Abstract
OBJECTIVES:
A number of trials related to critical care pharmacotherapy were published in 2022. We aimed to summarize the most influential publications related to the pharmacotherapeutic care of critically ill patients in 2022.
DATA SOURCES:
PubMed/Medical Literature Analysis and Retrieval System Online and the Clinical Pharmacy and Pharmacology Pharmacotherapy Literature Update.
STUDY SELECTION:
Randomized controlled trials, prospective studies, or systematic review/meta-analyses of adult critically ill patients assessing a pharmacotherapeutic intervention and reporting clinical endpoints published between January 1, 2022, and December 31, 2022, were included in this article.
DATA EXTRACTION:
Articles from a systematic search and the Clinical Pharmacy and Pharmacology Pharmacotherapy Literature Update were included and stratified into clinical domains based upon consistent themes. Consensus was obtained on the most influential publication within each clinical domain utilizing an a priori defined three-round modified Delphi process with the following considerations: 1) overall contribution to scientific knowledge and 2) novelty to the literature.
DATA SYNTHESIS:
The systematic search and Clinical Pharmacy and Pharmacology Pharmacotherapy Literature Update yielded a total of 704 articles, of which 660 were excluded. The remaining 44 articles were stratified into the following clinical domains: emergency/neurology, cardiovascular, gastroenterology/fluids/nutrition, hematology, infectious diseases/immunomodulation, and endocrine/metabolic. The final article selected from each clinical domain was summarized following a three-round modified Delphi process and included three randomized controlled trials and three systematic review/meta-analyses. Article topics summarized included dexmedetomidine versus other sedatives during mechanical ventilation, beta-blocker treatment in the critically ill, restriction of IV fluids in septic shock, venous thromboembolism prophylaxis in critically ill adults, duration of antibiotic therapy for Pseudomonas aeruginosa ventilator-associated pneumonia, and low-dose methylprednisolone treatment in severe community-acquired pneumonia.
CONCLUSIONS:
This concise review provides a perspective on articles published in 2022 that are relevant to the pharmacotherapeutic care of critically ill patients and their potential impact on clinical practice.
Keywords: critical, care, critically ill, pharmacotherapy, review, sedation, septic shock
KEY POINTS
Question: What were the most influential publications related to the pharmacotherapeutic care of critically ill patients in 2022?
Findings: This systematic search and modified Delphi process revealed three randomized controlled trials and three systematic review/meta-analyses. Article topics included a broad range of critical care topics stratified across various clinical domains including emergency/neurology, cardiovascular, gastroenterology/fluids/nutrition, hematology, infectious diseases/immunomodulation, and endocrine/metabolic.
Meaning: There has been substantial growth in the number of research articles published within the field of critical care annually. This concise review provides a perspective on articles published in 2022 that are relevant to the pharmacotherapeutic care of critically ill patients and their potential impact on clinical practice.
Clinicians face an inherent need to keep abreast with literature and implement evidence-based medicine into practice. However, given the substantial growth in research articles published annually, most are overcome with the sheer volume of publications (1). A meta-epidemiological study demonstrated an explosion in the number of publications in the field of critical care, concluding the number of publications exceeds the number that can be read (2). To mitigate information overload, strategies have been used to keep up with literature including journal surveillance, interaction with scientific and media communities, and services to journals including article review and editorial work (3). The Clinical Pharmacy and Pharmacology Literature Update (CPPLU) working group within the Society of Critical Care Medicine reviews major critical care journals, distributes a monthly summary to various sections of the Society, and reviews influential articles relevant to critical care pharmacotherapy annually (4–13). Therefore, we aimed to summarize the most influential publications related to the pharmacotherapeutic care of critically ill patients in 2022.
METHODS
A systematic search was conducted of PubMed/Medical Literature Analysis and Retrieval System Online from January 1, 2022, to December 31, 2022, to capture relevant articles related to the pharmacotherapeutic care of critically ill patients. Search criteria consistent with previous reviews are located in Appendix 1. Resulting articles were reviewed by two independent authors (P.K.G., P.M.W.) to assess eligibility for inclusion in the full-text review. A full-text review was performed to exclude any remaining articles that did not fulfill inclusion criteria including randomized controlled trial (RCT), prospective study, or systematic review/meta-analysis design, critically ill adult patient population, assessment of a pharmacotherapeutic intervention, and reporting of clinical endpoints. Eligible articles were categorized into clinical domains based upon a consistent theme and entered into a survey (Supplemental File, http://links.lww.com/CCX/B252).
An a priori defined three-round Delphi survey was performed to form consensus on influential publications relevant to the pharmacotherapeutic care of critically ill patients. The survey included the article title within each domain and full-text file to assist with ranking. A multiprofessional panel of authors (n = 15) independently ranked articles “within” each clinical domain in terms of overall contribution to scientific knowledge (morbidity/expense) and novelty to the literature. Each round of the Delphi process terminated if an 80% consensus was obtained in any clinical domain. If an 80% consensus was not obtained, articles with less than 50% agreement were removed and the remaining entered into the subsequent round. At the end of the third round, the article achieving the highest consensus agreement was included in this review. Each article selected was summarized including an analysis and applicability to critical care practice.
RESULTS
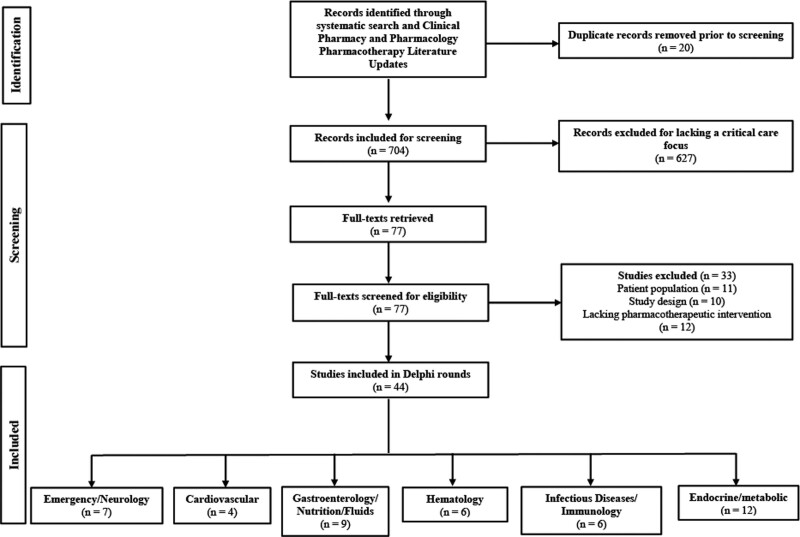
The systematic search and CPPLU revealed a total of 704 articles, of which 627 were excluded based upon aforementioned criteria, and 33 were excluded following full-text review. The remaining 44 articles were included in the modified Delphi process (Fig. 1; and Supplemental File, http://links.lww.com/CCX/B252). No article met the prespecified 80% or more consensus agreement in any round, and therefore, all articles were included in the a priori defined three-round Delphi process. At the completion of three rounds, one article from each clinical domain, achieved consensus agreement and were included in this review.
Figure 1.
Flow diagram of article screening.
DISCUSSION
Neurology/Emergency
Dexmedetomidine Versus Other Sedatives in Critically Ill Mechanically Ventilated Adults: A Systematic Review and Meta-Analysis of Randomized Trials
This systematic review and meta-analysis (SRMA) of 77 RCTs (n = 11,997) evaluated the efficacy and safety of dexmedetomidine versus other sedatives in mechanically ventilated adults in the ICU and found a significant reduction in the risk of delirium (relative risk [RR], 0.67; CI, 0.55–0.81), duration of mechanical ventilation (MV) (median difference [MD], –1.8 hr; 95% CI, –2.89 to –0.71 hr), and ICU length of stay (LOS) (MD, –0.32 d; 95% CI, –0.42 to –0.22 d) with dexmedetomidine use compared with other sedatives (14). Patients receiving dexmedetomidine were more likely to maintain lighter levels of sedation with an increased proportion of time spent at their target sedation goal (MD, 3.67 percentage points; 95% CI, 0.98–6.36). Dexmedetomidine was not found to reduce the risk of death at 30 days compared with other sedatives (RR, 0.94; 95% CI, 0.80–1.11) or hospital LOS (MD, –0.10 d; 95% CI, –0.72 to 0.91 d). In the safety analysis, dexmedetomidine increased the risk of bradycardia (RR, 2.39; 95% CI, 1.82–3.13) and hypotension (RR, 1.32; 95% CI, 1.07–1.63); there was not an increased need for interventions related to these adverse effects.
Delirium is prevalent in ICU patients and associated with an increased risk of morbidity and mortality (15). Adherence to the “A2F bundle,” including appropriate choice of sedation, increases delirium-free days, and reduces the risk of in-hospital mortality (16). Benzodiazepines are not recommended as first-line for sedation; however, the delirium risk with alternative sedatives remains unclear. In this SRMA, dexmedetomidine reduced the risk of delirium compared with benzodiazepines, propofol, and opioids, with an absolute risk reduction of 11% or number needed to treat of 23 (14). The proposed benefit of dexmedetomidine on delirium may include improved analgesia through central α2 agonism, attenuation of neuroinflammation, improved sleep quality, and facilitation of lighter sedation, thereby enhancing patient communication and interaction (17–19). Although a dose-response relationship could not be elicited from this study, most of the studies included limited doses to less than 0.7 µg/kg/hr with a significant reduction in the risk of delirium at these lower doses as compared with benzodiazepines, propofol, and opioids (RR, 0.46; CI, 0.34–0.62). Consistent with findings from the Sedation Practice in Intensive Care Evaluation III trial, a lower risk of delirium and duration of MV was demonstrated when dexmedetomidine initiation was within 12 hours of ICU admission (20). Of note, a subgroup analysis by duration of dexmedetomidine noted a shorter duration of use (≤ 24 hr) was associated with the largest reduction in delirium as compared with a longer duration of use (> 24 hr). This is relevant given the potential for tachyphylaxis with prolonged administration. While several studies used a bolus prior to infusion initiation, this strategy is not performed in clinical practice due to the risk of adverse effects. Low baseline mean arterial pressure (MAP), higher Acute Physiology and Chronic Health Evaluation (APACHE) II score, and history of coronary artery disease may increase the risk of hypotension; patient-specific factors should be considered prior to initiation (21).
Strengths of this SRMA included no correlation demonstrated between age and treatment effect, and increasing baseline APACHE II score and escalating doses of dexmedetomidine were associated with lower relative reductions in delirium. In addition, patients in medical, surgical, cardiovascular, and mixed medical/surgical ICUs were included; however, patients with alcohol withdrawal, requiring sedation for shivering, or deep sedation were excluded, thereby limiting the generalizability. A subgroup analysis by dose of dexmedetomidine was unable to be completed as no trial exclusively used doses greater than 0.7 µg/kg/hr. Last, significant heterogeneity in the reporting of results and a lack of individual patient data diminished predefined analyses to determine whether certain baseline factors influenced treatment effect (14).
Collectively, evidence suggests dexmedetomidine reduces the risk of delirium, ICU LOS, and increases liberation from the ventilator, especially when used for less than 24 hours. However, use may be limited by bradycardia and hypotension, with close monitoring required during treatment initiation and dose titration.
Cardiovascular
Beta-Blocker Treatment in the Critically Ill: A Systematic Review and Meta-Analysis
This SRMA of 16 RCTs (n = 2,410) evaluated the effect of β-blockers in critically ill patients (22). Studies including patients with sepsis/septic shock, any form of circulatory failure, burns (> 30% total body surface area), major trauma, and traumatic brain injury (TBI) were included. Eleven quantitative trials (n = 2,103) demonstrated a significant mortality reduction in patients treated with β-blockers compared with placebo or standard of care (SOC) (RR, 0.65; 95% CI, 0.53–0.79; p < 0.0001; I2 = 0%; high certainty of evidence). When separated into short-term (< 14 d) and long-term (> 14 d) mortality, only a significant reduction in long-term mortality with β-blockers was noted (RR, 0.60; 95% CI, 0.48–0.74; p < 0.00001; I2 = 0%; high certainty of evidence). Of the planned secondary endpoints, there was a significant reduction in heart rate (HR) with β-blocker treatment compared with control or SOC (MD at 24 hr, –11.96 beats/min; 95% CI, –20.86 to –3.06; p = 0.008; I2 = 91% and MD at 48 hr, –13.66 beats/min; 95% CI, –26.10 to –1.22; p = 0.03; I2 = 93%; moderate certainty of evidence) and no difference in vasopressor requirements and MAP between groups (high certainty of evidence). Although the effect on HR reduction is expected, additional patient-centered outcomes such as organ dysfunction and quality of life cannot be inferred from this surrogate endpoint. Endpoints, such as ejection fraction and lactate, were unable to be assessed due to heterogeneity of outcome reporting.
β-blockers are ubiquitously used in a variety of disease states but often avoided in critically ill patients due to negative inotropic, chronotropic, and blood pressure (BP) lowering effects, potentially compromising organ perfusion. It has been hypothesized, however, that β-blockers blunt the adrenergic response and may improve outcomes despite lowering of BP (23, 24). β-blockers, specifically propranolol, have been shown to improve survival in TBI (25). A previous meta-analysis, focusing on the addition of an esmolol infusion in septic patients, demonstrated improved survival (RR, 2.06; 95% CI, 1.52–2.79; p = 0.006) with a 31.1% absolute reduction in mortality (26, 27). Conversely, a cohort of general ICU patients found no association between β-blockers and mortality (adjusted odds ratio [aOR], 1.56; 95% CI, 0.83–2.9; p = 0.16); however, when analyzing patients without diabetes only, an increased association with β-blockers and ICU mortality was demonstrated (aOR, 2.93; 95% CI, 1.19–7.23). This finding of an increase in mortality with β-blocker use in patients without diabetes should be interpreted with caution given the retrospective nature of this study and post hoc study design (28).
While this is the first SRMA of RCTs on β-blockers in various critically ill patients, the conclusion is limited by small sample sizes and quality of RCTs included, qualitative heterogeneity of β-blockers and endpoints studied and inclusion criteria, and lack of reporting of other hemodynamically active drugs (nonstudy β-blockers).
Based on the evidence, considerable variation remains on the optimal timing of initiation, withholding, and restarting of β-blocker therapy in critically ill patients (29). In addition, choice of agent, patient selection, and optimal hemodynamic targets remain unanswered warranting further research. Therefore, initiation of β-blockers in the ICU with careful monitoring for bradycardia and hypotension should be determined on a case-by-case basis.
Gastroenterology/Fluids/Nutrition
Restriction of IV Fluid in ICU Patients With Septic Shock
The Conservative versus Liberal Approach to Fluid Therapy of Septic Shock in Intensive Care (CLASSIC) trial was an international, open-label, randomized trial evaluating the restriction of fluids on mortality and serious adverse events in adult patients with septic shock (30). Patients were included if the onset of shock was within 12 hours of screening and received at least 1 L of IV fluids (IVFs) within 24 hours of screening. The restrictive fluid (RF) group (n = 764) could have received fluids for any of the following conditions: severe hypoperfusion (lactate ≥ 4 mmol/L, MAP < 50 mm Hg despite vasopressor or inotropic support, mottling beyond the edge of the kneecap, or urinary output < 0.1 mL/kg/hr during the first 2 hr after randomization), to replace documented fluid losses, to correct hydration or electrolyte deficiency if the enteral route was contraindicated, or to ensure a total daily fluid intake of 1 L. The standard fluid (SF) group (n = 781) had no set limit on the amount of IVF administration and fluids could have been administered for any of the following conditions: fluids given as long as the patient had an improvement in hemodynamic factors, fluids to replace expected or observed losses or to correct dehydration or electrolyte derangements, or maintenance fluid per ICU protocol. Enteral and oral fluids, enteral or parenteral nutrition, and fluids used as a carrier for medication administration were allowed in both groups. Patients in both intervention groups remained in the ICU for a median of 5 days. In the ICU, the RF group received a median 1,798 mL of IVFs compared with 3,811 mL in the SF group, excluding fluids administered with medication and nutrition, during the 90-day trial period. There was no difference in 90-day mortality in the RF group compared with the SF group (42.3% vs 42.1%; 95% CI, –4.7 to 4.9; p = 0.96). Secondary outcomes were not significantly different, including serious adverse events, number of days alive without life support, and days alive and out of the hospital at 90 days.
The findings of the CLASSIC trial are in contrast to previous studies demonstrating higher volumes of IVF to be associated with worsening kidney injury, respiratory failure, and mortality in patients with septic shock (31–33). Nevertheless, comparison of the CLASSIC trial to previous studies is limited given differences in study design, patient severity of illness, sources of infection, and fluid protocols. Hjortrup et al (34) demonstrated that a RF group had a lower mean resuscitation fluid volume (MD, –1.2 L; 95% CI, –2.0 to –0.4 L) at day 5 compared with the standard care group (MD, –1.4 L; 95% CI, –2.4 to –0.4 L) with the higher resuscitation volumes associated with worsening acute kidney injury (odds ratio [OR], 0.46; 95% CI, 0.23–0.92; p = 0.03); however, interpretation of this study was limited by major protocol violations occurring in 37% of patients in the RF group. The findings of the CLASSIC trial are consistent with the Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis trial which demonstrated no difference in mortality before discharge home by day 90 with a RF strategy compared with a liberal fluid (LF) strategy (35).
Strengths of the CLASSIC trial include a relatively large sample size and randomized study design. However, the study was unblinded and did not provide data on tools used to guide fluid administration. Additionally, patients received fluids prior to enrollment, with the majority of fluids administered outside the study protocol (e.g., blood products, IVFs from with medication and nutrition, and oral intake), which may have impacted the results. Finally, the 7% absolute difference in 90-day mortality between groups may be considered quite large (36).
Based on the available literature, clinical equipoise exists regarding a RF versus LF volume approach in sepsis. The current evidence leaves many questions regarding the optimal volume of initial (within the first 24 hr) fluid resuscitation, the effect of additional resuscitation on targets other than MAP and lactate and its associated outcomes, which patient populations would benefit most from a RF versus LF strategy, and when fluid deresuscitation be implemented (37). Therefore, future studies should not only aim to assess the impact of all IVFs administered on mortality and other outcomes but also address these questions.
Hematology
Venous Thromboembolism Prophylaxis in Critically Ill Adults: A Systematic Review and Network Meta-Analysis
This systematic review and network meta-analysis of 13 RCTs from inception to January 2021 (n = 9,619) evaluated: 1) the efficacy of pharmacologic thromboprophylaxis with low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH), 2) mechanical thromboprophylaxis, 3) a combination of pharmacologic and mechanical thromboprophylaxis, 4) placebo, or 5) no thromboprophylaxis in adult critically ill patients (38). A majority of patients included were from mixed medical/surgical and trauma ICUs. LMWH prophylaxis reduced the incidence of deep vein thrombosis (DVT) compared with UFH prophylaxis (OR, 0.72; 95% credible interval [CrI], 0.46–0.98; moderate certainty) or control (OR, 0.59; 95% CrI, 0.33–0.90; high certainty) and UFH prophylaxis may reduce the incidence of DVT (OR, 0.82; 95% CrI, 0.47–1.37; low certainty) compared with control. Furthermore, LMWH prophylaxis may reduce the incidence of pulmonary embolism (PE) (OR, 0.47; 95% CrI, 0.03–3.91; low certainty) compared with control; however, the effect of UFH prophylaxis on PE and LMWH prophylaxis compared with UFH prophylaxis on PE was uncertain (OR, 0.70; 95% CrI, 0.05–7.95; low-certainty and OR, 0.65; 95% CrI, 0.08–3.65, respectively). Compared with control, both LMWH prophylaxis (OR, 0.63; 95% CrI, 0.18–1.59; low certainty) and UFH prophylaxis (OR, 0.79; 95% CrI, 0.22–2.28; low certainty) may reduce the incidence of any venous thromboembolism (VTE) defined as any upper or lower extremity DVT or any segmental or proximal PE. Data were insufficient to perform a network meta-analysis for several secondary outcomes including major bleeding, heparin-induced thrombocytopenia, ICU LOS, and mortality.
The rate of VTE varies greatly among patients in the ICU, with the highest rates reported in surgical or trauma patients (39). Thromboembolism prophylaxis is a foundational intervention in critically ill patients (40). VTE is associated with increased healthcare costs and mortality (41). This systematic review has limitations, including a lack of standardized screening protocols to identify DVTs; therefore, the effect of these modalities on clinically significant DVTs is uncertain. Additionally, a majority of patients analyzed were from a mixed medical/surgical and trauma population, thereby limiting generalizability to strictly medical patients. Finally, doses of pharmacological VTE prophylaxis were not standardized, limiting any conclusion of the impact of drug dose on efficacy.
Overall, while this analysis suggests LMWH may be preferable over UFH in reducing the incidence of DVT, caution should be exercised in generalizing these results to patients with concern for bleeding or impaired renal function (creatinine clearance < 30 mL/min). In addition, given the low certainty of evidence for reducing the incidence of PE and effect of these agents on major bleeding, further studies are warranted.
Infectious Disease/Immunomodulation
Comparison of 8 Versus 15 Days of Antibiotic Therapy for Pseudomonas aeruginosa Ventilator-Associated Pneumonia in Adults: A Randomized, Controlled, Open-Label Trial
The Impact of the Duration of Antibiotics on clinical events in Patients with Pseudomonas aeruginosa ventilator-associated pneumonia (iDIAPASON) study was a randomized, open-label, noninferiority trial evaluating short duration (8 d) versus prolonged antibiotic therapy (15 d) in adult patients with documented ventilator-associated pneumonia (VAP) caused by P. aeruginosa (PsA) (42). Patients were excluded if PsA was isolated from respiratory cultures prior to the current hospitalization, pregnancy, receiving immunosuppression (HIV, immunosuppressive therapy, corticosteroids > 0.5 mg/kg/d for > 1 mo), chronic pulmonary colonization with PsA or bronchiectasis, or received antibiotic therapy active against PsA for an extrapulmonary infection. Antibiotic therapy was initiated following respiratory sampling and choice of therapy was left to the discretion of the treating physician. The primary outcome of PsA recurrence occurring during the ICU stay until day 90 was defined by a post hoc diagnosis as clinical suspicion of VAP after greater than or equal to 48 hours without effective antibiotic therapy for PsA with fever greater than 38.5°C, leukocytosis greater than 109/L or leukopenia less than 4.108/L, purulent tracheobronchial secretions, and a new or persistent infiltrate on chest radiograph, then confirmed with a positive quantitative culture. The study was stopped early due to low enrollment and did not include the prespecified number of patients needed to meet statistical power. In the intention-to-treat group, 25 patients (25.5%) in the 15-day group and 31 patients (35.2%) in the 8-day group had a VAP recurrence or were dead in the ICU at 90 days. Noninferiority was not demonstrated as the upper bound of the 90% CI was greater than the predefined criteria of 10% (difference, 9.7%; 90% CI, –1.9% to 21.2%). Similar results were observed in the per protocol group and post hoc adjusted analyses. Furthermore, a higher rate of recurrence was observed in the 8-day group compared with the 15-day group (17% vs 9.2%; difference, 7.9%; 90% CI, –0.5% to 16.8%). There were no differences between groups in duration of MV, ICU LOS, and acquisition of multidrug-resistant organisms.
VAP is a major cause of morbidity and mortality in critically ill patients. While guidelines exist for management of VAP, there continue to be uncertainties regarding optimal treatment duration of VAP caused by PsA (43). This study was consistent in methodology and findings compared with the Comparison of 8 vs 15 Days of Antibiotic Therapy for Ventilator-Associated Pneumonia in Adults trial (44). Subsequent trials, however, evaluated treatment duration with conflicting results (45–47).
The iDIAPASON study was strengthened by inclusion of infections only caused by PsA, relatively long 90-day outcomes assessment (minimizing the impact of a differential time of follow-up due to one group receiving a longer duration of therapy), and a standardized definition of recurrence. However, this trial was limited by the open-label design, inability to meet the predefined sample size, and exclusion criteria limiting generalizability of the results.
Notably, while this trial suggested an increased risk of recurrence of PsA-VAP with 8 days of therapy, it remains unknown if a short or long duration should be used; therefore, clinicians should individualize PsA-VAP treatment duration based on clinical response.
Endocrine/Metabolic
Low-Dose Methylprednisolone Treatment in Critically Ill Patients With Severe Community-Acquired Pneumonia
Methylprednisolone in Hospitalized Veterans with Severe Community-Acquired Pneumonia (ESCAPe) was a multicenter, double-blind, RCT evaluating the effects of methylprednisolone on all-cause mortality and secondary endpoints of morbidity and mortality in patients with severe community-acquired pneumonia (CAP) admitted to the ICU within 72–96 hours of hospital presentation from January 2012 to April 2016 (48). A total of 584 patients were randomized 1:1 to receive methylprednisolone (IV loading dose of 40 mg on day 0 followed by a continuous infusion of 40 mg/d on days 1–7, 20 mg/d on days 8–14, 12 mg/d on days 15–17, and 4 mg/d on days 18–20) (n = 297) or placebo (n = 287). The study drug was administered by continuous infusion during the ICU stay and changed to bid via the enteral or IV route after ICU discharge. Of note, 34% of patients met healthcare-associated pneumonia (HCAP) criteria, 11% had acute respiratory distress syndrome (ARDS), and 33% of patients received MV at enrollment. There was no difference in 60-day all-cause mortality between groups (16% vs 18%; OR, 0.89; 95% CI, 0.58–1.38; p = 0.61), 180-day (21% vs 24%; OR, 0.86; 95% CI, 0.58–1.29; p = 1.00) or 1-year all-cause mortality (30% vs 33%; OR, 0.88; 95% CI, 0.61–1.27; p = 1.00). No difference in 60-day mortality was found when adjusted for site and MV (aOR, 0.90; 95% CI, 0.57–1.40; p = 0.63) and baseline patient characteristics (aOR, 0.87; 95% CI, 0.53–1.42; p = 0.58). Additionally, there were no significant differences between groups in the median MV-free days up to day 8 or 28, development of ARDS or vasopressor dependent shock, ICU and hospital LOS, or hospital mortality. In patients receiving MV at randomization, there was a 3-day reduction in duration of MV (median 4 vs 7 d; HR, 1.44; CI, 1.04–1.99; p = 0.21) in the methylprednisolone group.
Benefits to the use of glucocorticoids in CAP include a reduction in need for MV, progression to ARDS, and time to clinical stability, at the expense of hyperglycemia and secondary infection (49). Torres et al (50) demonstrated that use of methylprednisolone in patients with severe CAP and high initial inflammatory response resulted in a decrease in radiographic progression and decreased treatment failure. The Community-Acquired Pneumonia: Evaluation of Corticosteroids trial concluded ICU patients with severe CAP receiving hydrocortisone had a lower risk of death at 28 days versus placebo with a similar incidence of hospital-acquired infections and gastrointestinal bleeding (51). Of note, patients with a lower acuity were included in these trials as compared with the ESCAPe trial.
Although prior analyses have suggested a mortality benefit with glucocorticoids, significant heterogeneity exists in both the study quality and definition of severe CAP used (52–54). The 2019 CAP guidelines recommend against the routine use of glucocorticoids in CAP, with a higher quality of evidence against its use in nonsevere CAP (55).
Several strengths of the ESCAPe trial include a large population and long-term follow-up. However, interpretation of trial findings is limited by low-recruitment and generalizability given the inclusion of HCAP patients, selection bias as patients were excluded at the discretion of the physician, baseline comorbidities/severity scores, and racial, gender, and age composition of the Veterans Affairs population as compared with the general population. In addition, in-hospital adverse events related to study treatment such as hyperglycemia and gastrointestinal bleeding were not reported in the trial. The lack of benefit may be a result of low doses used or delayed timing of administration (72–96 hr after hospital admission) leading to an inadequate anti-inflammatory response. Nevertheless, a variable response to glucocorticoid treatment in CAP are likely multifactorial (e.g., different agent, dosing, route, duration). Therefore, the use of steroids in ICU patients with severe CAP should be individualized.
CONCLUSIONS
This concise review provides perspective on articles relevant to the pharmacotherapeutic care of critically ill patients. The studies included in this review add to the current body of critical care literature on pharmacotherapy interventions in the ICU and provide insight on areas of future research.
ACKNOWLEDGMENTS
We would like to acknowledge members of the Clinical Pharmacy and Pharmacology Pharmacotherapy Literature Update for their contributions: Diana Altshuler, NYU Langone; Mahmoud Ammar, Yale; Laura Brickett Andrick, Geisinger Med Center; Laura Baumgartner, Touro University California; Michael Behal, UK Health; Miranda Bowers, Duke University Hospital; Paige Bradshaw, UC Health; Judah Brown, NY Presbyterian; Sophia Brown, Northwestern Memorial; Lisa Burry, Mount Sinai; Tyler Chanas, Vidant Med Center; Laura Coles, Novant Health Forsyth; Reagan Collins, Pfizer; Patrick Costello, U of Chicago; Lauren Dehne, UCMC Cincinnati; Dharati Desai, Advocate Christ Med Center; Payal Desai, Morristown Med Center; Elisabeth Donahey, Lexicomp; Chris Droege, UCMC Cincinnati; Roxana Dumitru, NY Presbyterian-Columbia University Irving; Mary Eche, Beth Israel Deaconess; Pansy Elsamadisi, Beth Israel Deaconess; Claire Eng, Memorial Hermann; David Gagnon, Maine Med Center; Brian Gilbert, Wesley Med Center; Phil Grgurich, Massachusetts College of Pharmacy and Health Sciences; Mai Hashhoush, King Fahad Spec Hosp Dammam; Michelle Henninger, Cleveland Clinic; Olivia Henton, Ohio Health Riverside Methodist; Emily Highsmith, MD Anderson; Benjamin Hohlfelder, Cleveland Clinic; Randy Hollins, Tufts; Nicole Hume, UK Healthcare; Ah Hyun Jun, Northside Hosp Cherokee; Ada Jutba, UF Health Shands; Kristi Kim, Cooper University Healthcare; Brian Kopp, Banner—UMC Tucson; Liz Kowalik, UMass Memorial Med Center; Kinsey Kowalski, UC Health Mem Central; Elizabeth Langenstroer, NY Presbyterian; Steven Lemieux, Connecticut VA; Melanie Madorsky, Memorial Hermann; Courtney Makowski, Northwestern Memorial; Chris Miller, St Anthony Hospital; Megan Moore, Sanford Medical Center; Kendall Mores, Northwestern Memorial; Bill Olney, UK Healthcare; Jaimini Patel, Otsuka; Sarah Peppard, Concordia University; Caitlin Pfaff, UC Health; Carolyn Philpott, UCMC; Angela Plewa-Rusiecki, Stroger of Cook County; Nicole Reardon, HCA Florida North; Alicia Sacco; Mayo Clinic Arizona; Sara Saldana; Mike Semanco, Lakeland Regional Health; Elizabeth Shald, University of New Mexico Hospital; Angela Slampak-Cindric, Geisinger Med Center; Katherine Spezzano, UK Healthcare; Tori Thompson, University of Iowa Hospitals & Clinics; William Tidwell, Vanderbilt; Rachel LaBianca Toler, Duke Regional; Megan Van Berkel Patel, Erlanger Health System; Amy West, Orlando Health—Dr. P. Phillips Hospitals; Amanda Wiebe, Memorial Regional Med Center; and Jason Yerke, Cleveland Clinic.
Supplementary Material
APPENDIX 1
Search terms: ((((critical care[MeSH Terms]) OR (intensive care[MeSH Terms])) OR (intensive care unit[MeSH Terms])) OR (critical illness[MeSH Terms])) OR (critically ill[MeSH Terms]).
Filters: Full text, Clinical Study, Clinical Trial, Clinical Trial, Phase I, Clinical Trial, Phase II, Clinical Trial, Phase III, Clinical Trial, Phase IV, Comparative Study, Controlled Clinical Trial, Meta-Analysis, Pragmatic Clinical Trial, Randomized Controlled Trial, Validation Study.
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
This study was a work product of the Society of Critical Care Medicine and endorsed by the Section on Clinical Pharmacy and Pharmacology.
The content of this article contains previously peer-reviewed and published literature and not original data.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Maggio LA, Artino AR: Staying up to date and managing information overload. J Grad Med Educ 2018; 10:597–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsutsumi Y, Tsuchiya A, Kawahara T: Publication hyper-inflation in the field of intensive care. Intensive Care Med 2023; 49:706–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamtchum-Tatuene J, Zafack JG: Keeping up with the medical literature: Why, how, and when? Stroke 2021; 52:e746–e748 [DOI] [PubMed] [Google Scholar]
- 4.Turck CJ, Frazee E, Kram B, et al. ; Critical Care Pharmacotherapy Literature Update Group: Major publications in the critical care pharmacotherapy literature: February 2012 through February 2013. Am J Health Syst Pharm 2014; 71:68–77 [DOI] [PubMed] [Google Scholar]
- 5.Rech MA, Day SA, Kast JM, et al. ; Critical Care Pharmacotherapy Literature Update Group: Major publications in the critical care pharmacotherapy literature: January-December 2013. Am J Health Syst Pharm 2015; 72:224–236 [DOI] [PubMed] [Google Scholar]
- 6.Day SA, Cucci M, Droege ME, et al. : Major publications in the critical care pharmacotherapy literature: January-December 2014. Am J Health Syst Pharm 2015; 72:1974–1985 [DOI] [PubMed] [Google Scholar]
- 7.Wong A, Erdman M, Hammond DA, et al. : Major publications in the critical care pharmacotherapy literature in 2015. Am J Health Syst Pharm 2017; 74:295–311 [DOI] [PubMed] [Google Scholar]
- 8.Horner D, Altshuler D, Droege C, et al. : Major publications in the critical care pharmacotherapy literature: January-December 2016. J Crit Care 2018; 43:327–339 [DOI] [PubMed] [Google Scholar]
- 9.Hammond DA, Baumgartner L, Cooper C, et al. : Major publications in the critical care pharmacotherapy literature: January-December 2017. J Crit Care 2018; 45:239–246 [DOI] [PubMed] [Google Scholar]
- 10.Newsome AS, Bissell BD, Burry LD, et al. : Major publications in critical care pharmacotherapy literature in 2018. J Crit Care 2019; 52:200–207 [DOI] [PubMed] [Google Scholar]
- 11.Condeni MS, Basting AT, Costello PG, et al. : Major publications in the critical care pharmacotherapy literature: 2019. J Crit Care 2021; 62:197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bissell BD, Campbell J, Collins R, et al. : Major publications in the critical care pharmacotherapy literature: 2020. Crit Care Explor 2021; 3:e0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wieruszewski PM, Brickett LM, Dayal L, et al. : Major publications in the critical care pharmacotherapy literature: 2021. Crit Care Explor 2022; 4:e0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis K, Alshamsi F, Carayannopoulos KL, et al. ; GUIDE group: Dexmedetomidine vs other sedatives in critically ill mechanically ventilated adults: A systematic review and meta-analysis of randomized trials. Intensive Care Med 2022; 48:811–840 [DOI] [PubMed] [Google Scholar]
- 15.Rahimi-Bashar F, Abolhasani G, Manouchehrian N, et al. : Incidence and risk factors of delirium in the intensive care unit: A prospective cohort. Biomed Res Int 2021; 2021:6219678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pun BT, Balas MC, Barnes-Daly MA, et al. : Caring for critically ill patients with the ABCDEF bundle: Results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med 2019; 47:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao J, Sun Z, Xiao Z, et al. : Dexmedetomidine modulates neuroinflammation and improves outcome via alpha2-adrenergic receptor signaling after rat spinal cord injury. Br J Anaesth 2019; 123:827–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skrobik Y, Duprey MS, Hill NS, et al. : Low-dose nocturnal dexmedetomidine prevents ICU delirium. A randomized, placebo-controlled trial. Am J Respir Crit Care Med 2018; 197:1147–1156 [DOI] [PubMed] [Google Scholar]
- 19.Jakob SM, Ruokonen E, Grounds RM, et al. ; Dexmedetomidine for Long-Term Sedation Investigators: Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA 2012; 307:1151–1160 [DOI] [PubMed] [Google Scholar]
- 20.Shehabi Y, Howe BD, Bellomo R, et al. ; ANZICS Clinical Trials Group and the SPICE III Investigators: Early sedation with dexmedetomidine in critically ill patients. N Engl J Med 2019; 380:2506–2517 [DOI] [PubMed] [Google Scholar]
- 21.Gerlach AT, Blais DM, Jones GM, et al. : Predictors of dexmedetomidine-associated hypotension in critically ill patients. Int J Crit Illn Inj Sci 2016; 6:109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heliste M, Pettilä V, Berger D, et al. : Beta-blocker treatment in the critically ill: A systematic review and meta-analysis. Ann Med 2022; 54:1994–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coppola S, Froio S, Chiumello D: β-blockers in critically ill patients: From physiology to clinical evidence. Crit Care 2015; 19:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagiwara S, Iwasaka H, Maeda H, et al. : Landiolol, an ultrashort-acting beta1-adrenoceptor antagonist, has protective effects in an LPS-induced systemic inflammation model. Shock 2009; 31:515–520 [DOI] [PubMed] [Google Scholar]
- 25.Ley EJ, Leonard SD, Barmparas G, et al. ; Beta Blockers TBI Study Group Collaborators: Beta blockers in critically ill patients with traumatic brain injury: Results from a multicenter, prospective, observational American Association for the Surgery of Trauma study. J Trauma Acute Care Surg 2018; 84:234–244 [DOI] [PubMed] [Google Scholar]
- 26.Liu P, Wu Q, Tang Y, et al. : The influence of esmolol on septic shock and sepsis: A meta-analysis of randomized controlled studies. Am J Emerg Med 2018; 36:470–474 [DOI] [PubMed] [Google Scholar]
- 27.Morelli A, Ertmer C, Westphal M, et al. : Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: A randomized clinical trial. JAMA 2013; 310:1683–1691 [DOI] [PubMed] [Google Scholar]
- 28.Al Harbi SA, Al Sulaiman KA, Tamim H, et al. : Association between β-blocker use and mortality in critically ill patients: A nested cohort study. BMC Pharmacol Toxicol 2018; 19:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senussi MH: Beta-blockers in the critically ill: Friend or foe? J Am Coll Cardiol 2021; 78:1012–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyhoff TS, Hjortrup PB, Wetterslev J, et al. ; CLASSIC Trial Group: Restriction of intravenous fluid in ICU patients with septic shock. N Engl J Med 2022; 386:2459–2470 [DOI] [PubMed] [Google Scholar]
- 31.Silversides JA, Major E, Ferguson AJ, et al. : Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: A systematic review and meta-analysis. Intensive Care Med 2017; 43:155–170 [DOI] [PubMed] [Google Scholar]
- 32.van Mourik N, Geerts BF, Binnekade JM, et al. : A higher fluid balance in the days after septic shock reversal is associated with increased mortality: An observational cohort study. Crit Care Explor 2020; 2:e0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrews B, Semler MW, Muchemwa L, et al. : Effect of an early resuscitation protocol on in-hospital mortality among adults with sepsis and hypotension: A randomized clinical trial. JAMA 2017; 318:1233–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hjortrup PB, Haase N, Bundgaard H, et al. ; CLASSIC Trial Group: Restricting volumes of resuscitation fluid in adults with septic shock after initial management: The CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensive Care Med 2016; 42:1695–1705 [DOI] [PubMed] [Google Scholar]
- 35.Shapiro NI, Douglas IS, Brower RG, et al. ; National Heart, Lung, and Blood Institute Prevention and Early Treatment of Acute Lung Injury Clinical Trials Network: Early restrictive or liberal fluid management for sepsis-induced hypotension. N Engl J Med 2023; 388:499–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridgeon EE, Bellomo R, Aberegg SK, et al. : Effect sizes in ongoing randomized controlled critical care trials. Crit Care 2017; 21:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McIntyre LA, Marshall JC: Intravenous fluids in septic shock - more or less? N Engl J Med 2022; 386:2518–2519 [DOI] [PubMed] [Google Scholar]
- 38.Fernando SM, Tran A, Cheng W, et al. : VTE prophylaxis in critically ill adults: A systematic review and network meta-analysis. Chest 2022; 161:418–428 [DOI] [PubMed] [Google Scholar]
- 39.Tadesse TA, Kedir HM, Fentie AM, et al. : Venous thromboembolism risk and thromboprophylaxis assessment in surgical patients based on Caprini risk assessment model. Risk Manag Healthc Policy 2020; 13:2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vincent J-L: Give your patient a fast hug (at least) once a day. Crit Care Med 2005; 33:1225–1229 [DOI] [PubMed] [Google Scholar]
- 41.Lyman GH, Culakova E, Poniewierski MS, et al. : Morbidity, mortality and costs associated with venous thromboembolism in hospitalized patients with cancer. Thromb Res 2018; 164:S112–S118 [DOI] [PubMed] [Google Scholar]
- 42.Bouglé A, Tuffet S, Federici L, et al. ; iDIAPASON Trial Investigators: Comparison of 8 versus 15 days of antibiotic therapy for Pseudomonas aeruginosa ventilator-associated pneumonia in adults: A randomized, controlled, open-label trial. Intensive Care Med 2022; 48:841–849 [DOI] [PubMed] [Google Scholar]
- 43.Kalil AC, Metersky ML, Klompas M, et al. : Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63:e61–e111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chastre J, Wolff M, Fagon J-Y, et al. ; PneumA Trial Group: Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: A randomized trial. JAMA 2003; 290:2588–2598 [DOI] [PubMed] [Google Scholar]
- 45.Pugh R, Grant C, Cooke RPD, et al. : Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev 2015; 2015:CD007577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fekih Hassen M, Ayed S, Ben Sik Ali H, et al. : [Duration of antibiotic therapy for ventilator-associated pneumonia: Comparison of 7 and 10 days. A pilot study]. Ann Fr Anesth Reanim 2009; 28:16–23 [DOI] [PubMed] [Google Scholar]
- 47.Capellier G, Mockly H, Charpentier C, et al. : Early-onset ventilator-associated pneumonia in adults randomized clinical trial: Comparison of 8 versus 15 days of antibiotic treatment. PLoS One 2012; 7:e41290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meduri GU, Shih M-C, Bridges L, et al. ; ESCAPe Study Group: Low-dose methylprednisolone treatment in critically ill patients with severe community-acquired pneumonia. Intensive Care Med 2022; 48:1009–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lloyd M, Karahalios A, Janus E, et al. ; Improving Evidence-Based Treatment Gaps and Outcomes in Community-Acquired Pneumonia (IMPROVE-GAP) Implementation Team at Western Health: Effectiveness of a bundled intervention including adjunctive corticosteroids on outcomes of hospitalized patients with community-acquired pneumonia: A stepped-wedge randomized clinical trial. JAMA Intern Med 2019; 179:1052–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres A, Sibila O, Ferrer M, et al. : Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: A randomized clinical trial. JAMA 2015; 313:677–686 [DOI] [PubMed] [Google Scholar]
- 51.Dequin P-F, Meziani F, Quenot J-P, et al. ; CRICS-TriGGERSep Network: Hydrocortisone in severe community-acquired pneumonia. N Engl J Med 2023; 388:1931–1941 [DOI] [PubMed] [Google Scholar]
- 52.Confalonieri M, Urbino R, Potena A, et al. : Hydrocortisone infusion for severe community-acquired pneumonia: A preliminary randomized study. Am J Respir Crit Care Med 2005; 171:242–248 [DOI] [PubMed] [Google Scholar]
- 53.Siemieniuk RAC, Meade MO, Alonso-Coello P, et al. : Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med 2015; 163:519–528 [DOI] [PubMed] [Google Scholar]
- 54.Briel M, Spoorenberg SMC, Snijders D, et al. ; Ovidius Study Group: Corticosteroids in patients hospitalized with community-acquired pneumonia: Systematic review and individual patient data metaanalysis. Clin Infect Dis 2018; 66:346–354 [DOI] [PubMed] [Google Scholar]
- 55.Metlay JP, Waterer GW, Long AC, et al. : Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45–e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.