Abstract
Aims
High- (HR) and intermediate-high risk (IHR) pulmonary embolisms (PEs) are related to high early mortality and long-term sequelae. We aimed to describe clinical outcomes and adverse events in IHR and HR pulmonary embolism (PE) treated with catheter-directed mechanical thrombectomy (CDMT) in a real-world population.
Methods and Results
This study is a multicenter, prospective registry enrolling 110 PE patients treated with CDMT between 2019 and 2022. The CDMT was performed using the 8F Indigo (Penumbra, Alameda, CA, USA) system bilaterally in pulmonary arteries (PAs). The primary safety endpoints included device or PE-related death during the 48-h after CDMT, procedure-related major bleeding, or other major adverse events. Secondary safety outcomes were all-cause mortality during hospitalization or the follow-up. The primary efficacy outcomes were the reduction of PA pressures and change in the right-to-left ventricular (RV/L) ratio assessed in the imaging 24–48 h after the CDMT.
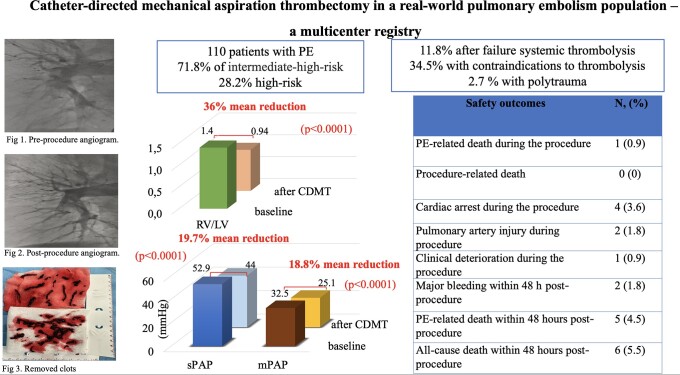
71.8% of patients had IHR PE and 28.2% HR PE. 11.8% of patients had a failure and 34.5% had contraindications to thrombolysis, and 2.7% had polytrauma. There was 0.9% intraprocedural death related to RV failure and 5.5% deaths within the first 48 h. CDMT was complicated by major bleeding in 1.8%, pulmonary artery injury in 1.8%, and ischaemic stroke in 0.9%. Immediate haemodynamic improvements included a 10.4 ± 7.8 mmHg (19.7%) drop in systolic PAP (P < 0.0001), a 6.1 ± 4.2 mmHg (18.8%) drop in mean PAP, and 0.48 ± 0.4 (36%) drop in RV/LV ratio (P < 0.0001).
Conclusion
These observational findings suggest that CDMT may improve hemodynamics with an acceptable safety profile in patients with IHR and HR PE.
Keywords: Catheter-directed mechanical aspiration thrombectomy, High-risk, Intermediate-high risk, Mortality, Pulmonary embolism, Safety
Graphical Abstract
Graphical Abstract.
Introduction
Over the last decade, the role of endovascular therapies in the management of acute pulmonary embolism (PE) has rapidly evolved.1–6 Catheter-directed thrombolysis and catheter-directed mechanical thrombectomy (CDMT) techniques have been developed to counter the bleeding risk associated with systemic thrombolysis (ST).3,7–9 While CDMT for the treatment of intermediate-high risk (IHR) and HR PE is becoming increasingly implemented, data supporting its safety, and efficacy have been limited.8,10–12 The paucity of clinical outcomes research in advanced stages of PE (IHR and HR), especially for endovascular therapies limits its implementation by the current recommendations’ guidelines.2,3,9,13 CDMT uses a vacuum source providing aspiration through a catheter to achieve a percutaneous thrombectomy.8 The Indigo system (Penumbra, Alameda, CA, USA) is an 8F CDMT device designed to remove clots from PAs without using thrombolysis. The effectiveness of the Indigo system in the PE treatment was demonstrated in the EXTRACT-PE study, but the patient cohort was restricted to intermediate-high-risk (submassive) PE patients.8 This report aims to evaluate outcomes of the Indigo aspiration system, including immediate changes in hemodynamics, acute safety, and effectiveness, and longer-term clinical outcomes during 90-day follow-up.
Methods
Study design
The study is a prospective multicenter registry that enrolled 110 patients at four centres in Poland between January 2019 and August 2022. The study protocol was in accordance with the Declaration of Helsinki and was approved by the coordinating centre’s institutional bioethics committee (Poznan University of Medical Sciences Bioethics Committee; approval number 271/2021). Data for this study were obtained from the larger prospective, multicenter registry of patients treated by Polish PERTs; the Polish Multicenter PE Response Teams Outcomes Registry registered in ClinicalTrials.gov: NCT04879069. All patients provided informed consent (if they were unconscious, close relatives approved the treatment).
Inclusion criteria were patients older than 18 years old with symptoms of acute PE for less than 14 days who were categorized as IHR, or HR in accordance with the guidelines of the European Society of Cardiology (ESC), and qualified for CDMT with the Indigo System per the local PE response team (PERT) discretion.2,14–16 Enrolled patients had proximal clots located in at the minimum one main or lobar pulmonary artery (PA) confirmed by computed tomography pulmonary angiography (CTPA). Patients with IHR PE were enrolled if they demonstrated at least one of the listed signs of clinically severe PE longer than 24 h despite proper parenteral anticoagulation: heart rate (HR) ≥ 100 bpm, systolic blood pressure (SBP) 90–100 mmHg, arterial blood saturation (SaO2) ≤ 90% on room air and were considered to be at increased risk of bleeding complications when treated with full-dose ST. Patients with IHR PE and haemodynamic deterioration (sudden occurrence of one or more of the abovementioned signs of severe PE) were also included. HR PE patients with SBP < 90 mmHg or requiring catecholamine support to keep SBP > 90 mmHg, or after cardiac arrest, were enrolled if they had absolute contraindications to ST, or previously administered thrombolysis failed.
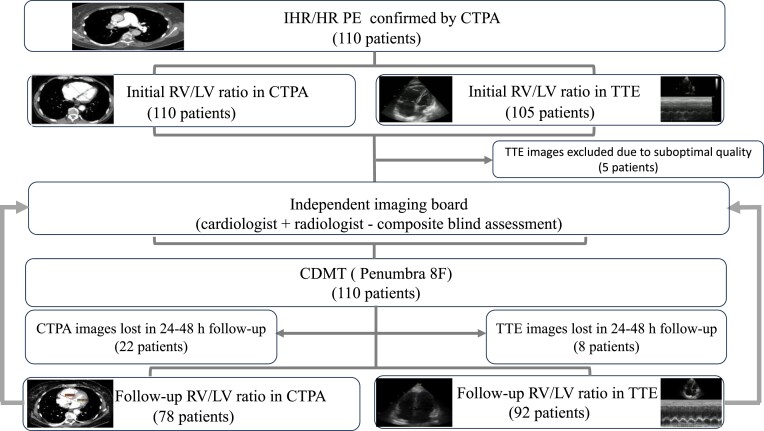
Exclusion criteria were pregnancy, refusal to sign the informed consent form, presence of intracardiac thrombus, history of severe or chronic pulmonary hypertension, known serious, and uncontrolled sensitivity to radiographic agents, a life expectancy of less than a month due to co-morbidities (as determined by the physician), severe thrombocytopenia (platelets count below 20 000/µL), or active major bleeding according to the Bleeding Academic Research Consortium (BARC).17 Follow-up evaluations were performed at 24–48 h, 30 days (±7 days), and 90 days (±21 days). Demographic, clinical, laboratory, and mandatory imaging studies (echocardiographic and CTPA) data were collected in all patients. The analysis of the imaging studies was performed by two independent reviewers from the coordinating centre, a cardiologist with an experience in echocardiography and a radiologist with experience in interpreting the CTPA of the PAs. All participating centres supported the study with internal funding from statutory funds.
Catheter-directed mechanical aspiration thrombectomy
The Indigo aspiration System comprises the 8F Indigo aspiration catheter and associated tubing, vacuum pump, and separator wire used for mechanical thrombus fragmentation.8 CDMT procedures were mostly performed via the common femoral vein access. To evaluate hemodynamics 7F Swan-Ganz catheter was used, and cardiac output and pulmonary vascular resistance were assessed with the Fick method. Then, a diagnostic pulmonary angiogram via a 6F pigtail catheter was performed to indicate detailed thrombi burden. Thrombi load was assessed according to the Miller Index.18,19
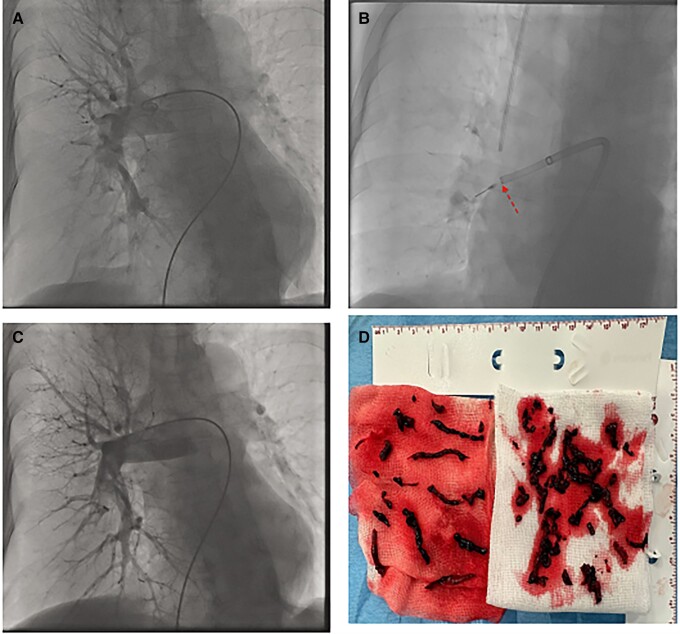
The 8F Indigo catheter was introduced over a 0.035-in guidewire and deployed proximal to the thrombus in the lobar artery of the right or left PA, and sustained aspiration was initiated to remove the clot. Then separator wire was repeatedly passed through the thrombus to break it up and facilitate suction. The above manoeuvres could be repeated in multiple vessels. A final angiogram and haemodynamic assessment, including PA pressures (PAPs) were performed directly after CDMT and recorded (see Figure 1). Procedural anticoagulation was provided with therapeutic doses of unfractionated heparin (UFH) under activated clotting time control (therapeutic range: 200–300 s.). Investigators determined when to terminate the intervention based on carefully evaluating the patient’s haemodynamic status, residual thrombus burden, and the total amount of aspirated blood (should not exceed 300 mL). After CDMT UFH or low-molecular-weight heparin (LMWH) in weight-adjusted dose was continued for 24–48 h depending on the patient’s clinical status, and the type of anticoagulation drug administered to each patient at discharge was at the investigator’s discretion.
Figure 1.
Catheter-directed mechanical aspiration thrombectomy procedure. (A) Pulmonary angiogram showing clots in right main and lobar pulmonary arteries with total occlusion of intermediate and lower lobe arteries. (B) Catheter-directed mechanical aspiration thrombectomy with the separator wire used in the right lower lobe artery. (C) Pulmonary angiogram showing clots removal from lower in the right lower lobe artery. (D) Image of clots removed from pulmonary arteries during the procedure.
Outcomes
The primary safety endpoints included device or PE-related death during the 48-h after CDMT, procedure-related major bleeding (BARC type 3a or greater), or other procedure-related major adverse events.17 Secondary safety outcomes were all-cause mortality during the index hospitalization or follow-up period. CDMT or device-related adverse events were specified as PA injury, cardiac injury, cardiac tamponade, sustained ventricular tachyarrhythmias, clinical deterioration defined by haemodynamic, or respiratory meeting specific thresholds, access-site pseudoaneurysm, arteriovenous fistula, peripheral ischaemia, or nerve injury. Clinical safety outcomes were assessed by trained independent physician reviewers not involved in performing the procedures.
The reduction of PAPs (systolic PAP and mean PAP) and the reduction in the right RV-to-left ventricular ratio (RV/LV ratio) assessed in the CTPA (using the reformatted four-chamber view)20 and/or echocardiography (using four-chamber view), 24–48 h after the CDMT were primary efficacy outcomes. Secondary efficacy measures were the improvement in RV strain [tricuspid annular plane excursion (TAPSE)] assessed in the echocardiography 24–48 h after the CDMT, reduction of heart rate (HR), oxygen demand, and change in blood pressure as well as symptoms relief. All CTPA studies were of good quality for analysis, but five initial transthoracic echocardiography (TTE) scans had suboptimal quality and were excluded from the analysis. Details regarding imaging acquisition and analysis are displayed in Figure 2.
Figure 2.
Flowchart of imaging studies analysis.
Statistical analysis
Patients’ characteristics are presented as absolute and percentage frequencies for categorical variables and mean with standard deviation (SD) for continuous variables with normal distribution. The normality distribution was assessed with the Shapiro–Wilk test. Variables with a non-normal distribution are presented as the median and interquartile range (IQR). The differences between the parameters at baseline, after CDMT, and follow-up were analysed using the paired Student’s t-test or Wilcoxon signed-rank test where appropriate. McNemar’s test was applied to compare categorical variables using available pairwise values. The associations between baseline characteristics and RV/LV ratio reduction 48 h post-procedure were evaluated using multiple linear regression models. Statistical analysis was performed using MedCalc Software Ltd. version 20.215 (Ostend, Belgium).
Results
Baseline patients’ characteristics
The mean age was 57.3 ± 13.5 years, and 38.2% of patients were female. According to current ESC guidelines, 28.2% were categorized as HR PE, and 71.8% as IHR PE. The mean Pulmonary Embolism Severity Index (PESI) score among IHR patients was 120.3 ± 32.4 points. Notably, 11.8% of patients underwent unsuccessful ST before CDMT, 4.5% deteriorated on parenteral anticoagulation, and 34.5% were assessed to have absolute contraindications to thrombolysis according to local PERT. The baseline composite RV/LV ratio was 1.4 ± 0.3. All patients with initial laboratory work-up (100 of 110) had elevated highly sensitive (hs) Troponin I and N-terminal pro-brain natriuretic peptide (NT-proBNP) levels. The mean baseline hs Troponin I level was 2.4 ± 7.3 ng/mL (normal value <0.05 ng/mL), and the mean baseline NT-proBNP level was 5609.7 ± 5805.3 pg/mL (normal value <125 pg/mL). Concomitant deep vein thrombosis was present in 60.9% of patients. Moreover, 14.5% of patients underwent surgery within 2 weeks before PE diagnosis and 2.7% were polytrauma patients. Details are presented in Table 1.
Table 1.
Baseline patients’ demographics and clinical characteristics
| Variables | Baseline |
|---|---|
| Demographics | |
| Age, years | 57.3 ± 13.5 |
| Female/male sex | 42 (38.2)/68 (61.8) |
| BMI, kg/m2 | 29.0 ± 5.0 |
| Co-morbidities | |
| Arterial hypertension | 40 (36.4) |
| Congestive heart failure | 3 (2.7) |
| Diabetes mellitus | 11 (10.0) |
| Concomitant deep vein thrombosis | 67 (60.9) |
| Coronary artery disease | 4 (3.6) |
| Chronic obstructive pulmonary disease | 10 (9.1) |
| COVID-19 | 1 (0.9) |
| Chronic kidney disease | 10 (9.1) |
| Risk factors for PE | |
| Obesity, BMI ≥ 30 kg/m2 | 35 (31.8) |
| Malignancy | 19 (17.3) |
| Surgery within last 2 weeks | 16 (14.5) |
| Polytrauma | 3 (2.7) |
| Immobilization | 14 (12.7) |
| Thrombophilia | 20 (18.2) |
| History of PE | 25 (22.7) |
| Oral contraceptive | 7 (6.4) |
| PE clinical presentation | |
| Syncope | 13 (11.8) |
| Dyspnea at rest | 100 (90.9) |
| Chest pain | 5 (4.5) |
| Symptoms duration, days | 3 ± 2.5 |
| PE severity | |
| Intermediate-high risk | 79 (71.8) |
| High risk | 31 (28.2) |
| PESI score | 120.3 ± 32.4 |
| PESI class | |
| PESI class | |
| I-II | 3 (2.7) |
| III | 6 (5.5) |
| IV | 41 (37.3) |
| V | 29 (26.4) |
| Heart rate, bpm | 110.7 ± 16.8 |
| Systolic blood pressure, mmHg | 115 ± 22.2 |
| Diastolic blood pressure, mmHg | 74.9 ± 15.4 |
| Respiratory rate, pm | 29.5 ± 5.4 |
| Oxygen supplementation (to keep SaO2 > 90%) | 80 (72.7) |
| Arterial oxyhaemoglobin saturation, % | 90.2 ± 6.0 |
| Arterial pO2, mmHg | 66.9 ± 21.2 |
| FiO2, | 0.6 ± 0.3 |
| Oxygenation index (pO2/FiO2) | 122.5 ± 113.5 |
| Biomarkers | |
| hs Troponin I level, ng/mL (normal value <0.05 ng/mL) | 2.4 ± 7.3 |
| NT-proBNP level, pg/mL (normal value < 125 pg/mL) | 5609.7 ± 5805.3 |
| Lactate level, mmol/L (normal value <2.2 mmo/L) | 2.8 ± 2.0 |
| RV dysfunction | |
| RV/LV ratio, mm/mm (CTPA) | 1.4 ± 0.3 |
| RV/LV ratio, mm/mm (echo) | 1.3 ± 0.3 |
| Composite RV/LV ratio, mm/mm (CTPA/echo)a | 1.4 ± 0.3 |
| TAPSE, mm | 15 ± 4.1 |
| S’ wave, cm/s | 11.5 ± 3.3 |
| RVSP, mmHg | 51.1 ± 12.6 |
| Failed therapy prior CDMT | |
| ST | 13 (11.8) |
| Anticoagulation (clinical deterioration during therapy) | 5 (4.5) |
| Absolute contraindication to thrombolysis | 38 (34.5) |
Composite RV/LV ratios using either CTPA or TTE measurements, with CTPA prioritized if both were available.
Abbreviations: BMI, Body mass index; CDMT, catheter-directed mechanical thrombectomy; FiO2, oxygen demand; hs, high sensitive; NT-proBNP, N-terminal-pro-B-type natriuretic peptide; pO2, oxygen partial pressure; PE, pulmonary embolism; PESI, pulmonary embolism severity index; RV/LV ratio, right ventricular-to-left ventricular ratio; RVSP, right ventricular systolic pressure; ST, systemic thrombolysis; TAPSE, tricuspid annular systolic excursion.
Procedural characteristics
All patients presented with bilateral PE, and 50.9% of patients had clots mostly located in the main and lobar PAs. Most patients (56.4%) received UFH as initial anticoagulation. In the vast majority of cases (98.2%), the CDMT was performed via common femoral vein access. There were two (1.8%) major access-site haematomas that were treated conservatively but required transfusion of two units of packed red blood cells. The mean total procedure duration time (skin-to-skin) was 64.7 ± 22.0 min, with the mean estimated blood loss per procedure of 296 ± 79.2 mL. In three HR PE patients (2.7%), the CDMT was performed on extracorporeal membrane oxygenation (ECMO) support. An inferior vena cava filter was implanted in four patients (3.6%) due to absolute contraindications to anticoagulation. In one case (0.9%) due to the co-existence of chronic thromboembolic lesions, the CDMT was escalated to open surgical thrombendarterectomy. 10.9% of patients were hospitalized in the intensive care unit (ICU) after the procedure, with a mean ICU stay of 5 ± 4 days. Patients were mostly discharged on direct oral anticoagulants 59% followed by low-molecular-weight-heparins in 18.2% and vitamin K antagonists in 8.2%. Procedural characteristics are summarized in Table 2.
Table 2.
Procedural characteristics and clinical outcomes
| Variables | |
|---|---|
| Time from diagnosis to procedure, days, mean ±SD | 1.6 ± 1.4 |
| Initial anticoagulant | |
| Unfractionated heparin | 62 (56.4) |
| Low-molecular-weight heparin | 41 (37.3) |
| PE location | |
| Bilateral | 110 (100) |
| Saddle and main arteries | 56 (50.9) |
| Lobar and segmental | 54 (49.1) |
| Segmental | — |
| Access-site | |
| Common femoral vein | 108 (98.2) |
| Jugular vein | 2 (1.8) |
| Access-site complications | |
| Major a | 2 (1.8) |
| Minor | 8 (7.3) |
| Total procedure duration time (min), mean ±SD | 64.7 ± 22.0 |
| Amount of blood loss, ml, mean ±SD | 296.0 ± 79.2 |
| Periprocedural ECMO use | 3 (2.7) |
| Vena cava filter implantation | 4 (3.6) |
| Conversion to open surgery | 1 (0.9) |
| Length of hospitalization, days, mean ±SD | 10 ± 6.8 |
| Length of hospitalization after the procedure, days, mean ±SD | 8 ± 5.6 |
| Need for ICU stay | 12 (10.9) |
| Anticoagulation at discharge | 65 (59) |
| Direct oral anticoagulant | 9 (8.2) |
| Vitamin K antagonist | 20 (18.2) |
| Low-molecular-weight heparin None | 4 (3.6) |
No need for surgical or endovascular treatment.
Abbreviations: See Table 1. ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
Clinical and haemodynamic outcomes
Clinical and haemodynamic outcomes are presented in Table 3. The mean baseline RV/LV ratio was 1.4 ± 0.3 and the 48-h post-procedure RV/LV ratio was 0.94 ± 0.1 [−0.48 ± 0.4 (36%) mean change, P < 0.0001]. Immediately following the procedure, the sPAP decreased from 52.9 ± 16.0 mmHg to 44 ± 13.4 mmHg [−10.4 ± 7.8 mmHg (19.7%) mean change, P < 0.0001]. The mPAP also significantly decreased on-table from 32.5 ± 7 mmHg to 25.1 ± 8.5 mmHg [−6.1 ± 4.2 mmHg (18.8%) mean change, P < 0.0001]. There was also a significant on-table improvement in mean pulmonary artery pulsatility index (PAPI) from 4.2 ± 2.2 mmHg/mmHg to 7.6 ± 6.6 mmHg/mmHg [+3.0 ± 5.6 mmHg/mmHg (71.4%) mean change, P < 0.0001]. Notably, there was a significant oxygen demand (FiO2) reduction from 0.6 ± 0.3 to 0.43 ± 0.1 [− 0.27 ± 0.2 (45%) mean change, P < 0.0001].
Table 3.
Changes in hemodynamics and vitals following catheter-directed mechanical thrombectomy
| Initial Mean ± SD |
After CDMT Mean ± SD |
Mean Change Mean ± SD (%) |
P value | |
|---|---|---|---|---|
| RV/LV ratio (CTPA) | 1.4 ± 0.3 | 0.94 ± 0.1 | −0.48 ± 0.4 (36) | <0.0001 |
| RV/LV ratio (TTE) | 1.3 ± 0.3 | 0.97 ± 0.1 | −0.33 ± 0.1 (42.9) | <0.0001 |
| Composite RV/LV (CTPA/TTE) a | 1.4 ± 0.3 | 0.94 ± 0.1 | −0.46 ± 0.1 (32.9) | <0.0001 |
| sPAP, mmHg | 52.9 ± 16.0 | 44 ± 13.4 | −10.4 ± 7.8 (19.7) | <0.0001 |
| mPAP, mmHg | 32.5 ± 7 | 25.1 ± 8.5 | −6.1 ± 4.2 (18.8) | <0.0001 |
| dPAP, mmHg | 21.1 ± 5.7 | 16.8 ± 8.5 | −5.9 ± 6.1 (28.0) | <0.0001 |
| mRAP, mmHg | 14.1 ± 2.9 | 11.2 ± 2.4 | −3.9 ± 3.5 (27.7) | <0.0001 |
| PAPI, mmHg/mmHg | 4.2 ± 2.2 | 7.6 ± 6.6 | +3.0 ± 5.6 (71.4) | <0.0001 |
| CO | 4.8 ± 1.3 | 5.0 ± 1.2 | +0.2 ± 0.1 (4.2) | 0.0008 |
| TPVR, WU | 4.5 ± 2.4 | 3.3 ± 1.8 | −1.2± 0.9 (26.7) | 0.003 |
| TAPSE, mm | 15 ± 4.1 | 20.5 ± 4.6 | +6.1 ± 4.5 (40.7) | <0.0001 |
| Heart rate, bpm | 110.7 ± 16.8 | 89.5 ± 13.8 | −23.9 ± 15.3 (21.6) | <0.0001 |
| SBP, mmHg | 115.1 ± 22.3 | 122.3 ± 13.9 | +8.7 ± 20.9 (7.6) | 0.07 |
| DBP, mmHg | 74.9 ± 15.8 | 79 ± 10.6 | +5.4 ± 15.0 (7.2) | 0.15 |
| Arterial pO2, mmHg | 66.9 ± 5.5 | 76.9 ± 2.8 | +13.1 ± 17.2 (19.6) | 0.0009 |
| FiO2 | 0.6 ± 0.3 | 0.43 ± 0.1 | − 0.27 ± 0.2 (45.0) | <0.0001 |
| hs Troponin I, ng/mL | 2.4 ± 0.7 | 2.1 ± 1.2 | −1.6 ± 7.6 (66.7) | <0.0001 |
| NT-proBNP, pg/mL | 5609.7 ± 5805.3 | 3319.7 ± 415.9 | − 3277.4 ± 1667.1 (58.4) | <0.0001 |
| Lactate, mmol/L | 2.8 ± 0.2 | 1.9 ± 0.1 | −0.9 ± 2.4 (32.1) | <0.0001 |
| Haemoglobin concentration, mmol/L | 8.3 ± 0.1 | 7.7 ± 0.2 | −0.8 ± 1.5 (9.6) | <0.0001 |
Composite RV/LV ratios using either CTPA or TTE measurements, with CTPA prioritized if both were available.
Abbreviations: See Table 1. CO, cardiac output; DBP, diastolic blood pressure; dPAP, diastolic pulmonary arterial pressure; mPAP, mean pulmonary arterial pressure; mRAP, mean atrial pressure; PAPI, pulmonary artery pulsatility index; SBP, systolic blood pressure; sPAP, systolic pulmonary pressure; TPVR, total pulmonary vascular resistance; WU, Wood Units.
RV function also improved significantly from baseline to 48 h, with the TAPSE improvement from 15 ± 4.1 mm to 20.5 ± 4.6 mm [+6.1 ± 4.5 mm (40.7%) mean change, P < 0.0001]. There was also a significant reduction in RV strain markers levels at 48 h post-procedure assessment, hs troponin I dropped from 2.4 ± 0.7 ng/mL to 2.1 ± 1.2 [−1.6 ± 7.6 ng/mL (66.7%) mean change, P < 0.0001], and NT-proBNP dropped from 5609.7 ± 5805.3 pg/mL to 3319.7 ± 415.9 pg/mL (−3277.4 ± 1667.1 pg/mL [58.4%] mean change, P < 0.0001). No significant differences were found in clinical and haemodynamic improvements for patients with HR PE compared to those with IHR PE.
Safety outcomes
There was one (0.9%) intraprocedural death related to RV failure and four patients (3.6%) experienced sudden cardiac arrest in the catheterization suite with successful resuscitation. In two patients (1.8%) CDMT was complicated by distal subsegmental PA injury and non-massive haemoptysis. In both cases, a 7 Fr thermodilution balloon-tipped catheter (Edwards Lifesciences, USA) was immediately placed and inflated in the segmental artery in proximity to the injured subsegmental artery. The balloon was inflated for ten minutes, with periodic deflations, and angiograms were performed through the distal port of the catheter to determine if the rupture would seal, with final success and no recurrence of haemoptysis. There was no significant drop in haemoglobin concentration in both cases, in the first patient the haemoglobin concentration dropped from the initial 7.4 mmol/L to 6.4 mmol/L, and in the second patient, haemoglobin concentration dropped from the initial 8.0 mmol/L to 6.9 mmol/L after the procedure, respectively.
There were two (1.8%) massive groin haematomas requiring transfusion of two red blood cell packs and qualified as major bleedings within 48 h post-procedure. In both cases, ST was administered as an initial treatment of HR PE. One (0.9%) patient developed an ischaemic stroke with aphasia and left-side paralysis several hours after the CDMT procedure. There were five deaths (4.5%) at 48-h follow-up, three (2.7%) were due to RV failure progression, and 2 (1.8%) were unrelated to the procedure and PE (caused by brain injury due to long-lasting resuscitation). All five patients were HR PE with prior unsuccessful ST with ongoing cardiopulmonary resuscitation (CPR) in whom CDMT was performed as a bailout treatment.
Moreover, 14.5% of patients developed pneumonia or sepsis secondary to pneumonia. There were no additional PE-related deaths at the 30-day follow-up related to PE (overall PE-related 30-day mortality 4.5%), but there was an additional one 0.9% death due to multiorgan failure and two deaths due to disseminated ovarian malignancies (overall all-cause 30-day mortality 8.2%). There were no 90-day all-cause readmission and no further complications. Details are presented in Tables 4 and 5.
Table 4.
Safety and mortality outcomes
| Safety outcomes | N (%) |
|---|---|
| During CDMT procedure | |
| PE-related death during the procedure | 1 (0.9) |
| Procedure-related death | 0 (0) |
| Major bleeding during procedure | 0 (0) |
| Pulmonary artery injury during procedure | 2 (1.8) |
| Cardiac tamponade during procedure | 0 (0) |
| Clinical deterioration during the procedure | 1 (0.9) |
| Cardiac arrest during the procedure | 4 (3.6) |
| Sustained ventricular tachycardia during the procedure | 1 (0.9) |
| 48-h follow-up | |
| PE-related death within 48 h post procedure | 4 (3.6) |
| All-cause death within 48 h post procedure | 6 (5.5) |
| Major bleeding within 48 h post procedure | 2 (1.8) |
| Pulmonary artery injury within 48 h post procedure | 2 (1.8) |
| Cardiac tamponade within 48 h post procedure | 0 (0) |
| Clinical deterioration within 48 h post procedure | 1 (0.9) |
| Infection (pneumonia/sepsis) | 16 (14.5) |
| Stroke (ischaemic/haemorrhagic) | 1 (0.9) |
| 30-day follow-up | |
| PE-related mortality within 30 days post procedure | 4 (3.6) |
| All-cause mortality within 30 days post-procedure | 9 (8.2) |
| PE recurrence | 0 (0) |
| 90-day follow-up | |
| PE-related mortality within 90 days post procedure | 5 (4.5) |
| All-cause mortality within 90 days post procedure | 9 (8.2) |
| PE recurrence | 0 (0) |
Abbreviations: See Table 1.
Table 5.
Detailed characteristics of patients with adverse events
| Patient’s sex, Male/Female; Age, years | Clinical presentation | Indication for CDMT treatment | PE risk category | Adverse event | |
|---|---|---|---|---|---|
| 1 | Female; 53 | obstructive shock, cardiac arrest with ongoing CPR, intubated | Unsuccessful ST |
HR | PE-related death during CDMT |
| 2 | Female, 44 | Sudden cardiac arrest, witnessed CPR > 1 h during CDMT, intubated, ECMO delivery | Unsuccessful ST |
HR | Death within 48 h due to post-traumatic brain injury |
| 3 | Male, 50 | Sudden cardiac arrest, CPR > 1 h during CDMT, severe COVID-19 infection, ECMO delivery | Unsuccessful ST |
HR | multiorgan failure—COVID-19 progression |
| 4 | Female, 59 | Syncope, obstructive shock, CPR during CDMT ECMO delivery | Unsuccessful ST |
HR | PE-related death within 48 h |
| 5 | Male, 64 | Syncope, obstructive shock, | Unsuccessful ST |
HR | PE-related death within 48 h |
| 6 | Male, 70 | Obstructive shock | Unsuccessful ST |
HR | PE-related death within 48 h |
| 7 | Female, 84 | Presyncope, respiratory failure, ovarian cancer | Relative contraindications to ST | IHR | Death within 30 days due to ovarian cancer |
| 8 | Female, 48 | Presyncope, respiratory failure, ovarian cancer | Relative contraindications to ST | IHR | Death within 30 days due to ovarian cancer |
| 9 | Female, 53 | Presyncope, respiratory failure, 24 h after haemorrhagic stroke treatment | Absolute contraindications to ST | HR | Multiorgan failure |
| 10 | Male, 44 | Obstructive shock | Unsuccessful ST |
HR | Major bleeding- access-site haematoma BARC 3a |
| 10 | Female, 47 | Obstructive shock | Unsuccessful ST |
HR | Major bleeding- access-site haematoma BARC 3a |
| 11 | Female, 63 | Sudden cardiac arrest, witnessed CPR > 1 h during CDMT, intubated, ECMO delivery | Relative contraindications to ST | HR | Pulmonary artery injury during CDMT |
| 12 | Male, 48 | Obstructive shock | Absolute contraindications to thrombolysis | HR | Pulmonary artery injury during CDMT |
| 13 | Female, 60 | Presyncope, respiratory failure, cervical cancer | no improvement on low-molecular-weight heparin | IHR | Clinical deterioration during transfer on cath lab table before CDMT |
| 14 | Female, 59 | Syncope, obstructive shock, | Unsuccessful ST |
HR | Sustained ventricular tachycardia when passing through RV outflow tract |
Abbreviations: See Table 1; BARC, Bleeding Academic Consortium Research; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation, ST, systemic thrombolysis.
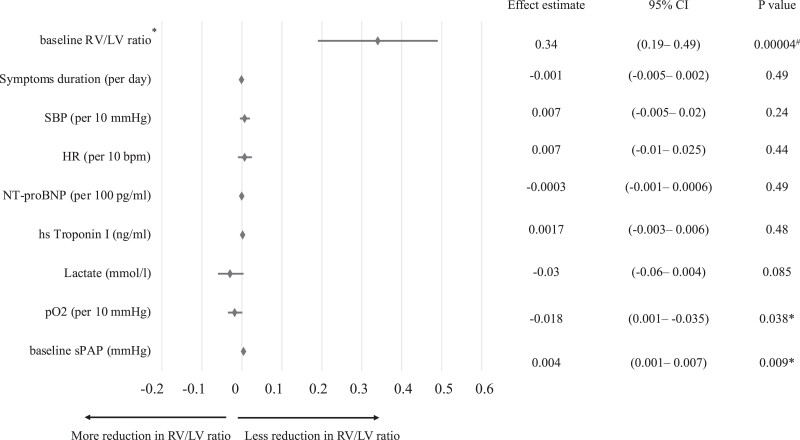
Factors associated with postprocedural RV/LV ratio reduction
Multiple linear regression application indicated that higher baseline sPAP was related to a more modest reduction in composite RV/LV ratio following CDMT (P = 0.009), while higher initial arterial pO2 was associated with a greater reduction of postprocedural RV/LV ratio (P = 0.038), respectively (see Figure 3).
Figure 3.
Multiple linear regression model of absolute reduction in RV/LV ratio. *composite RV/LV ratios using either CTPA or transthoracic echocardiography (TTE) measurements, with CTPA prioritized if both were available. #statistically significant.
Discussion
We observed that CDMT with an 8F Indigo catheter resulted in substantial clot removal and immediate improvement in haemodynamic conditions in PE patients with seemingly low periprocedural risk.
Early mortality in advanced PE is strongly related to haemodynamic compromise due to RV failure.21 Increased RV/LV ratio assessed by imaging studies is a reproducible and validated tool for identifying PE patients with an increased risk of early death.22 In addition to its prognostic role, the RV/LV ratio change plays a significant role as a marker of therapeutic effectiveness.21,23 The mean RV/LV ratio reduction of 0.48 (36%) during 48 h after the procedure is comparable to previous catheter-directed therapy studies including the EXTRACT-PE study (mean RV/LV reduction of 0.43), FLARE trial with the FlowTriever device (mean RV/LV change 0.38), SEATTLE II study (mean RV/LV change 0.42), RESCUE study with the Bashir catheter designed for pharmacomechanical local thrombolysis (mean RV/LV change 0.56) and initial results of mechanical-electric thrombectomy with Magneto 20F device (mean RV/LV change 0.45).8,21,23–25 In the recent FLASH registry also with the FlowTriever system, the mean RV/LV ratio drop was 0.25.10 What is more, the results of this study indicated a significant change in surrogate markers of RV dysfunction including tachycardia, troponin, NT-proBNP, and lactate levels 48 h after CDMT.
The 10.4 mmHg mean on-table drop in sPAP was like that obtained in the FLASH study (12.8 mmHg), but favourable as compared to the EXTRACT-PE study with the same Indigo aspiration system (4.3 mmHg).8,10 We also found that higher baseline sPAP was related to a more modest reduction in RV/LV ratio following CDMT. The mean on-table change in mPAP (−6.1 mmHg) was also similar to that in the FLASH study (−7.6 mmHg).10 While studies on the applicability of catheter-directed thrombolysis have also reported significant haemodynamic improvements, these approaches require more time to achieve pulmonary vascular bed decompression in the opposite of CDMT with fast thrombus debulking.23,26
In our real-world cohort, significantly more patients (28.2%) had HR PE as compared to previous studies regarding pulmonary CDMT 0.84% to 7.9%, respectively.8,10,21,27 This made our population sicker than in previous studies and more frequently excluded from clinical trials. It should be emphasized that our study included patients with polytrauma, malignancies during chemotherapy, and patients with ongoing CPR after the failure of ST in HR PE. Patients with these life-threatening conditions were typically excluded from previous studies. Nonetheless, this study showed a favourable safety profile of CDMT with 3.6% PE-related mortality during the first 48 h after the procedure.
CDMT is increasingly applied in PE treatment for the prevention of patient decompensation and potentially to impact mortality. In this patient cohort, the all-cause 48-h mortality was 5.5% (including 3.6% PE-related). All deaths were HR PE cases in whom CDMT was performed during ongoing CPR after unsuccessful ST. This is significantly lower than reported in a recent meta-analysis of 1517 HR PE patients with an in-hospital of 28.3% despite ST, catheter-directed thrombolysis, or surgical embolectomy.28 In the FLASH registry and the EXTRACT-PE study the 48-h mortality was 0.8%, respectively.8,10
The all-cause 30-day mortality in this study was 8.1%. One was due to multiorgan failure, and two of those deaths were due to malignancies and were not related to CDMT procedures. None of the previously published studies regarding CDMT devices enrolled patients with malignant neoplasms which significantly impact patients’ survival. A recent meta-analysis of 65 589 patients with PE treated with catheter-based therapy (CDMT or catheter-directed thrombolysis) or ST demonstrated a significantly lower 28–30-day mortality in patients treated with percutaneous techniques vs. ST (7.3% vs. 13.6%).29
The important pertinence of CDMT is its utility in patients with high bleeding risk, especially unstable patients with HR PE presentation. In these patients’ ST is absolutely contraindicated, but also catheter-directed local thrombolysis should not be used due to only partial reduction of bleeding risk.2,26 Simultaneously, current guidelines indicate surgical embolectomy as a salvage treatment option when other therapies failed.2,30 In this study, 34.5% of patients had absolute contraindications for thrombolytic therapy. The major bleedings occurred in 1.8% within 48 h post-procedure and were two access-site bleeds quantified as significant based on haemoglobin drop and necessity of blood products transfusion. Both these patients received ST as an initial treatment of HR PE. Our results are in line with the EXTRACT-PE study assessing the applicability of an 8F Indigo system in 119 IHR PE patients with a 1.7% major bleeding rate which included groin haematoma and vascular injury with the guidewire.8 In the present study, two non-massive vessel injuries (1.8%) during CDMT were successfully treated with prolonged balloon inflation and no need for blood product transfusion.
The major bleeding rate in this study was lower than in the ultrasound-assisted catheter-directed thrombolysis SEATTLE II study, where major bleeding occurred in 10% of patients.23 However, the overall major bleeding rate reported in recent studies with a lower dose of the locally administered lytic drug (total dose of alteplase ≤ 20 mg) was comparable to our study, 4.0% in the OPTALYSE study, 2.5% in the Standard vs. Ultrasound-assisted Catheter Thrombolysis for Submassive Pulmonary Embolism (SUNSET sPE) study, 2.1% in the CANARY study, and 0.9% in the RESCUE study PMID: 36121244, respectively.25,26,31,32 The results of the FLASH registry assessing the safety profile of large-bore CDMT were comparable to ours in terms of major bleeding rate (1.4%) despite FlowSaver blood return use (10.3% of patients).10
In the present study, CDMT with an 8F Indigo system was associated with a mean of 9.6% haemoglobin concentration drop due to blood aspiration during the procedure. Despite the lack of a blood return system in the applied 8F Indigo system, no patient needed the transfusion of packed red blood cells because of it. Of note, significant amounts of blood might be aspirated when the catheter is not embedded in the clot, particularly in the hands of inexperienced operators.33 Close and serial monitoring of the collection container must be performed to avoid excessive acute blood loss during the continuous aspiration because the system cannot recycle aspirate blood in contrast to the FlowSaver blood return system utilized in large-bore CDMT with FlowTriever since July 2021. The application of FlowSaver reduced the CDMT procedure-related blood loss from about 250 to 100 mL.10 In our cohort, the mean periprocedural blood loss was 296 mL. Recently, a new generation of Lightning 12F system (Penumbra Inc, Alameda, CA, USA) was introduced that includes new innovative mechanisms to prevent blood loss, efficiently regulate aspiration and enable aspiration of larger clots.12,34 What is more, the next-generation highly torqueable Lightning Flash 16 system (Penumbra Inc, Alameda, CA, USA) with dual clot detection algorithms and no necessity for separator use has just been released in the USA.35 The results of the ongoing STRIKE-PE study will show whether Lightning technology ultimately improves short- and long-term clinical outcomes.36
It should be emphasized that the CDMT procedure is more technically demanding than catheter-directed thrombolysis and requires familiarity with the PA tree anatomy and expertise to manoeuvre the currently available catheters of varying degrees of stiffness and size through the right heart. The present study focuses on 8F catheters with quite good deliverability into the distal main and lobar PAs. However, the relatively small size of the catheter makes it difficult to remove en bloc clots with a bigger diameter. The catheter may also obstruct with clot during the procedure, requiring the application of the separator to clear out the device, and if the separator fails, then the entire device needs to be removed and flushed.33 Furthermore, despite the high flexibility of the catheter ensuring manoeuvrability, a passage through the right heart may promote, and/or exacerbate cardiac and/or pulmonary intraprocedural injuries. Moreover, patient selection for CDMT is a work in progress, and next years need to develop trials that clarify the role of this and other catheter technologies in the treatment of severe PE.
Limitations
Several limitations need to be addressed. First, our study population was relatively small and heterogeneous. Second, this was a single-arm observational study without any comparison group with other treatment modalities or anticoagulation alone. Third, treatment indications were quite heterogeneous, as decisions to perform the CDMT were made by the local PERT. Despite the limited number of study patients, we believe that the consistency of the results validates our observations and helps to improve the understanding of the role of CDMT in IHR and HR PE management. Doubtless, future studies would have to identify, among IHR PE patients, the subgroup of patients with a higher risk of deterioration as potential candidates for urgent CDMT.
Conclusions
In our real-world single-arm observational registry study of patients treated with the 8F Penumbra system, we observed temporal improvements in RV dysfunction and pulmonary hemodynamics with low observed major bleeding and mortality rates. Currently, experts have no consensus about the optimal method for catheter-directed therapy of PE. Future prospective studies and randomized trials are needed to comparatively evaluate different catheter-directed and pharmacological approaches in PE.
Acknowledgements
None.
Contributor Information
Sylwia Sławek-Szmyt, Department of Cardiology, Poznan University of Medical Sciences, Długa 1/2 Street, 61-848 Poznan, Poland.
Jakub Stępniewski, Pulmonary Circulation Centre, Department of Cardiac and Vascular Disease, Jagiellonian University Medical College, John Paul II Hospital in Krakow, Prądnicka 80 Street, 31-202 Krakow, Poland; Department of Medical Education, Jagiellonian University Medical College, Medyczna 7 Street, 30-688 Krakow, Poland.
Marcin Kurzyna, Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology European Health Centre Otwock, Medical Centre for Postgraduate Education, Borowa 14/18 Street, 05-400 Otwock, Poland.
Wiktor Kuliczkowski, Department of Cardiology, Wroclaw Medical University, Borowska 213 Street, 50-556 Wroclaw, Poland.
Stanisław Jankiewicz, Department of Cardiology, Poznan University of Medical Sciences, Długa 1/2 Street, 61-848 Poznan, Poland.
Grzegorz Kopeć, Pulmonary Circulation Centre, Department of Cardiac and Vascular Disease, Jagiellonian University Medical College, John Paul II Hospital in Krakow, Prądnicka 80 Street, 31-202 Krakow, Poland.
Szymon Darocha, Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology European Health Centre Otwock, Medical Centre for Postgraduate Education, Borowa 14/18 Street, 05-400 Otwock, Poland.
Ewa Mroczek, Department of Cardiology, Wroclaw Medical University, Borowska 213 Street, 50-556 Wroclaw, Poland.
Arkadiusz Pietrasik, Department and Faculty of Cardiology, Medical University of Warsaw, Banacha 1A Street, 02-097 Warsaw, Poland.
Marek Grygier, Department of Cardiology, Poznan University of Medical Sciences, Długa 1/2 Street, 61-848 Poznan, Poland.
Maciej Lesiak, Department of Cardiology, Poznan University of Medical Sciences, Długa 1/2 Street, 61-848 Poznan, Poland.
Aleksander Araszkiewicz, Department of Cardiology, Poznan University of Medical Sciences, Długa 1/2 Street, 61-848 Poznan, Poland.
Funding
Statutory funding of all participating centres.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Sedhom R, Elbadawi A, Megaly M, Athar A, Bharadwaj AS, Prasad V, et al. Outcomes with catheter-directed thrombolysis vs. catheter-directed embolectomy among patients with high-risk pulmonary embolism: a nationwide analysis. Eur Heart J Acute Cardiovasc Care 2023;12:224–231. [DOI] [PubMed] [Google Scholar]
- 2. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020;41:543–603. [DOI] [PubMed] [Google Scholar]
- 3. Kopeć G, Araszkiewicz A, Kurzyna M, Sławek-Szmyt S, Stępniewski J, Roik M, et al. Role of catheter-directed therapies in the treatment of acute pulmonary embolism. Expert opinion of the Polish PERT Initiative, Working Group on Pulmonary Circulation, Association of Cardiovascular Interventions, and Association of Intensive Cardiac Care. Kardiol Pol 2023;81:423–440. [DOI] [PubMed] [Google Scholar]
- 4. Sedhom R, Elbadawi A, Megaly M, Jaber WA, Cameron SJ, Weinberg I, et al. Hospital procedural volume and outcomes with catheter-directed intervention for pulmonary embolism: a nationwide analysis. Eur Heart J Acute Cardiovasc Care 2022;11:684–692. [DOI] [PubMed] [Google Scholar]
- 5. Zuin M, Rigatelli G, Bongarzoni A, Enea I, Bilato C, Zonzin P, et al. Mean arterial pressure predicts 48 h clinical deterioration in intermediate-high risk patients with acute pulmonary embolism. Eur Heart J Acute Cardiovasc Care 2023;12:80–86. [DOI] [PubMed] [Google Scholar]
- 6. Araszkiewicz A, Sławek-Szmyt S, Jankiewicz S, Żabicki B, Grygier M, Mularek-Kubzdela T, et al. Continuous aspiration thrombectomy in high- and intermediate-high-risk pulmonary embolism in real-world clinical practice. J Interv Cardiol 2020;2020:4191079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pasha AK, Siddiqui MU, Siddiqui MD, Ahmed A, Abdullah A, Riaz I, et al. Catheter directed compared to systemically delivered thrombolysis for pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis 2022;53:454–466. [DOI] [PubMed] [Google Scholar]
- 8. Sista AK, Horowitz JM, Tapson VF, Rosenberg M, Elder MD, Schiro BJ, et al. Indigo aspiration system for treatment of pulmonary embolism: results of the EXTRACT-PE trial. JACC Cardiovasc Interv 2021;14:319–329. [DOI] [PubMed] [Google Scholar]
- 9. Pruszczyk P, Klok FA, Kucher N, Roik M, Meneveau N, Sharp ASP, et al. Percutaneous treatment options for acute pulmonary embolism: a clinical consensus statement by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function and the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention 2022;18:e623–ee38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toma C, Jaber WA, Weinberg MD, Bunte MC, Khandhar S, Stegman B, et al. Acute outcomes for the full US cohort of the FLASH mechanical thrombectomy registry in pulmonary embolism. EuroIntervention 2023;18:1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sławek-Szmyt S, Jankiewicz S, Grygier J, Mularek-Kubzdela T, Lesiak M, Araszkiewicz A. Acute-on-chronic pulmonary embolism-induced right ventricular failure salvaged by combined catheter-directed therapy and levosimendan infusion. Pol Arch Intern Med 2022;132:16310. [DOI] [PubMed] [Google Scholar]
- 12. Sławek-Szmyt S, Araszkiewicz A, Jankiewicz S, Grygier M, Mularek-Kubzdela T, Lesiak M. Intracranial hemorrhage in a patient with pulmonary embolism: how to overcome two elements? Pol Arch Intern Med 2023;33:16422. [DOI] [PubMed] [Google Scholar]
- 13. Giri J, Sista AK, Weinberg I, Kearon C, Kumbhani DJ, Desai ND, et al. Interventional therapies for acute pulmonary embolism: current Status and principles for the development of novel evidence: a scientific statement from the American Heart Association. Circulation 2019;140:e774–e801. [DOI] [PubMed] [Google Scholar]
- 14. Araszkiewicz A, Kurzyna M, Kopeć G, Roik M, Darocha S, Pietrasik A, et al. Expert opinion on the creating and operating of the regional pulmonary embolism response teams (PERT). Polish PERT initiative. Cardiol J 2019;26:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Araszkiewicz A, Kurzyna M, Kopeć G, Sławek-Szmyt S, Wrona K, Stępniewski J, et al. Pulmonary embolism response team: A multidisciplinary approach to pulmonary embolism treatment. Polish PERT initiative report. Kardiol Pol 2021;79:1311–1319. [DOI] [PubMed] [Google Scholar]
- 16. Sławek-Szmyt S, Jankiewicz S, Smukowska-Gorynia A, Janus M, Klotzka A, Puślecki M, et al. Implementation of a regional multidisciplinary pulmonary embolism response team: PERT-POZ initial 1-year experience. Kardiol Pol 2020;78:300–310. [DOI] [PubMed] [Google Scholar]
- 17. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 18. Miller GA, Sutton GC, Kerr IH, Gibson RV, Honey M. Comparison of streptokinase and heparin in treatment of isolated acute massive pulmonary embolism. Br Med J 1971;2:681–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qanadli SD, El Hajjam M, Vieillard-Baron A, Joseph T, Mesurolle B, Oliva VL, et al. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol 2001;176:1415–1420. [DOI] [PubMed] [Google Scholar]
- 20. Kipfmueller F, Quiroz R, Goldhaber SZ, Schoepf UJ, Costello P, Kucher N. Chest CT assessment following thrombolysis or surgical embolectomy for acute pulmonary embolism. Vasc Med 2005;10:85–89. [DOI] [PubMed] [Google Scholar]
- 21. Tu T, Toma C, Tapson VF, Adams C, Jaber WA, Silver M, et al. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: the FLARE study. JACC Cardiovasc Interv 2019;12:859–869. [DOI] [PubMed] [Google Scholar]
- 22. Meinel FG, Nance JW, Schoepf UJ, Hoffmann VS, Thierfelder KM, Costello P, et al. Predictive value of computed tomography in acute pulmonary embolism: systematic review and meta-analysis. Am J Med 2015;128:747–759.e2. [DOI] [PubMed] [Google Scholar]
- 23. Piazza G, Hohlfelder B, Jaff MR, Ouriel K, Engelhardt TC, Sterling KM, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv 2015;8:1382–1392. [DOI] [PubMed] [Google Scholar]
- 24. Andersen A, Musialek P, Araszkiewicz A, Schultz J, Nielsen-Kudsk JE, Tekieli L, et al. First-in-human trial of mechanical-electric thrombectomy in acute pulmonary embolism. JACC Cardiovasc Interv 2023;16:623–625. [DOI] [PubMed] [Google Scholar]
- 25. Bashir R, Foster M, Iskander A, Darki A, Jaber W, Rali PM, et al. Pharmacomechanical catheter-directed thrombolysis with the bashir endovascular catheter for acute pulmonary embolism: the RESCUE study. JACC Cardiovasc Interv 2022;15:2427–2436. [DOI] [PubMed] [Google Scholar]
- 26. Tapson VF, Sterling K, Jones N, Elder M, Tripathy U, Brower J, et al. A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: The OPTALYSE PE Trial. JACC Cardiovasc Interv 2018;11:1401–1410. [DOI] [PubMed] [Google Scholar]
- 27. Toma C, Bunte MC, Cho KH, Jaber WA, Chambers J, Stegman B, et al. Percutaneous mechanical thrombectomy in a real-world pulmonary embolism population: interim results of the FLASH registry. Catheter Cardiovasc Interv 2022;99:1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Silver MJ, Giri J, Duffy A, Jaber WA, Khandhar S, Ouriel K, et al. Incidence of mortality and complications in high-risk pulmonary embolism: a systematic review and meta-analysis. JSCAI 2023;1:100548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pietrasik A, Gąsecka A, Szarpak Ł, Pruc M, Kopiec T, Darocha S, et al. Catheter-Based therapies decrease mortality in patients with intermediate and high-risk pulmonary embolism: evidence from meta-analysis of 65,589 patients. Front Cardiovasc Med 2022;9:861307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rivera-Lebron B, McDaniel M, Ahrar K, Alrifai A, Dudzinski DM, Fanola C, et al. Diagnosis, treatment and follow up of acute pulmonary embolism: consensus practice from the PERT consortium. Clin Appl Thromb Hemost 2019;25:1076029619853037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Avgerinos ED, Jaber W, Lacomis J, Markel K, McDaniel M, Rivera-Lebron BN, et al. Randomized trial comparing standard versus ultrasound-assisted thrombolysis for submassive pulmonary embolism: the SUNSET sPE trial. JACC Cardiovasc Interv 2021;14:364–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sadeghipour P, Jenab Y, Moosavi J, Hosseini K, Mohebbi B, Hosseinsabet A, et al. Catheter-Directed thrombolysis vs anticoagulation in patients with acute intermediate-high-risk pulmonary embolism: The CANARY Randomized Clinical Trial. JAMA Cardiol 2022;7:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sedhom R, Abdelmaseeh P, Haroun M, Megaly M, Narayanan MA, Syed M, et al. Complications of penumbra indigo aspiration device in pulmonary embolism: insights from MAUDE database. Cardiovasc Revasc Med 2022;39:97–100. [DOI] [PubMed] [Google Scholar]
- 34. Araszkiewicz A, Sławek-Szmyt S, Jankiewicz S, Grygier M, Lesiak M. Lightning 12: A new player in the field of pulmonary percutaneous mechanical thrombectomy. Kardiol Pol 2022;80:956–957. [DOI] [PubMed] [Google Scholar]
- 35. [. Accessed 31 May 2023.]. https://evtoday.com/articles/2023-feb/lightning-flash-the-most-powerful-and-advanced-mechanical-thrombectomy-system-for-pe-and-venous-thrombus
- 36. [. Accessed 31 May 2023.]. https://clinicaltrials.gov/ct2/show/NCT04798261
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.