Abstract
Aims
The amount of fibrosis in the left atrium (LA) predicts atrial fibrillation (AF) recurrence after catheter ablation (CA). We aim to identify whether regional variations in LA fibrosis affect AF recurrence.
Methods and results
This post hoc analysis of the DECAAF II trial includes 734 patients with persistent AF undergoing first-time CA who underwent late gadolinium enhancement magnetic resonance imaging (LGE-MRI) within 1 month prior to ablation and were randomized to MRI-guided fibrosis ablation in addition to standard pulmonary vein isolation (PVI) or standard PVI only. The LA wall was divided into seven regions: anterior, posterior, septal, lateral, right pulmonary vein (PV) antrum, left PV antrum, and left atrial appendage (LAA) ostium. Regional fibrosis percentage was defined as a region’s fibrosis prior to ablation divided by total LA fibrosis. Regional surface area percentage was defined as an area’s surface area divided by the total LA wall surface area before ablation. Patients were followed up for a year with single-lead electrocardiogram (ECG) devices. The left PV had the highest regional fibrosis percentage (29.30 ± 14.04%), followed by the lateral wall (23.23 ± 13.56%), and the posterior wall (19.80 ± 10.85%). The regional fibrosis percentage of the LAA was a significant predictor of AF recurrence post-ablation (odds ratio = 1.017, P = 0.021), and this finding was only preserved in patients receiving MRI-guided fibrosis ablation. Regional surface area percentages did not significantly affect the primary outcome.
Conclusion
We have confirmed that atrial cardiomyopathy and remodelling are not a homogenous process, with variations in different regions of the LA. Atrial fibrosis does not uniformly affect the LA, and the left PV antral region has more fibrosis than the rest of the wall. Furthermore, we identified regional fibrosis of the LAA as a significant predictor of AF recurrence post-ablation in patients receiving MRI-guided fibrosis ablation in addition to standard PVI.
Keywords: Atrial fibrillation, Fibrosis, Regional, Left atrial wall, Scar
Graphical Abstract
Graphical abstract.
What’s new?
Atrial cardiomyopathy has regional variations in fibrosis within the left atrium, with prognostic value for predicting atrial fibrillation (AF) recurrence.
Patients with persistent AF have increased fibrosis in the left pulmonary vein region compared with the rest of the left atrium wall.
Preferential fibrosis of the left atrial appendage, i.e. regional fibrosis percentage, is a marker of more advanced atrial cardiomyopathy and is predictive of AF recurrence.
Introduction
Pulmonary vein isolation (PVI) is an effective treatment for persistent atrial fibrillation (AF).1 Despite advances in ablation techniques and modalities, AF recurrence after catheter ablation (CA) remains high.2 Atrial fibrosis has been identified as a predictor of incident AF and AF burden.3 Moreover, the amount of atrial fibrosis prior to CA predicts AF recurrence after the procedure.4 Cardiac late gadolinium enhancement magnetic resonance imaging (LGE-MRI) has been shown to identify atrial fibrosis and scar,3,5 and this has been correlated and validated with pathological specimens.6 The Efficacy of MRI-Guided Fibrosis Ablation vs. Conventional Catheter Ablation of Atrial Fibrillation (DECAAF II) trial showed that MRI-guided ablation of fibrosis does not decrease AF recurrence after CA when compared with conventional PVI alone.2 This prompts the question of whether atrial cardiomyopathy affects the left atrium (LA) uniformly, whether atrial remodelling is a homogenous process, and whether these variations provide prognostic value. For example, the posterior wall in particular is thought to be a source of arrhythmogenic triggers for patients with persistent AF and is readily amenable to electrical isolation.7 In addition, the left atrial appendage (LAA) has been identified as an important source of extra-pulmonary vein (PV) arrhythmogenic triggers in patients with persistent AF.8 Therefore, multiple adjunct therapies to standard PVI targeting these potentially arrhythmogenic regions have been described, with varying degrees of success.9–11
While the heterogeneity of myopathy distribution across the LA wall has been suggested in the literature,12–14 there exists a paucity of data regarding regional fibrosis as detected by LGE-MRI and its relationship with recurrence after CA. We sought to leverage the extensive imaging data of the DECAAF II trial database to study these regional variations and their associated prognostic values.
Methods
Study population
This is a post hoc analysis of patients enrolled in the DECAAF II clinical trial, which has been previously described.2 Eight hundred forty-three patients with persistent AF and undergoing AF CA were randomized to receive PVI plus MRI-guided ablation or PVI alone. Patients with contraindications to gadolinium or MRI and patients who had a previous AF ablation or valvular cardiac surgery were excluded from the study. Late gadolinium enhancement magnetic resonance imaging was performed in both groups within 1 month before the ablation procedure to assess baseline atrial fibrosis and at 3 months post-ablation to assess for ablation scar. Physicians were encouraged but not required to discontinue anti-arrhythmic drugs (AADs) after the 90-day blanking period. Participants were followed for a period of 12–18 months using daily smartphone electrocardiogram (ECG) device recordings (ECG Check Device, Cardiac Designs Inc.) to assess the primary outcome of AF recurrence after ablation. Patients who had non-regional LGE-MR images were excluded from this study. This study was approved by the Tulane University Biomedical IRB.
Image processing
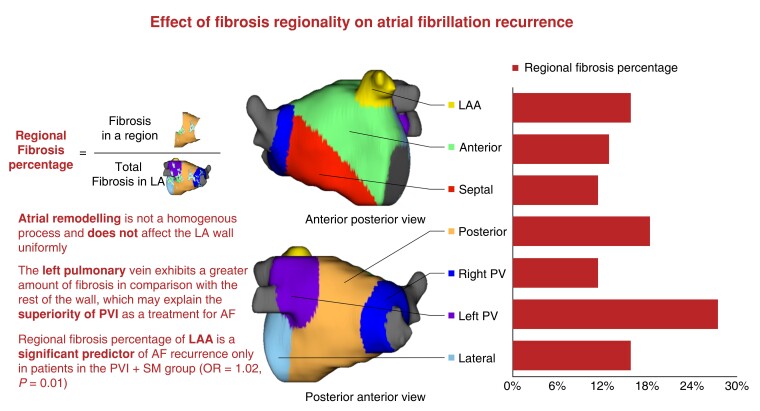
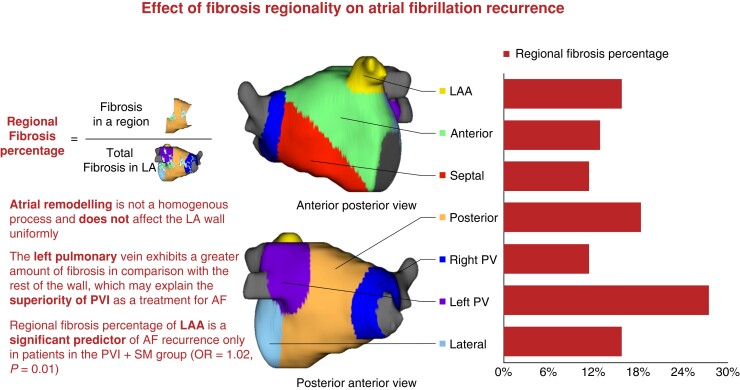
All patients underwent cardiac LGE-MR imaging using previously described methods.4,15–17 The LA wall was manually segmented, and regions of fibrosis were delineated using an intensity threshold set by expert inspection of each image. Regions exhibiting enhancement intensity two to three standard deviations (SD) above the mean intensity of normal tissues were considered fibrotic. The LA wall was divided into seven regions using previously described methods17: anterior, posterior, septal, lateral, right PV antrum, left PV antrum, and LAA ostium (Figure 1). The left PV antrum was defined as the LA wall extending 10 mm from the left PV–LA junction, the right PV antrum was defined as the LA wall extending 10 mm from the right PV–LA junction, the posterior wall as the posterior LA extending from the LA floor to the LA roof and bordered by both PV antra, the septum wall as the wall between LA and right atrium, the anterior wall as the anterior part of the LA, and the left lateral wall as the left side of the LA that is not covered by other areas. After sub-segmentation, the LGE area (mm2) and LGE coverage (%) in each LA sub-region were calculated using the Corview image analysis software (MARREK, Inc., Salt Lake City, Utah, USA).17 The amount of fibrosis in the LA was stratified into four Utah stages, as previously described.2 Baseline fibrosis was defined as the total amount of fibrosis in the LA wall prior to ablation divided by the surface area of the LA wall. Regional fibrosis percentage was defined as the amount of fibrosis in a particular region of the LA wall before ablation divided by the total amount of fibrosis in the LA wall (Figure 1). Surface area percentage was defined as the surface area of a particular region, divided by the total surface area of the LA wall.
Figure 1.
Central figure, effect of fibrosis regionality on atrial fibrillation recurrence.
Primary outcome
The primary outcome for this study was the first confirmed recurrence of AF lasting for at least 30 s after the 90-day blanking period, demonstrated by at least two consecutive 1-lead smartphone ECG device tracings, one positive reading on a clinical 12-lead ECG tracing, ambulatory monitor, or if the patient underwent repeat CA.2
Statistical analysis
Continuous variables were reported as mean ± SD. Binary logistic regression was used to perform the univariate and multivariate analysis with the dependent variable being the primary outcome and regional fibrosis percentages as independent variables. A P-value <0.05 was considered statistically significant. Statistical analysis was done using Software Package for Social Sciences (SPSS Inc., Chicago, Illinois) version 27.0.1.
Results
Baseline characteristics
The DECAAF II study included 843 patients. One hundred nine patients had inadequate MRI data and were excluded from this study. This study included 734 patients, and 333 (45.4%) of them achieved the primary outcome. The mean age of patients was 62.0 ± 9.0 years, and 78.2% of patients were males. At baseline, pre-ablation fibrosis percentage ranged from a minimum of 4.3% to a maximum of 37.6%, and had a mean of 18.6 ± 7.3%; 11.7% of patients had Utah Stage I fibrosis, 46.9% had Stage II, 32.7% had Stage III and 10% had Stage IV fibrosis. Age (β = 0.13, P < 0.001) and body mass index (β = 0.09, P = 0.047) were identified as significant predictors of baseline fibrosis. At baseline, 46.7% of patients were taking AADs, and 25.1% continued AADs through the 90-day blanking period post-ablation. Further description of baseline patient demographics can be found in Table 1.
Table 1.
Baseline characteristics of the study cohort
| N | 734 | |
|---|---|---|
| Sex (% males) | 78.2 | |
| Age (mean ± SD) | 62.0 ± 9.0 | |
| History of tobacco use (%) | 38.7 | |
| Congestive heart failure (%) | 18.8 | |
| Hypertension (%) | 59.1 | |
| Diabetes mellitus (%) | 9.4 | |
| Coronary artery disease (%) | 12.7 | |
| Stroke (%) | 8.4 | |
| Taking anti-arrhythmic medications (%) | 46.7 | |
| Long-standing persistent AF (%) | 61.1 | |
| Baseline fibrosis percentage (mean ± SD) | 18.6 ± 7.2 | |
| Baseline Utah stage (%) | I | 11.7 |
| II | 46.9 | |
| III | 32.7 | |
| IV | 10 | |
| Preablation LA size (mean ± SD) | 131.2 ± 41.0 | |
| Post-ablation scar percentage (mean ± SD) | 9.6 ± 5.1 | |
| Post-ablation LA Size (mean ± SD) | 108.1 ± 35.4 | |
Regional distribution of fibrosis
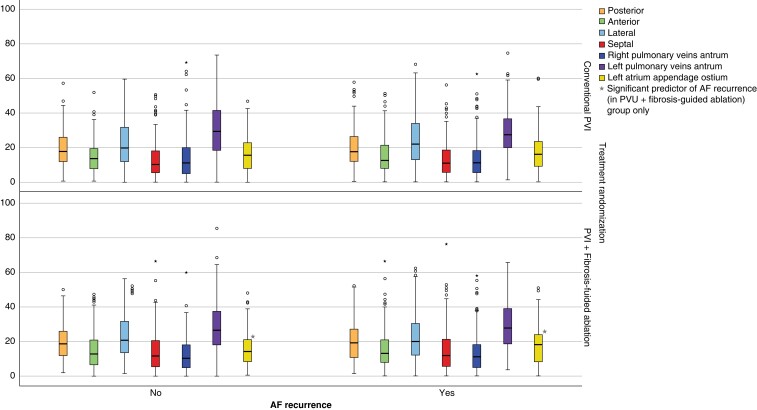
The mean fibrosis percentage prior to ablation was highest in the left PV antrum (29.30 ± 14.04%), followed by the lateral wall (23.23 ± 13.56%), and the posterior wall (19.80 ± 10.85%) (Figure 2). Time from first diagnosis with AF to CA and baseline AAD use did not significantly affect the distribution of fibrosis. There was no significant difference in the distribution of fibrosis between the two treatment arms. Further description of the regional distribution of fibrosis can be found in Table 2 and Figure 2.
Figure 2.
Boxplot figure showing regional fibrosis percentages of different regions of the LA wall, stratified by treatment randomization and AF recurrence.
Table 2.
Regional distribution of pre-ablation regional fibrosis percentages
| LA wall region | All patients (mean ± SD) | Standard PVI group (mean ± SD) | MRI-guided fibrosis ablation (mean ± SD) | P value |
|---|---|---|---|---|
| Anterior wall | 15.32 ± 10.48 | 15.35 ± 10.32 | 15.30 ± 10.65 | 0.941 |
| Posterior wall | 19.80 ± 10.85 | 19.69 ± 11.18 | 19.91 ± 10.53 | 0.793 |
| Lateral wall | 23.23 ± 13.56 | 23.46 ± 13.76 | 23.00 ± 13.38 | 0.648 |
| Septal wall | 14.02 ± 11.17 | 13.66 ± 10.61 | 14.38 ± 11.71 | 0.383 |
| Right pulmonary vein antrum | 13.75 ± 11.64 | 14.19 ± 11.97 | 13.31 ± 11.30 | 0.309 |
| Left pulmonary vein antrum | 29.30 ± 14.04 | 29.78 ± 13.84 | 28.82 ± 14.24 | 0.355 |
| LAA ostium | 16.70 ± 10.08 | 16.69 ± 10.04 | 16.71 ± 10.14 | 0.980 |
Effect of regional fibrosis on primary outcome
Univariate analysis showed that the pre-ablation regional fibrosis percentage of the LAA ostium affects the primary outcome {odds ratio [OR] = 1.021 [95% confidence interval (CI) 1.003–1.039], P = 0.019}. On multivariate analysis, the regional fibrosis percentage of the LAA ostium was predictive of the primary outcome [OR = 1.017 (CI 1.003–1.032), P = 0.021]. The rest of the results of the univariate and multivariate analyses of regional fibrosis percentages are shown in Table 3.
Table 3.
Regional fibrosis percentages as predictors of the primary outcome
| LA wall region | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Univariate analysis | |||
| Anterior wall | 1.009 | 0.991–1.027 | 0.332 |
| Posterior wall | 0.998 | 0.981–1.016 | 0.807 |
| Lateral wall | 1.002 | 0.990–1.014 | 0.682 |
| Septal wall | 0.993 | 0.976–1.011 | 0.419 |
| Right pulmonary vein antrum | 0.999 | 0.985–1.127 | 0.857 |
| Left pulmonary vein antrum | 0.994 | 0.981–1.008 | 0.322 |
| LAA ostium | 1.021 | 1.003–1.039 | 0.019 |
| Multivariate analysis | |||
| LAA ostium | 1.017 | 1.003–1.031 | 0.021 |
Effect of treatment randomization
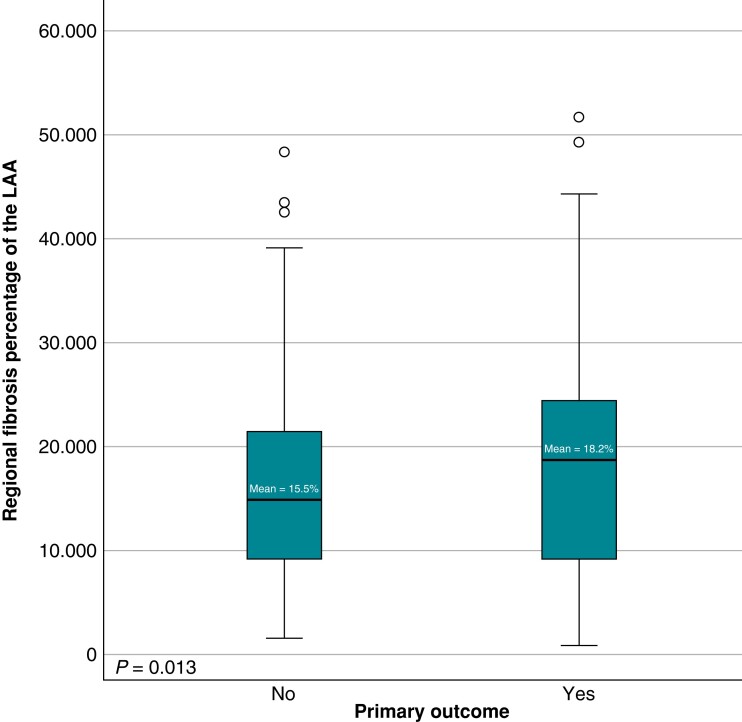
In patients randomized to receive MRI-guided fibrosis ablation, multivariate analysis showed that the pre-ablation regional fibrosis percentage of the LAA ostium remains a significant predictor of the primary outcome [OR = 1.02 (95% CI 1.006–1.047), P = 0.01] (Figure 3), while the regional distribution of fibrosis does not affect the primary outcome in patients receiving standard PVI only. Table 4 shows the results of the univariate and multivariate analyses stratified by the treatment randomization group.
Figure 3.
Difference in fibrosis percentage of the LAA in patients randomized to receive PVI + MRI-guided fibrosis ablation.
Table 4.
Regional fibrosis percentages as predictors of the primary outcome, stratified by treatment randomization
| LA wall region | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Univariate analysis (PVI-only group) | |||
| Anterior wall | 1.008 | 0.988–1.028 | 0.45784617 |
| Posterior wall | 1.004 | 0.986–1.023 | 0.65882351 |
| Lateral wall | 1.011 | 0.996–1.026 | 0.16228285 |
| Septal wall | 1.005 | 0.986–1.025 | 0.60722093 |
| Right pulmonary vein antrum | 0.993 | 0.976–1.010 | 0.40868463 |
| Left pulmonary vein antrum | 0.992 | 0.977–1.006 | 0.2653776 |
| LAA ostium | 1.010 | 0.989–1.031 | 0.35281304 |
| Univariate analysis (PVI + MRI-guided fibrosis ablation) | |||
| Anterior wall | 1.008 | 0.989–1.028 | 0.415 |
| Posterior wall | 1.006 | 0.987–1.026 | 0.554 |
| Lateral wall | 0.998 | 0.983–1.013 | 0.797 |
| Septal wall | 1.005 | 0.988–1.023 | 0.583 |
| Right pulmonary vein antrum | 1.009 | 0.991–1.027 | 0.344 |
| Left pulmonary vein antrum | 1.005 | 0.990–1.019 | 0.522 |
| LAA ostium | 1.026 | 1.006–1.048 | 0.013 |
| Multivariate analysis (PVI + MRI-guided fibrosis ablation) | |||
| LAA ostium | 1.02643567 | 1.006–1.048 | 0.0135 |
Regional surface area percentages
The posterior wall had the highest pre-ablation surface area percentage of the LA wall (20.71 ± 3.50%), followed by the anterior wall (17.71 ± 2.53%). Further description of these parameters can be found in Table 5. Regional surface area distribution did not affect the primary outcome, and this was preserved when stratifying patients by treatment randomization (Table 6).
Table 5.
Regional distribution of pre-ablation surface area percentages
| LA wall region | All patients (mean ± SD) | Standard PVI group (mean ± SD) | MRI-guided fibrosis ablation (mean ± SD) | P value |
|---|---|---|---|---|
| Anterior wall | 17.71 ± 2.53 | 17.8 ± 2.61 | 17.60 ± 2.61 | 0.411 |
| Posterior wall | 20.71 ± 3.50 | 20.61 ± 3.45 | 20.82 ± 3.54 | 0.432 |
| Lateral wall | 9.09 ± 2.21 | 9.11 ± 2.26 | 9.08 ± 2.17 | 0.861 |
| Septal wall | 12.04 ± 1.83 | 12.07 ± 1.82 | 12.01 ± 1.84 | 0.703 |
| Right pulmonary vein antrum | 17.05 ± 4.56 | 16.97 ± 4.65 | 17.13 ± 4.47 | 0.628 |
| Left pulmonary vein antrum | 10.74 ± 2.38 | 10.73 ± 2.47 | 10.75 ± 2.29 | 0.910 |
| LAA ostium | 12.69 ± 2.92 | 12.76 ± 3.10 | 12.61 ± 2.73 | 0.510 |
Table 6.
Regional surface area percentages as predictors of the primary outcome
| LA wall region | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Univariate analysis | |||
| Anterior wall | 0.886 | 0.135–5.831 | 0.900 |
| Posterior wall | 0.844 | 0.128–5.549 | 0.860 |
| Lateral wall | 0.854 | 0.130–5.616 | 0.870 |
| Septal wall | 0.888 | 0.135–5.841 | 0.961 |
| Right pulmonary vein antrum | 0.843 | 0.126–5.439 | 0.843 |
| Left pulmonary vein antrum | 0.797 | 0.121–5.244 | 0.813 |
| LAA ostium | 0.813 | 0.124–5.349 | 0.830 |
Discussion
In the present study, we confirmed the non-homogeneity of atrial remodelling and cardiomyopathy. Patterns of fibrosis variation between different LA wall regions in persistent AF patients were described using LGE-MRI. In addition, we demonstrated that pre-ablation fibrosis of the LAA is a positive predictor of AF recurrence after CA (Figure 1).
We have shown that the left PV antrum demonstrates a significant percentage of fibrosis when compared with the rest of the LA (Figure 1), followed by the lateral and posterior walls. Cochet et al.18 have also used LGE-MRI to describe fibrosis distribution in 190 patients and showed that fibrosis was more commonly found below the left inferior PV ostium than in any other region of the LA. However, it must be noted that this study included AF and non-AF patients, whereas our study cohort consists of persistent AF patients only. These findings are supported by previous histologic findings by Hassink et al.,19 who have exhibited greater amounts of fibrosis in the PV antra in AF patients than in non-AF patients. These findings may help explain the superiority of PVI as a treatment for patients with AF.1
While there is a paucity of data on regional LA wall fibrosis as detected by LGE-MRI, several studies have described it using electroanatomical voltage mapping (EAVM). A study by Teh et al.20 reported that patients with persistent AF had more atrial fibrosis in the septum and the roof of the LA wall than in any other region. Meanwhile, Lin et al.21 found more fibrosis in the anterior wall followed by the posterior wall in patients with persistent AF, and Chang et al.22 found the low anteroseptal wall to have more fibrosis than any other region, followed by the right PV. While there is no consensus across these studies, it must be noted that none of these studies have used LGE-MRI to assess for fibrosis. The discrepancy in results between these studies may be due to different operator techniques, contact force, different catheters, or interelectrode spacing. While fibrosis assessment using LGE-MRI is associated with low-voltage areas on EAVM,23 Sim et al.24 showed the use of LGE-MRI to identify atrial fibrosis may be a better predictor of AF recurrence than EAVM. Therefore, evaluating the relationship between regional fibrosis patterns and AF recurrence after CA might be better using LGE-MRI.
The LAA is an important source of arrhythmogenic triggers in patients with persistent AF.25 Electrical isolation of the LAA has been studied thoroughly, and the results are contradicting and non-confirmatory. A meta-analysis by AlTurki et al.26 showed a significant reduction in AF recurrence in patients who underwent electrical isolation of the LAA in addition to PVI when compared with those who underwent PVI alone. On the other hand, a more recent meta-analysis by Wang et al. showed that the addition of LAA isolation to PVI does not provide incremental benefit with respect to freedom from atrial arrhythmia.
While the benefit of LAA isolation remains unclear, the LAA seems to be of a significant role in the prediction of CA ablation outcomes, and the extent of fibrosis in the LAA may be indicative of advanced atrial myopathy.13 Our data show that a higher regional fibrosis percentage in the LAA, which may be indicative of advanced atrial myopathy,13 significantly predicts AF recurrence post-ablation in patients with persistent AF undergoing MRI-guided fibrosis ablation in addition to standard PVI. This finding was not observed in the patients treated with PVI only.
The differential effect observed between the two treatment groups may be explained by the association of advanced atrial myopathy with less lesion formation during fibrosis-guided ablation, as shown in previous work by our group.27 Therefore, patients with more preferential fibrosis of the LAA, indicating more advanced atrial myopathy, may derive less benefit from additional, fibrosis-guided ablation. These results highlight the need for further investigation into the role of LAA fibrosis and its implications for treatment strategies in AF.
A recent meta-analysis has identified multiple structural and functional attributes of the LAA to be predictive of AF recurrence post-ablation, namely LAA volume, orifice area, orifice long/short axis, and volume index, as well as LAA emptying flow velocity, filling flow velocity and ejection fraction.28 Pinto Teixeria et al. have also demonstrated that the volume of the LAA, as measured by computed tomography (CT) scanning, is a significant predictor of AF recurrence post-ablation in patients with paroxysmal or persistent AF.29 Moreover, Istratoaie et al.30 used echocardiography to demonstrate that a lower LAA emptying velocity predicts AF recurrence post-ablation in patients with paroxysmal AF. These studies, as well as our data, emphasize the predictive value intrinsic to the LAA as an indicator of advanced atrial cardiomyopathy.
The posterior wall of LA is considered an arrhythmogenic focus in patients with persistent AF. It has been postulated that posterior wall isolation (PWI) as an adjunct to standard PVI may improve outcomes. While multiple meta-analyses have confirmed the superiority of this approach in preventing AF recurrence in patients with persistent AF,31–33 other studies found opposing results.34,35 However, our data show that atrial fibrosis and dilation of the posterior wall do not predict AF recurrence after CA and may not explain why PWI decreases AF recurrence. This may mean that other factors specific to the posterior wall may be driving its arrhythmogenicity, and further studies on this matter are required.
There are several limitations to this study. First, only patients with persistent AF undergoing CA were included, which may be selected for patients with more advanced AF, and the effect of fibrosis regionality at earlier disease stages cannot be inferred from our data. Further studies should include other patient populations with and without AF to better understand the effect of the aforementioned factors on the development as well as the recurrence of AF. Furthermore, the technique used to segment LA fibrosis is dependent upon operator experience, and the follow-up period was relatively limited. In addition, 109 patients in the DECAAF II cohort had suboptimal image quality that hindered the extraction of regional fibrosis patterns due to technical issues and thus were excluded from this study.
Conclusion
In summary, our study has provided new insights into the heterogeneous nature of atrial cardiomyopathy and remodelling. We found significant variations in the distribution of atrial fibrosis across different regions of the LA, with the left PV antral region showing a higher propensity for fibrosis. Furthermore, our results suggest that regional fibrosis in the LAA may be associated with AF recurrence after ablation, particularly in patients who received substrate modification. These findings underscore the importance of taking into account the regional heterogeneity of atrial remodelling in developing personalized treatment strategies for AF patients.
Contributor Information
Ala Assaf, Tulane Research Innovation for Arrhythmia Discovery (TRIAD), Tulane University School of Medicine, 1324 Tulane Avenue, Suite A128, New Orleans, LA 70112, USA.
Mario Mekhael, Tulane Research Innovation for Arrhythmia Discovery (TRIAD), Tulane University School of Medicine, 1324 Tulane Avenue, Suite A128, New Orleans, LA 70112, USA.
Charbel Noujaim, Tulane Research Innovation for Arrhythmia Discovery (TRIAD), Tulane University School of Medicine, 1324 Tulane Avenue, Suite A128, New Orleans, LA 70112, USA.
Nour Chouman, Tulane Research Innovation for Arrhythmia Discovery (TRIAD), Tulane University School of Medicine, 1324 Tulane Avenue, Suite A128, New Orleans, LA 70112, USA.
Hadi Younes, Tulane Research Innovation for Arrhythmia Discovery (TRIAD), Tulane University School of Medicine, 1324 Tulane Avenue, Suite A128, New Orleans, LA 70112, USA.
Han Feng, Tulane Research Innovation for Arrhythmia Discovery (TRIAD), Tulane University School of Medicine, 1324 Tulane Avenue, Suite A128, New Orleans, LA 70112, USA.
Abdelhadi ElHajjar, Department of Internal Medicine, Cleveland Clinic, Cleveland, OH, USA.
Botao Shan, Tulane Research Innovation for Arrhythmia Discovery (TRIAD), Tulane University School of Medicine, 1324 Tulane Avenue, Suite A128, New Orleans, LA 70112, USA.
Peter Kistler, Clinical Electrophysiology Research Laboratory, Baker Heart and Diabetes Research Institute, Melbourne, Australia.
Omar Kreidieh, Tulane Research Innovation for Arrhythmia Discovery (TRIAD), Tulane University School of Medicine, 1324 Tulane Avenue, Suite A128, New Orleans, LA 70112, USA.
Nassir Marrouche, Tulane Research Innovation for Arrhythmia Discovery (TRIAD), Tulane University School of Medicine, 1324 Tulane Avenue, Suite A128, New Orleans, LA 70112, USA.
Eoin Donnellan, Tulane Research Innovation for Arrhythmia Discovery (TRIAD), Tulane University School of Medicine, 1324 Tulane Avenue, Suite A128, New Orleans, LA 70112, USA.
Funding
The DECAAF II trial was funded by Medtronic, GE, Siemens, Boston Scientific, Biosense Webster, and Abbott.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Jais P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah Ret al. . Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008;118:2498–505. [DOI] [PubMed] [Google Scholar]
- 2. Marrouche NF, Wazni O, McGann C, Greene T, Dean JM, Dagher Let al. . Effect of MRI-guided fibrosis ablation vs conventional catheter ablation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the DECAAF II randomized clinical trial. JAMA 2022;327:2296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gal P, Marrouche NF. Magnetic resonance imaging of atrial fibrosis: redefining atrial fibrillation to a syndrome. Eur Heart J 2017;38:14–9. [DOI] [PubMed] [Google Scholar]
- 4. Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski Fet al. . Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 2014;311:498–506. [DOI] [PubMed] [Google Scholar]
- 5. Sciacca V, Fink T, Körperich H, Bergau L, Guckel D, Nischik Fet al. . Magnetic resonance assessment of left atrial scar formation following a novel very high-power short-duration workflow for atrial fibrillation ablation. Europace 2023;25:1392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGann C, Akoum N, Patel A, Kholmovski E, Revelo P, Damal Ket al. . Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ: Arrhythmia Electrophysiol 2014;7:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sherif HM. The developing pulmonary veins and left atrium: implications for ablation strategy for atrial fibrillation. Eur J Cardiothorac Surg 2013;44:792–9. [DOI] [PubMed] [Google Scholar]
- 8. Takahashi Y, Sanders P, Rotter M, HaÏSsaguerre M. Disconnection of the left atrial appendage for elimination of foci maintaining atrial fibrillation. J Cardiovasc Electrophysiol 2005;16:917–9. [DOI] [PubMed] [Google Scholar]
- 9. Wang A, Jiang J, Xie Z, Zhong G. Efficacy and safety of catheter ablation combined with left atrial appendage closure in the treatment of atrial fibrillation: a systematic review and meta-analysis. Anatol J Cardiol 2022;26:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chieng D, Sugumar H, Ling L-H, Segan L, Azzopardi S, Prabhu Set al. . Catheter ablation for persistent atrial fibrillation: a multicenter randomized trial of pulmonary vein isolation (PVI) versus PVI with posterior left atrial wall isolation (PWI)—the CAPLA study. Am Heart J 2022;243:210–20. [DOI] [PubMed] [Google Scholar]
- 11. Quinto L, Cozzari J, Benito E, Alarcón F, Bisbal F, Trotta Oet al. . Magnetic resonance-guided re-ablation for atrial fibrillation is associated with a lower recurrence rate: a case–control study. Europace 2020;22:1805–11. [DOI] [PubMed] [Google Scholar]
- 12. Ravelli F, Masè M, Cristoforetti A, Avogaro L, D’Amato E, Tessarolo Fet al. . Quantitative assessment of transmural fibrosis profile in the human atrium: evidence for a three-dimensional arrhythmic substrate by slice-to-slice histology. Europace 2023;25:739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hopman LHGA, Frenaij IM, Solís-Lemus JA, el Mathari S, Niederer SA, Allaart CPet al. . Quantification of left atrial appendage fibrosis by cardiac magnetic resonance: an accurate surrogate for left atrial fibrosis in atrial fibrillation patients? Europace. 2023;25:euad084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Assaf A, Mekhael M, Noujaim C, Chouman N, Younes H, Khoury Cet al. . Effect of fibrosis regionality on atrial fibrillation recurrence. J Am Coll Cardiol 2023;81:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marrouche NF, Greene T, Dean JM, Kholmovski EG, Boer LM, Mansour Met al. . Efficacy of LGE-MRI-guided fibrosis ablation versus conventional catheter ablation of atrial fibrillation: the DECAAF II trial: study design. J Cardiovasc Electrophysiol 2021;32:916–24. [DOI] [PubMed] [Google Scholar]
- 16. Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish ENet al. . Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation 2009;119:1758–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higuchi K, Cates J, Gardner G, Morris A, Burgon NS, Akoum Net al. . The spatial distribution of late gadolinium enhancement of left atrial magnetic resonance imaging in patients with atrial fibrillation. JACC Clin Electrophysiol 2018;4:49–58. [DOI] [PubMed] [Google Scholar]
- 18. Cochet H, Mouries A, Nivet H, Sacher F, Derval N, Denis Aet al. . Age, atrial fibrillation, and structural heart disease are the main determinants of left atrial fibrosis detected by delayed-enhanced magnetic resonance imaging in a general cardiology population. J Cardiovasc Electrophysiol 2015;26:484–92. [DOI] [PubMed] [Google Scholar]
- 19. Hassink RJ, Aretz HT, Ruskin J, Keane D. Morphology of atrial myocardium in human pulmonary veins. J Am Coll Cardiol 2003;42:1108–14. [DOI] [PubMed] [Google Scholar]
- 20. Teh AW, Kistler PM, Lee G, Medi C, Heck PM, Spence SJet al. . Electroanatomic remodeling of the left atrium in paroxysmal and persistent atrial fibrillation patients without structural heart disease. J Cardiovasc Electrophysiol 2012;23:232–8. [DOI] [PubMed] [Google Scholar]
- 21. Lin Y, Yang B, Garcia FC, Ju W, Zhang F, Chen Het al. . Comparison of left atrial electrophysiologic abnormalities during sinus rhythm in patients with different type of atrial fibrillation. J Interv Card Electrophysiol 2013;39:57–67. [DOI] [PubMed] [Google Scholar]
- 22. Chang S-L, Tai C-T, Lin Y-J, Wongcharoen W, Lo L-W, Tuan T-Cet al. . Biatrial substrate properties in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2007;18:1134–9. [DOI] [PubMed] [Google Scholar]
- 23. Caixal G, Alarcón F, Althoff TF, Nuñez-Garcia M, Benito EM, Borràs Ret al. . Accuracy of left atrial fibrosis detection with cardiac magnetic resonance: correlation of late gadolinium enhancement with endocardial voltage and conduction velocity. Europace 2021;23:380–8. [DOI] [PubMed] [Google Scholar]
- 24. Sim I, Razeghi O, Solis Lemus JA, Mukherjee R, O’hare D, O’neill Let al. . Atrial tissue characterisation using electroanatomic voltage mapping and cardiac magnetic resonance imaging. Europace 2022;24:euac053. [Google Scholar]
- 25. Di Biase L, Burkhardt JD, Mohanty P, Sanchez J, Mohanty S, Horton Ret al. . Left atrial appendage. Circulation 2010;122:109–18. [DOI] [PubMed] [Google Scholar]
- 26. AlTurki A, Huynh T, Dawas A, AlTurki H, Joza J, Healey JSet al. . Left atrial appendage isolation in atrial fibrillation catheter ablation: a meta-analysis. J Arrhythm 2018;34:478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mekhael M, El Hajjar AH, Noujaim C, Dagher L, Ayoub T, Li DLet al. . Po-697-04 worse ablation-induced scar formation on fibrotic tissue: a sub analysis from the DECAAF II. Heart Rhythm 2022;19:S423. [Google Scholar]
- 28. Han S, Liu M, Jia R, Cen Z, Guo R, Liu Get al. . Left atrial appendage function and structure predictors of recurrent atrial fibrillation after catheter ablation: a meta-analysis of observational studies. Front Cardiovasc Med 2022;9:1009494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pinto Teixeira P, Martins Oliveira M, Ramos R, Rio P, Silva Cunha P, Delgado ASet al. . Left atrial appendage volume as a new predictor of atrial fibrillation recurrence after catheter ablation. J Interv Card Electrophysiol 2017;49:165–71. [DOI] [PubMed] [Google Scholar]
- 30. Istratoaie S, Vesa ȘC, Cismaru G, Pop D, Roșu R, Puiu Met al. . Value of left atrial appendage function measured by transesophageal echocardiography for prediction of atrial fibrillation recurrence after radiofrequency catheter ablation. Diagnostics 2021;11:1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee JM, Shim J, Park J, Yu HT, Kim T-H, Park J-Ket al. . The electrical isolation of the left atrial posterior wall in catheter ablation of persistent atrial fibrillation. JACC: Clinical Electrophysiology 2019;5:1253–61. [DOI] [PubMed] [Google Scholar]
- 32. Kanitsoraphan C, Rattanawong P, Techorueangwiwat C, Kewcharoen J, Mekritthikrai R, Prasitlumkum Net al. . The efficacy of posterior wall isolation in atrial fibrillation ablation: a systematic review and meta-analysis of randomized controlled trials. J Arrhythm 2022;38:275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu X, Gao X, Chen L, Shen L, Liu M, Xu Y. Clinical impact of posterior wall isolation in catheter ablation for persistent atrial fibrillation: a systematic review and meta-analysis. Pacing Clin Electrophysiol 2022;45:1268–76. [DOI] [PubMed] [Google Scholar]
- 34. Kistler PM, Chieng D, Sugumar H, Ling L-H, Segan L, Azzopardi Set al. . Effect of catheter ablation using pulmonary vein isolation with vs without posterior left atrial wall isolation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation. Jama 2023;329:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim D, Yu HT, Kim T-H, Uhm J-S, Joung B, Lee M-Het al. . Electrical posterior box isolation in repeat ablation for atrial fibrillation. JACC: Clinical Electrophysiology 2022;8:582–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.