Abstract
Although kinase inhibitors (KI) frequently portray large interpatient variability, a ‘one size fits all’ regimen is still often used. In the meantime, relationships between exposure-response and exposure-toxicity have been established for several KIs, so this regimen could lead to unnecessary toxicity and suboptimal efficacy. Dose adjustments based on measured systemic pharmacokinetic levels—i.e., therapeutic drug monitoring (TDM)—could therefore improve treatment efficacy and reduce the incidence of toxicities. Therefore, the aim of this comprehensive review is to give an overview of the available evidence for TDM for the 77 FDA/EMA kinase inhibitors currently approved (as of July 1st, 2023) used in hematology and oncology. We elaborate on exposure-response and exposure-toxicity relationships for these kinase inhibitors and provide practical recommendations for TDM and discuss corresponding pharmacokinetic targets when possible.
Key Points
| Therapeutic drug monitoring can be a practical tool for personalizing therapy with kinase inhibitors. |
| The potential of therapeutic drug monitoring for kinase inhibitors is mostly based on exposure-response and exposure-toxicity relationships. |
| Randomized, prospective studies are highly needed to confirm the beneficial effect of therapeutic drug monitoring before it could be incorporated into daily clinical practice. |
Introduction
Kinase inhibitors (KIs, or small molecule kinase inhibitors) have become increasingly important in the treatment of malignant diseases over the last decades. This is mainly due to the ease of oral administration and the rising number of targets available. These KIs are associated with a high inter-individual variability in drug exposure (typically ranging from 40 to 70%) [1]. Many factors account for this variability, such as variable absorption, genetic polymorphisms in metabolizing enzymes and interacting food, herbs and co-medication [2–4]. For many KIs, pharmacokinetic (PK) exposure (i.e., area under the curve plasma concentration [AUC] or plasma trough level [Cmin]) is associated with efficacy and/or toxicity, resulting in patients being at risk of systemic concentrations when drug levels are outside the therapeutic window [1, 5]. Despite high inter-individual variability and the association between exposure-response and exposure-toxicity, the “one dose fits all” regimen is still applied for all KIs [1].
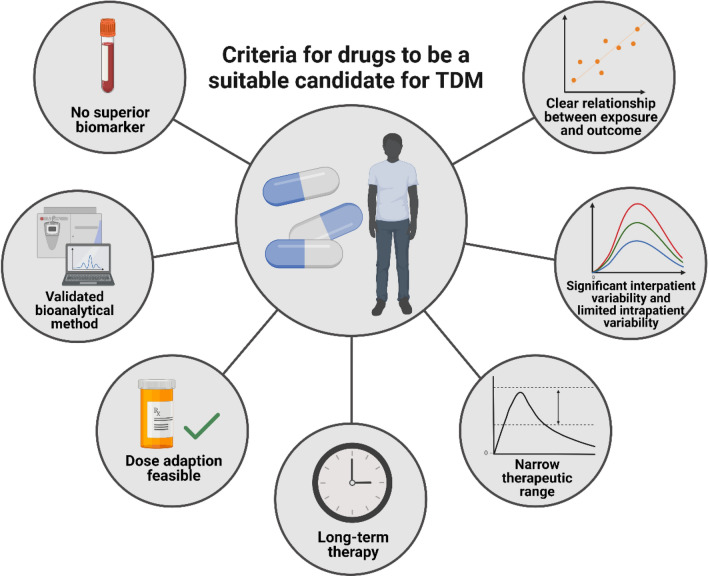
Therapeutic drug monitoring (TDM) has been proposed as a method to individualize KI treatment by adjusting the dose based on the measured drug concentration while targeting a certain threshold [1, 5]. It was recently shown that TDM is feasible in daily practice for commonly used oral targeted therapies (mostly KIs) and that TDM substantially reduces the number of underexposed patients [6]. Several factors are important for determining whether a drug is a suitable candidate for TDM (Fig. 1) [7]. First, the drug must have a defined exposure-response and/or -toxicity relationship at the approved dose with a considerable inter-individual variability in PK exposure and a narrow therapeutic range (i.e., the drug has a small difference between the therapeutic and toxic concentration). Moreover, the average time on treatment should be long enough to allow time to reach steady-state concentrations and dose modifications (i.e., at least several weeks) and these interventions should be feasible in daily practice. Last, bioanalytical methods to measure the plasma concentrations are required and no other superior biomarker for drug response should be available, as this would make TDM unnecessary [7].
Fig. 1.
An overview of the seven most important criteria for drugs to be a suitable candidate for therapeutic drug monitoring (TDM). Based on the requirements suggested by Groenland et al [7].
Created with Biorender.org
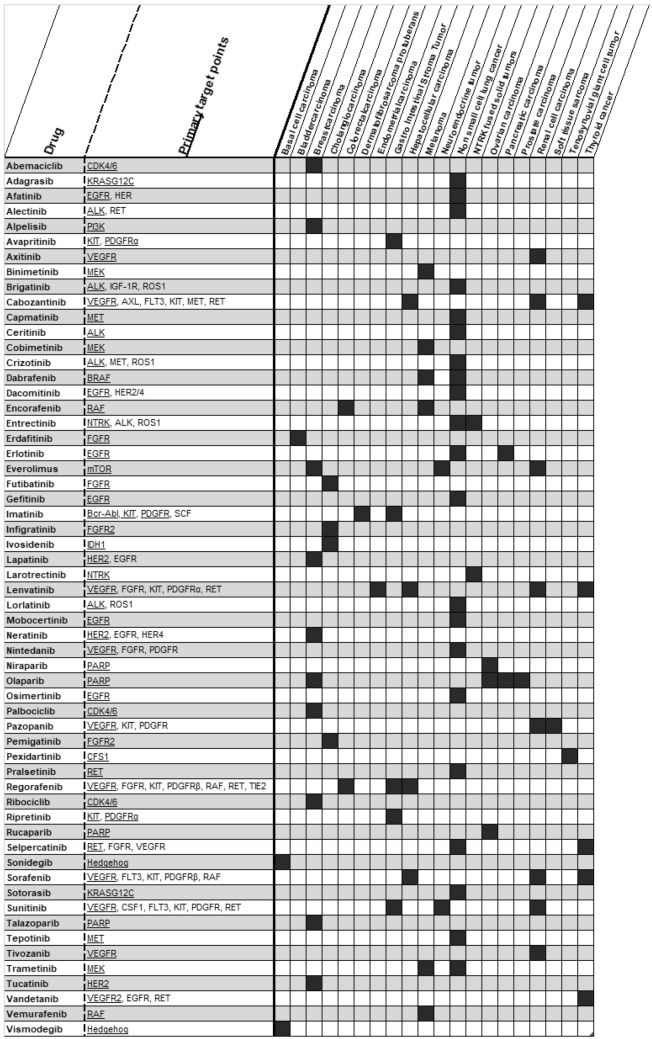
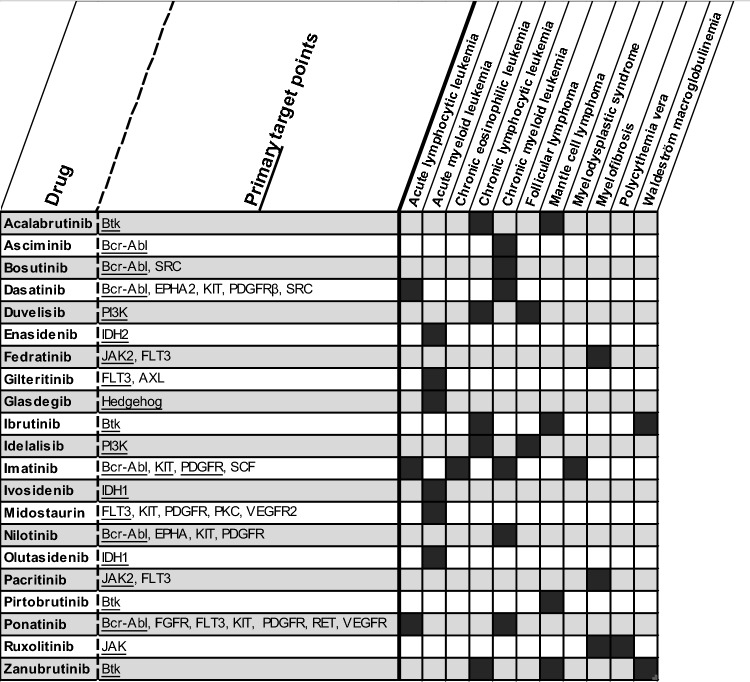
Several reviews describing TDM of KIs have been published in past years [1, 5, 8]. However, as the number of new compounds is expanding fast and additional data regarding TDM of previously described KIs have become available, an update is required. The aim of this review is to provide an overview of the current evidence on exposure-response and exposure-safety relationships of all KIs used in hematology and oncology approved by the FDA and/or the EMA up to July 1st, 2023 (see Figs 2 and 3 for an overview of these KIs with their targets and indications). Based on the previously proposed requirements for TDM and the available evidence per drug, we provide practical recommendations and evidence levels for TDM with corresponding PK targets that can be used to personalize KI treatment (Table 1) [7, 9]. We advise the use of TDM in daily clinical practice when a clear exposure-response and/or -toxicity relationship is established with a target threshold that is validated in at least one prospective clinical trial.
Fig. 2.
Overview of described KIs with target mechanism and indications used in solid tumors
Fig. 3.
Overview of described KIs with target mechanism and indications used in hematology
Table 1.
Overview for all KIs approved by the FDA/EMA with evidence and recommendations for TDM
| Drug | Reversible or irreversible binding | Exposure-response relationship | Exposure-toxicity relationship | Evidence level for TDM | Proposed TDM target | Comments | References | |
|---|---|---|---|---|---|---|---|---|
| Type | Target | |||||||
| Abemaciclib | Reversible | Yes (tumor shrinkage, PFS) | Yes (neutropenia) | Exploratory | NA | NA | Targeting Cmin of 169 ng/mL based on the geometric mean most appropriate for now | [65, 67] |
| Acalabrutinib | Irreversible | No | No | Not advised | NA | NA | [57–59] | |
| Adagrasib | Irreversible | Inconclusive | Inconclusive | Not advised | NA | NA | No reports on exposure-response and exposure-toxicity relationships yet. | [135, 136] |
| Afatinib | Irreversible | No | Yes (diarrhea, anorexia, rash) | Promising | Safety | Cmin ≤ 28.5 ng/mL | Monitoring Cmin on day 8 after start to prevent onset diarrhea grade 2 | [78–81] |
| Alectinib | Reversible | Yes (PFS, tumor response) | No | Promising | Efficacy | Cmin ≥ 435 ng/mL | A RCT regarding TDM of alectinib has recently started | [11, 18–21] |
| Alpelisib | Reversible | Inconclusive | Yes (hyperglycemia) | Exploratory | NA | NA | [167, 168] | |
| Asciminib | Reversible | Yes (only with tumor response in patients with T315I mutation) | No | Exploratory | NA | NA | [43] | |
| Avapritinib | Reversible | Inconclusive | Yes (adverse cognitive effects, any grade 3/4 AE) | Exploratory | NA | NA | The sample size was too small to draw conclusions about an E-R relationship | [131, 132] |
| Axitinib | Reversible | Yes (PFS, OS, response) | Yes (hypertension, proteinuria, fatigue, diarrhea) | Advised | Efficacy | Cmin ≥ 5 ng/mL | The Cmin ≥ 5 ng/mL requires further confirmation in larger studies | [172–178] |
| Binimetinib | Reversible | No | Inconclusive | Not advised | NA | NA | [140] | |
| Bosutinib | Reversible | Yes (CCyR, MMR and CHR at 1 year) | Yes (diarrhea, rash) | Exploratory | NA | NA |
- E-R relationship only observed in newly diagnosed CML patients - Targeting Cmin of 147 ng/mL based on geometric mean most appropriate for now |
[38, 39] |
| Brigatinib | Reversible | Yes (intracranial ORR) | Yes (elevated lipase and amylase levels) | Exploratory | NA | NA | [16, 17] | |
| Cabozantinib | Reversible | Inconclusive (if so, for PFS) | Yes (HFS, fatigue, diarrhea, hypertension, dose reduction) | Promising | Efficacy | Cmin ≥ 537 ng/mL | In the setting combined with nivolumab, there is no evidence for the use of TDM | [180–183, 243] |
| Safety | Cmin ≤ 618 ng/mL | |||||||
| Capmatinib | Reversible | Inconclusive | Yes (nausea, lipase and amylase levels) | Exploratory | NA | NA | [142] | |
| Ceritinib | Reversible | No | Yes (elevated liver enzymes, hyperglycemia) | Exploratory | NA | NA | [12, 13] | |
| Cobimetinib | Reversible | No | No | Not advised | NA | NA | [137] | |
| Crizotinib | Reversible | Yes (PFS, ORR) | No | Promising | Efficacy | Cmin ≥ 235 ng/mL | [10, 11] | |
| Dabrafenib | Reversible | No | No | Not advised | NA | NA | [49–52] | |
| Dacomitinib | Irreversible | No | Yes (rash, dermatitis, diarrhea) | Exploratory | NA | NA | [85] | |
| Dasatinib | Reversible | Yes (McyR) | Yes (pleural effusion) | Advised | Safety | Cmin ≤ 2.5 ng/mL | A study showed that TDM reduces the incidence of pleural effusion significantly | [31–35, 244] |
| Duvelisib | Reversible | No | No | Not advised | NA | NA | [166] | |
| Enasidenib | Reversible | Yes (only with tumor response in patients with R140 mutation) | Yes (bilirubin increase) | Exploratory | NA | NA | [116] | |
| Encorafenib | Reversible | No | Yes (any grade 3/4 AE, retinopathy) | Exploratory | NA | NA | [53] | |
| Entrectinib | Reversible | No | No | Not advised | NA | NA | [148, 149] | |
| Erdafitinib | Reversible | No | No | Not advised | NA | NA | Serum phosphate concentration is a better predictive marker of erdafitinib response and toxicity | [92, 93] |
| Erlotinib | Reversible | Inconclusive | Yes (rash, diarrhea) | Exploratory | NA | NA | Low dose erlotinib has been suggested as an effective tool for frail patients, improving toxicity and maintaining efficacy | [70–74, 245] |
| Everolimus | Reversible | Yes (tumor reduction, PFS) | Yes | Advised | Efficacy | Cmin ≥ 10 ng/mL | [228–232] | |
| Fedratinib | Reversible | Yes (spleen volume reduction, symptom score) |
Yes (any grade 3/4 AE, anemia, thrombocytopenia, nausea, vomiting) |
Exploratory | NA | NA | [119] | |
| Futibatinib | Irreversible | Inconclusive | Yes (hyperphosphatemia) | Exploratory | NA | NA | Measuring serum phosphate might be a better option for personalizing infigratinib treatment | [99] |
| Gefitinib | Reversible | Yes (OS) | Yes (interstitial lung disease, diarrhea) | Promising | NA | NA | A proposed target for efficacy of 200 ng/mL was later invalidated in a subsequent study | [68, 69, 76, 77] |
| Gilteritinib | Reversible | No | Yes (elevated liver enzymes, elevated creatine kinase) | Exploratory | NA | NA | [103] | |
| Glasdegib | Reversible | No | Yes (dysgeusia, muscle spasms, renal toxicity, QTc prolongation) | Exploratory | NA | NA | [107–109] | |
| Ibrutinib | Irreversible | No | No | Not advised | NA | NA | Limited accumulation of ibrutinib in plasma might hamper feasibility of TDM | [54–56] |
| Idelalisib | Reversible | No | No | Not advised | NA | NA | Exposure relationships for idelalisib were also not observed when used in combination therapy | [162–165] |
| Imatinib | Reversible | Yes (MMR, CcyR for CML, PFS for GIST) | Yes (neutropenia, rash, diarrhea, edema, arthralgia) | Advised | Efficacy |
CML: Cmin ≥ 1000 ng/mL GIST: Cmin ≥ 1100 ng/mL |
- TDM was found feasible and effective in a RCT and a cohort study - Several studies demonstrated higher QALY’s and lower costs due to TDM |
[22–25, 27–29, 122–130] |
| Infigratinib | Reversible | Inconclusive | Yes (hyperphosphatemia) | Exploratory | NA | NA | Measuring serum phosphate might be a better option for personalizing infigratinib treatment | [98] |
| Ivosidenib | Reversible | No | Yes (QTc-prolongation) | Exploratory | NA | NA | [114, 115] | |
| Lapatinib | Reversible | Inconclusive | No | Not advised | NA | NA | [111] | |
| Larotrectinib | Reversible | No | No | Not advised | NA | NA | [147] | |
| Lenvatinib | Reversible | No | Inconclusive (possibly for vomiting, nausea, hypertension, proteinuria, elevated bilirubin and liver enzymes) | Exploratory | Toxicity | Cmin ≤ 88 ng/mL | As results on a exposure-toxicity relationship are contradictory, this requires additional research before the target should be validated | [186–189] |
| Lorlatinib | Reversible | No | Yes (any grade 3/4 AE, hypercholes-terolemia) | Exploratory | NA | NA | [14, 15] | |
| Midostaurin | Reversible | Yes (PBBR for midostaurin and metabolite, OS only for metabolite) | No | Exploratory | NA | NA | Measuring the most active metabolite CGP2221 should be considered when exploring potential for TDM | [101, 102] |
| Mobocertinib | Irreversible | No | Yes (any grade 3/4 AE) | Exploratory | NA | NA | [86] | |
| Neratinib | Irreversible | Yes (ORR) | No | Exploratory | NA | NA | The E-R relationship was only observed in patients with metastatic breast cancer, and not in early-stage breast cancer | [112] |
| Nilotinib | Reversible | Yes (TTP) | Yes (elevated bilirubin and liver enzymes) | Promising | Efficacy | Cmin ≥ 469 ng/mL | [36, 37] | |
| Nintedanib | Reversible | Inconclusive | Yes (elevated liver enzymes) | Exploratory | NA | NA | The E-R relationship was only observed for the anti-angiogenic effect, not for survival endpoints | [191, 192] |
| Niraparib | Reversible | Yes (PFS only in patients with BRCA mutation) | Yes (hematological adverse events, fatigue) | Exploratory | NA | NA | Research regarding TDM should primarily focus on the BRCA mutated group. | [155, 157, 158] |
| Olaparib | Reversible | No | Yes (anemia, diarrhea, appetite loss, dysgeusia, fatigue, nausea, vomiting) | Promising | Safety | Cmin ≤ 2500 ng/mL | Threshold is applicable both for capsules and tablets | [150–154] |
| Olutasidenib | Reversible | Inconclusive | No | Not advised | NA | NA | No reports on E-R and exposure-toxicity relationships yet | [117] |
| Osimertinib | Irreversible | No | Yes (rash, diarrhea, QTc-prolongation) | Promising | Safety | Cmin ≤ 259 ng/mL | [87–91] | |
| Pacritinib | Reversible | Yes (spleen volume reduction) | No | Exploratory | NA | NA | [120] | |
| Palbociclib | Reversible | No | Yes (neutropenia) | Exploratory | NA | NA | [63, 66] | |
| Pazopanib | Reversible | Yes (PFS, disease free survival) | Yes (hypertension, diarrhea, hand-foot syndrome, stomatitis, ALT increase, fatigue) | Advised | Efficacy | Cmin ≥ 20.5 mg/L | [193–201] | |
| Safety | Cmin ≤ 46 mg/L | |||||||
| Pemigatinib | Reversible | Inconclusive | Yes (hyperphosphatemia) | Exploratory | NA | NA | Measuring serum phosphate might be a better option for personalizing infigratinib treatment | [96, 97] |
| Pexidartinib | Reversible | Inconclusive | Yes (elevated liver enzymes) | Exploratory | NA | NA | [233, 234] | |
| Pirtobrutinib | Reversible | Inconclusive | No | Exploratory | NA | NA | No reports on the exposure-response relationship yet | [62] |
| Ponatinib | Reversible | Inconclusive | Inconclusive | Exploratory | NA | NA | Only a relationship with dose was reported. No PK parameters were included in the studies | [40] |
| Pralsetinib | Reversible | No | Yes (pneumonia, anemia) | Exploratory | NA | NA | [169, 170] | |
| Regorafenib | Reversible | Inconclusive | Yes (HFS, rash, bilirubin increase) | Promising | Efficacy | Cmin ≥ 2900 ng/mL |
- Target levels consist of regorafenib + metabolites M2/M5 concentrations - A prospective study validating the target is currently ongoing |
[202–206] |
| Safety | Cmin ≤ 4300 ng/mL | |||||||
| Ribociclib | Reversible | Inconclusive | Yes (neutropenia, QTc-prolongation) | Exploratory | NA | NA | [64] | |
| Ripretinib | Reversible | Inconclusive | No | Exploratory | NA | NA | [133] | |
| Rucaparib | Reversible | Yes (radiological response only in patients with platinum-sensitive disease) | Yes (hematological adverse events, fatigue, elevated creatinine, elevated liver enzymes) | Exploratory | NA | NA | Research regarding TDM should primarily focus on group with platinum-sensitive disease | [156, 159] |
| Ruxolitinib | Reversible | Yes (spleen volume reduction, symptom score) | Yes (anemia, thrombocytopenia) | Exploratory | NA | NA | [118] | |
| Selpercatinib | Reversible | No | No | Not advised | NA | NA | [171] | |
| Sonidegib | Reversible | No | No | Not advised | NA | NA | [105] | |
| Sorafenib | Reversible | Yes (PFS, OS) | Yes (any grade 3/4 AE, HFS, hypertension) | Exploratory | NA | NA | A recent study found that a target of 3750 ng/mL for efficacy was not feasible due to toxicity | [207–214] |
| Sotorasib | Irreversible | No | No | Not advised | NA | NA | [134] | |
| Sunitinib | Reversible | Yes (PFS, OS) | Yes (grade 3/4 fatigue, vomiting, neutropenia, thrombocytopenia, grade ≥ 2 hypertension) | Advised | Efficacy |
Continuous Cmin ≥ 37.5 ng/mL Intermittent Cmin ≥ 50 ng/mL |
Target levels consist of sunitinib + metabolite N-desethyl sunitinib concentrations | [215–226] |
| Safety |
Continuous Cmin ≤ 60–75 ng/mL Intermittent Cmin ≤ 80–87.5 ng/mL |
|||||||
| Talazoparib | Reversible | Yes (PFS) | Yes (grade 3 AE, anemia, thrombocytopenia) | Exploratory | NA | NA | [160, 161] | |
| Tepotinib | Reversible | No | No | Not advised | NA | NA | [143–146] | |
| Tivozanib | Reversible | Yes (PFS) | Yes (HFS, hypertension) | Exploratory | NA | NA | [179] | |
| Trametinib | Reversible | Inconclusive (but most studies found a relationship between exposure and PFS). | Inconclusive | Promising | Efficacy | Cmin ≥ 10.6 ng/mL | The proposed target is still unsettled and requires additional confirmation | [49, 50, 138, 139] |
| Tucatinib | Reversible | No | No | Not advised | NA | NA | [113] | |
| Vandetanib | Reversible | No | Yes (diarrhea, fatigue, QTcF prolongation) | Exploratory | NA | NA | [227] | |
| Vemurafenib | Reversible | Yes (PFS, OS) | Yes (QTc prolongation, rash) | Promising | Efficacy | Cmin ≥ 50 mg/L | [44–48] | |
| Vismodegib | Reversible | No | No | Not advised | NA | NA | [104] | |
| Zanubrutinib | Irreversible | No | No | Not advised | NA | NA | [60, 61] | |
An evidence level for TDM is given per drug based on the available data, according to the following definitions: Not advised: No exposure-response or exposure-safety relationship has been identified, and/or there is evidence that TDM is not indicated; Exploratory: An exposure-response or exposure-safety relationship has been identified, but no target threshold has been proposed or investigated; Promising: Based on an exposure-response or exposure-toxicity relationship, a target threshold has been proposed, but this target still requires prospective validation. Advised: The target threshold for TDM is established and validated in a prospective study. Strongly advised: The positive effect of routine TDM to improve efficacy and/or safety is determined in a randomized, prospective study
AE adverse event, AUC area under the concentration-time curve, CCyR complete cytogenetic response, CHR complete hematologic response, Cmin minimum concentration at steady state, E-R exposure-response relationship, HFS hand-foot syndrome, McyR major cytogenetic response, MMR major molecular response, NA not available, OS overall survival, ORR overall response rate, PBBR peripheral blood blast response, PFS progression-free survival, QALY quality-adjusted life years, RCT randomized controlled trial, TDM therapeutic drug monitoring, TTP time to progression
Anaplastic Lymphoma Kinase (ALK) Inhibitors
For the first-generation ALK inhibitor crizotinib, an exposure-response relationship has been established for overall response rate (ORR) and progression-free survival (PFS) in two pivotal trials, whereas an exposure-toxicity relationship was not found for grade ≥ 3 adverse events (AEs). The ORR was 60% in patients with a Cmin ≥ 235 ng/mL compared to 47% in patients with a Cmin ≤ 235 ng/mL (n = 120) [10]. A recent observational study confirmed this with a significant improvement in PFS when comparing two groups divided by the Cmin of 235 ng/mL (median PFS: 5.7 vs 17.5 months) (n = 48) [11]. Despite these promising results, prospective studies investigating TDM for crizotinib are not expected because of the development of new and favorable ALK inhibitors. For ceritinib and lorlatinib, no exposure-response relationship was found for several clinical outcome measures (n = 54–328) [12–15]. For both drugs, positive exposure-toxicity relationships were established for grade ≥ 3 AEs, specifically elevated liver enzymes and hyperglycemia for ceritinib and hypercholesterolemia for lorlatinib [13, 15]. For brigatinib, an exposure-response relationship was found for intracranial overall response rate, but not for PFS and ORR (n = 202), and an exposure-toxicity relationship was found only for elevated lipase and amylase levels [16, 17]. Therefore, TDM guided dosing does not seem of added value for these three drugs.
For alectinib, TDM seems a more compelling step to improve treatment outcome. Improved PFS was found to be associated with exposure, with a significant difference in PFS when targeting a Cmin of 435 ng/mL (n = 52) [11]. A recent American Society of Clinical Oncology (ASCO) abstract including 334 patients confirmed the improved PFS across three Phase III studies in patients with high alectinib + metabolite M4 exposure (defined as a combined exposure above 1040 nM, i.e., 475 ng/mL) [18]. For other clinical outcomes, a relationship with exposure is less clearly established. A relationship between exposure and overall survival (OS) and best response was not found, but higher Cmin levels were significantly associated with tumor reduction (including 207 and 49 patients, respectively) [19, 20]. Higher alectinib exposure was not associated with an increased rate of AEs [19, 20]. A prospective randomized controlled trial has recently started evaluating the added value of TDM for alectinib targeting a Cmin of 435 ng/mL (NCT05525338), results are not expected before 2026 [21]. For now, targeting the Cmin ≥ 435 ng/mL seems most appropriate.
Break Point Cluster Region-Abelson (BCR-ABL) Oncoprotein Inhibitors
Many studies have established a positive exposure-response and exposure-toxicity relationship for the first-generation BCR-ABL inhibitor imatinib in the treatment of chronic myeloid leukemia (CML), with a target for exposure of 1000 ng/mL, with a total of 1096 included patients. Although no threshold for toxicity is clear, AEs have been associated with trough concentrations > 3000 ng/mL [22–26]. The International Association of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT) has provided guidelines for TDM of imatinib and advise the same efficacy target of 1000 ng/mL [26]. Furthermore, TDM was found to be feasible and effective, in terms of a higher major molecular response rate at 12 months for CML patients (n = 139) who received dose adjustments based on this target (63% vs 37%) [27, 28]. In addition, a recent US cost-effectiveness study suggests economic favorability for TDM in CML patients, describing higher quality-adjusted life years (QALY) and lower costs than with regular dosing [29]. Imatinib is also used in patients with gastro-intestinal stromal tumors (GIST), but with a different target (KIT/PDGFRA) and is therefore described in the paragraph with the KIT/PDGFRA inhibitors.
Three second-generation BCR-ABL inhibitors have been developed. For dasatinib, results from four Phase II trials with a combined enrollment of 445 patients showed no exposure-response relationship at the approved dose [30]. One study described a positive relationship between the weighted average steady-state plasma concentration and the probability of achieving major cytogenic response, but there was no relationship between Cmin and this response [31, 32]. However, monitoring the Cmax has been recommended, as it can reduce the risk of developing resistance, with a target Cmax ≥ 50 ng/mL measured at 2 h after intake [33, 34]. A relationship between exposure and risk for pleural effusion has been described, with the advice to not exceed a Cmin of 2.5 ng/mL to reduce this risk [1, 31–33]. Another study describes TDM as a tool to decrease this risk in 287 patients. Patients with a Cmin ≥ 1.5 ng/mL could choose between continuing their dose or TDM-based dose reductions until a Cmin of < 1.5 ng/mL was reached. Patients in the TDM group had a significantly lower incidence of pleural effusion than the control group (13.2% vs 42.8%) over the study period. With older age and higher Cmin values being the two risk factors of pleural effusion, they advised TDM to prevent toxicity for at least all patients aged over 50 years [35]. For nilotinib, relationships between exposure and longer time to progression and toxicity have been demonstrated, with a suggested efficacy Cmin target of ≥ 469 ng/mL (total n = 1035) [36, 37]. For bosutinib, a small study including 25 patients showed a significant difference in Cmin levels between patients with and without diarrhea (103 ng/mL vs 55 ng/mL) [38]. A study including 749 patients identified a weak relationship between both diarrhea and incidence of rash and exposure. This study also showed a relationship between exposure and multiple response endpoints, but only in patients with newly diagnosed chronic phase CML. This study did not propose an exposure target [39].
For ponatinib, a third-generation BCR-ABL inhibitor, significant relationships between dose intensity and response and toxicity were described in the approval report (n = 444), although PK parameters such as Cmin were not reported [40]. In more recent years, the dose intensity-toxicity relationship has been studied more thoroughly, due to the increased risk of cardiovascular events with higher doses [40, 41]. Results from the OPTIC trial, in which 282 patients were randomized between three dosing strategies with different starting doses, showed a significant relationship between higher dose and response rate (51.6% vs 25.3%). Patients starting at this higher dose (45 mg/day) also had a 6.4% higher AE risk, including thrombocytopenia and arterial occlusive events. We have to await the results from the long-term safety and efficacy follow-up from this trial before giving adjusted starting dose advice [42].
Asciminib, the most recently approved BCR-ABL inhibitor, only showed a significant relationship between exposure (in terms of higher daily AUC) and response in patients with a T315I mutation (n = 67) in the approval report. In all patients (n = 303), higher Cmax, but not Cmin, was associated with a slightly higher frequency of AEs, primarily the increase of liver enzymes [43].
In conclusion, there are sufficient data that support TDM for imatinib and nilotinib targeting Cmin levels of ≥ 1000 ng/mL and ≥ 469 ng/mL, respectively. For dasatinib, TDM could be especially useful to prevent excessive toxicity. The recommended Cmin threshold for toxicity is ≤ 2.5 ng/mL and for patients aged over 50, a threshold Cmin ≤ 1.5 ng/mL could help prevent pleural effusion. A target Cmax of ≥ 50 ng/mL could be used to reduce the risk of resistance. For ponatinib, although results from the OPTIC trial suggest both a dose-response and dose-toxicity relationship, no exposure was measured in this trial, so the value of TDM remains to be elucidated. For bosutinib and asciminib, studies on TDM should focus on certain patient groups, namely newly diagnosed chronic phase CML patients for bosutinib, and patients with a T315I mutation for asciminib.
B-Raf (BRAF) Inhibitors
Significant relationships for exposure-response and exposure-toxicity for vemurafenib have been clearly described with an advised target Cmin of ≥ 42 mg/L, based on data of 110 patients using monotherapy vemurafenib [44–47]. A more recent study, containing data from 402 patients using either monotherapy vemurafenib or combined therapy with the MEK inhibitor cobimetinib, focused on validating this target. In this study, the 42 mg/L target did not demonstrate a significant association with OS and PFS, but a 50 mg/L target did show a significant relationship to PFS and OS and was advised to be used as threshold. This target was reached by approximately 60% of included patients [48]. No studies on dabrafenib showed an exposure-response relationship at the approved dose (both monotherapy and combined with the MEK inhibitor trametinib) [49–52]. However, Goldwirt et al (n = 50) did report a significantly higher PFS when dabrafenib concentrations (in a combination therapy with trametinib) were between 30.4 and 71.8 mg/L, potentially explained by bias caused by early drop-out due to toxicity [49]. Neither Goldwirt et al nor Groenland et al (n = 140) showed an association between exposure and toxicity in combination therapy with trametinib. Unfortunately, neither took hydroxyl-dabrafenib – the most active metabolite—into account, despite the fact that it was previously shown that higher exposure of this metabolite leads to higher incidence of grade ≥ 3 AEs [49–51]. Another study including both monotherapy and combined therapy patients showed higher mean Cmin levels in patients with AEs, although only 27 patients were included [52]. For encorafenib, the latest approved BRAF inhibitor given in combination with the MEK inhibitor binimetinib, no exposure-response was reported, but an exposure-toxicity relationship for occurrence of grade 3 or 4 AEs was described in the approval report (n = 449) [53]. No additional research has been performed hereafter. As a conclusion, TDM cannot be advised for encorafenib and dabrafenib with the current lack of an E-R relationship at the approved dose. For vemurafenib, there is more evidence for the Cmin target of ≥ 50 mg/L than for the previously reported target of ≥ 42 mg/L and should be considered as threshold for TDM.
Bruton’s Tyrosine Kinase (BTK) Inhibitors
For ibrutinib, no relationship between exposure and response has been established in patients with mantle cell lymphoma (n = 34) [54]. The US Food and Drug Administration (FDA) report found no relationship between exposure and grade ≥ 3 infections or neutropenia, but it was demonstrated that higher plasma levels of ibrutinib were associated with more frequent discontinuation of therapy due to AEs [54]. A recent population PK-model found that mantle cell lymphoma patients who discontinued treatment because of disease progression (n = 5) had a non-significant lower ibrutinib AUC (0.45 × lower) than patients who continued treatment (n = 2) [55]. Therapeutic drug monitoring has not yet been studied for ibrutinib, but might be hampered by the limited accumulation of ibrutinib in plasma. It was recently shown that at least three samples are necessary within 4 h after ingestion to estimate an accurate exposure, and one Cmin sample might not be sufficient [56]. For both acalabrutinib and zanubrutinib, exposure was not associated with clinical outcomes (such as PFS or ORR), nor with a higher incidence of toxicity (n = 45–573) [57–61]. In the Phase I/II trial of the recently approved KI pirtobrutinib, no relationship was found between exposure and grade 3 AEs for all dose levels, including the now registered dose (n = 323) [62]. To date, no reports on the exposure-response relationship have been published. For these three compounds, there is therefore no rationale for TDM.
Cyclin-Dependent Kinase 4/6 (CDK4/6) Inhibitors
A recent exposure-response analysis using a Cox proportional hazard model found no association between palbociclib exposure and PFS (n = 421) [63]. For ribociclib, the approval report includes an exposure-response analysis between cycle 1 Day 15 Cmin levels and PFS in a group of 44 patients, and no clear trend was found. The FDA states that no definite conclusion on an exposure-response relationship could be drawn based on this analysis, due to the small patient group and the limited available drug exposure data [64]. For abemaciclib, multiple analyses described in the approval report found a positive relationship between exposure and PFS and tumor shrinkage (n = 446) [65]. For all CDK 4/6 inhibitors, positive exposure-toxicity relationships have been reported, mostly regarding neutropenia [64–66]. Moreover, for ribociclib a relationship between QTc prolongation and exposure has been established [64]. Therapeutic drug monitoring for palbociclib and ribociclib does not seem logical for now due to the absence of an established exposure-response relationship. Future studies should focus on abemaciclib and determine a target. For now, targeting abemaciclib Cmin of 169 ng/mL based on the geometric mean at the standard dose of 150 mg BID seems the most appropriate [67].
Epidermal Growth Factor Receptor (EGFR) Inhibitors
For the first-generation EGFR KI gefitinib, Zhao et al identified a Cmin of 200 ng/mL as a threshold for improved OS in 30 patients [68]. A later study in 87 non-small lung cancer (NSCLC) patients could not confirm this threshold for PFS [69]. This threshold needs further examination, before the value of TDM for gefinitib can be further elucidated. For erlotinib, two studies on an E-R relationship have recently been published and these could not identify a relationship between exposure and PFS or OS (resp. in 70 and 122 NSCLC patients) [70, 71]. It has been suggested that reduced doses (e.g., 100 or 50 mg/day for erlotinib) could reduce toxicity while maintaining efficacy, especially in frail patients [72, 73]. Exposure-toxicity relationships are more evident, with a relationship between exposure and higher grades of interstitial lung disease and diarrhea for gefitinib, and with rash and diarrhea for erlotinib [70, 71, 74–77].
For the second- and third-generation EGFR KIs (afatinib, dacomitinib, mobocertinib, and osimertinib), the lack of an exposure-response relationship is more clearly established. For afatinib, higher exposure did not result in improved PFS, but was associated with an increased rate of grade ≥ 3 toxicity in several studies, most commonly for diarrhea and rash (n = 31–93) [78–81]. Therapeutic drug monitoring was suggested as a potential tool to prevent the onset of diarrhea grade ≥ 2, with a threshold of 28.5 ng/mL (n = 31) [81]. The authors therefore recommended monitoring the Cmin of afatinib on Day 8 of treatment, and adjust the dose if necessary based on that measurement [81]. Reducing the dose to 20 mg/day compared to the regular dose of 40 mg/day did not result in impaired efficacy with improved tolerability in several studies, but these findings need confirmation in future prospective trials [82–84]. For dacomitinib (n = 272) and mobocertinib (n = 114), the approval reports showed that exposure was associated with an increased probability of experiencing several grade ≥ 3 toxicities, but not with efficacy [85, 86]. For osimertinib, no exposure-response relationship was found for several clinical outcomes, namely PFS, OS, or duration of response (n = 159–748) [87–91]. However, increased osimertinib exposure is correlated with an increased probability of rash, diarrhea, and cardiac QTc-time prolongation [87–90]. Therapeutic drug monitoring could therefore be useful as a tool to prevent excessive toxicity, like for afatinib. A Cmin threshold for toxicity of 259 ng/mL for osimertinib was suggested and could result in a 53% reduction of severe toxicity in the patients with high exposure (26% of the patients in the study, n = 159) [90]. As a conclusion, improving efficacy with TDM in EGFR inhibitors is less obvious due to the lack of an exposure-efficacy relationship. Therapeutic drug monitoring could on the other hand be useful to prevent excessive toxicity by monitoring the drug concentrations.
Fibroblast Growth Factor Receptor (FGFR) Inhibitors
For erdafitinib, no relationship between drug exposure and response or toxicity has been established (n = 156) [92]. Interestingly, the serum phosphate concentration was found to be a more predictive marker of erdafitinib response than the drug concentration, as inhibition of the fibroblast growth factor (FGF) pathway leads to an increase in serum phosphate concentration [93, 94]. Doubling erdafitinib free-drug concentrations resulted in a 1.8-fold increase in drug-related phosphate changes [95]. A phosphate level – response relationship for erdafitinib was established (n = 99), with higher levels being predictive for better OS, PFS and ORR [92, 93]. Moreover, serum phosphate concentrations were associated with a higher incidence of several AEs (several eye, nail and skin disorders) [92, 93]. Therapeutic drug monitoring will have a less profound role in personalizing erdafitinib treatment, as future research will mainly focus on serum phosphate monitoring. For the other FGFR inhibitors pemigatinib, infigratinib and futibatinib, only exploratory results on the exposure-response relationships have been described in the approval reports and the relationship between response and serum phosphate level is not yet known, but is expected based on the similar mechanism of action. No relationships were found for PFS and response rate, but the analyses were hampered by confounding factors (frequent dose reductions and small sample size) and an inadequate PopPK model [96–100]. More precise updated E-R analyses and serum phosphate analyses are awaited before the potential of TDM can be investigated for pemigatinib, infigratinib and futibatinib.
FMS-like Tyrosine Kinase 3 Receptor (FLT3) Inhibitors
For midostaurin, lower exposure showed a trend towards increased risk of death in patients with acute myeloid leukemia in combination with daunorubicin and cytarabine, but this was not statistically significant, as described in the approval report (n = 360) [101]. For the active metabolite CGP62221, the relationship between exposure and the overall survival was found to be significant (p = 0.0009). No exposure-toxicity relationship was established [101]. A PK/PD analysis including data from 115 patients determined a correlation between both the plasma trough levels of midostaurin/metabolites and blast response (reduction of ≥ 50% in percentage of bone marrow blasts or absolute number of peripheral blood blast) [102]. Given these findings, the potential of TDM for midostaurin should be further explored by focusing on defining a threshold and identifying its feasibility, to see whether TDM for midostaurin (or for the metabolite) is of added value. For gilteritinib, AUC and Cmin levels did not differ between responders and non-responders in the approval report (n = 38), but exposure was associated with a higher incidence of liver enzyme disturbances and creatine kinase increase. For other grade 3 or higher toxicity endpoints, higher exposure was not predictive [103]. Based on these data, there is no rationale to investigate TDM for gilteritinib, but future research on TDM regarding FLT3 inhibitors could be considered for midostaurin.
Hedgehog Pathway Inhibitors
For vismodegib, no relationships between exposure and efficacy (defined as ORR) and toxicity were found in the approval report; however, analyses were hampered by the small sample size (n = 96) [104]. An exposure-response was also absent for sonidegib, with the Cmin, Cmax and AUC overlapping for responders and non-responders (n = 109) [105]. An exposure-toxicity analysis was conducted for creatine kinase increase, but no relationship was observed [105, 106]. For glasdegib, no exposure-response relationship was seen for OS and radiological response, but exposure was significantly associated with dysgeusia, muscle spasms, renal toxicity and QTc-time prolongation (n = 75–272) [107–109] Therapeutic drug monitoring in hedgehog pathway inhibitors could therefore primarily be of valuable in preventing excessive toxicity in patients using glasdegib [108].
Human Epidermal Growth Factor Receptor 2 (HER2) Inhibitors
Lapatinib has a large inter-individual variability in exposure (6-fold for AUC and for Cmin). Although TDM could be useful and lapatinib has been FDA and EMA approved for over 10 years, there are no available reports on an exposure-response relationship [110, 111]. For neratinib, the E-R analysis suggested a positive correlation between exposure and ORR (n = 284). However, this study as described in the approval report was conducted in patients with metastatic breast cancer, although it is only registered for patients with early-stage breast cancer [112]. The exposure-response relationship of tucatinib was described in the Phase III HER2CLIMB study (n = 373). There was a positive trend towards exposure-response for PFS [113]. For all three drugs, no exposure-toxicity relationship was found [111–113]. To conclude, due to the lack of proven exposure-response relationships, there is not yet enough evidence to use TDM for these oral HER2 inhibitors.
Isocitrate Dehydrogenase (IDH) Inhibitors
For ivosidenib, exposure was comparable for responders and non-responders and no relationship with toxicity, except for QTc-prolongation, was found, decreasing the need for TDM (n = 205) [114, 115]. For enasidenib, the approval report included an exposure-response analysis, which was performed separately for patients with an IDH2 R140 or an IDH2 R172 mutation. There was a strong positive relationship between exposure (AUC) and objective response rate in patients with a R140 mutation (n = 131, p = 0.02). A positive relationship between exposure and response was suggested in patients with a R172 mutation as well, but this effect was not statistically significant (p = 0.07), which might be due to a small sample size in the R172 mutated subgroup (n = 46) [116]. Moreover, an increase in enasidenib exposure was associated with a higher incidence of total bilirubin elevation [116]. Therapeutic drug monitoring for enasidenib could be a valuable addition for improving response rates, but no specific threshold has yet been suggested. Further studies should focus on determining a target Cmin for enasidenib and repeating the exposure-response analysis specifically for patients with a R172 mutation in a larger sample size. Olutasidenib has recently been approved by the FDA, but currently published data do not include exposure-response and exposure-toxicity relationship analyses [117].
Janus Kinase (JAK) Inhibitors
Exposure-response relationships have been established in all three compounds; ruxolitinib (n = 309), fedratinib (n = 289), and pacritinib (n = 129). Myelofibrosis-related symptoms and associated splenomegaly are clinical determinants of response. A relationship between exposure and spleen volume reduction was proven for all drugs [118–120]. For ruxolitinib and fedratinib, exposure was also associated with a lower score on the myeloproliferative neoplasm symptom assessment form; a widely used assessment for the symptoms in patients with myeloproliferative neoplasms [118, 119, 121]. This ruxolitinib and fedratinib exposure was also associated with the probability of toxicity, including thrombocytopenia and anemia, as described in the approval reports [118, 119]. For pacritinib, no exposure-safety relationship was found [120]. Despite the existence of exposure-response relationships and therefore a possibility for TDM, no studies determining a target Cmin have yet been performed.
KIT/Platelet-Derived Growth Factor Receptor α (KIT/PDGFRα) Inhibitors
Imatinib was previously described in the BCR-ABL inhibitor section in this review article for the use in CML. As a KIT/PDGFRα tyrosine kinase inhibitor, it is used in GIST with an activating KIT/PDGRFα mutation. A target Cmin of ≥ 1100 ng/mL was previously proposed, based on a positive relationship between exposure and PFS (n = 35–96) demonstrated in a RCT and a TDM feasibility study [122–128]. This target was also advised by the IATDMCT guideline [26]. This feasibility was confirmed in a more recent retrospective study (n = 169), where a PK intervention was possible in 63% of patients with PK levels below Cmin of ≥ 1100 ng/mL [129]. Remarkably, no difference in PFS was found between patients with low and adequate Cmin levels in this study. A possible explanation for this was that the mean exposure in the low-group was only marginally below the target of 1100 ng/mL (i.e., 1050 ng/mL) [129]. Additionally, a cost-effectiveness study from the Netherlands comparing TDM-guided dosing versus fixed dosing suggested that TDM is a financially feasible intervention, described in gained QALYs [130].
The next-generation KIT/PDGFRα inhibitors avapritinib and ripretinib were approved by the FDA/EMA in more recent years. Avapritinib has shown large interpatient PK variability (an 8- to 10-fold range in exposure following 300 mg doses), which could make it a suitable candidate for TDM [131]. The approval report of avapritinib suggests a negative exposure-response relationship at the approved dose, with patients with stable disease having higher average steady state concentrations compared to patients with response. However, this correlation should be interpreted with caution, considering the high response rate (ORR >80%) and small sample size in the study (n = 62) [132]. The exposure-toxicity analysis showed a significant association between avapritinib concentrations and adverse cognitive effects (all < grade 4) and all grade 3/4 AEs [132]. The exposure-response analyses in the approval reports of ripretinib showed a positive trend between higher trough concentrations and longer PFS. However, no definitive conclusion can be drawn due to the small sample size (n = 83) [133]. No significant relationship was found between ripretinib levels and toxicity, although trough levels were lower in patient without AEs compared to patients with any grade of hand-foot syndrome and myalgia [133].
Based on the available data on KIT/PDGFRα inhibitors, TDM seems to be a feasible and cost-effective way of dosing for imatinib, with a target Cmin of ≥ 1100 ng/mL. Studies on the exposure-response relationships of avapritinib and ripretinib are too small to draw conclusions and before deciding on the value of TDM, exposure-response analyses with larger patient groups should be awaited.
Kirsten Rat Sarcoma (KRAS) Inhibitors
The FDA has recently approved the first KRAS inhibitors sotorasib and adagrasib for patients with KRAS G12C mutated NSCLC. For sotorasib, increased exposure was not yet associated with increased efficacy nor with toxicity, by the FDA [134]. Thus far, published reports on adagrasib do not include exposure-response nor exposure-toxicity analyses [135, 136]. Therapeutic drug monitoring is therefore not an obvious option in improving the treatment with sotorasib and adagrasib, and data from ongoing trials of potential other KRAS inhibitors have to be awaited before their potential for TDM could be evaluated.
Mitogen-Activated Protein Kinase (MEK) Inhibitors
Hitherto, three MEK inhibitors have been approved and are available on the market. For cobimetinib, most often combined with vemurafenib, no relationship between exposure and response or toxicity has been found and described in the approval report [137]. For trametinib, exposure was associated with PFS where patients with a Cmin ≥ 10.6 ng/mL had a significantly improved PFS (n = 493) [138]. It is important to note that in this study patients were treated with monotherapy trametinib only, where normally trametinib is most often combined with dabrafenib. A recent study on 140 patients using dabrafenib/trametinib described a significantly prolonged OS and PFS, with a median OS of 22.8 months for patient with a Cmin ≥ 15.6 ng/mL compared to 12.6 months in patients with a Cmin below that level and with a median PFS of 10.9 months for patients with a Cmin ≥ 15.6 ng/mL compared to 6.0 months in patients with a Cmin below that target. This was advised as the optimal threshold for PFS and OS. Noteworthy is that only 37% of patients reached this threshold, and the median exposure was 13.8 ng/mL [50]. No evident relationship between exposure and toxicity was described [50, 139]. Goldwirt et al on the other hand described no significant relationship between trametinib exposure and prolonged OS and PFS in 50 patients using dabrafenib/trametinib. They did describe significantly higher AUC and Cmin levels in patients with toxicity, suggesting an exposure-toxicity relationship [49]. For binimetinib, most often combined with encorafenib, the approval report showed no relationship between exposure and PFS, OS and ORR in 499 patients using encorafenib/binimetinib. The exposure-toxicity analysis showed a positive relationship between exposure and risk of dose reductions. On the contrary, patients with a lower exposure (< 52 ng/mL) had a higher serious AE risk compared to patients a with high exposure [140]. Therefore, TDM in MEK inhibitors is not ready for implementation in clinical practice. Most TDM potential is seen for trametinib, where it could improve efficacy, although the target is still debatable. For now, a Cmin ≥ 10.6 ng/mL seems the most feasible target, considering the possible increase of toxicities at higher exposure. Therapeutic drug monitoring for cobimetinib and binimetinib is not advised based on the currently available data.
Mesenchymal-Epithelial Transition (MET) Inhibitors
Targeting the METex14 skipping mutation, present in 3–4% of pulmonary adenocarcinoma, two highly selective KIs have been FDA approved: capmatinib and tepotinib [141]. Only the approval report discusses the exposure-response relationship of capmatinib. While there was an inconclusive result of the exposure and PFS analysis in capmatinib, no relationship could be established between capmatinib levels and the duration of response and tumor size change (n = 94) [142]. There was, however, a positive correlation between incidence of nausea and elevated lipase and amylase levels and capmatinib exposure [142]. More studies have been conducted on the exposure-response relationship of tepotinib, and all reported an absence of an exposure-response relationship (n = 146–438) [143–146]. These same studies showed no relationship between tepotinib exposure and toxicity, including peripheral edema, a common AE of tepotinib [143–146]. Since there is no evidence for an exposure-response relationship for these MET inhibitors, the use of TDM is not indicated.
Neurotrophic Tyrosine Receptor Kinase (NTRK) Fusion Inhibitors
No trend has been found for better response with rising exposure for larotrectinib and entrectinib, although a small study (n = 66) showed a counter-intuitive relationship for larotrectinib exposure, where a higher response rate was seen with lower exposure [147–149]. This was explained by the small sample size, heterogeneous tumor types, and high response in the pediatric population compared to the adult population, and due to these study characteristics no conclusion on the exposure-response relationship can be drawn. For larotrectinib, no relationship between exposure and toxicity was described in the approval report (n = 66); in exposure-toxicity analyses for entrectinib there is a weak trend between higher exposure and frequency of serious AEs, although this was not significant after correction for gender in the analysis (n = 263). As serious AE rates were higher in female patients than in male patients (18.8% vs 10.1%), and female patients were not evenly distributed between the exposure quartiles, this higher rate of SAEs was confounded by the higher number of female patients in the highest quartile [147, 148]. In another study on entrectinib, a higher incidence of SAEs was found with higher exposure; however, this was mainly at exposures higher than normally reached with the standard 600 mg once-daily dose (n = 89) [149]. Therapeutic drug monitoring for NTRK fusion inhibitors is therefore not yet recommended due to the lack of an exposure-response relationship.
Poly(ADP Ribose) Polymerase (PARP) Inhibitors
Olaparib, the first PARP inhibitor, is available in both capsule and tablet formulation. In the initial EMA and FDA report, no exposure-response relationship was observed for PFS and OS (n = 30) for the capsule or for the tablet [150, 151]. A more recent meta-analysis combining PK data of 398 ovarian cancer patients, identified AUC and Cmax as significant predictors of PFS for tablets [152]. The Cmin value was not significantly correlated with efficacy in this meta-analysis but was associated with early onset of grade III or IV AEs (most commonly anemia) in another retrospective study (n = 27) [153]. The authors found an increased risk of severe toxicity (OR = 1.31, 95% CI 1.10–1.57) for each additional 1000 ng/mL and determined a Cmin of 2500 ng/mL as a threshold for prediction of severe toxicity in both patients using capsules and tablets [153]. A recent study confirmed the absence of a relationship between Cmin and PFS (n = 35) [154]. The association between exposure and a more severe grade of anemia was also confirmed in other exposure-toxicity studies [150, 152]. For the more recently approved PARP inhibitors niraparib and rucaparib, the existence of an exposure-response relationship is still uncertain. In both approval reports, exposure (defined as steady state AUC) was not associated with PFS (n = 480 and n = 375) [155, 156]. However, in patients using niraparib and rucaparib, there were specific subgroups for whom an exposure-response relationship could be determined [155, 157, 158]. In the BReast CAncer (BRCA)-mutated patients (n = 150) using niraparib, PFS was significantly shorter in patients with an AUC lower than 16.1 µg*h/mL. An additional Cox proportional hazards model confirmed this statistically significant difference for this group [155]. In the subgroup of patients with platinum-sensitive disease using rucaparib (n = 75), a significant correlation was observed between exposure and radiological response according to RECIST [159]. Moreover, exposure was associated with several safety endpoints such as hematological AEs and fatigue (for niraparib and rucaparib) and with liver enzyme and creatinine increase (for rucaparib), although the latter is less likely because of an impaired renal function, but because of rucaparib’s interaction with the transporters responsible for the reabsorption of creatinine in the renal tubular cells [158, 159]. For the latest PARP inhibitor talazoparib, the relationship between exposure and response is more evident. Average Cmin of talazoparib was found to be an independent covariate for longer PFS in a multivariate analysis (n = 285) and was also associated with a higher risk of anemia and thrombocytopenia [160, 161]. In conclusion, TDM could be a valuable tool in personalizing the treatment for niraparib, rucaparib and talazoparib, especially for preventing excessive toxicity. A threshold Cmin should first be determined to assess the feasibility, and more in-depth analyses are needed to demonstrate an exposure-response relationship in specific subgroups. Therapeutic drug monitoring could also be a valuable addition in the treatment with olaparib for both formulations, but the threshold should be further validated before it can be implemented in daily practice.
Phosphoinositide 3-kinase (PI3K) Inhibitors
In hematology, idelalisib and duvelisib are used. For idelalisib, no relationships between exposure and response nor toxicity have been described in the literature (n = 59) [162, 163]. When administered in combination with other drugs (e.g., rituximab or ofatumumab), no exposure-response nor -toxicity relationships were found (n = 207 and n = 171) [164, 165]. For duvelisib, the approval report did not state a relationship between exposure and response nor with toxicity (n = 552) [166]. Due to the lack of these relationships, TDM does not seem a useful tool to personalize therapy for both idelalisib and duvelisib. Alpelisib is used in oncology, and the presence or absence of an exposure-response relationship at the approved dose is less conclusive. In the FDA and EMA reports, a trend was found between exposure (defined as Cmin) and PFS, although not significant (n = 169–254) [167, 168]. However, it was stated that a definitive conclusion was difficult to make as frequent dose reductions and the short half-life of alpelisib might be a confounding factor. The exposure-toxicity analysis showed a significant correlation between exposure and risk of hyperglycemia (for both grade ≥ 2 and grade ≥ 3) [168]. Further TDM research in PI3K inhibitors should therefore primarily focus on alpelisib by confirming a potential exposure-response relationship and subsequently determining a target threshold.
Rearranged during Transfection (RET) Fusion Inhibitors
For selpercatinib and pralsetinib, two RET fusion-specific KIs, exposure has no significant relationship with tumor response as described in the approval reports (n = 160 and n = 149) [169–171]. With regard to toxicity, a significant relationship between the occurrence of pneumonia and anemia grade 3 and exposure of pralsetinib was established. Moreover, higher pralsetinib levels were associated with a decrease in absolute neutrophil count and minor increases of aspartate transaminase (AST) and alkaline phosphatase (ALT), but these associations were not statistically significant [170]. The exposure-toxicity analysis for selpercatinib showed no significant correlation [171]. TDM cannot be advised, considering the lack of exposure-response relationships.
Vascular Endothelial Growth Factor Receptor (VEGFR) Inhibitors
Selective VEGFR Inhibitors
For axitinib, the presence of an exposure-response (for AUC) and exposure-toxicity relationship (for hypertension, proteinuria, fatigue, and diarrhea) was clearly established in the approval reports (n = 233) [172, 173]. The finding of the exposure-response relationship was supported by multiple other studies, although the suggested targets (Cmax ≥ 12.4ng/mL [n = 20], AUC0–12 150 ng*h/mL [n = 26] and AUC ≥ 300 ng*h/mL [n =168], respectively) are less convenient for TDM as these require several blood samples [174–176]. Two small studies proposed a target Cmin of 5 ng/mL, but this requires confirmation in more patients (n = 24 and n = 35) [177, 178]. Moreover, no data on an exposure-response nor -toxicity relationship for axitinib in combination with immunotherapy is yet available. For tivozanib, an exposure-response relationship between PFS and average exposure (Cavg) (26 weeks in lowest quartile vs 72 weeks in highest quartile) was described in the approval report, which makes it a promising candidate for TDM (n = 364). Moreover, exposure was associated with occurrence of more severe hand-foot syndrome and hypertension [179]. Future studies should explore the potential of TDM for tivozanib by determining a target and to further confirm the axitinib target Cmin of ≥ 5 ng/mL in a larger patient group and investigate the exposure-response relationship for axitinib/immunotherapy combination.
Multi-kinase Inhibitors Targeting VEGFR
Cabozantinib Exposure-response analyses in renal-cell carcinoma (RCC) and hepatocellular carcinoma (HCC) patients using cabozantinib monotherapy showed a positive correlation between cabozantinib concentration and PFS (n = 76–452) [180–182]. These studies also described an increased risk of grade ≥ 3 AEs with higher cabozantinib levels. For both tumor types, higher exposure was correlated with more dose reductions [180–182]. A study with 76 metastatic RCC (mRCC) patients suggested an efficacy target of Cmin ≥ 537 ng/mL and a threshold for toxicity of Cmin ≤ 618 ng/mL, as these were the best predictors for disease progression and relevant toxicity based on ROC curves [182]. A TDM study with 59 mRCC patients targeting a Cmin of 750 ng/mL (based on the average exposure of the 40 mg dose) showed no difference in either PFS or OS. With a median exposure of 543 ng/mL at the best tolerated dose level, this suggests that the 750 ng/mL target might be too high [183]. In a study where cabozantinib was given combined with nivolumab (n = 33), no association was shown between cabozantinib exposure and PFS, but there was a significant relationship between incidence of diarrhea and hand-foot syndrome and cabozantinib levels [184]. In conclusion, studies investigating the exposure-response relationship of cabozantinib have produced conflicting results, potentially due to the use of a non-feasible high target in some of these studies. In monotherapy cabozantinib, TDM could be a useful tool, but with a Cmin target of around 537 ng/mL. In the setting combined with nivolumab there is no evidence for the use of TDM. In both settings, TDM might be useful to prevent toxicity.
Lenvatinib Although both approval reports describe no exposure-response relationship at the approved dose (n = 260) [185, 186], a PK/PD model suggested a correlation between exposure and reduction in tumor size (n = 50) [187]. Current studies suggest an exposure-toxicity relationship, with higher exposure leading to a higher incidence of grade 3 or higher nausea, hypertension and proteinuria (n = 45–260) [186–190]. One study including 48 patients did not find an exposure-toxicity relationship besides increased ALT/AST and bilirubin levels. The authors suggest a threshold for toxicity of ≤ 88 ng/mL [188]. With no exposure-response relationship established, no efficacy target for TDM can be proposed.
Nintedanib Only exploratory PK analyses have been completed and described in the approval reports. Exploratory logistic regressions showed a significant relationship between exposure and the anti-angiogenic effect of nintedanib, but this was not associated with survival endpoints [191]. These first PK-analyses showed an association between increased liver enzymes and exposure [191]. In a group of idiopathic pulmonary fibrosis patients, exposure was also associated with increased risk of gastrointestinal disorders [192]. For now, there is no evidence to use TDM for nintedanib.
Pazopanib There have been several studies on the exposure-response and exposure-toxicity relationships of pazopanib, with a suggested efficacy target Cmin of ≥ 20.5 mg/L [193–201]. The largest study, including samples of 315 advanced RCC patients, showed that patients with a Cmin ≥ 20.5 mg/L had a significantly longer disease-free survival than patients below this target. This target was achieved by 82% of patients at Weeks 3 or 5 of treatment and 75% of patients at Weeks 16 or 20 of treatment [199]. Patients with higher trough levels at the beginning of treatment had more AEs of all grades, although this correlation was not found for exposure and grade ≥ 3 AEs, with the exception of grade ≥ 3 hypertension [199]. Another study including both RCC and soft tissue sarcoma patients (n = 61) confirmed the previously proposed target Cmin of ≥ 20.5 mg/L for longer PFS for both tumor types. No significant relationship between exposure and safety was found in this study [200]. A study with 205 mRCC patients suggested a relationship between exposure (≥ 46 mg/L) and toxicity, e.g., diarrhea, hypertension and hand-foot syndrome [196]. A smaller study (n = 28) showed that Cmin levels above >50.3 mg/L were predictive for grade ≥ 3 toxicity [201]. Therapeutic drug monitoring feasibility was demonstrated in a study (n = 30) in which dose adjustments successfully increased the mean Cmin above the target of 20 mg/L [195]. Taking all the studies into consideration, TDM for pazopanib can be recommended, with a Cmin target for efficacy of ≥ 20.5 mg/L and a threshold for toxicity of ≤ 46 mg/L.
Regorafenib The PK analysis of a Phase III study showed no significant exposure-response relationship at the approved dose (n = 327); only a tendency for the OS and time to progression (TTP) to be longer with higher PK levels, in correspondence with the FDA report (n = 41) [202, 203]. However, this study did not show an association between toxicity and regorafenib concentrations, while the EMA and FDA reported otherwise for GIST and colorectal cancer (CRC) [202, 204]. A recent dose-escalation study in patients with metastatic CRC (mCRC) showed a significant association between regorafenib levels combined with its active metabolites M2/M5 and the highest grade of AEs (n = 70) [205]. A study on regorafenib in CRC, GIST and HCC patients (n = 34), published in 2021, reported that the PFS was significantly longer when the combined trough level of regorafenib + metabolites M2/M5 was ≥ 2900 ng/mL and the dose-limiting toxicity (DLT) incidence (mostly hand-foot syndrome) was significantly higher when this level was ≥ 4300 ng/mL [206]. A clinical intervention study in mCRC patients (clinicaltrials.gov: NCT04874207) aiming to find optimal regorafenib levels with TDM to improve OS has been open since 2021, and results are being awaited for. This study could provide more information on the need and efficacy of TDM, as long as M2/M5 concentrations are considered.
Sorafenib Previous small studies suggested an exposure-response and exposure-toxicity relationships, but more confirmation, a proposed exposure target and research on the metabolite N-oxide were needed [207–212]. A recent study described a correlation between trough levels and serious AEs (≥ grade 3), with a threshold of 3450 ng/mL, although this study is confounded by the small sample size (n = 20). Moreover, there was a significant relationship between higher concentrations and response, with a threshold of 1400 ng/mL. Patients between 1400 and 3450 ng/mL tended to have longer PFS and OS [213]. A feasibility study on TDM in patients using sorafenib showed that 83% of the 36 patients had at least one sample below the target of 3750 ng/mL [214]. This target was based on the mean Cmin at the approved dose of 400 mg twice daily. Of these patients, only 11 were able to undergo a dose adjustment, of which 3 interventions resulted in a trough level above the target without excessive additional toxicity. In the other patients, toxicity restricted the possibility to adjust the dose [214]. For now, there is evidence that TDM is not feasible, mainly because TDM driven dose increases are limited by (severe) toxicity.
Sunitinib For sunitinib and its active metabolite N-desethyl sunitinib, a clear exposure-response and exposure-toxicity relationship has been described, with a suggested efficacy sum target Cmin of ≥ 37.5 ng/mL when dosed continuously and a sum target Cmin of ≥ 50 ng/mL when dosed intermittently (once daily in a 4-weeks on 2-weeks off schedule) (n = 20–443) [215–224]. A meta-analysis containing PK levels of 443 patients, found a significant relationship between sunitinib + metabolite AUC exposure and PFS and OS [216]. A real-world study demonstrating PK-guided dosing in 71 mRCC and mGIST patients found that for patients with low PK, who received a dose intervention and had an adequate PK afterwards (n = 23), median time on treatment was significantly longer. All Cmin levels were dose regimen normalized to a continuous dosing schedule, to make comparison between intermittent and continuous dosed patients possible, which was possible because of sunitinib’s dose-proportional PK. A significant difference was found in median dosing regimen normalized combined sunitinib + metabolite exposure between patients with and without toxicity (60 ng/mL and 44 ng/mL) [222]. Although previously, Cmin toxicity thresholds of 75 ng/mL (continuous) or 87.5 ng/mL (intermittent) have been suggested [219, 220, 223], this real-world study suggests slightly lower thresholds of Cmin of 60 ng/mL (continuous) and 80 ng/mL (intermittent). A recent systematic review reports on the cost effectiveness of TDM-guided dosing of sunitinib in mRCC patients in the USA and China. This article suggests that TDM guided dosing is cost effective, described in an increase of QALYs by 0.83, especially when an intervention is advised with high exposure to prevent toxicity [225]. In conclusion, based on the apparent exposure-response and exposure-toxicity relationship and the cost effectiveness, TDM should be considered for sunitinib targeting a combined sunitinib + metabolite Cmin of ≥ 37.5 ng/mL when dosing continuously and a Cmin of ≥ 50 ng/mL when dosing intermittently. Toxicity thresholds are around 60–75 ng/mL (continuous dosing) and 80–87.5 ng/mL (intermittent dosing). A RCT studying traditional dosing versus TDM-guided dosing is reportedly underway, which can be the much-needed confirmation to use of TDM-guided dosing in patients using sunitinib [226].
Vandetanib The approval report describes the absence of an exposure-response relationship, and the presence of an exposure-toxicity relationship for fatigue, diarrhea, and QTc prolongation (n = 231) [227]. Since then, no more data have become available and the use of TDM is therefore not yet indicated for patients using vandetanib.
Other Kinase Inhibitors Used in Oncology
Everolimus Multiple studies have described the positive exposure-response and exposure-toxicity relationships for everolimus in several tumor types (both n = 42) [228, 229]. A meta-analysis from Phase II/III studies with data of 945 patients showed a relationship between everolimus exposure and tumor size reduction, and between exposure and grade > 3 stomatitis, pulmonary and metabolic events [230]. In the pancreatic neuroendocrine tumors (pNET) and RCC patients included in this meta-analysis, Cmin values ≥ 10 ng/mL were associated with longer PFS. No toxicity threshold was suggested [230]. A study in 42 patients showed that patients with Cmin < 11.9 ng/mL had a 3-fold increased disease progression risk and patients ≥ 26.2 ng/mL had a 4-fold increased toxicity risk [231]. Another study including 44 patients suggested a toxicity threshold of 19.2 ng/mL with 11 of 13 patients above this threshold needing a dose reduction [232]. Based on the described studies, there is substantial evidence for both a positive exposure-response and an exposure-toxicity relationship. The TDM efficacy target Cmin of >10 ng/mL seems most apparent. For toxicity, no definite conclusions can be drawn on the target due to the small patient numbers in the reported studies, but current evidence suggests a threshold between 19.2 and 26.2 ng/mL.
Pexidartinib Pexidartinib was studied in a recent E-R analysis using a PopPK model. This study showed a trend towards better efficacy with higher pexidartinib exposure, although only a small difference was observed with a small sample size (n = 65) [233, 234]. This analysis showed a significant relationship between the pexidartinib concentrations and ALT/AST elevation. This association was most apparent during the first 14 days of treatment [233, 234]. With insufficient data on this drug, TDM cannot be advised.
Discussion
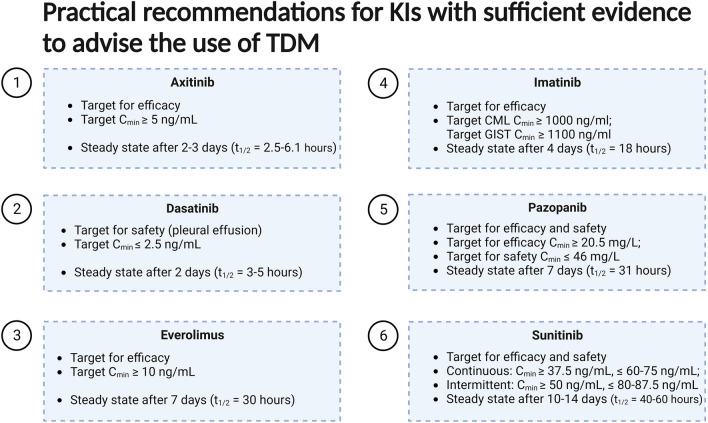
The aim of this review was to give an overview of the available evidence for TDM of the currently approved KIs. Exposure-response and/or exposure-toxicity relationships at the approved dose have been established for many KIs used in hematology and oncology. In recent years, this has been translated into several clinical studies investigating the use of TDM. Recently, a prospective multicenter study showed that TDM is feasible in daily clinical practice for a selection of commonly used KIs. In this study, adequate exposure and manageable tolerability were achieved after a pharmacokinetically guided intervention in the majority of the patients [6]. This current review showed that for six KIs, sufficient data from prospective studies are already available to advise the use of TDM in daily clinical practice (Fig. 4). Ongoing studies, such as a RCT on TDM-guided dosing for alectinib [21] and a Dutch nationwide implementation project for TDM of imatinib, sunitinib and pazopanib [235], could be the final step for these six KIs to incorporate TDM in (inter)national guidelines. For imatinib, the International Association of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT) released a consensus guideline in 2021 with clear recommendations for the use of TDM [26]. This guideline could serve as an example for other oncology organizations to incorporate TDM in their guidelines, in order to foster the widespread use of TDM for KIs for which sufficient evidence is available. At this time, the current evidence provided in Fig. 4 could already serve as a tool for clinicians to implement TDM in their current practice. For a substantial number of KIs, especially for the recently approved KIs (Table 2), data on potential exposure relationships are lacking, which hampers the further investigations regarding TDM for that drug. It is important to routinely incorporate exposure-response and -toxicity analyses in all Phase III trials for new KIs, so a potential for TDM can be observed early. Additionally, 11 currently used KIs were classified as promising in this review as a TDM threshold was proposed based on retrospective data. For these drugs, prospective studies will likely follow to validate their thresholds and further establish the role of TDM in treatment with these drugs.
Fig. 4.
Summary figure with TDM targets for drugs with enough evidence to advise the use of TDM in clinical practice.
Created with Biorender.org
Table 2.
Distribution between exposure-response or -toxicity relationship per drug
| Exposure-toxicity relationship | No exposure-toxicity relationship observed | Exposure-toxicity relationship inconclusive | |||
|---|---|---|---|---|---|
| Exposure-response relationship | 20 drugs | 6 drugs | 0 drugs | ||
| Abemaciclib | Nilotinib | Alectinib | |||
| Axitinib | Niraparib | Asciminib | |||
| Bosutinib | Pazopanib | Crizotinib | |||
| Brigatinib | Rucaparib | Midostaurin | |||
| Dasatinib | Ruxolitinib | Neratinib | |||
| Enasidenib | Sorafenib | Pacritinib | |||
| Everolimus | Sunitinib | ||||
| Fedratinib | Talazoparib | ||||
| Gefitinib | Tivozanib | ||||
| Imatinib | Vemurafenib | ||||
| No exposure-response relationship observed | 14 drugs | 16 drugs | 2 drugs | ||
| Afatinib | Lorlatinib | Acalabrutinib | Larotrectinib | Binimetinib | |
| Ceritinib | Mobocertinib | Cobimetinib | Selpercatinib | Lenvatinib | |
| Dacomitinib | Olaparib | Dabrafenib | Sonidegib | ||
| Encorafenib | Osimertinib | Duvelisib | Sotorasib | ||
| Gilteritinib | Palbociclib | Entrectinib | Tepotinib | ||
| Glasdegib | Pralsetinib | Erdafitinib | Tucatinib | ||
| Ivosidenib | Vandetanib | Ibrutinib | Vismodegib | ||
| Idelalisib | Zanubrutinib | ||||
| Exposure-response relationship inconclusive | 12 drugs | 4 drugs | 3 drugs | ||
| Alpelisib | Infigratinib | Lapatinib | Adagrasib | ||
| Avapratinib | Nintedanib | Olutasidenib | Ponatinib | ||
| Cabozantinib | Pemigatinib | Pirtobrutinib | Trametinib | ||
| Capmatinib | Pexidartinib | Ripretinib | |||
| Erlotinib | Regorafenib | ||||
| Futibatinib | Ribociclib | ||||
Exposure-response/-toxicity relationship inconclusive means that no data is yet available for that drug regarding this relationship
Different PK parameters could be used as target thresholds for TDM, such as the Ctrough, the Cmax or the AUC. Most available studies have solely focused on targeting the Ctrough, as measuring this parameter is the most simple and feasible method in daily practice. In addition, the Ctrough is pharmacologically a relevant measure for drugs with reversible inhibition of a certain target as it reflects the lowest level of inhibition in a dosing interval. Targeting the Cmax or AUC is logistically more challenging as this would require specific time sampling (for the Cmax) or multiple sampling in a short amount of time (for the AUC). Therefore, in this review we specifically chose to focus on the Ctrough as a target for TDM, making it more implementable in routine patient care. However, it is important to note that intrapatient variability can impact the interpretation of a single Ctrough measured over time. Moreover, both the analytical methods used for measurement and the establishment of target thresholds are associated with a certain degree of uncertainty. This is particularly relevant in cases where the concentration falls just slightly above or below the defined threshold. By obtaining multiple measurements over time, clinicians can better assess the consistency and trend of drug concentrations, correcting for analytical and threshold uncertainties.
Traditionally, TDM has primarily focused on improving efficacy endpoints. However, many KIs were shown to only have an established exposure-toxicity relationship and no exposure-response relationship at the approved dose (Tables 1 and 2), potentially resulting from the fact that dose selection is primarily based on finding the maximum tolerated dose (MTD) of the drug [236]. Because of the MTD dose-finding model, it is thought that these drugs are relatively overdosed, explaining an absent exposure-response relationship at the approved dose. Therapeutic drug monitoring could therefore play an important role in improving drug safety by identifying patients who are at risk of toxicity, especially for these drugs with only an exposure-toxicity relationship.
For some other drug classes, such as antibiotics, TDM is already more commonly used for preventing excessive toxicity [237]. It is important to note that TDM for preventing toxicity requires a short turnaround time between the PK sample and the clinical intervention. A solid infrastructure between the treating physician, the laboratories and the pharmacy is therefore crucial. In addition, new methods like volumetric absorptive micro-sampling could provide adequate trough levels before outpatient appointments in a non-invasive way, with finger-pricks performed by the patient at home and shipment to the hospital per post. This method has been studied as a way of TDM in other research fields [238, 239], but its use in oncology remains to be elucidated [240].
Currently, the vast majority of available KIs form a reversible binding with the target kinase (Table 1), meaning that they bind through non-covalent interactions, such as hydrogen bonds, electrostatic interactions, or hydrophobic interactions, allowing the inhibitor to dissociate from the kinase [241]. On the other hand, some KIs are known to bind irreversibly (covalent bond) to their target, leading to a stable and long-lasting inhibition [241]. However, an analysis of available data, as presented in Table 1, indicates that clear E-R relationships have not been established for any irreversible KIs. This observation could theoretically be attributed to the irreversible binding characteristic of these inhibitors, which complicate the direct correlation between drug exposure and pharmacological response, making it more challenging to monitor drug effect solely on the blood concentration [242]. Therapeutic drug monitoring for irreversible inhibitors may require additional considerations, such as assessing target engagement through biopsy.
For KIs, for which there is insufficient evidence to routinely implement TDM, there are indications where TDM could still be a useful tool. For instance, it can provide more information on drug exposure in cases of potentially relevant drug-drug interactions or altered absorption (e.g. after a gastric bypass surgery). Therapeutic drug monitoring can also be helpful to differentiate between patients with toxicity due to high exposure and those with toxicity below expected exposure levels, which helps in deciding whether to lower the dose or switch therapy to tackle the toxicity.
Conclusion
Therapeutic drug monitoring can be a practical tool for personalizing therapy with KIs, which are often largely variable in exposure. This review gives a highly needed update on the potential of TDM for the currently available KIs and their exposure-response and exposure-toxicity relationships. We provide a corresponding evidence level and practical recommendations for each drug based on the available data. Randomized, prospective studies are needed to confirm the beneficial effect of TDM before it can be incorporated into daily clinical practice.
Declarations
Funding
No external funding was used in the preparation of this article.
Conflict of Interest
RHJM reported funding for investigator-initiated research from: Astellas, Bayer, Boehringer-Ingelheim, Cristal Therapeutics, Novartis, Pamgene, Pfizer, Roche, Sanofi & Servier. All outside the submitted work, and payment to the institute. NS provided consultation or attended advisory boards for Boehringer Ingelheim, Cogent Biosciences, Ellipses Pharma, Incyte, Luszana. N Steeghs received research grants from Abbvie, Actuate Therapeutics, Amgen, Array, Ascendis Pharma, AstraZeneca, Bayer, Blueprint Medicines, Boehringer Ingelheim, BridgeBio, Bristol-Myers Squibb, Cantargia, CellCentric, Cogent Biosciences, Cresecendo Biologics, Cytovation, Deciphera, Dragonfly, Eli Lilly, Exelixis, Genentech, GlaxoSmithKline, IDRx, Immunocore, Incyte, InteRNA, Janssen, Kinnate Biopharma, Kling Biotherapeutics, Luszana, Merck, Merck Sharp & Dohme, Merus, Molecular Partners, Navire Pharma, Novartis, Numab Therapeutics, Pfizer, Relay Pharmaceuticals, Revolution Medicin, Roche, Sanofi, Seattle Genetics, Taiho, Takeda. All outside the submitted work, all payment to the Netherlands Cancer Institute. MvdK, NG, JV, AH and SK have declared no conflicts of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors consent to publication of this article.
Data Availability
Not applicable because this is a review article.
Code Availability
Not applicable.
Author Contributions
MvdK and NG performed the literature review and wrote the first draft. All authors were involved in the critical revision of the data, and the final manuscript.
Footnotes
Maud B. A. van der Kleij, Niels A. D. Guchelaar share first-authorship.
Stijn L. W. Koolen, Neeltje Steeghs share last-authorship.
References
- 1.Yu H, Steeghs N, Nijenhuis CM, Schellens JH, Beijnen JH, Huitema AD. Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: focus on the pharmacokinetic targets. Clin Pharmacokinet. 2014;53(4):305–325. doi: 10.1007/s40262-014-0137-2. [DOI] [PubMed] [Google Scholar]
- 2.Hussaarts K, Veerman GDM, Jansman FGA, van Gelder T, Mathijssen RHJ, van Leeuwen RWF. Clinically relevant drug interactions with multikinase inhibitors: a review. Ther Adv Med Oncol. 2019;11:1758835918818347. doi: 10.1177/1758835918818347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathijssen RH, Sparreboom A, Verweij J. Determining the optimal dose in the development of anticancer agents. Nat Rev Clin Oncol. 2014;11(5):272–281. doi: 10.1038/nrclinonc.2014.40. [DOI] [PubMed] [Google Scholar]
- 4.Veerman GDM, Hussaarts K, Jansman FGA, Koolen SWL, van Leeuwen RWF, Mathijssen RHJ. Clinical implications of food-drug interactions with small-molecule kinase inhibitors. Lancet Oncol. 2020;21(5):e265–e279. doi: 10.1016/S1470-2045(20)30069-3. [DOI] [PubMed] [Google Scholar]
- 5.Verheijen RB, Yu H, Schellens JHM, Beijnen JH, Steeghs N, Huitema ADR. Practical recommendations for therapeutic drug monitoring of kinase inhibitors in oncology. Clin Pharmacol Ther. 2017;102(5):765–776. doi: 10.1002/cpt.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groenland SL, van Eerden RAG, Westerdijk K, Meertens M, Koolen SLW, Moes D, et al. Therapeutic drug monitoring-based precision dosing of oral targeted therapies in oncology: a prospective multicenter study. Ann Oncol. 2022;33(10):1071–1082. doi: 10.1016/j.annonc.2022.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Groenland SL, Mathijssen RHJ, Beijnen JH, Huitema ADR, Steeghs N. Individualized dosing of oral targeted therapies in oncology is crucial in the era of precision medicine. Eur J Clin Pharmacol. 2019;75(9):1309–1318. doi: 10.1007/s00228-019-02704-2. [DOI] [PubMed] [Google Scholar]
- 8.Mueller-Schoell A, Groenland SL, Scherf-Clavel O, van Dyk M, Huisinga W, Michelet R, et al. Therapeutic drug monitoring of oral targeted antineoplastic drugs. Eur J Clin Pharmacol. 2021;77(4):441–464. doi: 10.1007/s00228-020-03014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groenland SL, Verheijen RB, Joerger M, Mathijssen RHJ, Sparreboom A, Beijnen JH, et al. Precision dosing of targeted therapies is ready for prime time. Clin Cancer Res. 2021;27(24):6644–6652. doi: 10.1158/1078-0432.CCR-20-4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Food and Drug Administration. Center for Drug Evaluation and Research Crizotinib Clinical Pharmacology and Biopharmaceutics Review. 2011. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202570orig1s000clinpharmr.pdf. Accessed 1 Jul 2023.
- 11.Groenland SL, Geel DR, Janssen JM, de Vries N, Rosing H, Beijnen JH, et al. Exposure-response analyses of anaplastic lymphoma kinase inhibitors crizotinib and alectinib in non-small cell lung cancer patients. Clin Pharmacol Ther. 2021;109(2):394–402. doi: 10.1002/cpt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau YY, Gu W, Ho YY, Hong Y, Zhang X, Urban P. Application of time-dependent modeling for the exposure-efficacy analysis of ceritinib in untreated ALK-rearranged advanced NSCLC patients. Cancer Chemother Pharmacol. 2019;84(3):501–511. doi: 10.1007/s00280-019-03830-5. [DOI] [PubMed] [Google Scholar]
- 13.Food and Drug Administration. Center for Drug Evaluation and Research Ceritinib Clinical Pharmacology and Biopharmaceutics Review. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205755orig1s000clinpharmr.pdf. Accessed 1 Jul 2023.
- 14.Chen J, Ruiz-Garcia A, James LP, Peltz G, Thurm H, Clancy J, et al. Lorlatinib exposure-response analyses for safety and efficacy in a phase I/II trial to support benefit-risk assessment in non-small cell lung cancer. Clin Pharmacol Ther. 2021;110(5):1273–1281. doi: 10.1002/cpt.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Lorviqua European public assessment report. 2019. https://www.ema.europa.eu/en/documents/assessment-report/lorviqua-epar-public-assessment-report_en.pdf. Accessed 1 Jul 2023.
- 16.Food and Drug Administration. Center for Drug Evaluation and Research Brigatinib Clinical Pharmacology and Biopharmaceutics Review. 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208772Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 17.Gupta N, Reckamp KL, Camidge DR, Kleijn HJ, Ouerdani A, Bellanti F, et al. Population pharmacokinetic and exposure-response analyses from ALTA-1L: Model-based analyses supporting the brigatinib dose in ALK-positive NSCLC. Clin Transl Sci. 2022;15(5):1143-1154 [DOI] [PMC free article] [PubMed]
- 18.Smart K, Hsu JC, Jaminion F, Guerini E, Shaw AT, Zhou C, et al. Alectinib exposure-response (ER) in ALK-inhibitor naïve ALK-positive NSCLC patients: Pooled analysis across phase III studies. J Clin Oncol. 2019;37(15_suppl):e20575-e. [Google Scholar]
- 19.Food and Drug Administration. Center for Drug Evaluation and Research Alectinib Clinical Pharmacology and Biopharmaceutics Review. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/208434Orig1s000ClinPharmR.pd. Accessed 1 Jul 2023.
- 20.Morcos PN, Nueesch E, Jaminion F, Guerini E, Hsu JC, Bordogna W, et al. Exposure-response analysis of alectinib in crizotinib-resistant ALK-positive non-small cell lung cancer. Cancer Chemother Pharmacol. 2018;82(1):129–138. doi: 10.1007/s00280-018-3597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meertens M, Muntinghe-Wagenaar MB, Sikkema BJ, Lopez-Yurda M, Retèl VP, Paats MS, et al. Therapeutic drug monitoring guided dosing versus standard dosing of alectinib in advanced ALK positive non-small cell lung cancer patients: Study protocol for an international, multicenter phase IV randomized controlled trial (ADAPT ALEC). Front Oncol. 2023;13:1136221. [DOI] [PMC free article] [PubMed]
- 22.Picard S, Titier K, Etienne G, Teilhet E, Ducint D, Bernard MA, et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2007;109(8):3496–3499. doi: 10.1182/blood-2006-07-036012. [DOI] [PubMed] [Google Scholar]
- 23.Guilhot F, Hughes TP, Cortes J, Druker BJ, Baccarani M, Gathmann I, et al. Plasma exposure of imatinib and its correlation with clinical response in the Tyrosine Kinase Inhibitor Optimization and Selectivity Trial. Haematologica. 2012;97(5):731–738. doi: 10.3324/haematol.2011.045666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larson RA, Druker BJ, Guilhot F, O'Brien SG, Riviere GJ, Krahnke T, et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. 2008;111(8):4022–4028. doi: 10.1182/blood-2007-10-116475. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi N, Wakita H, Miura M, Scott SA, Nishii K, Masuko M, et al. Correlation between imatinib pharmacokinetics and clinical response in Japanese patients with chronic-phase chronic myeloid leukemia. Clin Pharmacol Ther. 2010;88(6):809–813. doi: 10.1038/clpt.2010.186. [DOI] [PubMed] [Google Scholar]
- 26.Clarke WA, Chatelut E, Fotoohi AK, Larson RA, Martin JH, Mathijssen RHJ, et al. Therapeutic drug monitoring in oncology: International Association of Therapeutic Drug Monitoring and Clinical Toxicology consensus guidelines for imatinib therapy. Eur J Cancer. 2021;157:428–440. doi: 10.1016/j.ejca.2021.08.033. [DOI] [PubMed] [Google Scholar]
- 27.Gotta V, Widmer N, Decosterd LA, Chalandon Y, Heim D, Gregor M, et al. Clinical usefulness of therapeutic concentration monitoring for imatinib dosage individualization: results from a randomized controlled trial. Cancer Chemother Pharmacol. 2014;74(6):1307–1319. doi: 10.1007/s00280-014-2599-1. [DOI] [PubMed] [Google Scholar]
- 28.Rousselot P, Johnson-Ansah H, Huguet F, Legros L, Escoffre-Barbe M, Gardembas M, et al. Personalized daily doses of imatinib by therapeutic drug monitoring increase the rates of molecular responses in patients with chronic myeloid leukemia final. Results of the randomized OPTIM imatinib study. Blood. 2015;126(23):133. [Google Scholar]
- 29.Conti RM, Padula WV, Becker RV, Salamone S. The cost-effectiveness of therapeutic drug monitoring for the prescription drug-based treatment of chronic myeloid leukemia. J Manag Care Spec Pharm. 2021;27(8):1077–1085. doi: 10.18553/jmcp.2021.27.8.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brave M, Goodman V, Kaminskas E, Farrell A, Timmer W, Pope S, et al. Sprycel for chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate. Clin Cancer Res. 2008;14(2):352–359. doi: 10.1158/1078-0432.CCR-07-4175. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Hochhaus A, Kantarjian HM, Agrawal S, Roy A, Pfister M, et al. Dasatinib pharmacokinetics and exposure-response (E-R): relationship to safety and efficacy in patients (pts) with chronic myeloid leukemia (CML) J Clin Oncol. 2008;26(15_suppl):3590. [Google Scholar]
- 32.Wang X, Roy A, Hochhaus A, Kantarjian HM, Chen TT, Shah NP. Differential effects of dosing regimen on the safety and efficacy of dasatinib: retrospective exposure-response analysis of a Phase III study. Clin Pharmacol. 2013;5:85–97. doi: 10.2147/CPAA.S42796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura M. Therapeutic drug monitoring of imatinib, nilotinib, and dasatinib for patients with chronic myeloid leukemia. Biol Pharm Bull. 2015;38(5):645–654. doi: 10.1248/bpb.b15-00103. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi N, Miura M, Scott SA, Niioka T, Sawada K. Pharmacokinetics of dasatinib for Philadelphia-positive acute lymphocytic leukemia with acquired T315I mutation. J Hematol Oncol. 2012;5:23. doi: 10.1186/1756-8722-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rousselot P, Mollica L, Guilhot J, Guerci A, Nicolini FE, Etienne G, et al. Dasatinib dose optimisation based on therapeutic drug monitoring reduces pleural effusion rates in chronic myeloid leukaemia patients. Br J Haematol. 2021;194(2):393–402. doi: 10.1111/bjh.17654. [DOI] [PubMed] [Google Scholar]
- 36.Larson RA, Yin OQ, Hochhaus A, Saglio G, Clark RE, Nakamae H, et al. Population pharmacokinetic and exposure-response analysis of nilotinib in patients with newly diagnosed Ph+ chronic myeloid leukemia in chronic phase. Eur J Clin Pharmacol. 2012;68(5):723–733. doi: 10.1007/s00228-011-1200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giles FJ, Yin OQ, Sallas WM, le Coutre PD, Woodman RC, Ottmann OG, et al. Nilotinib population pharmacokinetics and exposure-response analysis in patients with imatinib-resistant or -intolerant chronic myeloid leukemia. Eur J Clin Pharmacol. 2013;69(4):813–823. doi: 10.1007/s00228-012-1385-4. [DOI] [PubMed] [Google Scholar]
- 38.Mita A, Abumiya M, Miura M, Niioka T, Takahashi S, Yoshioka T, et al. Correlation of plasma concentration and adverse effects of bosutinib: standard dose or dose-escalation regimens of bosutinib treatment for patients with chronic myeloid leukemia. Exp Hematol Oncol. 2018;7:9. doi: 10.1186/s40164-018-0101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsyu PH, Mould DR, Upton RN, Amantea M. Pharmacokinetic-pharmacodynamic relationship of bosutinib in patients with chronic phase chronic myeloid leukemia. Cancer Chemother Pharmacol. 2013;71(1):209–218. doi: 10.1007/s00280-012-1998-4. [DOI] [PubMed] [Google Scholar]
- 40.Food and Drug Administration. Center for Drug Evaluation and Research Ponatinib Clinical Pharmacology and Biopharmaceutics Review. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203469orig1s000clinpharmr.pdf. Accessed 1 Jul 2023.
- 41.Dorer DJ, Knickerbocker RK, Baccarani M, Cortes JE, Hochhaus A, Talpaz M, et al. Impact of dose intensity of ponatinib on selected AEs: Multivariate analyses from a pooled population of clinical trial patients. Leuk Res. 2016;48:84–91. doi: 10.1016/j.leukres.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Cortes J, Apperley J, Lomaia E, Moiraghi B, Undurraga Sutton M, Pavlovsky C, et al. Ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia: a randomized, open-label phase 2 clinical trial. Blood. 2021;138(21):2042–2050. doi: 10.1182/blood.2021012082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Food and Drug Administration. Center for Drug Evaluation and Research Asciminib Clinical Pharmacology and Biopharmaceutics Review 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/215358Orig1s000,Orig2s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 44.Goldwirt L, Chami I, Feugeas JP, Pages C, Brunet-Possenti F, Allayous C, et al. Reply to 'Plasma vemurafenib concentrations in advanced BRAFV600mut melanoma patients: impact on tumour response and tolerance' by Funck-Brentano et al. Ann Oncol. 2016;27(2):363–364. doi: 10.1093/annonc/mdv538. [DOI] [PubMed] [Google Scholar]
- 45.Funck-Brentano E, Alvarez JC, Longvert C, Abe E, Beauchet A, Funck-Brentano C, et al. Plasma vemurafenib concentrations in advanced BRAFV600mut melanoma patients: impact on tumour response and tolerance. Ann Oncol. 2015;26(7):1470–1475. doi: 10.1093/annonc/mdv189. [DOI] [PubMed] [Google Scholar]
- 46.Kramkimel N, Thomas-Schoemann A, Sakji L, Golmard J, Noe G, Regnier-Rosencher E, et al. Vemurafenib pharmacokinetics and its correlation with efficacy and safety in outpatients with advanced BRAF-mutated melanoma. Target Oncol. 2016;11(1):59–69. doi: 10.1007/s11523-015-0375-8. [DOI] [PubMed] [Google Scholar]
- 47.Food and Drug Administration. Center for Drug Evaluation and Research Vemurafenib Clinical Pharmacology and Biopharmaceutics Review. 2011. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202429Orig1s000PharmR.pdf. Accessed 1 Jul 2023.
- 48.Kichenadasse G, Hughes JH, Miners JO, Mangoni AA, Rowland A, Hopkins AM, et al. Relationship between vemurafenib plasma concentrations and survival outcomes in patients with advanced melanoma. Cancer Chemother Pharmacol. 2020;85(3):615–620. doi: 10.1007/s00280-019-04002-1. [DOI] [PubMed] [Google Scholar]
- 49.Goldwirt L, Louveau B, Baroudjian B, Allayous C, Jouenne F, Da Meda L, et al. Dabrafenib and trametinib exposure-efficacy and tolerance in metastatic melanoma patients: a pharmacokinetic-pharmacodynamic real-life study. Cancer Chemother Pharmacol. 2021;88(3):427–437. doi: 10.1007/s00280-021-04299-x. [DOI] [PubMed] [Google Scholar]
- 50.Groenland SL, Janssen JM, Nijenhuis CM, de Vries N, Rosing H, Wilgenhof S, et al. Exposure-response analyses of BRAF- and MEK-inhibitors dabrafenib plus trametinib in melanoma patients. Cancer Chemother Pharmacol. 2023;91(6):447-456. [DOI] [PubMed]
- 51.Food and Drug Administration. Center for Drug Evaluation and Research Dabrafenib Clinical Pharmacology and Biopharmaceutics Review 2013. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/202806orig1s000clinpharmr.pdf. Accessed 1 Jul 2023.
- 52.Rousset M, Dutriaux C, Bosco-Lévy P, Prey S, Pham-Ledard A, Dousset L, et al. Trough dabrafenib plasma concentrations can predict occurrence of adverse events requiring dose reduction in metastatic melanoma. Clin Chim Acta. 2017;472:26–29. doi: 10.1016/j.cca.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 53.Food and Drug Administration. Center for Drug Evaluation and Research Encorafenib Clinical Pharmacology and Biopharmaceutics Review 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210496Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 54.Food and Drug Administration. Center for Drug Evaluation and Research Ibrutinib Clinical Pharmacology and Biopharmaceutics Review 2013. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/205552orig1s000clinpharmr.pdf. Accessed 1 Jul 2023.
- 55.Gallais F, Ysebaert L, Despas F, De Barros S, Dupré L, Quillet Mary A, et al. Population pharmacokinetics of ibrutinib and its dihydrodiol metabolite in patients with lymphoid malignancies. Clin Pharmacokinet. 2020;59(9):1171–1183. doi: 10.1007/s40262-020-00884-0. [DOI] [PubMed] [Google Scholar]
- 56.Le Louedec F, Gallais F, Thomas F, White-Koning M, Allal B, Protin C, et al. Limited sampling strategy for determination of ibrutinib plasma exposure: joint analyses with metabolite data. Pharmaceuticals. 2021;14(2):162. doi: 10.3390/ph14020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Calquence European public assessment report. 2020. https://www.ema.europa.eu/en/documents/assessment-report/calquence-epar-public-assessment-report_en.pdf. Accessed 1 Jul 2023.
- 58.Edlund H, Buil-Bruna N, Vishwanathan K, Wei H, Raman R, de Kock M, et al. Exposure–response analysis of acalabrutinib and its active metabolite, ACP-5862, in patients with B-cell malignancies. Br J Clin Pharmacol. 2022;88(5):2284–2296. doi: 10.1111/bcp.15087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin F, Yin M, Mandava V, Edlund H, Andrew M, Patel P, et al. Exposure-response of the bruton tyrosine kinase inhibitor, acalabrutinib, in the treatment of hematologic malignancies. Blood. 2017;130(Supplement 1):1268. [Google Scholar]
- 60.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Brukinsa European public assessment report. 2021. https://www.ema.europa.eu/en/documents/assessment-report/brukinsa-epar-public-assessment-report_en.pdf. Accessed 1 Jul 2023.
- 61.Ou Y, Wang K, Liu L, Jindal A, Gao Y, Sahasranaman S. Exposure-response relationship of the bruton tyrosine kinase inhibitor, zanubrutinib (BGB-3111) in patients with hematologic malignancies. Blood. 2019;134(Supplement 1):5063. [Google Scholar]
- 62.Mato AR, Shah NN, Jurczak W, Cheah CY, Pagel JM, Woyach JA, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. The Lancet. 2021;397(10277):892–901. doi: 10.1016/S0140-6736(21)00224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng J, Yu Y, Durairaj C, Amantea M, Dieras V, Finn R, et al. Palbociclib exposure-response analyses in the treatment of hormone-receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2-) advanced breast cancer (ABC). In: Cancer research. 2018;Conference: San Antonio Breast Cancer Symposium, SABCS 2017. United States. 78(4 Supplement 1).
- 64.Food and Drug Administration. Center for Drug Evaluation and Research Ribociclib Clinical Pharmacology and Biopharmaceutics Review. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209092orig1s000multidiscipliner.pdf. Accessed 1 Jul 2023.
- 65.Food and Drug Administration. Center for Drug Evaluation and Research Abemaciclib Clinical Pharmacology and Biopharmaceutics Review 2017. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208716Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 66.Food and Drug Administration. Center for Drug Evaluation and Research Palbociclib Clinical Pharmacology and Biopharmaceutics Review 2015. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207103orig1s000clinpharmr.pdf. Accessed 1 Jul 2023.
- 67.Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov. 2016;6(7):740–753. doi: 10.1158/2159-8290.CD-16-0095. [DOI] [PubMed] [Google Scholar]
- 68.Zhao YY, Li S, Zhang Y, Zhao HY, Liao H, Guo Y, et al. The relationship between drug exposure and clinical outcomes of non-small cell lung cancer patients treated with gefitinib. Med Oncol. 2011;28(3):697–702. doi: 10.1007/s12032-010-9541-0. [DOI] [PubMed] [Google Scholar]
- 69.Xin S, Zhao Y, Wang X, Huang Y, Zhang J, Guo Y, et al. The dissociation of gefitinib trough concentration and clinical outcome in NSCLC patients with EGFR sensitive mutations. Sci Rep. 2015;5:12675. doi: 10.1038/srep12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kenmotsu H, Imamura CK, Kawamura T, Oyakawa T, Omori S, Nakashima K, et al. Prospective evaluation of the relationship between response and exposure of total and unbound erlotinib in non-small cell lung cancer patients. Cancer Chemother Pharmacol. 2022;90(2):115–123. doi: 10.1007/s00280-022-04452-0. [DOI] [PubMed] [Google Scholar]
- 71.Fiala O, Hosek P, Pesek M, Finek J, Racek J, Stehlik P, et al. Serum concentration of erlotinib and its correlation with outcome and toxicity in patients with advanced-stage NSCLC. Anticancer Res. 2017;37(11):6469–6476. doi: 10.21873/anticanres.12102. [DOI] [PubMed] [Google Scholar]
- 72.Lampson BL, Nishino M, Dahlberg SE, Paul D, Santos AA, Jänne PA, et al. Activity of erlotinib when dosed below the maximum tolerated dose for EGFR-mutant lung cancer: Implications for targeted therapy development. Cancer. 2016;122(22):3456–3463. doi: 10.1002/cncr.30270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyamoto S, Azuma K, Ishii H, Bessho A, Hosokawa S, Fukamatsu N, et al. Low-dose erlotinib treatment in elderly or frail patients with EGFR mutation-positive non-small cell lung cancer: a multicenter phase 2 trial. JAMA Oncol. 2020;6(7):e201250. doi: 10.1001/jamaoncol.2020.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu JF, Eppler SM, Wolf J, Hamilton M, Rakhit A, Bruno R, et al. Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer. Clin Pharmacol Ther. 2006;80(2):136–145. doi: 10.1016/j.clpt.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 75.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Tarceva European public assessment report. 2010. https://www.ema.europa.eu/en/documents/variation-report/tarceva-h-c-618-ii-0017-epar-assessment-report-variation_en.pdf. Accessed 1 Jul 2023.
- 76.Kobayashi H, Sato K, Niioka T, Miura H, Ito H, Miura M. Relationship among gefitinib exposure, polymorphisms of its metabolizing enzymes and transporters, and side effects in japanese patients with non-small-cell lung cancer. Clin Lung Cancer. 2015;16(4):274–281. doi: 10.1016/j.cllc.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 77.Food and Drug Administration. Center for Drug Evaluation and Research Gefitinib Clinical Pharmacology and Biopharmaceutics Review. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/206995orig1s000clinpharmr.pdf. Accessed 1 Jul 2023.
- 78.Food and Drug Administration. Center for Drug Evaluation and Research Afatinib Clinical Pharmacology and Biopharmaceutics Review 2013. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/201292orig1s000clinpharmr.pdf. Accessed 1 Jul 2023.
- 79.Wind S, Schnell D, Ebner T, Freiwald M, Stopfer P. Clinical pharmacokinetics and pharmacodynamics of afatinib. Clin Pharmacokinet. 2017;56(3):235–250. doi: 10.1007/s40262-016-0440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakao K, Kobuchi S, Marutani S, Iwazaki A, Tamiya A, Isa S, et al. Population pharmacokinetics of afatinib and exposure-safety relationships in Japanese patients with EGFR mutation-positive non-small cell lung cancer. Sci Rep. 2019;9(1):18202. doi: 10.1038/s41598-019-54804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yokota H, Sato K, Sakamoto S, Okuda Y, Asano M, Takeda M, et al. Relationship between plasma concentrations of afatinib and the onset of diarrhea in patients with non-small cell lung cancer. Biology (Basel). 2021;10(10):1054. doi: 10.3390/biology10101054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen YC, Tsai MJ, Lee MH, Kuo CY, Shen MC, Tsai YM, et al. Lower starting dose of afatinib for the treatment of metastatic lung adenocarcinoma harboring exon 21 and exon 19 mutations. BMC Cancer. 2021;21(1):495. doi: 10.1186/s12885-021-08235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lim C-K, Wei Y-F, Tsai M-S, Chen K-Y, Shih J-Y, Yu C-J. Treatment effectiveness and tolerability of afatinib at different doses in patients with EGFR-mutated lung adenocarcinoma: how low can we go? Eur J Cancer. 2018;103:32–40. doi: 10.1016/j.ejca.2018.07.128. [DOI] [PubMed] [Google Scholar]
- 84.Yang JCH, Sequist LV, Zhou C, Schuler M, Geater SL, Mok T, et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann Oncol. 2016;27(11):2103–2110. doi: 10.1093/annonc/mdw322. [DOI] [PubMed] [Google Scholar]
- 85.Food and Drug Administration. Center for Drug Evaluation and Research Dacomitinib Clinical Pharmacology and Biopharmaceutics Review 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/211288Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 86.Gupta N, Largajolli A, Witjes H, Diderichsen PM, Zhang S, Hanley MJ, et al. Mobocertinib dose rationale in patients with metastatic NSCLC with EGFR exon 20 insertions: exposure-response analyses of a pivotal phase I/II study. Clin Pharm Therap. 2022;112(2):327–334. doi: 10.1002/cpt.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Tagrisso European public assessment report. 2016. https://www.ema.europa.eu/en/documents/assessment-report/tagrisso-epar-public-assessment-report_en.pdf. Accessed 1 Jul 2023.
- 88.Food and Drug Administration. Center for Drug Evaluation and Research Osimertinib Clinical Pharmacology and Biopharmaceutics Review 2015. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/208065orig1s000clinpharmr.pdf. Accessed 1 Jul 2023.
- 89.Brown K, Comisar C, Witjes H, Maringwa J, de Greef R, Vishwanathan K, et al. Population pharmacokinetics and exposure-response of osimertinib in patients with non-small cell lung cancer. Br J Clin Pharmacol. 2017;83(6):1216–1226. doi: 10.1111/bcp.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Agema BC, Veerman GDM, Steendam CMJ, Lanser DAC, Preijers T, van der Leest C, et al. Improving the tolerability of osimertinib by identifying its toxic limit. Ther Adv Med Oncol. 2022;14:17588359221103212. doi: 10.1177/17588359221103212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boosman RJ, Jebbink M, Veldhuis WB, Groenland SL, van Veggel B, Moeskops P, et al. Exposure-response analysis of osimertinib in EGFR mutation positive non-small cell lung cancer patients in a real-life setting. Pharm Res. 2022;39(10):2507–2514. doi: 10.1007/s11095-022-03355-2. [DOI] [PubMed] [Google Scholar]
- 92.Food and Drug Administration. Center for Drug Evaluation and Research Erdafitinib Clinical Pharmacology and Biopharmaceutics Review 2019. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212018Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 93.Dosne A-G, Valade E, Goeyvaerts N, De Porre P, Avadhani A, O'Hagan A, et al. Exposure-response analyses of erdafitinib in patients with locally advanced or metastatic urothelial carcinoma. Cancer Chemother Pharmacol. 2022;89(2):151–164. doi: 10.1007/s00280-021-04381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bahleda R, Italiano A, Hierro C, Mita A, Cervantes A, Chan N, et al. Multicenter phase I study of erdafitinib (JNJ-42756493), oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced or refractory solid tumors. Clin Cancer Res. 2019;25(16):4888–4897. doi: 10.1158/1078-0432.CCR-18-3334. [DOI] [PubMed] [Google Scholar]
- 95.Dosne A-G, Valade E, Stuyckens K, De Porre P, Avadhani A, O’Hagan A, et al. Erdafitinib’s effect on serum phosphate justifies its pharmacodynamically guided dosing in patients with cancer. CPT Pharmacomet Syst Pharmacol. 2022;11(5):569–580. doi: 10.1002/psp4.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Pemazyre European public assessment report. 2021. https://www.ema.europa.eu/en/documents/assessment-report/pemazyre-epar-public-assessment-report_en.pdf. Accessed 1 Jul 2023.
- 97.Food and Drug Administration. Center for Drug Evaluation and Research Pemigatinib Clinical Pharmacology and Biopharmaceutics Review 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213736Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 98.Food and Drug Administration. Center for Drug Evaluation and Research Infigratinib Clinical Pharmacology and Biopharmaceutics Review. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/214622Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 99.Hollebecque A, Bridgewater JA, Meric-Bernstam F, Goyal L, Arkenau HT, Yamamiya I, et al. 52P Assessment of futibatinib exposure–response (E–R) relationships in patients with advanced solid tumors, including cholangiocarcinoma (CCA) Ann Oncol. 2021;32:S378–S379. [Google Scholar]
- 100.Goyal L, Meric-Bernstam F, Hollebecque A, Valle JW, Morizane C, Karasic TB, et al. Futibatinib for FGFR2-rearranged intrahepatic cholangiocarcinoma. N Engl J Med. 2023;388(3):228–239. doi: 10.1056/NEJMoa2206834. [DOI] [PubMed] [Google Scholar]
- 101.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Rydapt European public assessment report. 2017. https://www.ema.europa.eu/en/documents/assessment-report/rydapt-epar-public-assessment-report_en.pdf. Accessed 1 Jul 2023.
- 102.Yin O, Wang Y, Lanza C, Schimansky T, Balez S, Schran HF, et al. Pharmacokinetics (PK) and pharmacodynamics (PD) of midostaurin (PKC412) in patients with acute myeloid leukemia (AML) J Clin Oncol. 2008;26(15_suppl):7064. [Google Scholar]
- 103.Food and Drug Administration. Center for Drug Evaluation and Research Gilterinib Clinical Pharmacology and Biopharmaceutics Review. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/211349Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 104.Food and Drug Administration. Center for Drug Evaluation and Research Vismodegib Clinical Pharmacology and Biopharmaceutics Review 2012. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203388Orig1s000SumR.pdf. Accessed 1 Jul 2023.
- 105.Zhou J, Quinlan M, Hurh E, Sellami D. Exposure-response analysis of sonidegib (LDE225), an oral inhibitor of the hedgehog signaling pathway, for effectiveness and safety in patients with advanced solid tumors. J Clin Pharmacol. 2016;56(11):1406–1415. doi: 10.1002/jcph.749. [DOI] [PubMed] [Google Scholar]
- 106.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Odomzo European public assessment report. 2015. https://www.ema.europa.eu/en/documents/assessment-report/odomzo-epar-public-assessment-report_en.pdf. Accessed 1 Jul 2023.
- 107.Lin S, Shaik N, Chan G, Cortes JE, Ruiz-Garcia A. An evaluation of overall survival in patients with newly diagnosed acute myeloid leukemia and the relationship with glasdegib treatment and exposure. Cancer Chemother Pharmacol. 2020;86(4):451–459. doi: 10.1007/s00280-020-04132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Food and Drug Administration. Center for Drug Evaluation and Research Glasdegib Clinical Pharmacology and Biopharmaceutics Review 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210656Orig1s000ClinPharmR.pdf. Accessed 1 Jul 2023.
- 109.Ruiz-Garcia A, Shaik N, Lin S, Jamieson C, Heuser M, Chan G. Evaluation of the relationship of glasdegib exposure and safety end points in patients with refractory solid tumors and hematologic malignancies. J Clin Pharmacol. 2021;61(3):349–359. doi: 10.1002/jcph.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roušarová J, Šíma M, Slanař O. Therapeutic drug monitoring of protein kinase inhibitors in breast cancer patients. Prague Med Rep. 2021;122(4):243–256. doi: 10.14712/23362936.2021.22. [DOI] [PubMed] [Google Scholar]
- 111.Food and Drug Administration. Center for Drug Evaluation and Research Lapatinib Clinical Pharmacology and Biopharmaceutics Review 2007. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022059s000_PharmR_P1.pdf. Accessed 1 Jul 2023.
- 112.Food and Drug Administration. Center for Drug Evaluation and Research Neratinib Clinical Pharmacology and Biopharmaceutics Review 2017. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208051Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 113.Food and Drug Administration. Center for Drug Evaluation and Research Tucatinib Clinical Pharmacology and Biopharmaceutics Review 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213411Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 114.Jiang X, Wada R, Poland B, Kleijn HJ, Fan B, Liu G, et al. Population pharmacokinetic and exposure-response analyses of ivosidenib in patients with IDH1-mutant advanced hematologic malignancies. Clin Transl Sci. 2021;14(3):942–953. doi: 10.1111/cts.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Food and Drug Administration. Center for Drug Evaluation and Research Ivosidenib Clinical Pharmacology and Biopharmaceutics Review 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/211192Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 116.Food and Drug administration. Center for Drug Evaluation and Research Enasidenib Clinical Pharmacology and Biopharmaceutics Review. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209606Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 117.Watts JM, Baer MR, Yang J, Prebet T, Lee S, Schiller GJ, et al. Olutasidenib alone or with azacitidine in IDH1-mutated acute myeloid leukaemia and myelodysplastic syndrome: phase 1 results of a phase 1/2 trial. Lancet Haematol. 2023;10(1):e46–e58. doi: 10.1016/S2352-3026(22)00292-7. [DOI] [PubMed] [Google Scholar]
- 118.Food and Drug administration. Center for Drug Evaluation and Research Ruxolitinib Clinical Pharmacology and Biopharmaceutics Review. 2011. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202192Orig1s000ClinPharmR.pdf. Accessed 1 Jul 2023.
- 119.Food and Drug administration. Center for Drug Evaluation and Research Fedratinib Clinical Pharmacology and Biopharmaceutics Review. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212327Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 120.Al-Fayoumi S, Wang L, Li H, Wada R, Dean JP. Exposure-response analysis for pacritinib (SB1518), a novel oral JAK2/FLT3 inhibitor, in patients with myelofibrosis. Blood. 2013;122(21):4080. [Google Scholar]
- 121.Emanuel RM, Dueck AC, Geyer HL, Kiladjian JJ, Slot S, Zweegman S, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30(33):4098–4103. doi: 10.1200/JCO.2012.42.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Widmer N, Decosterd LA, Leyvraz S, Duchosal MA, Rosselet A, Debiec-Rychter M, et al. Relationship of imatinib-free plasma levels and target genotype with efficacy and tolerability. Br J Cancer. 2008;98(10):1633–1640. doi: 10.1038/sj.bjc.6604355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Delbaldo C, Chatelut E, Ré M, Deroussent A, Séronie-Vivien S, Jambu A, et al. Pharmacokinetic-pharmacodynamic relationships of imatinib and its main metabolite in patients with advanced gastrointestinal stromal tumors. Clin Cancer Res. 2006;12(20 Pt 1):6073–6078. doi: 10.1158/1078-0432.CCR-05-2596. [DOI] [PubMed] [Google Scholar]
- 124.Eechoute K, Sparreboom A, Burger H, Franke RM, Schiavon G, Verweij J, et al. Drug transporters and imatinib treatment: implications for clinical practice. Clin Cancer Res. 2011;17(3):406–415. doi: 10.1158/1078-0432.CCR-10-2250. [DOI] [PubMed] [Google Scholar]
- 125.Demetri GD, Wang Y, Wehrle E, Racine A, Nikolova Z, Blanke CD, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009;27(19):3141–3147. doi: 10.1200/JCO.2008.20.4818. [DOI] [PubMed] [Google Scholar]
- 126.Lankheet NAG, Desar IME, Mulder SF, Burger DM, Kweekel DM, van Herpen CML, et al. Optimizing the dose in cancer patients treated with imatinib, sunitinib and pazopanib. Br J Clin Pharmacol. 2017;83(10):2195–2204. doi: 10.1111/bcp.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bouchet S, Poulette S, Titier K, Moore N, Lassalle R, Abouelfath A, et al. Relationship between imatinib trough concentration and outcomes in the treatment of advanced gastrointestinal stromal tumours in a real-life setting. Eur J Cancer. 2016;57:31–38. doi: 10.1016/j.ejca.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 128.Farag S, Verheijen RB, Martijn Kerst J, Cats A, Huitema AD, Steeghs N. Imatinib pharmacokinetics in a large observational cohort of gastrointestinal stromal tumour patients. Clin Pharmacokinet. 2017;56(3):287–292. doi: 10.1007/s40262-016-0439-7. [DOI] [PubMed] [Google Scholar]
- 129.IJzerman NS, Groenland SL, Koenen AM, Kerst M, van der Graaf WTA, Rosing H, et al. Therapeutic drug monitoring of imatinib in patients with gastrointestinal stromal tumours—results from daily clinical practice. Eur J Cancer. 2020;136:140–148. doi: 10.1016/j.ejca.2020.05.025. [DOI] [PubMed] [Google Scholar]
- 130.Zuidema S, Desar IME, van Erp NP, Kievit W. Optimizing the dose in patients treated with imatinib as first line treatment for gastrointestinal stromal tumours: a cost-effectiveness study. Br J Clin Pharmacol. 2019;85(9):1994–2001. doi: 10.1111/bcp.13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Ayvakyt European public assessment report. 2020. https://www.ema.europa.eu/en/documents/assessment-report/ayvakyt-epar-public-assessment-report_en.pdf. Accessed 1 Jul 2023.
- 132.Food and Drug administration. Center for Drug Evaluation and Research Avapratinib Clinical Pharmacology and Biopharmaceutics Review. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/212608Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 133.Food and Drug administration. Center for Drug Evaluation and Research Ripretinib Clinical Pharmacology and Biopharmaceutics Review. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213973Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 134.Food and Drug administration. Center for Drug Evaluation and Research Sotorasib Clinical Pharmacology and Biopharmaceutics Review. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/214665Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 135.Jänne PA, Riely GJ, Gadgeel SM, Heist RS, Ou S-HI, Pacheco JM, et al. Adagrasib in Non–small-cell lung cancer harboring a KRASG12C mutation. N Engl J Med. 2022;387(2):120–131. doi: 10.1056/NEJMoa2204619. [DOI] [PubMed] [Google Scholar]
- 136.Ou SI, Jänne PA, Leal TA, Rybkin II, Sabari JK, Barve MA, et al. First-in-human phase I/IB dose-finding study of Adagrasib (MRTX849) in patients with advanced KRAS(G12C) solid tumors (KRYSTAL-1) J Clin Oncol. 2022;40(23):2530–2538. doi: 10.1200/JCO.21.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Food and Drug administration. Center for Drug Evaluation and Research Cobimetinib Clinical Pharmacology and Biopharmaceutics Review. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/206192orig1s000clinpharmr.pdf. Accessed 1 Jul 2023.
- 138.Ouellet D, Kassir N, Chiu J, Mouksassi MS, Leonowens C, Cox D, et al. Population pharmacokinetics and exposure-response of trametinib, a MEK inhibitor, in patients with BRAF V600 mutation-positive melanoma. Cancer Chemother Pharmacol. 2016;77(4):807–817. doi: 10.1007/s00280-016-2993-y. [DOI] [PubMed] [Google Scholar]
- 139.Food and Drug administration. Center for Drug Evaluation and Research Trametinib Clinical Pharmacology and Biopharmaceutics Review. 2013. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204114Orig1s000ClinPharmR.pdf. Accessed 1 Jul 2023.
- 140.Food and Drug administration. Center for Drug Evaluation and Research Binimetinib Clinical Pharmacology and Biopharmaceutics Review. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210498Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 141.Drusbosky LM, Dawar R, Rodriguez E, Ikpeazu CV. Therapeutic strategies in METex14 skipping mutated non-small cell lung cancer. J Hematol Oncol. 2021;14(1):129. doi: 10.1186/s13045-021-01138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Food and Drug administration. Center for Drug Evaluation and Research Capmatinib Clinical Pharmacology and Biopharmaceutics Review. 2020 [cited; Available from: accessdata.fda.gov/drugsatfda_docs/nda/2020/213591Orig1s000MultidisciplineR.pdf.
- 143.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Tempetko European public assessment report. 2022. https://www.ema.europa.eu/en/documents/assessment-report/tepmetko-epar-public-assessment-report_en.pdf. Accessed 1 Jul 2023.
- 144.Food and Drug administration. Center for Drug Evaluation and Research Tepotinib Clinical Pharmacology and Biopharmaceutics Review. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/214096Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 145.Xiong W, Hietala SF, Nyberg J, Papasouliotis O, Johne A, Berghoff K, et al. Exposure–response analyses for the MET inhibitor tepotinib including patients in the pivotal VISION trial: support for dosage recommendations. Cancer Chemother Pharmacol. 2022;90(1):53-69. [DOI] [PMC free article] [PubMed]
- 146.Xiong W, Papasouliotis O, Jonsson EN, Strotmann R, Girard P. Population pharmacokinetic analysis of tepotinib, an oral MET kinase inhibitor, including data from the VISION study. Cancer Chemother Pharmacol. 2022;89(5):655–669. doi: 10.1007/s00280-022-04423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Food and Drug administration. Center for Drug Evaluation and Research Larotrectinib Clinical Pharmacology and Biopharmaceutics Review. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210861Orig1s000_211710Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 148.Food and Drug administration. Center for Drug Evaluation and Research Entrectinib Clinical Pharmacology and Biopharmaceutics Review. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212725Orig1s000,%20212726Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 149.Mercier F, Djebli N, González-Sales M, Jaminion F, Meneses-Lorente G. Efficacy and safety exposure–response analyses of entrectinib in patients with advanced or metastatic solid tumors. Cancer Chemother Pharmacol. 2022;89(3):363–372. doi: 10.1007/s00280-022-04402-w. [DOI] [PubMed] [Google Scholar]
- 150.Food and Drug administration. Center for Drug Evaluation and Research Olaparib Clinical Pharmacology and Biopharmaceutics Review. 2014 [cited; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206162Orig1s000ClinPharmR.pdf. Accessed 1 July 2023.
- 151.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Lynparza European public assessment report. 2018. https://www.ema.europa.eu/en/documents/variation-report/lynparza-h-c-3726-x-0016-g-epar-assessment-report-extension_en.pdf. Accessed 1 Jul 2023.
- 152.Zhou D, Li J, Learoyd M, Bui K, Berges A, Milenkova T, et al. Efficacy and safety exposure-response analyses of olaparib capsule and tablet formulations in oncology patients. Clin Pharm Therap. 2019;105(6):1492–1500. doi: 10.1002/cpt.1338. [DOI] [PubMed] [Google Scholar]
- 153.Velev M, Puszkiel A, Blanchet B, de Percin S, Delanoy N, Medioni J, et al. Association between olaparib exposure and early toxicity in BRCA-mutated ovarian cancer patients: results from a retrospective multicenter study. Pharmaceuticals. 2021;14(8):804. doi: 10.3390/ph14080804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mohmaed Ali MI, Bruin MAC, Dezentjé VO, Beijnen JH, Steeghs N, Huitema ADR. Exposure-response analyses of olaparib in real-life patients with ovarian cancer. Pharm Res. 2023;40(5):1239-1247. [DOI] [PubMed]
- 155.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Zejula European public assessment report. 2020. https://www.ema.europa.eu/en/documents/variation-report/zejula-h-c-003943-ii-0019-epar-assessment-report-variation_en.pdf. Accessed 1 Jul 2023.
- 156.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Rubraca European public assessment report. 2019. https://www.ema.europa.eu/en/documents/variation-report/rubraca-h-c-4272-ii-0001-epar-assessment-report-variation_en.pdf. Accessed 1 Jul 2023.
- 157.Wang J, Zhang ZY, Mirza M, Gilbert L, Fabbro M, Tinker AV, et al. 933PDThe exposure-response relationship of niraparib in patients with gBRCAmut and non-gBRCAmut: results from the ENGOT-OV16/NOVA Trial. Ann Oncol. 2017;28.
- 158.Bradley JM, Ignacio R, Whitney G, Cristina C, David MOM, Bente L, et al. Niraparib exposure-response relationship in patients (pts) with newly diagnosed advanced ovarian cancer (AOC) J Clin Oncol. 2020 doi: 10.1200/JCO.2020.38.15_suppl.6051. [DOI] [Google Scholar]
- 159.Konecny GE, Oza AM, Tinker AV, Oaknin A, Shapira-Frommer R, Ray-Coquard I, et al. Population exposure-efficacy and exposure-safety analyses for rucaparib in patients with recurrent ovarian carcinoma from Study 10 and ARIEL2. Gynecol Oncol. 2021;161(3):668–675. doi: 10.1016/j.ygyno.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yu Y, Elmeliegy M, Litton JK, Tudor IC, Czibere A, Zheng J, et al. Talazoparib exposure-efficacy analysis in patients with advanced breast cancer and germline BRCA1/2 mutations in the EMBRACA Trial. J Clin Pharmacol. 2020;60(10):1324–1333. doi: 10.1002/jcph.1623. [DOI] [PubMed] [Google Scholar]
- 161.Elmeliegy M, Yu Y, Litton JK, Czibere A, Wilson GG, Tudor IC, et al. Exposure-safety analyses of talazoparib in patients with advanced breast cancer and germline BRCA1/2 mutations in the EMBRACA and ABRAZO Trials. J Clin Pharmacol. 2020;60(10):1334–1343. doi: 10.1002/jcph.1626. [DOI] [PubMed] [Google Scholar]
- 162.Jin F, Zhou H, Fang L, Li X, Newcomb T, Dansey R, et al. Exposure-response of idelalisib, a novel PI3Kδ inhibitor, administered as monotherapy in the treatment of hematologic malignancies. Blood. 2013;122(21):5054. [Google Scholar]
- 163.Food and Drug Administration. Center for Drug Evaluation and Research Idelalisib Clinical Pharmacology and Biopharmaceutics Review. 2013. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206545Orig1s000ClinPharmR.pdf. Accessed 1 Jul 2023.
- 164.Daryani VM, Sharma S, Xing G, Silverman J, Adewoye AH, Mathias A. Exposure-response of idelalisib administered in combination with bendamustine and rituximab for the treatment of relapsed chronic lymphocytic leukemi. Blood. 2017;130:5344. [Google Scholar]
- 165.Sharma S, Guo Y, Jin F, Li X, Dubowy RL, Newcomb T, et al. Exposure-response of idelalisib administered in combination with ofatumumab for the treatment of relapsed chronic lymphocytic leukemia. Blood. 2015;126(23):4172. [Google Scholar]
- 166.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Copiktra European public assessment report. 2021. https://www.ema.europa.eu/en/documents/assessment-report/copiktra-epar-public-assessment-report_en.pdf. Accessed 1 Jul 2023.
- 167.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Piqray European public assessment report. 2020. https://www.ema.europa.eu/en/documents/assessment-report/piqray-epar-public-assessment-report_en.pdf. Accessed 1 Jul 2023.
- 168.Food and Drug Administration. Center for Drug Evaluation and Research Alpesilib Clinical Pharmacology and Biopharmaceutics Review. 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212526Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 169.Food and Drug Administration. Center for Drug Evaluation and Research Pralsetinib Clinical Pharmacology and Biopharmaceutics Review. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213721Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 170.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Gavreto European public assessment report. 2021. https://www.ema.europa.eu/en/documents/assessment-report/gavreto-epar-public-assessment-report_en.pdf. Accessed 1 Jul 2023.
- 171.Food and Drug Administration. Center for Drug Evaluation and Research Selpercatinib Clinical Pharmacology and Biopharmaceutics Review. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213246Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 172.Food and Drug Administration. Center for Drug Evaluation and Research Axitinib Clinical Pharmacology and Biopharmaceutics Review. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202324orig1s000clinpharmr.pdf. Accessed 1 Jul 2023.
- 173.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Inlyta European public assessment report. 2012. https://www.ema.europa.eu/en/documents/assessment-report/inlyta-epar-public-assessment-report_en.pdf. Accessed 1 Jul 2023.
- 174.Rini BI, Garrett M, Poland B, Dutcher JP, Rixe O, Wilding G, et al. Axitinib in metastatic renal cell carcinoma: results of a pharmacokinetic and pharmacodynamic analysis. J Clin Pharmacol. 2013;53(5):491–504. doi: 10.1002/jcph.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Miura Y, Imamura CK, Uchino K, Kishida T, Matsubara N, Shinojima T, et al. Individualized dosing of axitinib based on first-dose area under the concentration-time curve for metastatic renal-cell carcinoma. Clin Genitourin Cancer. 2019;17(1):e1–e11. doi: 10.1016/j.clgc.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 176.Fukudo M, Tamaki G, Azumi M, Kakizaki H, Matsumoto S, Tasaki Y. Absorption of the orally active multikinase inhibitor axitinib as a therapeutic index to guide dose titration in metastatic renal cell carcinoma. Invest New Drugs. 2021;39(2):595–604. doi: 10.1007/s10637-020-01023-z. [DOI] [PubMed] [Google Scholar]
- 177.Tsuchiya N, Igarashi R, Suzuki-Honma N, Fujiyama N, Narita S, Inoue T, et al. Association of pharmacokinetics of axitinib with treatment outcome and adverse events in advanced renal cell carcinoma patients. J Clin Oncol. 2015;33(7_suppl):506. [Google Scholar]
- 178.Synowiec Z, Sobańska K, Synowiec T, Teżyk A, Tomczak P, Jabłecka A. Axitinib trough concentration and its influence on the efficacy and toxicity of second-line renal cell carcinoma treatment. Clin Genitourin Cancer. 2022;20(4):390.e1–e8. doi: 10.1016/j.clgc.2022.03.006. [DOI] [PubMed] [Google Scholar]
- 179.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Fotivda European public assessment report. 2017. https://www.ema.europa.eu/en/documents/assessment-report/fotivda-epar-public-assessment-report_en.pdf. Accessed 1 Jul 2023.
- 180.Lacy S, Nielsen J, Yang B, Miles D, Nguyen L, Hutmacher M. Population exposure-response analysis of cabozantinib efficacy and safety endpoints in patients with renal cell carcinoma. Cancer Chemother Pharmacol. 2018;81(6):1061–1070. doi: 10.1007/s00280-018-3579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nguyen L, Chapel S, Tran BD, Lacy S. Cabozantinib exposure-response analyses of efficacy and safety in patients with advanced hepatocellular carcinoma. J Pharmacokinet Pharmacodyn. 2019;46(6):577–589. doi: 10.1007/s10928-019-09659-y. [DOI] [PubMed] [Google Scholar]
- 182.Cerbone L, Combarel D, Geraud A, Auclin E, Foulon S, Alves Costa Silva C, et al. Association of cabozantinib pharmacokinetics, progression and toxicity in metastatic renal cell carcinoma patients: results from a pharmacokinetics/pharmacodynamics study. ESMO Open. 2021;6(6):100312. [DOI] [PMC free article] [PubMed]
- 183.Krens SD, van Erp NP, Groenland SL, Moes DJAR, Mulder SF, Desar IME, et al. Exposure–response analyses of cabozantinib in patients with metastatic renal cell cancer. BMC Cancer. 2022;22(1):228. doi: 10.1186/s12885-022-09338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Aoyama T, Nakano K, Yuasa T, Sugiyama E, Okawa T, Ito K, et al. Association between pazopanib exposure and safety in Japanese patients with renal cell carcinoma or soft tissue sarcoma. Sci Rep. 2023;13(1):2099. doi: 10.1038/s41598-023-28688-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Lenvima European public assessment report. 2015. https://www.ema.europa.eu/en/documents/assessment-report/lenvima-epar-public-assessment-report_en.pdf. Accessed 1 Jul 2023.
- 186.Food and Drug Administration. Center for Drug Evaluation and Research Lenvatinib Clinical Pharmacology and Biopharmaceutics Review. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/206947orig1s000clinpharmr.pdf. Accessed 1 Jul 2023.
- 187.Hayato S, Shumaker R, Ferry J, Binder T, Dutcus CE, Hussein Z. Exposure-response analysis and simulation of lenvatinib safety and efficacy in patients with radioiodine-refractory differentiated thyroid cancer. Cancer Chemother Pharmacol. 2018;82(6):971–978. doi: 10.1007/s00280-018-3687-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nagahama M, Ozeki T, Suzuki A, Sugino K, Niioka T, Ito K, et al. Association of lenvatinib trough plasma concentrations with lenvatinib-induced toxicities in Japanese patients with thyroid cancer. Med Oncol. 2019;36(5):39. doi: 10.1007/s12032-019-1263-3. [DOI] [PubMed] [Google Scholar]
- 189.Tamai T, Hayato S, Hojo S, Suzuki T, Okusaka T, Ikeda K, et al. Dose finding of lenvatinib in subjects with advanced hepatocellular carcinoma based on population pharmacokinetic and exposure-response analyses. J Clin Pharmacol. 2017;57(9):1138–1147. doi: 10.1002/jcph.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Keizer RJ, Gupta A, Mac Gillavry MR, Jansen M, Wanders J, Beijnen JH, et al. A model of hypertension and proteinuria in cancer patients treated with the anti-angiogenic drug E7080. J Pharmacokinet Pharmacodyn. 2010;37(4):347–363. doi: 10.1007/s10928-010-9164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Vargatef European public assessment report. 2014. https://www.ema.europa.eu/en/documents/assessment-report/vargatef-epar-public-assessment-report_en.pdf. Accessed 1 Jul 2023.
- 192.Food and Drug Administration. Center for Drug Evaluation and Research Nintedanib Clinical Pharmacology and Biopharmaceutics Review. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205832Orig1s000ClinPharmR.pdf. Accessed 1 Jul 2023.
- 193.Food and Drug Administration. Center for Drug Evaluation and Research Pazopanib Clinical Pharmacology and Biopharmaceutics Review. 2009. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022465s000_clinpharmr.pdf. Accessed 1 Jul 2023.
- 194.Yu H, van Erp N, Bins S, Mathijssen RH, Schellens JH, Beijnen JH, et al. Development of a pharmacokinetic model to describe the complex pharmacokinetics of pazopanib in cancer patients. Clin Pharmacokinet. 2017;56(3):293–303. doi: 10.1007/s40262-016-0443-y. [DOI] [PubMed] [Google Scholar]
- 195.Verheijen RB, Bins S, Mathijssen RH, Lolkema MP, van Doorn L, Schellens JH, et al. Individualized pazopanib dosing: a prospective feasibility study in cancer patients. Clin Cancer Res. 2016;22(23):5738–5746. doi: 10.1158/1078-0432.CCR-16-1255. [DOI] [PubMed] [Google Scholar]
- 196.Lin Y, Ball HA, Suttle B, Mehmud F, Amado RG, Hutson TE, et al. Relationship between plasma pazopanib concentration and incidence of adverse events in renal cell carcinoma. J Clin Oncol. 2011;29(7_suppl):345. [Google Scholar]
- 197.de Wit D, van Erp NP, den Hartigh J, Wolterbeek R, den Hollander-van DM, Labots M, et al. Therapeutic drug monitoring to individualize the dosing of pazopanib: a pharmacokinetic feasibility study. Ther Drug Monit. 2015;37(3):331–338. doi: 10.1097/FTD.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 198.Suttle AB, Ball HA, Molimard M, Hutson TE, Carpenter C, Rajagopalan D, et al. Relationships between pazopanib exposure and clinical safety and efficacy in patients with advanced renal cell carcinoma. Br J Cancer. 2014;111(10):1909–1916. doi: 10.1038/bjc.2014.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sternberg CN, Donskov F, Haas NB, Doehn C, Russo P, Elmeliegy M, et al. Pazopanib exposure relationship with clinical efficacy and safety in the adjuvant treatment of advanced renal cell carcinoma. Clin Cancer Res. 2018;24(13):3005–3013. doi: 10.1158/1078-0432.CCR-17-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Verheijen RB, Swart LE, Beijnen JH, Schellens JHM, Huitema ADR, Steeghs N. Exposure-survival analyses of pazopanib in renal cell carcinoma and soft tissue sarcoma patients: opportunities for dose optimization. Cancer Chemother Pharmacol. 2017;80(6):1171–1178. doi: 10.1007/s00280-017-3463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Noda S, Yoshida T, Hira D, Murai R, Tomita K, Tsuru T, et al. Exploratory investigation of target pazopanib concentration range for patients with renal cell carcinoma. Clin Genitourin Cancer. 2019;17(2):e306–e313. doi: 10.1016/j.clgc.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 202.Food and Drug Administration. Center for Drug Evaluation and Research Regorafenib Clinical Pharmacology and Biopharmaceutics Review. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203085Orig1s000ClinPharmR.pdf. Accessed 1 Jul 2023.
- 203.Solms A, Reinecke I, Fiala-Buskies S, Keunecke A, Drenth HJ, Bruix J, et al. Exposure-response relationship of regorafenib efficacy in patients with hepatocellular carcinoma. Eur J Pharm Sci. 2017;109s:S149–S153. doi: 10.1016/j.ejps.2017.05.050. [DOI] [PubMed] [Google Scholar]
- 204.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Stivarga extension of indication variation assessment report. 2014. https://www.ema.europa.eu/en/documents/variation-report/stivarga-h-c-2573-ii-0001-epar-assessment-report-variation_en.pdf. Accessed 1 Jul 2023.
- 205.Suzuki T, Sukawa Y, Imamura CK, Masuishi T, Satake H, Kumekawa Y, et al. A phase II study of regorafenib with a lower starting dose in patients with metastatic colorectal cancer: exposure-toxicity analysis of unbound regorafenib and its active metabolites (RESET Trial) Clin Colorectal Cancer. 2020;19(1):13–21.e3. doi: 10.1016/j.clcc.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 206.Fukudo M, Asai K, Tani C, Miyamoto M, Ando K, Ueno N. Pharmacokinetics of the oral multikinase inhibitor regorafenib and its association with real-world treatment outcomes. Invest New Drugs. 2021;39(5):1422–1431. doi: 10.1007/s10637-021-01115-4. [DOI] [PubMed] [Google Scholar]
- 207.Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. Nexavar European public Assessment report. 2014. https://www.ema.europa.eu/en/documents/variation-report/nexavar-h-c-690-ii-35-epar-assessment-report-variation_en.pdf. Accessed 1 Jul 2023.
- 208.Shimada M, Okawa H, Kondo Y, Maejima T, Kataoka Y, Hisamichi K, et al. Monitoring serum levels of sorafenib and Its N-oxide is essential for long-term sorafenib treatment of patients with hepatocellular carcinoma. Tohoku J Exp Med. 2015;237(3):173–182. doi: 10.1620/tjem.237.173. [DOI] [PubMed] [Google Scholar]
- 209.Fukudo M, Ito T, Mizuno T, Shinsako K, Hatano E, Uemoto S, et al. Exposure-toxicity relationship of sorafenib in japanese patients with renal cell carcinoma and hepatocellular carcinoma. Clin Pharmacokinet. 2014;53(2):185–196. doi: 10.1007/s40262-013-0108-z. [DOI] [PubMed] [Google Scholar]
- 210.Boudou-Rouquette P, Narjoz C, Golmard JL, Thomas-Schoemann A, Mir O, Taieb F, et al. Early sorafenib-induced toxicity is associated with drug exposure and UGTIA9 genetic polymorphism in patients with solid tumors: a preliminary study. PLoS ONE. 2012;7(8):e42875. doi: 10.1371/journal.pone.0042875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Boudou-Rouquette P, Ropert S, Mir O, Coriat R, Billemont B, Tod M, et al. Variability of sorafenib toxicity and exposure over time: a pharmacokinetic/pharmacodynamic analysis. Oncologist. 2012;17(9):1204–1212. doi: 10.1634/theoncologist.2011-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Blanchet B, Billemont B, Cramard J, Benichou AS, Chhun S, Harcouet L, et al. Validation of an HPLC-UV method for sorafenib determination in human plasma and application to cancer patients in routine clinical practice. J Pharm Biomed Anal. 2009;49(4):1109–1114. doi: 10.1016/j.jpba.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 213.Noda S, Hira D, Osaki R, Fujimoto T, Iida H, Tanaka-Mizuno S, et al. Sorafenib exposure and its correlation with response and safety in advanced hepatocellular carcinoma: results from an observational retrospective study. Cancer Chemother Pharmacol. 2020;86(1):129–139. doi: 10.1007/s00280-020-04105-0. [DOI] [PubMed] [Google Scholar]
- 214.Guchelaar NAD, van Eerden RAG, Groenland SL, Doorn Lv, Desar IME, Eskens FALM, et al. Feasibility of therapeutic drug monitoring of sorafenib in patients with liver or thyroid cancer. Biomed Pharmacother. 2022;153:113393. doi: 10.1016/j.biopha.2022.113393. [DOI] [PubMed] [Google Scholar]
- 215.Houk BE, Bello CL, Michaelson MD, Bukowski RM, Redman BG, Hudes GR, et al. Exposure-response of sunitinib in metastatic renal cell carcinoma (mRCC): a population pharmacokinetic/pharmacodynamic (PKPD) approach. J Clin Oncol. 2007;25(18_suppl):5027. [Google Scholar]
- 216.Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol. 2010;66(2):357–371. doi: 10.1007/s00280-009-1170-y. [DOI] [PubMed] [Google Scholar]
- 217.Lankheet NA, Kloth JS, Gadellaa-van Hooijdonk CG, Cirkel GA, Mathijssen RH, Lolkema MP, et al. Pharmacokinetically guided sunitinib dosing: a feasibility study in patients with advanced solid tumours. Br J Cancer. 2014;110(10):2441–2449. doi: 10.1038/bjc.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lankheet NA, Knapen LM, Schellens JH, Beijnen JH, Steeghs N, Huitema AD. Plasma concentrations of tyrosine kinase inhibitors imatinib, erlotinib, and sunitinib in routine clinical outpatient cancer care. Ther Drug Monit. 2014;36(3):326–334. doi: 10.1097/FTD.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 219.Noda S, Otsuji T, Baba M, Yoshida T, Kageyama S, Okamoto K, et al. Assessment of sunitinib-induced toxicities and clinical outcomes based on therapeutic drug monitoring of sunitinib for patients with renal cell carcinoma. Clin Genitourin Cancer. 2015;13(4):350–358. doi: 10.1016/j.clgc.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 220.Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24(1):25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 221.Teo YL, Chue XP, Chau NM, Tan MH, Kanesvaran R, Wee HL, et al. Association of drug exposure with toxicity and clinical response in metastatic renal cell carcinoma patients receiving an attenuated dosing regimen of sunitinib. Target Oncol. 2015;10(3):429–437. doi: 10.1007/s11523-014-0349-2. [DOI] [PubMed] [Google Scholar]
- 222.Westerdijk K, Krens SD, van der Graaf WTA, Mulder SF, van Herpen CML, Smilde T, et al. The relationship between sunitinib exposure and both efficacy and toxicity in real-world patients with renal cell carcinoma and gastrointestinal stromal tumour. Br J Clin Pharmacol. 2021;87(2):326–335. doi: 10.1111/bcp.14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Takasaki S, Kawasaki Y, Kikuchi M, Tanaka M, Suzuka M, Noda A, et al. Relationships between sunitinib plasma concentration and clinical outcomes in Japanese patients with metastatic renal cell carcinoma. Int J Clin Oncol. 2018;23(5):936–943. doi: 10.1007/s10147-018-1302-7. [DOI] [PubMed] [Google Scholar]
- 224.Food and Drug Administration. Center for Drug Evaluation and Research Sunitinib Clinical Pharmacology and Biopharmaceutics Review. 2005. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021938_S000_Sutent_BioPharmR.pdf. Accessed 1 Jul 2023.
- 225.Chen T, Chen J, Chen C, Guo J, He X, Zheng S, et al. Systematic review and cost-effectiveness of pharmacokinetically guided sunitinib individualized treatment for patients with metastatic renal cell carcinoma. Ther Adv Med Oncol. 2022;14:17588359221085212. doi: 10.1177/17588359221085212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gandhi KA, Joshi A, Mehta P, Gurjar M, Rane P, Sharma J, et al. Feasibility of therapeutic drug monitoring of sunitinib and its implications on response and toxicity in patients with metastatic renal cell cancer. Cancer Chemother Pharmacol. 2022;89(6):751–759. doi: 10.1007/s00280-022-04432-4. [DOI] [PubMed] [Google Scholar]
- 227.Food and Drug Administration. Center for Drug Evaluation and Research Vandetanib Clinical Pharmacology and Biopharmaceutics Review. 2011. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022405Orig1s000ClinPharmR.pdf. Accessed 1 Jul 2023.
- 228.Thiery-Vuillemin A, Mouillet G, Nguyen Tan Hon T, Montcuquet P, Maurina T, Almotlak H, et al. Impact of everolimus blood concentration on its anti-cancer activity in patients with metastatic renal cell carcinoma. Cancer Chemother Pharmacol. 2014;73(5):999–1007. doi: 10.1007/s00280-014-2435-7. [DOI] [PubMed] [Google Scholar]
- 229.de Wit D, Schneider TC, Moes DJ, Roozen CF, den Hartigh J, Gelderblom H, et al. Everolimus pharmacokinetics and its exposure-toxicity relationship in patients with thyroid cancer. Cancer Chemother Pharmacol. 2016;78(1):63–71. doi: 10.1007/s00280-016-3050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ravaud A, Urva SR, Grosch K, Cheung WK, Anak O, Sellami DB. Relationship between everolimus exposure and safety and efficacy: meta-analysis of clinical trials in oncology. Eur J Cancer. 2014;50(3):486–495. doi: 10.1016/j.ejca.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 231.Deppenweiler M, Falkowski S, Saint-Marcoux F, Monchaud C, Picard N, Laroche M-L, et al. Towards therapeutic drug monitoring of everolimus in cancer? Results of an exploratory study of exposure-effect relationship. Pharmacol Res. 2017;121:138–144. doi: 10.1016/j.phrs.2017.04.029. [DOI] [PubMed] [Google Scholar]
- 232.Willemsen A, de Geus-Oei LF, de Boer M, Tol J, Kamm Y, de Jong PC, et al. Everolimus exposure and early metabolic response as predictors of treatment outcomes in breast cancer patients treated with everolimus and exemestane. Target Oncol. 2018;13(5):641–648. doi: 10.1007/s11523-018-0596-8. [DOI] [PubMed] [Google Scholar]
- 233.Yin O, Zahir H, French J, Polhamus D, Wang X, van de Sande M, et al. Exposure-response analysis of efficacy and safety for pexidartinib in patients with tenosynovial giant cell tumor. CPT Pharmacometrics Syst Pharmacol. 2021;10(11):1422–1432. doi: 10.1002/psp4.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Food and Drug Administration. Center for Drug Evaluation and Research Pexidartinib Clinical Pharmacology and Biopharmaceutics Review. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211810Orig1s000MultidisciplineR.pdf. Accessed 1 Jul 2023.
- 235.Westerdijk K, Desar IME, Steeghs N, van der Graaf WTA, van Erp NP. Imatinib, sunitinib and pazopanib: From flat-fixed dosing towards a pharmacokinetically guided personalized dose. Br J Clin Pharmacol. 2020;86(2):258–273. doi: 10.1111/bcp.14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Maliepaard M, Carree W, van Bussel MTJ. Dose selection and tolerability of anticancer agents evaluated by the European Medicines Agency in the period 2015–2020. ESMO Open. 2021;6(6):100301. doi: 10.1016/j.esmoop.2021.100301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Muller AE, Huttner B, Huttner A. Therapeutic drug monitoring of beta-lactams and other antibiotics in the intensive care unit: which agents, which patients and which infections? Drugs. 2018;78(4):439–451. doi: 10.1007/s40265-018-0880-z. [DOI] [PubMed] [Google Scholar]
- 238.Yoo S, Kim G, Kim S, Ha J, Cho BS, Joo DJ, et al. volumetric absorptive microsampling for the therapeutic drug monitoring of everolimus in patients who have undergone liver transplant. Ther Drug Monit. 2022. [DOI] [PubMed]
- 239.Velghe S, Stove CP. Volumetric absorptive microsampling as an alternative tool for therapeutic drug monitoring of first-generation anti-epileptic drugs. Anal Bioanal Chem. 2018;410(9):2331–2341. doi: 10.1007/s00216-018-0866-4. [DOI] [PubMed] [Google Scholar]
- 240.Mc Laughlin AM, Schmulenson E, Teplytska O, Zimmermann S, Opitz P, Groenland SL, et al. Developing a nationwide infrastructure for therapeutic drug monitoring of targeted oral anticancer drugs: the ON-TARGET study protocol. Cancers (Basel). 2021;13(24). [DOI] [PMC free article] [PubMed]
- 241.Boike L, Henning NJ, Nomura DK. Advances in covalent drug discovery. Nat Rev Drug Discovery. 2022;21(12):881–898. doi: 10.1038/s41573-022-00542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Strelow JM. A perspective on the kinetics of covalent and irreversible inhibition. SLAS Discov. 2017;22(1):3–20. doi: 10.1177/1087057116671509. [DOI] [PubMed] [Google Scholar]
- 243.Shah AY, Motzer RJ, Apolo AB, Powles T, Escudier B, Zhang J, et al. Cabozantinib (C) exposure-response (ER) analysis for the phase 3 CheckMate 9ER (CM 9ER) trial of nivolumab plus cabozantinib (N+C) versus sunitinib (S) in first-line advanced renal cell carcinoma (1L aRCC) J Clin Oncol. 2021;39(15_suppl):4561. [Google Scholar]
- 244.Ishida Y, Murai K, Yamaguchi K, Miyagishima T, Shindo M, Ogawa K, et al. Pharmacokinetics and pharmacodynamics of dasatinib in the chronic phase of newly diagnosed chronic myeloid leukemia. Eur J Clin Pharmacol. 2016;72(2):185–193. doi: 10.1007/s00228-015-1968-y. [DOI] [PubMed] [Google Scholar]
- 245.Tiseo M, Andreoli R, Gelsomino F, Mozzoni P, Azzoni C, Bartolotti M, et al. Correlation between erlotinib pharmacokinetics, cutaneous toxicity and clinical outcomes in patients with advanced non-small cell lung cancer (NSCLC) Lung Cancer. 2014;83(2):265–271. doi: 10.1016/j.lungcan.2013.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable because this is a review article.