Abstract
The combination of oxycodone and naloxone is useful for cancer pain management. Naloxone, as a pure opioid antagonist, cannot be used simultaneously with opioids. However, owing to its low bioavailability, it can be used in an oral composite formulation. We present the case of a 55-year-old man with gastric cancer who experienced severe opioid withdrawal syndrome (OWS) triggered by oxycodone/naloxone that was successfully managed with dexmedetomidine. He had been in a stable condition on intravenous morphine to alleviate cancer pain. Intravenous morphine was switched to oral oxycodone/naloxone for discharge from the hospital. The patient suddenly developed restlessness, heartburn, and violent behavior 30 minutes after taking oxycodone/naloxone. We attempted sedation with midazolam and propofol, but paradoxical agitation and desaturation occurred. Next, we tried dexmedetomidine and the patient showed a decreased heart rate and reduced agitation. The patient was eventually stabilized by increasing the dose of dexmedetomidine. This report informs clinicians of the possibility of OWS when switching from opioids to oxycodone/naloxone, which can be overcome with the appropriate use of sedatives and dexmedetomidine depending on the patient’s condition.
Keywords: Drug withdrawal symptoms, Oxycodone, Naloxone, Dexmedetomidine
INTRODUCTION
Oxycodone is a strong opioid with an affinity for both μ and κ opioid receptors. It has been widely used in clinical practice given its equivalence to other strong opioids [1]. Naloxone is a pure opioid antagonist that aids in reversing the side effects of oxycodone. The oxycodone/naloxone formulation is a modified formulation of oxycodone with the aim of reducing opioid-induced constipation while maintaining the analgesic effect of oxycodone [2]. Although this combination is seen as theoretically safe due to the low bioavailability of naloxone, the reality may be different.
Dexmedetomidine is an adrenergic α2 receptor agonist that provides a sedative effect with a low risk of respiratory depression; it is commonly used in the intensive care unit given its low risk. Here, we present the case of a patient with significant opioid withdrawal syndrome (OWS) after the use of oxycodone/naloxone, which was successfully managed with dexmedetomidine. This report was approved by the Institutional Review Board of Gyeongsang National University Changwon Hospital (GNUCH 2022-03-015).
CASE DESCRIPTION
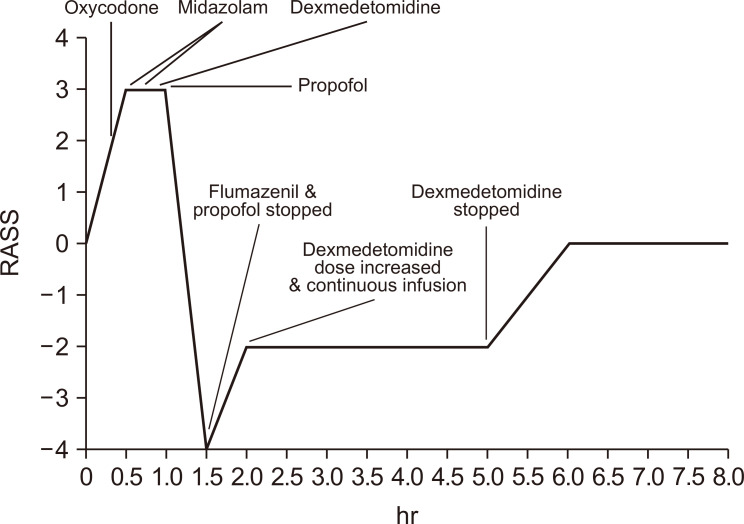
A 55-year-old man suddenly developed restlessness, heartburn, and violent behavior 30 minutes after taking oxycodone/naloxone. He had been diagnosed with gastric cancer with peritoneal seeding 9 months prior to presentation and was undergoing chemotherapy with paclitaxel/ramucirumab. He was on a fentanyl patch (300 μg/h) and morphine (30 mg/day, continuous infusion) for abdominal pain caused by cancer peritoneal metastasis; his pain was under control. Other oral medications included megestrol acetate, ursodeoxycholic acid, and esomeprazole. On the day of discharge, morphine was switched to oxycodone/naloxone (20 mg twice daily), and the fentanyl patch was continued. Approximately 30 minutes after taking oxycodone/naloxone, he complained of severe chest tightness. He began sweating and yawned several times. His blood pressure, pulse rate, and respiration rate were 160/80 mmHg, 116 beats/min, and 22 breaths/min, respectively. His pupils were moderately dilated. He remained confused and had a Richmond Agitation Sedation Scale (RASS) score of 3. Eventually, a four-point restraint was initiated. There was no sign of myocardial infarction on an electrocardiogram or desaturation on a pulse oximeter. Assuming the patient had oxycodone/naloxone-induced OWS, intravenous oxycodone (20 mg) and midazolam (2 mg) were administered immediately. Even after additional midazolam was administered 10 minutes later, the patient’s agitation worsened. Subsequent dexmedetomidine (1.0 μg/kg) over 10 minutes decreased his heart rate to 75 beats/min and reduced his agitation to a RASS score of 2. The patient tried to get out of bed although his limbs were bound. Then, to control agitation, propofol was initiated at a bolus dose of 1.0 mg/kg. After propofol administration, his consciousness decreased to a RASS score of 4, but his respiratory rate also dropped to 5 breaths/min; we also observed a desaturation to 60% on pulse oximetry. Flumazenil (0.3 mg) was immediately injected over 15 seconds to neutralize the sedatives, and rescue breathing with a bag valve mask was performed. The respiratory rate and oxygen saturation eventually returned to normal levels.
We decided to use only dexmedetomidine because of its antagonistic effect on OWS and the adverse effects of other sedatives. A maintenance dose of dexmedetomidine (0.6 μg/kg/h) was infused for 3 hours and stopped. Subsequently, his consciousness fully recovered, and the signs of augmented sympathetic nerve activity resolved (Figure 1).
Figure 1.
Consciousness change and medications after opioid withdrawal syndrome.
DISCUSSION
The combination of oral oxycodone and naloxone (Targin®) is a widely used opioid formulation for the management of cancer pain. Oral naloxone, a strong opioid antagonist, is administered to prevent opioid-induced constipation, mainly caused by receptors distributed in the intestines. The rationale for combining both an agonist and antagonist is that naloxone is almost entirely consumed through the process of extensive first-pass metabolism in the liver after absorption from the intestines [3]. When naloxone is administered orally, only 0.9% to 2% of the dose is absorbed [4]. Generally, naloxone is a safe drug with no potential for abuse by itself and does not provoke clinically significant problems in opioid-naive patients [5]. However, the safe threshold dose of naloxone to avoid OWS is unknown. Several studies have reported that acute OWS develops when oral naloxone is administered to patients taking opioids [6-8]. In our case, the patient developed moderate-severity OWS (score of 28 on the clinical opiate withdrawal scale) 30 minutes after ingesting oxycodone/naloxone.
When administering naloxone as an antidote for opioid intoxication, the American Heart Association recommended a dose of 0.04~0.4 mg of naloxone administered through an intravenous or intramuscular injection to avoid withdrawal complications [9]. As the commercially available oral oxycodone and naloxone combination is composed of a ratio of 2:1, the likelihood of oral absorption of naloxone increases as the dose of oxycodone increases. After passing through the blood-brain barrier, the binding affinity of naloxone to the μ receptor is stronger than that of opioids such as morphine and fentanyl; thus, naloxone competitively occupies this position [10].
Opioids bind to the μ receptor of the locus coeruleus (LC) in the brain, which is the largest group of noradrenergic neurons. The activation of the μ receptor in the LC subsequently inhibits adenylyl cyclase and cAMP, and eventually reduces norepinephrine levels. This leads to drowsiness, lower blood pressure, and decreased respiratory drive. However, when opioids are chronically used, the human body adapts to this imbalance of the autonomic system via neuroplasticity. The production of norepinephrine gradually returns to normal to maintain homeostasis through a compensatory increase in norepinephrine. Acute withdrawal from opioids by naloxone provokes a dramatic upregulation of the sympathetic nervous system and presents various symptoms, including tachycardia, irritability, violent behavior, yawning, restlessness, tremor, vomiting, piloerection, and sweating [11]. Even if a patient experiences acute and severe symptoms, they are rarely fatal [5].
The diagnosis of OWS is based on clinical manifestations and the temporal relationship between symptom development and medications. The treatment of withdrawal mainly comprises symptomatic therapy because the symptoms are temporary and nonfatal. Anesthetics or sedative agents can be administered to control agitation and intractable symptoms. A systematic review and phase III trials revealed that adrenergic α2 receptor agonists are helpful for the acute management of OWS [12,13]. Adrenergic α2 receptor agonists act as sympatholytic agents through cAMP reduction by inhibiting adenylated cyclase in the body, which reverses the OWS mechanism. Clonidine and lofexidine are the most widely investigated adrenergic α2 receptor agonists for OWS treatment. Unfortunately, these drugs are not accessible in Korea and oral medications are not a viable option for patients with OWS who are agitated, even if they were readily available. Dexmedetomidine (Precedex®) is a highly selective adrenergic α2 receptor agonist that is structurally similar to clonidine and is known to play a role in the acute management of OWS [14].
In the present case, we attempted sedation with midazolam and propofol to reduce the patient’s violent behavior and agitation. However, paradoxical agitation and desaturation due to decreased respiration occurred after the use of midazolam and propofol, respectively, making it difficult to maintain treatment with those drugs further. In comparison, the patient showed a decreased heart rate and reduced agitation after treatment with a loading dose of dexmedetomidine. The patient was eventually stabilized by increasing the dose of dexmedetomidine.
In conclusion, we emphasize two points based on our experience in this case. First, clinicians should be cautious regarding OWS when switching opioids to oxycodone/naloxone. Second, OWS can be overcome with the appropriate use of sedatives and dexmedetomidine depending on the patient’s condition. Prospective research is warranted to address the issues of OWS incidence, its severity, and the outcomes of symptomatic management after switching to oral naloxone-containing opioids.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.14475/jhpc.2023.26.1.18.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
PATIENT CONSENT
Written consent to publish was obtained from the patient.
AUTHOR’S CONTRIBUTIONS
Conception or design of the work: JHK. Data collection: all authors. Data analysis and interpretation: all authors. Drafting the article: all authors. Critical revision of the article: all authors. Final approval of the version to be published: all authors.
References
- 1.Reid CM, Martin RM, Sterne JA, Davies AN, Hanks GW. Oxycodone for cancer-related pain: meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:837–43. doi: 10.1001/archinte.166.8.837. [DOI] [PubMed] [Google Scholar]
- 2.Ahmedzai SH, Nauck F, Bar-Sela G, Bosse B, Leyendecker P, Hopp M. A randomized, double-blind, active-controlled, double-dummy, parallel-group study to determine the safety and efficacy of oxycodone/naloxone prolonged-release tablets in patients with moderate/severe, chronic cancer pain. Palliat Med. 2012;26:50–60. doi: 10.1177/0269216311418869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meissner W, Schmidt U, Hartmann M, Kath R, Reinhart K. Oral naloxone reverses opioid-associated constipation. Pain. 2000;84:105–9. doi: 10.1016/S0304-3959(99)00185-2. [DOI] [PubMed] [Google Scholar]
- 4.Smith K, Hopp M, Mundin G, Bond S, Bailey P, Woodward J, et al. Low absolute bioavailability of oral naloxone in healthy subjects. Int J Clin Pharmacol Ther. 2012;50:360–7. doi: 10.5414/CP201646. [DOI] [PubMed] [Google Scholar]
- 5.Rzasa Lynn R, Galinkin JL. Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther Adv Drug Saf. 2018;9:63–88. doi: 10.1177/2042098617744161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong A, Macleod D, Robinson J, Koutsogiannis Z, Graudins A, Greene SL. Oxycodone/naloxone preparation can cause acute withdrawal symptoms when misused parenterally or taken orally. Clin Toxicol (Phila) 2015;53:815–8. doi: 10.3109/15563650.2015.1060486. [DOI] [PubMed] [Google Scholar]
- 7.Kim JH, Song H, Lee G-W, Kang JH. Opioid withdrawal symptoms after conversion to oral oxycodone/naloxone in advanced cancer patients receiving strong opioids. Korean J Hosp Palliat Care. 2017;20:131–5. doi: 10.14475/kjhpc.2017.20.2.131. [DOI] [Google Scholar]
- 8.Kang JH, Lee GW, Shin SH, Bruera E. Opioid withdrawal syndrome after treatment with low-dose extended-release oxycodone and naloxone in a gastric cancer patient with portal vein thrombosis. J Pain Symptom Manage. 2013;46:e15–7. doi: 10.1016/j.jpainsymman.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Vanden Hoek TL, Morrison LJ, Shuster M, Donnino M, Sinz E, Lavonas EJ, et al. Part 12: cardiac arrest in special situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S829–61. doi: 10.1161/CIRCULATIONAHA.110.971069. [DOI] [PubMed] [Google Scholar]
- 10.Bellingan M. What drug?: Naloxone. Australian Pharmacist. 2017;36:54–7. [Google Scholar]
- 11.Pergolizzi JV, Jr, Raffa RB, Rosenblatt MH. Opioid withdrawal symptoms, a consequence of chronic opioid use and opioid use disorder: Current understanding and approaches to management. J Clin Pharm Ther. 2020;45:892–903. doi: 10.1111/jcpt.13114. [DOI] [PubMed] [Google Scholar]
- 12.Yu E, Miotto K, Akerele E, Montgomery A, Elkashef A, Walsh R, et al. A Phase 3 placebo-controlled, double-blind, multi-site trial of the alpha-2-adrenergic agonist, lofexidine, for opioid withdrawal. Drug Alcohol Depend. 2008;97:158–68. doi: 10.1016/j.drugalcdep.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gowing L, Farrell MF, Ali R, White JM. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2014:CD002024. doi: 10.1002/14651858.CD002024.pub4. [DOI] [PubMed] [Google Scholar]
- 14.Jung S, Rosini JM. Dexmedetomidine for treatment of refractory heroin withdrawal. J Emerg Nurs. 2017;43:182–4. doi: 10.1016/j.jen.2017.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.