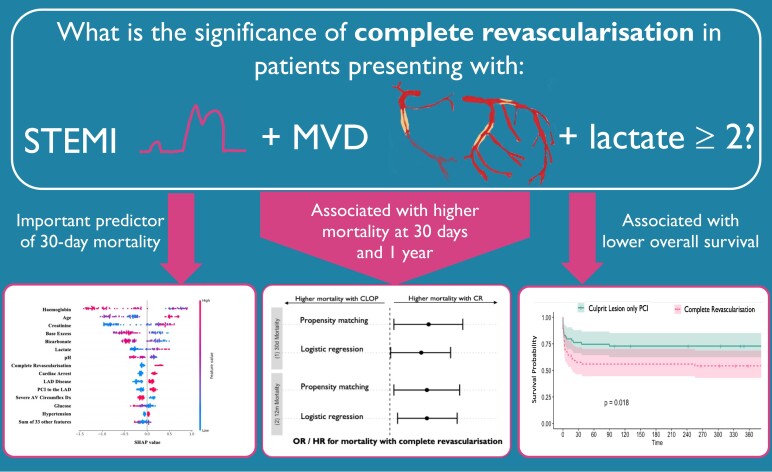
Abstract
Aims
Revascularization strategy for patients with ST-elevation myocardial infarction (STEMI) and multi-vessel disease varies according to the patient’s cardiogenic shock status, but assessing shock acutely can be difficult. This article examines the link between cardiogenic shock defined solely by a lactate of ≥2 mmol/L and mortality from complete vs. culprit-only revascularization in this cohort.
Methods and results
Patients presenting with STEMI, multi-vessel disease without severe left main stem stenosis and a lactate ≥2 mmol/L between 2011 and 2021 were included. The primary endpoint was mortality at 30 days by revascularization strategy for shocked patients. Secondary endpoints were mortality at 1 year and over a median follow-up of 30 months. Four hundred and eight patients presented in shock. Mortality in the shock cohort was 27.5% at 30 days. Complete revascularization (CR) was associated with higher mortality at 30 days [odds ratio (OR) 2.1 (1.02–4.2), P = 0.043], 1 year [OR 2.4 (1.2–4.9), P = 0.01], and over 30 months follow-up [hazard ratio (HR) 2.2 (1.4–3.4), P < 0.001] compared with culprit lesion-only percutaneous coronary intervention (CLOP). Mortality was again higher in the CR group after propensity matching (P = 0.018) and inverse probability treatment weighting [HR 2.0 (1.3–3.0), P = 0.001]. Furthermore, explainable machine learning demonstrated that CR was behind only blood gas parameters and creatinine levels in importance for predicting 30-day mortality.
Conclusion
In patients presenting with STEMI and multi-vessel disease in shock defined solely by a lactate of ≥2 mmol/L, CR is associated with higher mortality than CLOP.
Keywords: Cardiogenic shock, Machine learning, STEMI, Revascularisation strategy, XGBoost
Graphical Abstract
Graphical Abstract.
Introduction
Applying the results of clinical trials to real-world practice can be challenging due to restrictive entry criteria or under-representation of certain patient groups.1 This difficulty is increased if relatively subtle changes in the patient’s state place them in a different category that requires alternative treatment approaches. The management of multi-vessel disease in patients with ST-elevation myocardial infarction (STEMI) is an important example of this scenario.
Non-shock patients presenting with STEMI and multi-vessel disease benefit from complete revascularization (CR),2,3 even if the timing of CR is debateable.4 In contrast, patients presenting with acute coronary syndromes and cardiogenic shock are harmed by performing CR,5 and these are guideline recommendations on both sides of the Atlantic.6,7
However, applying these to a presenting STEMI patient can be difficult. For example, only 60% of patients in the seminal CULPRIT-SHOCK were admitted with STEMI, and the subgroup analysis was marginal for STEMI patients: the odds ratio (OR) upper limit was 0.99 for the composite endpoint of RRT and mortality at 30 days, and not significant for mortality at 1 year.
More pertinently, shock trials are difficult to undertake because of the dynamically unwell patients and hence the inclusion criteria are necessarily prescriptive to ensure comparable enrolment across centres. This can paradoxically lead to both the inclusion of patients who are not in cardiogenic shock and patients who are in deep shock refractory to any intervention. Common criteria for enrolment include any of: (i) systolic blood pressure <90 mmHg for 30 min or use of catecholamines, (ii) clinical signs of pulmonary congestion, (iii) altered mental status, (iv) cold and clammy skin, and (v) oliguria with urine <30 mL/h, or (6) lactate >2 mmol/L.8
Some are hard to assess in the immediate throes of a myocardial infarction (e.g. oliguria), some can be subjective (clammy skin and altered mental status), and some are operator-dependent (catecholamine administration). The problem of applying criteria requiring a greater depth of patient-level knowledge is exacerbated in the STEMI population where expediency is valuable.
Furthermore, there is a need to define early shock clearly and unambiguously because patients in early shock (Society for Cardiovascular Angiography and Interventions (SCAI) level B) often progress towards SCAI D/E and can be helped at this stage. This cause has been tackled by the Cardiogenic Shock Working Group (CSWG). Amongst other definitions, SCAI-CSWG level B can be defined solely by a lactate ≥2 mmol/L.9 This is a simple and sensitive means of defining shock, and a lactate of ≥2 mmol/L has been shown independently to predict adverse outcomes.10 However, in large, randomized trials, only two-thirds of patients had a lactate ≥2 mmol/L.8
Therefore, the aim of this study is to assess whether revascularization strategy affects mortality in patients presenting with STEMI, multi-vessel disease, and shock defined solely by a lactate of ≥2 mmol/L. If there is any difference in mortality observed in relation to revascularization strategy, then it reinforces the idea that using lactate as a single parameter can help to guide the management of such patients in the acute setting.
Due to the potential for confounding in observational studies, we apply a number of different methods to the shock cohort to examine this relationship further. These include multivariate analysis, propensity matching, inverse probability treatment weighted analysis, and explainable machine learning. This is the first paper applying this method of explainable machine learning to study revascularization strategy in patients presenting with cardiogenic shock, multi-vessel disease, and STEMI.
Methods
Study population and design
This was an observational study to determine associations between revascularization strategy and mortality in patients presenting to Harefield Hospital (Royal Brompton and Harefield Hospitals, Guy’s and St Thomas’ NHS Foundation Trust, London, UK) with STEMI, multi-vessel disease and shock defined by lactate ≥2 mmol/L between 2011 and 2021.
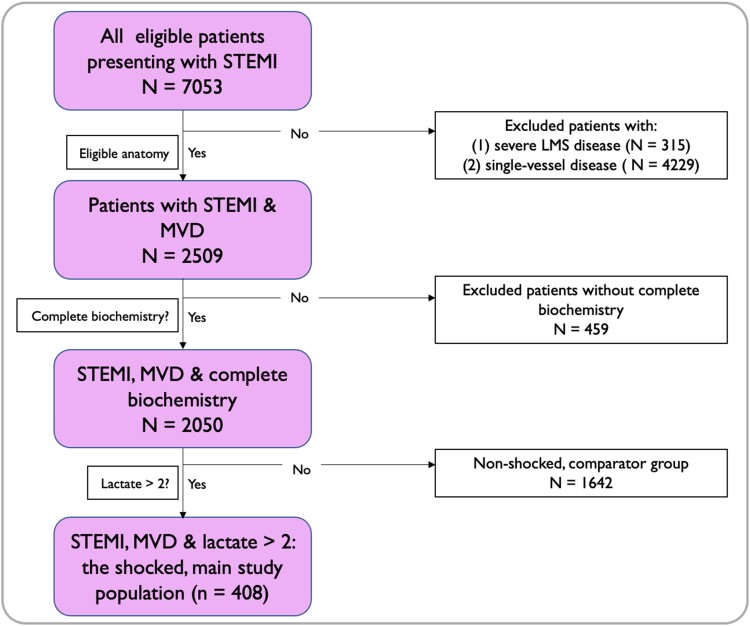
Patients included in the studied shock population: (i) were aged ≥18 years, (ii) were admitted to Harefield Hospital with an acute STEMI between 1 January 2011 and 1 January 2021 and underwent primary percutaneous coronary intervention (PCI), (iii) had multi-vessel disease as defined by ≥70% stenosis in two or more epicardial coronary vessels, and (iv) had a measured arterial lactate of ≥2 mmol/L prior to the index PCI. The study flow chart is shown in Figure 1.
Figure 1.
Study flow chart. LMS, left main stem; MVD, multi-vessel disease; STEMI, ST-elevation myocardial infarction.
Exclusion criteria were patients presenting with (i) ≥50% left main stem stenosis or (ii) cardiac arrest with ≥30 min downtime.
Complete revascularization was defined as the revascularization of all severe lesions during the index procedure. Patients who underwent staged PCI to all lesions later during the index admission were not classified as CR for the purposes of this study. All other patients were defined as culprit lesion-only percutaneous coronary intervention (CLOP).
Clinical and outcome data
The clinical data were taken from routine audit fields mandate for every patient undergoing PCI at our institution. Laboratory and blood gas tests were taken from our own hospital’s database. Survival data were obtained by linking patients’ NHS numbers to the NHS spine, in collaboration with the Office for National Statistics (ONS).
The primary endpoint was pre-assigned as mortality at 30 days. Secondary endpoints include mortality at 1 year and throughout the follow-up period (median 30 months).
Ethics
All patient-identifiable information was removed before analysis. Our local audit office assigned institutional support for this project. As this was the analysis of anonymized information taken from required audit data, we were advised that no further ethical approval was required.
Statistical analysis
Univariate analysis was performed using Student’s t-test for comparing the means of normally distributed data and Mann–Whitney U test if not normally distributed. Chi-squared and Fisher’s tests were used for categorical data. Fisher’s exact test was used if the expected value in any group was <5. Regression analysis was performed using binary logistic regression for dichotomous outcome variables and Cox proportional hazards and Kaplan–Meier curves for survival data, as appropriate.
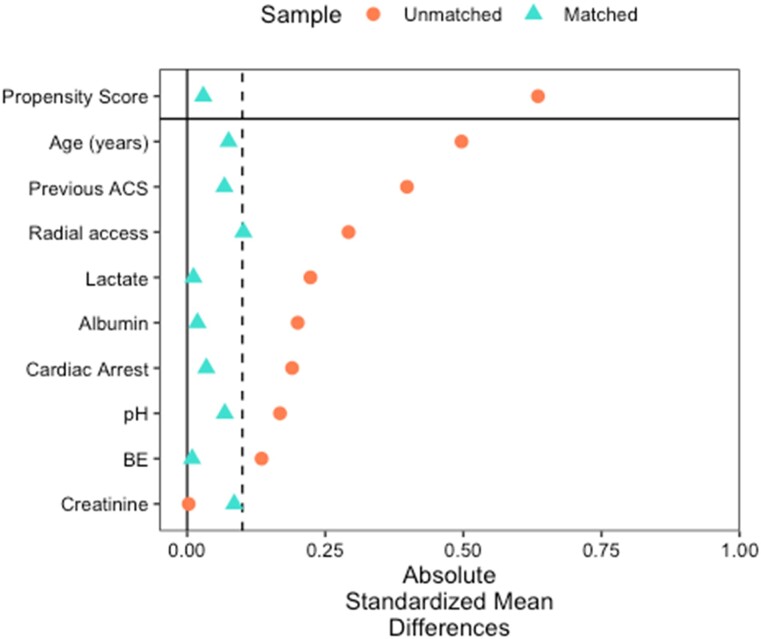
Propensity matching was performed via nearest-neighbour matching with a 1:1 ratio for the variables associated with both survival (via Cox regression) and treatment choice. The matched cohort comprised 118 patients, 59 in each arm. The absolute standardized mean difference for all matched variables and the propensity score between treatment groups was <0.1, indicating good matching (Figure 2).
Figure 2.
Propensity matching statistics for shock cohort. ACS, acute coronary syndrome, BE, base excess.
Inverse probability weighted analysis was carried out by incorporating inverse probability treatment weights into a Cox regression model. Inverse probability weighted scores were calculated using the propensity scores from the propensity-matched cohort above, and inverse probability weighted scores (IPTWi) were calculated where IPTWi = 1/PSi for patients who underwent CR and IPTWi is calculated as 1/(1 − PSi) for patients with CLOP.
A weighted Cox regression was then performed using these IPTWi as weights and age, pH, BE, creatinine, albumin, and ACS as covariates (as these were associated with mortality in the original adjusted multivariate model).
Statistical significance was established at P < 0.05 (two-tailed) for all tests. All data is reported according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines.11
Machine learning model development
We developed seven machine learning models using the shock cohort to predict 30-day mortality. These were decision trees, random forest, gradient boosting, extreme gradient boosting (XGBoost), support vector machines (SVM), logistic regression, and k-nearest neighbour. XGBoost has emerged as a highly effective algorithm for classification problems and involves using weak learners to boost training performance with regularization to reduce over-fitting and is especially effective for sparse datasets.12
The categorical data were first converted into numerical data using the One Hot Encoding technique. Numerical features were then normalized to a value between 0 and 1. We then applied random search with five-fold cross-validation to optimize the hyperparameters. Final model evaluation then was performed using these hyperparameters, again using five-fold cross-validation to partition the data.
ML algorithms have been limited in their explanation of predictions by being something of a ‘black box’. Recent advances in explainable ML have led to the development of the unified SHapley Additive exPlanations (SHAP) method.13 We then applied to SHAP to the best-performing model to provide an explanation of important factors in predicting 30-day mortality.
All calculations and statistical analysis were performed using R version 4.2.0 and Python version 3 (The Python Software Foundation) for machine learning.
Results
Baseline characteristics
The studied shock cohort comprised 408 patients who presented with STEMI, an arterial lactate ≥2 mmol/L, multi-vessel disease, and no significant left main stem disease. Their baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics and unadjusted outcomes for the shock cohort.
| All patients (n = 408) | CR (n = 74) | CLOP (n = 334) | P | |
|---|---|---|---|---|
| Patient factors | ||||
| Age (years) | 67.9 (13.3) | 63.9 (12.6) | 68.8 (13.3) | 0.005 |
| Male (n, %) | 304 (74.5) | 56 (75.7) | 248 (74.3) | 0.799 |
| Previous ACS (n, %) | 66 (16.2) | 6 (8.1) | 60 (18.0) | 0.037 |
| Previous PCI (n, %) | 51 (12.5) | 3 (4.1) | 48 (14.4) | 0.008 |
| Diabetes (n, %) | 119 (29.2) | 15 (20.3) | 104 (31.1) | 0.063 |
| HTN (n, %) | 209 (51.2) | 36 (48.6) | 173 (51.8) | 0.624 |
| Hypercholesterolaemia (n, %) | 130 (31.9) | 20 (27.0) | 110 (32.9) | 0.324 |
| Current smoker (n, %) | 78 (19.1) | 19 (25.7) | 59 (17.7) | 0.113 |
| Any smoking (n, %) | 157 (42.5) | 32 (45.1) | 125 (41.9) | 0.632 |
| LVEF (%) | 44.2 (12.4) | 46.8 (8.7) | 43.9 (12.8) | 0.674 |
| Systolic BP (mmHg) | 119.4 (31.3) | 117.1 (38.6) | 119.9 (29.7) | 0.57 |
| Cardiac arrest (n, %) | 127 (31.1) | 30 (40.5) | 97 (29.0) | 0.05 |
| OOHCA (n, %) | 85 (20.8) | 17 (23.0) | 68 (20.4) | 0.616 |
| Ventilated (n, %) | 72 (17.6) | 25 (33.8) | 47 (14.1) | <0.001 |
| Blood tests | ||||
| pH | 7.36 (0.1) | 7.34 (0.1) | 7.37 (0.1) | 0.167 |
| Lactate (mmol/L) | 4.2 (3.1) | 5.0 (4.0) | 4.0 (2.9) | 0.012 |
| Albumin (g/dL) | 35.7 (6.2) | 34.6 (7.2) | 35.9 (6.0) | 0.286 |
| BE (mmol/L) | −4.4 (4.9) | −5.1 (5.1) | −4.3 (4.8) | 0.252 |
| Hb (g/L) | 123.4 (36.5) | 117.6 (44.1) | 124.6 (34.5) | 0.478 |
| Creatinine (μmol/L) | 101.9 (50) | 100.6 (37.8) | 102.2 (52.4) | 0.729 |
| Troponin (ng/L) | 12 813 (27 856) | 9715.1 (19 287) | 13 332.5 (29 051) | 0.875 |
| Coronary anatomy | ||||
| LAD all (n, %) | 346 (84.8) | 61 (82.4) | 285 (85.3) | 0.53 |
| Proximal LAD (n, %) | 241 (59.1) | 46 (62.2) | 195 (58.4) | 0.55 |
| Non-proximal LAD (n, %) | 223 (54.7) | 37 (50.0) | 186 (55.7) | 0.374 |
| LCx (n, %) | 255 (62.5) | 47 (63.5) | 208 (62.3) | 0.842 |
| RCA (n, %) | 335 (82.1) | 50 (67.6) | 285 (85.3) | <0.001 |
| Vessels affected | <0.001 | |||
| 2 (n, %) | 288 (70.6) | 64 (86.5) | 224 (67.1) | |
| 3 (n, %) | 120 (29.4) | 10 (13.5) | 110 (32.9) | |
| Procedural characteristics | ||||
| Radial access (n, %) | 267 (65.4) | 39 (52.7) | 228 (68.3) | 0.011 |
| Impella (n, %) | 6 (1.5) | 4 (5.4) | 2 (0.6) | 0.01 |
| ECMO (n, %) | 8 (2.0) | 4 (1.2) | 4 (5.4) | 0.039 |
| GP 2b/3a (n, %) | 170 (41.7) | 35 (47.3) | 135 (40.4) | 0.278 |
| Outcomes | ||||
| Death at 30 days (n, %) | 112 (27.5) | 29 (39.2) | 83 (24.9) | 0.012 |
| Death at 1 year (n, %) | 138 (33.8) | 35 (47.3) | 103 (30.8) | 0.007 |
ACS, acute coronary syndrome; PCI, percutaneous intervention, HTN = hypertension, OOHCA, out of hospital cardiac arrest, LVEF, left ventricular ejection fraction; LAD, left anterior descending artery; LCx, left circumflex; RCA, right coronary artery; BP, blood pressure; Hb, haemoglobin; ECMO, extra corporeal membrane oxygenation; Continuous variables are presented as the mean (SD), categorical as count (percentage).
Of the shock cohort of 408 patients, 74 (18.1%) underwent CR, the remainder CLOP. One patient had three coronary vessels affected but underwent PCI to only two vessels, and they were assigned to the CLOP group.
Patients undergoing CR were more likely to be younger and less likely to have had a previous myocardial infarction. However, the CR group was more likely to have suffered a cardiac arrest and be ventilated prior to the procedure, with corresponding higher levels of blood lactate and more likely to be supported by extra-corporeal membranous oxygenation (ECMO). The baseline characteristics univariate analysis between treatment groups is shown in Table 1.
As a comparator group, the primary and secondary outcomes were also assessed for a non-shock cohort of 1642 patients. The baseline characteristics of the non-shock cohort are shown in the Supplementary material online (Table S1).
Unadjusted outcome measures
The unadjusted 30-day mortality was 39.2% in the CR group vs. 27.5% in the CLOP group (P = 0.007). Similarly, 1-year mortality was also higher in the CR group at 47.3 vs. 33.8% in the CLOP group (Table 1, P = 0.007) (Table 2).
Table 2.
Performance of machine learning algorithms for predicting 30-day mortality
| Model name | F1 | ROC AUC | Recall | Brier score |
|---|---|---|---|---|
| XGBoost | 0.65 | 0.78 | 0.84 | 0.25 |
| SVM | 0.64 | 0.75 | 0.63 | 0.19 |
| Decision tree | 0.59 | 0.72 | 0.61 | 0.23 |
| Gradient boosting | 0.58 | 0.72 | 0.56 | 0.22 |
| Logistic regression | 0.58 | 0.71 | 0.51 | 0.21 |
| Random forest | 0.55 | 0.69 | 0.46 | 0.20 |
| KNN | 0.27 | 0.55 | 0.20 | 0.29 |
F1 score is the harmonic mean of the precision (positive predictive value) and the recall (sensitivity), where higher scores are better. ROC AUC, receiver operator characteristic area under the curve, which is a marker of discrimination between classes and higher scores are preferable. The Brier score is a marker of calibration and discrimination, where lower scores are better.
KNN, k-nearest neighbours; SVM, support vector machines.
The overall mortality for the non-shocked comparator cohort was 3.6% at 30 days and 7.3% at 1 year. There was no significant difference in mortality at either timepoint between either revascularization strategy.
Adjusted outcome measures
Binary logistic regression for mortality at 30 days and 1 year
All factors that were significantly different between the groups on univariate analysis were entered into a binary logistic regression using the forward Wald method. Significant covariates were CR, age, lactate, radial access, and cardiac arrest prior to the procedure. When adjusted for significant confounders, the mortality rate at 30 days and 1 year remained higher with CR vs. CLOP [30 days: OR 2.1 (1.02–4.2), P = 0.043 and 1 year: OR 2.4 (1.2–4.9), P = 0.01].
In contrast, in the non-shocked control group, there was no association between CR and 30-day mortality [OR 1.2 (0.37–3.8), P = 0.791] nor 1-year mortality [OR 0.731 (0.264–2.0), P = 0.546].
Cox proportional hazards regression analysis in shocked patients
Cox regression was performed for all covariates in Table 1 using a stepwise conditional approach. All significant covariates were fed into the final model. These were: CR, age, pH, base excess, creatinine, albumin, and previous ACS. Complete revascularization was associated with higher mortality over a median follow-up of 2.6 years [hazard ratio (HR) 2.2 (95% confidence interval (CI) 1.4–3.4), P < 0.001]. Using CR as a covariate did not violate the proportional hazards assumption as shown by the lack of correlation between Schoenfeld residuals and survival time (P = 0.25).
There was no significant difference in survival by revascularization strategy in the non-shocked cohort [HR 0.90 (95% CI 0.6–1.3), P = 0.577].
Propensity-matched analysis for shocked patients
Propensity matching was performed for the shock cohort via nearest-neighbour matching with a 1:1 ratio for the variables associated with both survival (via Cox regression) and treatment choice. The matched cohort comprised 118 patients, 59 in each arm. The absolute standardized mean difference for all matched variables and the propensity score between treatment groups was <0.1, indicating good matching (Figure 2).
Basic unadjusted statistics for the propensity match group again showed increased mortality in the CR group at both 30 days (42.3 vs. 23.7%, P = 0.031) and 1 year (45.8 vs. 27.1%, P = 0.035).
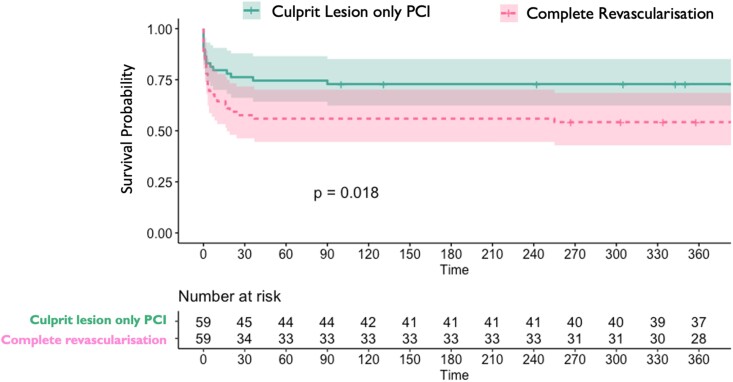
Survival was significantly worse in the CR group via the Kaplan–Meier method (χ2 5.581, P = 0.018, Figure 3). Double robust logistic regression models using PSi as a covariate showed increased mortality at 30 days (OR 2.5, 95% CI 1.1–5.6; P = 0.031) and 1 year in the CR group (OR 2.4, 95% CI 1.1–5.2; P = 0.036).
Figure 3.
Kaplan–Meier survival curves for propensity-matched shock cohort over 1 year.
Cox proportional hazards regression incorporating PSi and CR showed similar findings with significantly increased chance of dying throughout the follow-up period in the CR cohort (HR 2.0, 95% CI 1.1–3.6; P = 0.02).
Inverse probability weighted analysis for shocked patients
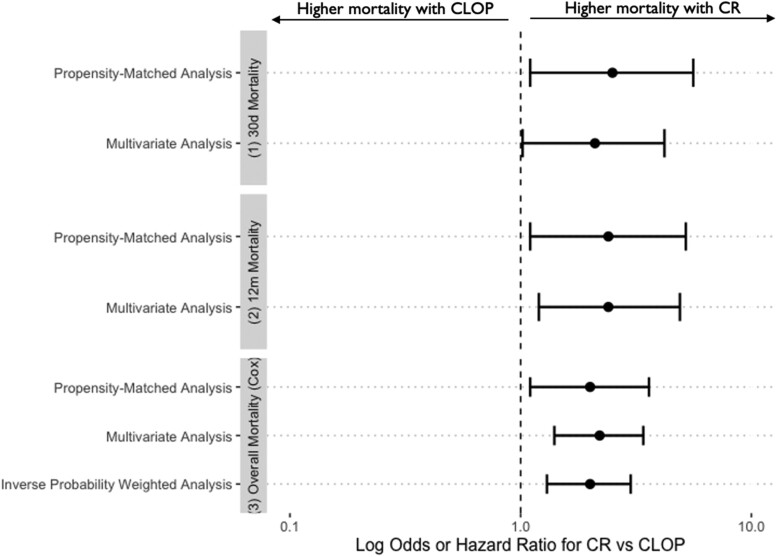
Inverse probability weighted analysis was carried out by incorporating inverse probability treatment weights into a Cox regression model. Complete revascularization was again associated with increased mortality over the duration of follow-up (HR 2.0, 95% CI 1.3–3.0; P = 0.001). The results of the adjusted and matched analyses are summarized in Figure 4.
Figure 4.
Complete revascularization is associated with higher mortality compared with culprit lesion-only percutaneous coronary intervention in all adjusted and weighted analyses for shocked patients.
Explainable machine learning algorithms for shocked patients
Seven machine learning algorithms were applied to the data with the aim of predicting 30-day mortality (Table 2). The best-performing algorithm was XGBoost in terms of F1 score, area under the curve, and recall. However, the main aim of this analysis was not to build an accurate model to predict 30-day mortality in this cohort per se, but rather to see which factors contributed to the mortality prediction.
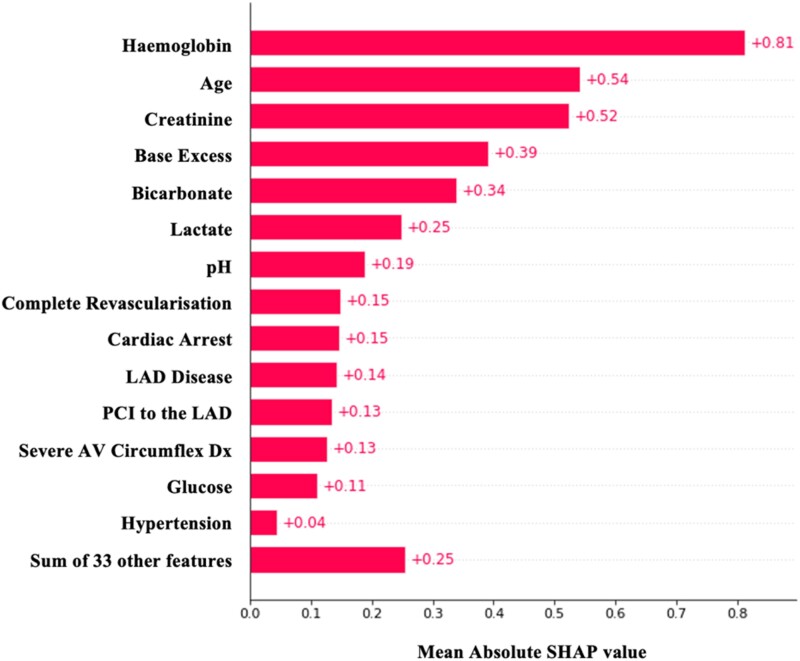
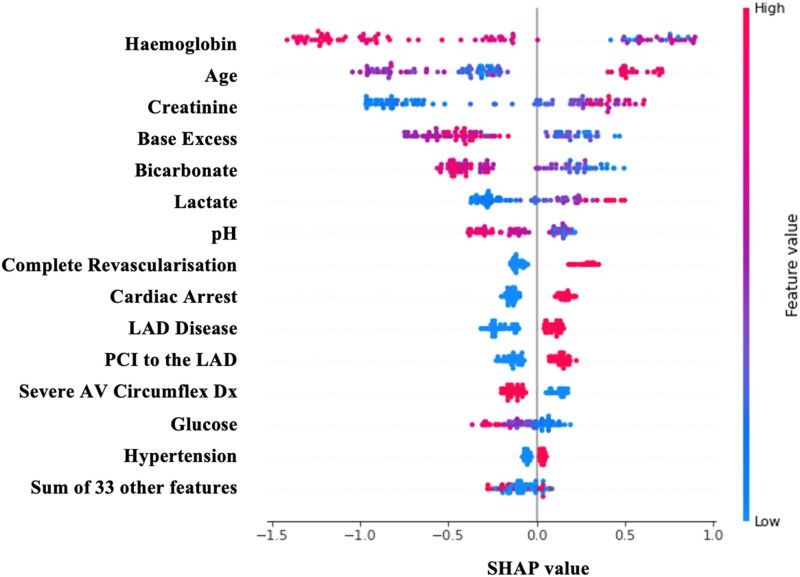
The contribution of each variable to the model is shown in Figure 5. These contributions are calculated using the SHapley Additive exPlanations (SHAP) method.13 Complete revascularization was ranked only behind age, creatinine levels, and blood gas values as a predictor of 30-day mortality. The direction of the effect of each variable on the prediction of 30-day mortality can be seen in Figure 6.
Figure 5.
Absolute SHapley Additive exPlanations (SHAP) values showing the importance of each variable in the XGBoost model predicting 30-day mortality in the shock cohort.
Figure 6.
Actual SHapley Additive exPlanations (SHAP) values showing the importance and direction of influence of each variable in the XGBoost model predicting 30-day mortality in the shock cohort.
Discussion
In this observational study, we have shown that patients with STEMI complicated by cardiogenic shock (defined as lactate ≥2 mmol/L) and multi-vessel disease have higher mortality when treated with CR rather than targeting the culprit vessel alone. This was demonstrated using a variety of adjusted statistical methods including regression analyses, propensity matching, and inverse probability treatment weighting. Regardless of the method used, the OR or HR for mortality was around 2 for complete vs. culprit lesion-only revascularization at 30 days, 1 year, and over a median follow-up of 30 months.
This was further explored by the novel use of explainable machine learning. This showed that revascularization strategy was behind only age, creatinine, and blood gas parameters in predicting 30-day mortality.
This finding is in keeping with other published studies looking at complete vs. culprit-lesion revascularization in patients with acute coronary syndromes, the most important being CULPRIT-SHOCK,5 which enrolled an all-comers ACS cohort. Indeed, as referenced in the introduction, this is now the guideline-based recommendation in Europe and the USA. In patients with shock and acute coronary syndromes, the potential benefits of CR appear to be outweighed by longer procedural times and increased contrast load. Although the mechanism of this is not yet clear, proposed causes have included renal dysfunction, platelet aggregation, and prothrombotic states during cardiogenic shock leading to more ischaemia and ventricular pump dysfunction during an extended multi-vessel procedure.14
A second finding of this article is that using a lactate threshold of ≥2 mmol/L could potentially be used as a straightforward heuristic guiding revascularization strategy. In comparison with the study shocked cohort, the non-shocked cohort did not have a difference in mortality observed in relation to either revascularization strategy. This is in keeping with previous trials,3,15–17 where the benefits of CR in non-shocked STEMI patients were not through mortality but through reduced repeat revascularization or MI. Therefore, using lactate to arbitrate shocked status divided this population into subgroups that exhibited different responses to revascularization strategy.
This is important because the classic hypotension-based criteria prevalent in the shock literature can result in neglect of the ∼50% of hypoperfused ACS patients who present as normotensive.18,19
This supposition is supported by the analysis of shock patients by the CSWG. Although a lactate of ≥2 mmol/L in isolation would place a patient in SCAI-CSWG Stage B, 90% of patients presenting at this level progress to a higher stage during hospital admission, and the mortality listed in the paper of 39.9% is comparable to the mortality in our shock cohort.9 Thus, this accurate and easy-to-implement categorization can help to define the most appropriate strategy for treating patients with STEMI and multi-vessel disease.
Finally, the importance of revascularization strategy on outcome was supported by using explainable machine learning in addition to traditional statistical methods. Tree-based machine learning models are the most commonly used non-linear models.20 XGBoost has emerged as a highly effective predictive algorithm and is integral to the winning solution in the majority of classification problems set by Kaggle.12 Although accurate, machine learning models have suffered from a lack of transparency and poor interpretability. In practice, this means that although predictions emerging from these models are accurate when applied to new testing sets, it is difficult to define the significance of each variable in making that prediction. This is particularly important in medical predictions, because making treatment decisions based upon a black box algorithm is inappropriate from both ethical and legal standpoints.
Recent work has allowed the explanation of tree-based models using game-theoretic SHAP values.21 This allows both local feature interaction effects and global model structure to be explained. By applying SHAP values to the XGBoost model, we could better explain the factors that predict 30-day mortality in the shock cohort. XGBoost does not require (or indeed allow) us to try to weight the data, rather it provides objective links between inputs and outputs. The fact that revascularization strategy was such a strong predictor of 30-day mortality using this method supports the findings of the traditional statistical methods applied to earlier sections of this article. This is the first time that SHAP values have been applied to assessing revascularization strategy on cardiogenic shock patients presenting with STEMI.
The most important limitation of this study is its non-randomized design. We have made numerous different attempts to adjust for confounding using statistical methods, but these can only ever be as effective as the inputted data and cannot take into account metrics that are used by clinicians to make decisions but are not captured in tabular data. The multiple analytical approaches yielded broad point estimates and therefore this study should be viewed primarily as hypothesis-generating. Furthermore, this analysis is based upon data from a single centre with a high proportion of shocked patients, which may limit its applicability to other settings. There is also likely to be an indication bias with regards to patients having measured arterial lactates.
Conclusions
This study has shown that CR of patients presenting with STEMI, multi-vessel disease, and a lactate ≥2 mmol/L is associated with increased mortality compared with culprit vessel-only PCI. This relationship held true when the data were analysed using both traditional statistical techniques and explainable machine learning.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care.
Supplementary Material
Acknowledgements
The authors would like to thank Andy Ryan in the Harefield audit office and the clinical informatics team for the Royal Brompton and Harefield clinical informatics team for help with collecting the relevant data.
Contributor Information
Alexander Tindale, Department of Cardiology, Harefield Hospital, Royal Brompton and Harefield Hospitals, Guy’s and St Thomas’ NHS Foundation Trust, Hill End Road, Harefield, UB9 6JH, UK; National Heart and Lung Institute, Imperial College London, Harefield Hospital, Hill End Road, UB9 6JH, London, UK.
Ioana Cretu, College of Engineering, Design and Physical Sciences, Brunel University London, Kingston Lane, Uxbridge, UB8 3PH, UK.
Hongying Meng, College of Engineering, Design and Physical Sciences, Brunel University London, Kingston Lane, Uxbridge, UB8 3PH, UK.
Vasileios Panoulas, Department of Cardiology, Harefield Hospital, Royal Brompton and Harefield Hospitals, Guy’s and St Thomas’ NHS Foundation Trust, Hill End Road, Harefield, UB9 6JH, UK; National Heart and Lung Institute, Imperial College London, Harefield Hospital, Hill End Road, UB9 6JH, London, UK.
Author contributions
A.T.: concept, design, data collection, data analysis, statistics, data interpretation, drafted article, critical revision of article, statistics. I.C.: data analysis, critical revision of article. H.M.: data analysis, critical revision of article. V.P.: concept, design, analysis, interpretation of data, critical revision.
Funding
British Heart Foundation (FS/19/73/34690 to I.C.).
Data availability
Data sharing is available upon request and approval by the local ethics review board.
References
- 1. Mas-Llado C, González-Del-Hoyo M, Siquier-Padilla J, Blaya-Peña L, Coughlan JJ, de la Villa BG, et al. Representativeness in randomised clinical trials supporting acute coronary syndrome guidelines. Eur Heart J Qual Care Clin Outcomes 2023:qcad007. doi: 10.1093/ehjqcco/qcad007. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. El-Hayek GE, Gershlick AH, Hong MK, Casso Dominguez A, Banning A, Afshar AE, et al. Meta-analysis of randomized controlled trials comparing multivessel versus culprit-only revascularization for patients with ST-segment elevation myocardial infarction and multivessel disease undergoing primary percutaneous coronary intervention. Am J Cardiol 2015;115:1481–1486. [DOI] [PubMed] [Google Scholar]
- 3. Mehta SR, Wood DA, Storey RF, Mehran R, Bainey KR, Nguyen H, et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med 2019;381:1411–1421. [DOI] [PubMed] [Google Scholar]
- 4. Gershlick AH, Price MJ. Full revascularization in the patient with ST-segment elevation myocardial infarction: the story so far. J Am Coll Cardiol 2019;74:2724–2727. [DOI] [PubMed] [Google Scholar]
- 5. Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer-Saraei R, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med 2017;377:2419–2432. [DOI] [PubMed] [Google Scholar]
- 6. Ibanez B, Halvorsen S, Roffi M, Bueno H, Thiele H, Vranckx P, et al. Integrating the results of the CULPRIT-SHOCK trial in the 2017 ESC ST-elevation myocardial infarction guidelines: viewpoint of the task force. Eur Heart J 2018;39:4239–4242. [DOI] [PubMed] [Google Scholar]
- 7. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e4–e17. Erratum in: Circulation 2022;145(11):e771. [DOI] [PubMed] [Google Scholar]
- 8. Thiele H, Desch S, Piek JJ, Stepinska J, Oldroyd K, Serpytis P, et al. Multivessel versus culprit lesion only percutaneous revascularization plus potential staged revascularization in patients with acute myocardial infarction complicated by cardiogenic shock: design and rationale of CULPRIT-SHOCK trial. Am Heart J 2016;172:160–169. [DOI] [PubMed] [Google Scholar]
- 9. Kapur NK, Kanwar M, Sinha SS, Thayer KL, Garan AR, Hernandez-Montfort J, et al. Criteria for defining stages of cardiogenic shock severity. J Am Coll Cardiol 2022;80:185–198. [DOI] [PubMed] [Google Scholar]
- 10. Tindale A, Vela MM, Panoulas V. Using base excess, albumin, lactate, and renal function to predict 30-day mortality in patients requiring impella monotherapy for left-sided mechanical circulatory support: the BALLAR score. Cardiovasc Revasc Med 2022;41:129–135. [DOI] [PubMed] [Google Scholar]
- 11. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573–577. [DOI] [PubMed] [Google Scholar]
- 12. Chen T, Guestrin C. XGBoost: a scalable tree boosting system. In: Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining (KDD’16), p785–794. Association for Computing Machinery, New York, NY, USA. [Google Scholar]
- 13. Lundberg S, Lee SI. A unified approach to interpreting model predictions. In: Advances in Neural Information Processing Systems 30 (NIPS 2017), p4765–4774. Curran Associates, New York, USA.
- 14. Reyentovich A, Barghash MH, Hochman JS. Management of refractory cardiogenic shock. Nat Rev Cardiol 2016; 13:481–492. [DOI] [PubMed] [Google Scholar]
- 15. Wald DS, Morris JK, Wald NJ, Chase AJ, Edwards RJ, Hughes LO, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med 2013;369:1115–1123. [DOI] [PubMed] [Google Scholar]
- 16. Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol 2015;65:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smits P, Omerovic E, Abdel-Wahab M, Boxma-de Klerk B, Lunde K, Neumann FJ, et al. Two year results from the compare-acute trial. J Am Coll Cardiol 2018;71:A1158. [Google Scholar]
- 18. Panoulas V, Ilsley C. Rapid classification and treatment algorithm of cardiogenic shock complicating acute coronary syndromes: the SAVE ACS classification. J Interv Cardiol 2022;2022:9948515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Da Rocha R C, Picarra B, Fernandes R, Dias Claudio F, Carrington M, Pais J, et al. Cardiogenic shock without hypotension in acute myocardial infarction. Eur Heart J Acute Cardiovasc Care 2021;10:zuab020.039. [Google Scholar]
- 20. Kaggle . The state of data science & machine learning. 2017. https://www.kaggle.com/datasets/kaggle/kaggle-survey-2017(15 May 2023).
- 21. Lundberg SM, Erion G, Chen H, DeGrave A, Prutkin JM, Nair B, et al. From local explanations to global understanding with explainable AI for trees. Nat Mach Intell 2020;2:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is available upon request and approval by the local ethics review board.