Abstract
A procedure for molecular identification of Burkholderia gladioli is described. Specific 16S and 23S rRNA gene signature sequences were defined as primers for PCR. The method allows rapid and specific discrimination of B. gladioli from related species (B. cepacia, B. multivorans, B. vietnamiensis, B. mallei, B. pseudomallei, Ralstonia pickettii, and R. eutropha) and should contribute to the clarification of its role as a human pathogen, e.g., in cystic fibrosis.
Burkholderia gladioli (26), previously Pseudomonas gladioli (20), was described by McCulloch in 1921 (17, 18) as P. marginata, a phytopathogen causing a leaf and corm disease of gladioli and other flowers (e.g., iris species). It has been associated with human pulmonary infections, namely, of chronic granulomatous disease (10, 21) and cystic fibrosis (3, 6, 12, 19, 22, 25). The assessment of the clinical significance of B. gladioli in cystic fibrosis is controversial. Clarification has been impaired by uncertain differentiation of B. gladioli and B. cepacia by phenotypic procedures (3). Therefore, we developed a molecular procedure to identify B. gladioli. 16S and 23S rRNA gene (rDNA) sequences were screened for signatures specific for B. gladioli. They were used to establish a rapid and specific PCR procedure which discriminates B. gladioli from related species.
Organisms.
For characterizations of the strains used in this study, see Table 3.
TABLE 3.
Strains and PCR results
| Organism | Strain desig- nation or origina | Size (bp) of product obtained with primer CGM-16-1 or CGM-23-1 plus primer:
|
||||
|---|---|---|---|---|---|---|
| G16-2 | G23-2 | CM16-2458 | CM23-2 | C16-21011 | ||
| B. gladioli | DSM 4285 | 468 | 388 | |||
| CF isolatesb | 468 | 388 | ||||
| B. cepacia | LMG 1222T | 468 | 388 | 1,021 | ||
| DSM 50181 | 468 | 388 | 1,021 | |||
| CF isolatesc | 468 | 388 | 1,021 | |||
| B. multivorans | LMG 14280 | 468 | 388 | |||
| LMG 14293 | 468 | 388 | ||||
| CF isolatesd | 468 | 388 | ||||
| B. mallei | ATCC 23344T | |||||
| ATCC 15310 | ||||||
| B. pseudomallei | ATCC 23343T | |||||
| ATCC 15682 | ||||||
| NCTC 1691 | ||||||
| R. pickettii | ATCC 27511T | |||||
| R. eutropha | ATCC 17697T | |||||
DSM, Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, FRG. CF, cystic fibrosis. LMG, Laboratorium voor Microbiologie Universiteit Gent, Gent. Belgium. ATCC, American Type Culture Collection, Rockville, Md. NCTC, National Collection of Type Cultures. Central Public Health Laboratory, London, England.
Strains from Germany (n = 2) and Canada (n = 3).
Strains from Germany (n = 3), the United Kingdom (n = 2), and Canada (n = 1).
Strains from Germany (n = 16), Denmark (n = 6), and The Netherlands (n = 4).
Nucleic acid preparation.
Genomic DNA was prepared and purified by using the QiaAmp purification kit (Qiagen, Hilden, Federal Republic of Germany [FRG]).
PCR.
Custom oligonucleotide primers (see Table 2) were purchased from MWG Biotech, Ebersberg, FRG. Amplification reactions were performed in a 50-μl final volume with 1 U of Taq polymerase (Boehringer, Mannheim, FRG), 5 μl of the reaction buffer supplied by the manufacturer (diluted 1:10), each deoxynucleoside triphosphate at 10 μM, and each oligonucleotide primer 50 pM. Approximately 50 to 100 ng of DNA was used as the template. To avoid reading mistakes, the Expand High Fidelity PCR System (Boehringer) with a proofreading polymerase was used. The PCR was performed with GeneAmp PCR System 9600 (Applied Biosystems, Weiterstadt, FRG) under the following conditions: denaturation for 5 min at 95°C and then 25 amplification cycles of 30 s at 95°C, 30 s at the annealing temperature specific for the respective primers, and 45 s at 72°C. The samples were then incubated at 72°C for another 7 min and cooled to 4°C. Pyrogen-free water that had been shown to be free of contaminating DNA was used throughout the study. To inactivate contaminating DNA, the PCR mixture was exposed to UV light for 15 min prior to enzyme and template addition. To ascertain reproducibility, all species identifications by PCR were performed in duplicate, each time starting with new cultures.
TABLE 2.
Primer combinations and specificity and PCR product size
| Primer combination | PCR product size (bp)
|
Annealing temp (°C) | |
|---|---|---|---|
| B. gladioli | B. cepacia | ||
| CMG-16-1 + G-16-2 | 468 | 57 | |
| CMG-23-1 + CM-23-2 | 388 | ||
| CMG-16-1 + CM-16-2458 | 468 | 58 | |
| CMG-23-1 + G-23-2 | 388 | ||
The amplification products were checked by agarose gel electrophoresis and purified of salt and excess primers by using a PCR purification kit from Qiagen.
Agarose gel electrophoresis.
Agarose gel electrophoresis was performed as described previously (2).
Sequence determination.
The amplified rDNA was sequenced as described previously (14). The two DNA strands were sequenced from different PCR products. A 373A DNA Sequencer (Applied Biosystems) was used, and the protocol of the manufacturer for dye terminator reactions was followed.
Analysis of the sequence data.
The nucleotide sequences were aligned with reference rDNA sequences provided in the noncommercial software program package ARB (beta-version 2.4). Secondary-structure analysis was done as described by Ludwig et al. (15).
Nucleotide sequence accession number.
The 23S rDNA sequence of B. gladioli DSM 4285 has been deposited in the EMBL database under accession no. Y17182.
16S and 23S rDNA sequences.
16S rDNA sequences of three B. cepacia strains (9, 15, 16) and two B. gladioli strains (13, 27) had already been published. The 23S rDNA of B. cepacia DSM 50181 (X16368) was also available (11). No 23S rDNA sequence of B. gladioli had yet been described. We therefore determined the 23S rDNA gene sequence of B. gladioli DSM 4285 (2,882 bp). Partial 16S and 23S rDNA sequences of additional strains (four of B. gladioli and two of B. cepacia) from various sources were also determined (Fig. 1 and 2).
FIG. 1.
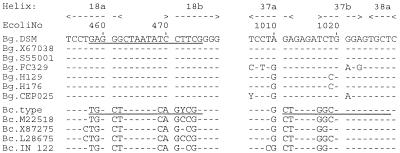
Alignment of the 16S rDNA within the variable helix 18 region and the variable helix 37 and 38 region. The underlined bases are the target signatures for B. gladioli- and B. cepacia-specific PCR primers. Nucleotide numbering is in accordance with Escherichia coli rDNA numbering (4). Bg.DSM, B. gladioli DSM 4285; Bg.X67038, B. gladioli ATCC 10248T (13); Bg.S55001, B. gladioli EY3258 (27); Bg.FC329, Bg.H129, Bg.H176, and Bg.CEP025, B. gladioli cystic fibrosis isolates (this study); Bc.type, B. cepacia LMG 1222T (this study); Bc.M22518, B. cepacia LMG 1222T (8); Bc.X87275, B. cepacia DSM 50181 (15); Bc.L28675, B. cepacia G4 (16); Bc.IN 122, B. cepacia cystic fibrosis isolate (this study). Wobble base: Y = C or T.
FIG. 2.
Alignment of the 23S rDNA within the helix 9 and 10 regions and within the variable helix 45 region. The underlined bases are the target signature for B. gladioli- and B. cepacia-specific PCR primers. Nucleotide numbering is in accordance with E. coli rDNA numbering (5). Bg.DSM, B. gladioli DSM 4285; Bg.FC329, Bg.H129, Bg.H176, and Bg.CEP025, B. gladioli cystic fibrosis isolates (this study); Bc.type, B. cepacia LMG 1222T; Bc.X16368, B. cepacia DSM 50181 (11); Bc.IN 122 and Bc.C5424, B. cepacia cystic fibrosis isolates (this study). The asterisks indicate that there was no nucleotide at this position, in contrast to E. coli.
Analysis of 16S and 23S rDNAs for signature sequences.
The prerequisite for discrimination of species by their S rDNAs are interspecies sequence deviations and their consistency among different strains of one species. There should be no or only low intraspecies sequence variability within the signature sequence section. Both conditions were checked by multiple sequence alignment of the hypervariable regions within the 16S and 23S rDNAs. Interspecies sequence deviations were identified in the helix 18a/18b region of the 16S rDNA. Within a region of the appropriate length for a primer (e.g., 18-mer), no intraspecies variability was detected among the seven B. gladioli strains and only one variable position (472) was found in B. cepacia. In the helix 37a/37b region, B. cepacia presents both consistent interspecies deviations (at four positions) and only minor intraspecies sequence variations. Therefore, this region might be exploited for a signature sequence specific for B. cepacia. Within the 23S rDNA hypervariable regions, only the helix 45a/b section carries useful interspecies differences (for B. gladioli at five positions and for B. cepacia at six positions) without major intraspecies variability (at two positions each for B. gladioli and B. cepacia). Altogether, five candidates for species-specific sequences were identified. They are characterized in Table 1.
TABLE 1.
Primers selected for PCR
| Target species | Primer designationa | Target position within tRNA helix | Sequence |
|---|---|---|---|
| B. cepacia, B. multivorans, B. gladioli | CMG-16-1 | 16S: 1a/2a | 5′-AGA GTT TGA TCM TGG CTC AG-3′ |
| B. cepacia, B. multivorans | CM-16-2458 | 16S: 18ab | 5′-CCG RCT GTA TTA GAG CCA-3′ |
| B. cepacia | C-16-21011 | 16S: 37ab | 5′-AGC ACT CCC RCC TCT CAG-3′ |
| B. gladioli | G-16-2458 | 16S: 18ab | 5′-CGA AGG ATA TTA GCC CTC-3′ |
| B. cepacia, B. multivorans, B. gladioli | CMG-23-1 | 23S: 31b/36b | 5′-ATA GCT GGT TCT CTC CGA A-3′ |
| B. cepacia, B. multivorans | CM-23-2 | 23S: 45ab/36b | 5′-CTC TCC TAC CAT GCG YGC-3′ |
| B. gladioli | G-23-2 | 23S: 45ab/36b | 5′-CCT ACC ATG CAY ATA AAT-3′ |
1, sense (forward) primer; 2, antisense (reverse) primer. Wobble bases: M = A or C, R = A or G, and Y = C or T.
Selection of primers for PCR.
The aim was to establish a procedure which allows discrimination of strains of both species by a single PCR. Thus, the selection of primers was guided towards PCR products of different lengths for either species. Therefore, a constant sense primer (annealing to a DNA target common to both species) was paired with a primer specific for either B. gladioli or B. cepacia or B. cepacia plus B. multivorans that, however, anneals at a distance different from that of the sense primer (Table 2). The PCR products obtained with different sets of primers are shown in Fig. 3, which demonstrates the efficacy and specificity of the proposed primers both alone and in combination.
FIG. 3.
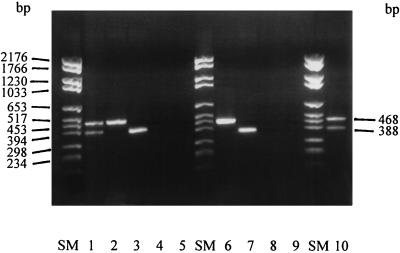
Primer combinations and PCR products of two different sizes. Lanes: SM, DNA molecular size markers; 1, CMG-16-1 plus G-16-2 and CMG-23-1 plus CM-23-2 (PCR products obtained with B. gladioli [468 bp] and B. cepacia [388 bp]); 2, CMG-16-1 plus G-16-2 (B. gladioli product [468 bp]); 3, CMG-23-1 plus G-23-2 (B. gladioli product [388 bp]); 4, CMG-16-1 plus G-16-2 (no PCR product obtained with B. cepacia); 5, CMG-23-1 plus G-23-2 (no PCR product obtained with B. cepacia); 6, CMG-16-1 plus CM-16-2 (B. cepacia product [468 bp]); 7, CMG-23-1 plus CM-2-2 (B. cepacia product [388 bp]); 8, CMG-16-1 plus CM-16-2 (no PCR product obtained with B. gladioli); 9, CMG-23-1 plus CM-23-2 (no PCR product obtained with B. gladioli); 10, CMG-16-1 plus CM-16-2 and CMG-23-1 plus G-23-2 (PCR products obtained with B. cepacia [468 bp] and B. gladioli [388 bp]). The first letters(s) of the species from which PCR products were obtained is underlined.
Evaluation of primer specificity.
The abilities of the primers to initiate the synthesis of species-specific PCR products were evaluated with strains of various Burkholderia and Ralstonia species from type culture collections (Table 3). Either no PCR products or amplification products of the expected sizes were found (Table 3). Furthermore, three strains each of P. aeruginosa, Stenotrophomonas maltophilia, and Acinetobacter baumannii were investigated for cross-reactivity with the selected primers. No PCR product was detected with these strains (data not shown).
Comparison of species identifications by PCR and by biochemical tests of clinical isolates.
A number of Burkholderia strains isolated from cystic fibrosis patients at various centers in Europe and Canada were identified to the species level by biochemical reactions as proposed by Vandamme et al. (24). Their phenotypic identification was confirmed by the PCR procedure (Table 3). However, three strains sent to our laboratory as B. cepacia were found to be B. gladioli by PCR.
B. gladioli has been associated with lung infections in humans with an impaired host defense, e.g., in chronic granulomatous disease (10, 21), where a genetic defect causes ineffective killing mainly of pathogens producing catalase (such as B. gladioli). B. gladioli was first isolated from sputa of patients suffering from cystic fibrosis by Mortensen et al. (19) and Christenson et al. (6) and was then regarded as a commensal. In contrast, Khan et al. (12) described the growth of B. gladioli (without P. aeruginosa) in the sputum of a cystic fibrosis patient before lung transplantation, in the explant, and on postoperative day 40 in abscess drainage fluid, pleural fluid, and two sets of blood cultures. The patient died 6 months after lung transplantation. Those authors considered B. gladioli to be a pathogen of cystic fibrosis patients who undergo lung transplantation. Wilsher et al. (26) reported on six patients with cystic fibrosis in whose sputa the same ribotype of B. gladioli grew. All six patients died of respiratory failure between 1 and 20 months after acquisition of B. gladioli, one from fulminant B. gladioli septicemia. This is the first report of B. gladioli septicemia in a patient with cystic fibrosis. Simpson et al. (22) and Baxter et al. (3) described strains isolated from cystic fibrosis patients which could not unambiguously be identified either as B. cepacia or B. gladioli and therefore were supposed possibly to represent intermediates between the two species.
These reports demonstrate the controversy about the role of B. gladioli in the clinical course of cystic fibrosis. This is partly due to a deficit of procedures for unequivocal identification of B. gladioli in routine diagnostic laboratories (9, 25). Baxter et al. (3) reported that none of nine tests selected from the literature for their potential to differentiate between B. gladioli and B. cepacia produced clear-cut results. Furthermore, 7 of the 32 strains investigated possessed about equal characteristics of both species and were therefore regarded as hybrids. The API 20NE kit falsely identifies B. gladioli as B. cepacia. More reliable identification is achievable by analysis of the fatty acid methyl ester profile (3, 7, 22, 23). In addition, DNA-DNA hybridization was used to differentiate B. gladioli and B. cepacia (1, 6, 25). These procedures are, however, usually not established in laboratories for routine diagnostic use.
Our approach for discrimination of B. gladioli and B. cepacia is based upon the genetic diversity of S rDNAs between the two species. The primers designed for distinction between B. gladioli and B. cepacia were selected to generate amplicons of clearly different sizes for the two species and for not cross-reacting with other Burkholderia (B. pseudomallei and B. mallei) and Ralstonia (Ralstonia pickettii and Ralstonia eutropha) species (Table 3). Therefore, a PCR-based procedure for specific identification of B. gladioli was established. Its main utility in clinical laboratories is more rapid identification of strains grown on selective media (5 h versus up to 5 days) and, furthermore, clarification of uncertain species identification by biochemical reactions. The PCR technique has been established in the majority of clinical laboratories, so there is adequate equipment and technical know-how to allow the use of this procedure in a reliable way.
The procedure is inadequate for discrimination of B. cepacia genomovars I, III, and IV. It should, however, contribute to the clarification of the role of B. gladioli and other Burkholderia species in cystic fibrosis.
Acknowledgments
We thank H. Bärmeier, Erlangen, Germany; J. Dankert, Amsterdam, The Netherlands; J. Govan, Edinburgh, Scotland; N. Høiby, Copenhagen, Denmark; F. Ratjen, Essen, Germany; D. P. Speert, E. Mahenthiralingam, and D. Henry, Vancouver, British Columbia, Canada; and B. Tümmler, Hannover, Germany, for providing Burkholderia sp. isolates from cystic fibrosis patients. We particularly thank P. Vandamme, Gent, Belgium, for stimulating discussions and verification of the species identifications obtained by the PCR procedure described.
REFERENCES
- 1.Ballard R W, Palleroni N J, Doudoroff M, Stanier RY. Taxonomy of the aerobic pseudomonads: Pseudomonas cepacia, P. marginata, P. alliicola, and P. caryophylli. J Gen Microbiol. 1970;60:199–214. doi: 10.1099/00221287-60-2-199. [DOI] [PubMed] [Google Scholar]
- 2.Bauernfeind A, Roller C, Meyer D, Jungwirth R, Schneider I. Molecular procedure for rapid detection of Burkholderia mallei and Burkholderia pseudomallei. J Clin Microbiol. 1998;36:2737–2741. doi: 10.1128/jcm.36.9.2737-2741.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter I A, Lambert P A, Simpson I N. Isolation from clinical sources of Burkholderia cepacia possessing characteristics of Burkholderia gladioli. J Antimicrob Chemother. 1997;39:169–175. doi: 10.1093/jac/39.2.169. [DOI] [PubMed] [Google Scholar]
- 4.Brosius J, Palmer M L, Kennedy P J, Noller H F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci USA. 1978;75:4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brosius J, Dull T, Noller H F. Gene organisation and primary structure of a ribosomal RNA operon of Escherichia coli. J Mol Biol. 1981;148:107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- 6.Christenson J C, Welch D F, Mukwaya G, Muszynski M J, Weaver R E, Brenner D J. Recovery of Pseudomonas gladioli from respiratory tract specimens of patients with cystic fibrosis. J Clin Microbiol. 1989;27:270–273. doi: 10.1128/jcm.27.2.270-273.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dees S B, Hollis D G, Weaver R E, Moss C W. Cellular fatty acid composition of Pseudomonas marginata and closely associated bacteria. J Clin Microbiol. 1983;18:1073–1078. doi: 10.1128/jcm.18.5.1073-1078.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dewhirst, F. E., B. J. Paster, and P. L. Bright. 1991. EMBL database accession no. M 22518.
- 9.Henry D A, Campbell M E, LiPuma J J, Speert D P. Identification of Burkholderia cepacia isolates from patients with cystic fibrosis and use of a simple new selective medium. J Clin Microbiol. 1997;35:614–619. doi: 10.1128/jcm.35.3.614-619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoare S, Cant A J. Chronic granulomatous disease presenting as severe sepsis due to Burkholderia gladioli. Clin Infect Dis. 1996;23:411. doi: 10.1093/clinids/23.2.411. [DOI] [PubMed] [Google Scholar]
- 11.Höpfl P, Ludwig W, Schleifer K-H, Larsen N. The 23S ribosomal RNA higher-order structure of Pseudomonas cepacia and other procaryotes. Eur J Biochem. 1989;185:355–364. doi: 10.1111/j.1432-1033.1989.tb15123.x. [DOI] [PubMed] [Google Scholar]
- 12.Khan S U, Gordon S M, Stillwell P C, Kirby T J, Arroliga A C. Empyema and bloodstream infection caused by Burkholderia gladioli in a patient with cystic fibrosis after lung transplantation. Pediatr Infect Dis J. 1996;15:637–639. doi: 10.1097/00006454-199607000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Del Dot T, Dorsh M, Stackebrandt E, Sly L I, Hayward A C. Phylogenetic studies on rRNA group II Pseudomonas using 16S rRNA sequences. J Appl Bacteriol. 1993;74:324–329. [Google Scholar]
- 14.Ludwig W, Kirchhof G, Klugbauer N, Weizenegger M, Betzl D, Ehrmann M, Hertl C, Jilg S, Tatzel R, Zitzelsberger H, Liebl S, Hochberger M, Lane D, Wallnöfer P R, Schleifer K-H. Complete 23S ribosomal RNA sequences of Gram-positive bacteria with a low DNA G+C content. Syst Appl Microbiol. 1992;15:487–501. [Google Scholar]
- 15.Ludwig W, Rosselló-Mora R, Aznar R, Klugbauer S, Spring S, Reetz K, Beimfohr C, Brockmann E, Kirchhof G, Dorn S, Bachleitner M, Klugbauer N, Springer N, Lane D, Nietupsky R, Weizenegger M, Schleifer K-H. Comparative sequence analysis of 23S rRNA from Proteobacteria. Syst Appl Microbiol. 1995;18:164–188. [Google Scholar]
- 16.Massol-Deya A, Weller R, Rios-Hernandez L, Zhou J Z, Hickey R F, Tiedge J M. Succession and convergence of biofilm communities in fixed-film reactors treating aromatic hydrocarbons in groundwater. Appl Environ Microbiol. 1997;63:270–276. doi: 10.1128/aem.63.1.270-276.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCulloch L. A bacterial disease of gladiolus. Science. 1921;54:115–116. doi: 10.1126/science.54.1388.115. [DOI] [PubMed] [Google Scholar]
- 18.McCulloch L. A bacterial blight of gladioli. J Agric Res. 1924;27:225–230. [Google Scholar]
- 19.Mortensen J B, Schidlow D V, Stahl E M. Pseudomonas gladioli (marginata) isolated from a patient with cystic fibrosis. Clin Microbiol Newsl. 1988;10:29–30. [Google Scholar]
- 20.Palleroni N J. Genus I. Pseudomonas Migula 1894, 237AL. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins, Co.; 1984. pp. 141–199. [Google Scholar]
- 21.Ross J P, Holland S M, Gill V J, DeCarlo E S, Gallin J I. Severe Burkholderia (Pseudomonas) gladioli infection in chronic granulomatous disease: report of two successfully treated cases. Clin Infect Dis. 1995;21:1291–1293. doi: 10.1093/clinids/21.5.1291. [DOI] [PubMed] [Google Scholar]
- 22.Simpson I N, Finlay J, Winstanley D J, Dewhurst N, Nelson J W, Butler S L, Govan J R W. Multi-resistance isolates possessing characteristics of both Burkholderia (Pseudomonas) cepacia and Burkholderia gladioli from patients with cystic fibrosis. J Antimicrob Chemother. 1994;34:353–361. doi: 10.1093/jac/34.3.353. [DOI] [PubMed] [Google Scholar]
- 23.Stead D E. Grouping of plant-pathogenic and some other Pseudomonas spp. by using cellular fatty acid profiles. Int J Syst Bacteriol. 1992;42:281–295. [Google Scholar]
- 24.Vandamme P, Holmes B, Vancannyet M, Coenye T, Hoste B, Coopman R, Revets H, Lauwers S, Gillis M, Kersters K, Govan J R W. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int J Syst Bacteriol. 1997;47:1188–1200. doi: 10.1099/00207713-47-4-1188. [DOI] [PubMed] [Google Scholar]
- 25.Welch D F, Muszynski M J, Pai C H, Marcon M J, Hribar M M, Gilligan P H, Matsen J M, Ahlin P A, Hilman B C, Chartrand S A. Selective and differential medium for recovery of Pseudomonas cepacia from the respiratory tracts of patients with cystic fibrosis. J Clin Microbiol. 1987;25:1730–1734. doi: 10.1128/jcm.25.9.1730-1734.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilsher M L, Kolbe J, Morris A J, Welch D F. Nosocomial acquisition of Burkholderia gladioli in patients with cystic fibrosis. Am J Respir Crit Care Med. 1997;155:1436–1440. doi: 10.1164/ajrccm.155.4.9105090. [DOI] [PubMed] [Google Scholar]
- 27.Yabuuchi E, Kosako Y, Oyaizu H, Yano I, Hotta H, Hashimoto Y, Ezaki T, Arakawa M. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes, 1981) comb. nov. Microbiol Immunol. 1992;36:1251–1275. doi: 10.1111/j.1348-0421.1992.tb02129.x. [DOI] [PubMed] [Google Scholar]