Abstract
A 10-month-old male American Staffordshire terrier was presented to the Autonomous University of Barcelona Veterinary Teaching Hospital because of a 6-month history of a mucopurulent bilateral nasal discharge. The dog had not responded to antibiotics. A follow-up X ray revealed a mixed pattern of osteolysis and increased radiodensity confined to the nasal cavity. Histologic sections of the biopsy specimens revealed the presence of granules containing numerous septate hyphae that were hyaline to pale brown and smooth, one-celled, subspherical-to-elongate conidia that were hyaline to brownish green, and bacteria. Cultures yielded numerous colonies belonging to Scedosporium apiospermum. Susceptibility tests were performed on the isolated strain. The isolate was sensitive to ketoconazole, intermediate to clotrimazole, and resistant to amphotericin B, 5-fluorocytosine, fluconazole, and itraconazole. The dog was treated with oral ketoconazole. During the treatment a general improvement in the lesions was observed. To our knowledge, S. apiospermum has not been implicated previously as an etiologic agent of nasal disease in dogs. This report provides its first description as such.
Fungal infections are a common cause of nasal disease in dogs and cats. Aspergillus fumigatus is the species most commonly isolated from infections in the nasal cavities of these animals. However, Aspergillus niger, Aspergillus nidulans, and Aspergillus flavus have also been recovered from this location. Penicillium spp. are rarely reported as causing invasive nasal disease (12, 16).
Pseudallescheria boydii is an ubiquitous saprophytic ascomycete that has two anamorphs. It has been isolated from soil, water, vegetation, and sewage. The Scedosporium apiospermum synanamorph is most commonly isolated from clinical cultures (1) and from all strains of P. boydii (5).
To our knowledge, S. apiospermum has not been implicated previously as an etiologic agent of nasal disease in dogs. This report provides its first description as such.
Case report.
A 10-month-old male American Staffordshire terrier was presented to the Autonomous University of Barcelona Veterinary Teaching Hospital because of a 6-month history of a mucopurulent bilateral nasal discharge and some sneezing. The disorder started 10 days after the dog suffered a parvoviral enteritis, and the rinitis persisted for the 6 months before admission. The dog had not responded to antibiotics.
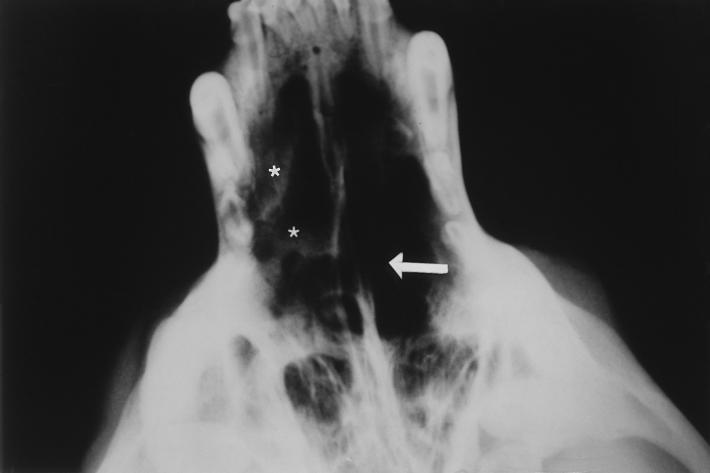
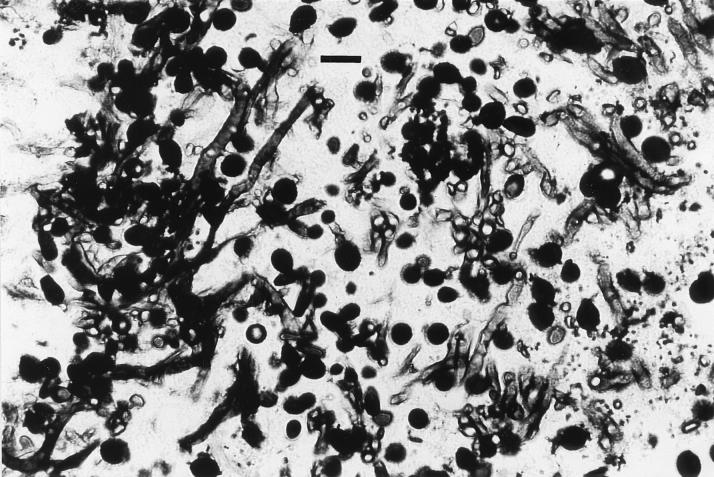
The animal was mildly depressed and had a temperature of 39.6°C. There was bilateral thick yellow discharge and deformated external nares. The dog had a mature neutrophilic leukocytosis (24,000 leukocytes/μl). The serum chemistry profile and urinalysis were normal. A follow-up X ray revealed a mixed pattern of osteolysis and an increased radiodensity confined to the nasal cavity that was greater on the right side. The turbinate pattern was less apparent than in normal dogs on both sides (Fig. 1). Thoracic and abdominal radiographies were normal. Rhinoscopic examination of the nasal chambers revealed the destruction of the vomer bone and a large mass completely occluding the nasal cavity. Swabs and biopsy specimens were collected for microbiologic and histologic examinations. KOH-lactophenol and histologic sections (Fig. 2) of the biopsy specimens revealed the presence of granules containing numerous septate hyphae that were hyaline to pale brown, smooth, one-celled, subspherical-to-elongate conidia that were hyaline to brownish green, and bacteria. Fungal growth in close contact with bone tissues was observed.
FIG. 1.
Intraoral radiograph showing lysis of the vomer bone (arrow) and increased radiodensity confined to the nasal cavity, which is more pronounced on the right side (asterisks). The turbinate pattern is less apparent than in normal dogs on both sides (black areas in the nasal cavity).
FIG. 2.
Detail of a Grocott-methenamine-silver-stained section of a tissue biopsy sample showing a granule containing hyphal and conidial elements. Bar = 10 μm.
Microbiology.
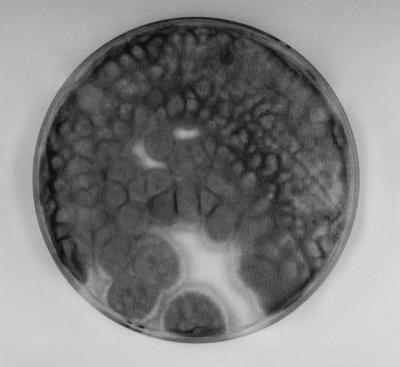
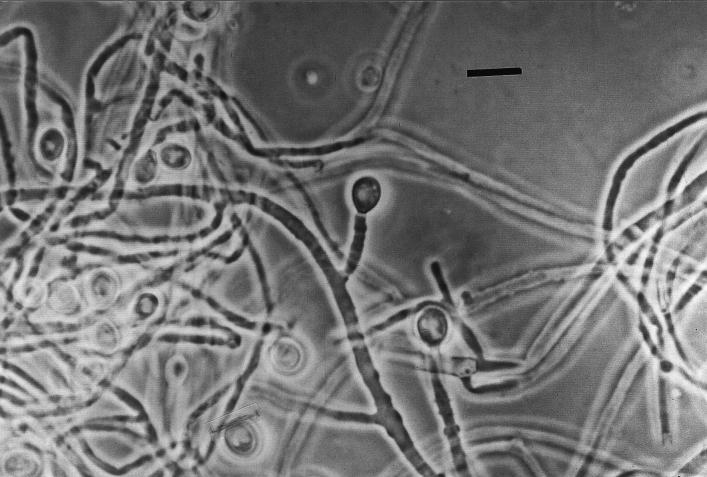
Cultures on Sabouraud glucose agar (SGA; Difco Laboratories, Detroit, Mich.) supplemented with chloramphenicol (Fig. 3) and Mycosel agar (BBL Microbiology Systems, Cockeysville, Md.) yielded numerous cottony colonies in pure culture that initially were grayish white and became greenish gray and brownish gray, consistent with what occurs in infection with Scedosporium species. Culturing of the fungal isolate on SGA generated colonies which presented characteristic conidial structures, with annellidic conidiogenesis and with conidiogenous cells without inflated bases (Fig. 4). S. apiospermum, the nonsynnematous synanamorph of P. boydii (5) was identified. The isolate failed to produce the sexual state on corn meal agar (Oxoid Ltd., Basingstoke, England) and potato dextrose agar (Difco) culture media. Bacteriological cultures yielded Escherichia coli and Proteus mirabilis. Susceptibility tests on the isolated strain were performed with antifungal tablets (Neo-Sensitabs; Rosco Diagnostica, Taastrup, Denmark) and Shadomy agar (4). The strain was sensitive to ketoconazole, intermediate to clotrimazole, and resistant to amphotericin B, 5-fluorocytosine, fluconazole, and itraconazole.
FIG. 3.
Pure culture of S. apiospermum growing on an SGA plate supplemented with chloramphenicol and inoculated with material from a biopsy after 8 days of incubation at 37°C.
FIG. 4.
Conidiogenesis of S. apiospermum. Note the conidia situated terminally on annellides without swollen bases. Phase-contrast microscopy was used. Bar = 10 μm.
Treatment.
The dog was treated with oral ketoconazole (10 mg/kg of body weight, twice a day) and amoxicillin plus clavulanic acid (20 mg/kg of body weight, orally, twice a day for 30 days). The baseline laboratory analysis (complete blood count, chemistry profile, and urinalysis) were normal. After a month, the nasal discharge decreased and the sneezing disappeared. During treatment (the last examination was at 7 months) a general improvement of the lesions was observed. Unfortunately, 1 month after the last checkup, the dog died in a car crash and the owners did not want to do the necropsy.
Discussion.
Reported cases of S. apiospermum infections in dogs are few. This species has been recognized as a causative agent of mycosis mainly in the abdominal cavity (1, 6, 8, 15). A disseminated infection has been reported (2). It has been cited in other locations such as the epidermis (chronic eczematous lesion) (10), eyes (keratomycosis) (14), and testicles (1). Additionally, in horses, two reports of P. boydii causing infection of the nasal chamber have been published (3, 7).
In humans, the wide spectrum of diseases caused by P. boydii includes sinusitis, brain abscess, meningitis, pulmonary colonization, fungus ball, invasive pneumonitis, endocarditis, arthritis, osteomyelitis, thyroid abscess, endophthalmitis, disseminated systemic disease, cutaneous and subcutaneous granulomata, keratomycosis, and mycetoma (9). Infection in humans most frequently occurs in soil-contaminated wounds on the hands or feet. However, especially in a compromised host, infection by inhalation causing sinusitis is not uncommon. Pulmonary lesions may also occur, and the angioinvasive nature of the fungus may result in spread to other areas of the body (3).
In our report, except for the parvoviral enteritis process and antibiotic treatment, no other predisposing factors such as neoplasia, immunodeficiency, depression, trauma, and exposure to high levels of fungal spores were identified. No sign of disseminated systemic disease was detected. The infection was limited to the nasal chambers. Nevertheless, lysis of the vomer bone was observed.
Some authors (13) have stated that in the treatment of canine nasal aspergillosis, topically administered enilconazole (via indwelling tubes, implanted bilaterally through trephine holes in each frontal sinus) is more effective than the oral administration of thiabendazole, ketoconazole, fluconazole, or itraconazole. In our case the isolate belonging to S. apiospermum was sensitive only to ketoconazole, and for this reason the dog was treated with oral ketoconazole.
Although antifungal susceptibility tests for filamentous fungi remain unstandardized and the in vivo outcome cannot always be extrapolated from the in vitro results, the resistance of P. boydii to different antifungal agents such as amphotericin B, clotrimazole, 5-fluorocytosine, griseofulvin, and nystatin and its mixed responses to ketoconazole, miconazole, and natamycin have been mentioned (14). On the other hand, different strains of S. apiospermum and P. boydii appear to have various degrees of sensitivity to antifungal agents (11) (for these strains the MICs of miconazole, ketoconazole, and itraconazole are lowest) and they appear to be resistant to fluconazole.
REFERENCES
- 1.Allison N, McDonald R K, Guist S R, Bentinck-Smith J. Eumycotic mycetoma caused by Pseudallescheria boydii in a dog. J Am Vet Med Assoc. 1989;194:797–799. [PubMed] [Google Scholar]
- 2.Baszler T, Chandler F W, Bertoy R W, Smith C W, Whiteley H E. Disseminated pseudallescheriasis in a dog. Vet Pathol. 1988;25:95–97. doi: 10.1177/030098588802500117. [DOI] [PubMed] [Google Scholar]
- 3.Brearley J C, McCandlish I A P, Sullivan M, Dawson C O. Nasal granuloma caused by Pseudallescheria boydii. Equine Vet J. 1986;18:151–153. doi: 10.1111/j.2042-3306.1986.tb03576.x. [DOI] [PubMed] [Google Scholar]
- 4.Casals J B. Tablet sensivity testing of pathogenic fungi. J Clin Pathol. 1979;32:719–722. doi: 10.1136/jcp.32.7.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guého E, de Hoog G S. Taxomomy of the medical species of Pseudallescheria and Scedosporium. J Mycol Med. 1991;118:3–9. [Google Scholar]
- 6.Jang S S, Popp J A. Eumycotic mycetoma in a dog caused by Allescheria boydii. J Am Vet Med Assoc. 1970;157:1071–1076. [PubMed] [Google Scholar]
- 7.Johnson G R, Schiefer B, Pantekoek J F C A. Maduromycosis in a horse in Western Canada. Can Vet J. 1975;16:341–344. [PMC free article] [PubMed] [Google Scholar]
- 8.Kurtz H J, Finco D R, Perman V. Maduromycosis (Allescheria boydii) in a dog. J Am Vet Med Assoc. 1970;157:917–921. [PubMed] [Google Scholar]
- 9.Kwon-Chung K J, Bennett J E. Medical mycology. Philadelphia, Pa: Lea and Febiger; 1992. pp. 678–694. [Google Scholar]
- 10.Pezenburg E. Allescheria boydii Shear 1921 isoliert aus einer Hautveränderung beim Hund. Mykosen. 1958;1:172–183. [Google Scholar]
- 11.Salkin I F, McGinnis M R, Dykstra M J, Rinaldi M G. Scedosporium inflatum, an emerging pathogen. J Clin Microbiol. 1988;26:498–503. doi: 10.1128/jcm.26.3.498-503.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharp N J H, Harvey C E, Sullivan M. Canine nasal aspergillosis and penicilliosis. Compend Contin Educ Pract Vet. 1991;13:41–49. [Google Scholar]
- 13.Sharp N J H, Sullivan M, Harvey C E, Webb T. Treatment of canine nasal aspergillosis with enilconazole. J Vet Intern Med. 1993;7:40–43. doi: 10.1111/j.1939-1676.1993.tb03167.x. [DOI] [PubMed] [Google Scholar]
- 14.Smedes S L, Miller P E, Dubielzig R R. Pseudallescheria boydii keratomycosis in a dog. J Am Vet Med Assoc. 1992;200:199–202. [PubMed] [Google Scholar]
- 15.Walker R L, Monticello T M, Ford R B, English R V. Eumycotic mycetoma caused by Pseudallescheria boydii in the abdominal cavity of a dog. J Am Vet Med Assoc. 1988;192:67–70. [PubMed] [Google Scholar]
- 16.Wolf A M. Fungal diseases of the nasal cavity of the dog and cat. Vet Clin N Am Small Anim Pract. 1992;36:1119–1132. doi: 10.1016/s0195-5616(92)50304-7. [DOI] [PubMed] [Google Scholar]