Summary
Background
Social isolation and loneliness pose significant public health challenges globally. The objective of this study is to investigate the association between social isolation, loneliness, and the risk of type 2 diabetes mellitus (T2DM).
Methods
423,503 UK adults from the UK Biobank (UKB) and 13,800 Chinese adults from the China Health and Retirement Longitudinal Study (CHARLS) were analyzed. The exposures of interest were social isolation and loneliness. Social isolation was evaluated based on the number of household members, frequency of social activities, contact with others, and marriage status (CHARLS only). Loneliness was evaluated by the subjective feeling of loneliness and the willingness to confide in others (UKB only). The primary endpoint was incident T2DM. The two-sample Mendelian randomization (MR) analysis was based on the genome-wide association studies of UKB (n = 463,010) and the European Bioinformatics Institute (n = 655,666).
Findings
The UKB cohort study documented 15,072 T2DM cases during a mean follow-up of 13.5 years, and the CHARLS cohort study recorded 1,249 T2DM cases during a mean follow-up of 5.8 years. Social isolation and loneliness showed significant associations with an elevated risk of T2DM in both UKB (social isolation [most vs least]: HR 1.17, 95% CI 1.11–1.23; loneliness [yes vs no]: HR 1.21, 95% CI 1.13–1.30) and CHARLS cohorts (social isolation [yes vs no]: HR 1.22, 95% CI 1.06–1.40; loneliness [yes vs no]: HR 1.21, 95% CI 1.07–1.36). These associations remained significant after accounting for baseline glucose status and genetic susceptibility to T2DM. Two-sample MR analyses determined that feeling lonely (OR 1.04, 95% CI 1.02–1.06) and engaging in fewer leisure/social activities (OR 1.03, 95% CI 1.02–1.05) were associated with increased T2DM risk, whereas more contact with friends or family (OR 0.99, 95% CI 0.98–0.99) was associated with reduced T2DM risk.
Interpretation
Social isolation and loneliness are each associated with an elevated risk of T2DM, with MR analyses suggesting potential causal links. These associations remain significant after considering genetic susceptibility to T2DM. The findings highlight the importance of promoting initiatives to address social isolation and loneliness as part of T2DM prevention strategies.
Funding
CAMS Innovation Fund for Medical Sciences (No. 2021-I2M-1-008) and National Natural Science Foundation of China (No. 72103187).
Keywords: Social isolation, Loneliness, Type 2 diabetes mellitus, Public health, Cohort study, Mendelian randomization
Research in context.
Evidence before this study
Social isolation and loneliness are prevalent global public health problems. Previous studies have established significant associations between social isolation, loneliness, and an increased risk of various diseases, including cardiovascular disease, in-hospital infection, and dementia. To investigate the research on the association between loneliness, social isolation, and type 2 diabetes mellitus (T2DM), we conducted a comprehensive search on PubMed for English-language studies published up to June 4, 2023. The search terms (in title) included “social isolation AND diabetes” (5 publications), “loneliness AND diabetes” (13), “social support AND diabetes” (251), “social relations AND diabetes” (6), and “social networks AND diabetes” (17). In general, the association between loneliness and an increased risk of T2DM has been affirmed in ongoing observational studies, including cohorts from England (n = 4,112), Denmark (n = 24,687), and Norway (n = 24,024). However, the link between social isolation and T2DM risk remains uncertain; a significant association was observed in the aforementioned Danish cohort study but not in most other European cohorts. Moreover, available observational data have several shortcomings, including limited generalizability to non-European populations, insufficient control of T2DM genetic susceptibility, and inadequacies in cohort methodologies, such as the lack of specific event dates during the follow-up and the inaccurate diagnosis of T2DM. Moreover, as conventional observational studies cannot completely rule out reverse causality and confounding, the causal nature of the association between loneliness/social isolation and T2DM remains uncertain.
Added value of this study
In the current study, we determined significant associations of social isolation and loneliness with an increased risk of T2DM in two large-scale cohorts from Europe and East Asia. Furthermore, our study revealed a causal relation between loneliness, social isolation, and heightened T2DM risk through MR analyses and documented the significant associations between social isolation, loneliness, and increased T2DM risk among participants with diverse genetic predispositions to T2DM. In addition, we suggested the significant associations between social isolation, loneliness, and increased T2DM risk across participants with varying baseline glucose metabolism status, and showed that there was no interaction between social isolation and loneliness in the risk of T2DM.
Implications of all the available evidence
This study found that social isolation and loneliness were associated with an increased risk of T2DM, with results of the MR analysis suggesting a causal relationship. These results raise the possibility that strategies to prevent or alleviate social isolation and loneliness in vulnerable populations may be an effective strategy to prevent T2DM. Future research should include randomized controlled trials that enroll participants with loneliness and social isolation, test interventions to alleviate these conditions, and compare T2DM incidence between intervention and control groups, thereby allowing for a direct assessment of this hypothesis.
Introduction
Type 2 diabetes mellitus (T2DM) is a widespread public health concern with high global prevalence. According to the International Diabetes Federation Diabetes Atlas Tenth Edition,1 the worldwide diabetes population quadrupled between 1980 and 2021, reaching 537 million,2 of which nearly 95% is T2DM.3 This number is projected to rise to 578 million (9.3% global prevalence) by 2030 and 700 million (10.2%) by 2045.2,4 Social isolation and loneliness are pervasive global concerns, intertwined yet distinct in their concepts. Social isolation is defined as an objective deficiency in the quantity of relationships and the frequency of interactions with family, friends, and the broader community,5,6 whereas loneliness is defined as the subjective adverse emotion of solitude or the distressing sensation of being socially isolated, regardless of objectively being socially isolated or not.7 The estimated prevalence of social isolation is 25% in community-dwelling older adults,8 and the estimated prevalence of loneliness, as reported in a recent meta-analysis, ranged from 1.8% to 12.0% in young or middle-aged adults and 4.2%–24.2% in older adults in Europe.9 Studies have shown that loneliness and social isolation are associated with activation of the hypothalamic–pituitary–adrenal axis, leading to enhanced sympathetic nervous system activity, increased release of inflammatory cytokines, and increased oxidative stress,10,11 which directly contribute to the development of T2DM. In addition, social isolation and loneliness are associated with poor weight control,12 high tobacco uses,13,14 low physical activity,15 and poor sleep quality,16,17 which indirectly increase the risk of T2DM.18,19
A significant association between loneliness and a heightened risk of T2DM has consistently been seen in recent longitudinal studies, including in cohorts in England (n = 4,112),20 Denmark (n = 24,687),21 and Norway (n = 24,024).22 Conversely, findings on the relation between social isolation and risk of T2DM have been less consistent, since the significant association between social isolation and an increased risk of T2DM was only observed in a Danish cohort study,21 but not reported in other European cohort research.20,23,24 In general, although recent research has demonstrated a positive connection between loneliness and the risk of T2DM, the relationship between social isolation and T2DM risk remains a subject of debate. The existing studies have provided promising insights into the associations of social isolation and loneliness with T2DM risk. However, the available data have several limitations, including a focus on European populations, which limits generalizability; reliance on a traditional cohort study design, which limits causal inference; and a lack of consideration for genetic predisposition to glucose metabolism or T2DM, which may be important confounders or effect modifiers. Furthermore, weaknesses such as insufficient follow-up (absence of specific event time) and less accurate T2DM diagnostic methods, may also introduce potential bias. Also, evidence from Mendelian randomization (MR) analyses, which could help to establish the causal associations between social isolation, loneliness, and the risk of T2DM, is lacking. The role played by genetic susceptibility to T2DM in the relations between social isolation, loneliness, and T2DM risk has yet to be thoroughly elucidated. Hence, despite the range of studies investigating the correlation between social isolation, loneliness, and T2DM, the existing evidence remains insufficient, demanding further evaluation.
To address these gaps, we analyzed the data on social isolation, loneliness, and incident T2DM in two large-scale prospective cohorts from Europe and Asia and conducted two-sample MR analysis to address causality of the associations. We integrated genetic susceptibilities to T2DM, insulin resistance, and beta cell function in the analysis, with the aim of bolstering the study's robustness through comprehensive consideration of genetic factors our overarching aim is to reveal connections between social isolation, loneliness, and T2DM risk, thereby enriching the landscape of T2DM prevention through a nuanced consideration of social dimensions.
Methods
Study design and participants
Two prospective cohort studies are based on data from the UK Biobank (UKB) and the China Health and Retirement Longitudinal Study (CHARLS) databases, respectively. The UKB is a comprehensive biomedical database of population health and genetic research resources. Between 2006 and 2010, more than 500,000 participants aged 37–73 years were recruited from 22 assessment centers across the UK. Participants completed touchscreen questionnaires and physical measurements and provided blood samples at recruitment.25 Multiple follow-ups were conducted, and the database is frequently updated. The full UKB study protocol is available at https://www.ukbiobank.ac.uk/media/gnkeyh2q/study-rationale.pdf. The study was approved by the North West Multicenter Research Ethics Committee (REC reference for UK Biobank 11/NW/0382), and all participants provided written informed consent.25 The CHARLS is a continuous nationwide survey that seeks to gather comprehensive data on middle-aged and older adults to support research and inform policy decisions related to aging in China.26 In CHARLS, individuals aged 45 years and older from 150 county-level units and 450 village-level units completed baseline interviews in 2011. Follow-up interviews were conducted using face-to-face computer-assisted personal interviews (CAPI) in 2013, 2015, and 2018. Ethical approval for CHARLS was granted by the Institutional Review Board at Peking University (IRB00001052-11015), and all participants provided written informed consent.26 Additional details can be found at http://charls.pku.edu.cn/.
In the current study, 502,369 UK adults and 25,533 Chinese were initially screened. In the UKB cohort, we excluded those with missing data on loneliness or social isolation (n = 35,707), a prior diagnosis of diabetes at baseline (n = 32,562), and missing data on the polygenic risk score (PRS) for T2DM (n = 10,597), resulting in 423,503 participants in our analyses (Supplementary Figure S1). Supplementary Table S1 and Supplementary Table S2 present a comparison of baseline characteristics between enrolled participants and excluded individuals, along with those with missing exposure data, in the UKB cohort. In general, participants excluded from the study, including those with missing exposure data, tended to be older, belong to the ethnic group of white, have a higher percentage of males, and exhibit lower socioeconomic statuses (including lower household income, lower percentage of college degree attainment, and unemployment). Additionally, this group also tended to have unhealthy lifestyle patterns (such as smoking, low levels of physical activity, unhealthy diet, and poor sleep patterns), and experience poorer health conditions (with a higher prevalence of prediabetes, cardiovascular disease [CVD], hypertension, high cholesterol, chronic lung diseases [CLD], cancer, and obesity) compared with the enrolled subjects. Within the CHARLS cohort, a total of 13,800 participants were included in the final analysis after excluding individuals with missing data on social isolation or loneliness (n = 10,034), baseline diagnosis of diabetes mellitus (n = 1,105), with censoring of those lost to follow-up (n = 594) (Supplementary Figure S1). Further information on the participant selection process can be found in Appendix 1.
Assessment of exposure
The exposures of interest are social isolation and loneliness. The evaluation of social isolation primarily centers on objective social connections, encompassing factors such as frequency of social interactions or engagement in social activities,5 whereas the assessment of loneliness concentrates on the subjective perception of isolation.7 To maintain consistency with previous studies conducted within the UKB and CHARLS cohorts, distinct assessment methods were employed between the two cohorts. In the current study, social isolation was evaluated based on the number of people in the household, frequency of social activities, contact with others, and marriage status (CHARLS only); whereas loneliness was evaluated by subjective feelings of loneliness and the willingness to confide in others (UKB only).
In the UKB cohort, social isolation was evaluated through responses to three questions on questionnaires: 1) “How often do you visit friends or family or have them visit you?” (1 point for the response of “once a month”, “once every few months”, “never or almost never”, or “no friends or family outside the household” and 0 point for the response of “once a week”, “2–4 times a week”, and “Almost daily”); 2) “Which of the following leisure or social activities do you engage in once a week or more often? You may select more than one of them: sports club or gym, pub or social club, religious group, adult education class, or other group activities” (1 point for the response of “none of above” and 0 point for the response of one of activities mentioned above); and 3) “Including yourself, how many people live in your household? Include those who usually live in the house such as students living away from home during term time, and partners in the armed forces or in professions such as pilots” (1 point for the response of “0” and 0 point for other numbers higher than 0). The social isolation score was calculated by summing up the points of three questions (ranging from 0 to 3). Participants were allocated into three groups according to the social isolation score: least isolated group (0 point), moderate isolated group (1 point), and most isolated group (2 or 3 points). Loneliness was assessed by two questions from UKB questionnaires: “Do you often feel lonely?” (1 point for the response of “yes” and 0 point for “no”) and “How often are you able to confide in someone close to you?” (1 point for the response of “never or almost never” and 0 point for the response of “once every few months”, “once a month”, “once a week”, “2–4 times a week”, or “almost daily”). The loneliness score was determined by adding up the scores from two questions, resulting in a range of 0–2 points. Individuals were allocated into two groups according to the loneliness score: the no-loneliness group (0 or 1 point) and the loneliness group (2 points). The field ID of the questions above is presented in Supplementary Table S5.
In the CHARLS cohort, social isolation and loneliness were also assessed through self-reported questionnaires. For the social isolation evaluation, participants received 1 point for each of the following: being unmarried (including separated, divorced, widowed, or never married), living alone, having less than weekly contact with their children (via phone, in person, or email), and not participating in any social activities over the past month (including interacting with friends, playing chess or cards, attending sports, social, or other clubs). Scores on the social isolation index ranged from 0 to 4, with higher scores indicating a higher level of social isolation. Participants with ≥2 points were allocated to the social isolation group.27 For the loneliness assessment, a single question from the Center for Epidemiological Studies Depression Scale (CESD), “In the past week, how often did you feel lonely?”, was used. Participants were classified as lonely if they said they felt lonely occasionally (1–2 days per week), frequently (3–4 days per week), or most of the time (5–7 days per week). Those respondents who said they felt lonely infrequently or never (less than one day) were categorized as not feeling lonely.28,29
Although the assessment methods were not the same in the two cohorts, the assessment methods for loneliness and social isolation in both cohorts were consistent with published studies focusing on the topic of loneliness and social isolation within the UKB and CHARLS cohorts. In the UKB cohort, researchers utilized methods above to document the association of loneliness and social isolation with heart failure,30 myocardial infarction,31 stroke,31 and in-hospital infection.32 The assessment methods utilized in CHARLS have also been applied in prior research that explored the associations of loneliness and social isolation with cognitive function27 and functional disability.29
Assessment of outcome
T2DM was the primary outcome in both cohorts. In the UKB cohort, the diagnosis of T2DM was based on hospital admission data and cause of death registry records. The diagnosis of T2DM was based on hospital admission data from the Hospital Episode Statistics for England (up to 31 October 2022), Scottish Morbidity Record data for Scotland (up to 31 July 2021), and Patient Episode Database for Wales (up to 28 February 2018), and the death cause registry records from the National Health Service (NHS) Information Centre (England and Wales, up to 30 November 2022) and the NHS Central Register, National Records of Scotland (Scotland, up to 30 November 2022). T2DM was confirmed when there was a medical diagnosis in the hospital admission data or when T2DM was listed as a cause of death in the death register. We collected the diagnostic data by following the International Classification of Diseases-Tenth Revision (ICD-10); the code for T2DM was ICD E11.33 In the CHARLS cohort, DM was confirmed by the questions “Have you been diagnosed with diabetes by a doctor?” and “Are you now taking any of the following treatments to treat or control your diabetes?”. Participants who responded affirmatively to either of the two questions were considered to have a diagnosis of DM.
Assessment of covariates
Covariates for this study were collected at baseline. In the UKB cohort, covariates included age, sex, race/ethnicity, assessment center, household income, educational level, employment status, smoking, alcohol drinking, physical activity, sun exposure time, healthy diet score, healthy sleep pattern, body mass index (BMI), self-reported medical conditions (history of prediabetes, CVD, hypertension, dyslipidemia, CLD, and cancer), and glucose or lipid metabolism measures from laboratory tests on blood samples. Physical activity was measured by the metabolic equivalent task (MET).30 A healthy sleep pattern was defined as a healthy sleep score ≥4.34 Prediabetes was defined as a fasting blood glucose (FBG) of 5.6–6.9 mmol/L or HbA1c of 5.7–6.4%.35 Family history of T2DM was defined by the presence of T2DM diagnosis in at least one first-degree relative. Grades of genetic risk (low, moderate, and high genetic risk) were defined according to the tertiles of the PRS for T2DM, insulin resistance, and beta cell function. The PRS for T2DM from the UKB database has been previously described.36 The calculation of PRS for insulin resistance and beta cell function is presented in Appendix 2. Information on single nucleotide polymorphisms (SNPs) utilized in the calculation of PRS for insulin resistance and beta cell function is presented in Supplementary Tables S3 and S4, respectively.
In the CHARLS cohort, covariates included demographic characteristics (age, sex, ethnicity, and residential location), socioeconomic information (household income, educational level, and employment), lifestyle (smoking, alcohol intake, physical activity), BMI, medical conditions (hypertension, dyslipidemia, heart disease, and stroke), and treatments including anti-hypertension and anti-hyperlipidemia therapies.
Specific information on covariates in the two cohorts is presented in Appendix 3 and the field ID of the questions above in the UKB is presented in Supplementary Table S5.
Mendelian randomization
Our study utilized a two-sample Mendelian randomization (MR) approach using summary-level data from genome-wide association studies (GWAS) conducted on individuals of European ancestry. The exposures of MR analysis included all items of social isolation and loneliness, with instrumental variables derived from the UKB GWAS. For the outcome of T2DM, instrumental variables were identified from two independent GWAS: UKB and European Bioinformatics Institute (EBI). Detailed information on the MR design is presented in Appendix 4.
Statistical analyses
Baseline variates were presented as means ± standard error or median (interquartile range) for continuous variables and frequency (percentages) for categorical variables. Continuous variables were assessed for statistical differences using two-sample T-tests, ANOVA tests, or Mann–Whitney U tests. Categorical variables were evaluated for differences using the χ2 test. For missing data, we imputed the median for variables with a missingness rate <5%, and treated missing data as a separate category labeled “unknown” for variables with a missingness rate ≥5%. Detailed information on the covariates with a missingness rate ≥5% in the UKB and CHARLS cohorts is presented in Supplementary Table S6.
The association of exposure with incident T2DM was assessed in multivariable Cox proportional hazards and presented as hazard ratios (HR) with 95% confidence intervals (CI). The association of social isolation and loneliness with T2DM risk was evaluated in the main-effect and interaction models. Three models were generated for the analysis in the UKB cohort: Model 1 adjusted for age, sex, and race/ethnicity; Model 2 further adjusted for BMI (continuous), grip strength, assessment center, household income, college/university degree, employed, currently smoking, current alcohol drinking, physical activity, sun exposure time, healthy diet score, and healthy sleep score. Model 3 further adjusted for family history of T2DM, prediabetes, history of CVD, history of hypertension, dyslipidemia, chronic lung disease, cancer, and PRS for T2DM. In the CHARLS cohort, we also built three models for the Cox regression: Model 1 adjusted for age, sex, and ethnicity; Model 2 further adjusted for location, income, educational level, currently employed, current smoking, drinking frequency, physical activity, and BMI; Model 3 further adjusted for heart problems, hypertension, hyperlipidemia, stroke, and medication for hypertension and hyperlipidemia. The assessment of the proportional hazard's assumption for the Cox models in both cohorts is provided in Supplementary Table S7, and the P values for all covariates within the models, as well as the P-values for the global models, exceeded 0.05. The population-attributable fraction (PAF) was calculated to reflect the proportion of the events that could be avoided by eliminating the exposure. In addition, the associations of loneliness and social isolation with T2DM were further explored in participants with different levels of genetic susceptibility to T2DM (low, moderate, and high genetic risk).
Beyond the analyses above, we conducted several supplementary analyses to enhance the comprehensiveness of our study. In supplementary analyses, the cumulative risk of incident T2DM among different groups is presented in Kaplan–Meier (KM) curves with a log-rank test. As a supplement to the baseline information, baseline characteristics of the CHARLS cohort and the comparisons between participants who developed T2DM during the follow-up and those who did not in the UKB cohort are presented. Moreover, we delved into the associations of specific items of social isolation and loneliness with T2DM risk and examined the trends in T2DM risk associated with increasing scores of loneliness and social isolation. Furthermore, we explored potential interactions between social isolation and loneliness in relation to T2DM risk. In addition to the whole population, the separate and joint associations of social isolation and loneliness with the risk of T2DM, the interaction between social isolation and loneliness in the risk of T2DM, and the validation for their associations among participants with different levels of genetic susceptibility to T2DM are further investigated in participants with different glucose metabolism statuses (normal glucose tolerance and prediabetes). Furthermore, we performed subgroups for the separate and joint associations of social isolation and loneliness with T2DM risk. Subgroup analyses were performed by stratifying by age (<65 years old or ≥65 years old), sex (male or female), race/ethnicity (white or others), BMI(<25 kg/m2, 25–29.9 kg/m2, and ≥30 kg/m2), high household income (yes or no), educational level (college/university degree or others), employment status (currently employed or not), smoking status (currently smoking or not), drinking status (currently drinking or not), physical activity (MET Score less than the cohort median or above the median), healthy diet (healthy diet score ≥4 or not), healthy sleep pattern (yes or no), less sun exposure (sun exposure time <3 h or not), CVD (yes or no), hypertension (yes or no), dyslipidemia (yes or no), chronic lung disease (yes or no), cancer (yes or no), PRS for insulin resistance (low, intermediate, and high), and PRS for beta cell function (low, intermediate, and high). Sensitivity analyses were further performed to validate the results, including the independent and joint associations of social isolation and loneliness with T2DM risk and the interaction between social isolation and loneliness concerning T2DM risk. In sensitivity analyses, we validated results in new models with further adjustment for physical measurements of blood pressure (systolic and diastolic blood pressure) and laboratory tests on glucose metabolism (FBG and HbA1C), and lipid metabolism (total cholesterol (TC), total triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and lipoprotein a (Lp (a))) to evaluate the independence of the findings from the established biological pathways for T2DM. Further, we excluded participants with a family history of T2DM to eliminate hereditary effects and those with a personal history of cancer due to shortened survival commonly associated with cancer. At last, we excluded those diagnosed with T2DM within the first year of follow-up to mitigate the potential impact of reverse causality. Also, we further utilized the Fine–Gray sub-distribution hazard model to account for the potential impact of mortality as a competing event and performed mediation analyses with bootstrapping (2000 replications) to confirm significant mediating covariates between social isolation, loneliness, and T2DM incidence.
In MR analysis, we used odds ratios (OR) and 95% CI to estimate the T2DM risk caused by increased levels of loneliness and social isolation. For the primary MR analysis, we utilized the random-effects inverse-variance weighted (RE-IVW) method to estimate the causal effect.37 Several other MR analyses, including IVW, fixed effects IVW, MR-Egger regression, weighted median (WM), and penalized WM, were performed to assess the robustness of the results. We used the MR-Egger intercept test to assess for horizontal pleiotropy38 and Cochran's Q statistic for population heterogeneity.39 To confirm the causal effect of any single SNP, we conducted a leave-one-out (LOO) analysis by discarding each exposure-associated SNP and repeatedly performing IVW analysis. We also performed a bidirectional two-sample Mendelian randomization analysis from the reverse direction. Additional details on the MR analyses are presented in Appendix 5.
We performed analyses with the R version (R Foundation for Statistical Computing, Vienna, Austria). P < 0.05 was used to indicate statistical significance in 2-sided statistical testing. Because of the absence of adjustment for multiple comparisons, secondary and subgroup analyses should be interpreted cautiously.
Role of the funding source
The funders played no part in the design of the study, collection, analysis, interpretation of data, or the writing of the report.
Results
Baseline characteristics
In the UKB cohort, the average age of the population was 56.3 years, and 44.7% of the participants were male. In the CHARLS cohort, the population had an average age of 58.3 years, with 46.7% of the participants being male. The baseline characteristics of participants from the UKB and CHARLS databases are presented in Table 1 and Supplementary Table S5, respectively. In the UKB cohort, individuals with higher levels of social isolation or loneliness tended to be male and had unhealthier lifestyle behaviors, including smoking, less physical activity, lower healthy diet scores, and unhealthier sleep patterns. They were also more likely to have obesity, prediabetes, and a history of cardiovascular disease, hypertension, dyslipidemia, chronic liver disease, or cancer (Table 1). In the CHARLS cohort, participants with social isolation and loneliness were more likely to be female, live in urban regions, have lower socioeconomic status and less physical activity, have obesity, and have comorbidities such as hypertension, heart disease, and stroke (Supplementary Table S8).
Table 1.
Baseline characteristics grouped by social isolation and loneliness in the UKB cohort.
| All (n = 423,503) | Social isolation |
Loneliness |
||||||
|---|---|---|---|---|---|---|---|---|
| Least isolated (n = 193,693) | Moderately isolated (n = 170,641) | Most isolated (n = 59,169) | Pvalue | No loneliness (n = 404,016) | Loneliness (n = 19,487) | Pvalue | ||
| Age, years | 56.3 ± 8.1 | 56.6 ± 8.1 | 56.2 ± 8.1 | 55.8 ± 7.9 | <0.001 | 56.3 ± 8.1 | 55.7 ± 8.0 | <0.001 |
| Male, n (%) | 189,117 (44.7) | 84,575 (43.7) | 75,405 (44.2) | 29,137 (49.2) | <0.001 | 179,461 (44.4) | 9,656 (49.6) | <0.001 |
| Ethnicity, white, n (%) | 406,435 (96.0) | 187,319 (96.7) | 163,380 (95.7) | 55,736 (94.2) | <0.001 | 14,968 (3.7) | 963 (4.9) | <0.001 |
| Assessment center | <0.001 | 0.006 | ||||||
| English, n (%) | 374,869 (88.5) | 170,758 (88.2) | 151,296 (88.7) | 52,815 (89.3) | 357,757 (88.6) | 17,112 (87.8) | ||
| Scotland, n (%) | 30,878 (7.3) | 14,321 (7.4) | 12,353 (7.2) | 4,204 (7.1) | 29,383 (7.3) | 1,495 (7.7) | ||
| Wales, n (%) | 17,756 (4.2) | 8,614 (4.4) | 6,992 (4.1) | 2,150 (3.6) | 16,876 (4.2) | 880 (4.5) | ||
| Household income | <0.001 | <0.001 | ||||||
| Low, n (%) | 78,417 (18.5) | 25,035 (12.9) | 36,237 (21.2) | 17,145 (29.0) | 72,315 (17.9) | 6,102 (31.3) | ||
| Medium, n (%) | 191,139 (45.1) | 90,955 (47) | 75,803 (44.4) | 24,381 (41.2) | 183,097 (45.3) | 8,042 (41.3) | ||
| High, n (%) | 99,623 (23.5) | 51,258 (26.5) | 37,357 (21.9) | 11,008 (18.6) | 96,973 (24) | 2,650 (13.6) | ||
| Unknown, n (%) | 54,324 (12.8) | 26,445 (13.7) | 21,244 (12.4) | 6,635 (11.2) | 51,631 (12.8) | 2,693 (13.8) | ||
| College/university degree, n (%) | 142,166 (33.6) | 65,182 (33.7) | 56,671 (33.2) | 20,313 (34.3) | <0.001 | 137,434 (34) | 4,732 (24.3) | <0.001 |
| Employed, n (%) | 250,564 (59.2) | 110,163 (56.9) | 103,166 (60.5) | 37,235 (62.9) | <0.001 | 239,835 (59.4) | 10,729 (55.1) | <0.001 |
| Current smoker, n (%) | 43,721 (10.3) | 15,365 (7.9) | 18,901 (11.1) | 9,455 (16.0) | <0.001 | 40,218 (10.0) | 3,503 (18.0) | <0.001 |
| Current drinker, n (%) | 387,809 (91.6) | 177,485 (91.6) | 156,150 (91.5) | 54,174 (91.6) | 0.400 | 369,968 (91.6) | 17,841 (91.6) | 0.925 |
| Physical activity (MET) | 10.7 ± 4.8 | 11.2 ± 4.6 | 10.5 ± 4.9 | 9.7 ± 5.0 | <0.001 | 10.7 ± 4.8 | 10.0 ± 5.2 | <0.001 |
| Sun exposure time, hours/day | 2.8 ± 1.9 | 2.8 ± 1.9 | 2.7 ± 1.9 | 2.6 ± 2.0 | <0.001 | 2.8 ± 1.9 | 2.8 ± 2.1 | 0.725 |
| Healthy diet score | 3.0 (2.0, 3.0) | 3.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | <0.001 | 3.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | <0.001 |
| Healthy sleep patterna, n (%) | 246,372 (58.2) | 11,7784 (60.8) | 97,823 (57.3) | 30,765 (52.0) | <0.001 | 238,629 (59.1) | 7,743 (39.7) | <0.001 |
| Family history of T2DM, n (%) | 84,692 (20.0) | 38,160 (19.7) | 34,488 (20.2) | 12,044 (20.4) | <0.001 | 80,457 (19.9) | 4,235 (21.7) | <0.001 |
| Prediabetes, n (%) | 85,864 (20.3) | 37,526 (19.4) | 35,222 (20.6) | 13,116 (22.2) | <0.001 | 81,428 (20.2) | 4,436 (22.8) | <0.001 |
| History of CVD, n (%) | 20,547 (4.9) | 9,023 (4.7) | 8,288 (4.9) | 3,236 (5.5) | <0.001 | 19,022 (4.7) | 1,525 (7.8) | <0.001 |
| History of hypertension, n (%) | 108,671 (25.7) | 48,360 (25) | 44,189 (25.9) | 16,122 (27.2) | <0.001 | 102,784 (25.4) | 5,887 (30.2) | <0.001 |
| History of dyslipidemia, n (%) | 182,085 (43.0) | 83,200 (43.0) | 73,316 (43.0) | 25,569 (43.2) | 0.511 | 173,546 (43.0) | 8,539 (43.8) | 0.017 |
| History of CLDa, n (%) | 6,451 (1.5) | 2,353 (1.2) | 2,778 (1.6) | 1,320 (2.2) | <0.001 | 5,832 (1.4) | 619 (3.2) | <0.001 |
| History of cancer, n (%) | 31,968 (7.6) | 14,557 (7.5) | 13,006 (7.6) | 4,405 (7.5) | 0.283 | 30,552 (7.6) | 1,416 (7.3) | 0.154 |
| Obesitya, n (%) | 93,748 (22.1) | 40,506 (20.9) | 38,849 (22.8) | 14,393 (24.3) | <0.001 | 88,106 (21.8) | 5,642 (29.0) | <0.001 |
| Grip strength, kg | 30.8 ± 11.0 | 30.9 ± 10.9 | 30.7 ± 11.0 | 30.9 ± 11.1 | <0.001 | 30.8 ± 11.0 | 30.3 ± 11.4 | <0.001 |
| SBP, mmHg | 139.4 ± 19.7 | 139.6 ± 19.7 | 139.2 ± 19.6 | 138.9 ± 19.6 | <0.001 | 139.4 ± 19.7 | 138.2 ± 19.2 | <0.001 |
| DBP, mmHg | 82.2 ± 10.7 | 82.1 ± 10.6 | 82.2 ± 10.7 | 82.5 ± 10.9 | <0.001 | 82.2 ± 10.7 | 82.2 ± 11.0 | 0.789 |
| FBG, mmol/L | 4.9 (4.6, 5.2) | 4.9 (4.6, 5.2) | 4.9 (4.6, 5.2) | 4.9 (4.6, 5.2) | <0.001 | 4.9 (4.6, 5.2) | 4.9 (4.6, 5.2) | 0.026 |
| HbA1C, % | 5.3 (5.1, 5.6) | 5.3 (5.1, 5.6) | 5.4 (5.1, 5.6) | 5.4 (5.1, 5.6) | <0.001 | 5.3 (5.1, 5.6) | 5.4 (5.2, 5.6) | <0.001 |
| TC, mmol/L | 5.7 (4.9, 6.4) | 5.6 (4.9, 6.4) | 5.7 (4.9, 6.4) | 5.6 (4.9, 6.4) | 0.134 | 5.6 (4.9, 6.4) | 5.7 (4.9, 6.4) | 0.427 |
| TG, mmol/L | 1.5 (1.0, 2.1) | 1.4 (1.0, 2.1) | 1.5 (1.0, 2.1) | 1.5 (1.1, 2.2) | <0.001 | 1.4 (1.0, 2.1) | 1.6 (1.1, 2.3) | <0.001 |
| LDL, mmol/L | 1.4 (1.2, 1.7) | 1.4 (1.2, 1.7) | 1.4 (1.2, 1.7) | 1.4 (1.2, 1.6) | 0.468 | 1.4 (1.2, 1.7) | 1.3 (1.1, 1.6) | 0.454 |
| HDL, mmol/L | 3.6 (3.0, 4.2) | 3.6 (3.0, 4.1) | 3.6 (3.0, 4.2) | 3.6 (3.0, 4.2) | <0.001 | 3.6 (3.0, 4.1) | 3.6 (3.0, 4.2) | <0.001 |
| Lp (a), nmol/L | 21.0 (9.6, 61.6) | 20.9 (9.6, 61.5) | 21.1 (9.6, 61.9) | 21.0 (9.5, 61.3) | 0.148 | 21.0 (9.6, 61.7) | 21.3 (9.6, 60.6) | 0.705 |
Abbreviations: CVD, cardiovascular disease; CLD, chronic lung disease; DM, diabetes mellitus; DBP, diastolic blood pressure; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Lipoprotein (a), Lp (a); MET, metabolic equivalent of task; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, Triglyceride; UKB, UK biobank.
MET: Range 0–21; positively correlated with weekly physical activity.
Sun exposure time: Range 0–24 h/day.
Healthy diet score: Range 0–5 points; positively linked with the level of adherence to a healthy diet.
Healthy sleep score: Range 0–5 points; positively connected with the degree of adherence to a healthy sleep pattern.
Healthy sleep pattern: the healthy sleep score ≥4; Chronic lung disease: chronic bronchitis, emphysema, chronic obstructive pulmonary disease; Obesity: BMI ≥30 kg/m2.
Social isolation, loneliness, and incident T2DM: results from two prospective cohorts
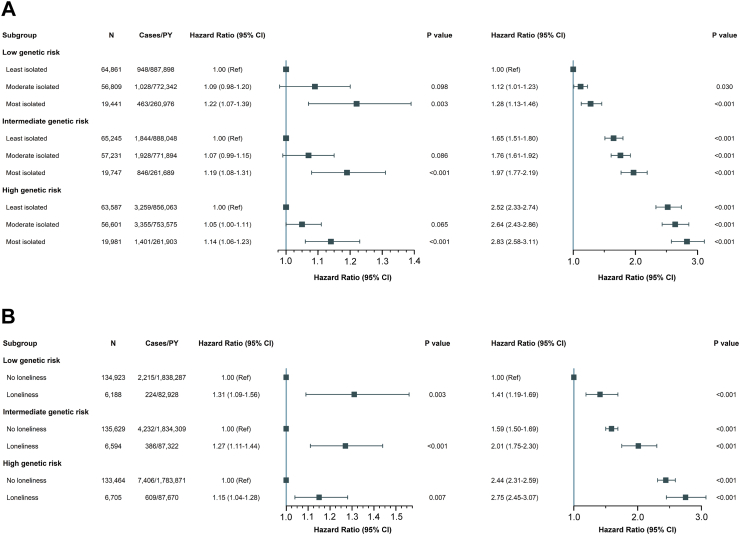
In the UKB cohort, 15,072 study participants were diagnosed with T2DM during a mean follow-up period of 13.5 years. Compared with those without a T2DM diagnosis, those with T2DM exhibited higher levels of social isolation and loneliness, as well as more unhealthy lifestyle factors such as smoking, low levels of physical activity, and unhealthy sleep patterns (Supplementary Table S9). In comparison to participants in the least isolated group, those in the moderately and most isolated groups had a significantly higher risk of T2DM (moderately isolated: HR 1.06, 95% CI 1.02–1.11; most isolated: HR 1.17, 95% CI 1.11–1.23) (Table 2). Similarly, individuals in the loneliness group had a significantly higher risk of T2DM compared with those in the no-loneliness group (HR 1.21, 95% CI 1.13–1.30). With increasing levels of both social isolation and loneliness, participants had a progressively and significantly higher risk of T2DM (P trend < 0.001) (Table 2). Consistent outcomes were obtained from Kaplan–Meier plots in supplementary materials (social isolation: Supplementary Figure S2; Loneliness: Supplementary Figure S3; Joint effects: Supplementary Figure S4). Further, we identified a positive dose–gradient association of social isolation score and loneliness score with T2DM risk (P trend < 0.001), and found that participants with less social contact (HR 1.14, 95% CI 1.07–1.21), living alone (HR 1.08, 95% CI 1.04–1.13), feeling lonely (HR 1.22, 95% CI 1.17–1.27), and being unwilling to confide in others (HR 1.06, 95% CI 1.01–1.11) were at significantly enhanced risk of T2DM (Supplementary Table S10). The loneliness and social isolation scores were positively associated with the risk of T2DM (P trend < 0.001) (Supplementary Table S10). A significant positive gradient of association between social isolation and T2DM risk was found in participants who did not experience loneliness (P trend < 0.001) but not in participants with loneliness (P trend = 0.391), whereas the association of loneliness with increased T2DM risk was significant regardless of social isolation level (P trend < 0.001) (Supplementary Table S11). The significant association between social isolation, loneliness, and increased risk of T2DM was observed across strata of genetic susceptibility for T2DM (social isolation: P value = 0.003 [low], <0.001 [moderate and high]; loneliness: P value = 0.003 [low], <0.001 [moderate], and 0.003 [high]), as depicted in Fig. 1, and these association was not modified by genetic susceptibility (P interaction = 0.420 for social isolation and 0.070 for loneliness).
Table 2.
Separate and joint association of social isolation and loneliness with long-term risk of T2DM in the UKB cohort.
| N | Cases/Person-years | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) | PAF (%) (95% CI [%]) | |
|---|---|---|---|---|---|---|
| Separate effects | ||||||
| Social isolation | 12.61 (10.93–14.28) | |||||
| Least isolated | 193,693 | 6,051/2,632,008 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | |
| Moderate isolated | 170,641 | 6,311/2,297,812 | 1.21 (1.17–1.25) | 1.10 (1.05–1.14) | 1.06 (1.02–1.11) | |
| Most isolated | 59,169 | 2,710/784,568 | 1.50 (1.43–1.56) | 1.22 (1.16–1.29) | 1.17 (1.11–1.23) | |
| Ptrend | <0.001 | <0.001 | <0.001 | |||
| Loneliness | 8.47 (7.64–9.29) | |||||
| No loneliness | 404,016 | 13,853/5,456,468 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | |
| Loneliness | 19,487 | 1,219/257,920 | 1.97 (1.86–2.09) | 1.29 (1.20–1.38) | 1.21 (1.13–1.30) | |
| Joint effects | 14.39 (12.68–16.09) | |||||
| No loneliness | ||||||
| Least isolated | 188,725 | 5,776/2,565,150 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | |
| Moderate isolated | 162,297 | 5,805/2,187,049 | 1.19 (1.15–1.24) | 1.09 (1.05–1.14) | 1.06 (1.02–1.10) | |
| Most isolated | 52,994 | 2,272/704,269 | 1.43 (1.36–1.50) | 1.21 (1.15–1.28) | 1.16 (1.10–1.23) | |
| Loneliness | ||||||
| Least isolated | 4,968 | 275/66,858 | 1.90 (1.68–2.14) | 1.37 (1.19–1.57) | 1.29 (1.12–1.49) | |
| Moderate isolated | 8,344 | 506/110,762 | 2.07 (1.89–2.26) | 1.36 (1.23–1.52) | 1.23 (1.11–1.37) | |
| Most isolated | 6,175 | 438/80,299 | 2.38 (2.16–2.63) | 1.43 (1.28–1.61) | 1.34 (1.19–1.50) | |
| Ptrend | <0.001 | <0.001 | <0.001 |
Abbreviations: CVD, cardiovascular disease; CLD, chronic lung disease; DM, diabetes mellitus; PAF, population attributed fraction; PRS, polygenic risk score; T2DM, type 2 diabetes mellitus; UKB, UK biobank.
Model 1: adjusted for age, sex, and ethnicity.
Model 2: adjusted for age, sex, ethnicity, BMI, grip strength, assessment center, household income, college/university degree, employed, currently smoking, currently drinking, physical activity, sun exposure time, healthy diet score, and healthy sleep score.
Model 3: age, sex, ethnicity, BMI, grip strength, assessment center, household income, college/university degree, employed, currently smoking, currently drinking, physical activity, sun exposure time, healthy diet score, healthy sleep score, family history of T2DM, prediabetes, history of CVD, history of hypertension, history of dyslipidemia, history of CLD,a history of cancer, and PRS for T2DM.
Chronic lung disease: chronic bronchitis, emphysema, and chronic obstructive pulmonary disease.
Fig. 1.
Separate (A) and joint (B) association of social isolation and loneliness with the long-term risk of T2DM among subjects with different levels of PRS for T2DM in the UKB. P interaction of genic risk subgroups for social isolation: 0.420. P interaction of genic risk subgroups for loneliness: 0.070. The analysis was performed in Model 3 (adjusted with age, sex, ethnicity, BMI, grip strength, assessment center, household income, college/university degree, employed, healthy sleep score, current smoking, current drinking, physical activity, sun exposure, healthy diet score, family history of T2DM, prediabetes, history of CVD, history of hypertension, history of dyslipidemia, history of CLD, and history of cancer). Abbreviations: BMI, body mass index; CVD, cardiovascular disease; CLD, chronic lung disease; PRS, polygenic risk score; T2DM, type 2 diabetes mellitus; UKB, UK biobank.
In the CHARLS cohort, 1,247 T2DM cases were documented during the mean follow-up of 5.8 years. Consistent with the UKB cohort, participants with social isolation (HR 1.22, 95% CI 1.06–1.40) and loneliness (HR 1.21, 95% CI 1.07–1.36) had a significantly higher risk of T2DM when compared with those without social isolation/loneliness (Table 3). Similar to the UKB cohort, a progressively increased risk of T2DM was observed with increasing levels of social isolation and loneliness (P trend < 0.001), and no interaction between social isolation and loneliness was observed (P interaction = 0.758) (Table 3).
Table 3.
Separate and joint association of social isolation and loneliness with long-term risk of T2DM in the CHARLS cohort.
| N | Cases/Person-years | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) | PAF (%) (95% CI [%]) | |
|---|---|---|---|---|---|---|
| Separate effects | ||||||
| Social isolation | 3.90 (1.03–6.76) | |||||
| No isolated | 10,816 | 947/63,145 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | |
| Isolated | 2,984 | 302/16,957 | 1.17 (1.02–1.33) | 1.19 (1.04–1.36) | 1.22 (1.06–1.40) | |
| Loneliness | 6.65 (3.17–10.12) | |||||
| No loneliness | 9,733 | 826/56,803 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | |
| Loneliness | 4,067 | 423/23,299 | 1.22 (1.09–1.38) | 1.24 (1.10–1.40) | 1.21 (1.07–1.36) | |
| Pinteraction | 0.610 | 0.627 | 0.758 | |||
| Joint effects | 8.41 (3.99–12.83) | |||||
| No loneliness | ||||||
| No isolated | 8,060 | 674/47,202 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | |
| Isolated | 1,673 | 152/9,601 | 1.09 (0.91–1.31) | 1.12 (0.93–1.34) | 1.17 (0.97–1.40) | |
| Loneliness | ||||||
| No isolated | 2,756 | 273/15,943 | 1.18 (1.02–1.36) | 1.20 (1.04–1.38) | 1.17 (1.02–1.35) | |
| Isolated | 1,311 | 150/7,356 | 1.39 (1.16–1.67) | 1.44 (1.19–1.73) | 1.42 (1.18–1.71) | |
| Ptrend | <0.001 | <0.001 | <0.001 |
Abbreviations: BMI, body mass index; CHARLS, China Health and Retirement Longitudinal Study; DM, diabetes mellitus; PAF, population attributed fraction; T2DM, type 2 diabetes mellitus.
Model 1: adjusted for age, sex, ethnicity.
Model 2: adjusted for age, sex, ethnicity, location, income, educational level, currently employed, currently smoking, drinking frequency, physical activity, and BMI.
Model 3: adjusted for age, sex, ethnicity, location, income, educational level, currently employed, currently smoking, drinking frequency, physical activity, BMI, heart problems, hypertension, hyperlipidemia, stroke, and medication for hypertension and hyperlipidemia.
Social isolation, loneliness, and incident T2DM in individuals with different glucose statuses: results from the UKB cohort
Regardless of participants' glucose status, the association between social isolation, loneliness, and increased risk of T2DM remained statistically significant (social isolation: P value < 0.001 [normal glucose tolerance], = 0.001 [prediabetes]; loneliness: P value < 0.001 [normal glucose tolerance], = 0.005 [prediabetes]) (Supplementary Figure S5A, Supplementary Table S12). Glucose status had significant interactions with the effects of social isolation and loneliness in T2DM risk (P interaction = 0.009 for social isolation and <0.001 for loneliness) (Supplementary Table S12). No interaction was found between social isolation and loneliness for T2DM risk among participants with normal glucose tolerance or prediabetes (Supplementary Tables S13 and S14). Joint analysis for social isolation and loneliness showed a positive gradient association with T2DM risk in both the normal glucose tolerance and prediabetes groups (P trend < 0.001) (Supplementary Figure S5B, Supplementary Table S15). Among participants with normal glucose tolerance, the association of social isolation with increased T2DM risk was significant regardless of genetic risk (P value = 0.032 [low], 0.014 [moderate], and <0.001 [high]), whereas, among participants with prediabetes, the association was significant only in those with low and intermediate genetic risk (P value = 0.016 [low], 0.006 [moderate], and 0.180 [high]) (Supplementary Table S16). Loneliness was significantly associated with enhanced T2DM risk in each group of genetic risk among participants with normal glucose tolerance (P value = 0.002 [low], 0.001 [moderate], and 0.002 [high]), but only in the low genetic risk group among participants with prediabetes (P value = 0.011 [low], 0.162 [moderate], and 0.115 [high]) (Supplementary Table S17).
Subgroups, sensitivity, and mediation analyses: results from the UKB cohort
Subgroup analyses for the separate and joint associations of social isolation and loneliness with T2DM risk are presented in Supplementary Tables S18–S20. No interaction was found between most of the covariates and social isolation or loneliness in the risk of T2DM, except for ethnicity (P interaction = 0.001 for social isolation and 0.014 for loneliness). The results were similar in the models that further adjusted for blood pressure, glucose metabolic, and lipid metabolic biomarkers (Supplementary Tables S21–S23). In the sensitivity analyses excluding participants with a family history of T2DM (Supplementary Tables S24–S26), cancers (Supplemental Tables S27–S29), and those diagnosed with T2DM within the first year (Supplementary Tables S30–S32), the association between loneliness and social isolation and T2DM remained significant. Moreover, in the analysis of the Fine–Gray models (Supplemental Tables S33 and S34), the results were consistent with the main findings. In mediation analyses, we confirmed that BMI, current smoking, low physical activity, and an unhealthy sleep pattern were significant mediators between social isolation, loneliness, and incident T2DM (all P value < 0.001) (Supplemental Table S35).
Social isolation, loneliness, and incident T2DM: results from MR analysis
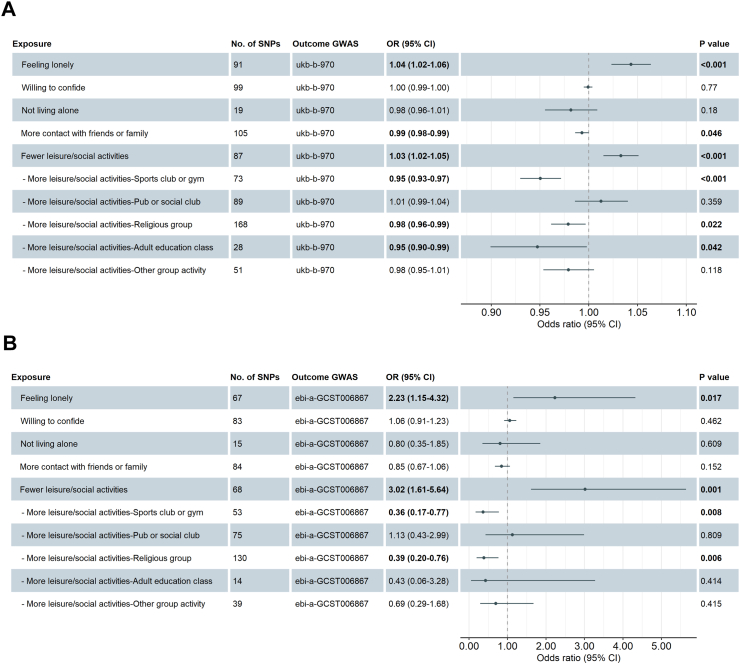
In the two-sample MR analyses based on UKB GWAS for T2DM, feeling lonely (OR 1.04, 95% CI 1.02–1.06, P value < 0.001), and engaging in less leisure/social activities (OR 1.03, 95% CI 1.02–1.05, P value < 0.001) were associated with an increased risk of T2DM, while more contact with friends or family (OR 0.99, 95% CI 0.98–0.99, P value = 0.046) was associated with a reduced risk of T2DM (Fig. 2 A). Furthermore, the analysis identified that participating in more sports clubs or gym (OR 0.95, 95% CI 0.93–0.97, P value < 0.001), religious activities (OR 0.98, 95% CI 0.96–0.99, P value = 0.022), and/or adult education classes (OR 0.95, 95% CI 0.90–0.99, P value = 0.042) were each associated with a reduced risk of T2DM (Fig. 2A). After validating the causal associations above in the EBI GWAS for T2DM, the causal impacts of feeling lonely (OR 2.23, 95% CI 1.15–4.32, P value = 0.017), taking less leisure/social activities (OR 3.02, 95% CI 1.61–5.64, P value = 0.001), more sports club or gym (OR 0.36, 95% CI 0.17–0.77, P value = 0.008) and more religious activities (OR 0.39, 95% CI 0.20–0.76, P value = 0.006) on the risk of T2DM were still robust (Fig. 2B). Scatter plots and LOO plots are presented in Supplementary Figures S6–S25. Estimates from MR analyses with RE-IVW, MR-Egger intercept test for horizontal pleiotropy, and heterogeneity tests with Cochran's Q statistic are presented in Supplementary Table S36 (UKB GWAS) and Supplementary Table S37 (EBI GWAS). In the reverse direction, we found evidence of a causal effect of T2DM on the tendency to participate in less leisure/social activities (pleiotropy-corrected MR-PRESSO OR 1.18, 95% CI 1.024–1.32, P value = 0.009) and a lower tendency to participate in sports club or gym activities (pleiotropy-corrected MR-PRESSO OR 0.85, 95% CI 0.74–0.96, P value = 0.014), suggesting potential bidirectional causality between the traits (Supplementary Table S38).
Fig. 2.
MR analyses for the causal effects of loneliness and social isolation on the risk of T2DM by using IVW method based on GWAS of UKB (A) and EBI (B). Abbreviations: GWAS, genome-wide association studies; EBI, European Bioinformatics Institute; MR, Mendelian randomization; IVW, inverse-variance weighted; T2DM, type 2 diabetes mellitus; UKB, UK biobank.
Discussion
The key findings from this prospective cohort study can be summarized as follows: Loneliness and social isolation were found to be independently and jointly associated with a higher risk of developing long-term T2DM. Two-sample MR analysis provided evidence to support a causal link between social isolation, loneliness, and the incidence of T2DM. Associations between social isolation, loneliness, and increased T2DM risk remained significant regardless of genetic susceptibility to T2DM. The link between loneliness, social isolation, and an elevated risk of T2DM was seen across participants with varying baseline glucose metabolism status. There was no interaction between loneliness and social isolation with respect to T2DM risk.
The association of loneliness and social isolation with the risk of T2DM has been previously explored. In 2020, Hackett et al. conducted a cohort study involving 4,112 participants who were initially free from DM, using data from the English Longitudinal Study of Ageing database. Their study concluded that loneliness, but not social isolation, was associated with an increased risk of developing T2DM.20 Christiansen et al. explored the association between loneliness, social isolation, and T2DM risk in a Danish cohort study involving 24,687 participants and found a significant increase in T2DM risk among individuals experiencing loneliness over a 5-year follow-up period.21 In contrast to Hackett et al.'s study, Christiansen et al. also reported a significant association between social isolation and an elevated risk of T2DM. Recently, Henriksen et al. conducted a cohort study involving 24,024 participants from the Trøndelag Health Study and found that individuals experiencing loneliness had twice the risk of developing T2DM compared with those who did not report feelings of loneliness.22 However, the association between social isolation and T2DM risk was not analyzed. Overall, the research on the association between loneliness and T2DM risk appears to be more consistent than the research on social isolation and T2DM risk. Although existing studies provided novel insights into the association of loneliness and social isolation with risk of T2DM, some limitations remain. For example, previous studies mainly focused on European populations, which may constrain the broader applicability of their findings. Moreover, previous studies mainly used cohort settings, limiting causal inference, and did not consider genetic predisposition, a critical determinant of T2DM incidence.40 Additional concerns, such as inadequate follow-up periods (lacking specific event date) and less accurate diagnostic criteria for T2DM (relying solely on immediate blood glucose biomarkers or self-report questionnaires), may further weaken the solidity of previous conclusions. In the current study, we aimed to address these limitations. We incorporated both European and East Asian populations to improve generalizability of the results. We used two-sample MR analyses to allow for investigation of the causal relationship between loneliness, social isolation, and T2DM risk. Additionally, we incorporated PRS for T2DM, insulin resistance, and beta cell function into our analyses, thus factoring in genetic determinants of glucose metabolism. Furthermore, use of the UKB cohort, with its large sample size (n > 400,000), prolonged follow-up (mean duration of 13.5 years, with specific event dates), and precise T2DM diagnosis (relying on inpatient hospital records and death registers), substantially reinforced the validity of our findings. Beyond these robust methodological enhancements, our study results also exhibit several compelling features. First, our research provides additional evidence to support a link between social isolation and increased T2DM risk. Second, the MR analyses provide novel evidence for a causal relationship between loneliness, social isolation, and the heightened risk of T2DM. Third, the research found significant positive associations between loneliness, social isolation, and T2DM risk regardless of participants’ underlying genetic susceptibility to T2DM. Fourth, the study is the first to demonstrate significant associations between loneliness, social isolation, and risk of T2DM across participants with diverse baseline glucose metabolism statuses. Finally, the study showed that social isolation and loneliness have joint positive effects on T2DM risk, while also confirming the absence of an interaction effect between them in relation to T2DM risk. In summary, our study provides a more nuanced understanding of the association between social isolation, loneliness, and T2DM risk.
Distinct facets of social isolation (reduced social contact and solitary living) and loneliness (emotional loneliness and hesitance to confide) were examined separately. Intriguingly, within the UKB cohort, each of these components—living alone, reduced social contact, emotional loneliness, and reluctance to confide–exhibited a significant connection with heightened T2DM risk. These findings generally align with prior research. In 2009, a study of 8,804 participants in Germany underscored that living alone was an independent predictor of T2DM, particularly among males.41 Similarly, a Swedish cohort study involving 4,963 participants highlighted that individuals engaged in social contact and activities were less prone to T2DM development.42 The significance of feeling lonely in relation to increased T2DM risk was demonstrated in the cohort study by Hackett et al., as described earlier.20 Furthermore, a study involving 139,924 postmenopausal women revealed that social strain, characterized by unease in social interactions and reduced confiding, correlated with elevated T2DM risk.43 Beyond these indications, the current study employed the factors of social isolation and loneliness discussed above to compute the scores for both loneliness and social isolation. The outcomes revealed that as the scores for loneliness and social isolation increased, a corresponding upward trend in T2DM risk was evident. This outcome underscores a direct correlation between the degree of loneliness and social isolation and the susceptibility to T2DM, once more highlighting the potential utility of addressing these factors to mitigate T2DM risk.
To the best of our knowledge, this study is the first to use a two-sample MR analysis to evaluate the causal relationship between loneliness, social isolation, and the risk of T2DM, further reinforcing the strength of these associations. We also explored differential associations of various social activities with T2DM risk in MR analyses. Specifically, engagement in sports clubs or gyms and religious activities was associated with significant risk reductions in T2DM, whereas other social activities did not significantly correlate with the long-term risk of this outcome. This distinction may be attributed to the multifaceted nature of sports clubs or gyms, which involve not only social interactions but also physical activities known to be effective in T2DM prevention. Religious activities have been previously hypothesized to provide preventive and protective roles against the development of T2DM. In a qualitative study in British Bangladeshis, religious leaders highlighted the significant role of religious education in T2DM prevention, emphasizing the T2DM-preventive support from religious teaching regarding dietary and physical activity principles.44 In 2022, a cross-sectional study of participants in Ghana revealed that a higher frequency of engagement in religious activities was linked to reduced HbA1C levels in individuals with T2DM, providing additional evidence for the beneficial impact of religious activities on blood glucose control.45 As suggested by earlier studies carried out in Ghana, involvement in religious activities may have the potential to diminish stress and counteract the influence of stress-triggering factors on health.46,47 Furthermore, consistent engagement in religious activities may foster a sense of hope, which may lead to better-coping strategies to prevent and manage T2DM.48 In addition, it is worth noting that the additional bidirectional MR results suggest that increasing leisure/social activities, such as sports club or gym activities, are inversely related to T2DM risk, and that reducing incident T2DM may lead to additional health benefits by increasing sports/gym activities, such as weight loss.
Loneliness and social isolation were found to have no significant interaction in relation to T2DM risk. Notably, the association between loneliness and increased T2DM risk remained significant regardless of social isolation, whereas the positive link between social isolation and T2DM risk was contingent on the presence of non-loneliness. This outcome highlights the pivotal role of the subjective feeling of social disconnection compared with the objective state of social isolation. Consistent conclusions on the interaction between loneliness and social isolation have been drawn in relation to the risk of heart failure30 and mental disorders.49 This may be attributed to loneliness exerting a stronger adverse psychological impact,50 potentially leading to stress or depression that directly impacts health.51 Conversely, social isolation is an objective condition and may not evoke negative emotions in all individuals. Consequently, prioritizing psychological intervention, beyond mere alleviation of objective social isolation, is critical.
The precise mechanisms underlying the behavioral and emotional alterations caused by social isolation, and loneliness, and their subsequent impact on health outcomes are not yet fully understood.52 A previous study determined that T2DM patients with loneliness had significantly higher levels of monocyte chemoattractant protein-1, indicating a potential association between loneliness and increased inflammation.11 Research has also found that both loneliness and social isolation can trigger the activation of the hypothalamic–pituitary–adrenal axis, enhance sympathetic activity, impair parasympathetic function, and stimulate a pro-inflammatory immune response and oxidative stress.10 These are potential pathways, beyond unfavorable changes in lifestyle behaviors, through which social isolation and loneliness increase the risk of T2DM and metabolic disease.10,53
According to our study, loneliness and social isolation pose significant public health challenges that mental health professionals and clinicians should consider. Unfortunately, there is a lack of evidence-based interventions available to address and improve loneliness and social isolation,54 and preventive measures for T2DM do not currently include strategies for reducing loneliness and social isolation.55 Tackling loneliness requires a collective effort from society as a whole to provide protective interventions and support for individuals who feel lonely and socially isolated. Effective societal strategies include the establishment of community engagement initiatives aimed at fostering social interactions in public domains, such as local events, workshops, and clubs.56 Furthermore, the implementation of elderly care services, including home visits, transportation aid, and senior centers, can provide older individuals with avenues to connect with peers and engage in activities.57 Additionally, the promotion of information and communication technology can be crucial for individuals experiencing social isolation or loneliness.6 This approach has the potential to transcend social and geographical barriers by enabling convenient and affordable communication through various means regardless of time and location, especially among older people with limited mobility.6 As we navigate the post-pandemic recovery, it is crucial to establish a comprehensive response to address these challenges, supported by appropriate national policies.58
Certain limitations in this study need to be declared. First, there were limitations in the assessment of loneliness and social isolation. Specifically, there were differences between the UKB and CHARLS studies in the assessment of these exposures. CHARLS incorporated an evaluation of marital status for assessing social isolation, a component not present in the UKB. Conversely, the UKB evaluated loneliness based on the willingness to confide in others, a criterion not included in CHARLS. Although these assessment methods have been previously used, the differences might have introduced bias. Hence, we recommend that future studies employ consistent assessment methods for loneliness and social isolation across diverse populations. In addition, the exposure assessments did not rely on validated scales. Although these methods have been employed in previous research, the lack of validation introduces the possibility of bias. Second, there were limitations in the assessment of T2DM. The diagnostic methods for assessing T2DM in the UKB and CHARLS cohorts were not consistent, and each method had certain shortcomings. In the UKB cohort, the diagnosis of T2DM was based on in-patient data and the death register. Individuals diagnosed with T2DM in outpatient settings and who underwent outpatient treatment during follow-up might not have been captured, potentially leading to an underestimation of T2DM incidence. Future studies should utilize diagnostic methods that capture data from both outpatient and inpatient systems. In the CHARLS cohort, the diagnosis of DM was made via self-report on questionnaires, without subsequent corroboration from medical records. Thus, the diagnostic accuracy was less than ideal, and the precise type of DM could not be confirmed. Moreover, the DM diagnosis questionnaire in the CHARLS database was administered biennially and did not ask about a specific date of diagnosis during the follow-up. This design allowed for time-to-event data to be presented only in the form of even-numbered years within the follow-up, potentially introducing bias into the analysis. Additionally, the variation in outcome definitions between the two cohorts could introduce potential bias. Third, there was a large discrepancy in sample sizes between the two cohorts. The UKB cohort included more than 400,000 participants, whereas the CHARLS cohort included 13,800 participants. Although associations between loneliness, social isolation, and heightened T2DM risk were seen in both cohorts, the robustness of evidence within the Asian population remains comparatively limited. Future research should include larger-scale Asian cohorts. Fourth, the baseline disparities between the included and excluded participants in the UKB cohort, and not using survey weights in the CHARLS cohort analysis might limit the representation of the population and introduce potential bias. Fifth, the study did not capture changes in social isolation and loneliness during the follow-up period in either cohort, which could lead to underestimates of the observed associations. Sixth, although we controlled for several potential covariates in the analysis, residual confounding may still be present. Finally, the absence of randomized interventions limits the strength of evidence in drawing causal conclusions. Future research should include randomized controlled trials of interventions to mitigate loneliness and social isolation among individuals experiencing or at high risk of these conditions. This approach will allow for more definitive answers regarding the causality of the relationships between loneliness, social isolation, and T2DM.
In conclusion, our findings demonstrate a significant association between social isolation, loneliness, and an increased risk of T2DM. Implementing strategies aimed at mitigating these factors warrants further investigation.
Contributors
Prof. Kefei Dou attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. Yanjun Song and Chen Zhu have directly accessed and verified the underlying data. All the authors were responsible for the decision to submit the manuscript. Concept and design: Kefei Dou and Yanjun Song. Acquisition of data: Yanjun Song (UKB and CHARLS), Chen Zhu (CHARLS), Chenxi Song (UKB), and Zhen'ge Chang (CHARLS). Formal analysis and interpretation of data: Yanjun Song, Chen Zhu, Boqun Shi, Chenxi Song, Kongyong Cui, Zhen'ge Chang, Guofeng Gao, Lei Jia, Rui Fu, Qiuting Dong, Lei Feng, Chenggang Zhu, Dong Yin. Statistical analysis: Yanjun Song and Chen Zhu. Drafting of the manuscript: Yanjun Song, Chen Zhu, and Boqun Shi. Critical revision of the manuscript for important intellectual content: JoAnn E. Manson and Kefei Dou. Obtained funding: Kefei Dou and Chen Zhu. Administrative, technical, or material support: Kefei Dou. Supervision: JoAnn E. Manson and Kefei Dou.
Data sharing statement
Researchers interested in accessing the data used in this study can apply for access to the UK Biobank by visiting their website (https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access) and submitting an application that includes a research protocol summary and requested data fields. Upon approval by the UK Biobank management team and payment of applicable fees, researchers will be granted access to the database. As for the CHARLS resource, it is freely available to the public, and researchers can directly apply for data access on the website (http://charls.pku.edu.cn/). Please note that no additional data beyond what was used in this study are available.
Declaration of interests
We declare no competing interests.
Acknowledgements
This work was supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (Grant No. 2021-I2M-1-008) and the National Natural Science Foundation of China (Grant No. 72103187). The authors thank the participants and staff of the UK Biobank for their dedication and contribution to the research, as well as Shari S. Bassuk (Brigham and Women's Hospital) for careful review and editorial assistance. This research has been conducted using the UK biobank resource under application number 97155. The UK Biobank study obtained ethical approval from the North West Multi-centre Research Ethics Committee (REC reference: 11/NW/03820). Ethical approval for the data collection in CHARLS was granted by the Ethical Review Committee of Peking University (IRB00001052-11015). Written informed consent was obtained from all participants before their enrollment in the study, and the research was conducted in accordance with the principles outlined in the Declaration of Helsinki.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102236.
Appendix A. Supplementary data
References
- 1.Sun H., Saeedi P., Karuranga S., et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183 doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet (London, England) 2016;387(10027):1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan M.A.B., Hashim M.J., King J.K., Govender R.D., Mustafa H., Al Kaabi J. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107–111. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saeedi P., Petersohn I., Salpea P., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157 doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 5.Escalante E., Golden R.L., Mason D.J. Social isolation and loneliness: imperatives for health care in a post-COVID world. JAMA. 2021;325(6):520–521. doi: 10.1001/jama.2021.0100. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y.R., Schulz P.J. The effect of information communication technology interventions on reducing social isolation in the elderly: a systematic review. J Med Internet Res. 2016;18(1):e18. doi: 10.2196/jmir.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cacioppo S., Grippo A.J., London S., Goossens L., Cacioppo J.T. Loneliness: clinical import and interventions. Perspect Psychol Sci. 2015;10(2):238–249. doi: 10.1177/1745691615570616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teo R.H., Cheng W.H., Cheng L.J., Lau Y., Lau S.T. Global prevalence of social isolation among community-dwelling older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. 2023;107 doi: 10.1016/j.archger.2022.104904. [DOI] [PubMed] [Google Scholar]
- 9.Surkalim D.L., Luo M., Eres R., et al. The prevalence of loneliness across 113 countries: systematic review and meta-analysis. BMJ (Clinical research ed) 2022;376 doi: 10.1136/bmj-2021-067068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H., Xia N. The role of oxidative stress in cardiovascular disease caused by social isolation and loneliness. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hackett R.A., Poole L., Hunt E., Panagi L., Steptoe A. Loneliness and biological responses to acute stress in people with Type 2 diabetes. Psychophysiology. 2019;56(6) doi: 10.1111/psyp.13341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Queen N.J., Huang W., Komatineni S., et al. Social isolation exacerbates diet-induced obesity and peripheral inflammation in young male mice under thermoneutrality. iScience. 2023;26(3) doi: 10.1016/j.isci.2023.106259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wootton R.E., Greenstone H.S.R., Abdellaoui A., et al. Bidirectional effects between loneliness, smoking and alcohol use: evidence from a Mendelian randomization study. Addiction. 2021;116(2):400–406. doi: 10.1111/add.15142. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda T., Cable N., Saito M., et al. Association between social isolation and smoking in Japan and England. J Epidemiol. 2021;31(10):523–529. doi: 10.2188/jea.JE20200138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazo G.Z., Fank F., Franco P.S., Capanema B., Pereira F.D.S. Impact of social isolation on physical activity and factors associated with sedentary behavior in older adults during the COVID-19 pandemic. J Aging Phys Act. 2022;30(1):148–152. doi: 10.1123/japa.2020-0456. [DOI] [PubMed] [Google Scholar]
- 16.Matthews T., Danese A., Gregory A.M., Caspi A., Moffitt T.E., Arseneault L. Sleeping with one eye open: loneliness and sleep quality in young adults. Psychol Med. 2017;47(12):2177–2186. doi: 10.1017/S0033291717000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi X., Pei Y., Malone S.K., Wu B. Social isolation, sleep disturbance, and cognitive functioning (hrs): a longitudinal mediation study. J Gerontol A Biol Sci Med Sci. 2023 doi: 10.1093/gerona/glad004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolb H., Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15(1):131. doi: 10.1186/s12916-017-0901-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ley S.H., Ardisson Korat A.V., Sun Q., et al. Contribution of the nurses' health studies to uncovering risk factors for type 2 diabetes: diet, lifestyle, biomarkers, and genetics. Am J Public Health. 2016;106(9):1624–1630. doi: 10.2105/AJPH.2016.303314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hackett R.A., Hudson J.L., Chilcot J. Loneliness and type 2 diabetes incidence: findings from the English Longitudinal Study of Ageing. Diabetologia. 2020;63(11):2329–2338. doi: 10.1007/s00125-020-05258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christiansen J., Lund R., Qualter P., Andersen C.M., Pedersen S.S., Lasgaard M. Loneliness, social isolation, and chronic disease outcomes. Ann Behav Med. 2021;55(3):203–215. doi: 10.1093/abm/kaaa044. [DOI] [PubMed] [Google Scholar]
- 22.Henriksen R.E., Nilsen R.M., Strandberg R.B. Loneliness increases the risk of type 2 diabetes: a 20 year follow-up - results from the HUNT study. Diabetologia. 2023;66(1):82–92. doi: 10.1007/s00125-022-05791-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laursen K.R., Hulman A., Witte D.R., Terkildsen Maindal H. Social relations, depressive symptoms, and incident type 2 diabetes mellitus: the English Longitudinal Study of Ageing. Diabetes Res Clin Pract. 2017;126:86–94. doi: 10.1016/j.diabres.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Altevers J., Lukaschek K., Baumert J., et al. Poor structural social support is associated with an increased risk of Type 2 diabetes mellitus: findings from the MONICA/KORA Augsburg cohort study. Diabet Med. 2016;33(1):47–54. doi: 10.1111/dme.12951. [DOI] [PubMed] [Google Scholar]
- 25.Sudlow C., Gallacher J., Allen N., et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3) doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y., Hu Y., Smith J.P., Strauss J., Yang G. Cohort profile: the China health and retirement longitudinal study (CHARLS) Int J Epidemiol. 2014;43(1):61–68. doi: 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin L., Cao B., Chen W., Li J., Zhang Y., Guo V.Y. Association of adverse childhood experiences and social isolation with later-life cognitive function among adults in China. JAMA Netw Open. 2022;5(11) doi: 10.1001/jamanetworkopen.2022.41714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q., Zhang S., Wang Y., Zhao D., Zhou C. Dual sensory impairment as a predictor of loneliness and isolation in older adults: national cohort study. JMIR Public Health Surveill. 2022;8(11) doi: 10.2196/39314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo L., An L., Luo F., Yu B. Social isolation, loneliness and functional disability in Chinese older women and men: a longitudinal study. Age Ageing. 2021;50(4):1222–1228. doi: 10.1093/ageing/afaa271. [DOI] [PubMed] [Google Scholar]
- 30.Liang Y.Y., Chen Y., Feng H., et al. Association of social isolation and loneliness with incident heart failure in a population-based cohort study. JACC Heart Fail. 2023;11(3):334–344. doi: 10.1016/j.jchf.2022.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hakulinen C., Pulkki-Råback L., Virtanen M., Jokela M., Kivimäki M., Elovainio M. Social isolation and loneliness as risk factors for myocardial infarction, stroke and mortality: UK Biobank cohort study of 479 054 men and women. Heart. 2018;104(18):1536–1542. doi: 10.1136/heartjnl-2017-312663. [DOI] [PubMed] [Google Scholar]
- 32.Elovainio M., Komulainen K., Sipilä P.N., et al. Association of social isolation and loneliness with risk of incident hospital-treated infections: an analysis of data from the UK Biobank and Finnish Health and Social Support studies. Lancet Public Health. 2023;8(2):e109–e118. doi: 10.1016/S2468-2667(22)00253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen G.C., Arthur R., Qin L.Q., et al. Association of oily and nonoily fish consumption and fish oil supplements with incident type 2 diabetes: a large population-based prospective study. Diabetes Care. 2021;44(3):672–680. doi: 10.2337/dc20-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X., Zhou T., Ma H., et al. Healthy sleep patterns and risk of incident arrhythmias. J Am Coll Cardiol. 2021;78(12):1197–1207. doi: 10.1016/j.jacc.2021.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ElSayed N.A., Aleppo G., Aroda V.R., et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S19–s40. [Google Scholar]
- 36.Thompson D.J., Wells D., Selzam S., et al. UK Biobank release and systematic evaluation of optimised polygenic risk scores for 53 diseases and quantitative traits. medRXiv. 2022 doi: 10.1101/2022.06.16.22276246. [DOI] [Google Scholar]
- 37.Bowden J., Davey Smith G., Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgess S., Thompson S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–389. doi: 10.1007/s10654-017-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greco M.F., Minelli C., Sheehan N.A., Thompson J.R. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34(21):2926–2940. doi: 10.1002/sim.6522. [DOI] [PubMed] [Google Scholar]
- 40.Grarup N., Sandholt C.H., Hansen T., Pedersen O. Genetic susceptibility to type 2 diabetes and obesity: from genome-wide association studies to rare variants and beyond. Diabetologia. 2014;57(8):1528–1541. doi: 10.1007/s00125-014-3270-4. [DOI] [PubMed] [Google Scholar]
- 41.Meisinger C., Kandler U., Ladwig K.H. Living alone is associated with an increased risk of type 2 diabetes mellitus in men but not women from the general population: the MONICA/KORA Augsburg Cohort Study. Psychosom Med. 2009;71(7):784–788. doi: 10.1097/PSY.0b013e3181ae5770. [DOI] [PubMed] [Google Scholar]
- 42.Hilding A., Shen C., Östenson C.G. Social network and development of prediabetes and type 2 diabetes in middle-aged Swedish women and men. Diabetes Res Clin Pract. 2015;107(1):166–177. doi: 10.1016/j.diabres.2014.09.057. [DOI] [PubMed] [Google Scholar]
- 43.Hendryx M., Nicholson W., Manson J.E., et al. Social relationships and risk of type 2 diabetes among postmenopausal women. J Gerontol B Psychol Sci Soc Sci. 2020;75(7):1597–1608. doi: 10.1093/geronb/gbz047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grace C., Begum R., Subhani S., Kopelman P., Greenhalgh T. Prevention of type 2 diabetes in British Bangladeshis: qualitative study of community, religious, and professional perspectives. BMJ (Clinical research ed) 2008;337:a1931. doi: 10.1136/bmj.a1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Botchway M., Davis R.E., Appiah L.T., Moore S., Merchant A.T. The influence of religious participation and use of traditional medicine on type 2 diabetes control in urban Ghana. J Relig Health. 2022;61(3):1966–1979. doi: 10.1007/s10943-021-01187-9. [DOI] [PubMed] [Google Scholar]
- 46.Aikins A.D. Living with diabetes in rural and urban Ghana: a critical social psychological examination of illness action and scope for intervention. J Health Psychol. 2003;8(5):557–572. doi: 10.1177/13591053030085007. [DOI] [PubMed] [Google Scholar]
- 47.de-Graft Aikins A. Healer shopping in Africa: new evidence from rural-urban qualitative study of Ghanaian diabetes experiences. BMJ. 2005;331(7519):737. doi: 10.1136/bmj.331.7519.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Unantenne N., Warren N., Canaway R., Manderson L. The strength to cope: spirituality and faith in chronic disease. J Relig Health. 2013;52(4):1147–1161. doi: 10.1007/s10943-011-9554-9. [DOI] [PubMed] [Google Scholar]
- 49.Santini Z.I., Jose P.E., York Cornwell E., et al. Social disconnectedness, perceived isolation, and symptoms of depression and anxiety among older Americans (NSHAP): a longitudinal mediation analysis. Lancet Public Health. 2020;5(1):e62–e70. doi: 10.1016/S2468-2667(19)30230-0. [DOI] [PubMed] [Google Scholar]
- 50.Levine G.N., Cohen B.E., Commodore-Mensah Y., et al. Psychological health, well-being, and the mind-heart-body connection: a scientific statement from the American heart association. Circulation. 2021;143(10):e763–e783. doi: 10.1161/CIR.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 51.Hackett R.A., Steptoe A. Type 2 diabetes mellitus and psychological stress - a modifiable risk factor. Nat Rev Endocrinol. 2017;13(9):547–560. doi: 10.1038/nrendo.2017.64. [DOI] [PubMed] [Google Scholar]
- 52.Xiong Y., Hong H., Liu C., Zhang Y.Q. Social isolation and the brain: effects and mechanisms. Mol Psychiatry. 2023;28(1):191–201. doi: 10.1038/s41380-022-01835-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halim M., Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes) Diabetes Metabol Syndr. 2019;13(2):1165–1172. doi: 10.1016/j.dsx.2019.01.040. [DOI] [PubMed] [Google Scholar]
- 54.Hoang P., King J.A., Moore S., et al. Interventions associated with reduced loneliness and social isolation in older adults: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(10) doi: 10.1001/jamanetworkopen.2022.36676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.ElSayed N.A., Aleppo G., Aroda V.R., et al. 3. Prevention or delay of type 2 diabetes and associated comorbidities: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S41–S48. doi: 10.2337/dc23-S003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Erku D., Khatri R., Endalamaw A., et al. Community engagement initiatives in primary health care to achieve universal health coverage: a realist synthesis of scoping review. PLoS One. 2023;18(5) doi: 10.1371/journal.pone.0285222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo J., Ling W. The relationship between the mental health status and social support of the lonely elderly with government participation in the Internet context. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.1013069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Haboubi Y., Oladimeji K. Awareness, loneliness, and demand for mental health services in the NHS. BMJ (Clinical research ed) 2022;377:o1178. doi: 10.1136/bmj.o1178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.