Abstract
Structural and functional alterations in the Ca2+ regulatory proteins present in the sarcoplasmic reticulum have recently been shown to be strongly involved in the pathogenesis of heart failure. Chronic activation of the sympathetic nervous system or of the renin-angiotensin system induces abnormalities in both the function and structure of these proteins. We review here the considerable body of evidence that has accumulated to support the notion that such abnormalities contribute to a defectiveness of contractile performance and hence to the progression of heart failure.
Introduction
Heart failure (HF) is characterized by a complex disorder that leads to a disturbance of the normal pumping of blood to the peripheral organs to meet the metabolic demands of the body. In a heart that has suffered myocardial damage, regardless of the initial cause of the damage (hypertension, myocardial ischemia, cardiomyopathy, etc.), HF eventually occurs if such damage persists for a prolonged period (1, 2). In the initial stages, compensation for the myocardial damage and maintenance of hemodynamics can occur via activation of both the sympathetic nervous system and the renin-angiotensin system, resulting in LV dilatation and/or hypertrophy. However, if the depressed cardiac function persists, with a parallel activation of neurohumoral factors, the myocardial damage becomes progressive and irreversible, and the heart can no longer meet the metabolic demand of the body, resulting in the phenotype of HF (1, 2).
A growing body of evidence has accumulated concerning the altered intracellular Ca2+ cycling that plays a key role in the development of HF (3–5). Recent advances in the field of molecular biology have shed light on the close relationship between Ca2+ cycling abnormalities and the progression of HF. In many cases, altered Ca2+ cycling precedes the observed depression of mechanical performance; consequently, an amelioration of the disorder of Ca2+ cycling has potential as a new and intriguing therapeutic strategy against HF (5). In this review, we focus on the role of Ca2+ regulatory proteins in the pathogenesis of HF and on the possibility of developing a new therapeutic strategy against HF using Ca2+ regulatory proteins as the target.
Intracellular Ca2+ handling in normal cardiomyocytes
In the normal heart, intracellular Ca2+ movements critically regulate subsequent mechanical contractions. In cardiac excitation-contraction (E-C) coupling (Figure 1), a small amount of Ca2+ first enters through the L-type Ca2+ channel (LTCC) during membrane depolarization. This Ca2+ influx triggers a large-scale Ca2+ release through the Ca2+ release channel of the sarcoplasmic reticulum (SR), referred to as the ryanodine receptor (RyR). The released Ca2+ then binds to the troponin C within the myofilaments, which induces activation of the myofilaments and a consequent muscle contraction (6–8). Relaxation is initiated by dissociation of Ca2+ from troponin C, followed by its reuptake into the SR through phospholamban-regulated (PLN-regulated) Ca2+-ATPase (SERCA2a) and subsequent trans-sarcolemmal Ca2+ removal through the Na+/Ca2+ exchanger (NCX) operating in its forward mode (7–9). The whole process of Ca2+ movement is characterized by a transient increase in intracellular [Ca2+] from 100 nM to about 1 μM (8). For termination of Ca2+ release, RyR adaptation (10), RyR inactivation (11), and SR Ca2+ depletion may play important roles by acting in a synergistic manner.
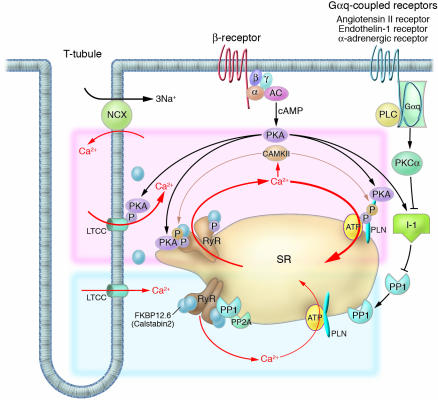
Figure 1.
Intracellular Ca2+ cycling and associated signaling pathway in cardiomyocytes. On a beat-by-beat basis, a calcium transient is evoked by the initial influx of a small amount of Ca2+ through the LTCC and the subsequent large-scale Ca2+ release from the SR through the RyR. During diastole, cytosolic Ca2+ is taken up into the SR by the PLN-regulated SERCA2a pump. β receptor–mediated PKA stimulation regulates this Ca2+ cycling by phosphorylating LTCC, RyR, and PLN. In normal hearts, sympathetic stimulation activates β1-adrenergic receptor, which in turn stimulates the production of cAMP by adenylyl cyclase and thereby activates PKA. PKA phosphorylates PLN and RyR, both of which contribute to an increased intracellular Ca2+ transient and enhanced cellular contractility (pink zone signal). PP1 and PP2A regulate the dephosphorylation process of these Ca2+ regulatory proteins (RyR, PLN, LTCC) (blue zone signaling). Activation of the Gαq-coupled receptors (angiotensin II receptor, endothelin 1 receptor, or α-adrenergic receptor) activates PLC, which in turn activates PKC-α. The PKC-α phosphorylates I-1, augmenting the activity of PP1 and causing hypophosphorylation of PLB. The PLB hypophosphorylation inhibits SERCA2a activity, thereby decreasing SR Ca2+ uptake. The increased Ca2+ level in the cytosol activates CAMKII, which affects the functions of RyR and PLN. Activation or deactivation of these molecules at a node in the signaling cascade affects beat-by-beat Ca2+ cycling, and such maneuvers have recently been highlighted as potential new therapeutic strategies against HF. α, G protein subunit α; β, G protein subunit β; γ, G protein subunit γ; AC, adenylyl cyclase; PLC, phospholipase C.
Triggers for Ca2+ release and defective E-C coupling in HF
In most types of HF, the density of LTCC seems to be either unaltered or reduced (12). However, there is evidence that the function of LTCC may be altered in human HF. For instance, Shröder et al. (13) demonstrated increases in both the availability and open probability of LTCC isolated from failing human hearts, possibly due to a defect in dephosphorylation. Moreover, Chen et al. (14) recently reported that the density of LTCC was reduced in human HF although the current was maintained due to an increase in the phosphorylation level. The efficiency of the trigger (the size of the inward Ca2+ current) needed to cause Ca2+ release from the SR has been termed E-C coupling gain (15). In many cases of HF, the E-C coupling gain seems to be reduced by several factors: (a) a functional defect in LTCC (16–18); (b) an increase in the space between LTCC and RyR (19); (c) a decrease in SR Ca2+ (20–22); and/or (d) an abnormality in the channel-gating property of RyR (23–26). Not only the amount of Ca2+ released for a given Ca2+ release trigger but also the rate of Ca2+ release may be important for the contractility of the myofilaments. Since crossbridge cycling is considered to occur very rapidly from the beginning of the rising phase of the Ca2+ transient (27), a faster elevation of the cytosolic Ca2+ concentration might accelerate crossbridge attachment, resulting in faster and/or higher tension development. In contrast, the dyssynchronous Ca2+ release seen in HF might lead to a slower rate of rise in the Ca2+ transient (28, 29), probably leading to a dyssynchronous binding of Ca2+ to troponin C, and thereby to a slower velocity of contraction (30). However, since there is no direct evidence to support this notion, it remains to be determined whether the reduced velocity of contraction in HF really is caused by an altered gain or efficiency of E-C coupling.
In HF, the SR Ca2+ content is reportedly decreased (20–22) although the fraction of Ca2+ released to Ca2+ sequestered during Ca2+ uptake seems to be increased (25). Both an upregulation of NCX and a reduction in SERCA2a activity may be responsible for the reduced SR Ca2+ content observed in HF (12). The depressed SR Ca2+ load would reduce the E-C coupling gain, leading to contractile dysfunction as described above. In the normal contractile state, the greater SR Ca2+ content leads to a large fraction of the SR Ca2+ being released for a given Ca2+ trigger (7). This may be attributable to a stimulatory effect of the high intraluminal [Ca2+] ([Ca2+]SR) on the channel open probability of RyRs (7, 31). Since the SR Ca2+ content is reduced in HF, the threshold SR content for a fractional Ca2+ release may be reduced, leading to a susceptibility to aberrant Ca2+ release (or spontaneous Ca2+ leak) at lower cytosolic [Ca2+]. RyRs are coupled to proteins at the luminal SR surface (triadin, junctin, and calsequestrin) (32). Since these proteins buffer luminal Ca2+ and modulate the Ca2+ release process (32), structural and functional alterations in these proteins may be causally involved in the development of defective intraluminal [Ca2+] regulation seen in HF. A hypersensitivity of RyR2 channel opening to cytosolic [Ca2+] may contribute to the presence of a spontaneous Ca2+ leak at much lower levels of cytosolic [Ca2+] in HF than in the normal SR (i.e., approximately 100 nM during diastole). This spontaneous Ca2+ leak may lead to a delayed after-depolarization (DAD), which can trigger arrhythmia (33).
Altered function of SR Ca2+ regulatory proteins in HF
Defective FKBP12.
6-mediated stabilization of RyR as a cause of HF. RyR is a Ca2+ release channel existing as a huge homotetramer transversing the SR membrane (34) (Figure 2). Three mammalian isoforms of RyR have been identified. Of these, RyR1 is found in skeletal muscle while RyR2 is predominantly expressed in cardiac muscle (35, 36). RyR3 is ubiquitously expressed at low levels and has functional properties that differ from those of both RyR1 and RyR2 (37).
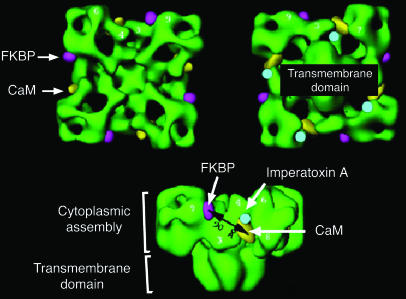
Figure 2.
Three-dimensional structure of the skeletal muscle RyR, with some key sites of protein interactions. FKBP, FK506-binding protein; CaM, calmodulin. Image reprinted with permission from the Journal of Biological Chemistry (115).
Each monomer contains approximately 5,000 amino acids and has a molecular weight of 565 kDa. RyR is also a scaffolding protein to which numerous key regulatory proteins are bound, thus forming the junctional complex (38–40). It associates with FK506–binding protein (FKBP), calmodulin, protein kinase A (PKA), protein phosphatase 1 (PP1), and protein phosphatase 2A (PP2A). The RyRs are closely associated with LTCC, and this spatial association of the 2 channels forms a key functional unit in cardiac E-C coupling (32).
One of these accessory proteins, FKBP12.6, plays an important role in the stabilization of the channel (in other words, in the maintenance of the closed state of the channel). FKBP12.6 binds to RyR2 with a stoichiometric ratio of 1 FKBP12.6 to 1 RyR2 monomer, or 4 FKBP12.6s to 1 tetramer (41). Marx et al. (39) reported that in human HF, and in an experimental model of HF, PKA-mediated hyperphosphorylation of RyR2 occurs, and this in turn dissociates FKBP12.6 from RyR2, leading to a diastolic Ca2+ leak through RyR2. Long-term hyperphosphorylation of RyR2 can be maintained through a reduction in the protein abundance of PP1 and PP2A, both of which are tightly coupled to RyR2 (39). In a lipid-bilayer experiment, single-channel activity was found to be hypersensitized to [Ca2+], owing to a partial loss of FKBP12.6 from RyR2, thus causing (in HF) a diastolic Ca2+ leak at a concentration of [Ca2+] (approximately 100 nM) at which no significant Ca2+ release is induced in normal hearts (39). This diastolic Ca2+ leak depresses the SR Ca2+ load and serves as a substrate for DAD, which can trigger cardiac arrhythmia and lead to sudden death (42–44). The dissociation of FKBP12.6 from RyR2 also functionally uncouples multiple RyR2s and disturbs both the simultaneous opening of RyR2s during systole and their simultaneous closing during diastole (26, 39). In vivo, Shannon et al. (45) did indeed find a diastolic Ca2+ leak in a rabbit model of myocardial infarction. Earlier, we found that in a canine model of pacing-induced HF, PKA-hyperphosphorylation of RyR2 occurred in association with a conformational change in RyR2 and a subsequent prominent Ca2+ leak through RyR2 (23), although Jiang et al. later obtained conflicting results using the same model (46).
Attempts to reproduce the altered channel-gating property seen in HF have not been successful in intact myocytes. Independent groups have reported that phosphorylation at serine2808 or serine2809 does not cause FKBP12.6 dissociation from RyR2 and that the constitutive phosphorylation of serine2808 or serine2809 by mutations (S2808D or S2809D) fails to disrupt the FKBP12.6-RyR2 interaction (47, 48). To explain these apparently contradictory findings, Wehrens et al. (49) recently provided data suggesting that overexpression of FKBP12.6 outside the physiological range (47, 48) overwhelms the shift in FKBP12.6-binding affinity induced by PKA phosphorylation, allowing FKBP12.6 to bind to PKA-phosphorylated RyR2. Regarding other findings that seem to conflict with the PKA-hyperphosphorylation theory of HF (39), Li et al. (50) found that PKA phosphorylation of RyR did not increase calcium sparks in permeabilized myocytes. However, this study was performed under conditions in which cytosolic Ca2+ is clamped at 50 or 10 nM, which is lower than diastolic Ca2+ concentrations. It appears that this may match the physiology of E-C coupling because increased Ca2+ release under resting diastolic conditions would cause a serious problem. Valdivia et al. (51) showed that PKA phosphorylation caused an initial transient RyR2 opening in response to a jump in [Ca2+], followed by a rapid deactivation of channel gating. Eisner et al. (52) showed that the abrupt increase in RyR2 opening induced by caffeine in intact cells has only transient effects on the amplitude of the Ca2+ transient (due to autoregulation). That is to say, the additional Ca2+ released by enhanced RyR2 opening will be rapidly removed by NCX during the subsequent beat, thereby reducing the SR Ca2+ available for the next beat. In the steady state, the reduced SR Ca2+ content offsets the effects of increased RyR2 opening with the result that Ca2+ transients are almost unchanged. These studies may provide a mechanism for transiently increasing systolic SR Ca2+ release in a physiological manner to increase cardiac contractility. An important problem in HF is that RyR2s are chronically PKA hyperphosphorylated with a partial loss of FKBP12.6 and that as a result the channels become leaky. These leaky RyR2 channels may reduce the SR Ca2+ load and in turn lead to the reduced contractility of cardiac muscle in failing hearts.
Mutations within RyR as a cause of defective channel opening.
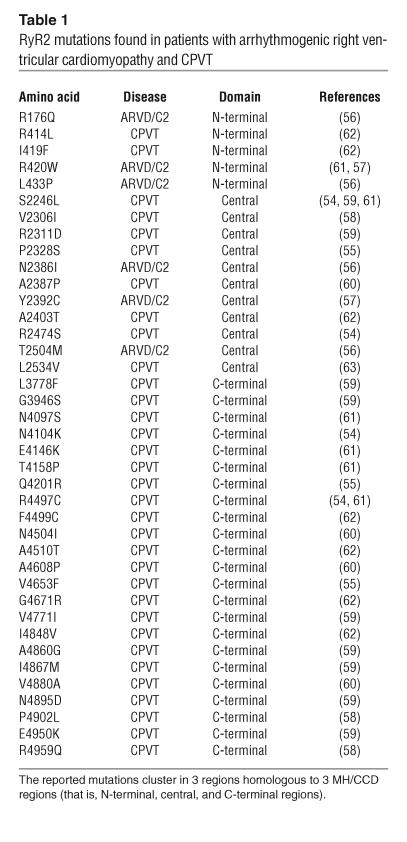
More than 40 RyR1 mutations have been found in patients with malignant hyperthermia (MH) or central core disease (CCD) (33). It has been shown that such RyR1 mutations in MH and CCD produce an abnormal mode of channel gating that alters the Ca2+ inactivation process and makes the channel hyper- and hyposensitive to activating and inactivating ligands, respectively (53). The mutation sites cluster into 3 major regions (N-terminal, central, and C-terminal). To date, more than 30 mutations have been found in the analogous RyR2 regions in patients with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD/C2) or catecholaminergic polymorphic ventricular tachycardia (CPVT) (54–63) (Table 1). This suggests that these 3 regions represent domains that are critical for the regulation of both RyR1 and RyR2 and that these domains are also involved in the pathogenesis of RyR-linked skeletal and cardiac muscle diseases. Interestingly, 1 of the cardiomyopathy (ARVD/C2) mutations in the N-terminal domain of RyR2, Arg176Gln, corresponds exactly to the Arg163Cys human MH mutation of RyR1. Likewise, some RyR2 mutations either match exactly or are located very close to some of the mutations found in RyR1.
Table 1.
RyR2 mutations found in patients with arrhythmogenic right ventricular cardiomyopathy and CPVT
The above findings strongly suggest that each mutation site is crucial for the maintenance of the normal channel-gating property. Marks and colleagues recently investigated the pathogenic role of RyR2 mutations by evaluating channel activity in recombinant RyR2 containing the same single-point mutation as that seen in CPVT patients (64). They found that FKBP12.6-deficient mice and CPVT-associated RyR2 mutants exhibited a significantly increased open probability of the channel only during exercise or in the PKA-phosphorylated state, that these RyR2 mutants displayed a reduced affinity of FKBP12.6 for RyR2, and that a constitutively active recombinant FKBP12.6 (FKBP12.6-D37S, a mutant form of FKBP12.6 with serine residue 37 substituted for aspartic acid) that can bind to PKA-phosphorylated RyR2 reversed the hyperactivity of channel gating seen in PKA-phosphorylated mutated RyR2 (64). These finding are compatible with the clinical finding that CPVT patients do not exhibit arrhythmia at rest but may suffer lethal arrhythmia during exercise (65).
Recently, Jiang et al. (66) reported that the mutant RyR2 linked to CPVT and sudden death increased the sensitivity of single RyR2 channels to activation by luminal Ca2+ and enhanced the basal level of [3H]ryanodine binding, even without PKA-phosphorylation. The discrepancies between these reports might be partly explained by FKBP12.6 being absent from the RyR2 mutant studied by Jiang et al. (66) but present in the RyR2 mutant studied by Wehrens et al. (64). It remains to be determined whether the resting channel-gating property of the FKBP12.6-depleted, mutant RyR2 linked to CPVT and sudden cardiac death can be altered even without PKA phosphorylation and how PKA phosphorylation affects the channel activity in the FKBP12.6-depleted, mutant RyR2.
Domain-domain interaction: a key mechanism for stabilization of RyR.
On the grounds that mutations in either the N-terminal or the central domain produce abnormal modes of RyR channel gating, generally referred to as hyperactivation and hypersensitization effects (33, 53), Ikemoto et al. (67) proposed an intriguing hypothesis. In this hypothesis, the 2 domains (N-terminal and central domain) interact with each other to act as a regulatory switch for channel-gating activity, with a tight zipping of the interacting domains serving to stabilize the channel. A mutation in either domain weakens the interdomain interaction, thus increasing the tendency toward unzipping, which causes activation and leakiness of the Ca2+ channel (67). For instance, 1 of the domain peptides, DP4, which corresponds to the Leu2442-Pro2477 region of the central domain, has been found to enhance [3H]ryanodine binding and to induce Ca2+ release from the SR, thereby inducing contraction, in skinned muscle fibers at an inhibitory Mg2+ concentration (68). DP4 is also known to increase the frequency of Ca2+ sparks in saponin-permeabilized fibers (69) and to increase the open probability of single channels (69). In addition, a cardiac domain peptide corresponding to the Gly2459-Pro2494 region of RyR2 (DPc10) has been shown to produce significant activation of the RyR2 Ca2+ channel at low Ca2+ concentrations in a way similar to that described for DP4 (70). An Arg-to-Ser mutation in the peptide that mimics the Arg2474-to-Ser2474 human CPVT mutation completely abolished both the hyperactivation and the hypersensitization effects seen with DPc10 (70). In light of these data, it might be anticipated that a mutation in the Gly2460-Pro2495 domain of RyR2 would not only make the Ca2+ channel leaky but also increase its sensitivity to various pharmacological agonists, leading to diastolic Ca2+ overload, as widely seen in failing hearts. Since the binding region of FKBP12.6 to RyR2, which seems to reside in residues 2361–2496 according to Marx et al. (39), is included in this sequence in DPc10 (residues 2460–2495), there may be a close mechanistic relationship between PKA-mediated FKBP12.6 dissociation and domain-domain interaction.
NCX.
In intact hearts, trans-sarcolemmal Ca2+ removal occurs through the NCX, acting in its forward mode (8). In hypertrophied or failing hearts, cytosolic [Na+] has been shown to be elevated (71–73), leading to activation of the reverse mode of NCX, which causes a Ca2+ influx (74). Although this Ca2+ influx via NCX is not as efficient for triggering Ca2+ release as the inward Ca2+ current through LTCC (75), it may contribute to an increase in SR Ca2+ content that is favorable for an increase in contractility. In failing hearts, the increased Ca2+ influx via the reverse mode of NCX may not be completely taken up during diastole by SERCA2a, owing to its decreased activity, resulting in the increase in diastolic [Ca2+] that leads to impairment of relaxation (74). As heart rate increases through an activation in sympathetic tone, cytosolic [Na+] is elevated, and the subsequent rise in Ca2+ influx via NCX contributes to a frequency-dependent increase in contractility (positive staircase) (72). In failing hearts, this response is blunted, and the diastolic [Ca2+] is elevated due to the combined effect of a decreased SR Ca2+ uptake and an additional Ca2+ influx via NCX in its reverse mode (74). Moreover, the increased Ca2+ influx that occurs during the later phase of the action potential causes a tail Ca2+ transient that induces DAD and triggers arrhythmia (76). The abundance of NCX protein is reportedly increased in both experimental and human failing hearts although some studies showed an unchanged level (12). This may possibly be explained by differences in the stage and/or severity of the HF.
SERCA2a and PLN.
Many studies have demonstrated a reduced expression of SERCA2a protein in failing hearts, although some studies have shown an unaltered expression (12). Consistently, previous studies have indicated that SR Ca2+ uptake (or SERCA2a activity) is reduced in the failing animal or human myocardium (25, 72, 77). A decrease in the PLN mRNA level has been consistently observed in failing hearts. However, some studies report that the PLN protein level was unchanged (12), resulting in the protein expression of SERCA2a relative to PLN being diminished (3, 4). This ratio (i.e., the protein expression of SERCA2a relative to PLN) indicates the extent of Ca2+ pump inhibition, and hence the basal level of SERCA2a activity is at a lower level in failing hearts than in normal hearts (3, 4). Regarding the phosphorylation of PLN, the level of serine 16 phosphorylation has variously been reported to be reduced (78–80) or unaltered (81, 82) in HF whereas the level of threonine 17 phosphorylation has consistently been reported to be decreased (82, 83). Threonine 17 phosphorylation is affected by the decreased calmodulin-dependent kinase II (CAMKII) activity in HF whereas serine 16 phosphorylation is mainly affected by PKA activity. The level of CAMKII activity also affects the ser-38 residue, which is the calcium-binding domain of SERCA2a, thereby regulating calcium uptake (84). The altered phosphorylation state of PLN may be responsible for the reduced SR calcium-uptake activity seen in HF. Type-1 PP1, which makes up a major protein of the serine/threonine protein phosphatases present in the cardiac myocyte, may also play an important role in regulating PLN phosphorylation since PP1 has been shown to be hyperactivated concurrently with a reduced level of serine 16 phosphorylation in PLN in several models of HF (85–87) and since overexpression of PP1 catalytic subunit α in the mouse heart was shown to lead to marked left ventricular dilation and premature death due to severe HF (88). Decreased threonine 35 phosphorylation in inhibitor-1 (I-1), an endogenous inhibitor of PP1, is further associated with increased PP1 activity in the failing heart (88).
A recent report by Braz et al. (89) demonstrated that PKC regulation of PP1 activity is critical. In HF, not only is the β-adrenergic system stimulated, but the receptor-operated signalings triggered by angiotensin II, endothelin, and the α-adrenergic system are chronically activated, contributing to depressed contractility and to a progression of both remodeling and apoptosis (1, 2). The common key enzyme in the downstream events in these receptor-operated systems is PKC. It has been demonstrated that PKC-α, which is the predominant PKC isoenzyme expressed in the heart (90), plays a key role in regulating cardiac contractility and Ca2+ handling in myocytes (89). PKC-α directly phosphorylates serine 67 in I-1, thereby augmenting the activity of PP1 and causing hypophosphorylation of PLN (89). This finding may in part answer the question of why SERCA2a activity is found to be reduced in HF, apart from the more obvious possibility of a reduced abundance of SERCA2a protein.
New treatment for HF by modulation of Ca2+ regulatory proteins
Stabilization of RyR.
The RyR2 has been shown to be hyperphosphorylated by PKA in both human and experimental HF (23, 39, 91–94), although admittedly Jiang et al. did not observe PKA hyperphosphorylation of RyR2 in a canine model of HF (46). Many large clinical trials have shown that treatment with a β blocker restores cardiac function and reduces the rate of mortality in patients with HF (2, 95). Several researchers have reported recently that in experimental and human HF, β blockers reversed PKA-mediated hyperphosphorylation of RyR2, restored the stoichiometry of the RyR2 macromolecular complex, restored normal single-channel function, and inhibited the Ca2+ leak (91–93). These findings may provide a molecular basis for the common clinical observation that the use of β receptor blockers improves the prognosis of patients with HF. In a canine model of HF, we found that the angiotensin II–receptor blocker valsartan, which has been used in the treatment of HF in the clinical setting, also normalizes the Ca2+ regulatory process through a β blocker–like action (94). By acting on the presynaptic angiotensin II receptor, valsartan inhibited norepinephrine release and stimulated norepinephrine uptake back into the synaptic pool, with the result that adrenergic signals were not overtransmitted into the cell. This would lead to a reduction in the PKA-hyperphosphorylation of RyR2 and to an inhibition of the Ca2+ leak in the failing heart (94).
Since a conformational change in RyR2 precedes the Ca2+ leak (23), an amelioration of this conformational change could be a new therapeutic strategy against HF (Figure 3). Using a canine model of HF, we recently found that chronic administration of a new compound, the 1,4-benzothiazepine derivative JTV519, improved contractility and prevented the development of LV remodeling and HF, presumably by stabilization of RyR2 (80). In JTV519-untreated hearts, RyR2 was PKA-hyperphosphorylated with a dissociation of FKBP12.6 whereas the reverse of these states was true of JTV519-treated hearts, in which channel phosphorylation returned toward the levels seen in the normal heart (80). Using FKBP12.6+/– mice, Wehrens et al. (49) demonstrated that JTV519 increased the affinity of FKBP12.6 for RyR2, which stabilized the closed state of RyR2 and prevented the Ca2+ leak that triggers arrhythmias. In their study, FKBP12.6–/– mice showed an increase in RyR2 open probability, ventricular tachycardia, and sudden cardiac death upon either exercise or PKA-phosphorylation. JTV519 did not prevent arrhythmias in FKBP12.6–/– mice, indicating that the presence of FKBP12.6 in the heart is required for the therapeutic effects of JTV519 to be expressed (49), although it needs to be determined whether the same is true in FKBP12.6-depleted (by PKA-phosphorylation or FK506) RyR2. Lehnart et al. (96) found that recombinant RyR2 channels containing the missense mutations seen in CPVT patients (RyR2-P2328S, RyR2-Q4201R, and RyR2-V4653F) showed defective channel-gating properties (that is, an increase in open probability and resistance to Mg2+-induced inhibition after PKA phosphorylation) and that JTV519 normalized this abnormal channel gating via a rebinding of FKBP12.6 to the channel complex. Collectively, the above data suggest that stabilization of RyR2 may represent a new molecular target for the treatment or prevention of exercise-induced arrhythmias and sudden death in patients with CPVT mutations and HF.
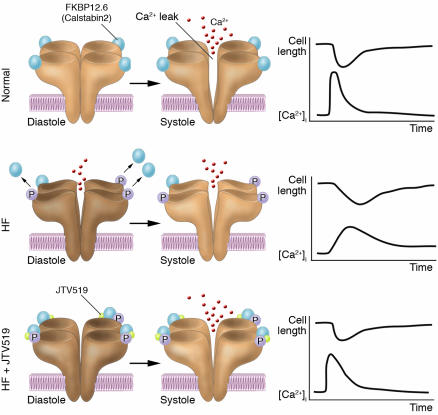
Figure 3.
Therapeutic strategy involving FKBP12.6-mediated stabilization of RyR. A small influx of Ca2+ through the LTCC leads to the release of a large amount of Ca2+ from the SR through RyR in the normal heart. In HF, however, PKA-mediated hyperphosphorylation of RyR2 occurs, and this in turn dissociates FKBP12.6 from RyR2, leading to a diastolic Ca2+ leak through RyR2. This results in the Ca2+ transient being diminished (due to the reduced SR Ca2+ content and dyssynchronous Ca2+ release). Administration of a new compound, the 1,4-benzothiazepine derivative JTV519, normalizes this abnormal channel gating by restoring the conformational state of RyR and by rebinding FKBP12.6 to the channel complex. Thereby, JTV519 normalizes Ca2+ cycling and contractile function in failing cardiac myocytes and hence provides chronic suppression of progressive left ventricular dysfunction in HF. P, PKA phosphorylation at serine 2809; [Ca2+]i, intracellular [Ca2+].
Overexpression of SERCA2a and PLN inhibition.
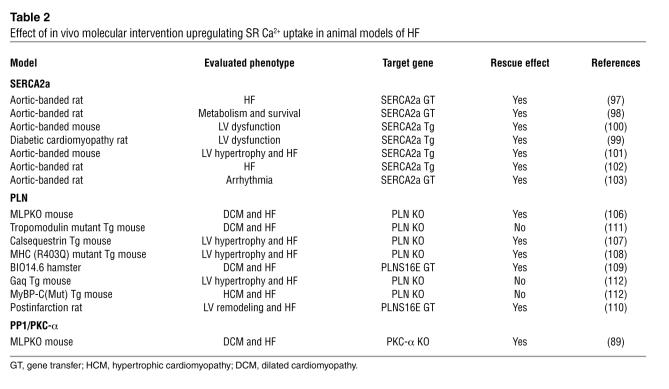
One intriguing therapeutic approach to the treatment of HF may be to restore the depressed Ca2+ uptake activity that is generally considered to play a key pathogenic role in the development of this condition. The enhanced contractility associated with SERCA2a overexpression has been reported to be protective against both HF and cardiac hypertrophy (97–102). For example, in a pressure-overload rat model of HF, adenovirus-mediated overexpression of SERCA2a has been found to rescue depressed contractility and survival without adverse effects on energy metabolism (97, 98) and cardiac arrhythmia (103). The enhanced contractility induced by SERCA2a overexpression is due to an enhanced SR Ca2+ content and the resulting increase in Ca2+ efflux during systole (104, 105). An inhibition of PLN and the subsequent increase in SERCA2a activity appear to be promising strategies for the treatment of HF. Indeed, PLN gene ablation has been shown to prevent ventricular dysfunction, fibrosis, and development of HF both in dilated cardiomyopathy and hypertrophic cardiomyopathy (106–108). An in vivo gene transfer of a dominant-negative PLN mutant (which leads to greater SERCA2a activity) rescued HF both in a cardiomyopathic hamster model (109) and in rats with myocardial infarction (110). In contrast to these studies showing the functional benefit of enhanced SERCA2a activity in failing myocardium, other studies showed that PLN ablation failed to rescue the cardiomyopathic phenotype in several models of cardiomyopathy (i.e., tropomodulin-overexpressing transgenic [TOT] mouse [ref. 111], Gaq-transgenic mouse, and myosin-binding protein C–mutant mouse [MyBP-Cmut] [ref. 112]). These data suggest that the ablation or inhibition of PLN may not always be effective for the treatment of HF (Table 2). Independent groups have reported that human PLN mutations lead to dilated cardiomyopathy with Arg9Cys (113) and Leu39stop mutations (114). The PLN with Arg9Cys mutation was found to interact abnormally with PKA and lack the ability to be phosphorylated at Ser16, thereby dominantly inhibiting SERCA2a function (113). These data support the notion that PLN inhibition is a promising therapeutic approach for human HF. On the other hand, the fact that PLN with Leu39stop mutation lacks transmembrane peptide and thereby disables PLN retention in the SR (114) raises the possibility that innate absence of PLN in the SR may cause long-term adverse effects in the human heart. However, the lod score for the linkage between Leu39stop mutation and HF was low, and moreover, the physiological significance of Leu39stop mutation was not evaluated in the animal model. Further investigation is still needed to determine whether either overexpression of SERCA2a or PLN inhibition can be a new therapeutic strategy against human HF. It is also of great interest that, as recently demonstrated, overexpression of I-1 (88) or ablation of PKC-α (89) leads to increased myocyte contractility in the human failing myocyte and muscle LIM protein (MLP)–deficient cardiomyopathy, respectively, presumably by inhibiting the increased PP1 activity seen in the failing heart. Further assessment will be needed to determine whether PP1 inhibition might be beneficial in the long-term setting of HF.
Table 2.
Effect of in vivo molecular intervention upregulating SR Ca2+ uptake in animal models of HF
Conclusions and perspectives
Recent progress in molecular cardiology makes it possible to envision a new therapeutic approach to HF, targeting key molecules involved in intracellular Ca2+ handling (such as RyR, SERCA2a, PLN, and others). Controlling these molecular functions has been found to be beneficial in certain experimental conditions. However, not all investigators are agreed that such therapies can usefully be extended to all types of failing hearts. Depending on the experimental conditions or on the model of HF, both positive and negative results (i.e., benefit or no benefit with the above therapies) have been obtained. With regard to this, it is important to notice whether the animal models accurately reflect the human disorder or the underlying human biology. Because of the heterogeneous nature of human HF examined in various studies (i.e., different stages and etiologies), caution should be exercised when trying to decide whether this approach might be generally applicable to the treatment of HF. In this regard, further investigation is clearly needed. Moreover, in contrast to many experimental situations, in which treatments are administered before HF develops, human HF has to be cured after it has developed. At present, little information is available to indicate whether manipulations targeting Ca2+ regulatory proteins are effective after HF has developed as well as before. Nevertheless, new forms of therapy targeting Ca2+ regulatory proteins should open up a new chapter in the potential treatment of HF.
Acknowledgments
This work was supported by grants-in-aid for scientific research from The Ministry of Education in Japan (16590689, 16209026, 14370228, and 15590754 to M. Yano, Y. Ikeda, and M. Matsuzaki) and by grants-in-aid from the Takeda Science Foundation (to M. Yano and Y. Ikeda).
Footnotes
Nonstandard abbreviations used: ARVD/C2, arrhythmogenic right ventricular cardiomyopathy type 2; CAMKII, calmodulin-dependent kinase II; CCD, central core disease; CPVT, catecholaminergic polymorphic ventricular tachycardia; DAD, delayed after-depolarization; E-C, excitation-contraction; FKBP, FK506–binding protein; HF, heart failure; I-1, inhibitor-1; LTCC, L-type Ca2+ channel; MH, malignant hyperthermia; MyBP-Cmut, myosin-binding protein C-mutant mouse; NCX, Na+/Ca2+exchanger; PKA, protein kinase A; PLN, phospholamban; PP1, protein phosphatase 1; PP2A, protein phosphatase 2A; RyR, ryanodine receptor; SERCA2a, Ca2+-ATPase; SR, sarcoplasmic reticulum.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Francis GS. Pathophysiology of chronic heart failure. Am. J. Med. 2001;110:37S–46S. doi: 10.1016/s0002-9343(98)00385-4. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E, Bristow MR. Congestive heart failure: fifty years of progress. Circulation. 2000;102:IV14–IV23. doi: 10.1161/01.cir.102.suppl_4.iv-14. [DOI] [PubMed] [Google Scholar]
- 3.Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J. Mol. Cell. Cardiol. 2002;34:951–969. doi: 10.1006/jmcc.2002.2037. [DOI] [PubMed] [Google Scholar]
- 4.Meyer M, et al. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation. 1995;92:778–784. doi: 10.1161/01.cir.92.4.778. [DOI] [PubMed] [Google Scholar]
- 5.Houser SR, Margulies KB. Is depressed myocyte contractility centrally involved in heart failure? Circ. Res. 2003;92:350–358. doi: 10.1161/01.RES.0000060027.40275.A6. [DOI] [PubMed] [Google Scholar]
- 6.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 7.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 8.Bers, D.M. 2001. Excitation-contraction coupling and cardiac contractile force. Kluwer Academic Publishers. Dordrecht, The Netherlands. 427 pp.
- 9.Bassani JW, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J. Physiol. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fill M, et al. Ryanodine receptor adaptation. J. Gen. Physiol. 2000;116:873–882. doi: 10.1085/jgp.116.6.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sitsapesan R, Williams AJ. Do inactivation mechanisms rather than adaptation hold the key to understanding ryanodine receptor channel gating? J. Gen. Physiol. 2000;116:867–872. doi: 10.1085/jgp.116.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasenfuss G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc. Res. 1998;37:279–289. doi: 10.1016/s0008-6363(97)00277-0. [DOI] [PubMed] [Google Scholar]
- 13.Shröder F, et al. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation. 1998;98:969–976. doi: 10.1161/01.cir.98.10.969. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, et al. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ. Res. 2002;91:517–524. doi: 10.1161/01.res.0000033988.13062.7c. [DOI] [PubMed] [Google Scholar]
- 15.Wier WG, Balke CW. Ca2+ release mechanisms, Ca2+ sparks, and local control of excitation-contraction coupling in normal heart muscle. Circ. Res. 1999;85:770–776. doi: 10.1161/01.res.85.9.770. [DOI] [PubMed] [Google Scholar]
- 16.Piot C, et al. High frequency-induced upregulation of human cardiac calcium currents. Circulation. 1996;93:120–128. doi: 10.1161/01.cir.93.1.120. [DOI] [PubMed] [Google Scholar]
- 17.Barrere-Lemaire S, Piot C, Leclercq F, Nargeot J, Richard S. Facilitation of L-type calcium currents by diastolic depolarization in cardiac cells: impairment in heart failure. Cardiovasc. Res. 2000;47:336–349. doi: 10.1016/s0008-6363(00)00107-3. [DOI] [PubMed] [Google Scholar]
- 18.He J, et al. Reduction in density of transverse tubules and L-type Ca2+ channels in canine tachycardia-induced heart failure. Cardiovasc. Res. 2001;49:298–307. doi: 10.1016/s0008-6363(00)00256-x. [DOI] [PubMed] [Google Scholar]
- 19.Gomez AM, et al. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- 20.O’Rourke B, et al. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ. Res. 1999;84:562–570. doi: 10.1161/01.res.84.5.562. [DOI] [PubMed] [Google Scholar]
- 21.Lindner M, Erdmann E, Beuckelmann DJ. Calcium content of the sarcoplasmic reticulum in isolated ventricular myocytes from patients with terminal heart failure. J. Mol. Cell. Cardiol. 1998;30:743–749. doi: 10.1006/jmcc.1997.0626. [DOI] [PubMed] [Google Scholar]
- 22.Hobai IA, O’Rourke B. Decreased sarcoplasmic reticulum calcium content is responsible for defective excitation-contraction coupling in canine heart failure. Circulation. 2001;103:1577–1584. doi: 10.1161/01.cir.103.11.1577. [DOI] [PubMed] [Google Scholar]
- 23.Yano M, et al. Altered stoichiometry of FKBP12.6 versus ryanodine receptor as a cause of abnormal Ca2+ leak through ryanodine receptor in heart failure. Circulation. 2000;102:2131–2136. doi: 10.1161/01.cir.102.17.2131. [DOI] [PubMed] [Google Scholar]
- 24.Ono K, et al. Altered interaction of FKBP12.6 with ryanodine receptor as a cause of abnormal Ca2+ release in heart failure. Cardiovasc. Res. 2000;48:323–331. doi: 10.1016/s0008-6363(00)00191-7. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto T, et al. Abnormal Ca2+ release from cardiac sarcoplasmic reticulum in tachycardia-induced heart failure. Cardiovasc. Res. 1999;44:146–155. doi: 10.1016/s0008-6363(99)00200-x. [DOI] [PubMed] [Google Scholar]
- 26.Marx SO, et al. Coupled gating between cardiac calcium release channels (ryanodine receptors) Circ. Res. 2001;88:1151–1158. doi: 10.1161/hh1101.091268. [DOI] [PubMed] [Google Scholar]
- 27.Blinks, J.R., and Hannon, J.D. 1996. Evaluation of changes in myofibrillar Ca2+ sensitivity in intact cardiac cells. In Molecular physiology and pharmacology of cardiac ion channels and transporters. M. Morad, S. Ebashi, W. Trautwein, and K. Kurachi, editors. Kluwer Academic. Dordrecht, The Netherlands/London, United Kingdom. 513–529.
- 28.Litwin SE, Zhang D, Bridge JH. Dyssynchronous Ca2+ sparks in myocytes from infarcted hearts. Circ. Res. 2000;87:1040–1047. doi: 10.1161/01.res.87.11.1040. [DOI] [PubMed] [Google Scholar]
- 29.Sah R, Ramirez RJ, Backx PH. Modulation of Ca2+ release in cardiac myocytes by changes in repolarization rate: role of phase-1 action potential repolarization in excitation-contraction coupling. Circ. Res. 2002;90:165–173. doi: 10.1161/hh0202.103315. [DOI] [PubMed] [Google Scholar]
- 30.Sjaastad I, Wasserstrom JA, Sejersted OM. Heart failure — a challenge to our current concepts of excitation-contraction coupling. J. Physiol. 2003;546:33–47. doi: 10.1113/jphysiol.2002.034728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shannon TR, Bers DM. Integrated Ca2+ management in cardiac myocytes. Ann. N. Y. Acad. Sci. 2004;1015:28–38. doi: 10.1196/annals.1302.003. [DOI] [PubMed] [Google Scholar]
- 32.Bers DM. Macromolecular complexes regulating cardiac ryanodine receptor function. J. Mol. Cell. Cardiol. 2004;37:417–429. doi: 10.1016/j.yjmcc.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 33.Brini M. Ryanodine receptor defects in muscle genetic diseases. Biochem. Biophys. Res. Commun. 2004;322:1245–1255. doi: 10.1016/j.bbrc.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 34.Wagenknecht T, et al. Three-dimensional architecture of the calcium channel/foot structure of sarcoplasmic reticulum. Nature. 1989;338:167–170. doi: 10.1038/338167a0. [DOI] [PubMed] [Google Scholar]
- 35.Marks AR, et al. Molecular cloning and characterization of the ryanodine receptor/junctional channel complex cDNA from skeletal muscle sarcoplasmic reticulum. Proc. Natl. Acad. Sci. U. S. A. 1989;86:8683–8687. doi: 10.1073/pnas.86.22.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otsu K, et al. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J. Biol. Chem. 1990;265:13472–13483. [PubMed] [Google Scholar]
- 37.Takeshima H, et al. Ca2+-induced Ca2+ release in myocytes from dyspedic mice lacking the type-1 ryanodine receptor. EMBO J. 1995;14:2999–3006. doi: 10.1002/j.1460-2075.1995.tb07302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brillantes AB, et al. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 39.Marx SO, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 40.Marks AR, Marx SO, Reiken S. Regulation of ryanodine receptors via macromolecular complexes: a novel role for leucine/isoleucine zippers. Trends Cardiovasc. Med. 2002;12:166–170. doi: 10.1016/s1050-1738(02)00156-1. [DOI] [PubMed] [Google Scholar]
- 41.Marks AR. Ryanodine receptors, FKBP12, and heart failure. Front. Biosci. 2002;7:d970–d977. doi: 10.2741/A822. [DOI] [PubMed] [Google Scholar]
- 42.Marks AR, Reiken S, Marx SO. Progression of heart failure: is protein kinase a hyperphosphorylation of the ryanodine receptor a contributing factor? Circulation. 2002;105:272–275. [PubMed] [Google Scholar]
- 43.Lehnart SE, Wehrens XH, Marks AR. Calstabin deficiency, ryanodine receptors, and sudden cardiac death. Biochem. Biophys. Res. Commun. 2004;322:1267–1279. doi: 10.1016/j.bbrc.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 44.Wehrens XH, Marks AR. Altered function and regulation of cardiac ryanodine receptors in cardiac disease. Trends Biochem. Sci. 2003;28:671–678. doi: 10.1016/j.tibs.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ. Res. 2003;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 46.Jiang MT, et al. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ. Res. 2002;91:1015–1022. doi: 10.1161/01.res.0000043663.08689.05. [DOI] [PubMed] [Google Scholar]
- 47.Stange M, Xu L, Balshaw D, Yamaguchi N, Meissner G. Characterization of recombinant skeletal muscle (Ser-2843) and cardiac muscle (Ser-2809) ryanodine receptor phosphorylation mutants. J. Biol. Chem. 2003;278:51693–51702. doi: 10.1074/jbc.M310406200. [DOI] [PubMed] [Google Scholar]
- 48.Xiao B, Sutherland C, Walsh MP, Chen SR. Protein kinase A phosphorylation at serine-2808 of the cardiac Ca2+-release channel (ryanodine receptor) does not dissociate 12.6-kDa FK506-binding protein (FKBP12.6) Circ. Res. 2004;94:487–495. doi: 10.1161/01.RES.0000115945.89741.22. [DOI] [PubMed] [Google Scholar]
- 49.Wehrens XH, et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 50.Li Y, Kranias EG, Mignery GA, Bers DM. Protein kinase A phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ. Res. 2002;90:309–316. doi: 10.1161/hh0302.105660. [DOI] [PubMed] [Google Scholar]
- 51.Valdivia HH, Kaplan JH, Ellis-Davies GC, Lederer WJ. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995;267:1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eisner DA, Choi HS, Diaz ME, O’Neill SC, Trafford AW. Integrative analysis of calcium cycling in cardiac muscle. Circ. Res. 2000;87:1087–1094. doi: 10.1161/01.res.87.12.1087. [DOI] [PubMed] [Google Scholar]
- 53.McCarthy TV, Quane KA, Lynch PJ. Ryanodine receptor mutations in malignant hyperthermia and central core disease. Hum. Mutat. 2000;15:410–417. doi: 10.1002/(SICI)1098-1004(200005)15:5<410::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 54.Priori SG, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 55.Laitinen PJ, et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485–490. doi: 10.1161/01.cir.103.4.485. [DOI] [PubMed] [Google Scholar]
- 56.Tiso N, et al. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2) Hum. Mol. Genet. 2001;10:189–194. doi: 10.1093/hmg/10.3.189. [DOI] [PubMed] [Google Scholar]
- 57.Bauce B, et al. Screening for ryanodine receptor type 2 mutations in families with effort-induced polymorphic ventricular arrhythmias and sudden death: early diagnosis of asymptomatic carriers. J. Am. Coll. Cardiol. 2002;40:341–349. doi: 10.1016/s0735-1097(02)01946-0. [DOI] [PubMed] [Google Scholar]
- 58.Laitinen PJ, Swan H, Kontula K. Molecular genetics of exercise-induced polymorphic ventricular tachycardia: identification of three novel cardiac ryanodine receptor mutations and two common calsequestrin 2 amino-acid polymorphisms. Eur. J. Hum. Genet. 2003;11:888–891. doi: 10.1038/sj.ejhg.5201061. [DOI] [PubMed] [Google Scholar]
- 59.Priori SG, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 60.Bagattin A, et al. Denaturing HPLC-based approach for detecting RYR2 mutations involved in malignant arrhythmias. Clin. Chem. 2004;50:1148–1155. doi: 10.1373/clinchem.2003.030734. [DOI] [PubMed] [Google Scholar]
- 61.Tester DJ, Spoon DB, Valdivia HH, Makielski JC, Ackerman MJ. Targeted mutational analysis of the RyR2-encoded cardiac ryanodine receptor in sudden unexplained death: a molecular autopsy of 49 medical examiner/coroner’s cases. Mayo Clin. Proc. 2004;79:1380–1384. doi: 10.4065/79.11.1380. [DOI] [PubMed] [Google Scholar]
- 62.Choi G, et al. Spectrum and frequency of cardiac channel defects in swimming-triggered arrhythmia syndromes. Circulation. 2004;110:2119–2124. doi: 10.1161/01.CIR.0000144471.98080.CA. [DOI] [PubMed] [Google Scholar]
- 63.Hasdemir C, Priori SG, Overholt E, Lazzara R. Catecholaminergic polymorphic ventricular tachycardia, recurrent syncope, and implantable loop recorder. J. Cardiovasc. Electrophysiol. 2004;15:729. doi: 10.1046/j.1540-8167.2004.03408.x. [DOI] [PubMed] [Google Scholar]
- 64.Wehrens XH, et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 65.Marks AR, Priori S, Memmi M, Kontula K, Laitinen PJ. Involvement of the cardiac ryanodine receptor/calcium release channel in catecholaminergic polymorphic ventricular tachycardia. J. Cell. Physiol. 2002;190:1–6. doi: 10.1002/jcp.10031. [DOI] [PubMed] [Google Scholar]
- 66.Jiang D, et al. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc. Natl. Acad. Sci. U. S. A. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ikemoto N, Yamamoto T. Regulation of calcium release by interdomain interaction within ryanodine receptors. Front. Biosci. 2002;7:d671–d683. doi: 10.2741/A803. [DOI] [PubMed] [Google Scholar]
- 68.Lamb GD, Posterino GS, Yamamoto T, Ikemoto N. Effects of a domain peptide of the ryanodine receptor on Ca2+ release in skinned skeletal muscle fibers. Am. J. Physiol. Cell Physiol. 2001;281:C207–C214. doi: 10.1152/ajpcell.2001.281.1.C207. [DOI] [PubMed] [Google Scholar]
- 69.Shtifman A, et al. Interdomain interactions within ryanodine receptors regulate Ca2+ spark frequency in skeletal muscle. J. Gen. Physiol. 2002;119:15–32. doi: 10.1085/jgp.119.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamamoto T, Ikemoto N. Peptide probe study of the critical regulatory domain of the cardiac ryanodine receptor. Biochem. Biophys. Res. Commun. 2002;291:1102–1108. doi: 10.1006/bbrc.2002.6569. [DOI] [PubMed] [Google Scholar]
- 71.Gray RP, McIntyre H, Sheridan DS, Fry CH. Intracellular sodium and contractile function in hypertrophied human and guinea-pig myocardium. Pflugers Arch. 2001;442:117–123. doi: 10.1007/s004240000512. [DOI] [PubMed] [Google Scholar]
- 72.Pieske B, et al. Alterations in intracellular calcium handling associated with the inverse force-frequency relation in human dilated cardiomyopathy. Circulation. 1995;92:1169–1178. doi: 10.1161/01.cir.92.5.1169. [DOI] [PubMed] [Google Scholar]
- 73.Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ. Res. 1999;85:38–46. doi: 10.1161/01.res.85.1.38. [DOI] [PubMed] [Google Scholar]
- 74.Pieske B, Houser SR. [Na+]i handling in the failing human heart. Cardiovasc. Res. 2003;57:874–886. doi: 10.1016/s0008-6363(02)00841-6. [DOI] [PubMed] [Google Scholar]
- 75.Sipido KR, Maes M, Van de Werf F. Low efficiency of Ca2+ entry through the Na(+)-Ca2+ exchanger as trigger for Ca2+ release from the sarcoplasmic reticulum. A comparison between L-type Ca2+ current and reverse-mode Na(+)-Ca2+ exchange. Circ. Res. 1997;81:1034–1044. doi: 10.1161/01.res.81.6.1034. [DOI] [PubMed] [Google Scholar]
- 76.Pieske B, et al. Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation. 2002;106:447–453. doi: 10.1161/01.cir.0000023042.50192.f4. [DOI] [PubMed] [Google Scholar]
- 77.Limas CJ, et al. Calcium uptake by cardiac sarcoplasmic reticulum in human dilated cardiomyopathy. Cardiovasc. Res. 1987;21:601–605. doi: 10.1093/cvr/21.8.601. [DOI] [PubMed] [Google Scholar]
- 78.Schwinger RH, et al. Reduced Ca2+-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J. Mol. Cell. Cardiol. 1999;31:479–491. doi: 10.1006/jmcc.1998.0897. [DOI] [PubMed] [Google Scholar]
- 79.Sande JB, et al. Reduced level of serine(16) phosphorylated phospholamban in the failing rat myocardium: a major contributor to reduced SERCA2 activity. Cardiovasc. Res. 2002;53:382–391. doi: 10.1016/s0008-6363(01)00489-8. [DOI] [PubMed] [Google Scholar]
- 80.Yano M, et al. FKBP12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation. 2003;107:477–484. doi: 10.1161/01.cir.0000044917.74408.be. [DOI] [PubMed] [Google Scholar]
- 81.Bohm M, Reiger B, Schwinger RH, Erdmann E. cAMP concentrations, cAMP dependent protein kinase activity, and phospholamban in non-failing and failing myocardium. Cardiovasc. Res. 1994;28:1713–1719. doi: 10.1093/cvr/28.11.1713. [DOI] [PubMed] [Google Scholar]
- 82.Netticadan T, Temsah RM, Kawabata K, Dhalla NS. Sarcoplasmic reticulum Ca2+/Calmodulin-dependent protein kinase is altered in heart failure. Circ. Res. 2000;86:596–605. doi: 10.1161/01.res.86.5.596. [DOI] [PubMed] [Google Scholar]
- 83.Mishra S, Sabbah HN, Jain JC, Gupta RC. Reduced Ca2+-calmodulin-dependent protein kinase activity and expression in LV myocardium of dogs with heart failure. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H876–H883. doi: 10.1152/ajpheart.00266.2002. [DOI] [PubMed] [Google Scholar]
- 84.Frank KF, Bolck B, Erdmann E, Schwinger RH. Sarcoplasmic reticulum Ca2+-ATPase modulates cardiac contraction and relaxation. Cardiovasc. Res. 2003;57:20–27. doi: 10.1016/s0008-6363(02)00694-6. [DOI] [PubMed] [Google Scholar]
- 85.Neumann J, et al. Increased expression of cardiac phosphatases in patients with end-stage heart failure. J. Mol. Cell. Cardiol. 1997;29:265–272. doi: 10.1006/jmcc.1996.0271. [DOI] [PubMed] [Google Scholar]
- 86.Huang B, Wang S, Qin D, Boutjdir M, El-Sherif N. Diminished basal phosphorylation level of phospholamban in the postinfarction remodeled rat ventricle: role of beta-adrenergic pathway, G(i) protein, phosphodiesterase, and phosphatases. Circ. Res. 1999;85:848–855. doi: 10.1161/01.res.85.9.848. [DOI] [PubMed] [Google Scholar]
- 87.Gupta RC, et al. Cardiac SR-coupled PP1 activity and expression are increased and inhibitor 1 protein expression is decreased in failing hearts. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2373–H2381. doi: 10.1152/ajpheart.00442.2003. [DOI] [PubMed] [Google Scholar]
- 88.Carr AN, et al. Type 1 phosphatase, a negative regulator of cardiac function. Mol. Cell. Biol. 2002;22:4124–4135. doi: 10.1128/MCB.22.12.4124-4135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Braz JC, et al. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat. Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 90.Pass JM, et al. Enhanced PKC beta II translocation and PKC beta II-RACK1 interactions in PKC epsilon-induced heart failure: a role for RACK1. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H2500–H2510. doi: 10.1152/ajpheart.2001.281.6.H2500. [DOI] [PubMed] [Google Scholar]
- 91.Reiken S, et al. Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation. 2003;107:2459–2466. doi: 10.1161/01.CIR.0000068316.53218.49. [DOI] [PubMed] [Google Scholar]
- 92.Reiken S, et al. Beta-adrenergic receptor blockers restore cardiac calcium release channel (ryanodine receptor) structure and function in heart failure. Circulation. 2001;104:2843–2848. doi: 10.1161/hc4701.099578. [DOI] [PubMed] [Google Scholar]
- 93.Doi M, et al. Propranolol prevents the development of heart failure by restoring FKBP12.6-mediated stabilization of ryanodine receptor. Circulation. 2002;105:1374–1379. doi: 10.1161/hc1102.105270. [DOI] [PubMed] [Google Scholar]
- 94.Okuda S, et al. Valsartan restores sarcoplasmic reticulum function with no appreciable effect on resting cardiac function in pacing-induced heart failure. Circulation. 2004;109:911–919. doi: 10.1161/01.CIR.0000115526.92541.D2. [DOI] [PubMed] [Google Scholar]
- 95.Bristow MR. Beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 96.Lehnart SE, et al. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation. 2004;109:3208–3214. doi: 10.1161/01.CIR.0000132472.98675.EC. [DOI] [PubMed] [Google Scholar]
- 97.Miyamoto MI, et al. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc. Natl. Acad. Sci. U. S. A. 2000;97:793–798. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.del Monte F, et al. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca2+-ATPase in a rat model of heart failure. Circulation. 2001;104:1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Trost SU, et al. Overexpression of the sarcoplasmic reticulum Ca2+-ATPase improves myocardial contractility in diabetic cardiomyopathy. Diabetes. 2002;51:1166–1171. doi: 10.2337/diabetes.51.4.1166. [DOI] [PubMed] [Google Scholar]
- 100.Ito K, et al. Transgenic expression of sarcoplasmic reticulum Ca2+ atpase modifies the transition from hypertrophy to early heart failure. Circ. Res. 2001;89:422–429. doi: 10.1161/hh1701.095522. [DOI] [PubMed] [Google Scholar]
- 101.Nakayama H, et al. Cardiac-specific overexpression of a high Ca2+ affinity mutant of SERCA2a attenuates in vivo pressure overload cardiac hypertrophy. FASEB J. 2003;17:61–63. doi: 10.1096/fj.02-0474fje. [DOI] [PubMed] [Google Scholar]
- 102.Muller OJ, et al. Transgenic rat hearts overexpressing SERCA2a show improved contractility under baseline conditions and pressure overload. Cardiovasc. Res. 2003;59:380–389. doi: 10.1016/s0008-6363(03)00429-2. [DOI] [PubMed] [Google Scholar]
- 103.del Monte F, et al. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5622–5627. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.He H, et al. Overexpression of the rat sarcoplasmic reticulum Ca2+ ATPase gene in the heart of transgenic mice accelerates calcium transients and cardiac relaxation. J. Clin. Invest. 1997;100:380–389. doi: 10.1172/JCI119544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baker DL, et al. Targeted overexpression of the sarcoplasmic reticulum Ca2+-ATPase increases cardiac contractility in transgenic mouse hearts. Circ. Res. 1998;83:1205–1214. doi: 10.1161/01.res.83.12.1205. [DOI] [PubMed] [Google Scholar]
- 106.Minamisawa S, et al. Chronic phospholamban-sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell. 1999;99:313–322. doi: 10.1016/s0092-8674(00)81662-1. [DOI] [PubMed] [Google Scholar]
- 107.Sato Y, et al. Rescue of contractile parameters and myocyte hypertrophy in calsequestrin overexpressing myocardium by phospholamban ablation. J. Biol. Chem. 2001;276:9392–9399. doi: 10.1074/jbc.M006889200. [DOI] [PubMed] [Google Scholar]
- 108.Freeman K, et al. Alterations in cardiac adrenergic signaling and calcium cycling differentially affect the progression of cardiomyopathy. J. Clin. Invest. 2001;107:967–974. doi: 10.1172/JCI12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hoshijima M, et al. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat. Med. 2002;8:864–871. doi: 10.1038/nm739. [DOI] [PubMed] [Google Scholar]
- 110.Iwanaga Y, et al. Chronic phospholamban inhibition prevents progressive cardiac dysfunction and pathological remodeling after infarction in rats. J. Clin. Invest. 2004;113:727–736. doi:10.1172/JCI200418716. doi: 10.1172/JCI18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Delling U, Sussman MA, Molkentin JD. Re-evaluating sarcoplasmic reticulum function in heart failure. Nat. Med. 2000;6:942–943. doi: 10.1038/79592. [DOI] [PubMed] [Google Scholar]
- 112.Song Q, et al. Rescue of cardiomyocyte dysfunction by phospholamban ablation does not prevent ventricular failure in genetic hypertrophy. J. Clin. Invest. 2003;111:859–867. doi:10.1172/JCI200316738. doi: 10.1172/JCI16738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schmitt JP, et al. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science. 2003;299:1410–1413. doi: 10.1126/science.1081578. [DOI] [PubMed] [Google Scholar]
- 114.Haghighi K, et al. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J. Clin. Invest. 2003;111:869–876. doi:10.1172/JCI200317892. doi: 10.1172/JCI17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wagenknecht T, et al. Locations of calmodulin and FK506-binding protein on the three-dimensional architecture of the skeletal muscle ryanodine receptor. J. Biol. Chem. 1997;272:32463–32471. doi: 10.1074/jbc.272.51.32463. [DOI] [PubMed] [Google Scholar]