Summary:
This practical review critically evaluates the evidence behind the widespread use of postoperative compression therapy intended to improve surgical outcomes, such as reduced edema, ecchymosis, pain, and seroma formation. A literature search of PubMed was conducted to identify relevant studies concerning the use of compression garments after aesthetic surgery, including rhinoplasty, facelift, neck lift, mammoplasty, abdominoplasty, limb contouring, and others. Additionally, reconstructive and therapeutic procedures closely related in anatomy or technique to these cosmetic operations, such as breast reconstruction, mastectomy, and hernia repair, were also considered to provide further perspective. After study extraction, the volume, quality, and agreement of the evidence found was highly heterogenous depending on the context of specific operations and outcomes evaluated. The most well-supported indications for the use of postoperative compression garments are to mitigate edema and ecchymosis after rhinoplasty and to reduce postoperative pain after breast and abdominal procedures, although no effect on seroma rate was demonstrated. Any potential benefit must be balanced against the associated costs and possible complications of compression, including patient discomfort, increased venous stasis, and skin defects. Thus, we encourage surgeons to critically reassess their use of compression garments. In many settings, such as brachioplasty, there is limited high-quality evidence to inform best practice, and we urge the community to continue researching this important topic so that more definitive and comprehensive guidelines may be established.
INTRODUCTION
Compression garments are widely used across a spectrum of patients and procedures for everything from the treatment of hypertrophic scars to decreasing edema and seroma formation.1 Plastic surgeons, in particular, use various compression garments all over the body after both aesthetic and reconstructive procedures. For example, abdominal binders are used for abdominoplasties and hernia repairs, compressive bras for aesthetic breast procedures and breast reconstruction, and compressive masks for facelifts and neck lifts. Although previous reviews have evaluated the efficacy of compression therapy in specific settings, such as the role of nasal splinting after rhinoplasty,2 there is a dearth of existing literature that has consolidated, evaluated, and synthesized the broad landscape of compression garment use in plastic surgery. Considering that compression garments add financial burden and may, themselves, lead to complications, it is important to critically examine whether their commonplace employment in postoperative care is supported by evidenced-based decision-making. Thus, this comprehensive practical review considers the literature regarding postoperative compression garment use in plastic surgery to determine its value in modern-day practice.
HISTORICAL PERSPECTIVE
The first use of compression therapy is thought to be by Hippocrates roughly 2500 years ago (460–370 BC) to treat sequelae of venous insufficiency.1,3 Although plastic surgeons initially described the use of compression garments to treat facial injuries in WWI,4 it was not until the 1970s that Dr. Yves Gerard Illouz described its first use in cosmetic surgery for his liposuction patients.5 He worked closely with Medical Z, a compression garment company from Houston, Texas, to assist in the development of their products. In 1989, they reported a study in which 189 lipectomy patients were given Lipo-Pantys of varying pressures and instructed to wear them for 3–8 weeks. They concluded that 17–20 mm Hg provided optimal skin outcomes while still minimizing postoperative edema.6 However, this preliminary report had notable flaws that weakened their endorsement of postoperative compression. The analysis was void of statistical tests of significance, the design was unclear on what specific procedures the patients received, and importantly, the study was conducted for the Medical Z corporation and was not published in a peer-reviewed journal.6
Since this initial report, Illouz has described complications that could be associated with compression garment use. In 2006, he noted how excessive or poorly distributed compression could result in venous stasis and thrombosis along with skin folding and bulging.7 Depending on the site and severity of insult, garment alterations or even total discontinuation could be required. He acknowledged that removing a garment early does not result in worse outcomes and stated that “a good operation with no compressive garment is better than [an ill-fitting garment or] a mediocre operation followed by prolonged use of a compressive garment that simply masks the defects.”7 Nonetheless, the early work of Illouz has led to the widespread integration of compression garments throughout plastic surgery. Considering their tenuous origin in clinical practice, further scrutiny on their modern-day value is warranted.
MECHANISM OF ACTION
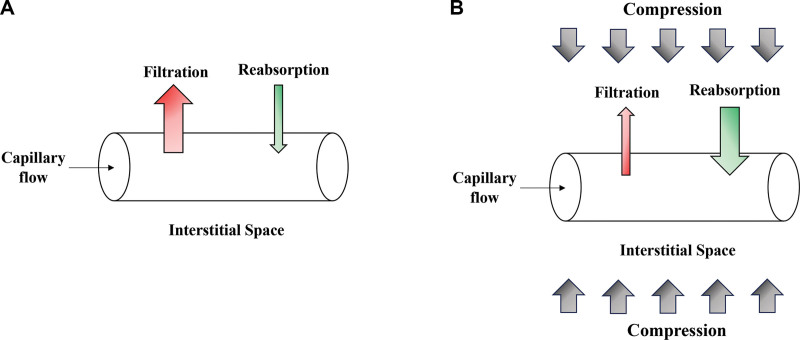
The Starling equation demonstrates the basic physiologic principle underlying compression garment therapy. By increasing the hydrostatic pressure of tissue surrounding blood vessels, less fluid exits in those vessels, and more interstitial fluid is reabsorbed (Fig. 1). Increasing pressure of the affected area also decreases venous pooling and increases venous outflow, which can further help reduce edema.8 A 1974 foundational study used a rabbit model to show how compressive air splints applied after tibial fractures significantly reduced swelling. Hind limb volume was measured using water displacement before injury and nine times over a week after being fractured. All rabbits had both legs broken, and one treatment limb was randomly chosen to receive a unilateral air splint (10 mm Hg). The maximum increase in limb volume was 30% at 2–3 days after injury, but this was limited significantly by air splinting. Even rebound swelling after splint removal was less than that of the control group.9 Compression garments have also been noted to reduce the chance of dehiscence by holding postoperative wounds in place and may decrease dead space within the surgical site, but whether this has a significant effect on postoperative complications remains to be proven.10
Fig. 1.
The effect of compression therapy on fluid accumulation in the interstitial space. The direction of fluid movement is determined by the balance of hydrostatic and oncotic forces exerted by both the intracapillary and interstitial spaces. A, The net result is initial fluid filtration out from the vessel into the interstitial space followed by fluid reabsorption. B, Applying compression therapy increases the hydrostatic forces, pushing fluid into the capillary, and leads to greater net absorption out of the interstitial space, thus decreasing clinical edema.
SEARCH METHODS
An electronic search was conducted to identify relevant articles indexed in PubMed any time through July 2023. The search strategy was designed to focus on aesthetic surgery of the head and neck, breast, abdomen, and extremities. Reconstructive operations closely related in anatomy or technique were also included to provide additional perspective. The terms used to generate the search strategy are shown in Supplemental Digital Content 1. (See table, Supplemental Digital Content 1, which displays the PubMed search strategy. http://links.lww.com/PRSGO/C782.) The primary inclusion criterion was any study investigating the effects of compression therapy on postoperative outcomes. Studies that reported the use of compression therapy but did not report their influence on postoperative outcomes were excluded. Studies published in languages other than English were excluded. The titles and abstracts were screened to determine whether studies met criteria, and the relevant articles were selected for full-text review. Two authors (B.O. and N.L.) independently reviewed search results, and any discrepancies were resolved through consensus by a third author (A.S.). The bibliographies of the included studies were also queried for additional sources. Following study selection, two authors (B.O. and N.L.) extracted relevant study variables, including type of study, level of evidence, type of operation, postoperative outcomes, and type of compression therapy used.
USES IN FACIAL SURGERY
The volume of literature regarding compression therapy after cosmetic facial surgery varies depending on the operation of interest. Regarding rhinoplasty, a 2020 randomized controlled trial (RCT) evaluated the effects of pressure and vibration treatments in the early postoperative period. Groups were split into one control group receiving nasal casting, one intervention group receiving elastic bandaging, and another intervention group receiving both elastic bandaging and vibration treatment.11 On postoperative days 3 and 7, both intervention groups had significantly lower edema and ecchymosis scores than the control group, although optimal outcomes were achieved with both compression and vibration therapy in tandem. Comparatively, compression in the form of nasal splinting has not been shown to improve postoperative outcomes. Khan et al previously conducted a meta-analysis of four studies investigating the value of splinting after rhinoplasty.2 In an RCT of 40 patients randomized to receive either nasal splinting or only surgical taping postoperatively, the levels of periorbital edema and ecchymosis were significantly lower in the patients without splints (P < 0.05 for each).12 The other three studies reviewed by Khan et al were retrospective case series of rhinoplasty patients who did not receive postoperative splints.13–15 Across 2385 total patients, the collective results demonstrated that splinting was not necessary to achieve desirable aesthetic outcomes. Taken in consideration with the previous study described, the efficacy of compression therapy after rhinoplasty may depend on the method of application, with evidence favoring elastic bandaging over nasal splinting to reduce edema and ecchymosis postoperatively.
Shifting the focus to other head and neck procedures, the evidence is relatively sparse and low quality. Regarding facelifts, only two relevant publications were identified. The first was an isolated case series from 1995 in which Teimourian et al noted zero occurrences of either ecchymosis or hematoma after 100 facelifts. Although they attributed these favorable results to postoperative compression achieved by their MicroFoam tape dressing technique,16 this deduction was not supported by a stronger comparative study published by Jones and Grover in 2005. When comparing hematoma rates between 331 patients receiving compressive dressings versus 341 who did not, they found no significant difference (P > 0.05), with an overall incidence of 4.4%.17 Otherwise, beyond these two studies, no others were found on facelift procedures, and the current evidence only permits a weak conclusion that compression likely does not reduce hematoma formation.
Research on compression after neck lifts has been even more limited, as only one study was identified on this topic. Herein, Noodleman and Harris described favorable qualitative results using compressive mineral oil polymer disc dressings after 350 laser-assisted neck lifts, although objective outcome reporting was severely limited.18 Similarly, only one relevant study was found regarding buccal fat pad removal. This retrospective study showed that the overall complication rate was significantly lower for patients who received compressive bandaging versus those who did not across 643 operations (P < 0.05).19
Thus, considering the collective head and neck literature beyond rhinoplasty, there are no RCTs to inform strong recommendations on the use of postoperative compression therapy. Although it may be more beneficial after neck lifts and buccal fat pad removals than facelifts in reducing complications, further research is necessary to inform best practice.
USES IN BREAST SURGERY
When considering the efficacy of compression therapy after breast surgery, the literature results have varied depending on the outcome of interest. Regarding postoperative pain, a recent RCT from 2023 involving 201 patients found that, of patients who still had postoperative pain at 3 weeks after breast cancer surgery, those provided compressive bras had significantly lower pain scores than those provided soft bras, according to the numeric pain scale (2.0 ± 1.0 versus 2.7 ± 1.5, respectively, P = 0.018).20 Additionally, Camirand et al separately emphasized the utility of manual compression combined with compressive brassieres after breast augmentation to reduce the incidence of capsular contracture.21,22 In a report of their clinical experience, the authors encouraged patients to apply periodic manual breast compression throughout the day, lie prone while watching television, and sleep in the prone position for three months. In theory, compression causes the scar surrounding the implant to stretch, and after two or more months of regular compression, the scar ceases to contract and leaves the breast soft.22 The authors reported that zero of approximately 1000 implants required surgical treatment for contracture after introducing manual compression recommendations. Although they heavily attributed this result to compression therapy, without a properly designed study, it is difficult to ascertain the validity and relevance of this conclusion.
Beyond pain and capsular contracture, the literature has shown no added benefit to compression therapy in reducing complication rates after breast surgery. A 2001 RCT involving 130 patients studied the effects of compressive wrapping after breast augmentation.23 Foam compression was applied to the treatment group postoperatively and removed the next morning, and patients were examined for hematoma, bruising, induration, and infection immediately after surgery and on postoperative day 10. No significant differences were found between groups in any of the four tracked outcomes, and 39% of women in the treatment group complained of discomfort. Additionally, when considering seroma formation and drain output, studies have generally demonstrated no increased benefit of compression. One retrospective study involving 200 patients undergoing mastectomy did find a statistically significant decrease in the seroma rate for patients receiving pressure dressings versus those not (2.5% versus 8%, respectively, P value not reported), along with a faster time to drain removal (4.9 days versus 5.5 days, P value not reported).24 However, across three different RCTs investigating compression after various breast cancer procedures that collectively involved 337 patients, no significant reduction in seroma development was demonstrated in any study.25–27 Although one investigation did show a faster time to drain removal,27 this was not corroborated by the other two,25,26 and none of these studies found an improvement in overall drain output.
When considering this collective evidence in concert, comparative studies indicate that compression after breast surgery may reduce postoperative pain but otherwise does not enhance recovery or prevent complications.
USES IN ABDOMINAL SURGERY
In 2014, Rothman et al published a systematic review of the literature investigating the effect of postoperative abdominal binders on pain, physical function, pulmonary function, seroma formation, and intraabdominal pressure after abdominoplasty, laparotomy, or laparoscopic hernia repair.28 Regarding pain outcomes, they reviewed three RCTs that together provided modest evidence in support of binders to reduce early postoperative pain. Although two of the studies demonstrated nonsignificant associations with pain relief,29,30 the third found a statistically significant reduction in pain between those wearing binders versus those not on postoperative day 5.31 These results were later corroborated by a 2016 RCT conducted by Arici et al (n = 84), in which patients wearing binders after laparotomy had significantly lower pain scores on postoperative days 1, 4, and 7 (P < 0.001).32 Collectively, these studies point toward a possible utility of binders in reducing postoperative pain in the acute postoperative period.
Rothman et al also identified two studies investigating the association of abdominal binders with seroma formation. In one retrospective study on laparoscopic hernia repair, authors reported a reduction in seroma incidence from 32% to 18% after introducing routine postoperative binder use for 7–10 days in their practice, but the study design and historical controls preclude causal inference.33 In comparison, a post hoc reanalysis of a previous randomized study on incisional hernia repair demonstrated that binder use was not associated with decreased seroma incidence across 145 patients.34 More recently, two RCTs specifically within the setting of abdominoplasty have provided stronger evidence against the benefit of abdominal binders in reducing postoperative complications. In a 2022 study, Martins et al (n = 34) demonstrated that binder use neither reduced seroma formation at postoperative days 7 (P = 0.830) or 14 (P = 0.882), nor did they reduce the incidence of diastasis recti at 6 months (P = 1.000).35 Additionally, in 2023, Fontes de Moraes et al (n = 32) showed that patients who wore compression garments actually had more subcutaneous edema 35 days after surgery than those who did not (P < 0.001).36
Regarding changes in pulmonary function, Rothman et al described an RCT that found a significant increase in vital capacity postoperatively for patients wearing abdominal binders,37 yet this was contrasted with four RCTs showing no significant difference in pulmonary function based on static29 and dynamic spirometry measurements.30–32 Most recently, a 2023 study by Kosloski et al showed that patients wearing compression garments after abdominoplasty (n = 18) experienced postoperative ventilatory restriction compared with controls without binder use (n = 18), as indicated by reduced forced vital capacity, inspiratory capacity, and maximum expiratory pressure.38 A separate prospective clinical study found that binder use led to increased intraabdominal pressure after abdominoplasty.39 Venous thromboembolism is a feared complication of abdominal surgery, and it has been hypothesized that increased intraabdominal pressure may be a risk factor by impeding venous return from the lower extremity.40 Multiple studies have demonstrated reduced blood flow in the femoral and popliteal veins of healthy volunteers wearing compressive abdominal binders, and the authors endorsed caution on the use of excessively tight garments while even suggesting the avoidance of compression garments altogether.41,42
In total, abdominal surgery had the highest volume of high-quality evidence regarding postoperative compression therapy. Existing literature currently suggests that abdominal binders may reduce postoperative pain in the acute postoperative period, but do not affect other postoperative sequela. Furthermore, this benefit in pain prevention should be balanced against potential risks in promoting venous thromboembolism.
USES IN BODY CONTOURING
Although plastic surgeons have frequently reported the use of compression garments after body contouring operations,43–45 no RCTs or comparative studies have been conducted to evaluate the efficacy of compression in this setting. Authors have only described the use of compression garments for corresponding contouring procedures, such as a surgical hose following calf and ankle liposuction,46 or the Tubigrip, a compressive tube of fabric fitted for the patient by cutting appropriate holes for the head and limbs, in the setting of brachioplasty.47 However, the influence of these garments across studies has not been evaluated with regard to patient outcomes or satisfaction rates. Thus, at present, there is little to no evidence available to objectively ascertain the value of compression in body contouring procedures.
DISCUSSION
Compression garments are widely used throughout plastic surgery as postoperative adjuncts intended to minimize complications and enhance recovery; however, a comprehensive analysis of the literature is necessary to evaluate the evidence behind this practice. This practical review thus aimed to fulfill this need and determine the value of compression therapy in modern-day plastic surgery, with an emphasis on cosmetic procedures. The identified studies are summarized in Supplemental Digital Content 2. (See table, Supplemental Digital Content 2, which displays the summary of articles reviewed. http://links.lww.com/PRSGO/C783.)
When considering the collective literature in concert, there is currently limited high-quality evidence supporting the efficacy of postoperative compression garments. To summarize positive findings from the identified RCTs, postoperative elastic bandaging reduced ecchymosis and edema following rhinoplasty, and compression therapy was found to improve postoperative pain following both breast20 and abdominal operations.29–32 Although further investigations have generally not yielded significant findings, an important consideration is whether the studied outcomes are appropriate measures to determine the efficacy of compression therapy. Initial rabbit model experiments demonstrated reduced edema following limb fractures with compressive therapy, likely by limiting the expansion of fluid in the superficial interstitial space.9 Although further RCTs have found that compression garments did not mitigate seroma formation, hematoma formation, drain output, or diastasis recti across breast and abdominal settings,23–27,33–35 these outcomes have not been explored as benefits of compression through scientific investigation, and they may be more related to surgical technique and/or postoperative activities. As such, the authors of the current study caution against an interpretation of the literature that compression garments should not be used after the highlighted plastic surgery operations. One consequence of this decision would be to then leave patients deciding for themselves as to whether and how to wear compression garments postoperatively. Without the clinical acumen of a practicing surgeon to guide garment selection and use, patients may be more susceptible to the established consequences of ill-fitting garments, such as discomfort,23 skin defects and necrosis,48 and increased venous stasis.42 Importantly, studies have found the incidence of poor garment fitting already to range from 4% to 44%.49 Thus, although empirical evidence to standardize compressive therapy across plastic surgery procedures is currently lacking, the authors recommend continuing patient-centered discussions on the known risks and benefits, and importantly, prioritizing early follow-up for surveillance of developing complications.
It is also important to note that, although the current study has focused on compression therapy in the settings of facial, breast, abdominal, and extremity contouring operations, compression garments have been used and demonstrated benefits across a wide range of further plastic surgery contexts. A recent systematic review from 2023 assessing 17 studies found that pressure therapy has both prophylactic and curative value for scar management in the setting of burn injuries.50 Additionally, compression garments are integral to the conservative management of limb lymphedema, and an RCT from 2013 found that compression garments alone offered benefits comparable to more involved decongestive therapy programs.51 Moreover, a recent multicenter, prospective study from 2023 found that postoperative compression could improve acute postoperative pain, quality of life, and even seroma incidence after groin hernia repair.52 Compression garments have further been implicated in fracture management, as a systematic review from 2017 demonstrated reduced edema and improved joint mobility with compression therapy after ankle fracture surgery.53 A separate narrative review of the orthopedic literature found that compression and cold in tandem resulted in less pain, less postoperative drainage, and faster return to function than compression alone.54 Thus, further research on augmenting compression with cryotherapy after plastic surgery operations may be warranted.
Considering the benefits observed in further settings, the dearth of high-quality evidence supporting compression garment use after facial, breast, abdominal, and limb contouring operations may more reflect a lack of research efforts than a true absence of efficacy. As such, future investigations should be aimed to fill the current gaps in knowledge. Specifically, a more granular understanding of what specific garments, at what pressure, and for what duration patients should wear them depending on the anatomical site of application would help standardize compression garment guidelines. Additionally, current studies in the rhinoplasty setting have not compared results of postoperative compression across groups with and without osteotomies, and research investigating this operative factor may further benefit patient outcomes. A patient-focused satisfaction survey evaluating the perceived value of surgeon instructions and experiences with subsequent garment use would further inform pragmatic decision-making. At present, the authors recommend surgeons review the risks, benefits, and associated financial burden of compression garments with patients along with assisting in garment wear recommendations and continuously reevaluating through close follow-up to ensure patient safety.
DISCLOSURES
Dr. Janis receives royalties from Thieme and Springer Publishing. All the other authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online 25 September 2023.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Gladfelter J. Compression garments 101. Plast Surg Nurs. 2007;27:73–77; quiz 78. [DOI] [PubMed] [Google Scholar]
- 2.Khan M, Mortada H, AlRajhi B, et al. Role of external nasal splinting following rhinoplasty: is it really important? A comprehensive systematic review of literature. Aesth Plast Surg. 2023. [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 3.Watkins WBC. Compression garment sizing: challenges, issues, and a solution. Plast Surg Nurs. 2010;30:85–87; quiz 88. [DOI] [PubMed] [Google Scholar]
- 4.Gilles H. Plastic Surgery of the Face. Hodder & Stoughton; 1920. [Google Scholar]
- 5.Illouz Y, de Villers G, Yves T. Body Sculpturing by Lipoplasty. Churchill-Livingstone; 1989. [Google Scholar]
- 6.Illouz Y, Gerard G, Masson CL, et al. Clinical evaluation of pressure therapy in conjunction with aesthetic and reconstructive surgery. 1989. Available at www.medicalz.com/studies. Accessed April 3, 2023.
- 7.Illouz YG. Complications of liposuction. Clin Plast Surg. 2006;33:129–163, viii. [DOI] [PubMed] [Google Scholar]
- 8.Partsch H. In: Understanding the pathophysiologic effects of compression. Understanding Compression Therapy. Medical Education Partnership Ltd; 2003:2–4. [Google Scholar]
- 9.Matsen FA, Krugmire RB. The effect of externally applied pressure on post-fracture swelling. J Bone Joint Surg Am. 1974;56:1586–1591. [PubMed] [Google Scholar]
- 10.Suh H, Jeong HH, Hong JP. Is early compression therapy after perforator flap safe and reliable? J Reconstr Microsurg. 2019;35:354–361. [DOI] [PubMed] [Google Scholar]
- 11.Taş S. The effects of vibration and pressure treatments in the early postoperative period of rhinoplasty. Aesthet Surg J. 2020;40:605–616. [DOI] [PubMed] [Google Scholar]
- 12.Cabbarzade C, Yücel OT, Sözen T, et al. External splinting is not mandatory after all rhinoplasties: a prospective randomized trial. Ann Plast Surg. 2021;86:376–380. [DOI] [PubMed] [Google Scholar]
- 13.Challita R, Shouman M, Ghanime G. Rhinoplasty and external nasal splinting: is it really a must? Plast Reconstr Surg Global Open. 2019;7:e2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vulovic D, Kozarski J, Radivojcevic U, et al. Rhinoplasty without nasal packing and splinting. VSP. 2018;75:352–358. [Google Scholar]
- 15.Vidal P, Berner JE. Is rigid nasal splinting needed after rhinoplasties? an 18-year experience using surgical tape. Plast Reconstr Surg Global Open. 2016;4:e1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teimourian B, Mankani M, Stefan M. A new dressing technique to minimize ecchymoses following face lifts. Plast Reconstr Surg. 1995;96:222–223. [DOI] [PubMed] [Google Scholar]
- 17.Jones BM, Grover R. Avoiding hematoma in cervicofacial rhytidectomy: a personal 8-year quest. Reviewing 910 patients. Plast Reconstr Surg. 2004;113:381–387; discussion 388. [DOI] [PubMed] [Google Scholar]
- 18.Noodleman FR, Harris DR. The laser-assisted neck lift: modifications in technique and postoperative care to improve results. Dermatol Surg. 2002;28:453–458. [DOI] [PubMed] [Google Scholar]
- 19.Grillo R, de la Puente Dongo JL, de Moura Moreira L, et al. Effectiveness of bandage in the incidence of major complications on bichectomy: literature review and case series of 643 bichectomies. Oral Maxillofac Surg. 2022;26:511–517. [DOI] [PubMed] [Google Scholar]
- 20.Backman M, Hassan-Nur M, Fridblom K, et al. OptiBra study, a randomized controlled trial on optimal postoperative bra support after breast cancer surgery. Eur J Oncol Nurs. 2023;63:102285. [DOI] [PubMed] [Google Scholar]
- 21.Camirand A, Doucet J, Harris J. Breast augmentation: compression—a very important factor in preventing capsular contracture. Plast Reconstr Surg. 1999;104:529–38; discussion 539–541. [DOI] [PubMed] [Google Scholar]
- 22.Camirand A, Doucet J. Breast augmentation: teaching our patients how compression can help prevent capsular contracture. Aesthetic Plast Surg. 2000;24:221–226. [DOI] [PubMed] [Google Scholar]
- 23.Nathan B, Singh S. Postoperative compression after breast augmentation. Aesthetic Plast Surg. 2001;25:290–291. [DOI] [PubMed] [Google Scholar]
- 24.Kontos M, Petrou A, Prassas E, et al. Pressure dressing in breast surgery: is this the solution for seroma formation? J BUON. 2008;13:65–67. [PubMed] [Google Scholar]
- 25.O’Hea BJ, Ho MN, Petrek JA. External compression dressing versus standard dressing after axillary lymphadenectomy. Am J Surg. 1999;177:450–453. [DOI] [PubMed] [Google Scholar]
- 26.Chen CY, Hoe AL, Wong CY. The effect of a pressure garment on post-surgical drainage and seroma formation in breast cancer patients. Singapore Med J. 1998;39:412–415. [PubMed] [Google Scholar]
- 27.Kottayasamy Seenivasagam R, Gupta V, Singh G. Prevention of seroma formation after axillary dissection—a comparative randomized clinical trial of three methods. Breast J. 2013;18:478–484. [DOI] [PubMed] [Google Scholar]
- 28.Rothman JP, Gunnarsson U, Bisgaard T. Abdominal binders may reduce pain and improve physical function after major abdominal surgery—a systematic review. Dan Med J. 2014;61:A4941. [PubMed] [Google Scholar]
- 29.Larson CM, Ratzer ER, Davis-Merritt D, et al. The effect of abdominal binders on postoperative pulmonary function. Am Surg. 2009;75:169–171. [PubMed] [Google Scholar]
- 30.Olsén M, Josefson K, Wiklund M. Evaluation of abdominal binder after major upper gastrointestinal surgery. Adv Physiother. 2009;11:104–110. [Google Scholar]
- 31.Cheifetz O, Lucy SD, Overend TJ, et al. The effect of abdominal support on functional outcomes in patients following major abdominal surgery: a randomized controlled trial. Physiother Can. 2010;62:242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arici E, Tastan S, Can MF. The effect of using an abdominal binder on postoperative gastrointestinal function, mobilization, pulmonary function, and pain in patients undergoing major abdominal surgery: a randomized controlled trial. Int J Nurs Stud. 2016;62:108–117. [DOI] [PubMed] [Google Scholar]
- 33.Chowbey PK, Sharma A, Khullar R, et al. Laparoscopic ventral hernia repair. J Laparoendosc Adv Surg Tech. 2000;10:79–84. [DOI] [PubMed] [Google Scholar]
- 34.Kaafarani HMA, Hur K, Hirter A, et al. Seroma in ventral incisional herniorrhaphy: incidence, predictors and outcome. Am J Surg. 2009;198:639–644. [DOI] [PubMed] [Google Scholar]
- 35.Martins MRC, Moraes BZF de, Fabri DC, et al. Do abdominal binders prevent seroma formation and recurrent diastasis following abdominoplasty? Aesthet Surg J. 2022;42:1294–1302. [DOI] [PubMed] [Google Scholar]
- 36.Fontes de Moraes BZ, Ferreira LM, Martins MRC, et al. Do compression garments prevent subcutaneous edema after abdominoplasty? Aesthet Surg J. 2023;43:329–336. [DOI] [PubMed] [Google Scholar]
- 37.Ali J. The effect of abdominal binders on post-operative pulmonary function. Surg Infect. 1983;2:875–881. [Google Scholar]
- 38.Kosloski FR, Barbosa MVJ, Rodrigues MA, et al. Effect of compression garments on the ventilatory function after abdominoplasty. Aesthet Surg J. 2023:sjad231 [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues MA, Nahas FX, Gomes HC, et al. Ventilatory function and intra-abdominal pressure in patients who underwent abdominoplasty with plication of the external oblique aponeurosis. Aesthet Plast Surg. 2013;37:993–999. [DOI] [PubMed] [Google Scholar]
- 40.Ivatury RR, Diebel L, Porter JM, et al. Intra-Abdominal Hypertension and the abdominal compartment syndrome. Surg Clin North Am. 1997;77:783–800. [DOI] [PubMed] [Google Scholar]
- 41.Clayman MA, Clayman ES, Seagle BM, et al. The pathophysiology of venous thromboembolism: implications with compression garments. Ann Plast Surg. 2009;62:468–472. [DOI] [PubMed] [Google Scholar]
- 42.Berjeaut RH, Nahas FX, dos Santos LKIL, et al. Does the use of compression garments increase venous stasis in the common femoral vein? Plast Reconstr Surg. 2015;135:85e–91e. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad MSI. Circumferential lipobrachioplasty is a safe procedure. Plast Reconstr Surg. 2021;147:337–344. [DOI] [PubMed] [Google Scholar]
- 44.Escandón JM, Vyas KS, Manrique OJ. High-definition lipoplasty in male patients: a systematic review of surgical techniques and outcomes. Aesthet Surg J. 2022;42:68–85. [DOI] [PubMed] [Google Scholar]
- 45.Kokosis G, Coon D. Safety in body contouring to avoid complications. Clin Plast Surg. 2019;46:25–32. [DOI] [PubMed] [Google Scholar]
- 46.Mladick R. Lipoplasty of the calves and ankles. Aesthet Surg J. 2003;23:121–124. [DOI] [PubMed] [Google Scholar]
- 47.Filobbos G, Chapman T. Compression dressing for brachioplasty. J Plast Reconstr Aesthet Surg. 2012;65:127. [DOI] [PubMed] [Google Scholar]
- 48.Callam MJ, Ruckley CV, Dale JJ, et al. Hazards of compression treatment of the leg: an estimate from Scottish surgeons. BMJ. 1987;295:1382–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohayon R, Rose R, Ebert K, et al. Incidence of incorrectly sized graduated compression stockings and lower leg skin irregularities in postoperative orthopedic patients. Medsurg Nurs. 2013;22:370–374. [PubMed] [Google Scholar]
- 50.De Decker I, Beeckman A, Hoeksema H, et al. Pressure therapy for scars: myth or reality? A systematic review. Burns. 2023;49:741–756. [DOI] [PubMed] [Google Scholar]
- 51.Dayes IS, Whelan TJ, Julian JA, et al. Randomized trial of decongestive lymphatic therapy for the treatment of lymphedema in women with breast cancer. JCO. 2013;31:3758–3763. [DOI] [PubMed] [Google Scholar]
- 52.Zou Z, Zhang D, Liu Y, et al. Postoperative compression in preventing early complications after groin hernia repair. Hernia. 2023;27:969–977. [DOI] [PubMed] [Google Scholar]
- 53.Winge R, Bayer L, Gottlieb H, et al. Compression therapy after ankle fracture surgery: a systematic review. Eur J Trauma Emerg Surg. 2017;43:451–459. [DOI] [PubMed] [Google Scholar]
- 54.Block J. Cold and compression in the management of musculoskeletal injuries and orthopedic operative procedures: a narrative review. OAJSM. 2010;105:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.