Steyerberg, 2019). Model fairness (Yuan, Kumar, Ahmad, & Teredesai, 2021) was evaluated by examining variation in the association of predicted probability of response with observed response across socio-demographic subgroups related to health disparities (age, sex, race/ethnicity, education) using robust Poisson regression models (Zou, 2004).
Predictor importance was examined using the model-agnostic kernel Shapley Additive Explanations (SHAP) method (Lundberg & Lee, 2017), which generates a predicted difference in outcome score for each patient based on changing one and only one predictor at a time from its observed score to the mean across all logically possible permutations of other predictors. The mean of this “SHAP value” for a given predictor across all patients is 0. However, the mean absolute SHAP value provides useful information about the average importance of the predictor. A bee swarm plot of the association between the individual-level SHAP value and the observed score for a given predictor was used to describe dominant direction of association. Mean absolute SHAP values can also be aggregated across subsets of predictors by summing SHAP values across the predictors at the individual level and then calculating the mean of the absolute value of this sum. Such aggregate scores estimate the expected change in prevalence of treatment response if all predictors for all patients changed from their observed values to the mean values.
SAS statistical software, version 9.4 (SAS Institute Inc, 2013), was used for data management, estimating prevalence of treatment response, and calculating SN, PPV, and AUC. R, version 4.0.5 (R Core Team, 2021) was used to estimate the SL model and SHAP values.
Reporting
We followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) guidelines (Collins, Reitsma, Altman, & Moons, 2015) in presenting results.
RESULTS
Sample characteristics and treatment response
Baseline QIDS-SR scores were transformed to approximate Hamilton Rating Scale of Depression (HRSD) categories using published transformation rules (Table 3 in Rush et al., 2003) to give a sense of the baseline symptom severity distribution. 30.1% of patients were classified as having baseline mild depression, 35.6% moderate, 21.4% severe, and 12.9% very severe (Supplementary Table 6). Given that the baseline assessment was not administered until after the initiation of treatment, there is a possibility that these severities were lower than if assessments had been carried out prior to beginning treatment. However, the Pearson correlation between the baseline QIDS-SR score and number of days between beginning treatment and taking the baseline assessment (Median=21 days; inter-quartile range=14–30 days) was nonsignificant (r=.013, p=.74).
The great majority (80.7%) of patients were prescribed a single ADM, most commonly SSRIs (57.0%), SNRIs (16.8%), NDRIs (15.7%), and SARIs (15.0%). The modal socio-demographic categories were between 35–49 years of age, male, non-Hispanic White, married, and living in a Major Metropolitan Area. Except for age (p = 0.009), no statistically significant differences were found in baseline socio-demographics, clinical severity, or ADM classes between the weighted baseline sample and the doubly weighted analytic sample. 35.7% of doubly weighted test sample patients responded to treatment as of the 3-month assessment.
Model performance
The SL test sample AUC (ROC) was 0.66 compared to 0.62 for the benchmark lasso model (Figure 1). SL had better SN than lasso for all values of specificity (SP) above 0.25. SL had much higher PPV than the lasso model for SN below 0.10 and somewhat higher PPV than lasso across most of the remaining SN range (Figure 2). SL test sample AUC (ROC) remained 0.66 when the analysis was limited to patients classified as having at least moderate baseline symptom severity compared to AUC (ROC) of 0.60 among patients classified as having mild baseline symptom severity.
Figure 1:
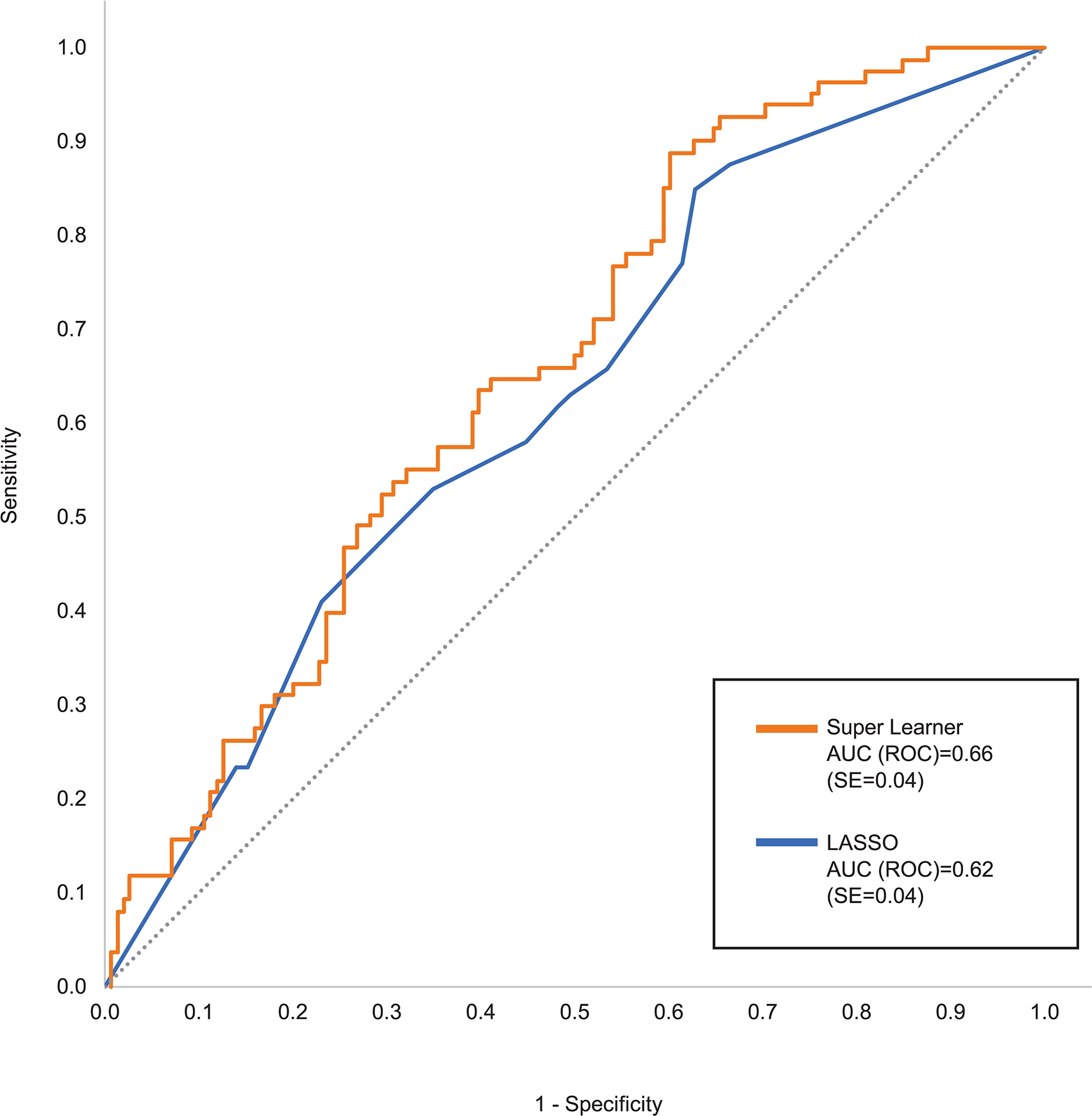
Receiver Operating Characteristic (ROC) curve comparing Super Learner with benchmark lasso in the test sample
Figure 2:
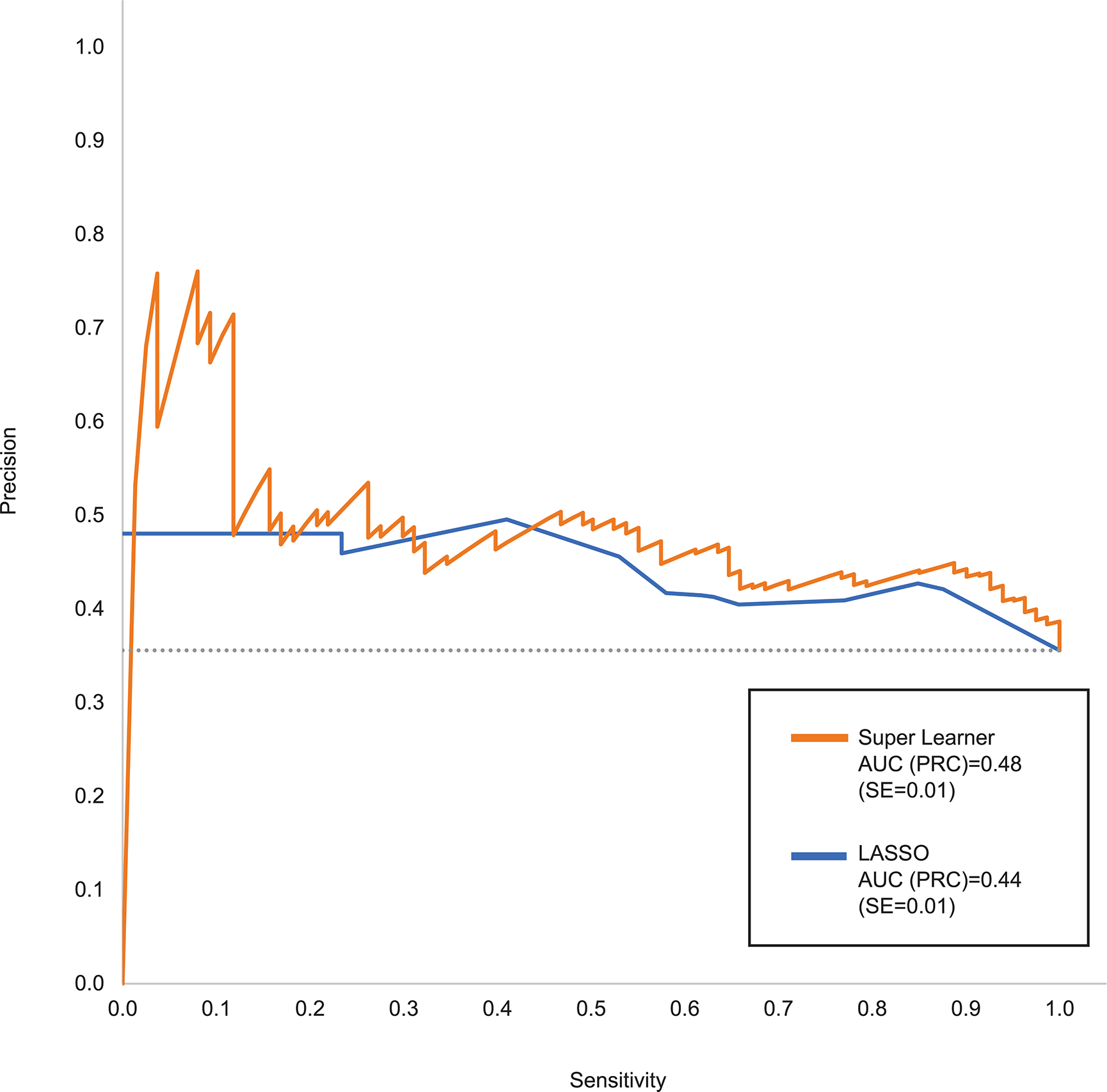
Precision Recall Curve (PRC) comparing Super Learner with benchmark lasso in the test sample
Calibration based on the isotonic regression transformation was (ICI=0.28 and ECE=0.34). The SL model also had comparable fairness across subgroups defined by age, sex, race/ethnicity, and education (Supplementary Table 7).
A monotonic gradient was found in the proportion of test sample patients that responded to treatment [i.e., PPV (SE)] across SL model quantiles defined in the training sample. These quantiles could be collapsed without meaningful loss of information into three groups of patients (Table 1). Among patients in the first group, those with high predicted probabilities of response, 45.7% (5.5) responded to treatment. In the intermediate predicted probably group, 34.5% (7.6) responded. In the low predicted probability group, 11.1% (4.9) responded. These predicted probabilities did not vary significantly between patients whose baseline symptom severity was mild versus more severe (Table 1). Although the thresholds to define these groups were for quintiles in the training sample, 50.4% of patients in the test sample fell into the high group, 30.1% the intermediate group, and 19.4% the low group.
Table 1.
Prediction of 3-Month ADM treatment response in the test sample in three group defined by predicted probabilities in the training sample (n=198)
| Distribution | PPV | Cumulative PPV | Sensitivity | Cumulative Sensitivity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | (SE) | % | (SE) | % | (SE) | % | (SE) | % | (SE) | |
|
|
|
|
|
|
||||||
| High | 50.4a | (4.1) | 45.7b | (5.5) | 45.7 | (5.5) | 64.7 | (6.7) | 64.7 | (6.7) |
| Intermediate | 30.1c | (3.9) | 34.5d | (7.6) | 41.5 | (4.5) | 29.2 | (6.6) | 93.9 | (2.7) |
| Low | 19.4 | (3.3) | 11.1 | (4.9) | 35.6 | (3.9) | 6.0 | (2.7) | 100.0 | -- |
Abbreviations: ADM, antidepressant medication; PPV, positive predictive value (i.e., predicted proportion with treatment response); SE, standard error; SN, sensitivity (i.e., proportion of all treatment responders).
80.9% among patients with mild baseline symptom severity versus 35.3% among other patients.
47.5% among patients with mild baseline symptom severity versus 45.7% among other patients.
13.7% in the subsample of patients with mild baseline symptom severity versus 38.3% among other patients.
30.3% among patients with mild baseline symptom severity versus 38.4% among other patients.
Predictor importance
The 2,768 predictors were highly redundant, as indicated by 750 (27.1%) of them having significant univariable associations with the outcome in the training sample at the .05 level but only 53 (1.9%) being selected by SL (Figure 3). Forty-six of these 53 were patient self-reports, 4 EHR variables, and 3 geospatial variables. The aggregate mean absolute SHAP value across all these predictors was 4.3%. This means the probability of treatment response would have changed by an estimated average of 4.3% if each patient’s scores on all selected predictors changed from observed to sample-wide mean values.
Figure 3:
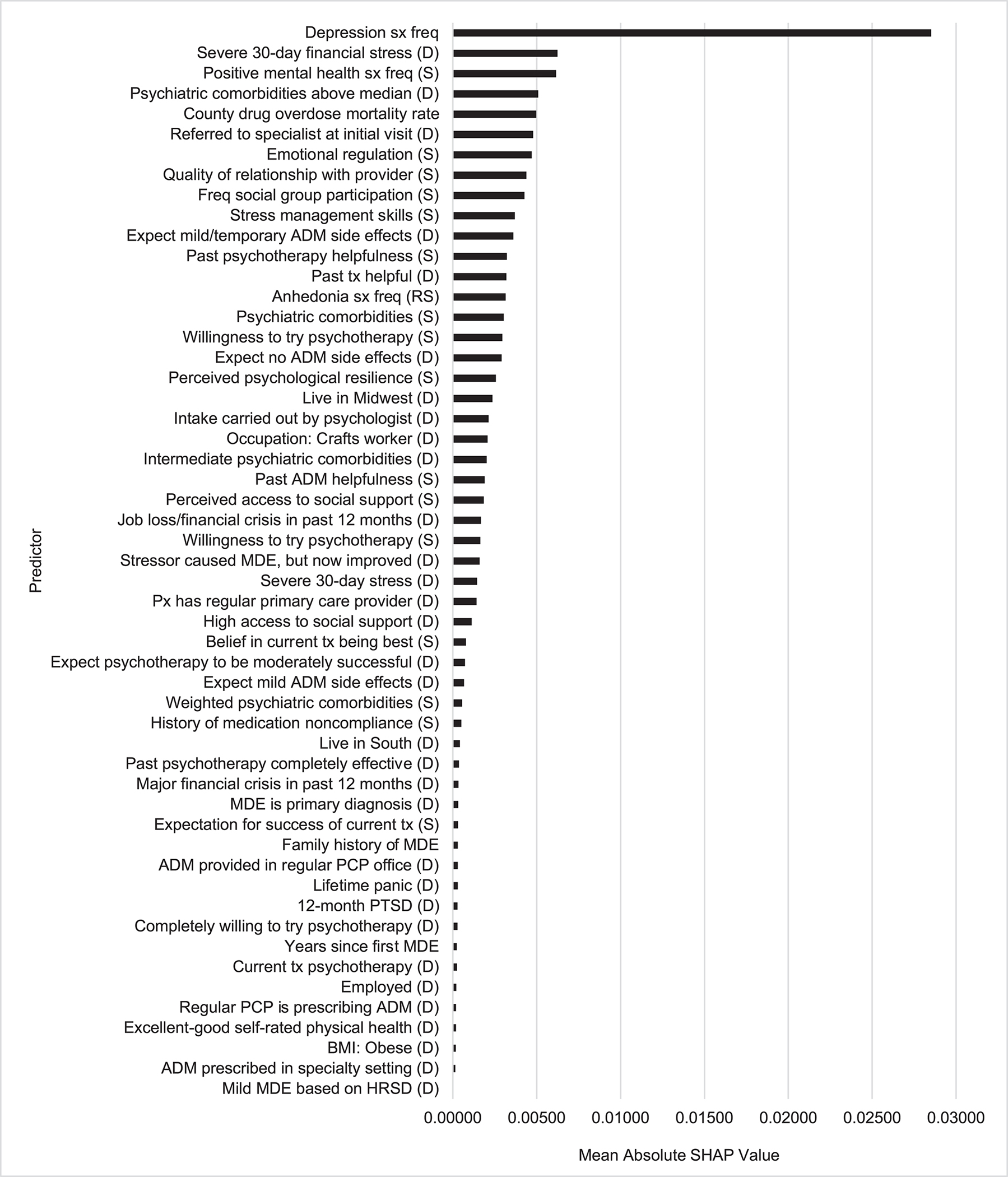
Predictor importance as determined by Shapley Additive Explanation (SHAP) values for the Super Learner Model in the test samplea
Abbreviations: sx, symptoms; freq, frequency; (D), dummy variable; (S), stabilized variable; ADM, antidepressant medication; tx, treatment; (RS), reverse stabilized; MDE, major depressive episode; px, patient; PCP, primary care provider; PTSD, post-traumatic stress disorder; BMI, body mass index; HRSD, Hamilton Rating Scale of Depression.
aSee Supplementary Table 8 for descriptions of the predictor labels.
The most important predictors were features of the episode (10 of 53 predictors), with an aggregate mean absolute SHAP value of 3.5% (81% of the total). This included the most important predictor, overall depressive symptom frequency in the 2 weeks before treatment, in addition to two other important symptom measures, frequency of being happy or at peace (3rd most important) and anhedonia (reverse coded, 14th most important), along with five indicators of current or recent comorbidity (4th, 15th, 22nd, 34th, 44th). These predictors were for the most part associated with reduced probability of treatment response. However, SHAP value distributions (Figure 4) show that some associations were nonmonotonic. For example, lower-than-average but not lowest overall depressive symptom frequency was associated with highest probability of treatment response.
Figure 4:
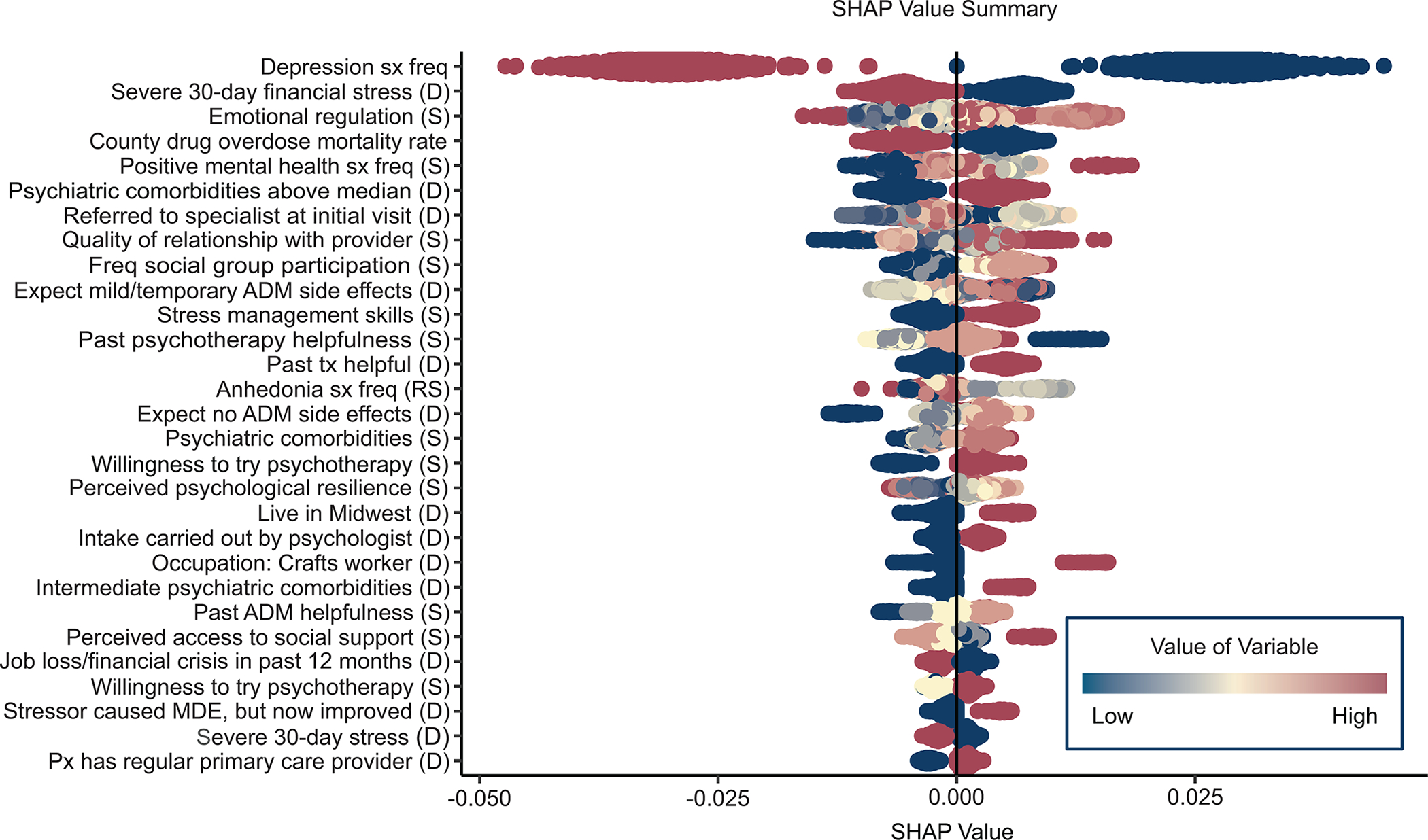
Bee swarm plot of individual-level predictor-specific SHAP values for the most important predictors in the Super Learner modela
Abbreviations: sx, symptoms; freq, frequency; (D), dummy variable; (S), stabilized variable; ADM, antidepressant medication; tx, treatment; (RS), reverse stabilized; MDE, major depressive episode; px, patient; PCP, primary care provider; PTSD, post-traumatic stress disorder; BMI, body mass index; HRSD, Hamilton Rating Scale of Depression.
aSee Supplementary Table 8 for descriptions of the predictor labels.
The 2nd most important predictor domain involved treatment characteristics (22 of 53 selected predictors), with an aggregate mean absolute SHAP value of 1.2% (25% of the total). Included were 10 indicators of positive treatment expectation/preference (e.g., 8th most important, expectation of having a good relationship with treatment provider), all positively associated with treatment response. Another 7 treatment-related predictors involved current treatment (e.g., 6th most important, referral to a mental health specialist or psychologist carried out intake). None of the ADM types was among the important predictors. The remaining treatment-related predictors involved treatment history (e.g., 12th most important, past psychotherapy was not helpful), all positively associated with treatment response.
There were only two other important predictor domains: recent stressors and protective/resilience factors, with aggregate mean absolute SHAP values of 0.9% (26% of the total) and 0.7% (17% of the total), respectively. The most important stressors were financial (2nd) and high mortality rate due to drug overdose in the patient’s county of residence (5th), both negatively associated with treatment response. The protective/resilience factors included 3 indicators of psychological resilience and 3 of social support. As shown in the bee swarm plot, most of these predictors had nonmonotonic associations with the outcome due to patients with higher-than-average but not highest reported protective/resilience scores having highest probabilities of treatment response.
DISCUSSION
The 35.7% 3-month ADM treatment response rate is comparable to previous VHA studies (Katz, Liebmann, Resnick, & Hoff, 2021) but lower than most civilian studies (Cuijpers et al., 2020), presumably reflecting the greater severity/complexity of depressed Veterans than civilians (Ziobrowski et al., 2021b). This highlights the potential importance of patients in the group with highest predicted probability of ADM response being more than four times as likely to respond as patients in the lowest group (45.7% versus 11.1%). Accurate discrimination of this sort is valuable as a first step in determining optimal treatments. However, multiple treatment-specific models need to be developed and combined to create a precision treatment rule for optimal assignment of patients across interventions (Kessler & Luedtke, 2021). For instance, psychotherapy or ADM plus psychotherapy might be prioritized for patients with low predicted probabilities of ADM treatment response, but only in the subset of patients with higher predicted probabilities of response in treatment-specific models for psychotherapy or combined therapy.
In this respect, our model might be compared to pharmacogenomic models used to determine pre-emptively whether ADMs are likely to be effective for individual patients (Greden et al., 2019). Our model performed at least as well as these pharmacogenomic models and could be implemented at a fraction of the cost of pharmacogenetic testing. The largest pharmacogenomic testing trial to date for ADM selection found that patients receiving test-congruent medications had a 12% higher probability of treatment response than patients receiving test-incongruent medications (29% versus 17%; Greden et al., 2019), whereas the differences we found were 34.6% (45.7% versus 11.1%) between our high and low groups, 11.2% (45.7% versus 34.5%) between our top and intermediate groups, and 23.4% (34.5% versus 11.1%) between our intermediate and low groups.
Caution is needed in interpreting results regarding predictor importance because predictor importance rankings can be very unstable when, as in our dataset, many predictors are highly correlated (Leeuwenberg et al., 2022). Several broad results about predictor importance are nonetheless noteworthy. The most striking is that baseline clinical characteristics of the episode were by far the most important predictors. This is consistent with a recent individual-level meta-analysis of over 6,000 patients in primary care depression treatment across 12 trials, where baseline depression symptom severity was by far the single most important predictor of treatment response independent of treatment type (Buckman et al., 2021b). Other significant clinical predictors in that recent meta-analysis included duration of the depressive episode before beginning treatment, comorbid panic, and duration of comorbid anxiety. We found a different set of important clinical predictors, including two secondary depressive symptom factors and several measures of psychiatric comorbidity, but, as in the meta-analysis, these were all much less important than overall baseline depression symptom frequency.
The secondary symptom factors (absence of positive emotions, anhedonia) are both central aspects of melancholic depression. Evidence in previous studies has been mixed for melancholic depression being less responsive to treatment than others (Maj et al., 2020). It is noteworthy that the baseline assessment included the other indicators of melancholia (i.e., deep feelings of despair, mood worse in the morning, early morning awakening, psychomotor changes, weight loss, excessive guilt), but we did not attempt to define this or any other theoretical (Benazzi, 2006) or data-driven (Buckman et al., 2021a) MDD subtype beyond those that emerged in an exploratory factor analysis of symptoms in the baseline assessment. The nonadditive models in the SL ensemble would have been expected to detect interactions across these factors if a strong data-driven episode subtype existed. Nonetheless, it might be useful in future investigations to use unsupervised ML methods to explore the possibility of detecting such clusters.
The importance of treatment characteristics, the next most important predictor domain in our sample, was striking in two ways. First, ADM type was unrelated to treatment response. Second, multiple aspects of treatment history and current treatment expectations were important. Although the literature on treatment expectations is inconsistent in its measures and controls for prior experiences, our finding that both process and outcome expectations were important predictors is broadly consistent with previous studies (Laferton, Kube, Salzmann, Auer, & Shedden-Mora, 2017). This is striking given that we controlled for and found significant associations of several measures of past treatment experiences that presumably underlie expectations. Taken together, these results argue for the potential value of shared decision making and patient-centered care for depression (Rush & Thase, 2018), for the potential value of expanding interventions to influence treatment expectations (Gruszka, Burger, & Jensen, 2019) and for the importance of including psychometrically sound and conceptually cohesive questions about treatment expectations and past treatment experiences in baseline patient assessments (e.g., Barth, Kern, Lüthi, & Witt, 2019).
The finding that recent stressors were important is broadly consistent with evidence documenting effects of stressful life experiences on depression treatment response (Buckman et al., 2022). The fact that financial stress was the 2nd most important predictor was especially striking and is consistent with prior studies showing that unemployment and low household income are top predictors of low ADM treatment response (Lee et al., 2018). The findings that baseline protective/resilience factors were important is also in line with much previous research (Buckman et al., 2021c; Laird, Lavretsky, St Cyr, & Siddarth, 2018). The fact that some of these associations were nonmonotonic is consistent with naturalistic evidence that moderate, compared to extremely low or high, levels of emotional reactivity to stress predict low future depression severity (Santee & Starr, 2021) and that baseline self-reported resilience is sometimes significant in predicting depression treatment response only in interaction with other predictors (Choi et al., 2021; Min, Lee, Lee, Lee, & Chae, 2012). These specifications might reflect the greater importance of protective/resilience factors in the subset of patients whose depressive episodes are triggered by stressful life experiences, which could be the subject of future investigation (Chromik, 2021).
Limitations:
The study had several noteworthy limitations. Three of these involve external validity. First, the baseline response rate was low, although comparable to response rates in other VHA studies examining mental health outcomes (King, Beehler, Buchholz, Johnson, & Wray, 2019; Stolzmann et al., 2019). However, as shown in a previous report (Puac-Polanco et al., 2021), there are minimal differences between our responders and non-responders on baseline administrative variables and equally modest differences in baseline self-reports between patients followed versus lost to follow-up, although response bias might nonetheless exist with respect to unmeasured variables. Second, we did not account for possible disruptions in care due to the COVID-19 pandemic, which involved 7.6% of study patients who completed assessments after February 2020. Third, although the model was validated in a separate test sample, it was not tested in an external validation sample. Nor is it clear whether findings would generalize to non-VHA patients.
A separate set of limitations involve design decisions that could have biased results. One of these is that study recruitment and assessment occurred only after the initial visit, during which time symptoms might have decreased, leading to distortion in our estimates of associations between baseline symptoms and treatment response. As reported above, the association between time between initiating treatment and completing the baseline assessment was unrelated to baseline QIDS-SR scores, somewhat reducing this concern, but it is nonetheless important that future replications and extensions of our work are carried out with baseline assessment administered before treatment selection is made. Another limitation that might have biased results was the use of a very large set of predictors, which could have resulted in over-fitting even though we used procedures to minimize this possibility.
A final set of noteworthy limitations involves the measures. The predictors excluded information about military experiences that might have led to the depression. And the outcomes were based on self-reports rather than clinical interviews.
Strengths:
The study also had several strengths, including an observational sample with greater external validity than clinical trial samples, a rich baseline predictor set that included a wide range of variables found in previous research to be prognostic predictors of depression treatment response, and use of a rigorous ML method to develop the model.
CONCLUSIONS
Within the context of these limitations, we found that a model to predict ADM treatment response could be developed based largely on a battery of self-report questions along with some administrative variables from EHRs and geospatial databases. The model had modest overall prediction strength but nonetheless provided enough discrimination across three broad groups of patients to have potential value in informing depressed patients pre-emptively about their likelihood of responding to ADM as part of a patient-centered shared decision-making process. The model had good calibration and fairness with respect to key indicators of health disparities. Our findings would need to be replicated in a sample where the baseline assessment occurred before the beginning of treatment, the model streamlined, and parallel models built for predicted response to other types of treatment before results could be useful. In addition, parallel models combined across different treatments would be needed to determine best treatment options for particular patients (Kessler & Luedtke, 2021).
Supplementary Material
ACKNOWLEDGMENTS
Financial Support:
This research was supported by the Office of Mental Health Services and Suicide Prevention and Center of Excellence for Suicide Prevention (Bossarte), the National Institute of Mental Health of the National Institutes of Health (R01MH121478, Kessler), the United States Department of Veterans Affairs Health Services Research & Development Service Career Development Award (IK2 HX002867, Leung), the PCORI Project Program Award (ME-2019C1-16172, Zubizarreta), and the Advanced Fellowship from the VISN 4 Mental Illness Research, Education, & Clinical Center (MIRECC, Cui, Oslin). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the U.S. Department of Veterans Affairs, or the United States Government.
Conflict of Interest:
In the past 3 years, Dr. Kessler was a consultant for Datastat, Inc., Holmusk, RallyPoint Networks, Inc., and Sage Therapeutics. He has stock options in Mirah, PYM, and Roga Sciences. Dr. Pigeon consulted for CurAegis Technologies and received clinical trial support from Pfizer, Inc. and Abbvie, Inc. Dr. Zubizarreta consulted for Johnson & Johnson Real World Data Analytics. Dr. Cipriani is supported by the National Institute for Health Research (NIHR) Oxford Cognitive Health Clinical Research Facility, by an NIHR Research Professorship (grant RP-2017-08-ST2-006), by the NIHR Oxford and Thames Valley Applied Research Collaboration and by the NIHR Oxford Health Biomedical Research Centre (grant BRC-1215-20005); he has also received research, educational and consultancy fees from INCiPiT (Italian Network for Paediatric Trials), CARIPLO Foundation and Angelini Pharma. The views expressed are those of the authors and not necessarily those of the UK National Health Service, the NIHR, or the UK Department of Health. The remaining authors report no conflict of interest.
Footnotes
Ethical Standards:
All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
REFERENCES
- Austin PC, & Steyerberg EW (2014) Graphical assessment of internal and external calibration of logistic regression models by using loess smoothers. Statistics in Medicine, 33(3), 517–535. doi: 10.1002/sim.5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC, & Steyerberg EW (2019) The Integrated Calibration Index (ICI) and related metrics for quantifying the calibration of logistic regression models. Statistics in Medicine, 38(21), 4051–4065. doi: 10.1002/sim.8281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth J, Kern A, Lüthi S, & Witt CM (2019) Assessment of patients’ expectations: development and validation of the Expectation for Treatment Scale (ETS). BMJ Open, 9(6), e026712. doi: 10.1136/bmjopen-2018-026712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benazzi F (2006) Various forms of depression. Dialogues in Clinical Neuroscience, 8(2), 151–161. doi: 10.31887/DCNS.2006.8.2/fbenazzi [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman JEJ, Cohen ZD, O’Driscoll C, Fried EI, Saunders R, Ambler G, . . . Pilling S (2021a) Predicting prognosis for adults with depression using individual symptom data: a comparison of modelling approaches. Psychological Medicine, Advance online publication. doi: 10.1017/s0033291721001616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman JEJ, Saunders R, Arundell LL, Oshinowo ID, Cohen ZD, O’Driscoll C, . . . Pilling S (2022) Life events and treatment prognosis for depression: A systematic review and individual patient data meta-analysis. Journal of Affective Disorders, 299, 298–308. doi: 10.1016/j.jad.2021.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman JEJ, Saunders R, Cohen ZD, Barnett P, Clarke K, Ambler G, . . . Pilling S (2021b) The contribution of depressive ‘disorder characteristics’ to determinations of prognosis for adults with depression: an individual patient data meta-analysis. Psychological Medicine, 51(7), 1068–1081. doi: 10.1017/s0033291721001367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman JEJ, Saunders R, O’Driscoll C, Cohen ZD, Stott J, Ambler G, . . . Pilling S (2021c) Is social support pre-treatment associated with prognosis for adults with depression in primary care? Acta Psychiatrica Scandinavica, 143(5), 392–405. doi: 10.1111/acps.13285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekroud AM, Bondar J, Delgadillo J, Doherty G, Wasil A, Fokkema M, . . . Choi K (2021) The promise of machine learning in predicting treatment outcomes in psychiatry. World Psychiatry, 20(2), 154–170. doi: 10.1002/wps.20882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipman HA, George EI, & McCulloch RE (2010) BART: Bayesian additive regression trees. The Annals of Applied Statistics, 4(1), 266–298. doi: 10.1214/09-AOAS285 [DOI] [Google Scholar]
- Choi W, Kim JW, Kang HJ, Kim HK, Kang HC, Lee JY, . . . Kim JM (2021) Synergistic effects of resilience and serum ghrelin levels on the 12-week pharmacotherapeutic response in patients with depressive disorders. Journal of Affective Disorders, 295, 1489–1493. doi: 10.1016/j.jad.2021.09.039 [DOI] [PubMed] [Google Scholar]
- Chromik M (2021) Making SHAP Rap: Bridging local and global insights through interaction and narratives. In Human-Computer Interaction – INTERACT 2021 (ed. Ardito C, Lanzilotti R, Malizia A, Petrie H, Piccinno A, Desolda G and Inkpen K), pp. 641–651. Springer: Cham. [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, . . . Geddes JR (2018) Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet (London, England), 391(10128), 1357–1366. doi: 10.1016/s0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GS, Reitsma JB, Altman DG, & Moons KG (2015) Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): the TRIPOD Statement. The British Journal of Surgery, 102(3), 148–158. doi: 10.1002/bjs.9736 [DOI] [PubMed] [Google Scholar]
- Constantino MJ, Vîslă A, Coyne AE, & Boswell JF (2018) A meta-analysis of the association between patients’ early treatment outcome expectation and their posttreatment outcomes. Psychotherapy (Chicago, Ill.), 55(4), 473–485. doi: 10.1037/pst0000169 [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Noma H, Karyotaki E, Vinkers CH, Cipriani A, & Furukawa TA (2020) A network meta-analysis of the effects of psychotherapies, pharmacotherapies and their combination in the treatment of adult depression. World Psychiatry, 19(1), 92–107. doi: 10.1002/wps.20701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day E, Shah R, Taylor RW, Marwood L, Nortey K, Harvey J, . . . Strawbridge R (2021) A retrospective examination of care pathways in individuals with treatment-resistant depression. British Journal of Psychiatry Open, 7(3), e101–e101. doi: 10.1192/bjo.2021.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermers NJ, Hagoort K, & Scheepers FE (2020) The Predictive Validity of Machine Learning Models in the Classification and Treatment of Major Depressive Disorder: State of the Art and Future Directions. Frontiers in Psychiatry, 11, 472. doi: 10.3389/fpsyt.2020.00472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa TA, Suganuma A, Ostinelli EG, Andersson G, Beevers CG, Shumake J, . . . Cuijpers P (2021) Dismantling, optimising, and personalising internet cognitive behavioural therapy for depression: a systematic review and component network meta-analysis using individual participant data. The Lancet Psychiatry, 8(6), 500–511. doi: 10.1016/s2215-0366(21)00077-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2019 Diseases and Injuries Collaborators (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. The Lancet, 396(10258), 1204–1222. doi: 10.1016/s0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greden JF, Parikh SV, Rothschild AJ, Thase ME, Dunlop BW, DeBattista C, . . . Dechairo B (2019) Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: A large, patient- and rater-blinded, randomized, controlled study. Journal of Psychiatric Research, 111, 59–67. doi: 10.1016/j.jpsychires.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Gruszka P, Burger C, & Jensen MP (2019) Optimizing Expectations via Mobile Apps: A New Approach for Examining and Enhancing Placebo Effects. Frontiers in Psychiatry, 10, 365. doi: 10.3389/fpsyt.2019.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenberry JM, Joski P, Yarbrough C, & Druss BG (2019) Trends in Treatment and Spending for Patients Receiving Outpatient Treatment of Depression in the United States, 1998–2015. JAMA Psychiatry, 76(8), 810–817. doi: 10.1001/jamapsychiatry.2019.0633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wang Y, Chen J, Zhang Y, Yuan Z, Yue L, . . . Fang Y (2018) Clinical outcomes of patients with major depressive disorder treated with either duloxetine, escitalopram, fluoxetine, paroxetine, or sertraline. Neuropsychiatric Disease and Treatment, 14, 2473–2484. doi: 10.2147/ndt.s159800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer TM, Bassett DS, Derntl B, Gruber O, Aleman A, Jardri R, . . . Bzdok D (2019) Brain-based ranking of cognitive domains to predict schizophrenia. Human Brain Mapping, 40(15), 4487–4507. doi: 10.1002/hbm.24716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz IR, Liebmann EP, Resnick SG, & Hoff RA (2021) Performance of the PHQ-9 across conditions and comorbidities: Findings from the Veterans Outcome Assessment survey. Journal of Affective Disorders, 294, 864–867. doi: 10.1016/j.jad.2021.07.108 [DOI] [PubMed] [Google Scholar]
- Kazdin AE, Wu C-S, Hwang I, Puac-Polanco V, Sampson NA, Al-Hamzawi A, . . . Kessler RC (2021) Antidepressant use in low- middle- and high-income countries: a World Mental Health Surveys report. Psychological Medicine, Advance online publication. doi: 10.1017/S0033291721003160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, & Luedtke A (2021) Pragmatic Precision Psychiatry-A New Direction for Optimizing Treatment Selection. JAMA Psychiatry, 78(12), 1384–1390. doi: 10.1001/jamapsychiatry.2021.2500 [DOI] [PubMed] [Google Scholar]
- King PR, Beehler GP, Buchholz LJ, Johnson EM, & Wray LO (2019) Functional concerns and treatment priorities among veterans receiving VHA Primary Care Behavioral Health services. Families, Systems & Health, 37(1), 68–73. doi: 10.1037/fsh0000393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus C, Kadriu B, Lanzenberger R, Zarate CA Jr, & Kasper S (2019) Prognosis and improved outcomes in major depression: a review. Translational Psychiatry, 9(1), 127. doi: 10.1038/s41398-019-0460-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laferton JAC, Kube T, Salzmann S, Auer CJ, & Shedden-Mora MC (2017) Patients’ Expectations Regarding Medical Treatment: A Critical Review of Concepts and Their Assessment. Frontiers in Psychology, 8, 233–233. doi: 10.3389/fpsyg.2017.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird KT, Lavretsky H, St Cyr N, & Siddarth P (2018) Resilience predicts remission in antidepressant treatment of geriatric depression. International Journal of Geriatric Psychiatry, 33(12), 1596–1603. doi: 10.1002/gps.4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson S, Nemoianu A, Lawrence DF, Troup MA, Gionfriddo MR, Pousti B, . . . Touya M (2021) Characterizing primary care for patients with major depressive disorder using electronic health records of a US-based healthcare provider. Journal of Affective Disorders, 300, 377–384. doi: 10.1016/j.jad.2021.12.096 [DOI] [PubMed] [Google Scholar]
- LeDell E, van der Laan MJ, & Petersen M (2016) AUC-Maximizing Ensembles through Metalearning. The International Journal of Biostatistics, 12(1), 203–218. doi: 10.1515/ijb-2015-0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Ragguett RM, Mansur RB, Boutilier JJ, Rosenblat JD, Trevizol A, . . . McIntyre RS (2018) Applications of machine learning algorithms to predict therapeutic outcomes in depression: A meta-analysis and systematic review. Journal of Affective Disorders, 241, 519–532. doi: 10.1016/j.jad.2018.08.073 [DOI] [PubMed] [Google Scholar]
- Leeuwenberg AM, van Smeden M, Langendijk JA, van der Schaaf A, Mauer ME, Moons KGM, . . . Schuit E (2022) Performance of binary prediction models in high-correlation low-dimensional settings: a comparison of methods. Diagnostic and Prognostic Research, 6(1), 1. doi: 10.1186/s41512-021-00115-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon AC, Olfson M, Portera L, Farber L, & Sheehan DV (1997) Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. International Journal of Psychiatry in Medicine, 27(2), 93–105. doi: 10.2190/t8em-c8yh-373n-1uwd [DOI] [PubMed] [Google Scholar]
- Lindhiem O, Petersen IT, Mentch LK, & Youngstrom EA (2020) The Importance of Calibration in Clinical Psychology. Assessment, 27(4), 840–854. doi: 10.1177/1073191117752055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little A (2009) Treatment-resistant depression. American Family Physician, 80(2), 167–172. [PubMed] [Google Scholar]
- Lundberg SM, & Lee S-I (2017). A Unified Approach to Interpreting Model Predictions. Retrieved from https://proceedings.neurips.cc//paper/2017/file/8a20a8621978632d76c43dfd28b67767-Paper.pdf.
- Maj M, Stein DJ, Parker G, Zimmerman M, Fava GA, De Hert M, . . . Wittchen HU (2020) The clinical characterization of the adult patient with depression aimed at personalization of management. World Psychiatry, 19(3), 269–293. doi: 10.1002/wps.20771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Whitton SW, Peckham AD, Welge JA, & Otto MW (2013) Patient preference for psychological vs pharmacologic treatment of psychiatric disorders: a meta-analytic review. The Journal of Clinical Psychiatry, 74(6), 595–602. doi: 10.4088/JCP.12r07757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JA, Lee NB, Lee CU, Lee C, & Chae JH (2012) Low trait anxiety, high resilience, and their interaction as possible predictors for treatment response in patients with depression. Journal of Affective Disorders, 137(1–3), 61–69. doi: 10.1016/j.jad.2011.12.026 [DOI] [PubMed] [Google Scholar]
- Noma H, Furukawa TA, Maruo K, Imai H, Shinohara K, Tanaka S, . . . Cipriani A (2019) Exploratory analyses of effect modifiers in the antidepressant treatment of major depression: Individual-participant data meta-analysis of 2803 participants in seven placebo-controlled randomized trials. Journal of Affective Disorders, 250, 419–424. doi: 10.1016/j.jad.2019.03.031 [DOI] [PubMed] [Google Scholar]
- Park T, & Casella G (2008) The Bayesian Lasso. Journal of the American Statistical Association, 103(482), 681–686. doi: 10.1198/016214508000000337 [DOI] [Google Scholar]
- Perlman K, Benrimoh D, Israel S, Rollins C, Brown E, Tunteng JF, . . . Berlim MT (2019) A systematic meta-review of predictors of antidepressant treatment outcome in major depressive disorder. Journal of Affective Disorders, 243, 503–515. doi: 10.1016/j.jad.2018.09.067 [DOI] [PubMed] [Google Scholar]
- Perna G, Alciati A, Daccò S, Grassi M, & Caldirola D (2020) Personalized Psychiatry and Depression: The Role of Sociodemographic and Clinical Variables. Psychiatry Investigation, 17(3), 193–206. doi: 10.30773/pi.2019.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley E, LeDell E, Kennedy C, Lendle S, & van der Laan MJ (2021). Superlearner: Super learner prediction, version 2.0–28. Retrieved from https://CRAN.R-project.org/package=SuperLearner.
- Polley EC, Rose S, & van der Laan MJ (2011) Super learning. In Targeted learning: Casual inference for observational and experimental data (ed. van der Laan MJ and Rose S), pp. 43–66. Springer: New York. [Google Scholar]
- Puac-Polanco V, Leung LB, Bossarte RM, Bryant C, Keusch JN, Liu H, . . . Kessler RC (2021) Treatment Differences in Primary and Specialty Settings in Veterans with Major Depression. Journal of the American Board of Family Medicine, 34(2), 268–290. doi: 10.3122/jabfm.2021.02.200475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaseem A, Barry MJ, & Kansagara D (2016) Nonpharmacologic Versus Pharmacologic Treatment of Adult Patients With Major Depressive Disorder: A Clinical Practice Guideline From the American College of Physicians. Annals of Internal Medicine, 164(5), 350–359. doi: 10.7326/m15-2570 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2021). R: A language and environment for statistical computing. Retrieved from https://www.R-project.org/.
- Rush AJ, & Thase ME (2018) Improving Depression Outcome by Patient-Centered Medical Management. American Journal of Psychiatry, 175(12), 1187–1198. doi: 10.1176/appi.ajp.2018.18040398 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, . . . Keller MB (2003) The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry, 54(5), 573–583. doi: 10.1016/s0006-3223(02)01866-8 [DOI] [PubMed] [Google Scholar]
- Santee AC, & Starr LR (2021) Examining Linear and Nonlinear Associations Between Negative Emotional Reactivity to Daily Events and Depression Among Adolescents. Clinical Psychological Science, Advance online publication. doi: 10.1177/21677026211045684 [DOI] [Google Scholar]
- SAS Institute Inc (2013) SAS ®Software 9.4 edn. Cary, NC. [Google Scholar]
- Stolzmann K, Meterko M, Miller CJ, Belanger L, Seibert MN, & Bauer MS (2019) Survey Response Rate and Quality in a Mental Health Clinic Population: Results from a Randomized Survey Comparison. The Journal of Behavioral Health Services & Research, 46(3), 521–532. doi: 10.1007/s11414-018-9617-8 [DOI] [PubMed] [Google Scholar]
- Wang G, You X, Wang X, Xu X, Bai L, Xie J, . . . Hu C (2018) Safety and effectiveness of escitalopram in an 8-week open study in Chinese patients with depression and anxiety. Neuropsychiatric Disease and Treatment, 14, 2087–2097. doi: 10.2147/ndt.s164673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Kumar V, Ahmad MA, & Teredesai A (2021). Assessing Fairness in Classification Parity of Machine Learning Models in Healthcare. Retrieved from https://arxiv.org/abs/2102.03717.
- Zilcha-Mano S, Wang X, Wajsbrot DB, Boucher M, Fine SA, & Rutherford BR (2021) Trajectories of Function and Symptom Change in Desvenlafaxine Clinical Trials: Toward Personalized Treatment for Depression. Journal of Clinical Psychopharmacology, 41(5), 579–584. doi: 10.1097/jcp.0000000000001435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziobrowski HN, Kennedy CJ, Ustun B, House SL, Beaudoin FL, An X, . . . van Rooij SJH (2021a) Development and Validation of a Model to Predict Posttraumatic Stress Disorder and Major Depression After a Motor Vehicle Collision. JAMA Psychiatry, 78(11), 1228–1237. doi: 10.1001/jamapsychiatry.2021.2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziobrowski HN, Leung LB, Bossarte RM, Bryant C, Keusch JN, Liu H, . . . Kessler RC (2021b) Comorbid mental disorders, depression symptom severity, and role impairment among Veterans initiating depression treatment through the Veterans Health Administration. Journal of Affective Disorders, 290, 227–236. doi: 10.1016/j.jad.2021.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou G (2004) A Modified Poisson Regression Approach to Prospective Studies with Binary Data. American Journal of Epidemiology, 159(7), 702–706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- Zubizarreta JR (2015) Stable Weights that Balance Covariates for Estimation With Incomplete Outcome Data. Journal of the American Statistical Association, 110(511), 910–922. doi: 10.1080/01621459.2015.1023805 [DOI] [Google Scholar]
- Zubizarreta JR, Li Y, Allouah A, & Greifer N (2021). sbw: Stable balancing weights for causal inference and estimation with incomplete outcome data (Version 1.1.1). Retrieved from https://cran.rstudio.com/web/packages/sbw/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


