ABSTRACT
Immune checkpoint inhibitors are becoming more commonly used for many forms of malignancy. With this class of medications being more heavily used, there has been an associated rise in medication-induced autoimmune hepatitis. This case involves a 35-year-old woman being treated with nivolumab/ipilimumab for renal cell carcinoma who developed a steroid-refractory autoimmune hepatitis.
KEYWORDS: autoimmune hepatitis, drug-induced autoimmune hepatitis, check point inhibitor, chemotherapy, renal cell carcinoma, hepatitis, drug induced liver injury, steroid refractory, tocilizumab, nivolumab, ipilimumab
INTRODUCTION
Immune checkpoint inhibitors (CPIs) are becoming increasingly common treatment options for several types of cancers.1 While their use in cancer treatment is very promising, these medications are not without side effects. Specifically, these drugs are commonly associated with hepatic adverse events, including hepatitis.2 In fact, in one study, hepatitis was found in approximately 5% of people treated with a CPI.3 While we know that CPI hepatitis is a potential outcome of treatment with CPIs, we have yet to establish the best treatment options for this adverse event. Based on expert opinion, the recommended course of treatment involves high-dose steroids and immunomodulators if needed.4 However, there is not much literature on the management of CPI hepatitis that is refractory to the typical treatments, although use of tocilizumab has shown some success previously in treatment of immune-related adverse events secondary to CPIs.5
CASE REPORTS
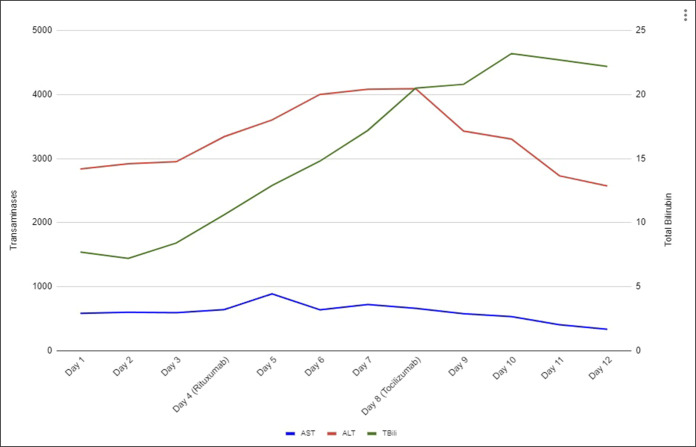
A 35-year-old woman with recurrent right kidney renal cell carcinoma, status post resection with nephrectomy 16 months earlier, on palliative treatment with nivolumab/ipilimumab, presented to the hospital for further evaluation of elevated liver function tests (LFTs) and abdominal magnetic resonance imaging concerning for hepatitis. She had received 4 cycles of treatment with nivolumab/ipilimumab at the time of presentation, but her last dose had been held the month earlier because of rising LFTs and concern for drug-induced autoimmune hepatitis from her CPI treatment. Because of this concern, she had been started on prednisone 120 mg daily 3 weeks before admission. However, her LFTs continued to worsen, prompting her oncologist to recommend admission to the hospital. Upon admission, her aspartate aminotransferase (AST) and alanine transaminase (ALT) were 582 and 2,828, respectively. Her total bilirubin level was also significantly elevated at 7.7. She was immediately started on intravenous methylprednisolone 125 mg every 6 hours and mycophenolate mofetil 1,000 mg twice daily. Initially, her LFTs and total bilirubin levels remained relatively stable on this treatment. However, on hospital day 4, her LFTs increased to an AST of 640 and ALT of 3,342, and her total bilirubin rose to 10.6. At this point, the decision was made to give a one-time dose of 1 g of rituximab in addition to her treatment with steroids and mycophenolate mofetil. On this day, she also went for a liver biopsy which showed significant lobular hepatitis, loss of zone 3 hepatocytes, acidophil bodies, and Kupffer cell hyperplasia, along with perivenular fibrosis with focal pericellular/perisinusoidal fibrosis, which are findings compatible with CPI hepatitis (Image 1). Despite treatment with rituximab, her LFTs and total bilirubin continued to rise, reaching an AST of 660, ALT of 4,091, and total bilirubin of 20.5. After discussion with several physicians at the nearest liver transplant center and a large literature review, the decision was made to administer a 4 mg/kg dose of tocilizumab. The day after the tocilizumab was given, her AST and ALT had both decreased. Three days later, her total bilirubin began to decrease as well. The fourth day after the tocilizumab was given, her AST was 333, her ALT was 2,573 (Figure 1), and her total bilirubin was 22.2 (Figure 2). At this point, she was stable for discharge with follow-up monitoring of her laboratory tests 3 times a week. She was discharged on a prolonged steroid taper. Laboratory values continued to decline over the next 6 months before stabilizing with an AST of 26, ALT of 34, and total bilirubin of 0.6.
Figure 1.
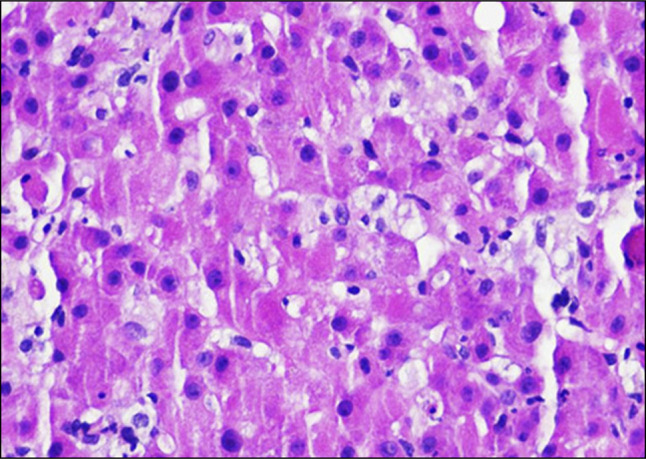
Liver biopsy with significant lobular hepatitis, loss of zone 3 hepatocytes, acidophil bodies, and Kupffer cell hyperplasia. Hematoxylin-eosin stain 200×.
Figure 2.
Time line of injury and medication administration with corresponding AST, ALT, and total bilirubin. ALT, alanine transaminase; AST, aspartate aminotransferase.
DISCUSSION
Checkpoint inhibitor hepatitis is a known side effect of CPI treatment that can affect anyone receiving treatment, although those with preexisting decompensated cirrhosis (Child-Pugh Class B7 or greater) and those taking a CPI in addition to other systemic chemotherapeutic agents are most susceptible.6,7 As the use of CPIs as treatment increases, so will the incidence of this potentially very serious side effect. Specifically, studies from 2019 reveal that 43.68% of all people with cancer in the United States are eligible for CPI therapy.8 This number translates to 7.9 million patients who are potentially eligible for CPI therapy. With the incidence of severe CPI hepatitis suspected to be up to 20%,9 this equates to 1.58 million people of whom could be affected. In addition to just the sheer number of people who could develop this condition, CPI hepatitis is also associated with significant mortality. Specifically, CPI hepatitis accounts for approximately 16% of all immunotherapy-related fatalities.9 Between the significant portion of the population who could be affected and the potentially significant morbidity and mortality associated with developing CPI hepatitis, it is imperative that alternative treatment options are found for when the standard practice fails. As previously mentioned, expert guidelines recommend a prolonged course of steroids with addition of mycophenolate mofetil in advanced CPI hepatitis, but there are no established guidelines with recommendations for CPI hepatitis that is refractory to these treatments. Data collected from various case reports have suggested utility to calcineurin inhibitors, antithymocyte globulin therapy, plasma exchange, tocilizumab, and infliximab in the setting of steroid and mycophenolate mofetil-refractory CPI hepatitis.9 The decision for using tocilizumab as the agent of choice was due to an interdisciplinary discussion between our oncology colleagues, those at the nearest transplant center, and the gastroenterology team. The reasoning came down to the mechanism of tocilizumab. While the pathogenesis of CPI hepatitis is still not clearly understood, biopsy specimens will have lobular or periportal inflammation mostly consisting of T cells, suggesting they play a key part in the condition.10 This patient's improvement with tocilizumab treatment is possibly because of the significant role of T cells in the pathogenesis of CPI hepatitis. This is unlike autoimmune hepatitis, which is predominantly B-cell driven.10 This key difference between the 2 conditions may also shed some light on while rituximab was ineffective in improving this patient's condition as rituximab primarily affects B cells, which appear to be less involved in CPI hepatitis. Calcineurin inhibitors, such as tacrolimus or cyclosporine, have also been shown to be very effective; however, because of the high risk of nephrotoxicity, this medication was not an ideal option because she was status post unilateral nephrectomy and was not a candidate for renal transplant. In addition to this specific case showing improvement with tocilizumab treatment, a small, previous study has shown a clinical improvement of 79% while on tocilizumab,5 further supporting the idea that tocilizumab may be efficacious in this condition. Overall, more studies are needed to understand the underlying pathogenesis of CPI hepatitis along with potential treatment options for refractory cases, but this case report does support the use of tocilizumab as a reasonable treatment option for refractory CPI hepatitis.
DISCLOSURES
Author contributions: R. Farmer and L. Lyles reviewed the literature and wrote and approved the manuscript. L. Lyles edited the manuscript. M. Abougergi approved the manuscript and is the article guarantor.
Financial disclosure: None to report.
Previous presentation: This case was presented at the American College of Gastroenterology Annual Scientific Meeting; October 21–25, 2022; Charlotte, North Carolina.
Informed consent was obtained for this case report.
Contributor Information
Reagan Farmer, Email: reaganfarmer12@gmail.com.
Marwan Abougergi, Email: mgeorgi@catalystclinic.com.
REFERENCES
- 1.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. [DOI] [PubMed] [Google Scholar]
- 2.Chan SL, Yip TC, Wong VW, et al. Pattern and impact of hepatic adverse events encountered during immune checkpoint inhibitors–a territory-wide cohort study. Cancer Med. 2020;9(19):7052–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sengul Samanci N, Cikman DI, Oruc K, et al. Immune-related adverse events associated with immune checkpoint inhibitors in patients with cancer. Tumori J. 2021;107(4):304–10. [DOI] [PubMed] [Google Scholar]
- 4.Dougan M, Wang Y, Rubio-Tapia A, Lim JK. AGA clinical practice update on diagnosis and management of immune checkpoint inhibitor colitis and hepatitis: Expert review. Gastroenterology. 2021;160(4):1384–93. [DOI] [PubMed] [Google Scholar]
- 5.Stroud CR, Hegde A, Cherry C, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract. 2019;25(3):551–7. [DOI] [PubMed] [Google Scholar]
- 6.Kudo M, Matilla A, Santoro A, Melero I, Sangro B. Checkmate-040: Nivolumab (NIVO) in patients (pts) with advanced hepatocellular carcinoma (aHCC) and Child-Pugh B (CPB) status. J Clin Oncol. 2019;37:327. [Google Scholar]
- 7.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: Results from a randomized, double-blind, multicenter phase II study [published correction appears in J Clin Oncol. 2012 Oct 10;30(29):3654]. J Clin Oncol. 2012;30(17):2046–54. [DOI] [PubMed] [Google Scholar]
- 8.Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remash D, Prince DS, McKenzie C, Strasser SI, Kao S, Liu K. Immune checkpoint inhibitor-related hepatotoxicity: A review. World J Gastroenterol. 2021;27(32):5376–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Martin E, Michot JM, Papouin B, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68(6):1181–90. [DOI] [PubMed] [Google Scholar]