Abstract
Background
Preclinical data suggest synergistic activity with the combination of programmed death-1 and cyclin-dependent kinase 4/6 blockade in oestrogen receptor-positive/human epidermal growth factor 2-negative (ER+/HER2–) breast cancer. The noncomparative phase 1b/2 CheckMate 7A8 study (NCT04075604) evaluated neoadjuvant treatment with nivolumab, palbociclib, and anastrozole in patients with ER+/HER2− breast cancer. Here, we report outcomes from the safety run-in phase.
Methods
Patients with histologically confirmed, untreated ER+/HER2− breast cancer, primary tumour ≥2 cm, ECOG performance status ≤1, and eligible for post-treatment surgery received nivolumab 480 mg intravenously every 4 weeks, palbociclib 125 mg or 100 mg orally once daily for 3 weeks per cycle, and anastrozole 1 mg orally once daily for five 4-week cycles, or until disease progression. The primary endpoint was the proportion of patients with dose-limiting toxicities (DLTs) within 4 weeks of treatment initiation.
Results
At safety data review, 21 patients were treated (palbociclib 125-mg group: n = 9; palbociclib 100-mg group: n = 12). DLTs were reported in 2 (22.2%) and 0 patients in the palbociclib 125-mg and 100-mg groups, respectively. Across both groups, 9 patients discontinued treatment due to toxicity (grade 3/4 hepatic adverse events [n = 6], grade 3 febrile neutropaenia [n = 1], grade 1 pneumonitis [n = 1], and grade 3 rash and grade 2 immune-mediated pneumonitis [n = 1]). Consequently, the study was closed early.
Conclusions
Neoadjuvant treatment with nivolumab, palbociclib, and anastrozole showed a high incidence of grade 3/4 hepatotoxicity and treatment discontinuation, indicating that this combination should not be further pursued for treatment of primary ER+/HER2− breast cancer.
Keywords: Anastrozole, Aromatase inhibitors, Breast neoplasms, Cyclin-dependent kinases, Immune checkpoint inhibitors, Neoadjuvant therapy, Nivolumab, Patient safety, Programmed cell death 1 receptor
Highlights
-
•
CheckMate 7A8 assessed neoadjuvant nivolumab plus palbociclib plus anastrozole (80/85 characters)
-
•
Patients had ER+/HER2− breast cancer with primary tumour ≥2 cm (64/85 characters)
-
•
Of the 21 patients in the safety run-in phase, 9 discontinued due to toxicity (79/85 characters)
-
•
The study was closed early due to grade 3/4 hepatotoxicity (60/85 characters)
-
•
Nivolumab combined with CDK4/6 inhibitors should not be pursued in this setting (79/85 characters)
1. Introduction
Approximately 60%–70% of patients with breast cancer have oestrogen receptor-positive (ER+) and human epidermal growth factor 2-negative (HER2−) tumours [1,2]. Historically, endocrine therapy, either alone or after adjuvant chemotherapy (depending on high-risk clinicopathological factors [eg, large tumour size, high histological grade, and greater extent of lymph node involvement]), has been the treatment option for patients with early-stage disease [[3], [4], [5]]. Following adjuvant treatment, a high risk of recurrence remains, with a 5-year recurrence rate of 17% reported in patients with lymph node involvement [6].
Based on recent data from randomised phase 3 trials [[7], [8], [9], [10], [11], [12], [13], [14]], the cyclin-dependent kinase (CDK) 4/6 inhibitors palbociclib, ribociclib, and abemaciclib have been approved for the treatment of patients with metastatic ER+/HER2− breast cancer; abemaciclib is also approved in the adjuvant setting for patients with high-risk, node-positive disease [[15], [16], [17]]. Additionally, recent data suggest that neoadjuvant treatment with the combination of CDK4/6 inhibitors and endocrine therapy improved efficacy compared with endocrine therapy alone [18,19].
Although immune checkpoint inhibitor-based therapy has been approved across multiple settings in various tumour types, immune checkpoint inhibitor monotherapy has shown only modest activity in metastatic ER+/HER2− breast cancer [20]. Preclinical data suggest synergistic activity of CDK4/6 blockade and immune checkpoint inhibition (eg, programmed death-1 [PD-1]) in ER+/HER2− breast cancer through multiple mechanisms, including upregulation of programmed cell death ligand 1 (PD-L1) and enhanced antitumour T-cell activity [[21], [22], [23], [24]]. In a phase 1b study, the combination of PD-1 inhibitor pembrolizumab and abemaciclib demonstrated antitumour activity in heavily pretreated patients with metastatic hormone receptor (HR+)/HER2− breast cancer [25].
Nivolumab is a fully human PD-1 inhibitor that has been approved as monotherapy or combination therapy for the treatment of various cancers [26]. The phase 1b/2 CheckMate 7A8 trial (NCT04075604) was designed to assess the safety and synergistic effect of nivolumab plus either abemaciclib (cohort 1) or palbociclib (cohort 2) plus anastrozole as neoadjuvant therapy in patients with ER+/HER2− breast cancer with primary tumour ≥2 cm. Enrolment into cohort 1 was stopped shortly after study initiation based on concerns of an increased risk of interstitial lung disease (ILD)/pneumonitis in trials assessing a PD-1 inhibitor plus abemaciclib in patients with metastatic ER+/HER2− breast cancer [25,27]. Based on results from the safety run-in phase, a decision to terminate the study was made by the sponsor (Bristol Myers Squibb) in collaboration with the study steering committee. Following this, enrolment into cohort 2 was stopped and the study did not proceed to the randomised phase. Here, we report outcomes in cohort 2 from the safety run-in phase.
2. Methods
2.1. Study design and patients
CheckMate 7A8 was a noncomparative, multicentre, phase 1b/2 study designed to assess the safety and efficacy of neoadjuvant treatment with nivolumab plus abemaciclib or palbociclib plus anastrozole in patients with ER+/HER2− breast cancer in a safety run-in phase, followed by a randomised phase. Eligible patients were men and postmenopausal women aged ≥18 years with newly diagnosed, histologically confirmed ER+/HER2− unilateral invasive breast carcinoma, primary tumour ≥2 cm in largest diameter (by ultrasound or mammogram; clinical stage T1c-3), Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and suitable to receive neoadjuvant endocrine treatment and standard-of-care breast surgery following completion of neoadjuvant treatment. Patients with a history of ipsilateral invasive breast cancer regardless of treatment, or breast cancer that was inflammatory, inoperable, multicentric, or bilaterally invasive were not included. Additionally, patients with autoimmune diseases, human immunodeficiency virus infection, or prior malignancies active within 3 years before enrolment, except for local cancers that have been apparently cured, were excluded.
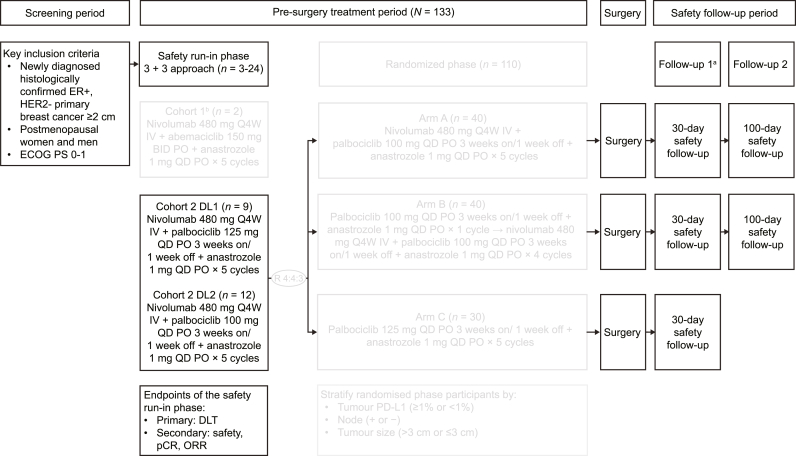
The safety run-in phase used a standard 3 + 3 approach to evaluate the tolerability of nivolumab plus abemaciclib or palbociclib and anastrozole as neoadjuvant therapy during a 4-week dose-limiting toxicity (DLT) period and determine the respective dose levels for the subsequent randomised phase. On completion or discontinuation of neoadjuvant treatment, patients in the safety run-in phase proceeded to safety follow-up. The first follow-up visit occurred 30 days (±7 days) after the last dose of a study drug; the second follow-up occurred 100 days (±7 days) after the last dose of nivolumab (Fig. 1).
Fig. 1.
Study design for CheckMate7A8. aFollow-up 1 begins at the end of study treatment. bCohort 1 was closed following enrolment of 2 patients, who received 1 and 2 doses of nivolumab, 41 and 69 doses of abemaciclib, and 56 and 138 doses of anastrozole. BID, twice a day; DL, dose level; DLT, dose-limiting toxicity; ECOG PS, Eastern Cooperative Oncology Group performance status; ER, oestrogen receptor; HER2, human epidermal growth factor receptor 2; IV, intravenous; ORR, objective response rate; pCR, pathological complete response; PD-L1, programmed death ligand 1; PO, orally; Q4W, every 4 weeks; QD, once daily.
Cohort 1 of the safety run-in phase assessed treatment with nivolumab (480 mg every 4 weeks [Q4W] intravenously) plus abemaciclib (150 mg twice daily orally) plus anastrozole (1 mg once daily [QD] orally). Following the enrolment of 2 patients, this cohort was permanently closed due to concerns of an increased risk of ILD/pneumonitis from trials assessing a PD-1 inhibitor plus abemaciclib in patients with metastatic ER+/HER2− breast cancer [25,27].
In cohort 2 of the safety run-in phase, patients received nivolumab (480 mg Q4W intravenously) plus palbociclib (125 or 100 mg QD orally; 3 weeks on and 1 week off) plus anastrozole (1 mg QD orally) for five 4-week cycles or until disease progression. After cycle 5 or end of treatment, patients continued to receive anastrozole until subsequent standard-of-care surgery for breast cancer within 4 weeks of the last neoadjuvant treatment administration.
After review of the safety data from the run-in phase, enrolment into cohort 2 was stopped and did not proceed to the planned randomised phase using the dose level tested; enrolled patients still underwent planned surgery and were followed up for safety. The methodology for the planned randomised phase is not detailed here.
CheckMate 7A8 was conducted in accordance with ethical principles from the Declaration of Helsinki, Council for International Organisations of Medical Sciences, International Council on Harmonisation Good Clinical Practice guidelines, and other applicable local requirements. All patients provided written informed consent before treatment initiation.
2.2. Endpoints and assessments
The primary endpoint of the safety run-in phase was the proportion of patients with DLTs, defined as any adverse events (AEs) meeting specific criteria and occurring during the first 4 weeks after start of treatment (ie, during cycle 1 of treatment) except for those that were due to disease progression or extraneous causes (DLT criteria in Supplementary Table 1). Beyond the first 4-week window, treatment-related adverse events (TRAEs) that met DLT criteria led to discontinuation of study treatment.
Secondary endpoints included overall safety and tolerability; rate of pathological complete response (pCR), defined as the absence of invasive residual disease in the breast or lymph nodes (ie, ypT0/Tis ypN0) in the American Joint Committee on Cancer staging system (8th edition); investigator-assessed objective response rate (ORR), defined as the proportion of patients with complete response (CR) or partial response (PR) using Response Evaluation Criteria in Solid Tumours (RECIST) v1.1; and breast-conserving surgery (BCS) rate, defined as the proportion of patients undergoing BCS after completion of study treatments. Efficacy analyses in the safety run-in phase were descriptive.
Additional descriptive analyses for the safety run-in phase included residual cancer burden (RCB) 0-I rate by central assessment, Ki67 levels, and Preoperative Endocrine Prognostic Index (PEPI) score for breast cancer-specific survival. RCB was assessed by independent central review from routine pathologic sections of the primary breast tumour site and the regional lymph nodes after surgery. Ki67 levels in tissue sections were assessed at baseline, intermediate biopsy (cycle 2 day 22), and time of surgery. PEPI score was determined at the time of surgery. Both Ki67 expression levels and PEPI scores were assessed by the central pathologist.
AEs were graded per National Cancer Institute Common Terminology Criteria for Adverse Events v5.0 using Medical Dictionary for Regulatory Activities v24.1 and reported within 30 days of last dose. Immune-mediated AEs (IMAEs) were defined as specific events occurring within 100 days of last dose, regardless of causality, treated with immune-modulating medication. Immune-mediated endocrine events (adrenal insufficiency, hypothyroidism/thyroiditis, hyperthyroidism, diabetes mellitus, and hypophysitis) were included regardless of whether treatment with immuno-modulating medication was administered.
Analyses of the primary and secondary endpoints of the safety run-in phase were based on the database lock date of February 3, 2022. Data for RCB, Ki67 levels, and PEPI scores were based on the database lock date of August 11, 2022.
2.3. Statistical considerations
Enrolment of 115–136 patients was planned for both phases, with 3–24 patients in each safety run-in combination cohort. In the safety run-in phase, the 3 + 3 design was used to determine the maximum tolerated dose, including approximately 3–6 DLT-evaluable patients per dose level, although the actual number of patients to be evaluated was dependent on the number of observed DLTs. Up to 12 DLT-evaluable patients were planned to be treated at the selected dose level (ie, the dose to be used in the randomised phase).
All safety and efficacy analyses in the safety run-in phase were conducted on patients receiving ≥1 dose of study drug (all treated). Additional analyses were based on all treated patients with evaluable data. Safety and tolerability were based on incidence of any-cause and TRAEs, serious AEs, AEs leading to discontinuation, IMAEs, death, and laboratory abnormalities. For pCR, ORR, BCS, and RCB, estimates of rates and the corresponding exact 2-sided 95% confidence intervals (CIs) were constructed using the Clopper-Pearson method. Ki67 level and PEPI score data were summarised as median values and ranges.
3. Results
3.1. Patients
Between October 18, 2019 and December 17, 2020, 9 and 12 patients were enrolled in the palbociclib 125-mg and 100-mg groups of cohort 2, respectively. Baseline characteristics are shown in Table 1. Across both dose levels, relative dose intensity was ≥90% for nivolumab and anastrozole and ≥70% for palbociclib (Table 2).
Table 1.
Demographic and baseline characteristics of all treated patients.
| Characteristic | NIVO 480 mg Q4W + Palbo 125 mg QD + ANZ 1 mg QD (n = 9) |
NIVO 480 mg Q4W + Palbo 100 mg QD + ANZ 1 mg QD (n = 12) |
|---|---|---|
| Age, median (range), years | 61.0 (45–79) | 70.0 (47–80) |
| Race, n (%) | ||
| White | 9 (100.0) | 11 (91.7) |
| Black | 0 | 1 (8.3) |
| Ethnicity, n (%) | ||
| Hispanic/Latino | 2 (22.2) | 2 (16.7) |
| Not Hispanic/Latino | 5 (55.6) | 7 (58.3) |
| Other | 2 (22.2) | 3 (25.0) |
| ECOG performance status, n (%) | ||
| 0 | 9 (100.0) | 10 (83.3) |
| 1 | 0 | 2 (16.7) |
| Tumour size, n (%) | ||
| 2–3 cm | 5 (55.6) | 7 (58.3) |
| >3 cm | 4 (44.4) | 5 (41.7) |
| Nodal status,an (%) | ||
| cN0 | 5 (55.6) | 4 (33.3) |
| cN1 | 2 (22.2) | 7 (58.3) |
| pN1 | 1 (11.1) | 0 |
| pN1mi | 0 | 1 (8.3) |
| Not reported | 1 (11.1) | 0 |
| Tumour grade, n (%) | ||
| G1 | 3 (33.3) | 1 (8.3) |
| G2 | 3 (33.3) | 9 (75.0) |
| G3 | 3 (33.3) | 1 (8.3) |
| Not reported | 0 | 1 (8.3) |
| PD-L1 expression on tumour-infiltrating cells,bn (%) | ||
| <1% | 5 (55.6) | 9 (75.0) |
| ≥1% | 2 (22.2) | 2 (16.7) |
| Not evaluable | 1 (11.1) | 1 (8.3) |
| Not reported | 1 (11.1) | 0 |
ANZ, anastrozole; ECOG, Eastern Cooperative Oncology Group; NIVO, nivolumab; Palbo, palbociclib; PD-L1, programmed death ligand 1; Q4W, every 4 weeks; QD, once daily.
cN0, no regional lymph node metastases; cN1, metastases to movable ipsilateral level I or II axillary lymph nodes; pN1, micrometastases; pN1mi, micrometastases (200 cells, >0.2 mm but none >2.0 mm). Reported as collected in the electronic case report forms. In CheckMate 7A8, axillary lymph node biopsy could be omitted at screening if there was no suspicion for positive axillary lymph nodes radiographically. Pathological nodal status was available for 2 patients. For the rest of the patients, nodal status was assessed clinically or was not reported.
Per Ventana PD-L1 SP142 assay.
Table 2.
Dose exposure summary in all treated patients.
| NIVO 480 mg Q4W + Palbo 125 mg QD + ANZ 1 mg QD (n = 9) |
NIVO 480 mg Q4W + Palbo 100 mg QD + ANZ 1 mg QD (n = 12) |
|||||
|---|---|---|---|---|---|---|
| NIVO | Palbo | ANZ | NIVO | Palbo | ANZ | |
| Expected doses per protocol, n | 5 | 105 | 140 | 5 | 105 | 140 |
| Median number of doses received, n (range) | 2.0 (1–5) | 42.0 (14–105) | 56.0 (28–141) | 4.5 (2–5) | 83.5 (52–105) | 129.0 (84–141) |
| Median average daily doses received, mg/day (range) | – | 83.93 (70.4–93.8) | 1.00 (1.0–1.0) | – | 71.43 (49.4–75.0) | 1.00 (1.0–1.0) |
|
Relative dose intensity,a n (%) | ||||||
| 90% to <110% | 7 (77.8) | 4 (44.4) | 9 (100.0) | 12 (100.0) | 7 (58.3) | 12 (100.0) |
| 70% to <90% | 2 (22.2) | 5 (55.6) | 0 | 0 | 4 (33.3) | 0 |
| 50% to <70% | 0 | 0 | 0 | 0 | 1 (8.3) | 0 |
ANZ, anastrozole; NIVO, nivolumab; Palbo, palbociclib; Q4W, every 4 weeks; QD, once daily.
Defined as (actual average daily dose)/(planned average daily dose) × 100.
Due to an increased risk of ILD/pneumonitis from trials assessing a PD-1 inhibitor plus abemaciclib [25,27], cohort 1 (nivolumab plus abemaciclib plus anastrozole) was closed on March 6, 2020. The 2 patients enrolled received 1 and 2 doses of nivolumab, 41 and 69 doses of abemaciclib, and 56 and 138 doses of anastrozole, respectively.
3.2. Safety
In cohort 2, DLTs were reported in 2 of 9 (22%) patients in the palbociclib 125-mg group: hepatitis (elevated transaminases) and febrile neutropaenia (1 patient each). No DLTs were reported in the palbociclib 100-mg group.
Any-cause grade 3/4 AEs were reported in 88.9% of patients in the palbociclib 125-mg group and 75.0% of patients in the palbociclib 100-mg group (Table 3). Across both groups, 9 patients discontinued treatment due to toxicity. In the palbociclib 125-mg group, 5 patients discontinued treatment due to toxicity (asymptomatic grade 3/4 hepatic AEs [n = 3], grade 3 rash and grade 2 immune-mediated pneumonitis [n = 1], grade 3 febrile neutropaenia [n = 1]). In the palbociclib 100-mg group, 4 patients discontinued treatment due to toxicity (asymptomatic grade 3/4 hepatic AEs [n = 3], grade 1 pneumonitis [n = 1]). After the 4-week DLT window, there were several grade 3/4 TRAEs, most commonly hepatoxicity, which led to treatment discontinuation in 4 (44.4%) patients in the palbociclib 125-mg group, and 3 (25.0%) patients in the palbociclib 100-mg group (Table 4). All grade 3/4 IMAEs leading to discontinuation were hepatic events and were reported in both the palbociclib 125-mg and 100-mg groups (Table 5). Of note, grade 3/4 hepatic IMAEs were reversible, with median time to resolution ranging from 7 to 9 weeks. No treatment-related deaths or grade 5 AEs were reported. This cohort was closed on May 6, 2021 after a safety data review.
Table 3.
Summary of any-cause AEs in all treated patients.
| Patients with an event, n (%)a | NIVO 480 mg Q4W + Palbo 125 mg QD + ANZ 1 mg QD (n = 9) |
NIVO 480 mg Q4W + Palbo 100 mg QD + ANZ 1 mg QD (n = 12) |
||
|---|---|---|---|---|
| Any grade | Grade 3/4 | Any grade | Grade 3/4 | |
| Any-cause AEs | 9 (100) | 8 (88.9) | 12 (100) | 9 (75.0) |
| Any-cause AEs (with incidence of grade 3/4 events) in either group | ||||
| Increased ALT | 4 (44.4) | 3 (33.3) | 2 (16.7) | 1 (8.3) |
| Increased AST | 4 (44.4) | 3 (33.3) | 2 (16.7) | 1 (8.3) |
| Neutropaenia | 3 (33.3) | 2 (22.2) | 3 (25.0) | 2 (16.7) |
| Decreased white blood cell count | 2 (22.2) | 2 (22.2) | 3 (25.0) | 1 (8.3) |
| Anaemia | 2 (22.2) | 1 (11.1) | 1 (8.3) | 0 |
| Decreased neutrophil count | 1 (11.1) | 1 (11.1) | 5 (41.7) | 5 (41.7) |
| Rash | 1 (11.1) | 1 (11.1) | 1 (8.3) | 0 |
| Febrile neutropaenia | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Hepatitis | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Thrombocytopaenia | 1 (11.1) | 0 | 1 (8.3) | 1 (8.3) |
| Increased transaminases | 1 (11.1) | 0 | 1 (8.3) | 1 (8.3) |
| Leucopenia | 0 | 0 | 2 (16.7) | 1 (8.3) |
| Decreased appetite | 0 | 0 | 1 (8.3) | 1 (8.3) |
| Hypertransaminasaemia | 0 | 0 | 1 (8.3) | 1 (8.3) |
| Secondary primary malignancy |
0 |
0 |
1 (8.3) |
1 (8.3) |
|
Any-cause AEs leading to treatment discontinuation |
5 (55.6) |
4 (44.4) |
4 (33.3) |
3 (25.0) |
|
Any-cause AEs (with incidence of grade 3/4 events) in either group leading to treatment discontinuation | ||||
| Increased ALT | 3 (33.3) | 3 (33.3) | 1 (8.3) | 1 (8.3) |
| Increased AST | 3 (33.3) | 3 (33.3) | 0 | 0 |
| Febrile neutropaenia | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Hepatitis | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Hypertransaminasaemia | 0 | 0 | 1 (8.3) | 1 (8.3) |
| Increased transaminases |
0 |
0 |
1 (8.3) |
1 (8.3) |
| Any-cause SAEs (with incidence of grade 3/4 events) in either group | ||||
| Anaemia | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Increased ALT | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Increased AST | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Febrile neutropaenia | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Hepatitis | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Rash | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Hypertransaminasaemia | 0 | 0 | 1 (8.3) | 1 (8.3) |
| Secondary primary malignancy | 0 | 0 | 1 (8.3) | 1 (8.3) |
AE, adverse event; ALT, alanine aminotransferase; ANZ, anastrozole; AST, aspartate aminotransferase; NIVO, nivolumab; Palbo, palbociclib; Q4W, every 4 weeks; QD, once daily; SAE, serious adverse event.
Includes events reported between first dose and 30 days after last dose of study treatment.
Table 4.
Summary of TRAEs in all treated patients.
| Patients with an event, n (%)a | NIVO 480 mg Q4W + Palbo 125 mg QD + ANZ 1 mg QD (n = 9) |
NIVO 480 mg Q4W + Palbo100 mg QD + ANZ 1 mg QD (n = 12) |
||
|---|---|---|---|---|
| Any grade | Grade 3/4 | Any grade | Grade 3/4 | |
| TRAEs | 9 (100.0) | 7 (77.8) | 12 (100.0) | 9 (75.0) |
| TRAEs (with incidence of grade 3/4 events) in either group | ||||
| Increased AST | 4 (44.4) | 3 (33.3) | 2 (16.7) | 1 (8.3) |
| Increased ALT | 4 (44.4) | 3 (33.3) | 1 (8.3) | 1 (8.3) |
| Neutropaenia | 2 (22.2) | 2 (22.2) | 3 (25.0) | 2 (16.7) |
| Decreased white blood cell count | 2 (22.2) | 2 (22.2) | 3 (25.0) | 1 (8.3) |
| Decreased neutrophil count | 1 (11.1) | 1 (11.1) | 5 (41.7) | 5 (41.7) |
| Febrile neutropaenia | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Hepatitis | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Rash | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Increased transaminases | 1 (11.1) | 0 | 1 (8.3) | 1 (8.3) |
| Leucopenia | 0 | 0 | 2 (16.7) | 1 (8.3) |
| Decreased appetite | 0 | 0 | 1 (8.3) | 1 (8.3) |
| Hypertransaminasaemia |
0 |
0 |
1 (8.3) |
1 (8.3) |
|
TRAEs leading to treatment discontinuation |
5 (55.6) |
4 (44.4) |
4 (33.3) |
3 (25.0) |
| TRAEs (with incidence of grade 3/4 events) in either group leading to treatment discontinuation | ||||
| Increased ALT | 3 (33.3) | 3 (33.3) | 1 (8.3) | 1 (8.3) |
| Increased AST | 3 (33.3) | 3 (33.3) | 0 | 0 |
| Febrile neutropaenia | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Hepatitis | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Hypertransaminasaemia | 0 | 0 | 1 (8.3) | 1 (8.3) |
| Increased transaminases |
0 |
0 |
1 (8.3) |
1 (8.3) |
| Treatment-related SAEs (with incidence of grade 3/4 events) in either group | ||||
| Febrile neutropaenia | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Hepatitis | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Increased ALT | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Increased AST | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Rash | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Hypertransaminasaemia | 0 | 0 | 1 (8.3) | 1 (8.3) |
ALT, alanine aminotransferase; ANZ, anastrozole; AST, aspartate aminotransferase; NIVO, nivolumab; Palbo, palbociclib; Q4W, every 4 weeks; QD, once daily; SAE, serious adverse event; TRAE, treatment-related adverse event.
Includes events reported between first dose and 30 days after last dose of study treatment.
Table 5.
Summary of IMAEs in all treated patients.
| Patients with an event, n (%)a | NIVO 480 mg Q4W + Palbo 125 mg QD + ANZ 1 mg QD (n = 9) |
NIVO 480 mg Q4W + Palbo 100 mg QD + ANZ 1 mg QD (n = 12) |
||
|---|---|---|---|---|
| Any grade | Grade 3/4 | Any grade | Grade 3/4 | |
| IMAEs in either group | ||||
| Endocrine | ||||
| Hyperthyroidism | 0 | 0 | 1 (8.3) | 0 |
| Non-endocrine | ||||
| Increased ALT | 3 (33.3) | 3 (33.3) | 1 (8.3) | 1 (8.3) |
| Increased AST | 3 (33.3) | 3 (33.3) | 1 (8.3) | 1 (8.3) |
| Hepatitis | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Rash | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Increased blood bilirubin | 1 (11.1) | 0 | 0 | 0 |
| Immune-mediated lung disease | 1 (11.1) | 0 | 0 | 0 |
| Rash maculopapular | 1 (11.1) | 0 | 0 | 0 |
| Increased transaminases | 0 | 0 | 1 (8.3) | 1 (8.3) |
| Pneumonitis |
0 |
0 |
1 (8.3) |
0 |
| IMAEs in either group leading to treatment discontinuation | ||||
| Non-endocrine | ||||
| Increased ALT | 3 (33.3) | 3 (33.3) | 1 (8.3) | 1 (8.3) |
| Increased AST | 3 (33.3) | 3 (33.3) | 0 | 0 |
| Hepatitis | 1 (11.1) | 1 (11.1) | 0 | 0 |
| Immune-mediated lung disease | 1 (11.1) | 0 | 0 | 0 |
| Increased transaminases | 0 | 0 | 1 (8.3) | 1 (8.3) |
| Pneumonitis | 0 | 0 | 1 (8.3) | 0 |
ALT, alanine aminotransferase; ANZ, anastrozole; AST, aspartate aminotransferase; IMAE, immune-mediated adverse event; NIVO, nivolumab; Palbo, palbociclib; Q4W, every 4 weeks; QD, once daily.
IMAEs were defined as specific events occurring within 100 days of last dose, regardless of causality, treated with immune-modulating medication, and includes endocrine events (adrenal insufficiency, hypothyroidism/thyroiditis, hyperthyroidism, diabetes mellitus, and hypophysitis), regardless of treatment since these events are often managed without immunosuppression.
In the discontinued cohort 1, 1 of the 2 treated patients experienced treatment-related grade 3/4 hypokalaemia; neither immune-mediated lung disease nor hepatitis were observed.
3.3. Efficacy
pCR was reported in 0 of 9 patients in the palbociclib 125-mg group and 1 of 12 (8.3%) patients in the palbociclib 100-mg group; ORR was 66.7% (6 PRs) and 75.0% (1 CR; 8 PRs), respectively. The RCB 0-I rate was 0% in the palbociclib 125-mg group and 8.3% (1 patient) in the 100-mg group. Of note, RCB was assessable in 2 of 9 patients in the palbociclib 125-mg group and 8 of 12 patients in the palbociclib 100-mg group. RCB II was 22.2% (2 patients) and 50.0% (6 patients), and RCB III was 0% and 8.3% (1 patient), respectively (Table 6). The BCS rates of both groups are shown in Table 7.
Table 6.
Summary of efficacy in all treated patients.
| Endpoint | NIVO 480 mg Q4W + Palbo 125 mg QD + ANZ 1 mg QD (n = 9) |
NIVO 480 mg Q4W + Palbo 100 mg QD + ANZ 1 mg QD (n = 12) | Total (n = 21) |
|---|---|---|---|
| pCR rate,a,bn (%) | 0 | 1 (8.3) | 1 (4.8) |
| (95% CI) |
(0–33.6) |
(0.2–38.5) |
(0.1–23.8) |
| BOR, n (%) | |||
| Complete response | 0 | 1 (8.3) | 1 (4.8) |
| Partial response | 6 (66.7) | 8 (66.7) | 14 (66.7) |
| Stable disease | 1 (11.1) | 3 (25.0) | 4 (19.0) |
| Progressive disease | 1 (11.1) | 0 | 1 (4.8) |
| Not evaluable | 1 (11.1) | 0 | 1 (4.8) |
| ORR,a,cn (%) | 6 (66.7) | 9 (75.0) | 15 (71.4) |
| (95% CI) |
(29.9–92.5) |
(42.8–94.5) |
(47.8–88.7) |
| RCBd | |||
| 0 | 0 | 1 (8.3) | 1 (4.8) |
| I | 0 | 0 | 0 |
| II | 2 (22.2) | 6 (50.0) | 8 (38.1) |
| III | 0 | 1 (8.3) | 1 (4.8) |
| Not assessed for RCB | 7 (77.8) | 4 (33.3) | 11 (52.4) |
| RCB 0-I rate | 0 (0) | 1 (8.3) | 1 (4.8) |
| (95% CI) | (0–33.6) | (0.2–38.5) | (0.1–23.8) |
ANZ, anastrozole; BOR, best overall response; CI, confidence interval; NIVO, nivolumab; ORR, objective response rate; Palbo, palbociclib; pCR, pathological complete response; Q4W, every 4 weeks; QD, once daily; RCB, residual cancer burden; RECIST, Response Evaluation Criteria in Solid Tumours.
Based on database lock February 3, 2022.
pCR was not assessed in 2 patients receiving NIVO 480 mg Q4W + Palbo 125 mg QD + ANZ 1 mg QD and 1 patient receiving NIVO 480 mg Q4W + Palbo 100 mg QD + ANZ 1 mg QD as these patients had withdrawn from the study due to toxicity before proceeding to surgery.
By investigator using RECIST v1.1.
Based on database lock August 11, 2022.
Table 7.
Surgical outcomes in all treated patients.
| Event, n (%) | NIVO 480 mg Q4W + Palbo 125 mg QD + ANZ 1 mg QD (n = 9) |
NIVO 480 mg Q4W + Palbo 100 mg QD + ANZ 1 mg QD (n = 12) |
|---|---|---|
| Planned surgery | ||
| BCS | 7 (77.8) | 6 (50.0) |
| Mastectomy | 2 (22.2) | 6 (50.0) |
| Actual surgery | ||
| BCS | 5 (55.6) | 6 (50.0) |
| Mastectomy | 2 (22.2) | 5 (41.7) |
| BCS rate | 5/9 (55.6) | 6/12 (50.0) |
| (95% CI) | (21.2–86.3) | (21.1–78.9) |
ANZ, anastrozole; BCS, breast-conserving surgery; CI, confidence interval; NIVO, nivolumab; Palbo, palbociclib; Q4W, every 4 weeks; QD, once daily.
3.4. Prognostic biomarker endpoints
At time of intermediate biopsy, median (range) Ki67 levels were 0.5% (0–5) in the palbociclib 125-mg group (n = 6) and 0.0% (0–10) in the 100-mg group (n = 9). At time of surgery, median (range) Ki67 levels were 5.0% (1–25) in the palbociclib 125-mg group (n = 4) and 1.0% (1–70) in the 100-mg group (n = 9; Supplementary Table 2). At time of surgery, median PEPI scores (range) were 2.5 (1–4) and 3.0 (0–6), respectively (Supplementary Table 3).
4. Discussion
Cohort 2 of the CheckMate 7A8 study was designed to evaluate nivolumab plus palbociclib plus anastrozole as neoadjuvant treatment for patients with ER+/HER2− primary breast cancer. DLTs were reported in 2 of 9 patients receiving the 125-mg palbociclib dose (hepatitis and febrile neutropaenia). While no DLTs were reported in patients receiving the 100-mg palbociclib dose, the safety profiles of the 125-mg and 100-mg palbociclib doses did not appear to be substantially different. After the 4-week DLT window, 9 of the 21 patients across both dose levels in cohort 2 discontinued treatment due to adverse events, the majority of which were asymptomatic and reversible during study follow-up. Additionally, hepatic AEs constituted the majority of grade 3/4 TRAEs and IMAEs. Previously published studies of nivolumab monotherapy and palbociclib combined with endocrine therapy have not shown significant hepatic toxicity profiles. The incidence of grade 3/4 immune-mediated hepatitis was ∼1.5% based on pooled data consisting of 1994 patients treated with nivolumab monotherapy [26]. Furthermore, in patients treated with palbociclib and endocrine therapy, the incidence of grade 3/4 alanine (ALT) and aspartate transaminase (AST) elevation was ∼2% and ∼3%, respectively [28]. This suggests that combining these treatments may have a synergistic effect on toxicity, but the exact mechanism remains unclear. After a review of the safety data, enrolment into cohort 2 was halted and did not proceed to the planned randomised phase.
Several trials have shown an increased risk of toxicity with the combination of PD-1/PD-L1 inhibitors plus CDK4/6 inhibitors in patients with metastatic ER+/HER2− breast cancer. An increased risk of ILD/pneumonitis was reported in clinical trials of PD-1 inhibitor plus abemaciclib in metastatic ER+/HER2− breast cancer [25,27]; results from both these studies do not support further investigation of this combination in patients with metastatic ER+/HER2− breast cancer. A phase Ib trial assessing the PD-1 inhibitor spartalizumab with ribociclib and fulvestrant in heavily pretreated metastatic HR+/HER2− breast cancer showed a high incidence of grade 3/4 ALT and AST elevation [29]. Also, the randomised phase 2 PACE trial, which evaluated the PD-L1 inhibitor avelumab with palbociclib and fulvestrant in patients with metastatic HR+/HER2− breast cancer with progression on CDK4/6 inhibitors, showed incidences of immune-mediated ALT and AST elevation, albeit most were grade 1/2 [30].
In all treated patients of cohort 2, we noted a pCR rate of 4.8% (1 patient in the palbociclib 100-mg group). Overall, due to small patient numbers, no conclusions can be drawn about the efficacy of combining nivolumab with palbociclib plus anastrozole based on the findings of this trial. Similarly, any correlation between Ki67 or PEPI score and efficacy were inconclusive due to the small number of patients with evaluable data.
Consistent with data reported in literature, our findings indicate that the combination of nivolumab plus palbociclib plus anastrozole was associated with increased risk of hepatotoxicity in patients with ER+/HER2− breast cancer, with 6 of 21 patients discontinuing treatment due to hepatotoxicity. Further development of nivolumab combined with CDK4/6 inhibitors should not be pursued in this setting.
Funding
This study and medical writing support of this manuscript were funded by Bristol Myers Squibb.
Author contributions
Guy Jerusalem: study design/conception, data acquisition, data interpretation; Aleix Prat: study design/conception, data interpretation; Roberto Salgado: study design/conception, data interpretation; Mattea Reinisch: data acquisition, data interpretation; Cristina Saura: data acquisition, data interpretation; Manuel Ruiz Borrego: data acquisition, data interpretation; Petros Nikolinakos: data acquisition, data interpretation; Felipe Ades: data analysis and interpretation; Jeiry Filian: data analysis and interpretation; Ning Huang: data analysis and interpretation; Antonella Mazzei-Abba: data analysis and interpretation; Sara M. Tolaney: study design/conception, data interpretation.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: G. Jerusalem declares acting in a consultancy or advisory role for AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Lilly, Novartis, Pfizer, Roche; receiving honoraria from Amgen, AstraZeneca, Bristol Myers Squibb, Lilly, Novartis, Pfizer, Roche, Seagen; research funding from Novartis, Pfizer, Roche; travel and accommodation expenses from Amgen, AstraZeneca, Bristol Myers Squibb, Lilly, Novartis, Pfizer, Roche; and medical writing support from Amgen, AstraZeneca, Bristol Myers Squibb, Lilly, Medimmune, Merck Sharp and Dohme Corp, Novartis, Roche. A. Prat declares acting in a consultancy or advisory role for AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, NanoString Technologies, Novartis, Oncolytics Biotech, Pfizer, Puma, Roche; receiving honoraria from Amgen, Daiichi Sankyo, Guardant Health, Lilly, Merck Sharp and Dohme Corp, Novartis, Pfizer, Roche; research funding from Incyte, Novartis, Puma, Roche; travel and accommodation expenses from Daiichi Sankyo; an employee of Novartis; and a stockholder of Reveal Genomics. R. Salgado declares acting in a consultancy or advisory role for Bristol Myers Squibb and Roche; and travel and accommodation expenses from Merck Sharp and Dohme Corp, Puma, and Roche. M. Reinisch declares acting in a consultancy or advisory role for Daiichi Sankyo, Somatex, and Roche; receiving honoraria from AstraZeneca, Daiichi Sankyo, Lilly, Merck Sharp and Dohme Corp, Novartis, Pfizer, Roche, Seagen, Somatex; and travel and accommodation expenses from Novartis and Pfizer. C. Saura declares acting in a consultancy or advisory role for AstraZeneca, Ax's Consulting, Byondis, Daiichi Sankyo, Eisai, Exact Sciences, Exeter, F. Hoffmann-La Roche Ltd., International Society for the Study and Exchange of evidence from Clinical research And Medical experience (ISSECAM), Medical Statistics Consulting, MediTech, Merck Sharp and Dohme Corp, Novartis, Pfizer, Philips, Pierre Fabre, PintPharma, Puma, Roche, Sanofi, Seagen, Zymeworks, and research funding from Aragon, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Byondis, CytomX, Daiichi Sankyo, F. Hoffmann-La Roche Ltd., Genentech, German Breast Group Forchungs, GlaxoSmithKline, Immunomedics, Innoup, International Breast Cancer Study Group (IBCSG), Lilly, Macrogenics, Medica Scientia Innovation Research, Menarini Ricerche, Merck Sharp and Dohme Corp, Merus, Millennium, Novartis, Pfizer, Piqur, Puma, Roche, Sanofi, Seagen, Synthon, and Zenith. F. Ades, J. Filian, and N. Huang are employees and stockholders of Bristol Myers Squibb. A. Mazzei-Abba is an employee of Bristol Myers Squibb. S.M. Tolaney declares honoraria or consultation fees from 4D Pharma, AstraZeneca, Athenex, Blueprint Medicines, Bristol Myers Squibb, CytomX, Daiichi Sankyo, Eisai, Eli Lilly, Ellipses Pharma, Genentech/Roche, G1 Therapeutics, Gilead, Merck, Mersana Therapeutics, Novartis, Odonate, OncoPep, OncoSec, OncXerna, Pfizer, Puma, Reveal Genomics, Sanofi, Seagen, Zentalis, Zymeworks; presented at a public event organised by Chugai Pharma; and received grants/research supports from AstraZeneca, Bristol Myers Squibb, Certara, Cyclacel, Eisai, Eli Lilly, Exelixis, Genentech/Roche, Gilead, Merck, Nanostring, Nektar, Novartis, Odonate, Pfizer, Sanofi, and Seagen. M. Ruiz-Borrego and P. Nikolinakos have no conflicts of interest to disclose.
Acknowledgements
This study was supported by Bristol Myers Squibb. We would like to thank the patients and families who made this study possible and the clinical study protocol manager, Lisa Hammond. All authors contributed towards the manuscript development and approved the final draft. Medical writing support was provided by Thai Cao, MS, and Meenakshi Subramanian, PhD, of Evidence Scientific Solutions Inc and was funded by Bristol Myers Squibb.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2023.103580.
Contributor Information
Guy Jerusalem, Email: g.jerusalem@chuliege.be.
Aleix Prat, Email: alprat@clinic.cat.
Roberto Salgado, Email: roberto@salgado.be.
Mattea Reinisch, Email: m.reinisch@kem-med.com.
Cristina Saura, Email: csaura@vhio.net.
Manuel Ruiz Borrego, Email: ruizborrego@gmail.com.
Petros Nikolinakos, Email: pnikolinakos@universitycancer.com.
Felipe Ades, Email: felipe.ades@bms.com.
Jeiry Filian, Email: jeiry.filian@bms.com.
Ning Huang, Email: ning.huang@bms.com.
Antonella Mazzei-Abba, Email: antonella.mazzei@bms.com.
Sara M. Tolaney, Email: sara_tolaney@dfci.harvard.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Andrahennadi S., Sami A., Manna M., Pauls M., Ahmed S. Current landscape of targeted therapy in hormone receptor-positive and HER2-negative breast cancer. Curr Oncol. 2021;28(3):1803–1822. doi: 10.3390/curroncol28030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Twelves C., Bartsch R., Ben-Baruch N.E., Borstnar S., Dirix L., Tesarova P., et al. The place of chemotherapy in the evolving treatment landscape for patients with HR-positive/HER2-negative MBC. Clin Breast Cancer. 2022;22(3):223–234. doi: 10.1016/j.clbc.2021.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Alexandre M., Maran-Gonzalez A., Viala M., Firmin N., D'Hondt V., Gutowski M., et al. Decision of adjuvant systemic treatment in HR+ HER2- early invasive breast cancer: which biomarkers could help? Cancer Manag Res. 2019;11:10353–10373. doi: 10.2147/CMAR.S221676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson D.R., Brown J., Morikawa A., Method M. Breast cancer-specific mortality in early breast cancer as defined by high-risk clinical and pathologic characteristics. PLoS One. 2022;17(2) doi: 10.1371/journal.pone.0264637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.All rights reserved. Accessed April 6, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. NCCN, National Comprehensive Cancer Network® (NCCN®); 2023. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V.4.2023. © National Comprehensive Cancer Network, Inc. [Google Scholar]
- 6.Salvo E.M., Ramirez A.O., Cueto J., Law E.H., Situ A., Cameron C., et al. Risk of recurrence among patients with HR-positive, HER2-negative, early breast cancer receiving adjuvant endocrine therapy: a systematic review and meta-analysis. Breast. 2021;57:5–17. doi: 10.1016/j.breast.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finn R.S., Rugo H.S., Dieras V.C., Harbeck N., Im S.-A., Gelmon K.A., et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): analyses from PALOMA-2. J Clin Oncol. 2022;40(17_suppl) doi: 10.1200/JCO.2022.40.17_suppl.LBA1003. LBA1003-LBA. [DOI] [Google Scholar]
- 8.Johnston S., Martin M., Di Leo A., Im S.-A., Awada A., Forrester T., et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5(1):5. doi: 10.1038/s41523-018-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rugo H.S., Finn R.S., Diéras V., Ettl J., Lipatov O., Joy A.A., et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719–729. doi: 10.1007/s10549-018-05125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cristofanilli M., Rugo H.S., Im S.-A., Slamon D.J., Harbeck N., Bondarenko I., et al. Overall survival with palbociclib and fulvestrant in women with HR+/HER2− ABC: updated exploratory analyses of PALOMA-3, a double-blind, phase III randomized study. Clin Cancer Res. 2022;28(16):3433–3442. doi: 10.1158/1078-0432.CCR-22-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hortobagyi G.N., Stemmer S.M., Burris H.A., Yap Y.-S., Sonke G.S., Hart L., et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386(10):942–950. doi: 10.1056/NEJMoa2114663. [DOI] [PubMed] [Google Scholar]
- 12.Lu Y.-S., Im S.-A., Colleoni M., Franke F., Bardia A., Cardoso F., et al. Updated overall survival (OS) results from the phase III MONALEESA-7 trial of pre- or perimenopausal patients with HR+/HER2- advanced breast cancer in MONALEESA-7: a phase III randomized clinical trial. Clin Cancer Res. 2022;28(5):851–859. doi: 10.1158/1078-0432.CCR-21-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slamon D.J., Neven P., Chia S., Jerusalem G., De Laurentiis M., Im S., et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol. 2021;32(8):1015–1024. doi: 10.1016/j.annonc.2021.05.353. [DOI] [PubMed] [Google Scholar]
- 14.Martin M., Hegg R., Kim S.-B., Schenker M., Grecea D., Garcia-Saenz J.A., et al. Treatment with adjuvant abemaciclib plus endocrine therapy in patients with high-risk early breast cancer who received neoadjuvant chemotherapy: a prespecified analysis of the monarchE randomized clinical trial. JAMA Oncol. 2022;8(8):1190–1194. doi: 10.1001/jamaoncol.2022.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfizer. Ibrance (palbociclib) prescribing information. New York, NY. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207103s015lbl.pdf. [Accessed 15 January 2023].
- 16.Novartis. Kisqali (ribociclib) prescribing information. East Hanover, NJ. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/209092s013,209935s021lbl.pdf. [Accessed 15 January 2023].
- 17.Lilly. Verzenio (abemaciclib) prescribing information. Indianapolis, IN. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/208716s006s007s008lbl.pdf. [Accessed 15 January 2023].
- 18.Gil-Gil M., Alba E., Gavilá J., de la Haba-Rodríguez J., Ciruelos E., Tolosa P., et al. The role of CDK4/6 inhibitors in early breast cancer. Breast. 2021;58:160–169. doi: 10.1016/j.breast.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong K., Yao L., Sheng X., Ye D., Guo Y. Cyclin-dependent kinase 4/6 inhibitor in combination with endocrine therapy versus endocrine therapy only for advanced breast cancer: a systematic review and meta-analysis. Oncol Res Treat. 2021;44(10):557–567. doi: 10.1159/000518573. [DOI] [PubMed] [Google Scholar]
- 20.Dirix L.Y., Takacs I., Jerusalem G., Nikolinakos P., Arkenau H.T., Forero-Torres A., et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 2018;167(3):671–686. doi: 10.1007/s10549-017-4537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goel S., DeCristo M.J., Watt A.C., BrinJones H., Sceneay J., Li B.B., et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548(7668):471–475. doi: 10.1038/nature23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng J., Wang E.S., Jenkins R.W., Li S., Dries R., Yates K., et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 2018;8(2):216–233. doi: 10.1158/2159-8290.Cd-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teh J.L.F., Aplin A.E. Arrested developments: CDK4/6 inhibitor resistance and alterations in the tumor immune microenvironment. Clin Cancer Res. 2019;25(3):921–927. doi: 10.1158/1078-0432.Ccr-18-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J., Bu X., Wang H., Zhu Y., Geng Y., Nihira N.T., et al. Cyclin D–CDK4 kinase destabilizes PD-L1 via cullin 3–SPOP to control cancer immune surveillance. Nature. 2018;553(7686):91–95. doi: 10.1038/nature25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rugo H.S., Kabos P., Beck J.T., Jerusalem G., Wildiers H., Sevillano E., et al. Abemaciclib in combination with pembrolizumab for HR+, HER2− metastatic breast cancer: phase 1b study. npj Breast Cancer. 2022;8(1):118. doi: 10.1038/s41523-022-00482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bristol Myers Squibb Opdivo (nivolumab) prescribing information. https://packageinserts.bms.com/pi/pi_opdivo.pdf
- 27.Masuda J., Tsurutani J., Masuda N., Tanabe Y., Iwasa T., Takahashi M., et al. Abstract PS12-10: Phase II study of nivolumab in combination with abemaciclib plus endocrine therapy in patients with HR+, HER2- metastatic breast cancer: WJOG11418B NEWFLAME trial. Cancer Res. 2021;81(4_Suppl):PS12-10. doi: 10.1158/1538-7445.SABCS20-PS12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diéras V., Rugo H.S., Schnell P., Gelmon K., Cristofanilli M., Loi S., et al. Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for HR+/HER2- advanced breast cancer. J Natl Cancer Inst. 2019;111(4):419–430. doi: 10.1093/jnci/djy109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herold C.I., Trippa L., Li T., Do K., Bardia A., Anderson L., et al. Abstract P3-14-03: A phase 1b study of the CDK4/6 inhibitor ribociclib in combination with the PD-1 inhibitor spartalizumab in patients with hormone receptor-positive metastatic breast cancer (HR+ MBC) and metastatic ovarian cancer (MOC) Cancer Res. 2020;80(4_Suppl):P3-14-03 doi: 10.1158/1538-7445.SABCS19-P3-14-03. [DOI] [Google Scholar]
- 30.Mayer E.L., Ren Y., Wagle N., Mahtani R., Ma C., DeMichele A., et al. PACE: palbociclib after CDK and endocrine therapy. A randomized phase II study of fulvestrant +/- palbociclib after progression on CDK4/6 inhibitor for HR+/HER2– metastatic breast cancer. Abstract GS3-06. San Antonio Breast Cancer Symposium 2022. San Antonio, TX, USA; December 6-10, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.