Abstract
There is growing evidence that the altered production and/or spatiotemporal distribution of reactive oxygen and nitrogen species creates oxidative and/or nitrosative stresses in the failing heart and vascular tree, which contribute to the abnormal cardiac and vascular phenotypes that characterize the failing cardiovascular system. These derangements at the integrated system level can be interpreted at the cellular and molecular levels in terms of adverse effects on signaling elements in the heart, vasculature, and blood that subserve cardiac and vascular homeostasis.
Cellular damage versus malfunction in signaling
Altered cellular production of ROS and/or reactive nitrogen species (RNS) is a ubiquitous feature of human disease. Many years of study, beginning with the discovery of superoxide (1) and superoxide dismutase (2), provide a sound understanding of the diverse chemical mechanisms by which these agents damage lipids, DNA, and proteins, and a pathophysiologic context is imparted by analyses of diseased tissues, which show the chemical footprints of oxidative and nitrosative stress (3–6). Thus, it has been widely assumed that direct chemical (oxidative and nitrosative) injury is a principal factor in the damage or disruption of cellular and subcellular structure–function that typifies such pathologic situations. A major difficulty with this hypothesis, however, lies in understanding how the salient features of chemical injury, which are common across organ systems, underlie the diverse pathophysiology of chronic disease. Moreover, irreversible oxidative damage cannot be easily reconciled with the acute restoration of cardiovascular performance by certain classes of antioxidants (see ref. 7 for example). It thus remains unclear to what extent the damage caused by RNS and ROS contributes to disease pathogenesis.
It was appreciated early on that NO and/or related congeners have a function in signal transduction, and over the past decade, the idea that additional redox-active species may have signaling roles has been strengthened (8–13). Although the molecular mechanisms by which RNS and ROS modulate cellular signal transduction remain incompletely understood, there is a general consensus that cysteine residues are principal sites of redox regulation (10, 13, 14). Crosstalk between ROS- and RNS-regulated pathways may occur at both the chemical interaction level (15, 16) and through their coordinate effects on target proteins (17, 18); this is reflected in a growing awareness that RNS and ROS may subserve conjoint signaling roles. Analysis of several such examples (17–22) has shown that S-nitrosylation (the covalent attachment of NO to cysteine thiol) may play a primary effector role while oxygen and other ROS may control the responsivity to S-nitrosylation (much as ROS control the strength of phosphorylation signaling by inhibiting phosphatases; ref. 11). This article addresses the physiologic and pathophysiologic consequences of the interplay between ROS and RNS in the cardiovascular system (Figure 1). We review work that directly implicates NO/redox-based signaling in the physiologic regulation of cardiac contractility and blood flow, and that consolidates the idea that malfunction in physiologic signaling plays a central role in the pathophysiology of heart failure (HF). It is further proposed that the impairments in NO-based signaling that contribute to the failing of the cardiovascular system can be ascribed to disequilibria between ROS and RNS in the heart, vasculature, and blood.
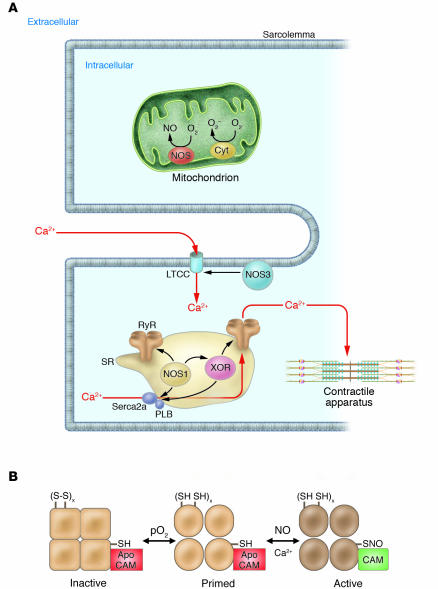
Figure 1.
(A) Spatial localization of NOSs and oxidases in the cardiac myocyte. NOS3 localizes to the sarcolemmal caveolae (85, 97), where it participates in regulation of L-type Ca2+ channel (LTCC) currents, mediated either by cGMP formation (S64) or by S-nitrosylation of the LTCC (S13). NOS1 localizes to the SR (32), where it facilitates the SR Ca2+ cycle (97) by S-nitrosylation of the RyR and possibly the SR Ca2+ ATPase (SERCA2a) (17, 33). XOR also localizes to the SR in the cardiac myocyte; upregulation of protein or activity (caused by SR NOS1 deficiency) disrupts SNO regulation of the RyR (22). Other oxidases (e.g., NADPH oxidase) have been described in the cardiac myocyte (50), but precise signaling roles, identities, and/or subcellular localizations have not been elucidated. The mitochondria are an additional source of both O2– and NO, which may participate in control of mitochondrial respiration (S65–S67) and apoptosis (S41). Cyt, cytochrome; PLB, phospholamban. (B) Regulation of the RyR by S-nitrosylation. S-nitrosylation occurs at a single cysteine residue (1 of approximately 50 free thiols), which resides within a calmodulin-binding domain of the cardiac RyR (17). NO binding (shown for RyR1 of skeletal muscle) occurs in an oxygen-concentration–dependent manner and primes the channel for calmodulin regulation (18). Higher pO2 oxidizes a small set of RyR-associated thiols that regulate the channel’s responsiveness to NO. SH, reduced thiol; S-S, oxidized thiols; CAM, calmodulin; x refers to a small set between 1 and 3.
ROS/RNS axis
Molecular basis of interaction.
ROS (e.g., O2–, H2O2, and OH•), may contribute to cardiac injury (4, 23) both by oxidizing cellular constituents, including proteins critical for excitation-contraction (E-C) coupling, (24, 25) and by diminishing NO bioactivity (22). One way that ROS may diminish the effect of NO is by directly inactivating it. But the extent to which interactions between RNS and ROS constitute a pathophysiologic mechanism in vivo is unclear. A second way that ROS may affect NO responses is by oxidizing sites in proteins with which NO reacts (direct competition) or which otherwise influence NO binding (allosteric modulation) (Figure 2). Evidence suggests that this mode of ROS action may contribute to cardiac pathophysiology.
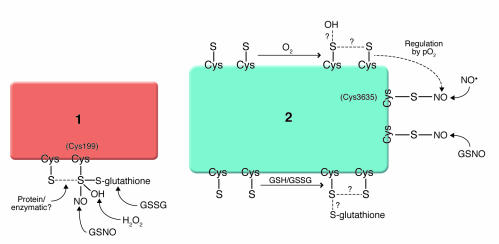
Figure 2.
NO/redox-based signaling and nitrosative stress. Molecular recognition by cysteine-containing proteins is achieved either through the existence of single classes of thiols that are adapted to differentiate NO modification (S-nitrosylation) from oxidations (S-glutathionylation, S-S [intramolecular disulfide] and/or sulfur oxides [SOx–, where x is 1–3]) – exemplified in protein 1 – or through the presence of multiple classes of thiols, each adapted to recognize different redox-related molecules, including NO, GSNO, H2O2, O2, and cellular redox potential (for protein 2, note that some classes of thiols may be functionally linked to others, exemplified in the pO2-dependent oxidation of RyR thiols that promotes S-nitrosylation). In model 1, thiol oxidation would adversely impact nitrosylation signaling. In model 2, signal malfunction may result from altered amounts, timing, and/or the nature of RNS/ROS-based modifications. S, cysteine thiol; GSH/GSSG, glutathione/glutathione disulfide; pO2, partial pressure of O2.
Signaling through cysteine thiols.
In addition to binding to transition metal centers in proteins such as guanylate cyclase and cytochrome c, NO-based modification of proteins is subserved by the attachment of a NO group to the thiol side chain of the amino acid cysteine. Well over a hundred proteins of all classes are substrates for S-nitrosylation (for review see refs. 20, 26), and the ubiquitous effects exerted by NO in cells are conveyed in large part by this mechanism of protein regulation (20, 27–30). Although early ideas about the chemistry of NO raised questions about the feasibility of S-nitrosylation reactions in vivo, a substantial body of recent work, which demonstrates the presence of multiple S-nitrosylated proteins at basal conditions and increases in S-nitrosylation that are coupled to activation of all isoforms of NO synthase (NOS) (stimuli include calcium, growth factors, cytokines, hormones, and multiple ligands), has established the physiologic relevance of this protein modification reaction (28–30). Moreover, recent studies in mice deficient in an S-nitrosothiol–metabolizing (SNO-metabolizing) enzyme (31) and analyses of S-nitrosylation in multiple cellular systems, including site-directed mutagenesis of cysteines in more than 20 proteins of different classes (20), has demonstrated that this NO-derived posttranslational modification serves as a major effector of NO bioactivity and an important mode of cellular signal transduction.
While the ubiquity of thiols across protein classes would appear to support a diversity of roles for S-nitrosylation, the conventional idea that NO acts promiscuously has posed a problem for understanding how specificity might be achieved. Recent literature, however, reveals a growing appreciation of the many determinants of RNS specificity (20), including colocalization of NOSs with their substrates (32, 33). In particular, spatial confinement of NO-signaling components (33, 34) and stimulus-coupled regulation of S-nitrosylation within the confines of signaling modules (26) contrast with ideas about free diffusion of NO and redox state alterations (affecting the cell or subellular compartment uniformly) and thus allow S-nitrosylation to serve as a prototype mechanism for redox-based cellular signal transduction. Compartmentalization of NOS isoforms is well exemplified in the cardiac myocyte and disrupted in HF.
Notably, ROS, in particular hydrogen peroxide (H2O2), share with NO/RNS a chemical reactivity toward thiols (13, 17, 35), and the concept of target-thiol specificity is reflected in the recent literature on both the differential reactivity of protein thiols toward H2O2 (13) and the colocalization of enzymatic sources of ROS (oxidases) with thiol-containing phosphatases (13, 36). However, while it has been convincingly demonstrated that inactivation of protein tyrosine phosphatases (PTPs) by endogenous ROS is required for full phosphorylation signaling by receptor tyrosine kinases (11–13, 36), the broader role of ROS in signal transduction remains to be elucidated, that is, the identity of additional proteins, sites of protein modification (by physiologic ROS), and nature of the in situ oxidations are still largely unknown. Furthermore, since ROS can easily upset the local and cellular redox milieu, the extent to which they elicit physiologic versus pathologic signaling is not always clear — the mitogenic activity that is potentiated by ROS inhibition of PTPs is a case in point. Resolution of this issue may be facilitated by experimental paradigms that replicate cellular O2 tensions rather than the oxidative stress represented by ambient pO2 (18, 37). One intriguing possibility consistent with available data is that ROS may operate to change the set points for initiation of phosphorylation and nitrosylation signaling (18, 20, 36) rather than signaling alone. Phosphorylation and nitrosylation would serve to propagate signals. Altered production of ROS, as will be described in the failing heart, would thus impair physiologic signal transduction.
Cardiovascular sources of O2–/ROS and NO/RNS
The major cardiovascular sources of ROS include the enzymes xanthine oxidoreductase (XOR) (38), NAD(P)H oxidase (multisubunit membrane complexes) (39), and NOS (40, 41) as well as the mitochondrial cytochromes (42) and hemoglobin (43, 44). NOSs and hemoglobin are also principal sources of RNS, including NO and SNOs (NO-modified cysteine thiols in amino acids, peptides, and proteins), which convey NO bioactivity. NO and SNOs can also be generated from nitrite in acidic compartments (10, 30), such as the lysosome or the inner membrane of the mitochondria, and from nitrite and nitrate by the actions of enzymes such as XOR (38).
Xanthine oxidoreductase.
XOR, a prominent cardiovascular source of ROS, exhibits increased abundance and activity in HF (7, 45, 46). XOR is upregulated in the heart (7) and vasculature (46) of patients with HF and contributes to mechanoenergetic uncoupling (7) and vasoconstriction (46), respectively. A molybdenum-containing enzyme, XOR is expressed as a 150-kDa homodimer that produces superoxide or hydrogen peroxide as byproducts of the terminal steps of purine metabolism (ref. 1; for review see ref. 38). The enzyme has 2 forms: xanthine oxidase (XO) and xanthine dehydrogenase (XDH) (47). XO is a variant of XDH, resulting from either irreversible proteolytic cleavage or reversible oxidation of sulfhydryl residues of XDH. Whereas XDH uses NAD+ as a cofactor (reducing it to NADH), XO utilizes molecular oxygen (reducing it to O2–/H2O2) (reviewed in refs. 38, 47, 48). The production of O2•– by XO has potential pathophysiologic relevance. XO can also generate NO from nitrite and nitrate at low pO2, but whether this is a physiologically relevant pathway in vivo is unclear (38, 49). Also unclear is whether XO-catalyzed production of NO is beneficial or deleterious in the heart.
NAD(P)H oxidase.
NAD(P)H oxidases are multisubunit enzymes that catalyze single-electron reductions of O2 using NAD(P)H as electron sources (39). The vascular enzymes are similar to the macrophage NAD(P)H oxidase and contain Nox family subunits, such as Nox1, Nox4, p22phox, and gp91phox. Like XOR, NAD(P)H oxidase subunits are increased in human HF myocytes (50) and in ischemia-reperfusion (51). There is crosstalk between NAD(P)H oxidase and XO: NAD(P)H oxidase activity maintains endothelial XO levels and participates in the conversion of XDH to XO (52). Mice deficient in gp91phox still exhibit NAD(P)H-dependent superoxide generation and develop pressure overload–induced hypertrophy (53), suggesting an alternative source of ROS. Interestingly, XO has a NADH oxidase activity, which can be inhibited by diphenyleneiodonium (DPI) (54) but not by allopurinol (54, 55). The idea that XOR may be the main pathophysiologic source of ROS generation is supported by the functional consequences of XO inhibition (XOI) in HF. Importantly, both XO (56, 57) and NADPH oxidases (58, 59) are inhibited by NO, providing a mechanism through which NOS activity regulates superoxide production and thereby maintains O2– /NO homeostasis.
NO synthases.
NOSs oxidize the terminal guanidino nitrogen of L-arginine to form NO and the amino acid L-citrulline (60). The NOS isoforms, neuronal NOS (nNOS or NOS1), iNOS or calcium-independent NOS (NOS2), and endothelial NOS (eNOS or NOS3), play modulatory roles in most cells, tissues and organs, including the nervous, immune, respiratory, urologic, and cardiovascular systems that collectively have an impact on cardiovascular performance. NOS1 and NOS3 can be activated by calcium and calmodulin whereas NOS2 is effectively calcium-independent by virtue of its high basal Ca2+/calmodulin affinity. NO activates soluble guanylate cyclase (S-GC) by binding to its heme prosthetic group (61). This activation leads to the production of 3′,5′-cyclic guanosine monophosphate (cGMP), which in turn activates protein kinase G and a cascade of biological signaling events (for reviews see refs. 62, 63).
While a role for cGMP in cardiac inotropy, lusitropy, and ion channel responsivity (64, 65) is reasonably well established, the extent to which natriuretic peptides versus NOS mediate these effects is less clear. It is also evident that NO has cGMP-independent effects in the heart and vasculature (26, 29, 66–69). In virtually all cases examined to date where sites of NO action have been identified in vivo, modulation of function by NO involves modification of 1 or very few cysteines, often contained within characteristic acid-base or hydrophobic motifs (20, 26), and as previously mentioned, site-directed mutagenesis experiments support the specificity of this cysteine modification (20, 69). Notably, the ryanodine receptor calcium–release channel (RyR) of cardiac myocytes is constitutively S-nitrosylated at a single cysteine in the rabbit heart (17), and NO circulates in the blood stream of mammals as a NO-derivative of single-reactive thiols in both albumin and hemoglobin (69, 70). Amino acids and small peptides (e.g., cysteine, glutathione) also undergo nitrosylation in vivo (15, 71), providing a reactive source of NO groups in equilibrium with SNO-proteins (72, 73). A putative motif for S-nitrosylation of proteins by S-nitrosoglutathione (GSNO) has been recently adduced (20). In addition, a GSNO reductase (GSNOR; formally alcohol dehydrogenase type III) has been discovered, which selectively metabolizes GSNO (31). Animals deficient in GSNOR show increased steady-state levels of circulating SNOs at basal conditions and widespread elevations of protein-SNO in tissues following challenge by cytokines (31, 74). These studies suggest that the role played by GSNOR is akin to that of phosphatases on the one hand (i.e., regulated denitrosylation) and of superoxide dismutase on the other (i.e., protection from nitrosative stress) and provide genetic evidence for the role of SNOs in physiology and disease.
Hemoglobin (SNO synthase and heme-oxidase).
Hemoglobin (a tetramer comprised of 2 α and 2 β subunits) is the largest reservoir of both O2 and NO in the body; O2 is carried at hemes, and NO at both hemes and cysteine thiols (S-nitrosohemoglobin [SNO-Hb]) (75, 76). The O2/NO-binding functions of hemoglobin (Hb) are governed, in significant part, by an equilibrium between 2 structures: deoxy (T) and oxy (R). An allosteric change from R to T structure lowers the affinity of hemes for O2 and promotes the transfer of NO groups from SNO-Hb to acceptor thiols (75). rbcs thereby provide a NO vasodilator activity that may enable increases in tissue blood flow (O2 delivery). Rebinding of O2 to Hb in the lungs helps to regenerate SNO-Hb by promoting intramolecular NO transfer from heme to thiol (75, 77, 78). Hemoglobin also possesses an intrinsic heme-oxidase activity, which leads to production of superoxide (43, 44, 79). The release of superoxide by Hb (with concomitant heme oxidation) is favored in the T structure (43, 75, 80, 81). Thus, sustained or excessive desaturation of hemoglobin, characteristic of HF, would increase ROS production. In addition, low O2 saturations may cause NO to migrate from the β chains (which dispense NO bioactivity) to the α chains (75, 82), which are impaired in their ability to support production of SNO (75). Indeed, it has recently been reported that HF is characterized by accumulations of heme-NO (relative to controls) that are correlated with venous desaturations (78). NO/redox disequilibrium in situ may impair rbc vasodilation and thereby contribute to tissue ischemia.
Cardiac NO signaling
Substrate specificity.
Ion channels of most classes are targets for S-nitrosylation (26); within the heart (63), the plasmalemmal L-type calcium channel and the sarcoplasmic reticulum (SR) RyR are notable examples (17, 18, 66) (Figure 1). cGMP is also involved in eliciting NO effects on myocardial E-C coupling (67, 83, 84). The range of NO effects in the heart is identified closely with the subcellular location of the NOS isoforms. NOS3 is found within membrane caveolae in proximity to the L-type channel (85), and NOS1 localizes to the SR (32, 86) in a complex with RyR (33) (Figure 1A). As discussed below, NOS1 can be targeted to caveolae under certain circumstances, where it is associated with the sarcolemmal calmodulin-dependent Ca2+ pump (87). Hydrophobic compartmentalization of the target Cys within RyR, coupled with allosteric regulation of thiol reactivity by O2/ROS (shown for RyR1), serves to direct NO to only 1 of approximately 50 channel thiols (66) (Figure 1B). More generally, NO specificity is conferred through spatial localization of NOSs to signaling modules (33, 34), direct interactions between NOSs and their targets (88), and the quaternary structure of the target protein, which influences the reactivity of its thiols and their access to nitrosylating reagents (20).
Functional specificity.
There is substantial physiologic support obtained from studies in NOS deletion mice for the notion that NOS1 and NOS3 exert independent and, in some cases, opposite effects on cardiac contractility (22, 33, 34, 89–91) — actions that are well rationalized by the spatial localization of the NO-signaling module (Figure 1). Specifically, NOS3 exerts its effects on signal-transduction events occurring at the plasmalemmal membrane, inhibiting the L-type Ca2+ channel and in turn attenuating β-adrenergic myocardial contractility (33, 92, 93). In contrast, NOS1 exerts its effects in the SR, facilitating the Ca2+ cycling between SR and cytosol and therefore enhancing myocardial contractility stimulated by either catecholamines (33) or increasing heart rate (22, 34). Such opposite effects of SR NOS1 versus plasmalemmal NOS3 on myocardial contractility indicate that NOS-derived NO does not act as a freely diffusible messenger within the myocyte. Additional support for the paradigm of specificity through compartmentalization of NOS and its targets comes from studies in ischemic (94) or failing myocardium (95) where NOS1 “translocates” from the SR to the plasmalemmal caveolae; there NOS1 exerts “NOS3-like” effects, inhibiting β-adrenergic inotropy (94). Thus NOS isoform specificity derives directly from its location within the cell. NOS structural motifs and fatty acylation enable such spatial localization: NOS1 has a PDZ-domain motif, which facilitates key protein-protein interactions (e.g., syntrophin, ref. 96; plasma membrane calcium ATPase, ref. 87), and NOS3 is myristoylated and palmitoylated, which targets it to the membrane (97). Both proteins can interact with caveolin, and an interaction of NOS1 with XO has been detected in the SR (22). In addition to their location-specific effects on contractility, the NOS isoforms contribute independently to other cardiac phenotypes — notably cardiac hypertrophy. For example, a cumulative hypertrophic phenotype emerges, at both structural and genetic levels, when both NOS isoforms are absent from myocardium (33, 89, 90). The cardiovascular phenotypes of these mice have been previously reviewed (63, 98).
Terminating NO signals.
Termination of NO-based signaling is partly governed by the actions of enzymes. cGMP is metabolized by phosphodiesterase-5 (PDE5), which is spatially localized in proximity to NOS (65); in myocytes, PDE5 is found at the cell membrane associated with caveolae. GSNOR regulates the levels of proteins-SNOs that are in equilibrium with GSNO by shifting the position of the equilibrium toward GSNO (31, 74). GSNOR, which shows activity in the endothelium and cardiac myocyte, degrades GSNO into glutathione disulphide (GSSG) and ammonia (NH3) (74).
Heart failure
NO/redox imbalance.
Oxidant-producing enzymes are upregulated in congestive HF (7, 50), and NO-producing enzymes — NOSs and XO — are altered in either their abundance or spatial localization (95). A relative NO deficiency may further promote oxidase activities (22, 59), which suggests that NO may be a global modulator of O2–/ROS production (22, 56, 57). In particular, vascular NADPH oxidases (99) are increased in abundance in the failing circulation, at least in part due to increased levels of angiotensin II, which suggests a link between neurohormonal activation and NO/redox disequilibrium (100, S1). Increases in superoxide may inactivate NO, reducing its control over the vascular oxidase (59). XO, which is produced in the liver, gut, and heart, is increased in abundance and activity throughout the cardiovascular system in HF (46), contributing to vasoconstriction and depressed cardiac function (7, 38, S2). In HF, increased XO activity is directly reflected in dysregulation of NO signaling (45). Furthermore, a hemoglobin oxidase activity may create a major oxidant burden in failing systems; venous desaturations characteristic of HF promote the T structure in Hb that supports the release of superoxide (43). The hypoxemia may be aggrevated by a deficiency in rbc-SNO that could reduce tissue perfusion (75).
Dysregulated reactions with cysteine thiols.
The relative flux of NO and O2– — a function of abundance and location of both NOSs and oxidases in the heart — determines the chemical fate of their interactions: NO > O2– (characteristic of the physiologic situation) favors S-nitrosylation, whereas NO/O2– disequilibrium (characteristic of HF) favors oxidation reactions (15, 69). More specifically, at low physiologic levels, NO may act as an antioxidant (S3), abating fenton-type reactions, terminating radical chain reactions, and inhibiting peroxidases and oxidases (e.g., by nitrosylation of allosteric thiols; ref. 59) (S3, S4). Further, NO reactions with O2– at basal conditions produce nitrosating reagents that react preferential with thiols (69). Thus, controlled production of RNS and ROS not only preserves an antioxidant environment (15), but may also serve as a mechanism of channeling NO to cysteine substrates. Conversely, when NO and/or O2– are elevated, both the nature of target modification and the specificity of targeting are impaired (S5, S6) (Figures 1 and 3). In other words, superoxide/ROS production may facilitate protein S-nitrosylation at basal conditions but disrupts this signaling mechanism at higher concentrations (17, 18, 22). Interestingly, superoxide dismutase, which preserves physiologic superoxide levels, may also catalyze S-nitrosylation reactions (75, S7).
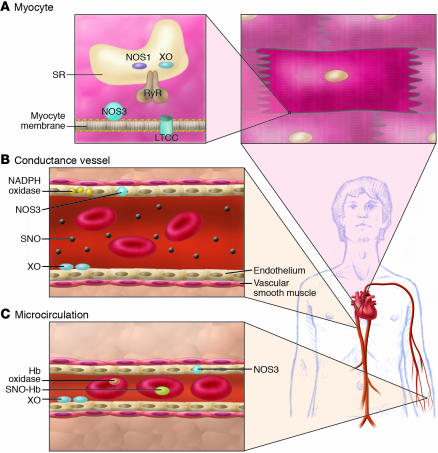
Figure 3.
Consequences of NO/redox disequilibrium in the cardiovascular system – congestive HF phenotype. The balance between nitric oxide (NOS and hemoglobin–based activities) and superoxide/ROS production (oxidase activity) plays a pivotal role in cell/organ function at key sites in the cardiovascular system, including the heart (A), large- and medium-sized conductance blood vessels (B), and the microvasculature (C) (S12). At each of these sites, NO/redox disequilibrium is identified with dysregulated NO-based signaling. (A) In the cardiac myocyte, NO regulates receptor-mediated signal transduction, the calcium cycle, mitrochondrial respiration, and myofilament contractility. Loss of NOS in the cardiac SR (95) impairs NO signaling and creates oxidative stress (by relieving inhibition of the oxidase) (22). Upregulation of inducible NOS (NOS2) may further disrupt physiologic NO regulation by producing a nitrosative stress. The NO/redox disequilibrium that ensues in HF is characterized by disruption and/or impairment of the cardiac calcium cycle, mitochondrial respiration, and myofilament responsiveness to activator calcium. (B) In conductance vessels, vasoconstriction may result from diminished endothelial NOS activity and/or impaired delivery of plasma-borne NO bioactivity. A NO/redox disequilibrium is linked to increased expression or activity of both vascular NADPH oxidase (Nox4) (99) and circulating XO (46). (C) In the microvasculature, rbcs govern NO bioactivity. Lower venous O2 saturation in HF may subserve a NO/redox disequilibrium by impairing NO release from rbcs (SNO-Hb) and promoting hemoglobin oxidase activity (44, 75). Impaired vasodilation by rbcs may exacerbate tissue ischemia.
Nitrosative stress.
In circumstances of acute ischemia, sepsis, or HF, iNOS (NOS2) abundance may increase, leading to nitrosative stress, a pathophysiologic situation characterized by accumulation of S-nitrosylated proteins to hazardous levels (amount and/or spatiotemporal distribution) (63). Nitrosative stress may be exacerbated in situ by oxidants (oxidative stress); stimuli that lead to iNOS induction may also upregulate oxidases, and concomitant elevations in NO/RNS and O2–/ROS may lead to formation of higher oxides (NOx), including peroxynitrite (38). Increased amounts of SNO/NOx favor polynitrosylation and oxidation of cysteine thiols as well as nitration of tyrosines in proteins (16, S8, S9). This situation is relevant to the failing heart, in which the loss of spatial confinement of NOS1, which redistributes from SR to the plasma membrane, may be a proximate cause of oxidative/nitrosative stress both by relieving the local (SR) control of XO (2, 56, 57, S10) and by altering NO/redox balance at the sarcolemma. At the molecular level, poly–S-nitrosylation, oxidation and nitration of the Ca2+ ATPase (S11) and RyR (17) may ensue with adverse effect on calcium homeostasis. Thus, a central pathophysiologic consequence of redox disequilibrium is the disruption of NO signaling by alteration of the occurrence or nature of the posttranslational modifications (S12).
Pathophysiologic impact of disruption in NO-based signaling
Cardiac function.
To the extent that nitric oxide governs key processes in the heart, NO/redox disequilibrium may adversely affect cardiac performance (Figure 3). In the myocyte, ion channels regulate the calcium cycle responsible for normal systolic and diastolic function (63), and the L-type and RyR, which subserve calcium homeostasis, are well-characterized NO targets (17, S13) (Figure 1). Lines of evidence supporting this view include: (a) cardiac NO levels have been shown to change on millisecond timescales, commensurate with physiologic E-C coupling (S14); (b) NOS1 is localized to the SR (32) in proximity to the RyR (NOS1 and the RyR coimmunoprecipitate in mice, ref. 33; and humans, ref. 95), and NOS3 is in the immediate vicinity of the L-type channel (97, S15); (c) NOS1 potentiates both contractility and SR Ca2+ release during inotropic stimulation by β-adrenergic agonists (33) or by pacing (the force-frequency response) (22, 34); (d) RyR isolated from hearts is constitutively S-nitrosylated (1 NO/RyR subunit) (17); (e) in lipid bilayer experiments, NO activates RyR by S-nitrosylating a single thiol, recapitulating the activity of NOS1 in vivo; likewise, in patch clamp experiments, NO activates the L-type channel independently of cGMP (63); (f) functional impairments observed in the NOS1 (and NOS3) knockout mice establish that NO regulates E-C coupling. Thus, there is substantial in vitro biochemical and in vivo functional data supporting the view that NOS1 positively modulates myocardial E-C coupling through RyR S-nitrosylation.
Disruption of the normal E-C coupling by ROS and RNS has been demonstrated for both the skeletal (18) and cardiac (17) RyRs. In vitro, the oxidatively modified channel exhibits sustained activation — directionally similar to the response with physiologic NO — but is unresponsive to physiologic effectors (17, 18). Polynitrosylation of RyR also results in dysregulated channel activity (17). Irreversible activation of the RyR by oxidants leads to SR Ca2+ leak (S16), depressed SR stores (S17), and a classic HF phenotype (S18) reminiscent of that described for RyR hyperphosphorylation (S19) and altered FKBP12.6 (calstabin2) interactions (S20–S22).
An additional body of work with inhibitors of NOSs and oxidases supports the concept of a critical balance between NO/SNO and O2–/ROS in physiologic vs. pathophysiologic cardiac function. XO upregulation in the failing heart (7, S23) represents a cause of oxidative stress (38). Inhibition of XO increases myofilament Ca2+ responsiveness in postischemic stunning (S24), improves abnormal mechanoenergetics in the failing heart (45, S25), and ameliorates remodeling postmyocardial infarction (S26, S27). Notably, the beneficial effects of XO inhibition are dependent on NOS, that is, reducing O2– levels is beneficial only if NO is available (45). Conversely, while XO inhibition does not affect the normal heart, it reverses the mechanoenergetic uncoupling caused by NOS inhibition. These data are consistent with the finding that NOS1 deficiency results in increased XO activity and impaired contractility (22), and they are well rationalized by the observation that NOS1 and XO interact within the SR (22). Thus, NO derived from NOS1 tonically inhibits XO, and loss of inhibition results in a HF phenotype. Pessah and colleagues have recently expanded this paradigm by showing that NO also regulates an NADH oxidase that inhibits SR Ca2+ release and couples SR calcium flux with mitochondrial energetics (S28). Collectively, the data support the idea that ROS pathophysiology does not result from overt oxidative injury to the heart but rather from disruption of physiologic NO signaling that controls E-C coupling and energetics. Thus, the balance between NO and ROS can be understood to have a direct impact upon cardiac regulation through its effects on Ca2+ signaling.
Vascular function.
NO/redox disequilibrium resulting from increased oxidase activity at the vascular wall may contribute to vasoconstriction, a central HF phenotype. Vascular ROS may originate from circulating XO (S2), NADPH oxidase (99), and/or NOS3 (if cofactors such as tetrahydrobiopterin are diminished) (40). The balance between NO and superoxide may be tipped in HF by a number of effectors, including endothelin, epinephrine, and angiotensin II, which stimulate ROS production, and by arginase (S29) and methylarginines (S30), which diminish NO production.
A function for SNOs in regulation of vasomotor tone has been established in GSNOR-deficient mice (31), and studies in humans demonstrate an association between abnormal SNO levels and adverse cardiovascular events (S31–S33). Notably, the turnover of circulating SNOs may be impaired in clinical situations characterized by oxidative stress (70). Thus, the increased oxidant production in HF may impair plasma-borne NO delivery.
Blood function.
A deficit in tissue O2 delivery is a key component of the HF syndrome. It has been recently appreciated that blood flow in the microcirculation is not linked primarily to eNOS activity or pO2, but rather to the O2 saturation of Hb (S34, S35), implicating a role for rbcs (75, 76). Impaired SNO-Hb function and/or vasodilation by rbcs has been observed in several disorders characterized by tissue O2 deficits, including HF (69, 78, S36, S37). Impaired vasodilation by rbcs may disrupt the O2 gradients (the normal decline in O2 saturation as vessel size decreases) in failing tissues. Interestingly these O2 gradients also regulate S-nitrosylation of RyR1, subserving increases in skeletal muscle force (37). Thus, low physiologic pO2 may provide a concerted mechanism for increasing blood flow to actively contracting muscle though S-nitrosylation of RyR and hemoglobin. Conversely, disruption of NO signaling in the microcirculation may be a major contributor to vasoconstriction as well as to the uncoupling of oxygen delivery from the work of skeletal muscle (S38), both of which are characteristics of HF.
Dysfunction of rbcs in the failing circulation (78) may result from both sustained hypoxemia and NO/redox disequilibrium within the rbc. Hypoxemia will favor retention of NO on the hemes of T structured (deoxygenated) Hb, inhibiting SNO-Hb production in the lung (75, S36). Moreover, the oxidase activity of Hb would be increased by chronic venous and tissue hypoxia (43, 79). The consequent production of ROS by hypoxic rbcs may disrupt the delivery of NO to the vessel wall by oxidizing thiol acceptors (73, 79, S39). Thus, the lowered venous pO2 in HF (78) may compromise the delivery of NO bioactivity that subserves regulation of blood flow.
Cardiac hypertrophy and apoptosis.
Apoptosis and cardiac hypertrophy are implicated in cardiac remodeling in HF. NO may exert antiapoptotic effects by S-nitrosylating and thereby inhibiting caspases 3 and 9 (S40, S41), the kinase activities of both apoptosis signaling kinase–1 (S42) and c-Jun N-terminal kinase (S43), and the transcriptional activity of jun (S44). NO can also activate the oxidoreductase activity of endothelial thioredoxin by S-nitrosylation of an allosteric thiol, thereby helping to preserve the NO/redox equilibrium (S45). In addition, denitrosylation of IκB kinase allows NF-κB to translocate to the nucleus and induce protective genes (S46). The mechanisms by which the different NOSs may exert their influence in modulating apoptosis in the failing heart are not known.
On the other hand, oxidative and nitrosative stress may promote apoptosis (S47). For example, increased levels of SNO may activate apoptosis signaling kinase–1 (by relieving an inhibitory association with thioredoxin) while also blocking the binding of NF-κB to DNA (20). Thus, S-nitrosylation of NF-κB would prevent transcription of antiapoptotic genes. In addition, Fas-associated apoptosis is associated with denitrosylation of caspases 3 and 9 (S48). Oxidative stress (mediated by both NADPH oxidase, ref. S49; and XO, ref. S26) is not only linked to apoptosis (through multiple mechanisms) but also to cardiac hypertrophy (23, S49), consistent with the involvement of ROS in mitogenic signaling (12), whereas NO is reported to be antihypertrophic (S50). The precise molecular targets of NO/ROS in cardiac hypertrophy remain to be determined (S49).
Implications for HF therapeutics
Angiotensin converting enzyme inhibitors, adrenergic receptor antagonists, and statins.
Insights into NO/redox-based signaling may offer new perspectives on the mechanism of action of existing therapies as well as guiding the development of novel therapeutic strategies for HF. Indeed, many currently used drug treatments influence NO/redox balance, including inhibitors of the renin-angiotensin aldosterone pathway (S51), the sympathetic nervous system, and the HMG-CoA reductase pathways (S52). Angiotensin-converting enzyme inhibitors stimulate NO production by increasing bradykinin formation and reduce O2–/ROS production by suppressing angiotensin II–stimulation of NADPH oxidase. The sympathetic nervous system is coupled intimately to NO/O2– at multiple levels, and the benefits of HMG-CoA reductases are mediated in significant part through increased expression of NOS (S53).
Hydralazine nitrate combinations.
The combination of isosorbide dinitrate and hydralazine as a treatment for HF may be relevant to NO/redox homeostasis (S54–S56). It has been reported that both hydralazine (S57, S58) and NO (59) can inhibit the NADPH oxidase, which is upregulated in failing hearts, and that hydralazine might also inhibit the production of ROS accompanying the use of nitrates. Some of these ideas, however, may need to be revisited in light of the finding that the mitochondria (not the NADH oxidase) are the principle source of ROS in nitroglycerin tolerance (S59), and the extent to which the NADPH oxidase contributes to the oxidant burden in HF is still unknown. It also remains to be shown that antioxidant effects of hydralazine (S57) can be achieved at the concentrations that are employed clinically. Future studies are needed to determine if the remarkable benefits of this drug combination result primarily from the nitrate or hydralazine components or from both.
XO inhibitors.
Allopurinol and oxypurinol were described as XO inhibitors in the 1950s by Gertrude Elion (S60, S61) and have enjoyed widespread clinical use in the treatment of a variety of conditions characterized by hyperuricemia, most notably gout and hematologic malignancies. As discussed above, XO is upregulated in HF, which leads to oxidative stress in both the heart (7) and vasculature (46, S62). Based on the findings that XO may serve as a primary source of the superoxide (38) implicated in myocyte apoptosis (4), endothelial dysfunction, and mechanoenergetic uncoupling (45, 90, S25), clinical trials of oxypurinol have been initiated in patients with symptomatic HF (S63).
rbc therapeutics.
The understanding that impaired vasodilation by rbcs may represent a primary cause of tissue O2 deficits that characterize HF (75) and, moreover, that rbcs are by far the largest source of oxidase activity in the cardiovascular system — yet one not previously considered — suggests that efforts directed at restoring the NO/redox homeostasis in red blood cells might have important implications for the treatment of the HF. Early efforts are underway to test these new concepts.
Conclusion
Insights into the integrated physiology of the cardiovascular system have been advanced by the understanding that S-nitrosylation is established as a route through which NO can modulate diverse cellular processes, including cardiac E-C coupling, endothelial/vascular function, and tissue oxygen delivery. This understanding supports a perspective in which oxidative and nitrosative stress disrupt physiologic signaling, and by inference, one in which malfunction in NO/redox-based signaling, rather than overt chemical injury, may subserve cardiovascular dysfunction, including structural alterations (e.g., cardiac hypertrophy and programmed cell death). The theme of “NO/redox disequilibrium” – reflecting increased oxidase activities in heart (XO), vasculature (NAD(P)H oxidase), and red blood cells (hemoglobin oxidase) — threads through our evolving understanding of a NO-deficient cardiovascular system. Viewed from this perspective, HF can be seen as a pathophysiologic state that is potentially amenable to therapeutic modulation through targeted restoration of NO/redox balance.
Supplementary Material
Acknowledgments
The authors are supported by a Paul Beeson Physician Faculty Scholar in Aging award (to J.M. Hare), the Donald W. Reynolds Foundation (to J.M. Hare), and by NIH grants HL-065455 (to J.M. Hare), PO1-HL75443 (to J.S. Stamler), and 5PO1-HL42444 (to J.S. Stamler).
Note: Due to space constraints, a number of important references could not be included. References S1–S67 are available online with this article; doi:10.1172/JCI200524459DS1.
Footnotes
Nonstandard abbreviations used: cGMP, 3′,5′-cyclic guanosine monophosphate; E-C, excitation-contraction; GSNO, S-nitrosoglutathione; GSNOR, S-nitrosoglutathione reductase; Hb, hemoglobin; HF, heart failure; NOS, NO synthase; NOS1, neuronal NOS; PTP, protein tyrosine phosphatase; RNS, reactive nitrogen species; RyR, ryanodine receptor calcium–release channel; SNO, S-nitrosothiol; SNO-Hb, S-nitrosohemoglobin; SR, sarcoplasmic reticulum; XDH, xanthine dehydrogenase; XO, xanthine oxidase; XOR, xanthine oxidoreductase.
Conflict of interest: J.M. Hare serves as a consultant to Cardiome Pharma, and J.S. Stamler has a financial interest in Nitrox LLC.
References
- 1.McCord JM, Fridovitch I. The reduction of cytochrome c by milk xanthine oxidase. J. Biol. Chem. 1968;243:5753–5760. [PubMed] [Google Scholar]
- 2.McCord JM, Fridovitch I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 3.Keith M, et al. Increased oxidative stress in patients with congestive heart failure. J. Am. Coll. Cardiol. 1998;31:1352–1356. doi: 10.1016/s0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 4.Cesselli D, et al. Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ. Res. 2001;89:279–286. doi: 10.1161/hh1501.094115. [DOI] [PubMed] [Google Scholar]
- 5.Dhalla AK, Hill MF, Singal PK. Role of oxidative stress in transition of hypertrophy to heart failure. J. Am. Coll. Cardiol. 1996;28:506–514. doi: 10.1016/0735-1097(96)00140-4. [DOI] [PubMed] [Google Scholar]
- 6.Vanderheyden M, et al. Hernodynamic effects of inducible nitric oxide synthase and nitrotyrosine generation in heart failure. J. Heart Lung Transplant. 2004;23:723–728. doi: 10.1016/j.healun.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Cappola TP, et al. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation. 2001;104:2407–2411. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- 8.Palmer RMJ, Ferrige AG, Moncada S. Nitric-oxide release accounts for the biological-activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 9.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric-oxide. Proc. Natl. Acad. Sci. U. S. A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric-oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 11.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth-factor signal-transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 12.Irani K, et al. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 13.Forman HJ, Fukuto JM, Torres M. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am. J. Physiol. Cell Physiol. 2004;287:C246–C256. doi: 10.1152/ajpcell.00516.2003. [DOI] [PubMed] [Google Scholar]
- 14.Stamler JS. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 15.Wink DA, et al. Superoxide modulates the oxidation and nitrosation of thiols by nitric oxide-derived reactive intermediates. J. Biol. Chem. 1997;272:11147–11151. doi: 10.1074/jbc.272.17.11147. [DOI] [PubMed] [Google Scholar]
- 16.Reiter CD, Teng RJ, Beckman JS. Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J. Biol. Chem. 2000;275:32460–32466. doi: 10.1074/jbc.M910433199. [DOI] [PubMed] [Google Scholar]
- 17.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 18.Eu JP, Sun J, Xu L, Stamler JS, Meissner G. The skeletal muscle calcium release channel: Coupled O2sensor and NO signaling functions. Cell. 2000;102:499–509. doi: 10.1016/s0092-8674(00)00054-4. [DOI] [PubMed] [Google Scholar]
- 19.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: A dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 20.Hess DT, Matsumoto A, Kim SO, Marshall H, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell. Biol. 2004;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 21.Abu-Soud H, Rousseau DL, Stuehr DJ. Nitric oxide binding to the heme of neuronal nitric-oxide synthase links its activity to changes in oxygen tension. J. Biol. Chem. 2005;271:32515–32518. doi: 10.1074/jbc.271.51.32515. [DOI] [PubMed] [Google Scholar]
- 22.Khan SA, et al. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc. Natl. Acad. Sci. U. S. A. 2004;101:15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siwik DA, et al. Inhibition of copper-zinc superoxide dismutase induces cell growth, hypertrophic phenotype, and apoptosis in neonatal rat cardiac myocytes in vitro. Circ. Res. 1999;85:147–153. doi: 10.1161/01.res.85.2.147. [DOI] [PubMed] [Google Scholar]
- 24.Chiamvimonvat N, et al. Functional consequences of sulfhydryl modification in the pore-forming subunits of cardiovascular Ca2+ and Na+channels. Circ. Res. 1995;76:325–334. doi: 10.1161/01.res.76.3.325. [DOI] [PubMed] [Google Scholar]
- 25.Thomas JA, Poland B, Honzatko R. Protein sulfhydryls and their role in the antioxidant function of protein S-thiolation. Arch. Biochem. Biophys. 1995;319:1–9. doi: 10.1006/abbi.1995.1261. [DOI] [PubMed] [Google Scholar]
- 26.Stamler JS, Lamas S, Fang FC. Nitrosylation. the prototypic redox-based signaling mechanism. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 27.Gow AJ, Buerk DG, Ischiropoulos H. A novel reaction mechanism for the formation of S-nitrosothiol in vivo. J. Biol. Chem. 1997;272:2841–2845. doi: 10.1074/jbc.272.5.2841. [DOI] [PubMed] [Google Scholar]
- 28.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat. Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 29.Gow AJ, et al. Basal and stimulated protein S-nitrosylation in multiple cell types and tissues. J. Biol. Chem. 2002;277:9637–9640. doi: 10.1074/jbc.C100746200. [DOI] [PubMed] [Google Scholar]
- 30.Rhee KY, Erdjument-Bromage H, Tempst P, Nathan C. S-nitroso proteome of Mycobacterium tuberculosis: enzymes of intermediary metabolism and antioxidant defense. Proc. Natl. Acad. Sci. U. S. A. 2005;102:467–472. doi: 10.1073/pnas.0406133102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 32.Xu KY, Huso DL, Dawson T, Bredt DS, Becker LC. NO synthase in cardiac sarcoplasmic reticulum. Proc. Natl. Acad. Sci. U. S. A. 1999;96:657–662. doi: 10.1073/pnas.96.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barouch LA, et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–340. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 34.Khan SA, et al. Nitric oxide regulation of myocardial contractility and calcium cycling: independent impact of neuronal and endothelial nitric oxide synthases. Circ. Res. 2003;92:1322–1329. doi: 10.1161/01.RES.0000078171.52542.9E. [DOI] [PubMed] [Google Scholar]
- 35.Stamler JS, Hausladen A. Oxidative modifications in nitrosative stress. Nat. Struct. Biol. 1998;5:247–249. doi: 10.1038/nsb0498-247. [DOI] [PubMed] [Google Scholar]
- 36.Kwon J, et al. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl. Acad. Sci. U. S. A. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eu JP, et al. Concerted regulation of skeletal muscle contractility by oxygen tension and endogenous nitric oxide. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15229–15234. doi: 10.1073/pnas.2433468100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berry CE, Hare JM. Xanthine oxidoreductase in the cardiovascular system: molecular mechanisms and pathophysiologic implications. J. Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 40.Landmesser U, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 2003;111:1201–1209. doi:10.1172/JCI200314172. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols — implications for uncoupling endothelial nitric-oxide synthase. J. Biol. Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 42.Ide T, et al. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ. Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 43.Balagopalakrishna C, Manoharan PT, Abugo OO, Rifkind JM. Production of superoxide from hemoglobin-bound oxygen under hypoxic conditions. Biochemistry. 1996;35:6393–6398. doi: 10.1021/bi952875+. [DOI] [PubMed] [Google Scholar]
- 44.Misra HP, Fridovitch I. The generation of superoxide radical during the autoxidation of hemoglobin. J. Biol. Chem. 1972;247:6960–6962. [PubMed] [Google Scholar]
- 45.Saavedra WF, et al. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ. Res. 2002;90:297–304. doi: 10.1161/hh0302.104531. [DOI] [PubMed] [Google Scholar]
- 46.Landmesser U, et al. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure - role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073–3078. doi: 10.1161/01.cir.0000041431.57222.af. [DOI] [PubMed] [Google Scholar]
- 47.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N. Eng. J. Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 48.Saugstad OD. Role of xanthine oxidase and its inhibitor in hypoxia: reoxygenation injury. Pediatrics. 1996;98:103–107. [PubMed] [Google Scholar]
- 49.Webb A, et al. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heymes C, et al. Increased myocardial NADPH oxidase activity in human heart failure. J. Am. Coll. Cardiol. 2003;41:2164–2171. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 51.Duilio C, et al. Neutrophils are primary source of O2 radicals during reperfusion after prolonged myocardial ischemia. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2649–H2657. doi: 10.1152/ajpheart.2001.280.6.H2649. [DOI] [PubMed] [Google Scholar]
- 52.McNally JS, et al. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2290–H2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- 53.Maytin M, et al. Pressure overload-induced myocardial hypertrophy in mice does not require gp91(phox) Circulation. 2004;109:1168–1171. doi: 10.1161/01.CIR.0000117229.60628.2F. [DOI] [PubMed] [Google Scholar]
- 54.Sanders SA, Eisenthal R, Harrison R. NADH oxidase activity of human xanthine oxidoreductase generation of superoxide anion. Eur. J. Biochem. 1997;245:541–548. doi: 10.1111/j.1432-1033.1997.00541.x. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Z, et al. A reappraisal of xanthine dehydrogenase and oxidase in hypoxic reperfusion injury: the role of NADH as an electron donor. Free Radic. Res. 1998;28:151–164. doi: 10.3109/10715769809065801. [DOI] [PubMed] [Google Scholar]
- 56.Cote CG, Yu FS, Zulueta JJ, Vosatka RJ, Hassoun PM. Regulation of intracellular xanthine oxidase by endothelial-derived nitric oxide. Am. J. Physiol. Lung Cell. Mol. Physiol. 1996;15:L869–L874. doi: 10.1152/ajplung.1996.271.5.L869. [DOI] [PubMed] [Google Scholar]
- 57.Hassoun PM, Yu FS, Zulueta JJ, White AC, Lanzillo JJ. Effect of nitric oxide and cell redox status on the regulation of endothelial cell xanthine dehydrogenase. Am. J. Physiol. 1995;268:L809–L817. doi: 10.1152/ajplung.1995.268.5.L809. [DOI] [PubMed] [Google Scholar]
- 58.Shinyashiki M, Pan CJG, Lopez BE, Fukuto JM. Inhibition of the yeast metal reductase heme protein Fre1 by nitric oxide (NO): A model for inhibition of NADPH oxidase by NO. Free Radic. Biol. Med. 2004;37:713–723. doi: 10.1016/j.freeradbiomed.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 59.Clancy RM, Leszczynskapiziak J, Abramson SB. Nitric-oxide, an endothelial-cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J. Clin. Invest. 1992;90:1116–1121. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Michel T, Feron O. Nitric oxide synthases: Which, where, how and why? J. Clin. Invest. 1997;100:2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wink DA, Mitchell JB. Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 1998;25:434–456. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt HHHW, Walter U. NO at work. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 63.Hare JM. Nitric oxide and excitation-contraction coupling. J. Mol. Cell. Cardiol. 2003;35:719–729. doi: 10.1016/s0022-2828(03)00143-3. [DOI] [PubMed] [Google Scholar]
- 64.Layland J, Li JM, Shah AM. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J. Physiol. 2002;540:457–467. doi: 10.1113/jphysiol.2001.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Senzaki H, et al. Cardiac phosphodiesterase 5 (cGMP-specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J. 2001;15:1718–1726. doi: 10.1096/fj.00-0538com. [DOI] [PubMed] [Google Scholar]
- 66.Sun J, Xin C, Eu JP, Stamler JS, Meissner G. Cysteine-3635 is responsible for skeletal muscle ryanodine receptor modulation by NO. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11158–11162. doi: 10.1073/pnas.201289098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paolocci N, et al. cGMP-independent inotropic effect of nitric oxide and peroxynitrite donors: Potential role for S-nitrosylation. Am. J. Physiol. 2000;279:H1982–H1988. doi: 10.1152/ajpheart.2000.279.4.H1982. [DOI] [PubMed] [Google Scholar]
- 68.Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric-oxide directly activates calcium-dependent potassium channels in vascular smooth-muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 69.Foster MW, McMahon TJ, Stamler JS. S-nitrosylation in health and disease. Trends Mol. Med. 2003;9:160–168. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 70.Foster MW, Pawloski JR, Singel D, Stamler JS. Role of circulating S-nitrosothiols in control of blood pressure. Hypertension. 2005;45:15–17. doi: 10.1161/01.HYP.0000150160.41992.71. [DOI] [PubMed] [Google Scholar]
- 71.Scharfstein JS, et al. In vivo transfer of nitric oxide between a plasma protein-bound resevoir and low molecular weight thiols. J. Clin. Invest. 1994;94:1432–1439. doi: 10.1172/JCI117480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stamler JS, et al. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc. Natl. Acad. Sci. U. S. A. 1992;89:7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lipton AJ, et al. S-nitrosothiols signal the ventilatory response to hypoxia. Nature. 2001;413:171–174. doi: 10.1038/35093117. [DOI] [PubMed] [Google Scholar]
- 74.Liu L, et al. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410:490–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 75.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: The role of nitric oxide and S-nitrosohemoglobin. Annu. Rev. Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 76.Singel DJ, Stamler JS. Blood traffic control. Nature. 2004;430:297. doi: 10.1038/430297a. [DOI] [PubMed] [Google Scholar]
- 77.McMahon TJ, et al. Nitric oxide in the human respiratory cycle. Nat. Med. 2002;8:711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 78.Datta B, et al. Red blood cell nitric oxide as an endocrine vasoregulator - A potential role in congestive heart failure. Circulation. 2004;109:1339–1342. doi: 10.1161/01.CIR.0000124450.07016.1D. [DOI] [PubMed] [Google Scholar]
- 79.Gow AJ, Stamler JS. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature. 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 80.Wallace WJ, Houtchens RA, Maxwell JC, Caughey WS. Mechanism of autoxidation for hemoglobins and myoglobins - promotion of superoxide production by protons and anions. J. Biol. Chem. 1982;257:4966–4977. [PubMed] [Google Scholar]
- 81.Mansouri A, Winterhalter KH. Nonequivalence of chains in hemoglobin oxidation and oxygen binding. Effect of organic phosphates. Biochemistry. 1974;13:3311–3314. doi: 10.1021/bi00713a021. [DOI] [PubMed] [Google Scholar]
- 82.Taketa F, Antholine WE, Chen JY. Chain nonequivalence in binding of nitric-oxide to hemoglobin. J. Biol. Chem. 1978;253:5448–5451. [PubMed] [Google Scholar]
- 83.Vila-Petroff MG, Younes A, Egan J, Lakatta EG, Sollott SJ. Activation of distinct cAMP-dependent and cGMP-dependent pathways by nitric oxide in cardiac myocytes. Circ. Res. 1999;84:1020–1031. doi: 10.1161/01.res.84.9.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chesnais JM, Fischmeister R, Mery PF. Positive and negative inotropic effects of NO donors in atrial and ventricular fibres of the frog heart. J. Physiol. 1999;518:449–461. doi: 10.1111/j.1469-7793.1999.0449p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feron O, Saldana F, Michel JB, Michel T. The endothelial nitric-oxide synthase-caveolin regulatory cycle. J. Biol. Chem. 1998;273:3125–3128. doi: 10.1074/jbc.273.6.3125. [DOI] [PubMed] [Google Scholar]
- 86.Sears C, et al. Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ. Res. 2003;92:e52–e59. doi: 10.1161/01.RES.0000064585.95749.6D. [DOI] [PubMed] [Google Scholar]
- 87.Schuh K, Uldrijan S, Telkamp M, Rothlein N, Neyses L. The plasmamembrane calmodulin-dependent calcium pump: a major regulator of nitric oxide synthase I. J. Cell Biol. 2001;155:201–205. doi: 10.1083/jcb.200104131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matsumoto A, Comatas KE, Liu L, Stamler JS. Screening for nitric oxide-dependent protein-protein interactions. Science. 2003;301:657–661. doi: 10.1126/science.1079319. [DOI] [PubMed] [Google Scholar]
- 89.Barouch LA, et al. Combined loss of cardiac neuronal and endothelial nitric oxide synthase causes premature mortality and age-associated hypertropic cardiac remodeling in mice. J. Mol. Cell. Cardiol. 2003;35:637–644. doi: 10.1016/s0022-2828(03)00079-8. [DOI] [PubMed] [Google Scholar]
- 90.Cappola TP, et al. Deficiency of different nitric oxide synthase isoforms activates divergent transcriptional programs in cardiac hypertrophy. Physiol. Genomics. 2003;14:25–34. doi: 10.1152/physiolgenomics.00156.2002. [DOI] [PubMed] [Google Scholar]
- 91.Sears CE, et al. Myocardial NOS1 controls the lusitropic response to β-adrenergic stimulation in vivo and in vitro [abstract] Circulation. 2003;108:IV–249. [Google Scholar]
- 92.Balligand J-L, et al. Nitric oxide-dependent parasympathetic signaling is due to activation of constitutive endothelial (type III) nitric oxide synthase in cardiac myocytes. J. Biol. Chem. 1995;270:14582–14586. doi: 10.1074/jbc.270.24.14582. [DOI] [PubMed] [Google Scholar]
- 93.Gyurko R, Kuhlencordt P, Fishman MC, Huang PL. Modulation of mouse cardiac function in vivo by eNOS and ANP. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H971–H981. doi: 10.1152/ajpheart.2000.278.3.H971. [DOI] [PubMed] [Google Scholar]
- 94.Damy T, et al. Up-regulation of cardiac nitric oxide synthase 1-derived nitric oxide after myocardial infarction in senescent rats. FASEB J. 2003;17:1934–1936. doi: 10.1096/fj.02-1208fje. [DOI] [PubMed] [Google Scholar]
- 95.Damy T, et al. Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet. 2004;363:1365–1367. doi: 10.1016/S0140-6736(04)16048-0. [DOI] [PubMed] [Google Scholar]
- 96.Brenman JE, et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 97.Feron O, et al. Modulation of endothelial nitric-oxide synthase-caveolin interaction in cardiac myocytes. J. Biol. Chem. 1998;273:30249–30254. doi: 10.1074/jbc.273.46.30249. [DOI] [PubMed] [Google Scholar]
- 98.Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function - Ten years after, and continuing. Circ. Res. 2003;93:388–398. doi: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- 99.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 100.Mollnau H, et al. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ. Res. 2002;90:E58–E65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.