Abstract
Background
Racemic ketamine consists of two enantiomers, namely (R)-ketamine and (S)-ketamine, with distinguishable pharmacological properties. Both enantiomers have been reported to show rapid antidepressant effects in rodents. Currently, the (S)-enantiomer has been approved for the treatment of major depression, whereas (R)-ketamine failed to show antidepressant effect in recent clinical studies. Major depressive disorder is frequently characterized by disinhibition of rapid eye movement (REM) sleep and disruption of non-REM (NREM) sleep. Racemic ketamine and most conventional antidepressants affect these parameters. However, it remains largely unknown which enantiomer is responsible for these effects.
Methods
Here, we compared acute effects of the two ketamine enantiomers (15 mg/kg i.p.) on different sleep-wake stages in freely moving, EEG-equipped rats. We also evaluated the antidepressant-like activity of the enantiomers in a chronic restraint stress model of depression.
Results
(S)-ketamine but not (R)-ketamine increased REM sleep latency and decreased REM sleep time at 2 and 3 hours, and increased electroencephalogram delta power during NREM sleep. In addition, only (S)-ketamine increased wakefulness and decreased NREM sleep in the first 2 hours. In the forced swimming test, only (S)-ketamine decreased the immobility time of chronically stressed rats.
Conclusion
Effects of the two ketamine enantiomers on rat sleep-wake architecture and behavior are markedly different when administered in the same dose. (S)-ketamine remarkably affects the sleep-wake cycle and very likely sleep-related neuroplasticity, which may be relevant for its antidepressant efficacy. Our results regarding (R)-ketamine’s lack of effect on vigilance and behavior are in line with recent clinical studies.
Keywords: Esketamine, arketamine, sleep, EEG delta power, antidepressive effect
Significance Statement.
Racemic ketamine consists of two enantiomers, in other words, optical isomers, and both have been reported to show rapid antidepressant effects in rodents. This is a unique property, since most antidepressants need a minimum of 2-3 weeks to show therapeutic actions. Currently, (S)-ketamine has been approved for the treatment of major depression, whereas (R)-ketamine failed to show antidepressant effect in recent clinical studies. Major depressive disorder is frequently characterized by early onset and overrepresented rapid eye movement (REM) sleep. Racemic ketamine and most conventional antidepressants affect these parameters. However, it remains largely unknown which enantiomer is responsible for these effects. Here we provide the first evidence, to our knowledge, that a 15-mg/kg dose of (S)-ketamine but not (R)-ketamine in rats shows antidepressant-like effects and suppresses REM sleep acutely. These results are consistent with the clinical antidepressive effects of (S)-ketamine and underline the importance of parallel behavioral, sleep, and EEG effects of antidepressants.
INTRODUCTION
Major depressive disorder is a debilitating mood disorder mainly characterized by depressed mood, loss of interest, impaired cognitive function, and disturbed sleep (Otte et al., 2016). Most patients with depression show characteristic sleep-electroencephalogram (EEG) alterations, including disinhibition of rapid eye movement (REM) sleep, disruption of sleep continuity, and changes in non-REM (NREM) sleep. Additionally, several depressed patients show reduced EEG delta power during sleep (Steiger and Pawlowski, 2019).
The majority of reuptake inhibitor antidepressants suppress REM sleep (increase REM sleep latency time and decrease REM sleep time), which is thought to be an important component of their therapeutic effect (Wilson and Argyropoulos, 2005; Palagini et al., 2013; Wichniak et al., 2017; Riemann et al., 2020). These characteristic sleep effects can be observed in both healthy volunteers and depressed patients (Dumont et al., 2005; Steiger and Kimura, 2010; Steiger and Pawlowski, 2019). Interestingly, some of the sleep-EEG effects of classical antidepressants are acute. However, the therapeutic effects only occur after an adaptive process, weeks after beginning of the treatment.
For decades, racemic ketamine has been used widely as a dissociative anesthetic in human and veterinary medicine (Kohtala, 2021). In 2000, Berman et al. were the first to show that a single administration of subanesthetic ketamine has a rapid-acting antidepressant effect in patients with major depressive disorder (Berman et al., 2000). Since then, several studies have shown that ketamine relieves the symptoms of depression or depressive-like states in humans and animals, respectively (Zarate et al., 2006; Abdallah et al., 2018).
(R,S)-ketamine consists of two enantiomers, namely (R)-ketamine and (S)-ketamine. In 2019 the (S)-enantiomer of ketamine (esketamine) was approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as an adjunctive treatment for treatment-resistant depression (Jelen et al., 2021). In addition, in 2020, the US Food and Drug Administration approved new supplemental application for (S)-ketamine to treat depressed patients with acute suicidal ideation/behavior to provide fast symptomatic relief (Liu et al., 2022). Thus, (S)-ketamine is the first officially approved and clinically used rapid-acting antidepressant. However, its wider usability is limited by its side effects, such as dissociation, and its abuse and misuse potential. The (R)-enantiomer has not been approved for clinical use; however, preclinical studies report a more favorable side-effect profile than that of (S)-ketamine (Yang et al., 2015). However, (R)-ketamine failed to show a significant antidepressant effect compared with placebo according to recently published clinical studies (Johnston et al., 2023; Leal et al., 2023).
The distinguishable effects and side effects of the two enantiomers are not surprising because the enantiomers can be characterized with a distinguishable pharmacological profile ranging from different affinities for several receptors,for example, (S)-ketamine has 4-fold higher binding affinity for NMDA (N-methyl-D-aspartate) receptors, and 2-fold higher affinity for muscarinic receptors than (R)-ketamine. Furthermore, differential effects on monoamine levels in various brain structures and different mechanisms of action at molecular level have been described (Zanos and Gould, 2018; Wei et al., 2020; Rafało-Ulińska and Pałucha-Poniewiera, 2022). Despite the fact that the use of ketamine enantiomers in the treatment of depression and other psychiatric or neurological disorders has been of great interest in recent years (Wang et al., 2022), enantiomer-specific effects of ketamine on sleep-EEG are scarce, and only the effects of racemic ketamine have been described in rodents. Racemic ketamine in subanesthetic, antidepressant-relevant doses affects sleep parameters that are characteristically altered in depressed individuals, namely increases REM sleep latency, decreases REM sleep time (Ahnaou et al., 2017; Banerjee et al., 2020), and increases delta EEG power during NREM sleep (Feinberg and Campbell, 1993). However, the enantiomer(s) responsible for these effects has not been investigated. Therefore, in this study, we investigated the effects of (R)- and (S)-ketamine in an antidepressant-relevant dose on sleep parameters affected by racemic ketamine in rodents. Antidepressive-like activities of the same doses of the two enantiomers also were tested in a chronic restraint depression model.
METHODS
Animals
All housing conditions and animal experiments were performed in accordance with the EU Directive 2010/63/EU, as well as specific national laws (the Hungarian Governmental Regulations on animal studies 40/2013). The experiments were approved by the National Scientific Ethical Committee on Animal Experimentation (permit no. PE/EA/292-7/2021). All surgeries were performed under anesthesia, and all efforts were made to minimize suffering, pain, and discomfort of the animals throughout the whole experiment.
Male Wistar rats (n = 60, Han:WIST, Toxi-Coop, Budapest, Hungary) were kept under controlled environmental conditions at an ambient temperature of 21°C ± 1°C and a 12-hour-light/-dark cycle (lights on at 10:00 am). Standard rodent food and tap water were available ad libitum for the animals during the whole study.
Surgery
Rats (9 weeks old), weighing 290–325 g at surgery, were equipped with electroencephalographic (EEG) and electromyographic (EMG) electrodes under 2% isoflurane anesthesia, using a Kopf stereotaxic instrument, as described earlier (Kantor et al., 2004; Papp et al., 2020). Briefly, for fronto-parietal EEG recordings, stainless-steel screw electrodes were placed epidurally over the left frontal cortex (1.5 mm lateral and 2.0 mm anterior to bregma) and over the left parietal cortex (1.5 mm lateral and 2.0 mm anterior to lambda), and a ground electrode was implanted over the cerebellum. A pair of EMG electrodes (stainless-steel spring electrodes embedded by silicon rubber, Plastics One Inc., Roanoke, VA, USA) were placed into the musculature of the neck for EMG recordings.
After recovery (7 days), rats were moved separately to a square, glass recording chamber and were attached and kept connected to the EEG system via a flexible recording cable during the whole study, permitting free movement to the animals. As a habituation to the recording conditions, the animals received i.p. injections of physiological saline for 5 days before the experiment.
EEG Recording and Analysis
After each treatment, EEG, EMG, and motor activity were monitored for 10 hours (Coulburn Lablinc System, Holliston, MA, USA), while the animals were undisturbed. The signals were filtered below 0.50 Hz and above 100 Hz and were amplified (EMG: 5000 times, EEG: 10,000 times). Analog-to-digital conversion was carried out at 256-Hz sampling rate.
The sleep-wake stages were classified using SleepSign for Animal sleep analysis software (Kissei Comtec America Inc., Fort Lee, NJ, USA), using conventional criteria (Kantor et al., 2004; Koncz et al., 2021). We used the automatic scoring feature of the software to score the vigilance stages at first; after that, visual supervision was conducted by researchers who were unaware of the treatment of the rats. 4-second periods (epochs) were differentiated as follows. In wakefulness, the EEG activity was characterized by low-amplitude at beta (14–29 Hz) and alpha (10–13 Hz) frequencies, along with high EMG and motor activity. In NREM sleep, the EEG was characterized by high-amplitude delta (0.5–4 Hz) frequency band activity and occasionally occurring spindles (6–15 Hz), with decreased EMG activity and minimal motor activity. In REM sleep, the EEG was characterized by low-amplitude, high-frequency activity and consistent theta waves (5–9 Hz), without any EMG or motor activity except from transient twitches.
The duration of wakefulness, NREM and REM sleep in each hour and the sum for the 10-hour period, as well as NREM sleep latency (time elapsed from the beginning of the administration until the occurrence of the first NREM sleep episode) and REM sleep latency (time elapsed between first NREM sleep episode and the beginning of the first REM sleep episode) were calculated.
For quantitative EEG (qEEG) delta power analysis, we used fast Fourier transformation (Hanning window, frequency resolution: 0.25 Hz) in the 0.5- to 4-Hz frequency range. The 1-Hz bins, marked by their upper limits, were created by summing the 0.25-Hz bins. Delta power values were averaged per hour in wakefulness and NREM sleep, then were averaged over the 1- to 4-Hz range. Epochs with artifacts or stage transitions were not included in the qEEG analysis.
Stress Procedures and Behavioral Tests
A chronic restraint stress procedure was used to induce depressive-like behaviors in rats, as previously described (Ampuero et al., 2015). Rats (7 weeks old) weighing 210–245 g on the first day of stress were used for the behavioral tests. Before starting the protocol, the rats were acclimatized to the housing conditions for 8 days. During this period, rats were handled for 2 minutes per day for 5 days. In the experimental days, rats were kept in their home cages, except for the 2 hours of restraint stress (between 12:00 pm and 2:00 pm). The restraint plastic bottles were adapted to prevent any movement of the animal, while a large hole in the nose and mouth zone allowed breathing. Nonstressed rats were handled for 2 minutes each day before being placed back in their cage. Rats were subjected to 10 days of stress (n = 27) or no stress (n = 9) before being assessed for depressive-like behaviors in the rat forced swimming test on day 12. All rats in a given cage received identical treatment, which was chosen using randomization for cages. As a habituation to the test conditions, the animals received i.p. injections of physiological saline for 5 days before the forced swimming test.
The modified rat forced swimming test was used to test the antidepressant-like activity of the administered drugs, as described earlier (Slattery and Cryan, 2012). The animals were forced to swim for 15 minutes (pretest) and 5 minutes (test) in a cylinder with 30-cm diameter and 60-cm height (MazeEngineers, Skokie, IL, USA) filled with 30 cm tap water at a temperature of 24°C ± 1°C. Four cylinders were used simultaneously, and each swimming session contained 1 animal from each treatment group in a randomized order. The swimming test was performed during the light cycle of the animals (between 1:00 pm and 5:00 pm). The behavior of the animals was video-recorded for 5 minutes and later scored for immobility, swimming, climbing, and diving manually using EthoVision XT 15 (Noldus Information Technology, Wageningen, Netherlands) by 2 blinded experimenters.
Drugs and Treatments
The animals received 15 mg/kg i.p. (S)-ketamine (Ketanest S, Pfizer Pharma GmbH, Berlin, Germany) or (R)-ketamine (Toronto Research Chemicals, Toronto, Canada) or vehicle (saline) in a volume of 1 mL/kg body weight. All treatments were injected by a male experimenter, while a female experimenter held the animals. For each animal the drugs were administered exactly at the beginning of the light (passive) phase in the EEG experiments or 1 hour before the swimming test in the behavioral experiments. The time of administration was selected based on previous research investigating the sleep effect of conventional antidepressants and racemic ketamine (Vas et al., 2013; Papp et al., 2018; Banerjee et al., 2020).
Statistics and Visualization
The statistical analyses were performed using Prism 8 (GraphPad, San Diego, CA, USA). To evaluate the effects of ketamine enantiomers on the time spent in wakefulness, NREM and REM sleep for each hour, 2-way repeated-measure ANOVA was used. For the analysis of the effects on overall time spent in wakefulness, NREM and REM sleep during 10 hours as well as NREM and REM sleep latencies, 1-way ANOVA was performed. For qEEG data analyses, mixed-model design ANOVA (2 main factors: treatment and time) was used. To analyze behaviors in the forced swimming test, 1-way ANOVA was performed. For multiple comparisons, Bonferroni post hoc test was performed. A customized script was used to construct Figure 3C using Matlab 9.13.0.2049777 (Mathworks, Natick, MA, USA).
Figure 3.
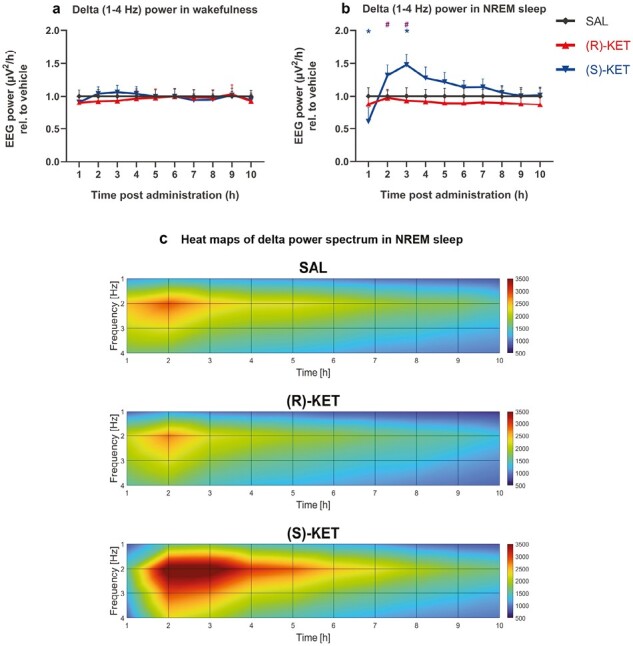
Delta (1-4 Hz) EEG power changes induced by (R)-ketamine [15 mg/kg i.p., (R)-KET] and (S)-ketamine [15 mg/kg i.p., (S)-KET] during wakefulness, and NREM sleep. Delta power of the two compounds compared with saline in wakefulness are shown in A and in NREM sleep in B. Significant results are indicated by * of the appropriate color (compared with vehicle, P < .05) and # (between the enantiomers, P < .05). Data are shown as mean ± SEM (n = 8 rats per group). Heat maps for saline, (R)-ketamine and (S)-ketamine treatment, as a function of time and frequency are shown in C.
Results
Effects of Ketamine Enantiomers on Sleep-Wake Architecture
Effects on Wakefulness
Acute (S)-ketamine treatment promoted wakefulness in the first 2 hours (treatment: F2,21 = 20.14, P < .0001, treatment × time interaction: F18,189 = 5.947, P < .0001; Figure 1A) compared with (R)-ketamine and saline treatment. The latter effect resulted in an overall increase in time spent in wakefulness during the recorded 10 hours of passive phase (treatment: F2,21 = 20.15, P < .0001; Figure 2A). (R)-ketamine showed no effect on wakefulness.
Figure 1.
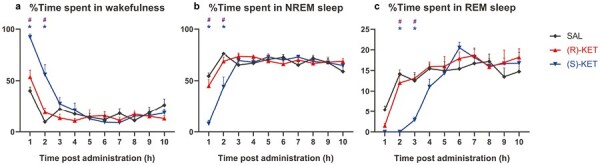
Effects of (R)-ketamine [15 mg/kg i.p., (R)-KET], (S)-ketamine [15 mg/kg i.p., (S)-KET], and vehicle (saline, SAL) in the amount of: (A) wake, (B) NREM sleep, and (C) REM sleep for 10 hours post administration, shown in percentages. Drugs were injected at the beginning of the light phase when all animals were awake. Significant results are indicated by * of the appropriate color (compared with vehicle, P < .05) and # (between two enantiomers, P < .05). Data are shown as mean ± SEM of 8 rats per group.
Figure 2.
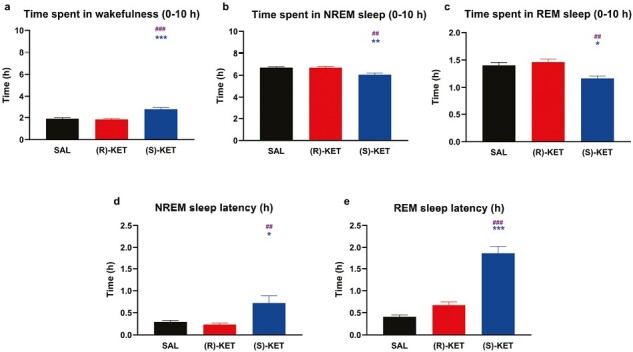
Effects of (R)-ketamine [15 mg/kg i.p., (R)-KET], (S)-ketamine [15 mg/kg i.p., (S)-KET], and vehicle (saline, SAL) on the quantity of time animals spent (A) awake, in (B) NREM sleep, and in (C) REM sleep for 10 hours following administration. Panels (D) and (E) show effects on NREM sleep latency and REM sleep latency, respectively. Significant results are indicated by * of the appropriate color (compared with vehicle, P < .05) and # (between two enantiomers, P < .05). Results are presented as mean ± SEM (n = 8 rats per group).
Effects on NREM Sleep
In parallel with its wake promoting effects, (S)-ketamine treatment decreased the time spent in NREM sleep in the first 2 hours (treatment: F2,21 = 11.13, P = .0005, treatment × time interaction: F18,189 = 5.596, P < .0001; Figure 1B) compared with (R)-ketamine and saline treatment. This resulted in an overall decrease in time spent in NREM sleep during the recorded passive phase (treatment: F2,21 = 11.13, P = .0005; Figure 2B) and an increase in NREM sleep latency (treatment: F2,21 = 7.242, P = .0041; Figure 2D). (R)-ketamine had no effect on any parameters of NREM sleep.
Effects on REM Sleep
(S)-ketamine treatment exerted a prominent REM suppression effect up to 3 hours after administration (treatment: F2,21 = 9.513, P = .0011, treatment × time interaction: F18,189 = 3.648, P < .0001; Figure 1C) compared with (R)-ketamine and saline treatment. Moreover, (S)-ketamine increased REM sleep latency (treatment: F2,21 = 59.48, P < .0001; Figure 2E) while decreasing the total time spent in REM sleep (treatment: F2,21 = 9.521, P = .0011; Figure 2C). (R)-ketamine had no effect on time spent in REM sleep or on REM sleep latency.
Effects of Ketamine Enantiomers on qEEG Delta Power During Wakefulness and NREM Sleep
The effects of ketamine enantiomers on qEEG delta power were sleep-wake stage dependent and enantiomer specific. Namely, while neither enantiomer had any effect on delta power during wakefulness (treatment: F2,21 = 0.04186, P = .9591, treatment × time interaction: F18,189 = 1.155, P = .3031; Figure 3A), during NREM sleep, (S)-ketamine reduced qEEG delta power in the first hour, which was followed by a delta power increase in the third hour (treatment × time interaction: F18,186 = 5.126, P < .0001; Figure 3B), although the treatment effect alone did not reach significance. This rebound effect was also observable between 4 and 7 hours but only at trend level (Figure 3B–C).
Effects of Ketamine Enantiomers on Depressive-Like Behaviors in Rat Forced Swimming Test
Ten days of chronic restraint stress induced depressive-like behaviors in saline-treated rats during the forced swimming test. Namely, saline-treated stressed animals spent more time in immobility compared with nonstressed animals (P < .05; Figure 4A). Administration of (S)-ketamine 1 hour before the behavioral test reversed depressive-like behaviors: it significantly reduced immobility compared with (R)-ketamine (P < .005) and saline (P < .005) treatment in stressed rats (Figure 4A). (S)-ketamine treatment also increased swimming and climbing compared with (R)-ketamine (P < .05) and saline (P < .05 and P < .0005, respectively) treatment (Figure 4B–C). (R)-ketamine had no effect on stressed rats, and thus, in this group similar depressive-like behaviors were observed as in saline treated stressed animals (Figure 4). Despite promoting wakefulness, (S)-ketamine failed to increase locomotor activity in the open field test (data not shown). Similarly, (R)-ketamine failed to affect locomotor activity in our in-house experiments.
Figure 4.
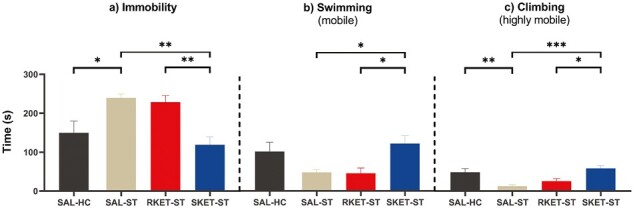
Effects of saline (SAL-ST), (R)-ketamine (15 mg/kg i.p., RKET-ST), and (S)-ketamine (15 mg/kg i.p., SKET-ST) compared with homecage (SAL-HC) on time of (A) immobility, (B) swimming, and (C) climbing in chronically stressed rats. Chronic restraint stress and forced swimming test were performed. Significant post-hoc results are marked by *P < .05, **P < .005, ***P < .001. Data are presented as mean ± SEM (n = 9 rats per group).
Discussion
The main finding of this study is that (S)-ketamine but not (R)-ketamine significantly alters the architecture of the sleep-wake cycle and modulates EEG delta power in a sleep-wake stage dependent manner in Wistar rats. Interestingly, these effects of the (S)-enantiomer on the vigilance states are similar to racemic ketamine described in previous studies (Ahnaou et al., 2017; Banerjee et al., 2020) (see also Table 1). Moreover, (S)-ketamine but not (R)-ketamine significantly decreased the depression-like behaviors induced by chronic stress at the same time interval, shortly after administration.
Table 1.
Preclinical evidence that the enantiomer (S)-ketamine, but not (R)-ketamine, has similar sleep-wake effects to (R,S)-ketamine
| Reference | Drugs | Dose and route of administration | Species | Time spent in wakefulness | Time spent in NREM sleep | Time spent in REM sleep | REM sleep latency time |
|---|---|---|---|---|---|---|---|
| Banerjee et al., 2020 | (R,S)-ketamine | 30 mg/kg i.p. | Wistar rat | ↑ | ↓ | ↓ | ↑ |
| This study | (S)-ketamine | 15 mg/kg i.p. | Wistar rat | ↑ | ↓ | ↓ | ↑ |
| This study | (R)-ketamine | 15 mg/kg i.p. | Wistar rat | ø | ø | ø | ø |
Abbreviations: ↑, increased, ↓, decreased, ø, no effect.
Effects on REM Sleep
Here we show that (S)-ketamine but not (R)-ketamine has a robust REM sleep suppression effect in freely moving rats. Most slow-acting antidepressants (e.g., reuptake inhibitors) increase REM sleep latency and decrease REM sleep time. These REM sleep suppression effects are thought to be important components of their therapeutic effects (Palagini et al., 2013). Indeed, these effects can be observed in depressed patients, healthy volunteers, and stressed and nonstressed animals as well (Dumont et al., 2005; Steiger and Kimura, 2010; Vas et al., 2013). Treatment with the rapid-acting antidepressant ketamine elicits a robust and fast-onset antidepressant effect both in clinical settings and in preclinical animal models of depression (Zanos et al., 2018). In rats, acute treatment with racemic ketamine (2.5, 5, 10 mg/kg s.c.) increased REM sleep latency and reduced REM sleep in the first 2 hours (Ahnaou et al., 2017). These effects were also observed at a higher dose (30 mg/kg i.p.) of racemic ketamine, namely, ketamine increased latency to REM sleep and significantly decreased the duration of REM sleep, when the first 4 hours were summarized (Banerjee et al., 2020). Here, our results show that only the (S)-enantiomer had similar REM suppressive effects at this dose (15 mg/kg i.p.), whereas the (R)-enantiomer had no effect on REM sleep (see Table 1).
Administration of subanesthetic doses of either (S)- or (R)-ketamine in mice resulted in similar brain levels of both drugs (Zanos et al., 2016). Therefore, the different effects of (S)- and (R)-ketamine treatment on REM sleep can be attributed to the different neurochemical effects of ketamine enantiomers on REM sleep regulating neuronal pathways. It has been shown that systemically administered cholinergic drugs induce REM sleep (Yamada and Ueda, 2020), whereas administration of cholinergic receptor antagonists (e.g., atropine and scopolamine) suppresses REM sleep in animals (Grace and Horner, 2015). In line with this, racemic ketamine is reported to bind as an antagonist to both muscarinic and nicotinic acetylcholine receptors. Moreover, a receptor binding assessment revealed that (S)-ketamine has a 2-fold higher affinity for mAChRs (muscarinic acetylcholine receptors) than (R)-ketamine (Zanos et al., 2018; Scotton et al., 2022). Thus, anticholinergic properties of (S)-ketamine might contribute to its REM sleep suppressive effects, moreover, could also be important in its rapid antidepressant effects, as antimuscarinic scopolamine’s rapid antidepressant effect has been described in depressed patients (Furey and Drevets, 2006; Jaffe et al., 2013). Serotonergic and noradrenergic neurotransmissions also play important roles in the regulation of REM sleep; mainly monoaminergic neurons activate the REM-off circuitry and suppress REM sleep (Ursin, 2002; Lu et al., 2006; Bacqué-cazenave et al., 2020). Interestingly, both ketamine enantiomers (both 10 and 20 mg/kg i.p.) acutely increased serotonin release in the prefrontal cortex (PFC) of mice, but the effects of (R)-ketamine were greater than that of (S)-ketamine (Ago et al., 2019). In our study, acute (R)-ketamine had no effect on REM sleep, suggesting that serotonergic effects of ketamine enantiomers at this dose are not key factors in its REM suppressing effects.
Effects on Wakefulness and NREM Sleep
In this study, we report that acute (S)-ketamine treatment promotes wakefulness and increases NREM sleep latency when administered at the onset of the inactive period. Earlier it was shown that racemic ketamine in subanesthetic doses elicits significant wake-enhancing properties (Ahnaou et al., 2017; Banerjee et al., 2020). Our results suggest that most likely the (S)-enantiomer is responsible for these wake-promoting effects. In line with this, a previous study showed that (S)-ketamine treatment (10 and 30 mg/kg i.p.) increased wakefulness and suppressed overall NREM sleep in the analyzed 1 hour post administration (Raith et al., 2020). The brain regions and neurotransmitters mediating these effects are not well known. Blockade of NMDAglutamatergic receptors in the PFC is known to promote the release of arousal-enhancing neurotransmitters (e.g., dopamine) and also increase motor activity (Del Arco et al., 2008). Previously it was shown that (S)-ketamine has a 4-fold higher binding affinity for NMDA receptors than (R)-ketamine (Zanos et al., 2018; Scotton et al., 2022). In line with this, an in vivo microdialysis experiment performed on mice showed that (S)-ketamine caused a robust increase in dopamine release in the PFC compared with (R)-ketamine. Interestingly, both ketamine enantiomers have been shown to increase noradrenaline release in the same manner (Ago et al., 2019). This effect does not seem to play an essential role in the wake-enhancing effect of racemic ketamine, as (R)-ketamine treatment had no effect on wakefulness in our study.
Effects on EEG Delta Power During NREM Sleep
The effects of ketamine enantiomers on delta power were sleep-wake stage dependent and enantiomer specific. During wakefulness, none of the enantiomers had any effect on EEG delta power. However, during NREM sleep, only the (S)-enantiomer affected qEEG delta power. In the first hour, (S)-ketamine, in parallel with its NREM suppressive effects, reduced EEG delta power. This was followed by a delta power rebound in the third hour, which was also observable at trend level during the following hours. It was previously reported that subanesthetic ketamine (10 mg/kg i.p.) administered during the passive phase evoked rebound delta oscillations in mice (Kohtala et al., 2019). Earlier it was also shown that racemic ketamine treatment (15, 25, and 50 mg/kg i.p.) increases EEG delta power during NREM sleep in rats (Feinberg and Campbell, 1993; Wang et al., 2023). This is similar to our findings with (S)-ketamine. The delta power–increasing effect seems to be important in clinical settings too, as low baseline delta-sleep ratio (i.e., the ratio of delta EEG intensity between the first two NREM sleep episodes) of depressed patients predicted a better antidepressive response to ketamine treatment (Duncan et al., 2013). Moreover, it has been shown that chronic treatment with slow-acting antidepressants, such as mirtazapine and sertraline, also increase delta activity during NREM sleep in depressed patients (Jindal et al., 2003; Schmid et al., 2006). The neuroplasticity hypothesis of depression suggests that major depressive disorder is characterized by deficient synaptic plasticity (Liu et al., 2017). Slow wave activity (EEG delta intensity) during NREM sleep has been proposed as a marker of synaptic plasticity (Esser et al., 2007; Duncan and Zarate, 2013; Dudysová et al., 2020). In line with this, patients with depression and rats exposed to chronic stress show reduced EEG delta power throughout sleep (Mrdalj et al., 2013; Steiger and Pawlowski, 2019). It is important to mention one of the limitations of our study: we evaluated the sleep-EEG characteristics only in nonstressed animals. Thus, further studies are needed with EEG-equipped chronically stressed animals to confirm the correlation between the sleep-EEG effects and the antidepressant effects of the ketamine enantiomers. Overall, acute effects of (S)-ketamine on delta frequency band during NREM sleep might have a role in its rapid and sustained antidepressant effects, but further studies are needed with depressed patients or animal models of depression.
Antidepressant-Like Activity of Ketamine Enantiomers
It was previously shown that a single dose of 15 mg/kg i.p. (S)-ketamine produced an acute (1 hour after dosing) and sustained (48 hours after dosing) antidepressant-like effects in the rat forced swimming test (du Jardin et al., 2016). Moreover, 14 days after the administration, the same dose of (S)-ketamine resulted in improved anhedonic behavior in adult rats following maternal deprivation (Réus et al., 2015). Interestingly, in mice and rat models of depression, it has been shown that (R)-ketamine (10 and 20 mg/kg, i.p.) produces a more potent antidepressant-like effect than (S)-ketamine (Fukumoto et al., 2017). In rats, a single administration of (R)-ketamine (10 mg/kg i.p.) 24 hours before the forced swimming test reversed the depressive-like behavior induced by repeated corticosterone treatments (Fukumoto et al., 2017). Moreover, in mice, (R)-ketamine has been reported to show antidepressant effects at lower doses than (S)-ketamine (Zanos et al., 2016). Overall, these data suggest that a 15-mg/kg i.p. dose of either (S)- or (R)-ketamine is relevant for the antidepressant effects in rats. Nevertheless, we found that in rats exposed to chronic restraint stress, only (S)-ketamine had antidepressant-like activity measured by the forced swimming test at the dose of 15 mg/kg i.p. Furthermore, we found that (S)-ketamine treatment reversed the depression-like behaviors induced by chronic stress, while (R)-ketamine effects on these parameters were similar to saline treatment in the applied dose. Our findings are in line with the results of recent clinical trials, which reported no significant differences between (R)-ketamine (PCN-101) and placebo treatment in depressed patients (Johnston et al., 2023; Leal et al., 2023). It should be mentioned however, that in these studies placebo exhibited a greater effect than the reported effects of ketamine in most of the clinical literature previously published.
In humans, the acute pharmacological effects of a single dose of racemic ketamine last for minutes to hours, whereas the antidepressive effects peak at approximately 24 hours and last up to 7 days. It has been hypothesized that the antidepressant effect of ketamine is also linked to its effects on sleep and circadian regulation (Duncan and Zarate, 2013; Rantamäki and Kohtala, 2020; Kohtala et al., 2021). As previously shown in preclinical studies, racemic ketamine markedly affects sleep parameters that can be crucial in the pathophysiology of depression. The present study revealed that in the studied dose only the enantiomer (S)-ketamine is responsible for these effects, such as REM sleep suppression and NREM sleep delta power increase. Furthermore, only the (S)-enantiomer showed antidepressant-like effects in this rat model of depression. Although (R)-ketamine in clinical studies appears to be safe with high tolerability (no sedation and dissociation), it seems to be ineffective in doses used for (S)-ketamine in the treatment of depression. Our study supports the notion that (R)-ketamine, with a lack of sleep-related effects, might show a better side-effect profile, but it might also indicate the lack of its therapeutic effects. In fact in our studies, sleep-EEG and behavioral effects were parallel, but the effects were clearly different for (R)- and (S)-ketamine.
Supplementary Material
Acknowledgments
We thank Ágnes Ruzsits for the excellent technical support in the EEG and behavioral experiments, and Dóra Török, Donát György Pál, Janka Horváth, and Györgyi Divikiné Gúth for the technical support in the behavioral experiments.
Contributor Information
Szabolcs Koncz, Department of Pharmacodynamics, Faculty of Pharmacy, Semmelweis University, Budapest, Hungary.
Noémi Papp, Department of Pharmacodynamics, Faculty of Pharmacy, Semmelweis University, Budapest, Hungary.
Dóra Pothorszki, Department of Pharmacodynamics, Faculty of Pharmacy, Semmelweis University, Budapest, Hungary.
György Bagdy, Department of Pharmacodynamics, Faculty of Pharmacy, Semmelweis University, Budapest, Hungary; NAP3.0-SE Neuropsychopharmacology Research Group, Hungarian Brain Research Program, Semmelweis University, Budapest, Hungary.
This work was supported by the Hungarian Academy of Sciences (MTA-SE Neuropsychopharmacology and Neurochemistry Research Group); the Hungarian Brain Research Program (grant: 2017-1.2.1-NKP-2017-00002); and the Hungarian Brain Research Program 3.0 (NAP2022-I-4/2022). The research was partially financed by the Thematic Excellence Programme (2020-4.1.1.-TKP2020, TKP2021-EGA-25) of the Ministry for Innovation and Technology in Hungary, within the framework of the Neurology and Translational Biotechnology thematic programs of the Semmelweis University and the National Research, Development and Innovation Fund. The contribution of Szabolcs Koncz was supported by the ÚNKP-22-4-I-SE-26 New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund; and by the Development of Scientific Workshops of Medical, Health Sciences and Pharmaceutical Educations (EFOP-3.6.3-VEKOP-16-2017-00009). The publication was prepared also with the support of the Richter Gedeon Talentum Foundation established by Richter Gedeon Plc. in concordance with the framework of the Richter Gedeon PhD Scholarship received by Dóra Pothorszki.
Interest Statement
G.B. was a member of the Board of directors at Gedeon Richter. Other authors declare no conflict of interest.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Author Contributions
G.B., S.K. and N.P. designed the experiments. S.K., N.P. and D.P. performed the experimental procedures. N.P and D.P. performed the scoring. S.K. and D.P performed the behavior analysis. S.K., N.P. and D.P. were involved in the analysis of the data with the supervision of G.B. G.B., S.K., and N.P. interpreted the results. S.K. and G.B. wrote, N.P. and D.P. corrected the manuscript. All authors have read and agreed to the published version of the manuscript.
References
- Abdallah CG, Sanacora G, Duman RS, Krystal JH (2018) The neurobiology of depression, ketamine and rapid-acting antidepressants: is it glutamate inhibition or activation? Pharmacol Ther 190:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ago Y, Tanabe W, Higuchi M, Tsukada S, Tanaka T, Yamaguchi T, Igarashi H, Yokoyama R, Seiriki K, Kasai A, Nakazawa T, Nakagawa S, Hashimoto K, Hashimoto H (2019) (R)-Ketamine induces a greater increase in prefrontal 5-HT release than (S)-ketamine and ketamine metabolites via an AMPA receptor-independent mechanism. Int J Neuropsychopharmacol 22:665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnaou A, Huysmans H, Biermans R, Manyakov NV, Drinkenburg WHIM (2017) Ketamine: differential neurophysiological dynamics in functional networks in the rat brain. Transl Psychiatry 7:e1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampuero E, Luarte A, Santibañez M, Varas-Godoy M, Toledo J, Diaz-Veliz G, Cavada G, Rubio FJ, Wyneken U (2015) Two chronic stress models based on movement restriction in rats respond selectively to antidepressant drugs: aldolase C as a potential biomarker. Int J Neuropsychopharmacol 18:pyv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacqué-cazenave J, Bharatiya R, Barrière G, Delbecque JP, Bouguiyoud N, Di Giovanni G, Cattaert D, De Deurwaerdère P (2020) Serotonin in animal cognition and behavior. Int J Mol Sci 21:1649–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P, Donello JE, Hare B, Duman RS (2020) Rapastinel, an NMDAR positive modulator, produces distinct behavioral, sleep, and EEG profiles compared with ketamine. Behav Brain Res 391:112706. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Mora F (2008) Blockade of NMDA receptors in the prefrontal cortex increases dopamine and acetylcholine release in the nucleus accumbens and motor activity. Psychopharmacol 201:325–338. [DOI] [PubMed] [Google Scholar]
- Dudysová D, Janků K, Šmotek M, Saifutdinova E, Kopřivová J, Bušková J, Mander BA, Brunovský M, Zach P, Korčák J, Andrashko V, Viktorinová M, Tylš F, Bravermanová A, Froese T, Páleníček T, Horáček J (2020) The effects of daytime psilocybin administration on sleep: implications for antidepressant action. Front Pharmacol 11:602590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Jardin KG, Liebenberg N, Müller HK, Elfving B, Sanchez C, Wegener G (2016) Differential interaction with the serotonin system by S-ketamine, vortioxetine, and fluoxetine in a genetic rat model of depression. Psychopharmacol 233:2813–2825. [DOI] [PubMed] [Google Scholar]
- Dumont G, De Visser S, Cohen A, Van Gerven J (2005) Biomarkers for the effects of selective serotonin reuptake inhibitors (SSRIs) in healthy subjects. Br J Clin Pharmacol 59:495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC, Selter J, Brutsche N, Sarasso S, Zarate CA (2013) Baseline delta sleep ratio predicts acute ketamine mood response in major depressive disorder. J Affect Disord 145:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC, Zarate CA (2013) Ketamine, sleep, and depression: current status and new questions. Curr Psychiatry Rep 15:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser SK, Hill SL, Tononi G (2007) Sleep homeostasis and cortical synchronization: I. Modeling the effects of synaptic strength on sleep slow waves. Sleep 30:1617–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I, Campbell IG (1993) Ketamine administration during waking increases delta EEG intensity in rat sleep. Neuropsychopharmacol 9:41–48. [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi J-i, Hashimoto K, Chaki S (2017) Antidepressant potential of (R)-Ketamine in rodent models: comparison with (S)-Ketamine. J Pharmacol Exp Ther 361:9–16. [DOI] [PubMed] [Google Scholar]
- Furey ML, Drevets WC (2006) Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry 63:1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace KP, Horner RL (2015) Evaluating the evidence surrounding pontine cholinergic involvement in REM sleep generation. Front Neurol 6:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe RJ, Novakovic V, Peselow ED (2013) Scopolamine as an antidepressant: a systematic review. Clin Neuropharmacol 36:24–26. [DOI] [PubMed] [Google Scholar]
- Jelen LA, Young AH, Stone JM (2021) Ketamine: a tale of two enantiomers. J Psychopharmacol 35:109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal RD, Friedman ES, Berman SR, Fasiczka AL, Howland RH, Thase ME (2003) Effects of sertraline on sleep architecture in patients with depression. J Clin Psychopharmacol 23:540–548. [DOI] [PubMed] [Google Scholar]
- Johnston JN, Henter ID, Zarate CA Jr (2023) The antidepressant actions of ketamine and its enantiomers. Pharmacol Ther 246:108431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor S, Jakus R, Balogh B, Benko A, Bagdy G (2004) Increased wakefulness, motor activity and decreased theta activity after blockade of the 5-HT 2B receptor by the subtype-selective antagonist SB-215505. Br J Pharmacol 142:1332–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtala S (2021) Ketamine—50 years in use: from anesthesia to rapid antidepressant effects and neurobiological mechanisms. Pharmacol Rep 73:323–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtala S, Theilmann W, Rosenholm M, Penna L, Karabulut G, Uusitalo S, Järventausta K, Yli-Hankala A, Yalcin I, Matsui N, Wigren H-K, Rantamäki T (2019) Cortical excitability and activation of TrkB signaling during rebound slow oscillations are critical for rapid antidepressant responses. Mol Neurobiol 56:4163–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtala S, Alitalo O, Rosenholm M, Rozov S, Rantamäki T (2021) Time is of the essence: coupling sleep-wake and circadian neurobiology to the antidepressant effects of ketamine. Pharmacol Ther 221:107741. [DOI] [PubMed] [Google Scholar]
- Koncz S, Papp N, Menczelesz N, Pothorszki D, Bagdy G (2021) EEG and sleep effects of tramadol suggest potential antidepressant effects with different mechanisms of action. Pharmaceuticals 14:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal GC, et al. (2023) Arketamine as adjunctive therapy for treatment-resistant depression: a placebo-controlled pilot study. J Affect Disord 330:7–15. [DOI] [PubMed] [Google Scholar]
- Liu B, Liu J, Wang M, Zhang Y, Li L (2017) From serotonin to neuroplasticity: evolvement of theories for major depressive disorder. Front Cell Neurosci 11:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Lan X, Wang C, Zhang F, Fu L, Li W, Ye Y, Hu Z, Chao Z, Ning Y, Zhou Y (2022) The efficacy and safety of esketamine in the treatment of major depressive disorder with suicidal ideation: study protocol for a randomized controlled trial. BMC Psychiatry 22:744–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB (2006) A putative flip-flop switch for control of REM sleep. Nature 441:589–594. [DOI] [PubMed] [Google Scholar]
- Mrdalj J, Pallesen S, Milde AM, Jellestad FK, Murison R, Ursin R, Bjorvatn B, Grønli J (2013) Early and later life stress alter brain activity and sleep in rats. PLoS One 8:e69923–e69910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, Schatzberg AF (2016) Major depressive disorder. Nat Rev Dis Primers 2:1–20. [DOI] [PubMed] [Google Scholar]
- Palagini L, Baglioni C, Ciapparelli A, Gemignani A, Riemann D (2013) REM sleep dysregulation in depression: state of the art. Sleep Med Rev 17:377–390. [DOI] [PubMed] [Google Scholar]
- Papp N, Vas S, Bogáthy E, Kátai Z, Kostyalik D, Bagdy G (2018) Acute and chronic escitalopram alter EEG gamma oscillations differently: relevance to therapeutic effects. Eur J Pharm Sci 121:347–355. [DOI] [PubMed] [Google Scholar]
- Papp N, Koncz S, Kostyalik D, Kitka T, Petschner P, Vas S, Bagdy G (2020) Acute 5-HT2C receptor antagonist SB-242084 treatment affects EEG gamma band activity similarly to chronic escitalopram. Front Pharmacol 10:1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafało-Ulińska A, Pałucha-Poniewiera A (2022) The effectiveness of (R)-ketamine and its mechanism of action differ from those of (S)-ketamine in a chronic unpredictable mild stress model of depression in C57BL/6J mice. Behav Brain Res 418:113633. [DOI] [PubMed] [Google Scholar]
- Raith H, Schuelert N, Duveau V, Roucard C, Plano A, Dorner-Ciossek C, Ferger B (2020) Differential effects of traxoprodil and S-ketamine on quantitative EEG and auditory event-related potentials as translational biomarkers in preclinical trials in rats and mice. Neuropharmacol 171:108072. [DOI] [PubMed] [Google Scholar]
- Rantamäki T, Kohtala S (2020) Encoding, consolidation, and renormalization in depression: synaptic homeostasis, plasticity, and sleep integrate rapid antidepressant effects. Pharmacol Rev 72:439–465. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Carlessi AS, Titus SE, Abelaira HM, Ignácio ZM, da Luz JR, Matias BI, Bruchchen L, Florentino D, Vieira A, Petronilho F, Quevedo J (2015) A single dose of S-ketamine induces long-term antidepressant effects and decreases oxidative stress in adulthood rats following maternal deprivation. Dev Neurobiol 75:1268–1281. [DOI] [PubMed] [Google Scholar]
- Riemann D, Krone LB, Wulff K, Nissen C (2020) Sleep, insomnia, and depression. Neuropsychopharmacol 45:74–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid DA, Wichniak A, Uhr M, Ising M, Brunner H, Held K, Weikel JC, Sonntag A, Steiger A (2006) Changes of sleep architecture, spectral composition of sleep EEG, the nocturnal secretion of cortisol, ACTH, GH, prolactin, melatonin, ghrelin, and leptin, and the DEX-CRH test in depressed patients during treatment with mirtazapine. Neuropsychopharmacol 31:832–844. [DOI] [PubMed] [Google Scholar]
- Scotton E, Antqueviezc B, Vasconcelos MF, Dalpiaz G, Paul Géa L, Ferraz Goularte J, Colombo R, Ribeiro Rosa A (2022) Is (R)-ketamine a potential therapeutic agent for treatment-resistant depression with less detrimental side effects? A review of molecular mechanisms underlying ketamine and its enantiomers. Biochem Pharmacol 198:114963. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Cryan JF (2012) Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc 7:1009–1014. [DOI] [PubMed] [Google Scholar]
- Steiger A, Kimura M (2010) Wake and sleep EEG provide biomarkers in depression. J Psychiatr Res 44:242–252. [DOI] [PubMed] [Google Scholar]
- Steiger A, Pawlowski M (2019) Depression and sleep. Int J Mol Sci 20:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin R (2002) Serotonin and sleep. Sleep Med Rev 6:55–69. [DOI] [PubMed] [Google Scholar]
- Vas S, Kátai Z, Kostyalik D, Pap D, Molnár E, Petschner P, Kalmár L, Bagdy G (2013) Differential adaptation of REM sleep latency, intermediate stage and theta power effects of escitalopram after chronic treatment. J Neural Transm 120:169–176. [DOI] [PubMed] [Google Scholar]
- Wang X, Yang J, Hashimoto K (2022) (R)-ketamine as prophylactic and therapeutic drug for neurological disorders: Beyond depression. Neurosci Biobehav Rev 139:104762. [DOI] [PubMed] [Google Scholar]
- Wang Y, Melgers M, Meijer JH, Deboer T (2023) Comparison of sleep deprivation and a low dose of ketamine on sleep and the electroencephalogram in Brown Norway rats. J Sleep Res e13863. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Wei Y, Chang L, Hashimoto K (2020) A historical review of antidepressant effects of ketamine and its enantiomers. Pharmacol Biochem Behav 190:172870. [DOI] [PubMed] [Google Scholar]
- Wichniak A, Wierzbicka A, Walęcka M, Jernajczyk W (2017) Effects of antidepressants on sleep. Curr Psychiatry Rep 19:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S, Argyropoulos S (2005) Antidepressants and sleep: a qualitative review of the literature. Drugs 65:927–947. [DOI] [PubMed] [Google Scholar]
- Yamada RG, Ueda HR (2020) Molecular mechanisms of REM sleep. Front Neurosci 13:1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K (2015) R-ketamine: A rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, et al. (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Gould T (2018) Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 23:801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA, Gould TD (2018) Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev 70:621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


