Abstract
Recently, type I interferons IFN-α and IFN-β (IFN-α/β) have been evaluated in pilot clinical trials for the treatment of active ulcerative colitis. However, the underlying mechanisms that may contribute to a potential therapeutic effect are incompletely understood. A new study in this issue demonstrates a protective role for IFN-α/β, induced by activation of a Toll-like receptor 9–dependent pathway, in a rodent model of experimental colitis.
The pathogenesis of Crohn disease and of ulcerative colitis (UC), the 2 major forms of inflammatory bowel disease (IBD), involve a complex interplay among certain genetic, environmental, and immunological factors. Research in the last decade resulted in considerable progress in defining key inflammatory pathways in the inflamed gut and identifying new potential therapeutic targets. In particular, administration or manipulation of immunomodulatory cytokines have been proposed as alternative therapeutic strategies to modulate or inhibit proinflammatory cytokine production in IBD. Although, in the case of Crohn disease, novel strategies to inhibit TNF-α (e.g., administration of the anti–TNF-α monoclonal antibody, infliximab), IFN-γ, and IL-12 have been used in clinical trials (1, 2), relatively few successful studies using anticytokine agents for the treatment of UC have been performed. Recently, type I IFN-α and IFN-β (IFN-α/β) have been evaluated in pilot clinical trials in active UC. In these studies, a subgroup of patients responded to therapy with IFN-α 2a or IFN-β; however, the results were too preliminary for final conclusions regarding efficacy to be drawn (3, 4).
Type I IFNs consist of the protein products of various, mainly intron-less, genes including 14 IFN-α genes and a single IFN-β gene. These molecules use a common heterodimeric receptor complex expressed on most cell types throughout the body. Due to their rapid and high level of production following viral infection, they were initially characterized as potent inhibitors of viral replication and hence have been used in the therapy of viral infections such as hepatitis B and C. However, it is now evident that IFN-α/β have important immunoregulatory functions, e.g., during inflammation or nonviral infections (5).
The role of IFN-α/β in the normal and inflamed gut
It is astonishing to realize that in spite of the existence of clinical trials on the use of IFN-α/β in the treatment of UC, there is only very limited information about their expression and biological function in the immune system of the human gut. Moreover, there is little published data regarding the activity of these molecules in animal models of IBD. In this issue of the JCI, Katakura and colleagues report that they have discovered a protective role for IFN-α/β in a murine model of experimental colitis (6). These results underscore a potentially important protective role for type I IFNs in intestinal homeostasis and suggest that strategies to modulate innate immunity may be of therapeutic value for intestinal inflammatory conditions.
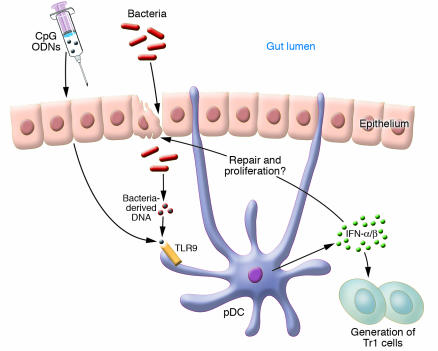
In previous reports, it was shown somewhat unexpectedly by the Katakura group and others that pretreatment of mice, before induction of dextran sulphate sodium (DSS) colitis with bacterial DNA or synthetic oligonucleotides containing unmethylated CpG dinucleotides, ameliorates colonic inflammation (7–9). Based on these earlier observations, Katakura et al. (6) have now explored the role of IFN-α/β, which are strongly induced by CpG-containing oligodeoxynucleotides (CpG ODNs), in CD11lowB220+Gr1+ plasmacytoid dendritic cells and macrophages in acute DSS-induced colitis in mice (Figure 1). They demonstrate in several experiments the existence of a Toll-like receptor 9–dependent (TLR9–dependent) mechanism of IFN-α/β induction, which accounts for CpG ODN–mediated protection. This effect is also evident in mice deficient in T and B lymphocytes and, hence, apparently independent of the presence of the adaptive immune system. Mice lacking the IFN-α/β receptor were resistant to the CpG ODN–mediated effect and, interestingly, in comparison to wild-type controls, these mice suffered from increased mortality rates in response to DSS treatment without CpG ODN pretreatment. This suggests that endogenous mechanisms, such as the entry of bacterial DNA into the mucosa after DSS-induced epithelial damage, induce production of antiinflammatory proteins such as IFN-α/β. These data now provide a rational basis for an in-depth analysis of IFN-α/β function in gut homeostasis. In this regard, real-time PCR experiments with specific IFN primer sets and analysis of the kinetics of the differential expression of IFN response genes could be helpful in the search for an optimal IFN-based therapy. However, there are potential concerns about the therapeutic window of CpG ODN or recombinant IFN-α/β (rIFN-α/β) treatment. There is evidence that CpG ODN treatment is only effective when given prior to DSS treatment while administration to mice with chronic colitis in fact worsens disease (8, 10). Thus, administration of CpG ODNs in chronically active UC may augment rather than suppress intestinal inflammation. In addition to IFN-α/β, CpG ODN–TLR9 signaling strongly induces the NF-κB–dependent expression of proinflammatory cytokines, such as IL-6 and IL-12, that exert potent proinflammatory effects in T cell–dependent colitis (11, 12). It will therefore be interesting to see whether rIFN-α/β administration has fewer of these unwanted side effects compared to CpG ODN administration and whether it will be effective in the chronic phase of DSS-induced colitis.
Figure 1.
Hypothetical model for a regulatory role of type I IFNs in the gut. Intestinal bacteria entering the mucosa after epithelial damage or exogenous administration of CpG ODNs are recognized by mucosal plasmacytoid dendritic cells (pDCs) via TLR9. Stimulated pDCs produce IFN-α/β, which promote epithelial regeneration or generation of regulatory T cell subsets (e.g., T regulatory 1 cells; Tr1).
It is well established that the adaptive immune system (in particular CD4+ T cells) is absolutely required for both gut homeostasis and a deregulated chronic immune response against the commensal microflora (13). Although the function of type I IFNs on T cells is incompletely understood, an important rationale for the trial of IFN treatment for UC was the known function of IFN-α/β on T cells. In fact, IFN-α/β was shown to stimulate production of the antiinflammatory cytokine IL-10 by human CD4+ T cells (14). Furthermore, IFN-α/β modulates Th1 responses (15) and inhibits production of Th2 cytokines (16), some of which (e.g., IL-5, IL-13) are upregulated in the mucosa of patients with UC (17). Interestingly, recent studies suggest that IFN-α/β is able to induce regulatory T cells (18) and, moreover, intestinal CD8+ plasmacytoid dendritic cells producing IFN-α/β after CpG ODN stimulation were shown to induce the differentiation of naive T cells to IL-10–secreting T regulatory 1–like cells (19).
The protective effect of CpG ODNs seems to be independent of IL-10, as they ameliorate spontaneous colitis in IL-10–deficient mice (7). However, a key remaining question is whether IFN-α/β treatment is beneficial in T cell–dependent animal models of colitis such as the well-established adoptive CD4+ CD45RBhigh transfer model (20). Such detailed characterization of the biological function of IFN-α/β in experimental animal models will undoubtedly be of great value for future clinical trials in IBD.
Footnotes
See the related article beginning on page 695.
Nonstandard abbreviations used: CpG ODN, CpG-containing oligodeoxynucleotide; DSS, dextran sulphate sodium; IBD, inflammatory bowel disease; TLR9, Toll-like receptor 9; UC, ulcerative colitis.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Targan SR, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N. Engl. J. Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 2.Mannon PJ, et al. Anti-interleukin-12 antibody for active Crohn’s disease. N. Engl. J. Med. 2004;351:2069–2079. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- 3.Madsen SM, et al. An open-labeled, randomized study comparing systemic interferon-alpha-2A and prednisolone enemas in the treatment of left-sided ulcerative colitis. Am. J. Gastroenterol. 2001;96:1807–1815. doi: 10.1111/j.1572-0241.2001.03875.x. [DOI] [PubMed] [Google Scholar]
- 4.Nikolaus S, et al. Interferon beta-1a in ulcerative colitis: a placebo controlled, randomised, dose escalating study. Gut. 2003;52:1286–1290. doi: 10.1136/gut.52.9.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogdan C, Mattner J, Schleicher U. The role of type I interferons in non-viral infections. Immunol. Rev. 2004;202:33–48. doi: 10.1111/j.0105-2896.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 6.Katakura K, et al. Toll-like receptor 9–induced type 1 IFN protects mice from experimental colitis. J. Clin. Invest. 2005;115:695–702. doi:10.1172/JCI200522996. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rachmilewitz D, et al. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology. 2002;122:1428–1441. doi: 10.1053/gast.2002.32994. [DOI] [PubMed] [Google Scholar]
- 8.Obermeier F, et al. Contrasting activity of cytosin-guanosin dinucleotide oligonucleotides in mice with experimental colitis. Clin. Exp. Immunol. 2003;134:217–224. doi: 10.1046/j.1365-2249.2003.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rachmilewitz D, et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520–528. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Obermeier F, et al. CpG motifs of bacterial DNA exacerbate colitis of dextran sulfate sodium-treated mice. Eur. J. Immunol. 2002;32:2084–2092. doi: 10.1002/1521-4141(200207)32:7<2084::AID-IMMU2084>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 11.Atreya R, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat. Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 12.Simpson SJ, et al. T cell-mediated pathology in two models of experimental colitis depends predominantly on the interleukin 12/signal transducer and activator of transcription (Stat)-4 pathway, but is not conditional on interferon gamma expression by T cells. J. Exp. Med. 1998;187:1225–1234. doi: 10.1084/jem.187.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wirtz S, Neurath MF. Animal models of intestinal inflammation: new insights into the molecular pathogenesis and immunotherapy of inflammatory bowel disease. Int. J. Colorectal Dis. 2000;15:144–160. doi: 10.1007/s003840000227. [DOI] [PubMed] [Google Scholar]
- 14.Aman MJ, et al. Interferon-alpha stimulates production of interleukin-10 in activated CD4+ T cells and monocytes. Blood. 1996;87:4731–4736. [PubMed] [Google Scholar]
- 15.Nguyen KB, et al. Interferon alpha/beta-mediated inhibition and promotion of interferon gamma: STAT1 resolves a paradox. Nat. Immunol. 2000;1:70–76. doi: 10.1038/76940. [DOI] [PubMed] [Google Scholar]
- 16.Kaser A, Molnar C, Tilg H. Differential regulation of interleukin 4 and interleukin 13 production by interferon alpha. Cytokine. 1998;10:75–81. doi: 10.1006/cyto.1997.0270. [DOI] [PubMed] [Google Scholar]
- 17.Fuss IJ, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J. Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- 18.Levings MK, et al. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J. Immunol. 2001;166:5530–5539. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 19.Bilsborough J, George TC, Norment A, Viney JL. Mucosal CD8alpha+ DC, with a plasmacytoid phenotype, induce differentiation and support function of T cells with regulatory properties. Immunology. 2003;108:481–492. doi: 10.1046/j.1365-2567.2003.01606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powrie F, Correa-Oliveira R, Mauze S, Coffman RL. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J. Exp. Med. 1994;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]