ABSTRACT
Intestinal transplant is an uncommon treatment of intestinal failure that has provided many patients with reduced morbidity and mortality. However, there are associated risks, including an increased risk of cancer, such as posttransplant lymphoproliferative disorder and solid-organ malignancy. Here, we report a unique case of primary jejunal adenocarcinoma presenting initially only with axillary lymphadenopathy in a patient with recurrent posttransplant lymphoproliferative disorder after multiple solid-organ transplants, including small intestine and 2 renal transplants.
KEYWORDS: small bowel transplant, jejunal adenocarcinoma, lymphadenopathy
INTRODUCTION
Intestinal transplant is a rare treatment of intestinal failure, with less than 100 transplants performed in 2021 in the United States.1 The most common indications for intestinal transplant in pediatric patients include gastroschisis, volvulus, and necrotizing enterocolitis, whereas in adults, the most common indications are ischemia, Crohn's disease, and trauma.2
Intestinal transplant has risks, including developing subsequent malignancy. The mechanism for an increased risk of malignancy after organ transplant is not fully understood but is believed to be related to long-term immunosuppression, leading to reduced immune surveillance of neoplastic cells and to opportunistic infections such as Epstein-Barr virus.3 Posttransplant lymphoproliferative disorder (PTLD) is one of the most common malignancies after transplant. However, solid-organ transplant recipients are also at increased risk of developing solid-organ cancers. We report a unique case of a 37-year-old patient with a history of small-bowel and kidney transplants, as well as recurrent PTLD, who presented with nonresolving left axillary lymphadenopathy. He was eventually found to have metastatic jejunal adenocarcinoma.
CASE REPORT
A 37-year-old man presented with a 1-month history of nonresolving left axillary lymphadenopathy. His medical history was significant for a small-bowel transplant as a child for necrotizing enterocolitis and intestinal atresia. He also received 2 living donor kidney transplants after developing end-stage renal disease secondary to calcineurin inhibitor toxicity. He was recipient Epstein-Barr virus positive. His posttransplant course was complicated by recurrent PTLD on 3 separate occasions. Six years before his current presentation, his second PTLD was diagnosed incidentally on examination of a resected jejunal segment after presenting with a small-bowel obstruction from jejunal ischemia. He was treated with 4 doses of rituximab, and his mycophenolate immunosuppression was discontinued. He then developed left cervical lymphadenopathy 1 year later and was diagnosed with stage 4 polymorphic PTLD, which was again treated with rituximab. A follow-up positron emission tomography/computed tomography scan 1 year later showed no evidence of recurrence (Deauville score of 1). He continued immunosuppression with tacrolimus and low-dose prednisone and was followed by medical oncology at regular intervals.
Approximately 4 years later, he contacted his oncologist because of a new palpable lymph node in his left axilla. He had no other clinical symptoms, including weight loss, fevers, night sweats, or any gastrointestinal symptoms. An ultrasound confirmed an enlarged left axillary lymph node measuring 10 × 26 mm with prominent fat and a thin hypoechoic cortex, favored to be benign. Because the patient had received a COVID-19 booster vaccine a few weeks before, benign vaccine-related lymphadenopathy was suspected. However, a follow-up ultrasound of the lymph node arranged 6 weeks later demonstrated interval growth in size to 29 × 19 × 13 mm. A subsequent positron emission tomography scan confirmed multiple fluorodeoxyglucose avid lymph nodes in the left neck, left axilla, mediastinum, and left inguinal region which were suspicious for PTLD. Notably, there was no abnormal fluorodeoxyglucose accumulation within solid organs, including the bowel. A following computed tomography scan of the abdomen and pelvis showed circumferential mural thickening of the proximal jejunal loops without any mass-like lesions or signs of obstruction (Figure 1).
Figure 1.
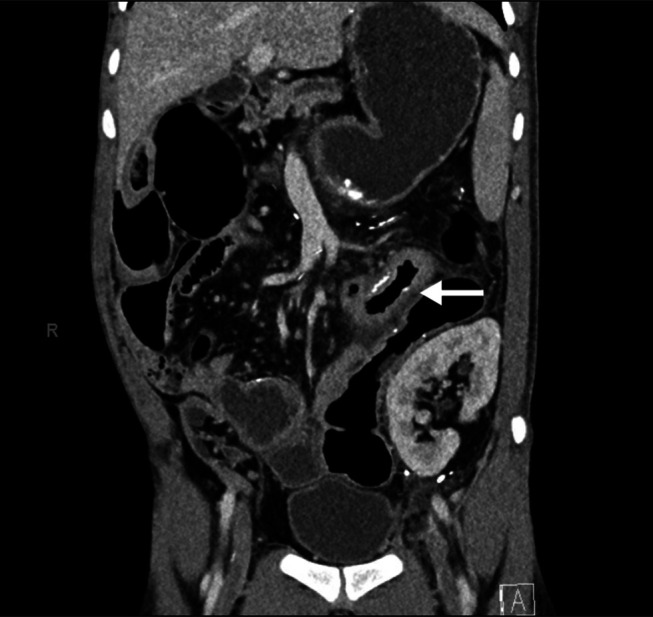
Computed tomography scan of the abdomen and pelvis showing jejunal thickening (arrow).
Pathologic examination of an open excisional biopsy of a left inguinal lymph node demonstrated involvement of poorly differentiated adenocarcinoma (Figure 2). Full immunohistochemical workup suggested primary origin from the gastrointestinal tract, with positive stains for CK7, CK20, and CDX2, as well as negative stains for SATB2, GATA3, S100, Melan-A, PAX8, CK5, NKX3.1, and TTF1. There was no evidence of PTLD. An esophagogastroduodenoscopy was unremarkable aside from LA grade C esophagitis. Duodenal and esophageal biopsies demonstrated reactive changes, without evidence of malignancy. A push enteroscopy was subsequently performed to investigate the jejunal thickening. Small, scattered erosions seen within the proximal jejunum were biopsied. To exclude a pancreatic primary, an endoscopic ultrasound was attempted but unsuccessful because of patient intolerance. A subsequent magnetic resonance cholangiopancreatography showed stable pancreatic duct dilation and pancreatic atrophy seen throughout an 11-year period, without any pancreatic mass or evidence of pancreatic ductal adenocarcinoma. Colonoscopy was deferred because it was last completed 2 years before and showed normal mucosa. Subsequently, biopsies from the push enteroscopy returned and confirmed primary, poorly differentiated jejunal adenocarcinoma, mismatch repair proficient, and human epidermal growth factor receptor 2 negative by immunohistochemistry, along with the positive CK7 and CK20 (Figure 3). The patient was started on systemic chemotherapy with leucovorin, fluorouracil, and irinotecan. Unfortunately, 2 months later, he was admitted to hospital for pancytopenia and rapid functional decline. He eventually died.
Figure 2.
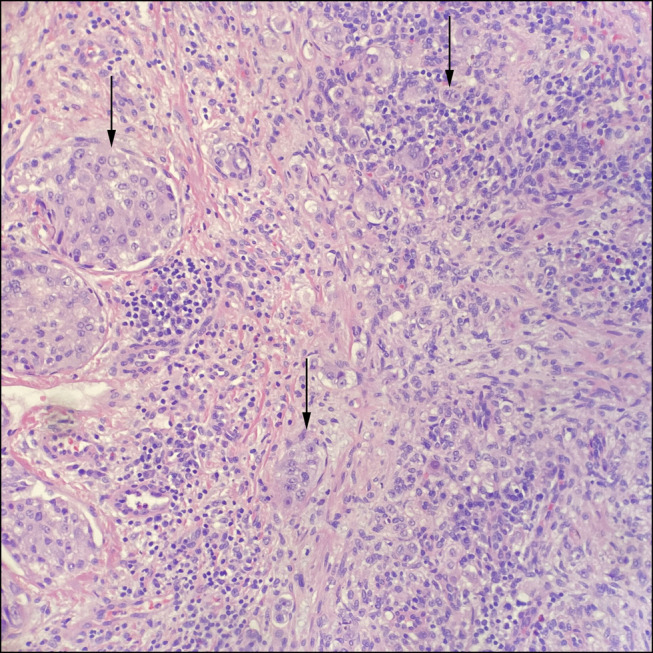
Excisional biopsy of a left inguinal lymph node infiltrated by poorly differentiated adenocarcinoma (arrows), hematoxylin and eosin, 200× original magnification.
Figure 3.
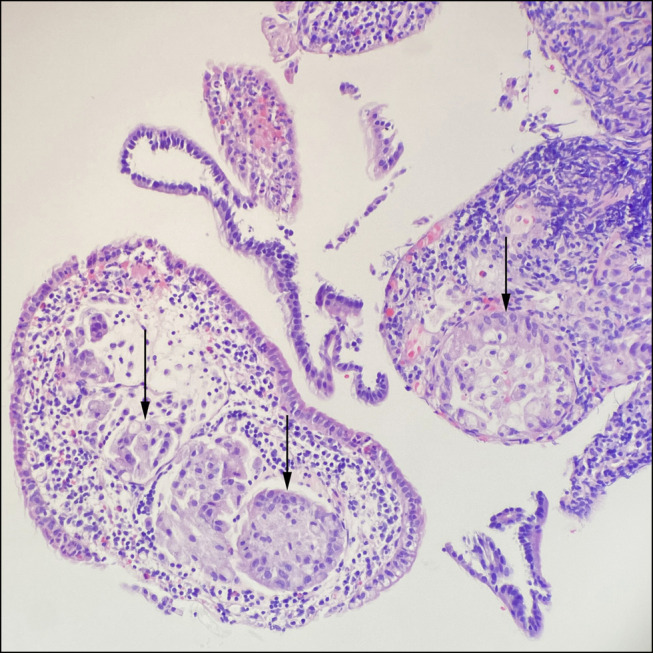
Endoscopic biopsies of jejunal thickening showing poorly differentiated jejunal adenocarcinoma (arrows), hematoxylin and eosin, 200× original magnification.
DISCUSSION
Solid-organ transplant can provide life-saving therapy for many patients with end-stage organ diseases. Intestinal transplant for intestinal failure can provide freedom from parenteral nutrition, reducing morbidity and mortality. Intestinal transplant, however, comes with associated risks, including an elevated risk of cancer, such as PTLD and solid-organ malignancy.3,4 The incidence of PTLD is up to 20% in small-intestinal transplant recipients.5 Therefore, PTLD is always considered when a new mass or lymphadenopathy is found in patients with a previous transplant. However, a broad differential should always be considered in these patients, including other forms of malignancy, such as adenocarcinoma. Although solid-organ transplantation may lead to an increased risk of malignancies, a history of PTLD is also associated with a greater risk of developing subsequent malignancies including cancer of the esophagus, colon, liver, kidney, central nervous system, and thyroid, as well as nonlymphocytic leukemia when compared to solid-organ transplant recipients who have not developed PTLD. This association is potentially due to underlying risk factors for cancer development or treatment-related risk.6 Although these results suggest an increased overall risk of cancer, there are insufficient data to conclude an increased risk of small-bowel adenocarcinoma in association with PTLD, potentially due to the low incidence of these malignancies in the general population.
Here, we present an interesting case of a primary jejunal adenocarcinoma masquerading as PTLD in a patient with recurrent PTLD after multi–solid-organ transplant, including a small intestine transplant. To our knowledge, this is the first reported case in the literature of primary jejunal adenocarcinoma after small-bowel transplant. This case highlights the importance of having a broad differential diagnosis initially when lymphadenopathy or a mass is discovered after solid-organ transplant, even if there is a history of PTLD.
DISCLOSURES
Author contributions: A. Hemy: drafting the manuscript. A. Fetz: patient evaluation and management and drafting the manuscript. HJ Kim: critical revision of the manuscript. D. Owen: Pathological expertise and provided pathology images. S. Jayakumar: patient evaluation and management, critical revision and is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Alexander Hemy, Email: alhemy@nosm.ca.
Hyun Jae Kim, Email: hyunjae.works@gmail.com.
Daniel Owen, Email: daniel.owen@vch.ca.
Saumya Jayakumar, Email: saumya.jayakumar@vch.ca.
REFERENCES
- 1.Horslen SP, Wood NL, Cafarella M, Schnellinger EM. Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN/SRTR 2021 Annual Data Report, 2023. [Google Scholar]
- 2.Grant D, Abu-Elmagd K, Reyes J, et al. 2003 report of the intestine transplant registry: A new era has dawned. Ann Surg. 2005;241(4):607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gogna S, Ramakrishna K, John S. Post Transplantation Cancer. StatPearls Publishing, 2022. (https://www.ncbi.nlm.nih.gov/books/NBK537256/). Accessed April 29, 2023. [Google Scholar]
- 4.Engels EA, Pfeiffer RM, Fraumeni JF, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaCasce AS. Post-transplant lymphoproliferative disorders. Oncologist. 2006;11(6):674–80. [DOI] [PubMed] [Google Scholar]
- 6.Mahale P, Engels EA, Lynch CF, Morton LM. Cancer risk following post-transplant lymphoproliferative disorders in solid organ transplant recipients. Br J Haematol. 2019;186(2):347–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


