Abstract
Background
Postoperative depression is not well characterised. We investigated the incidence of postoperative depression with the hypothesis that after controlling for confounders, new onset depression would vary significantly by surgical type.
Methods
We conducted a retrospective cohort study using the Optum Clinformatics Datamart. The primary outcome was new onset postoperative depression, defined by a new diagnosis of depression or new prescription for an antidepressant in the year after surgery using International Classification of Diseases (ICD) 9/10 codes and drug names. Adjustment for preoperative comorbidities and predictors of depression was with multivariable Cox regression and propensity score matching. Sensitivity analyses defining new onset depression as both a new diagnosis of depression and a new prescription for an antidepressant, or either outcome separately, were conducted.
Results
Data from 132 390 cardiac surgery, 12 538 thoracotomy, 32 630 video-assisted thoracoscopic surgery (VATS), 96 750 hip fracture surgery, 157 484 hip replacement, and 347 878 laparoscopic cholecystectomy patients from January 2004 to June 2021 were analysed. The incidence of new onset postoperative depression was 18.8% for hip fracture surgery, 16.1% for thoracotomy, 12.6% for cardiac surgery, 12.4% for VATS, 8.6% for laparoscopic cholecystectomy, and 6.8% for hip replacement. After multivariable adjustment, hip fracture surgery patients were most likely to develop new onset postoperative depression (hazard ratio [95% confidence interval]) 1.56 [1.45–1.68]), followed by thoracotomy (1.12 [1.03–1.22]), cardiac surgery (1.09 [1.04–1.12]), VATS (0.95 [0.90–1.00]), and hip replacement (0.55 [0.52–0.57]) compared with patients undergoing laparoscopic cholecystectomy (hazard ratio=1). Results from propensity score matched analyses and sensitivity analyses were similar.
Conclusions
The risk of postoperative depression differs by surgical type after controlling for preoperative characteristics.
Keywords: major surgery, national database, postoperative depression, propensity matching
Depression is the largest contributor to global disability, with an estimated 322 million people affected worldwide.1 The association between a new medical diagnosis and the risk of subsequent depression is well documented, leading to the prioritisation of early detection and intervention for patients with depression.2, 3, 4, 5, 6, 7, 8 However, the risk of depression after surgery is not as well characterised. Depression may develop after a new diagnosis for which treatment with surgery is recommended, but surgery and postoperative recovery can also be associated with physiological, cognitive, and emotional stressors which could potentially contribute to an increased risk of subsequent depression.6,9, 10, 11, 12
The incidence of postoperative depression and its association with adverse outcomes has been previously described in small cohort studies of patients undergoing cardiac, thoracic, or hip fracture surgery.9,10,13,14 These studies have been limited in their ability to identify new onset postoperative depression, either by not excluding patients with preoperative depression or by not controlling for baseline predictors of depression. Some of these predictors may confound the relationship between surgically treatable disease and depression, such as smoking.15, 16, 17, 18 Without controlling for these potential confounders, it is difficult to ascertain how the exposure to surgery and the postoperative period may influence the risk of subsequent depression. Furthermore, relying on data from small, single-centre and disease-specific cohorts makes it challenging to generalise these results to the larger population or to make comparisons of risk between surgical types. Given the substantial impact that depression has on postoperative recovery and future disability, investigation into the risk of postoperative depression after major surgery could lead to valuable insights for patients and providers.
To address these gaps in knowledge, we used healthcare utilisation data to investigate the incidence of postoperative depression after common major surgical procedures and to explore how the risk of postoperative depression may vary amongst them after controlling for confounders. Our hypothesis was that significant differences in risk would persist between the types of surgery after adjusting for preoperative variables.
Methods
We conducted a population-based cohort study using a national United States commercial insurance claims database (Optum© Clinformatics® Data Mart, Eden Prarie Minnesota, USA.), containing patient-level information on personal and physical characteristics, medical diagnoses, clinical procedures, and prescriptions for 81 796 156 patients with commercial health insurance or Medicare Advantage from January 2004 to June 2021. Regulatory approval was granted by the Institutional Review Board at Brigham and Women's Hospital (2022P001307, 6 September 2022, principal investigator: BOG). The types of surgery were chosen after a literature search identified previously published cohort studies describing postoperative depression (cardiac surgery, thoracic surgery, and hip fracture surgery), to explore the risks for procedures with similar anatomical or procedural characteristics (thoracoscopic surgery, hip replacement) and an active control group (laparoscopic cholecystectomy).9,10,13,14 Our methods are described according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist.19
Population
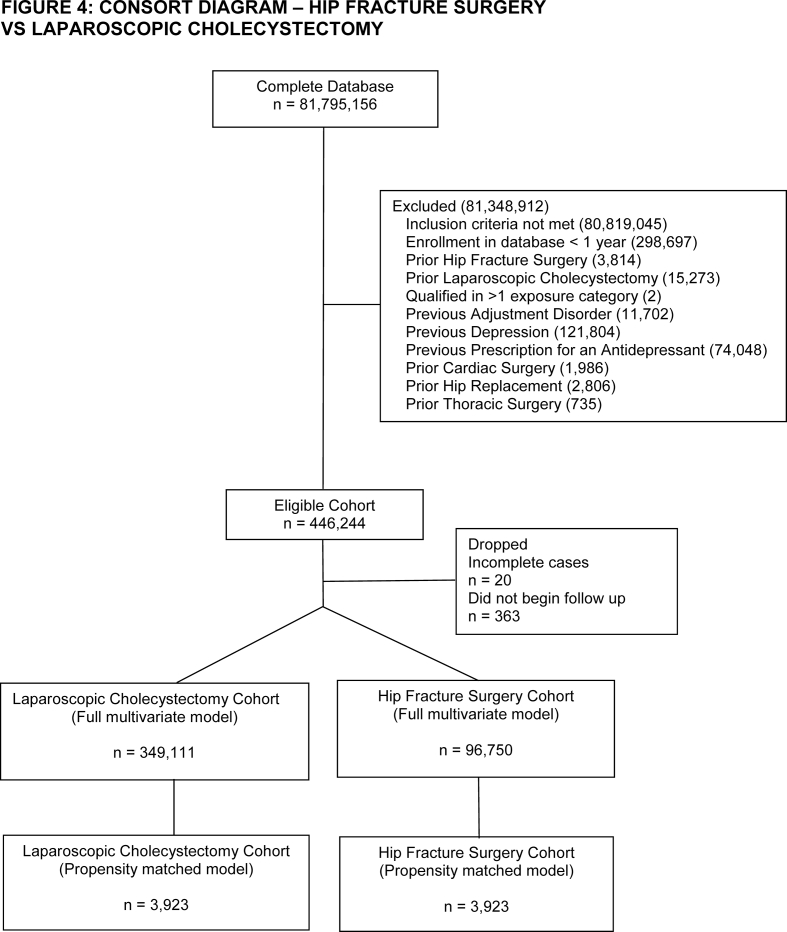
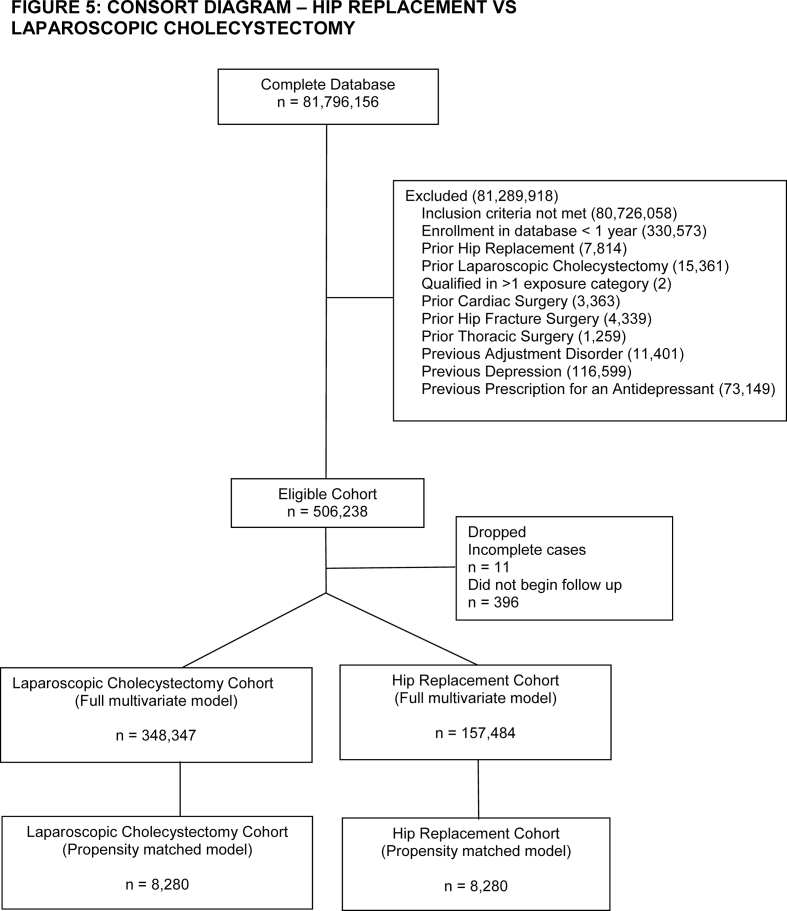
Patients were eligible for inclusion if they underwent cardiac, thoracic (thoracotomy or video-assisted thoracoscopic surgery [VATS]), hip (fracture surgery or replacement), or laparoscopic cholecystectomy surgery between 2004 and 2021, defined by a claim containing a Current Procedural Terminology (CPT) code for these procedures (Supplementary material: EMethods A). The cohort entry date was defined as date of surgery. Patients were required to be enrolled in the database on the day of surgery and for the preceding 365 days. Analyses were conducted in pairs to facilitate adjustment with propensity score matching, with laparoscopic cholecystectomy assigned as the reference group for each comparison (e.g. cardiac surgery vs laparoscopic cholecystectomy, thoracic surgery vs laparoscopic cholecystectomy, etc.). Laparoscopic cholecystectomy was chosen a priori as the reference group for all analyses as it is one of the most commonly performed surgical procedures in the USA in patients with a broad range of pre-existing comorbidities and has been previously linked to an increased risk of subsequent depression as opposed to medical management.20,21
Within each comparison, patients exposed to any of the other surgery types during either the baseline or follow-up period were excluded. For example, a patient who underwent cardiac surgery but then had a hip replacement within 1 yr would be excluded from the cardiac surgery cohort. If a patient qualified for the cohort multiple times, the patient entered the cohort after the first surgery for which the exclusion criteria could be applied. Other exclusion criteria included prior diagnosis of depression, prior diagnosis of adjustment disorder, or previously filled antidepressant prescription in the year before surgery. The follow-up period was 365 days after surgery. Death or disenrollment during follow-up were treated as censoring events. Patients who did not begin follow-up or who had missing covariate data aside from race were excluded.22
Outcomes
Our primary outcome was new onset postoperative depression, defined by either a claim containing a new International Classification of Diseases (ICD) 9/10 code for depression or a new prescription for an antidepressant in the 365 days after surgery (Supplementary material: EMethods B).23 As antidepressants can be prescribed for conditions other than depression, we performed sensitivity analyses with the following alternate definitions of new onset postoperative depression: (a) the overlapping presence of both a new diagnosis code for depression and a new prescription for an antidepressant, (b) new diagnosis of depression only, and (c) new prescription for an antidepressant only.
Covariate assessment
In the 365 days preceding surgery, we collected data on covariates relevant to baseline characteristics (age, sex, race), comorbidities, and known predictors of depression.4,24,25 Comorbidities were selected a priori based on clinical knowledge with an emphasis on conditions which could predispose a patient to requiring the types of major surgery being investigated (cardiac surgery: angina, atrial fibrillation, congestive heart failure, coronary heart disease, history of percutaneous coronary intervention, valvular disease; thoracic surgery: lung cancer, chronic obstructive pulmonary disease, metastatic cancer; hip surgery: arthritis, hip fracture; cholecystectomy: cholelithiasis, cholecystitis; and chronic conditions linked to depression: obesity, diabetes, hypertension, hyperlipidaemia, chronic kidney disease, alcohol abuse, smoking, opioid use). In addition, the sum Charlson Comorbidity Index Score over the preceding 365 days before surgery was included. These covariates were then used to adjust for confounders and to generate the propensity score used for matching in our analyses. The complete set of variables can be found in Table 1, and definitions for those variables can be found in the Supplementary material (EMethods C).
Table 1.
Baseline characteristics by surgery type. Values are reported as n (%) or mean (standard deviation) depending on variable type. ∗Values are reported from the cohort resulting from the comparison between laparoscopic cholecystectomy and cardiac surgery. †Sum of scores over the preceding 365 days. VATS, video-assisted thoracoscopic surgery.
| Cardiac surgery | Thoracotomy | VATS | Hip fracture surgery | Hip replacement | Laparoscopic cholecystectomy∗ | |
|---|---|---|---|---|---|---|
| N | 132 390 | 12 538 | 32 630 | 96 750 | 157 484 | 347 878 |
| Age | 67.18 (0-90) | 65.44 (0-90) | 62.19 (0-90) | 79.73 (1-90) | 67.19 (8-90) | 52.88 (0-90) |
| Sex, n (%) | ||||||
| Male | 98 535 (74.4) | 7728 (61.6) | 18 687 (57.3) | 31 890 (33.0) | 77 995 (49.5) | 124 769 (35.9) |
| Female | 33 829 (25.6) | 4808 (38.3) | 13 927 (42.7) | 64 852 (67.0) | 79 476 (50.5) | 223 077 (64.1) |
| Race, n (%) | ||||||
| White | 98 505 (74.4) | 9271 (73.9) | 23 343 (71.5) | 74 384 (76.9) | 127 452 (80.9) | 234 524 (67.4) |
| Asian | 3661 (2.8) | 281 (2.2) | 1322 (4.1) | 2221 (2.3) | 2024 (1.3) | 9248 (2.7) |
| Black | 11 330 (8.6) | 1251 (10.0) | 3229 (9.9) | 7270 (7.5) | 12 492 (7.9) | 31 069 (8.9) |
| Hispanic | 11 189 (8.5) | 865 (6.9) | 2550 (7.8) | 7301 (7.5) | 7568 (4.8) | 50 838 (14.6) |
| Comorbidities, n (%) | ||||||
| Charlson Comorbidity Index Score† | 297.81 (498.23) | 480.65 (679.22) | 402.40 (734.27) | 312.54 (567.04) | 147.78 (357.09) | 153.30 (368.73) |
| Angina | 41 981 (31.7) | 582 (4.6) | 874 (2.7) | 2507 (2.6) | 2198 (1.4) | 7533 (2.2) |
| Atrial fibrillation | 27 182 (20.5) | 1804 (14.4) | 2694 (8.3) | 11 849 (12.2) | 6633 (4.2) | 10 864 (3.1) |
| Congestive heart failure | 14 406 (10.9) | 451 (3.6) | 1095 (3.4) | 5279 (5.5) | 1707 (1.1) | 4069 (1.2) |
| Coronary heart disease | 42 151 (31.8) | 111 (0.9) | 158 (0.5) | 421 (0.4) | 230 (0.1) | 919 (0.3) |
| Myocardial infarction | 23 316 (17.6) | 321 (2.6) | 479 (1.5) | 2239 (2.3) | 855 (0.5) | 2480 (0.7) |
| Percutaneous coronary intervention | 103 881 (78.5) | 143 (1.1) | 181 (0.6) | 328 (0.3) | 353 (0.2) | 1106 (0.3) |
| Valvular disease | 33 034 (25.0) | 499 (4.0) | 987 (3.0) | 3431 (3.5) | 2538 (1.6) | 4355 (1.3) |
| Alzheimer's disease | 258 (0.2) | 35 (0.3) | 77 (0.2) | 4416 (4.6) | 288 (0.2) | 787 (0.2) |
| Parkinson's disease | 245 (0.2) | 16 (0.1) | 91 (0.3) | 1657 (1.7) | 424 (0.3) | 504 (0.1) |
| Cognitive disorders | 2521 (1.9) | 235 (1.9) | 719 (2.2) | 23 728 (24.5) | 2230 (1.4) | 4827 (1.4) |
| Chronic obstructive pulmonary disease | 38 090 (28.8) | 8244 (65.8) | 18 467 (56.6) | 28 303 (29.3) | 28 326 (18.0) | 59 677 (17.2) |
| Lung cancer | 692 (0.5) | 8453 (67.4) | 12 870 (39.4) | 2010 (2.1) | 727 (0.5) | 1339 (0.4) |
| Metastatic cancer | 1110 (0.8) | 3389 (27.0) | 8679 (26.6) | 3408 (3.5) | 1556 (1.0) | 3470 (1.0) |
| Rheumatoid arthritis | 2944 (2.2) | 460 (3.7) | 1207 (3.7) | 3694 (3.8) | 6977 (4.4) | 7042 (2.0) |
| Osteoarthritis | 25 861 (19.5) | 2384 (19.0) | 6353 (19.5) | 32 309 (33.4) | 153 351 (97.4) | 50 203 (14.4) |
| Hip fracture | 213 (0.2) | 39 (0.3) | 83 (0.3) | 92 005 (95.1) | 8182 (5.2) | 485 (0.1) |
| Cholelithiasis | 3020 (2.3) | 497 (4.0) | 1380 (4.2) | 2238 (2.3) | 1641 (1.0) | 294 537 (84.7) |
| Cholecystitis | 510 (0.4) | 76 (0.6) | 147 (0.5) | 279 (0.3) | 143 (0.1) | 214 213 (61.6) |
| Obesity | 15 524 (11.5) | 1146 (9.1) | 2020 (6.2) | 2332 (2.4) | 15 621 (9.9) | 45 094 (13.0) |
| Diabetes | 32 823 (24.8) | 2293 (18.3) | 4001 (12.3) | 13 364 (13.8) | 14 651 (9.3) | 40 108 (11.5) |
| Hypertension | 71 094 (53.7) | 6179 (49.3) | 10 535 (32.3) | 40 189 (41.5) | 53 901 (34.2) | 104 160 (29.9) |
| Hyperlipidaemia | 65 760 (49.7) | 5274 (42.1) | 9069 (27.8) | 26 428 (27.3) | 47 800 (30.4) | 92 910 (26.7) |
| Chronic kidney disease | 25 775 (19.5) | 2570 (20.5) | 4568 (14.0) | 16 747 (17.3) | 11 237 (7.1) | 33 340 (9.6) |
| Alcohol abuse | 1586 (1.2) | 285 (2.3) | 428 (1.3) | 1484 (1.5) | 1236 (0.8) | 2496 (0.7) |
| Smoking | 25 883 (19.6) | 5177 (41.3) | 7800 (23.9) | 8732 (9.0) | 17 134 (10.9) | 40 452 (11.6) |
| Opioid use | 62 579 (47.3) | 7600 (60.6) | 17 410 (53.4) | 33 095 (34.2) | 91 061 (57.8) | 268 702 (77.2) |
Statistical analysis
Cox proportional hazards regression was used to analyse potential differences in outcomes between groups. For unadjusted analyses, the outcomes were included in the model as dependent variables without additional covariates. We adjusted for confounders using both multivariable regression and propensity score matching.26 Analyses were performed in pairs to facilitate propensity score matching with laparoscopic cholecystectomy serving as the reference group for all comparisons. All models contained the complete list of preoperative covariates. Propensity scores were estimated using logistic regression. Surgery type was specified as the dependent variable. All covariates were entered as independent variables without further variable selection. Patients with missing covariate data, with the exception of race, were excluded from propensity score analysis. Patients' propensity score values were predicted using the resulting regression model. Propensity score matching was then performed using 1:1 nearest neighbour matching with a maximum matching calliper of 1%. In the matched propensity score analyses, multivariate adjustment was achieved through the matching process. After matching, treatment effect measures were directly derived from the balanced populations without any further adjustment. Inspection of pre- and post-matching C-statistics and absolute standardised differences between groups were used as metrics for confounder balance. A preliminary feasibility analysis with the cardiac surgery vs laparoscopic cholecystectomy comparison estimated an event rate of 11.2%. With this event rate and a sample exceeding 100 000 patients, it was possible to include the complete set of covariates without over-specifying the models. A power calculation using an estimate of the event rate in the laparoscopic cholecystectomy group of 6% was performed and revealed that the model would have a power of 1.0 to detect a relative risk difference of 5%. Hazard ratios and 95% confidence intervals are reported for all analyses. No adjustments were made for multiple comparisons. All analyses were conducted with the Aetion Evidence Generation Platform, New York, New York, USA, version r3.16. Statistical computations were conducted using R version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
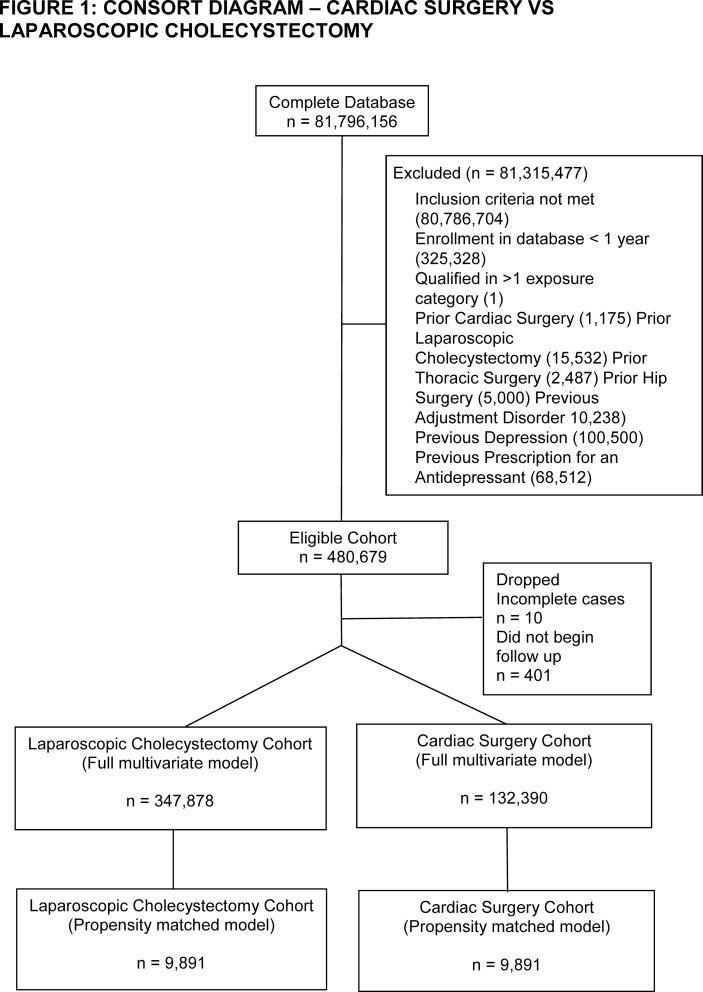
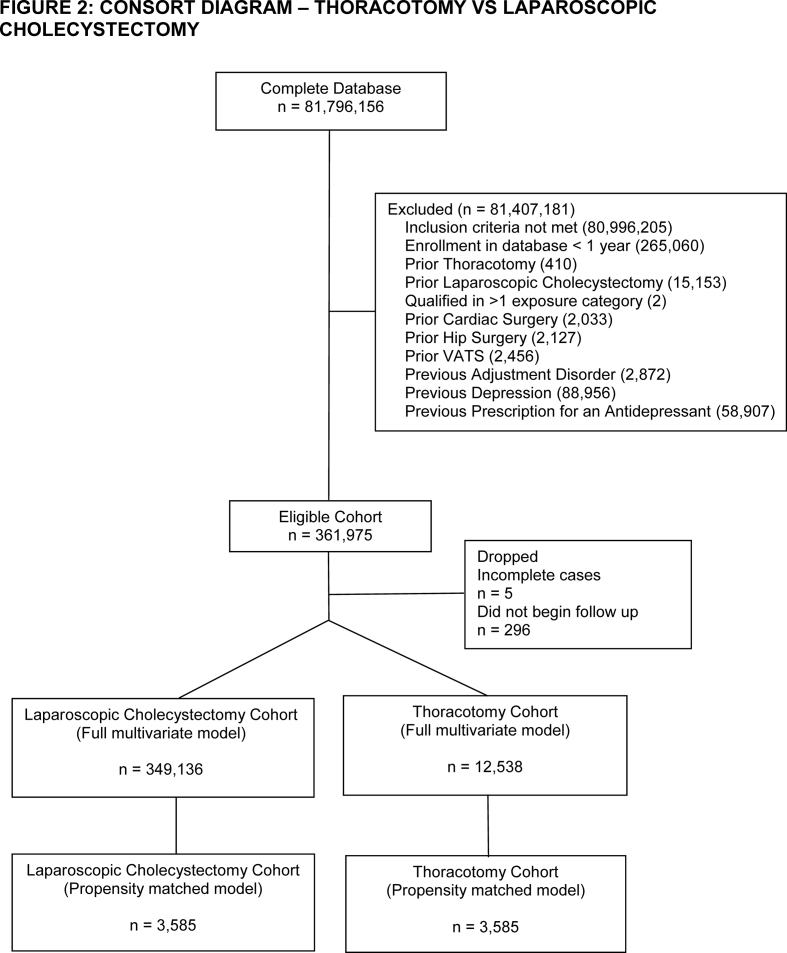
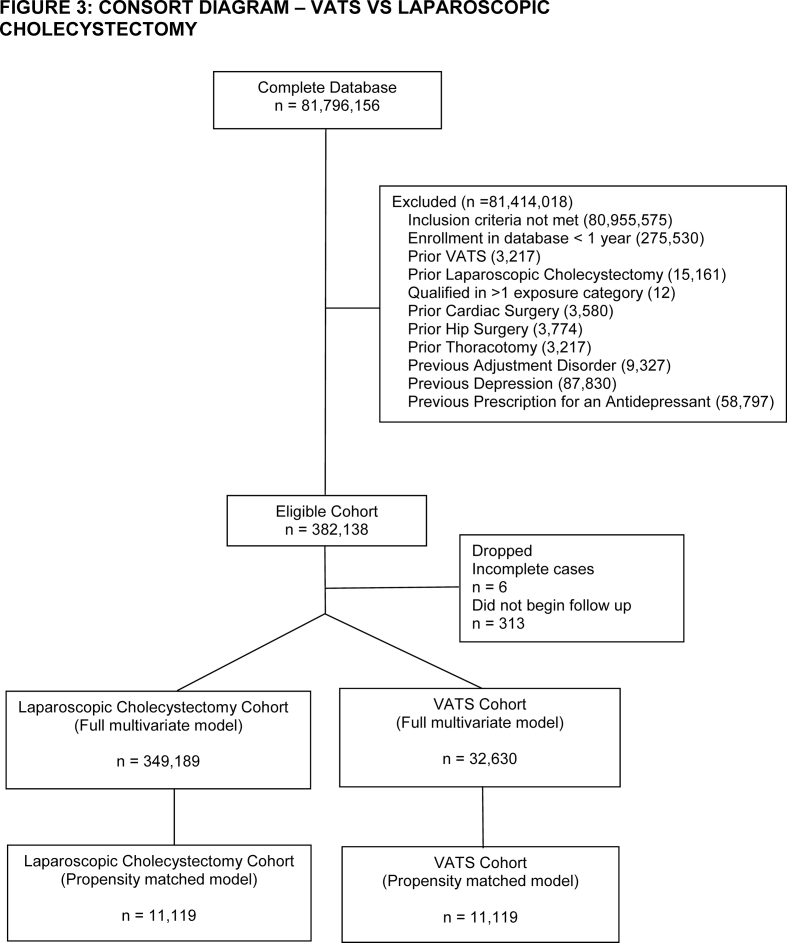
After inclusion and exclusion criteria were applied, we identified 132 390 cardiac surgery, 12 538 thoracotomy, 32 630 VATS, 96 750 hip fracture, and 157 484 hip replacement patients in the database. The number of laparoscopic cholecystectomy patients ranged between 347 878 and 349 136 depending on how many patients had the comparator surgery (Supplementary Figs S1–S5). Patients in the cardiac surgery group had a high incidence of coronary heart disease, valvular disease, and congestive heart failure (Table 1). Patients undergoing thoracic surgery had high rates of lung cancer. Hip fracture patients were older with a high incidence of cognitive disorders. Hip replacement patients had a high incidence of osteoarthritis. Laparoscopic cholecystectomy patients had high rates of obesity and were predominantly female. After matching, there were sizeable reductions in absolute standardised differences across all of the covariates analysed (Supplementary Table S1). The only variable that remained with a standardised difference >0.1 after matching was age in both the cardiac surgery–laparoscopic cholecystectomy (mean [standard deviation] 64.25 [14.41] vs 66.25 [14.15] yr, P<0.01) and thoracotomy–laparoscopic cholecystectomy comparisons (59.45 [18.06] vs 61.46 [17.4] yr, P<0.01). As these differences were unlikely to be clinically relevant and also as age was not found in subsequent analyses to be a predictor of postoperative depression in these cohorts (Supplementary Figs S6 and S7), we decided to not further adjust for the residual differences in age in our propensity matched analysis. Information on the frequency of censoring because of either death or disenrollment is available in Supplementary Table S2.
Primary outcome
The unadjusted incidences of new onset postoperative depression defined as a new diagnosis of depression or prescription for an antidepressant within 1 yr after surgery were 12.6% (16 747/132 390) for cardiac surgery, 16.1% (2018/12 538) for thoracotomy, 12.4% (4046/32 630) for VATS, 18.8% (18 231/96 750) for hip fracture surgery, and 6.8% (10 772/157 484) for hip replacement. The unadjusted incidence of new onset postoperative depression after laparoscopic cholecystectomy across all comparisons was between 8.5 and 8.6%. The mean (standard deviation) time until a new claim for depression or antidepressant prescription was 99 (96) days for cardiac surgery patients, 108 (96) days for thoracotomy patients, 117 (102) days for VATS patients, 90 (92) days for hip fracture surgery patients, 132 (109) days for hip replacement patients, and 153 (104) days for laparoscopic cholecystectomy patients.
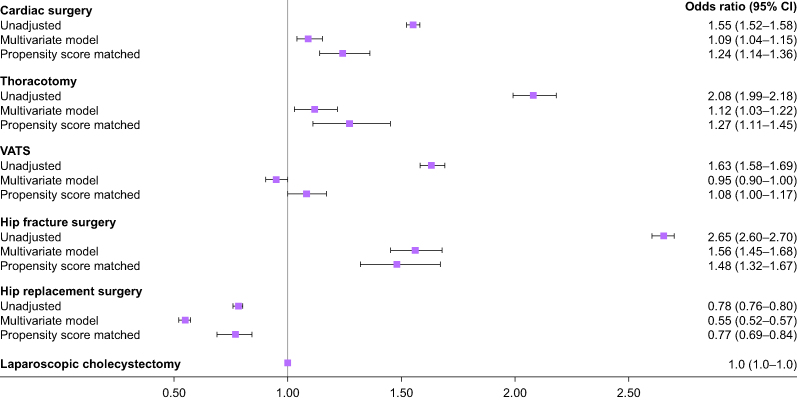
The unadjusted, multivariable adjusted, and propensity score matched adjusted hazard ratios for new onset postoperative depression by surgical type are presented in Figure 1.
Fig. 1.
Multivariable adjusted odds of new onset postoperative depression by surgery type. Laparoscopic cholecystectomy was assigned as the reference group (OR=1). Error bars represent 95% confidence intervals. CI, confidence interval; VATS, video-assisted thoracoscopic surgery.
Cox regression analysis
After multivariable adjustment for preoperative confounders, the hazard ratio for new onset postoperative depression was the highest for hip fracture surgery patients, followed by thoracotomy and cardiac surgery. There was no difference in the multivariable adjusted hazard ratio for new onset depression between VATS and laparoscopic cholecystectomy patients. Hip replacement patients had the lowest multivariable adjusted hazard ratio for new onset depression among the surgeries tested.
Propensity score analysis
Results of the propensity score matched analyses revealed a similar pattern, with hip fracture surgery having the highest adjusted hazard ratio for new onset depression, followed by thoracotomy and cardiac surgery. In the propensity score matched cohort, patients undergoing VATS had a significantly higher hazard ratio for new onset depression than patients undergoing laparoscopic cholecystectomy. Hip replacement surgery patients had the lowest adjusted hazard ratio for new onset depression among the types of surgery analysed in the propensity score matched analysis.
Sensitivity analyses
Compared with laparoscopic cholecystectomy patients, hip fracture surgery patients were the most likely to have a new claim for depression overlapping with a new prescription for an antidepressant after multivariable adjustment, followed by thoracotomy and cardiac surgery (Table 2). There was no significant difference in the incidence of new onset depression defined as both a new diagnosis of depression and a new prescription for an antidepressant between patients undergoing VATS and laparoscopic cholecystectomy in the multivariable model. However, VATS patients in the propensity score matched cohort were significantly more likely to develop new onset depression by this definition. Hip replacement patients were again least likely to experience new onset postoperative depression according to this definition. When analysed individually, the odds of (a) a new diagnosis code for depression or (b) a new prescription with an antidepressant demonstrated a similar pattern as when they were analysed jointly. Notable differences in the effect estimates between these definitions were slightly higher rates of a new diagnosis of depression than a new prescription for an antidepressant for most analyses, with the exception of the hip fracture surgery cohorts where this difference was notably larger.
Table 2.
Sensitivity analyses using alternate definitions of new onset postoperative depression. Laparoscopic cholecystectomy was the reference group for all comparisons (HR=1). CI, confidence interval; HR, hazard ratio; VATS, video-assisted thoracoscopic surgery.
| Unadjusted HR (95% CI) |
P | Multivariable model HR (95% CI) | P | Propensity score matched HR (95% CI) | P | |
|---|---|---|---|---|---|---|
| Cardiac surgery | ||||||
| New depression diagnosisAND a new antidepressant prescription | 1.57 (1.50–1.64) | <0.01 | 1.34 (1.20–1.50) | <0.01 | 1.66 (1.36–2.03) | <0.01 |
| New depression diagnosis | 1.67 (1.62–1.71) | <0.01 | 1.23 (1.14–1.32) | <0.01 | 1.31 (1.16–1.48) | <0.01 |
| New antidepressant prescription | 1.49 (1.46–1.52) | <0.01 | 1.07 (1.01–1.13) | 0.03 | 1.27 (1.15–1.41) | <0.01 |
| Thoracic surgerysThoracotomy | ||||||
| New depression AND a new antidepressant prescription | 1.98 (1.79–2.19) | <0.01 | 1.30 (1.08–1.56) | <0.01 | 1.36 (1.01–1.83) | 0.04 |
| New depression diagnosis | 2.09 (1.96–2.23) | <0.01 | 1.20 (1.06–1.35) | <0.01 | 1.24 (1.03–1.49) | 0.03 |
| New antidepressant prescription | 2.04 (1.94–2.14) | <0.01 | 1.13 (1.03–1.24) | 0.01 | 1.30 (1.12–1.52) | <0.01 |
| VATS | ||||||
| New depression diagnosisAND a new antidepressant prescription | 1.59 (1.48–1.71) | <0.01 | 1.02 (0.91–1.15) | 0.76 | 1.20 (1.00–1.45) | 0.05 |
| New depression diagnosis | 1.66 (1.58–1.74) | <0.01 | 1.01 (0.93–1.08) | 0.88 | 1.16 (1.03–1.30) | 0.01 |
| New antidepressant prescription | 1.59 (1.53–1.65) | <0.01 | 0.95 (0.89–1.00) | 0.06 | 1.04 (0.95–1.14) | 0.35 |
| Hip fracture surgery | ||||||
| New depression diagnosisAND a new antidepressant prescription | 2.35 (2.26–2.45) | <0.01 | 1.63 (1.36–1.95) | <0.01 | 1.37 (1.04–1.80) | 0.02 |
| New depression diagnosis | 3.40 (3.31–3.48) | <0.01 | 1.97 (1.79–2.18) | <0.01 | 1.71 (1.47–1.99) | <0.01 |
| New antidepressant prescription | 2.11 (2.07–2.16) | <0.01 | 1.34 (1.22–1.47) | <0.01 | 1.23 (1.07–1.43) | <0.01 |
| Hip replacement | ||||||
| New depression diagnosis AND a new antidepressant prescription | 0.61 (0.58–0.64) | <0.01 | 0.51 (0.46–0.56) | <0.01 | 0.70 (0.54–0.89) | <0.01 |
| New depression diagnosis | 0.90 (0.87–0.93) | <0.01 | 0.63 (0.59–0.67) | <0.01 | 0.84 (0.73–0.96) | <0.01 |
| New antidepressant prescription | 0.66 (0.65–0.68) | <0.01 | 0.50 (0.47–0.52) | <0.01 | 0.72 (0.64–0.81) | <0.01 |
Conditions associated with new onset depression
Model diagnostics including the adjusted effect of every covariate in the model on the risk of new onset depression according to the primary outcome definition were available for each analysis and are summarised graphically (Supplementary Figs S6–S10). Among the variables tested, cognitive disorders, Parkinson's disease, female sex, and metastatic cancer were consistently highly associated with new onset depression. Hyperlipidaemia, cholelithiasis, and Asian ethnicity were consistently associated with a lower risk of new onset depression.
Discussion
In a retrospective analysis of data from a national claims database, we found that the risk of new onset postoperative depression varied by surgical type after adjustment for a broad set of preoperative variables. Our incidence rates of new onset depression ranged from 6.8% to 18.8%. Although the incidence of new depression in the general population is not easily measured, for context, 21 million adults experienced a major depressive episode in 2020, giving a prevalence representing 8.4% of the US adult population.27 The results of our adjusted analyses suggest that after controlling for preoperative comorbidities and predictors of future depression, new onset postoperative depression may be associated with some types of surgery more than others. The consistency of these findings after different methods of confounder adjustment and sensitivity analyses suggest that these differences are not likely to be attributed mainly to preoperative characteristics or to alternate classifications of postoperative depression.
The existing literature describing depression and surgery focuses mainly on the association between preoperative depression and postoperative outcomes. There are a few studies from small samples which have described the incidence of new onset postoperative depression. In cardiac surgery, the rates of new postoperative depression are 13–20% using estimates from multiple small cohorts, the largest being 817 patients.9,13,28, 29, 30 One cohort study of 278 thoracic surgery patients described rates of 9% for VATS and 19% for thoracotomy.10 The rate of new depression after hip fracture is estimated between 14% and 30%, with the largest cohort equalling 482 patients.14,31,32 Our results both align with and add substantially to these established data. First, because our results are derived from a large contemporary national database, they provide updated estimates of the rates of postoperative depression using a large representative sample. Second, unlike previous studies, our analyses were performed after excluding patients with preoperative depression which provides a more accurate assessment of the risk of new onset depression.
We believe our results support a hypothesis that the risk of new onset postoperative depression may in part be influenced by complex relationships between surgically treatable disease processes and exposures occurring in the perioperative period. The relationships between stress/inflammation, pain, and cognitive impairment and the risk of subsequent depression have all been previously described and may help explain why the risk of new onset postoperative depression is higher after certain surgical procedures. For example, systemic inflammation may predispose to central nervous system changes in regions of the brain associated with depression.33,34 Cardiac surgery with cardiopulmonary bypass is associated with extreme exposure to systemic inflammation.35,36 Pain and depression are frequently comorbid and involve similar central and peripheral neural pathways.11 The influence of pain on subsequent depression may be evident in our results, where VATS was associated with a lower risk of new onset postoperative depression than thoracotomy, an operation in the same body cavity with notably higher rates of chronic postoperative pain.37 Depression frequently coexists with cognitive impairment, a common postoperative risk for both the older cardiac surgery and hip fracture populations.36,38,39 The indication and the context surrounding surgery may also contribute to the risk of subsequent depression, as hip fracture patients were at the highest risk for new onset postoperative depression, whereas those undergoing hip replacement were at the lowest risk despite undergoing a similar procedure on the same body part. Functional outcomes, disability either from the initial injury or perioperative complications, intensive care unit stay, or inability to return home after surgery could also influence the risk of subsequent depression for hip fracture patients, whereas the restoration of mobility and quality of life for hip replacement patients may be protective against future depression.12
These findings should be interpreted in the context of our study's limitations. Claims data lack clinical detail which can affect the accurate assessment of our outcomes, and our dataset does not capture data on care provided without a corresponding insurance claim, which can be a potential source of unmeasured confounding. This can be especially challenging for the accurate identification of depression, which relies on accurate assessments by clinicians, documentation, and prioritisation as billing diagnoses for claims. We accounted for this by defining depression broadly and by performing multiple sensitivity analyses using alternative definitions. We used a validated set of codes which are 99% specific for depression, however, they are poorly sensitive, a common limitation of identifying depression using claims data.40 Nevertheless, differential misclassification of the outcome across the various surgical cohorts selected for this study is unlikely. Thus, a lack of sensitivity in detecting the outcome would be expected to bias our results towards the null rather than in the direction of any particular surgical type.
The absence of detail in the database regarding disease severity or acuity is another limitation, as these are key factors in determining the indication for surgery. Along these lines, our attempts to control for other conditions related to surgically treatable diseases are also limited by this lack of context. Additionally, details of intraoperative exposures, such as the length of surgery/anaesthesia, perioperative factors including ICU admission, and postoperative events including complications were not available. We believe that our results suggest that these unmeasured perioperative exposures may potentially contribute to the differential risk of depression seen amongst the surgery types investigated rather than differences in any unmeasured preoperative characteristic, a hypothesis that should be investigated more thoroughly with more granular perioperative datasets. Lastly, although our data have been extracted from a national database, the population sampled may not be representative of the larger surgical population, especially patients on Medicaid, those who are uninsured, or patients who are not cared for in the USA.
We opted not to compare the risk of new onset depression after surgery to that of a non-surgical comparator group, which would introduce multiple sources of bias including latency bias, immortal time bias, and confounding by indication. Because none of these are easily corrected for in retrospective studies, and because we are primarily interested in postoperative depression rather than the relationship between surgically treatable disease and depression, we chose to analyse the risk of depression solely among patients requiring surgery. The question of how an exposure to one type of surgery vs another can influence the risk of postoperative depression is not feasibly assessed in a randomised controlled trial, therefore we attempted to address this question within the constraints of a retrospective analysis. Given the predictably large differences in the distribution of comorbidities between patients presenting for the different types of surgery, we addressed confounding by using both multivariable regression and propensity score matching. Propensity score matching in this context may be limited since it is best suited for investigations where there is a choice between therapies for the same indication. When analysing such a large population, propensity score matching offered an advantage by matching patients from the overlapping areas of the propensity score distribution, dropping those whose preoperative characteristics are very strongly associated with one type of surgery in particular and still retaining a sizeable cohort of patients with similar baseline risk profiles.
Our findings have significant implications for perioperative care, as postoperative depression has been linked with higher mortality and worse functional outcomes after major surgery.9,13,14,32 Based on our findings, we believe additional investigation is warranted into this potentially preventable source of postoperative disability. Further defining the perioperative predictors of postoperative depression could lead to more precise identification of vulnerable patients and opportunities to intervene before depression develops. Mechanistic studies could identify pharmacological, procedural, or behavioural interventions to prevent postoperative depression. As depression and anxiety frequently coexist, future studies could investigate the potential effect surgery and recovery may have on anxiety. Another potential interesting avenue for future study could be the downstream effects of postoperative depression on healthcare utilisation. Finally, although major surgery is often undertaken with the objective of reducing disability and preserving longevity, our findings suggest that exposure to certain types of major surgery may confer an increased risk of another highly debilitating condition in postoperative depression. Awareness of this risk may influence surgical planning and postoperative care so that optimal functional outcomes may be achieved for both the body and mind of vulnerable patients.
Authors’ contributions
Design of study or acquisition of data: BO, KR, MS, MF
Drafting and revising: all authors
Final approval: all authors
Acknowledgements
The authors would like to acknowledge the technical assistance provided by Sushama Kattinakere Sreedhara from the Brigham and Women's Department of Pharmacoepidemiology in the design and analysis of this study, and to Samantha Harrison from Beth Israel Deaconess Medical Center's Center for Anesthesia Research Excellence for their help with additional analyses for the study. A good deal of the work was done during a research training grant of Dr. O'Gara's (mentor = Dr. Talmor). The grant info: American Society of Anesthesiologists' Foundation for Anesthesia, Education, and Research. Mentored Research Training Grant.
Handling editor: Phil Hopkins
Footnotes
Prior presentations: O'Gara B, Talmor D, Fischer M. Postoperative depression after major surgery: a comparative analysis using a national claims database. International Anesthesia Research Society 2020 Annual Meeting, San Francisco, CA, United States. (Conference cancelled.)
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjao.2023.100223.
Declarations of interest
The authors declare that they have no conflicts of interest.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
figs1
figs2
figs3
figs4
figs5
References
- 1.World Health Organization . WHO; Geneva: 2017. Depression and other common mental disorders: global health estimates. [Google Scholar]
- 2.Bisschop M.I., Kriegsman D.M., Deeg D.J., Beekman A.T., van Tilburg W. The longitudinal relation between chronic diseases and depression in older persons in the community: the Longitudinal Aging Study Amsterdam. J Clin Epidemiol. 2004;57:187–194. doi: 10.1016/j.jclinepi.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Cole M.G., Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160:1147–1156. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- 4.Egede L.E. Major depression in individuals with chronic medical disorders: prevalence, correlates and association with health resource utilization, lost productivity and functional disability. Gen Hosp Psychiatry. 2007;29:409–416. doi: 10.1016/j.genhosppsych.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Lichtman J.H., Bigger J.T., Blumenthal J.A., et al. Depression and coronary heart disease. Circulation. 2008;118:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 6.Mehta R.D., Roth A.J. Psychiatric considerations in the oncology setting. CA Cancer J Clin. 2015;65:299–314. doi: 10.3322/caac.21285. [DOI] [PubMed] [Google Scholar]
- 7.Lichtman J.H., Froelicher E.S., Blumenthal J.A., et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129:1350–1369. doi: 10.1161/CIR.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 8.Runowicz C.D., Leach C.R., Henry N.L., et al. American cancer society/American society of clinical oncology breast cancer survivorship care guideline. CA Cancer J Clin. 2016;66:43–73. doi: 10.3322/caac.21319. [DOI] [PubMed] [Google Scholar]
- 9.Blumenthal J.A., Lett H.S., Babyak M.A., et al. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet. 2003;362:604–609. doi: 10.1016/S0140-6736(03)14190-6. [DOI] [PubMed] [Google Scholar]
- 10.Park S., Kang C.H., Hwang Y., et al. Risk factors for postoperative anxiety and depression after surgical treatment for lung cancer. Eur J Cardiothorac Surg. 2016;49:e16–e21. doi: 10.1093/ejcts/ezv336. [DOI] [PubMed] [Google Scholar]
- 11.Kleiber B., Jain S., Trivedi M.H. Depression and pain: implications for symptomatic presentation and pharmacological treatments. Psychiatry (Edgmont) 2005;2:12–18. [PMC free article] [PubMed] [Google Scholar]
- 12.Ghoneim M.M., O'Hara M.W. Depression and postoperative complications: an overview. BMC Surg. 2016;16:5. doi: 10.1186/s12893-016-0120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connerney I., Shapiro P.A., McLaughlin J.S., Bagiella E., Sloan R.P. Relation between depression after coronary artery bypass surgery and 12-month outcome: a prospective study. Lancet. 2001;358:1766–1771. doi: 10.1016/S0140-6736(01)06803-9. [DOI] [PubMed] [Google Scholar]
- 14.Lenze E.J., Munin M.C., Skidmore E.R., et al. Onset of depression in elderly persons after hip fracture: implications for prevention and early intervention of late-life depression. J Am Geriatr Soc. 2007;55:81–86. doi: 10.1111/j.1532-5415.2006.01017.x. [DOI] [PubMed] [Google Scholar]
- 15.Covey L.S., Glassman A.H., Stetner F. Cigarette smoking and major depression. J Addict Dis. 1998;17:35–46. doi: 10.1300/J069v17n01_04. [DOI] [PubMed] [Google Scholar]
- 16.Breslau N., Kilbey M.M., Andreski P. Nicotine dependence and major depression. New evidence from a prospective investigation. Arch Gen Psychiatry. 1993;50:31–35. doi: 10.1001/archpsyc.1993.01820130033006. [DOI] [PubMed] [Google Scholar]
- 17.Hecht S.S. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol. 2002;3:461–469. doi: 10.1016/s1470-2045(02)00815-x. [DOI] [PubMed] [Google Scholar]
- 18.Lakier J.B. Smoking and cardiovascular disease. Am J Med. 1992;93(Suppl 1):S8–S12. doi: 10.1016/0002-9343(92)90620-q. [DOI] [PubMed] [Google Scholar]
- 19.Vandenbroucke J.P., von Elm E., Altman D.G., et al. Strengthening the reporting of observational studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18:805–835. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 20.Fingar K.R.S.C., Weiss A.J., Steiner C.A. Healthcare cost and utlization project (HCUP) statistical brief #186, 2014. Agency for Healthcare Research and Quality (US); Rockville (MD): 2003-2012. Most frequent operating room procedures performed in U.S. hospitals. [PubMed] [Google Scholar]
- 21.Tsai M.-C., Chen C.-H., Lee H.-C., Lin H.-C., Lee C.-Z. Increased risk of depressive disorder following cholecystectomy for gallstones. PloS One. 2015;10 doi: 10.1371/journal.pone.0129962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schenker N., Raghunathan T.E., Chiu P.-L., Makuc D.M., Zhang G., Cohen A.J. Multiple imputation of missing income data in the national health interview survey. J Am Stat Assoc. 2006;101:924–933. [Google Scholar]
- 23.Spettell C.M., Wall T.C., Allison J., et al. Identifying physician-recognized depression from administrative data: consequences for quality measurement. Health Serv Res. 2003;38:1081–1082. doi: 10.1111/1475-6773.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djernes J.K. Prevalence and predictors of depression in populations of elderly: a review. Acta Psychiatr Scand. 2006;113:372–387. doi: 10.1111/j.1600-0447.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 25.Brody D.J., Pratt L.A., Hughes J.P. Prevalence of depression among adults aged 20 and older: United States, 2013-2016. NCHS Data Brief. 2018;303:1–8. [PubMed] [Google Scholar]
- 26.Rubin D.B. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8 Pt 2):757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 27.Substance Abuse and Mental Health Services Administration . Published 2019. Key substance use and mental health indicators in the United States: results from the 2018 national survey on drug use and health (HHS publication No. PEP19-5068, NSDUH series H-54). Rockville, MD: center for behavioral health statistics and quality, substance abuse and mental health services administration.https://www.samhsa.gov/data/ Available from. Accessed date January 10, 2023. [Google Scholar]
- 28.McKhann G.M., Borowicz L.M., Goldsborough M.A., Enger C., Selnes O.A. Depression and cognitive decline after coronary artery bypass grafting. Lancet. 1997;349:1282–1284. doi: 10.1016/S0140-6736(96)09466-4. [DOI] [PubMed] [Google Scholar]
- 29.Pirraglia P.A., Peterson J.C., Williams-Russo P., Gorkin L., Charlson M.E. Depressive symptomatology in coronary artery bypass graft surgery patients. Int J Geriatr Psychiatry. 1999;14:668–680. doi: 10.1002/(sici)1099-1166(199908)14:8<668::aid-gps988>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Rafanelli C., Roncuzzi R., Milaneschi Y. Minor depression as a cardiac risk factor after coronary artery bypass surgery. Psychosomatics. 2006;47:289–295. doi: 10.1176/appi.psy.47.4.289. [DOI] [PubMed] [Google Scholar]
- 31.Givens J.L., Sanft T.B., Marcantonio E.R. Functional recovery after hip fracture: the combined effects of depressive symptoms, cognitive impairment, and delirium. J Am Geriatr Soc. 2008;56:1075–1079. doi: 10.1111/j.1532-5415.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- 32.Cristancho P., Lenze E.J., Avidan M.S., Rawson K.S. Vol. 46. 2016. Trajectories of depressive symptoms after hip fracture; pp. 1413–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraynak T.E., Marsland A.L., Wager T.D., Gianaros P.J. Functional neuroanatomy of peripheral inflammatory physiology: a meta-analysis of human neuroimaging studies. Neurosci Biobehav Rev. 2018;94:76–92. doi: 10.1016/j.neubiorev.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wittenberg G.M., Stylianou A., Zhang Y., et al. Effects of immunomodulatory drugs on depressive symptoms: a mega-analysis of randomized, placebo-controlled clinical trials in inflammatory disorders. Mol Psychiatry. 2019;25:1275–1285. doi: 10.1038/s41380-019-0471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller B.E., Levy J.H. The inflammatory response to cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1997;11:355–366. doi: 10.1016/s1053-0770(97)90106-3. [DOI] [PubMed] [Google Scholar]
- 36.Newman M.F., Kirchner J.L., Phillips-Bute B., et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 37.Gottschalk A., Cohen S.P., Yang S., Ochroch E.A. Preventing and treating pain after thoracic surgery. Anesthesiology. 2006;104:594–600. doi: 10.1097/00000542-200603000-00027. [DOI] [PubMed] [Google Scholar]
- 38.Korczyn A.D., Halperin I. Depression and dementia. J Neurol Sci. 2009;283:139–142. doi: 10.1016/j.jns.2009.02.346. [DOI] [PubMed] [Google Scholar]
- 39.Berger M., Terrando N., Smith S.K., Browndyke J.N., Newman M.F., Mathew J.P. Neurocognitive function after cardiac surgery: from phenotypes to mechanisms. Anesthesiology. 2018;129:829–851. doi: 10.1097/ALN.0000000000002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiest K.M., Jette N., Quan H., et al. Systematic review and assessment of validated case definitions for depression in administrative data. BMC Psychiatry. 2014;14:289. doi: 10.1186/s12888-014-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.