Summary
Background
No standard maintenance treatment has been obtained to prolong the response duration of soft tissue sarcoma (STS) after first-line chemotherapy. In this study, we aimed to evaluate the efficacy and safety of anlotinib as a maintenance treatment after chemotherapy in STS.
Methods
In this multicentre, open-label, single-arm phase 2 trial, patients with advanced STS who achieved partial response or stable disease after first-line anthracycline-based chemotherapy were enrolled between April 2019 and January 2022. All patients received anlotinib as a maintenance treatment. The primary endpoint was progression-free survival (PFS) of anlotinib maintenance treatment. Other endpoints included overall survival (OS), objective response rate (ORR), disease control rate (DCR) and safety. This study is registered with ClinicalTrials.gov, NCT03890068.
Findings
At the data cut-off date (August 8, 2022), 49 patients were enrolled, including 17 with liposarcoma (35%) and 15 with leiomyosarcoma (31%). After a median follow-up of 17.1 months (IQR 9.0–27.2), the median PFS from the beginning of maintenance treatment was 9.1 months (95% CI 5.7–12.5), and the median OS was not reached, and the 1-year OS rate for anlotinib maintenance treatment was 98.0%. The best ORR and DCR were 16% (8/49, 95% CI 7–30) and 94% (46/49, 95% CI 83–99), respectively. Most of the treatment-related adverse events were grade 1–2. Of the grade 3–4 adverse events, the most common were hypertension (10%) and hand-foot syndrome reaction (6%).
Interpretation
Postchemotherapy maintenance treatment with anlotinib exhibits promising efficacy and tolerable toxicity in patients with advanced STS.
Funding
Chia Tai Tianqing Pharmaceutical Group Co., Ltd., the National Key Research and Development Program of China, and the National Natural Science Foundation of China.
Keywords: Anlotinib, Soft tissue sarcoma, Maintenance treatment, First-line chemotherapy
Research in context.
Evidence before this study
Till now, there is no recommended first-line post-chemotherapy maintenance treatment in advanced soft tissue sarcoma. In terms of the temporary response duration of first-line anthracycline-based chemotherapy, and that patients often lose their opportunities of prolonged survivals for the intolerance of long-term chemotherapy, appropriate regimens for efficacy maintenance require more attention. To explore, we searched PubMed for articles published from inception until April 28, 2023, on maintenance treatment after first-line standard chemotherapy in patients with soft tissue sarcoma, using the terms: “sarcoma”, “maintenance” and “first-line”. Reviews were excluded. We found one phase 2 trial discussed about anlotinib combining with chemotherapy followed by anlotinib continuous maintenance treatment. For switch maintenance, however, we found only one single-centre retrospective study, which mentioned the anlotinib therapy. We did not find any clinical trial regarding anlotinib as maintenance treatment after first-line chemotherapy in patients with soft tissue sarcoma.
Added value of this study
The standard maintenance treatments for advanced soft tissue sarcoma patients after first-line anthracycline-based chemotherapy are still lacking. Post-chemotherapy maintenance treatment with anlotinib shows significant efficacy and tolerable toxicity in patients with advanced soft tissue sarcoma. To our knowledge, this is the first prospective clinical trial assessing the value of switch maintenance strategy which could effectively prolong the progression-free survival after first-line standard chemotherapy in soft tissue sarcoma.
Implications of all the available evidence
This study demonstrated that anlotinib, as the first effective maintenance strategy after first-line standard chemotherapy, showing promising activity and remarkable progression-free survival with tolerable toxicities in patients with advanced soft tissue sarcoma. Our work lays the basis for subsequent larger randomized controlled studies. Once the conclusions confirmed, anlotinib could be recommended as one of the most optimal maintenance treatments after standard first-line chemotherapy, contributing to prolonging survivals and improving the life quality for advanced sarcoma patients.
Introduction
Soft tissue sarcoma (STS) encompasses a group of rare tumours; STS accounts for fewer than 1% of adult malignancies but is associated with a high mortality rate.1,2 It has been reported that 14.5%–26.5% of STS patients present with metastases at the initial diagnosis,3 while 40%–50% of patients who underwent localized resection developed distant metastases and showed a 5-year survival of less than 10%.4 Anthracycline-based chemotherapy is recommended as the standard first-line treatment for advanced STS5; this approach yields a median progression-free survival (PFS) of only approximately 6 months and a median overall survival (OS) of 12–16 months.6, 7, 8 However, the benefits of continuous chemotherapy have not been proven to be greater than those of surveillance once the maximal benefit has been obtained, and there have been concerns with anthracycline-mediated toxicities, such as cumulative cardiotoxicity.9, 10, 11, 12 Therefore, it is necessary to explore appropriate strategies of postchemotherapy maintenance treatment to prolong the duration of response and delay disease progression in patients with partial response (PR) or stable disease (SD) after a defined cycle of chemotherapy in advanced STS.13 Moreover, standard maintenance treatments for advanced or inoperable STS patients who benefit from first-line chemotherapy are still lacking.
Although one study demonstrated that maintenance treatment with a less intensive chemotherapy after standard chemotherapy could improve survival for patients with high-risk rhabdomyosarcoma,14 the benefit of maintenance treatment remains uncertain when used in advanced STS with non-specific pathological subtypes. The results of one of the largest international phase 3 trials to date showed that maintenance therapy with oral ridaforolimus in metastatic sarcoma patients (n = 711) after prior chemotherapy provided only a 3.1-week significant increase in median PFS.15 Currently, there is still no recommended standard maintenance treatment. Given the inherent toxicity associated with chemotherapy and the fact that agents used in maintenance therapy should be convenient and well tolerated, maintenance treatment with oral targeted drugs in advanced STS may be a preferred option and requires further exploration.
Anlotinib, a newly developed, orally multitargeted tyrosine kinase inhibitor (TKI), suppresses tumour growth and angiogenesis by mainly blocking the vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR) and platelet-derived growth factor receptor (PDGFR) pathways.16, 17, 18, 19, 20, 21, 22, 23 Exhibiting promising efficacy and manageable toxicity in various cancers,24, 25, 26, 27, 28, 29, 30 anlotinib was recommended as a third-line treatment for advanced NSCLC in 2018.24,25 Moreover, in 2019, based on the encouraging results of a phase 2 study (ALTER-0203) in patients with advanced STS,31 anlotinib was approved as the first second-line therapeutic agent by the China Food and Drug Administration (CFDA) for advanced STS. Although a retrospective study demonstrated that anlotinib may be an option for switch maintenance treatment after chemotherapy for advanced STS,32 whether anlotinib could be used as a maintenance treatment paradigm for patients with advanced STS after achieving disease control with first-line chemotherapy should be tested in a prospective study.
In this multicentre, open-label, single-arm, prospective phase 2 clinical trial, we aim to evaluate the efficacy and safety of anlotinib as a switch maintenance therapy after first-line chemotherapy in advanced STS to demonstrate whether anlotinib maintenance treatment after chemotherapy could improve survival of patients with advanced STS.
Methods
Study design and participants
This multicentre, single-arm, open-label, phase 2 clinical trial evaluating the antitumour activity and safety of anlotinib as a maintenance treatment in advanced STS patients who achieved partial response or stable disease with at least four cycles of first-line anthracycline-based chemotherapy was conducted at seven hospitals in China.
Eligible patients were 18–70 years old and had a histologically confirmed diagnosis of advanced STS, mainly leiomyosarcoma, synovial sarcoma, liposarcoma or angiosarcoma. Other inclusion criteria were an Eastern Cooperative Oncology Group (ECOG) performance status score of 0–2; expected survival time of over three months; at least one evaluable disease that can be accurately measured according to Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1; remained PR or SD after 6 cycles of anthracycline-based chemotherapy, or had disease controlled but intolerable to at least 4 cycles of chemotherapy; no more than 8 weeks from the last cycle of anthracycline administration to enrolment and with no prior anlotinib treatment or any systemic antitumour treatments within 4 weeks before enrolment. Patients who had used anlotinib were excluded. The full eligibility criteria are provided in the Clinical Trial Protocol.
Procedures
Anlotinib was given orally once daily at 12 mg on day 1–14, followed by one week off, every three weeks per cycle. The doses were chosen based on a previous study in advanced refractory solid tumours.16 The treatment continued until progressive disease (PD) or intolerable toxicity. Dose reduction to 10 mg per day or 8 mg per day of anlotinib was allowed due to grade 3 nonhaematologic or grade 4 haematologic toxicities, according to the protocol-defined dose modification criteria. Briefly, if the patient could not tolerate 12 mg per day, then the dose could be reduced to 10 mg per day or 8 mg per day. If the dose of 8 mg per day was not tolerated, then treatment was terminated. Once the anlotinib dose was reduced, it could no longer be increased.
In accordance with RECIST version 1.1, responses were assessed by an independent experienced radiologist at each site. Tumour assessment was performed using computed tomography (CT) or magnetic resonance imaging (MRI) within four weeks before treatment started at baseline, 3 weeks after treatment initiation, and every 2 cycles (6 weeks) thereafter. Tumour responses had to be confirmed at least 4 weeks later with a repeat scan. Adverse events were recorded throughout the treatment period and 21 days after the last dose and graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. The follow-up evaluations were conducted every 8 weeks after treatment was discontinued until the date of death or the data cut-off.
Outcomes
The primary end point was PFS for anlotinib maintenance treatment. Secondary endpoints included OS, objective response rate (ORR), the disease control rate (DCR), and safety. PFS was defined as the period from the start of anlotinib maintenance treatment until the date of disease progression or death due to any cause (which ever occur first). If the subject did not experience disease progression or death, PFS is defined as the period from initiation of treatment to the date of the last confirmed progression-free status. If no postbaseline tumour assessment is available, PFS was censored at the date of enrolment plus one day. The OS was defined as the period from the start of anlotinib maintenance treatment until death due to any cause. According to RECIST version 1.1, the ORR was defined as the proportion of patients with a confirmed complete response (CR) or PR prior to progression or any further therapy, and the DCR was defined as the proportion of patients with a confirmed CR, PR, and SD.
Statistical analysis
Based on a previous study,15 the median maintenance time after first-line chemotherapy in STS was 3.3 months as the null hypothesis. We expected that the PFS for anlotinib maintenance was 5.0 months. To detect this increase in PFS, a power of 80% was assumed at a one-sided 5% significance level, with a 12-month enrolment period and a 12-month follow-up period. Considering a 10% drop-out rate, 48 patients were required to be enrolled in this study.
The baseline data were collected and analysed according to the full analysis set, who took at least one oral dose of anlotinib, as shown by descriptive data. Categorical data are summarized as frequencies (percentages), while continuous data are summarized as medians (interquartile ranges, IQRs). The Kaplan–Meier method was used to present survival curves, with the estimated median time and 95% confidence intervals (CIs). The PFS for subgroups were calculated as a post hoc analysis. The 95% CIs for the overall ORR and DCR were calculated using the Clopper-Pearson method. Cox regression was used to analyse the associations between baseline characteristics and subgroups. A p value < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS software version 26.0. This study is registered with ClinicalTrials.gov, NCT03890068.
Ethics statement
The study protocol was reviewed and approved by the Ethics Committees of all participating hospitals, following the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent was obtained from all patients prior to study enrolment.
Role of the funding source
Chia Tai Tianqing Pharmaceutical Group Co., Ltd., provided the study drug for research. The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. BSX, QZP, HP, HML, XAL, JC, DMP, BQZ, DSW, MYF, and XZ had full access to the dataset of the study. The corresponding authors (XZ and MYF) are responsible for the decision to submit for publication.
Results
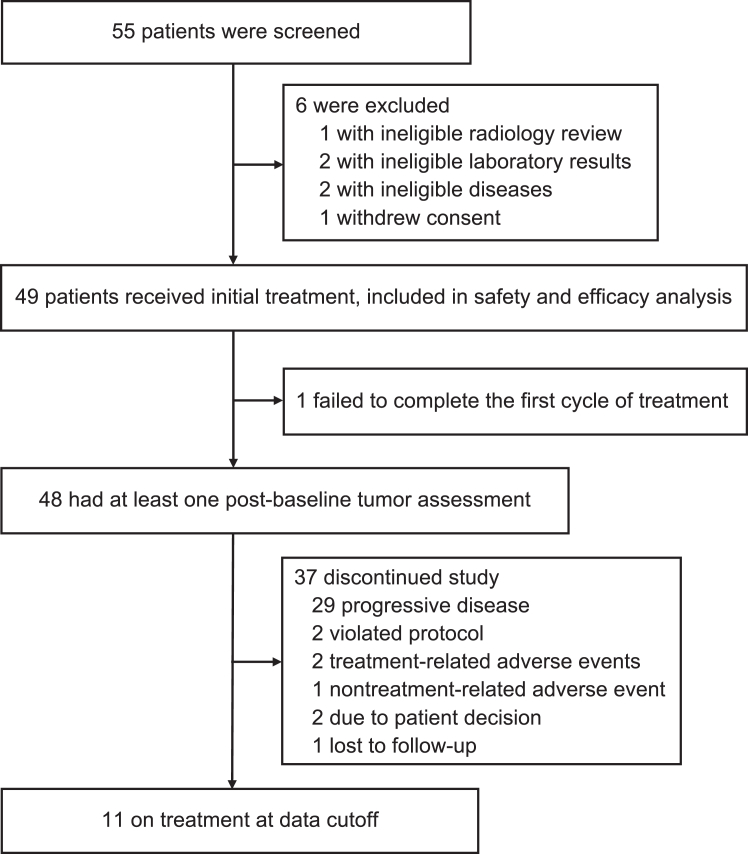
Between 15 April 2019 and 5 January 2022, we screened 55 patients. A total of 49 patients met the inclusion criteria and were enrolled (Fig. 1). One patient (2%) was excluded from the analysis of treatment response due to being lost to follow-up before the first scheduled postbaseline assessment. Thirty-seven patients (76%) discontinued treatment because of disease progression (29, 59%), protocol violation (2, 4%), adverse events (3, 6%), patient decision (2, 4%) or missing data (1, 2%). Eleven patients (22%) were still under treatment as of the cut-off date and had at least one postbaseline tumour assessment. Therefore, 48 patients were eligible for the analysis of treatment response, while all 49 patients were eligible for the analysis of survival and safety. Anlotinib was administered orally once daily at 12 mg for two weeks, followed by one week off, every three weeks per cycle, with a median number of cycles of 9 (IQR 3–20). During the anlotinib treatment, 10 patients (20%) had their dose adjusted to 10 mg per day, among which the dose of 2 patients (4%) was reduced to 8 mg per day.
Fig. 1.
Study profile. The data cut-off date was August 8, 2022.
The patients’ baseline characteristics are listed in Table 1. The data cut-off date was August 8, 2022. Of the 49 patients enrolled, 32 (65%) were female and 17 (35%) were male, with a median age of 49 years (IQR 39–60). All of them had an acceptable ECOG performance status score (≤2). The main histologic subtypes included liposarcoma (17, 35%), leiomyosarcoma (15, 31%), synovial sarcoma (4, 8%), fibrosarcoma (3, 6%), and unclassified sarcoma (3, 6%). Seven patients had other histologic subtypes, including one (2%) patient each with angiosarcoma, undifferentiated pleomorphic sarcoma, low-grade myofibroblastic sarcoma, sclerosing epithelioid fibrosarcoma, epithelioid sarcoma, epithelioid haemangioendothelioma or extraskeletal myxoid chondrosarcoma. Among the 17 patients with liposarcoma, 4 were confirmed to have well-differentiated liposarcoma (WDLS, 8%), 11 were confirmed to have dedifferentiated liposarcoma (DDLS, 22%) and 2 were confirmed to have myxoid liposarcoma (4%). The primary tumour sites of these patients included the retroperitoneum/intra-abdomen (22, 45%), trunk (9, 18%), viscera (8, 16%), extremities (7, 14%) and head and neck (3, 6%). At baseline, 43 (88%) patients had at least one distant metastasis, and the other 6 (12%) had confirmed locally advanced disease. The number of metastatic sites was one in 25 (51%) patients, two in 9 (18%) patients, and at least three simultaneously in 9 (18%) patients, respectively. Lung (25, 51%) and liver (10, 20%) were the most common sites of metastases. Eighteen percent (9/49) of patients had reached PR after first-line chemotherapy, while 82% (40/49) had reached SD.
Table 1.
Baseline characteristics (N = 49).
| Characteristic | Patients |
|---|---|
| Age, years | 49 (39–60) |
| Sex | |
| Female | 32 (65) |
| Male | 17 (35) |
| ECOG performance status | |
| 0 | 15 (31) |
| 1 | 33 (67) |
| 2 | 1 (2) |
| Histologic subtype | |
| Liposarcoma | 17 (35) |
| WDLS | 4 (8) |
| DDLS | 11 (22) |
| Myxoid liposarcoma | 2 (4) |
| Leiomyosarcoma | 15 (31) |
| Synovial sarcoma | 4 (8) |
| Fibrosarcoma | 3 (6) |
| Unclassified sarcoma | 3 (6) |
| Angiosarcoma | 1 (2) |
| Undifferentiated pleomorphic sarcoma | 1 (2) |
| Low-grade myofibroblastic sarcoma | 1 (2) |
| Sclerosing epithelioid fibrosarcoma | 1 (2) |
| Epithelioid sarcoma | 1 (2) |
| Epithelioid haemangioendothelioma | 1 (2) |
| Extraskeletal myxoid chondrosarcoma | 1 (2) |
| Primary site | |
| Head and neck | 3 (6) |
| Trunk | 9 (18) |
| Extremities | 7 (14) |
| Retroperitoneum/Intra-abdominal | 22 (45) |
| Viscera | 8 (16) |
| Metastatic site | |
| Lung | 25 (51) |
| Liver | 10 (20) |
| Bone | 5 (10) |
| Lymph node | 2 (4) |
| Othersa | 18 (37) |
| Number of metastatic sites | |
| 0 | 6 (12) |
| 1 | 25 (51) |
| 2 | 9 (18) |
| ≥3 | 9 (18) |
| First-line chemotherapy regimen | |
| MAIDb | 21 (43) |
| AIc | 24 (49) |
| CAV/IEd | 1 (2) |
| Otherse | 3 (6) |
| Cycles of first-line chemotherapy | 5 (4–6) |
| 4 | 24 (49) |
| 5 | 5 (10) |
| 6 | 15 (31) |
| 7 | 1 (2) |
| 8 | 4 (8) |
| Best response to first-line chemotherapy | |
| PR | 9 (18) |
| SD | 40 (82) |
| Previous radiotherapy | |
| Yes | 3 (6) |
| No | 46 (94) |
Data are median (IQR) or n (%).
ECOG = Eastern Cooperative Oncology Group. WDLS = well-differentiated liposarcoma. DDLS = dedifferentiated liposarcoma. PR = partial response. SD = stable disease.
Others refer to metastasis on peritoneum, abdomen or pelvic cavity, pancreas, spleen, kidney, gall bladder, inferior vena cava, extremities and muscles.
MAID refers to combined chemotherapy regimen of mesna, doxorubicin, ifosfamide, dacarbazine.
AI refers to combined chemotherapy regimen of doxorubicin and ifosfamide.
CAV/IE refers to an alternating regimen of cyclophosphamide, doxorubicin and vincristine (CAV), and ifosfamide and etoposide (IE).
Others refer to chemotherapy containing doxorubicin followed by AI, pegylated liposomal doxorubicin plus albumin-bound paclitaxel, doxorubicin plus dacarbazine.
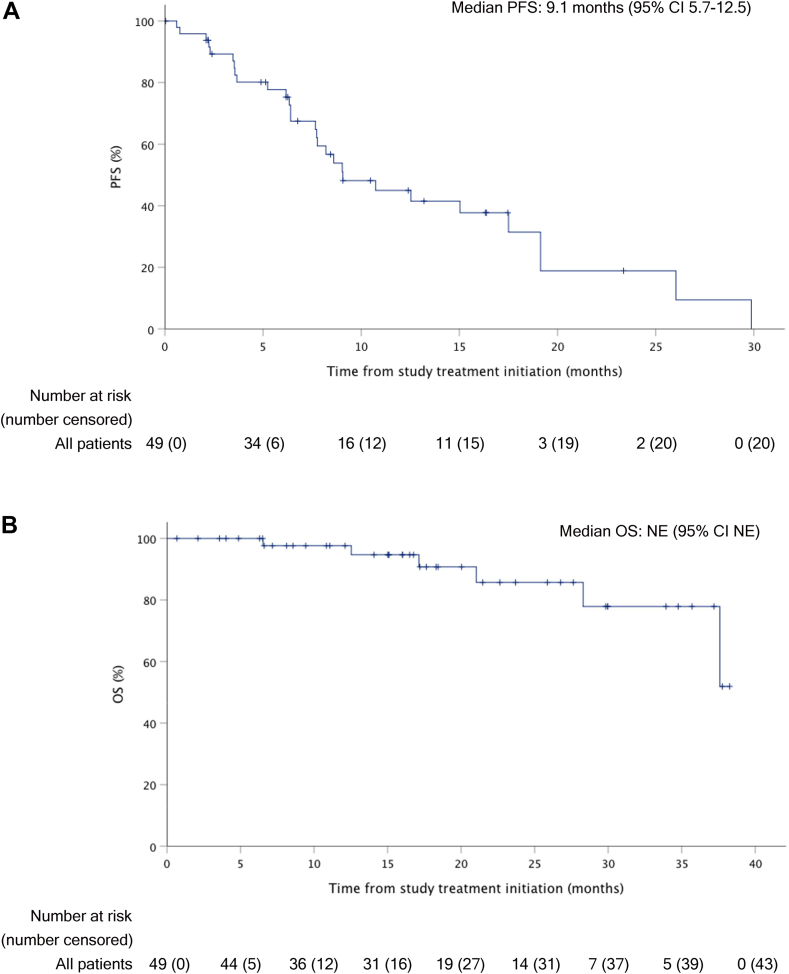
With a median follow-up of 17.1 months (IQR 9.0–27.2), for anlotinib maintenance treatment, the median PFS was 9.1 months (95% CI 5.7–12.5; Fig. 2A). Since the lower bound of the 95% CI exceeded the null hypothesis PFS of 3.3 months, the study met its primary objective. The median PFS for liposarcoma (n = 17), leiomyosarcoma (n = 15), synovial sarcoma (n = 4), fibrosarcoma (n = 3) and other subtypes (n = 7) was 12.5 months (95% CI 7.1–18.0), 7.7 months (95% CI 5.7–9.6), 19.1 months (95% CI 0–48.8), 3.6 months (95% CI 3.5–3.6) and 7.8 months (95% CI 0–18.5), respectively (Table 2). Among patients with liposarcoma, the median PFS for WDLS (n = 4) and DDLS (n = 11) was 19.1 months (95% CI 3.0–35.2) and 9.0 months (95% CI 4.9–13.2), respectively, while which was not reached in patients with myxoid liposarcoma (n = 2). Moreover, we also assessed the favourably biased median PFS for chemotherapy plus anlotinib maintenance treatment, which was 14.0 months (95% CI 10.1–17.8; Appendix Fig. 1). At the time of data cut-off, 6 of 49 patients (12%) had died, and the median OS was not reached (Fig. 2B). For anlotinib maintenance treatment, the 1-year OS rate was 98.0%. Through Cox regression analysis, patients over 40 years old benefited from both anlotinib maintenance treatment (≤40 vs. >40 years old, HR 2.632 [95% CI 1.165–5.945], p = 0.020). In addition, no other significant correlations between patient characteristics and PFS were found (Appendix Table 1).
Fig. 2.
Survival after anlotinib maintenance treatment. (A) Kaplan–Meier curves of PFS. The median PFS for anlotinib maintenance treatment was 9.1 months (95% CI 5.7–12.5). (B) Kaplan–Meier curves of OS. The median OS was not reached (NE, 95% CI NE) for the anlotinib maintenance treatment. The crosses represent censored patients. PFS = progression-free survival. OS = overall survival. NE = not evaluable.
Table 2.
Responses and survival analysis according to different histological subtypes.
| Histologic subtype | Patients | Best response |
Median PFSb (95% CI) | 1-year OS Rate (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CR | PR | SD | PD | NAa | ORR (%) | DCR (%) | ||||
| Liposarcoma | 17 (35) | 0 | 2 (12) | 15 (88) | 0 | 0 | 12 | 100 | 12.5 (7.1–18.0) | 100.0 |
| WDLS | 4 (8) | 0 | 1 (25) | 3 (75) | 0 | 0 | 25 | 100 | 19.1 (3.0–35.2) | 100.0 |
| DDLS | 11 (22) | 0 | 1 (9) | 10 (91) | 0 | 0 | 9 | 100 | 9.0 (4.9–13.2) | 100.0 |
| Myxoid liposarcoma | 2 (4) | 0 | 0 | 2 (100) | 0 | 0 | 0 | 100 | NE (NE) | 100.0 |
| Leiomyosarcoma | 15 (31) | 0 | 2 (13) | 12 (80) | 1 (7) | 0 | 13 | 93 | 7.7 (5.7–9.6) | 100.0 |
| Synovial sarcoma | 4 (8) | 1 (25) | 0 | 1 (25) | 1 (25) | 1 (25) | 25 | 50 | 19.1 (0–48.8) | 100.0 |
| Fibrosarcoma | 3 (6) | 0 | 1 (33) | 2 (67) | 0 | 0 | 33 | 100 | 3.6 (3.5–3.6) | 100.0 |
| Unclassified sarcoma | 3 (6) | 0 | 1 (33) | 2 (67) | 0 | 0 | 33 | 100 | NE (NE) | 100.0 |
| Other subtypesc | 7 (14) | 0 | 1 (14) | 6 (86) | 0 | 0 | 14 | 100 | 7.8 (0–18.5) | 85.7 |
| Overall | 49 (100) | 1 (2) | 7 (14) | 38 (78) | 2 (4) | 1 (2) | 16 (95% CI 7–30) | 94 (95% CI 83–99) | 9.1 (5.7–12.5) | 98.0 |
Data are n (%), n/N (%) and median survival (95% CI). Responses were assessed in accordance with Response Evaluation Criteria in Solid Tumours (RECIST, version 1.1). Only confirmed responses were included.
CR = complete response. PR = partial response. SD = stable disease. PD = progressive disease. NA = not assessed. ORR = objective response rate. DCR = disease control rate. PFS = progression-free survival. CI = confidence interval. OS = overall survival. WDLS = well-differentiated liposarcoma. DDLS = dedifferentiated liposarcoma. NE = not evaluable.
The patient who lost to follow-up before the first scheduled post-baseline evaluation.
The median PFS for anlotinib maintenance treatment.
Other subtypes: angiosarcoma, undifferentiated pleomorphic sarcoma, low-grade myofibroblastic sarcoma, sclerosing epithelioid fibrosarcoma, epithelioid sarcoma, epithelioid haemangioendothelioma and extraskeletal myxoid chondrosarcoma.
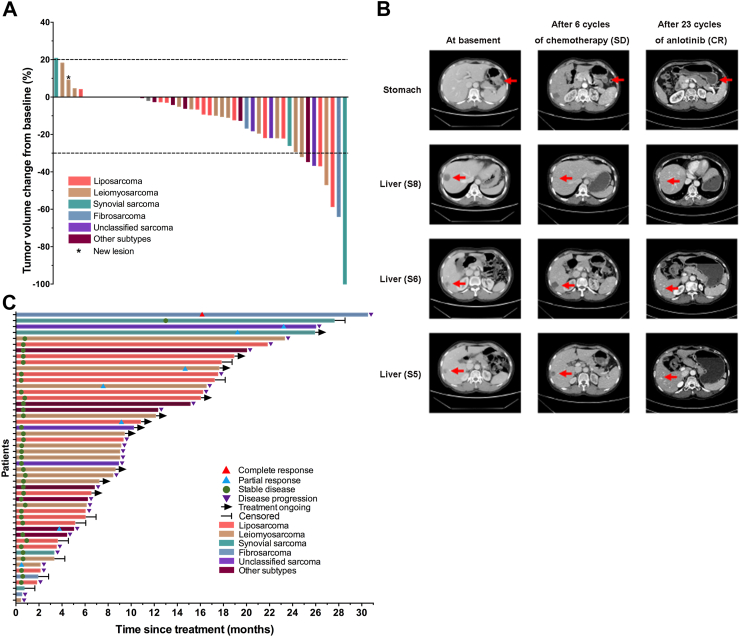
Forty-eight patients were assessable for response to anlotinib maintenance treatment. As shown in Fig. 3A, seven (14%) patients who had a maximum tumour regression of over 30% were confirmed to have PR. Thirty-eight (78%) patients had reached SD, while two (4%) patients had PD at the first assessment. One (2%) patient (female, 53 years old), who had a primary synovial sarcoma arising in the stomach with metastases in the liver reached CR after receiving 23 cycles of anlotinib maintenance treatment (Fig. 3B). Therefore, the overall ORR and DCR for anlotinib maintenance treatment after first-line chemotherapy were 16% (8/49, 95% CI 7–30) and 94% (46/49, 95% CI 83–99), respectively. As shown in Fig. 3C, the median time to achieve the best responses was 11.9 months (IQR 4.8–18.4) for the eight patients who had confirmed objective responses. Treatments for three (38%) of eight patients with responses were ongoing.
Fig. 3.
Clinical efficacy of anlotinib maintenance treatment. (A) The best percentage change in target lesions by RECIST version 1.1 (n = 48). Bars with different colours represent patients with different subtypes. Patients were defined as partial response (PR) with bars of over 30% reduction, progressive disease (PD) with bars of over 20% increase, and as stable disease (SD) with bars between 30% reduction and 20% increase (cut-offs represented by the dashed lines). Asterisks refer to patients with new lesions who were also assessed as PD. (B) Tumour regression of the patient with gastric synovial sarcoma. Computed tomography (CT) scans showed that the patient, who had primary synovial sarcoma in the stomach and metastasis in the liver, achieved SD after 6 cycles of first-line anthracycline-based chemotherapy and reached complete response (CR) after receiving 23 cycles of anlotinib maintenance treatment. Red arrows indicate the lesions. (C) Swimmer plot represents the duration of responses. The length of each bar represents each patient's treatment duration. The red triangles, blue triangles and green circles indicate the point in time when the best CR, PR and SD responses are first achieved. The purple triangles indicate the progression time. Other subtypes included angiosarcoma (n = 1), undifferentiated pleomorphic sarcoma (n = 1), low-grade myofibroblastic sarcoma (n = 1), sclerosing epithelioid fibrosarcoma (n = 1), epithelioid sarcoma (n = 1), epithelioid haemangioendothelioma (n = 1) and extraskeletal myxoid chondrosarcoma (n = 1). SD = stable disease. CR = complete response.
For each histologic subtype, the best ORRs and DCRs were 12% (2/17) and 100% (17/17) for liposarcoma, 13% (2/15) and 93% (14/15) for leiomyosarcoma, 25% (1/4) and 50% (2/4) for synovial sarcoma (one reached CR), 33% (1/3) and 100% (3/3) for fibrosarcoma, 33% (1/3) and 100% (3/3) for unclassified sarcoma, and 14% (1/7) and 100% (7/7) for other histologic subtypes (Table 2). Additionally, the ORRs for WDLS and DDLS were 25% (1/4) and 9% (1/11), respectively, while the DCRs for both reached 100%. No CR or PR but 2 SD was observed in patients with myxoid liposarcoma, with a DCR of 100%.
Most of the anlotinib maintenance treatment-related adverse events were mild or modest. Table 3 summarizes the adverse events that occurred in more than 10% of the patients in our study. Of all the grade 1–2 adverse events, the most common were hand-foot skin (HFS) reaction (57%), cholesterol elevation (47%), proteinuria (43%), triglyceride elevation (41%), hypertension (39%), thyroid stimulating hormone (TSH) elevation (35%), diarrhoea (33%), urine occult blood (31%), faecal occult blood (29%), hypothyroidism (27%), arthralgia (27%) and pharyngalgia (20%). Of the grade 3–4 adverse events, the most common were hypertension (10%) and HFS reaction (6%). The major reasons for dose reduction were proteinuria, neutropenia and HFS reaction. No anlotinib treatment-related deaths were reported.
Table 3.
Treatment-related adverse events in all treated patients (N = 49).
| Adverse events | All patients | Grade 1–2 | Grade 3–4 |
|---|---|---|---|
| HFS reaction | 28 (57) | 25 (51) | 3 (6) |
| Cholesterol elevation | 23 (47) | 23 (47) | 0 |
| Proteinuria | 21 (43) | 21 (43) | 0 |
| Triglyceride elevation | 20 (41) | 19 (39) | 1 (2) |
| Hypertension | 19 (39) | 14 (29) | 5 (10) |
| TSH elevation | 17 (35) | 17 (35) | 0 |
| Diarrhoea | 16 (33) | 16 (33) | 0 |
| Urine occult blood | 15 (31) | 15 (31) | 0 |
| Faecal occult blood | 14 (29) | 14 (29) | 0 |
| Hypothyroidism | 13 (27) | 13 (27) | 0 |
| Arthralgia | 13 (27) | 13 (27) | 0 |
| Pharyngalgia | 10 (20) | 10 (20) | 0 |
| ALT elevation | 9 (18) | 9 (18) | 0 |
| AST elevation | 9 (18) | 9 (18) | 0 |
| Leukopenia | 9 (18) | 9 (18) | 0 |
| Hyperuricemia | 8 (16) | 8 (16) | 0 |
| Serum creatinine elevation | 8 (16) | 8 (16) | 0 |
| Neutropenia | 7 (14) | 6 (12) | 1 (2) |
| Voice hoarse | 7 (14) | 7 (14) | 0 |
| Gingivitis | 6 (12) | 6 (12) | 0 |
| Abdominal pain | 6 (12) | 6 (12) | 0 |
| Headache | 6 (12) | 6 (12) | 0 |
| Hyperbilirubinemia | 6 (12) | 5 (10) | 1 (2) |
| Fatigue | 5 (10) | 5 (10) | 0 |
| Hypokalemia | 5 (10) | 5 (10) | 0 |
| Oral mucositis | 5 (10) | 5 (10) | 0 |
| Dizziness | 5 (10) | 5 (10) | 0 |
| Nausea | 5 (10) | 5 (10) | 0 |
| Thrombocytopenia | 5 (10) | 5 (10) | 0 |
Data presented as n (%). Each patient is counted once for a specific adverse event. Only the highest grade of a given adverse event is reported for each patient. Listed are events of any grade reported in at least 10% of patients.
HFS = hand-foot skin. TSH = thyroid stimulating hormone. ALT = alanine aminotransferase. AST = aspartate aminotransferase.
Discussion
To our knowledge, this is the first prospective study to evaluate the efficacy of anlotinib switch maintenance therapy in patients with advanced STS after benefiting from first-line anthracycline-based chemotherapy. This phase 2 study demonstrated the efficacy of anlotinib as maintenance treatment for patients with advanced STS after achieving partial or stable response to first-line anthracycline-based regimens. With a median PFS of 9.1 months, the study met its primary objective.
After first-line chemotherapy, the tumour lesions remain stable for a relatively brief period of time.15 Patients who achieved the maximal benefits after adequate doses of anthracycline-based chemotherapy were scheduled to stop chemotherapy due to concern with anthracycline-associated cumulative cardiotoxicity or treatment-related toxicity in previous clinical trials.5,33,34 In addition, the median PFS of first-line anthracycline-based chemotherapy in advanced STS was approximately 6 months, which is far from fulfilling clinical demand. Currently, with the exception of maintenance chemotherapy (cyclophosphamide/vinorelbine) for patients with high-risk rhabdomyosarcoma,14 there is no recommended maintenance treatment for patients with advanced STS who achieve benefits from first-line anthracycline-based chemotherapy. Therefore, it is necessary to explore a safe, effective and convenient strategy to consolidate the benefits achieved from first-line chemotherapy for advanced STS patients. Demetri et al. first reported and demonstrated the potential value of postchemotherapy maintenance therapy with targeted drugs in patients with advanced STS.15 However, the improvement in median PFS of maintenance therapy with the mTOR inhibitor ridaforolimus was clinically small (3.1 weeks).15 In addition, second-line continuous maintenance therapy with pazopanib alone following gemcitabine plus pazopanib treatment failed to improve progression-free survival compared with chemotherapy.35 Therefore, it is urgent to explore other targeted drugs that can significantly prolong the PFS of maintenance therapy after chemotherapy.
Anlotinib, a novel multitargeted TKI,17, 18, 19, 20, 21 has been proven to be effective in a variety of tumours,24,25,27, 28, 29, 30,36 including advanced STS (Appendix Table 2). The results of a phase 2 study26 and a phase 2b study (ALTER-0203)31 demonstrated that anlotinib exhibited a significant efficacy and tolerable safety in advanced STS after the failure of anthracycline-based chemotherapy, which also contributed to the CFDA's approval of anlotinib for the treatment of advanced STS. A real-world retrospective study, which analysed the data of anlotinib in 209 patients with unresectable locally advanced or metastatic STS, showed that the median PFS was 6.1 months (95% CI 4.9–7.2) and the ORR was 13.4%.37 Thereafter, two retrospective studies showed that anlotinib combined with chemotherapy followed by anlotinib continuous maintenance treatment was effective and tolerable in STS.38,39 These results were further confirmed by a recently reported prospective phase 2 clinical study.40 However, the evidence supporting anlotinib as a switch maintenance treatment in patients with advanced STS who have achieved disease control with first-line chemotherapy is not sufficient. Only one single-centre, retrospective, small-sample (n = 21) study reported the efficacy of anlotinib maintenance treatment for patients with unresectable or metastatic STS who benefited from chemotherapy.32 A prospective, multicentre clinical study to investigate the efficacy and safety of anlotinib as a maintenance treatment after standard chemotherapy is warranted.
In this study, we recruited 49 patients with advanced STS across different histologic subtypes, mainly liposarcoma, leiomyosarcoma, synovial sarcoma and fibrosarcoma. In terms of dose selection, we continued the conventional clinical use of anlotinib (12 mg for 2 weeks on-treatment followed by 1 week off-treatment).16 We observed the striking efficacy of maintenance anlotinib with respect to the median PFS, whereas patients remained progression-free after discontinuing chemotherapy for a median of 9.1 months. Collectively, these results suggest that anlotinib maintenance treatment may improve the median PFS in patients with advanced STS who benefited from prior chemotherapy when compared with the previously reported median PFS of placebo maintenance treatment.15 Generally, optimal effects were achieved in STS after 6–8 cycles of first-line chemotherapy, with a median PFS of approximately 6 months.6,41,42 It is unfortunate that intolerance to the toxicities of chemotherapy makes patients who have reached PR or SD lose the opportunity to sustain benefits. In this regard, switching to oral targeted drugs as a maintenance treatment may not only further consolidate the therapeutic effects but also reduce injuries to chemotherapy and improve quality of life. To explore the efficacy of maintenance therapy in this population, patients who responded to 4 cycles of chemotherapy but were unable to tolerate further chemotherapy were also enrolled in this study. The median PFS of all patients who underwent 4 cycles of first-line chemotherapy were also promising (n = 24, 49%; median PFS: 9.1 months, 95% CI 5.3–12.8), indicating that 4 cycles of chemotherapy might be enough to achieve satisfactory therapeutic effects for patients who are scheduled to receive anlotinib maintenance treatment after intolerance to prior chemotherapy. However, further randomized phase 3 study is required. Furthermore, subgroup analysis was performed to identify which of the factors were correlated with the patients’ survival. The results showed that with the exception of age, no other predictive factors, such as sex, ECOG performance status, histologic subtype, site/number of primary lesion or metastases, number of first-line chemotherapy cycles and the best responses to first-line chemotherapy, were found to be related to PFS.
Interestingly, among the disease-controlled patients (n = 46), more than half of them (32/46, 70%) maintained disease control for at least 6 months, and 30% (14/46) of them even sustained for over one year, which indicated that the efficacy of anlotinib maintenance treatment was long-lasting for some patients with advanced STS. Because liposarcoma exhibited limited efficacy to other TKIs,43, 44, 45 we enrolled 17 patients with liposarcoma. Although the efficacy of first-line chemotherapy for liposarcoma was unsatisfactory (median PFS: 4 months, 95% CI 3–6),46 patients with liposarcoma exhibited prolonged median PFS in our study (for anlotinib maintenance: 12.5 months, 95% CI 7.1–18.0; for chemotherapy plus anlotinib maintenance: 16.0 months, 95% CI 8.8–23.2); more than a half of them were with DDLS (65%, 11/17). Improved survival was also observed in synovial sarcoma (PFS: 19.1 months, 95% CI 0–48.8) and leiomyosarcoma (PFS: 7.7 months, 95% CI 5.7–9.6). Primary synovial sarcoma rarely presents in the stomach with poor survival and limited therapeutic options, especially when metastasis occurs.47,48 Inspiringly, we observed the dramatic effects of anlotinib maintenance treatment after first-line chemotherapy in a patient with metastatic gastric synovial sarcoma, which may provide evidence for novel therapeutic strategies for advanced gastric synovial sarcoma. However, anlotinib maintenance treatment failed to prolong the survival of patients with fibrosarcoma, with a median PFS of 3.6 months. These results indicated that liposarcoma, synovial sarcoma, and leiomyosarcoma remained the dominant pathological subtypes when treated with anlotinib maintenance treatment after first-line chemotherapy and would be the candidates of concern in future practice and clinical trials.
The common adverse events of anlotinib maintenance treatment included HFS reaction, cholesterol elevation, proteinuria, triglyceride elevation, hypertension, TSH elevation, diarrhoea, urine occult blood, faecal occult blood, hypothyroidism, arthralgia and pharyngalgia, most of which were mild or modest (grade 1–2), similar to those previously reported.16,24, 25, 26, 27, 28,30,36,49 The most frequent grade 3–4 adverse events were hypertension and HFS reaction, while HFS reaction was one of the major reasons for dose reduction. No anlotinib treatment related deaths were reported. Overall, anlotinib maintenance treatment was generally well tolerated.
The results in this study present great potential for the strategy of first-line chemotherapy followed by anlotinib maintenance treatment in STS. However, we have realized that PFS from the beginning of chemotherapy is affected by the immortal bias due to the study design that progressive patients were excluded before the start of anlotinib treatment, which should be interpreted with caution in our study. Moreover, exploration analysis with blood and tissue samples to conduct next-generation sequencing and molecular biological experiments will be performed on a larger cohort in the future studies.
In conclusion, this multicentre, open-label, single-arm, phase 2 trial showed the promising efficacy and acceptable toxicity of anlotinib as maintenance treatment after first-line anthracycline-based chemotherapy, thus revealing the promising efficacy of anlotinib as maintenance therapy in patients with advanced STS who benefited from standard chemotherapy.
Contributors
BSX and XZ conceived and designed the study. QZP, MYF, HML, XAL, JC, DMP, and BQZ recruited patients. DSW and RQP collected the clinical data. BSX, QZP, HP and MYF analysed and interpreted these data. BSX and XZ wrote the manuscript. BSX, QZP, and XZ critically reviewed the manuscript. XZ and MYF contributed to the study supervision. All authors approved the final draft. BSX, QZP, HP, and XZ have directly accessed and verified the underlying data reported in the manuscript. All the authors had full access to all the data in the study and the corresponding authors had final responsibility for the decision to submit for publication.
Data sharing statement
The authenticity of this study has been validated by uploading the raw data that support the findings onto the Research Data Deposit public platform (No. RDDA2023373178). Data are available from the corresponding authors upon reasonable request and with the permission of Sun Yat-sen University Cancer Centre.
Declaration of interests
The authors declare no competing interests.
Acknowledgements
This work was supported by grants from the National Key Research and Development Program of China (No. 2021YFC2400600/2021YFC2400601) and the National Natural Science Foundation of China (No. 82102776, 82072958, and 82272699). Study drug was provided by Chia Tai Tianqing Pharmaceutical Group Co., Ltd. We thank the patients who participated in this study, and also be grateful to all the colleagues for their contributions to the work.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102240.
Contributor Information
Meiyu Fang, Email: fangmy@zjcc.org.cn.
Xing Zhang, Email: zhangxing@sysucc.org.cn.
Appendix A. Supplementary data
References
- 1.Gamboa A.C., Gronchi A., Cardona K. Soft-tissue sarcoma in adults: an update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin. 2020;70:200–229. doi: 10.3322/caac.21605. [DOI] [PubMed] [Google Scholar]
- 2.Sbaraglia M., Bellan E., Dei Tos A.P. The 2020 WHO classification of soft tissue tumours: news and perspectives. Pathologica. 2021;113:70–84. doi: 10.32074/1591-951X-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Italiano A., Mathoulin-Pelissier S., Cesne A.L., et al. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer. 2011;117:1049–1054. doi: 10.1002/cncr.25538. [DOI] [PubMed] [Google Scholar]
- 4.Cormier J.N., Pollock R.E. Soft tissue sarcomas. CA Cancer J Clin. 2004;54:94–109. doi: 10.3322/canjclin.54.2.94. [DOI] [PubMed] [Google Scholar]
- 5.Santoro A., Tursz T., Mouridsen H., et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1995;13:1537–1545. doi: 10.1200/JCO.1995.13.7.1537. [DOI] [PubMed] [Google Scholar]
- 6.Judson I., Verweij J., Gelderblom H., et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415–423. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 7.Elias A., Ryan L., Sulkes A., Collins J., Aisner J., Antman K. Response to mesna, doxorubicin, ifosfamide, and dacarbazine in 108 patients with metastatic or unresectable sarcoma and no prior chemotherapy. J Clin Oncol. 1989;7:1208–1216. doi: 10.1200/JCO.1989.7.9.1208. [DOI] [PubMed] [Google Scholar]
- 8.Edmonson J.H., Ryan L.M., Blum R.H., et al. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol. 1993;11:1269–1275. doi: 10.1200/JCO.1993.11.7.1269. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho C., Santos R.X., Cardoso S., et al. Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem. 2009;16:3267–3285. doi: 10.2174/092986709788803312. [DOI] [PubMed] [Google Scholar]
- 10.Launchbury A.P., Habboubi N. Epirubicin and doxorubicin: a comparison of their characteristics, therapeutic activity and toxicity. Cancer Treat Rev. 1993;19:197–228. doi: 10.1016/0305-7372(93)90036-q. [DOI] [PubMed] [Google Scholar]
- 11.Shan K., Lincoff A.M., Young J.B. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996;125:47–58. doi: 10.7326/0003-4819-125-1-199607010-00008. [DOI] [PubMed] [Google Scholar]
- 12.Von Hoff D.D., Layard M.W., Basa P., et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 13.Verschoor A.J., Litiere S., Marreaud S., et al. Survival of soft tissue sarcoma patients after completing six cycles of first-line anthracycline containing treatment: an EORTC-STBSG database study. Clin Sarcoma Res. 2020;10:18. doi: 10.1186/s13569-020-00137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bisogno G., De Salvo G.L., Bergeron C., et al. Vinorelbine and continuous low-dose cyclophosphamide as maintenance chemotherapy in patients with high-risk rhabdomyosarcoma (RMS 2005): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20:1566–1575. doi: 10.1016/S1470-2045(19)30617-5. [DOI] [PubMed] [Google Scholar]
- 15.Demetri G.D., Chawla S.P., Ray-Coquard I., et al. Results of an international randomized phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapy. J Clin Oncol. 2013;31:2485–2492. doi: 10.1200/JCO.2012.45.5766. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y., Niu W., Du F., et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. 2016;9:105. doi: 10.1186/s13045-016-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin B., Song X., Yang D., Bai D., Yao Y., Lu N. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene. 2018;654:77–86. doi: 10.1016/j.gene.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Zhong C.C., Chen F., Yang J.L., et al. Pharmacokinetics and disposition of anlotinib, an oral tyrosine kinase inhibitor, in experimental animal species. Acta Pharmacol Sin. 2018;39:1048–1063. doi: 10.1038/aps.2017.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song F., Hu B., Cheng J.W., et al. Anlotinib suppresses tumor progression via blocking the VEGFR2/PI3K/AKT cascade in intrahepatic cholangiocarcinoma. Cell Death Dis. 2020;11:573. doi: 10.1038/s41419-020-02749-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie C., Wan X., Quan H., et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018;109:1207–1219. doi: 10.1111/cas.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He C., Wu T., Hao Y. Anlotinib induces hepatocellular carcinoma apoptosis and inhibits proliferation via Erk and Akt pathway. Biochem Biophys Res Commun. 2018;503:3093–3099. doi: 10.1016/j.bbrc.2018.08.098. [DOI] [PubMed] [Google Scholar]
- 22.Wang G., Sun M., Jiang Y., et al. Anlotinib, a novel small molecular tyrosine kinase inhibitor, suppresses growth and metastasis via dual blockade of VEGFR2 and MET in osteosarcoma. Int J Cancer. 2019;145:979–993. doi: 10.1002/ijc.32180. [DOI] [PubMed] [Google Scholar]
- 23.Syed Y.Y. Anlotinib: first global approval. Drugs. 2018;78:1057–1062. doi: 10.1007/s40265-018-0939-x. [DOI] [PubMed] [Google Scholar]
- 24.Han B., Li K., Zhao Y., et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302) Br J Cancer. 2018;118:654–661. doi: 10.1038/bjc.2017.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han B., Li K., Wang Q., et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 2018;4:1569–1575. doi: 10.1001/jamaoncol.2018.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chi Y., Fang Z., Hong X., et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma. Clin Cancer Res. 2018;24:5233–5238. doi: 10.1158/1078-0432.CCR-17-3766. [DOI] [PubMed] [Google Scholar]
- 27.Sun Y., Du F., Gao M., et al. Anlotinib for the treatment of patients with locally advanced or metastatic medullary thyroid cancer. Thyroid. 2018;28:1455–1461. doi: 10.1089/thy.2018.0022. [DOI] [PubMed] [Google Scholar]
- 28.Ma J., Song Y., Shou J., et al. Anlotinib for patients with metastatic renal cell carcinoma previously treated with one vascular endothelial growth factor receptor-tyrosine kinase inhibitor: a phase 2 trial. Front Oncol. 2020;10:664. doi: 10.3389/fonc.2020.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang J., Xiao J., Fang W., et al. Anlotinib for previously treated advanced or metastatic esophageal squamous cell carcinoma: a double-blind randomized phase 2 trial. Cancer Med. 2021;10:1681–1689. doi: 10.1002/cam4.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chi Y., Shu Y., Ba Y., et al. Anlotinib monotherapy for refractory metastatic colorectal cancer: a double-blinded, placebo-controlled, randomized phase III trial (ALTER0703) Oncologist. 2021;26:e1693–e1703. doi: 10.1002/onco.13857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chi Y., Yao Y., Wang S., et al. Anlotinib for metastasis soft tissue sarcoma: a randomized, double-blind, placebo-controlled and multi-centered clinical trial. J Clin Oncol. 2018;36 [Google Scholar]
- 32.Liu J., Deng Y.T., Jiang Y. Switch maintenance therapy with anlotinib after chemotherapy in unresectable or metastatic soft tissue sarcoma: a single-center retrospective study. Invest New Drugs. 2021;39:330–336. doi: 10.1007/s10637-020-01015-z. [DOI] [PubMed] [Google Scholar]
- 33.Grünwald V., Karch A., Schuler M., et al. Randomized comparison of pazopanib and doxorubicin as first-line treatment in patients with metastatic soft tissue sarcoma age 60 Years or older: results of a German intergroup study. J Clin Oncol. 2020;38:3555–3564. doi: 10.1200/JCO.20.00714. [DOI] [PubMed] [Google Scholar]
- 34.Judson I., Radford J., Harris M., et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXILI/CAELYXI) versus doxorubicin. Eur J Cancer. 2001;37:870–877. doi: 10.1016/s0959-8049(01)00050-8. [DOI] [PubMed] [Google Scholar]
- 35.Pautier P., Penel N., Ray-Coquard I., et al. A phase II of gemcitabine combined with pazopanib followed by pazopanib maintenance, as second-line treatment in patients with advanced leiomyosarcomas: a unicancer French Sarcoma Group study (LMS03 study) Eur J Cancer. 2020;125:31–37. doi: 10.1016/j.ejca.2019.10.028. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y., Zhou A., Zhang W., et al. Anlotinib in the treatment of advanced hepatocellular carcinoma: an open-label phase II study (ALTER-0802 study) Hepatol Int. 2021;15:621–629. doi: 10.1007/s12072-021-10171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang R.S., Liu J., Deng Y.T., Wu X., Jiang Y. The real-world clinical outcomes and treatment patterns of patients with unresectable locally advanced or metastatic soft tissue sarcoma treated with anlotinib in the post-ALTER0203 trial era. Cancer Med. 2022;11:2271–2283. doi: 10.1002/cam4.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng K., Liu X., Chen Y., Zhou K., Li Z. Response to chemotherapy combined with anlotinib plus anlotinib maintenance in intra-abdominal desmoplastic small round cell tumors (IADSRCT): a case report and literature review. BMC Gastroenterol. 2022;22:388. doi: 10.1186/s12876-022-02463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H.Y., Chu J.F., Zhang P., et al. Safety and efficacy of chemotherapy combined with anlotinib plus anlotinib maintenance in Chinese patients with advanced/metastatic soft tissue sarcoma. Onco Targets Ther. 2020;13:1561–1568. doi: 10.2147/OTT.S235349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z., Zhuang R., Guo X., et al. Anlotinib plus epirubicin followed by anlotinib maintenance as first-line treatment for advanced soft tissue sarcoma: an open-label, single-arm, phase 2 trial. Clin Cancer Res. 2022;28:5290–5296. doi: 10.1158/1078-0432.CCR-22-1903. [DOI] [PubMed] [Google Scholar]
- 41.Maurel J., Lopez-Pousa A., de Las Penas R., et al. Efficacy of sequential high-dose doxorubicin and ifosfamide compared with standard-dose doxorubicin in patients with advanced soft tissue sarcoma: an open-label randomized phase II study of the Spanish group for research on sarcomas. J Clin Oncol. 2009;27:1893–1898. doi: 10.1200/JCO.2008.19.2930. [DOI] [PubMed] [Google Scholar]
- 42.Seddon B., Strauss S.J., Whelan J., et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1397–1410. doi: 10.1016/S1470-2045(17)30622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samuels B.L., Chawla S.P., Somaiah N., et al. Results of a prospective phase 2 study of pazopanib in patients with advanced intermediate-grade or high-grade liposarcoma. Cancer. 2017;123:4640–4647. doi: 10.1002/cncr.30926. [DOI] [PubMed] [Google Scholar]
- 44.Riedel R.F., Ballman K.V., Lu Y., et al. A randomized, double-blind, placebo-controlled, phase II study of regorafenib versus placebo in advanced/metastatic, treatment-refractory liposarcoma: results from the SARC024 study. Oncologist. 2020;25:e1655–e1662. doi: 10.1634/theoncologist.2020-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Mehren M., Rankin C., Goldblum J.R., et al. Phase 2 Southwest Oncology Group-directed intergroup trial (S0505) of sorafenib in advanced soft tissue sarcomas. Cancer. 2012;118:770–776. doi: 10.1002/cncr.26334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stacchiotti S., Van der Graaf W.T.A., Sanfilippo R.G., et al. First-line chemotherapy in advanced intra-abdominal well-differentiated/dedifferentiated liposarcoma: an EORTC Soft Tissue and Bone Sarcoma Group retrospective analysis. Cancer. 2022;128:2932–2938. doi: 10.1002/cncr.34264. [DOI] [PubMed] [Google Scholar]
- 47.Makhlouf H.R., Ahrens W., Agarwal B., et al. Synovial sarcoma of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 10 cases. Am J Surg Pathol. 2008;32:275–281. doi: 10.1097/PAS.0b013e31812e6a58. [DOI] [PubMed] [Google Scholar]
- 48.Kamata K., Wada R., Yajima N., Sawada M., Wakasa H., Yagihashi S. Primary gastric synovial sarcoma: molecular diagnosis and prediction of prognosis. Clin J Gastroenterol. 2013;6:303–308. doi: 10.1007/s12328-013-0403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chi Y., Ji G., Zhang J., et al. Efficacy and safety of anlotinib in patients with advanced malignancy: a single-center, single-arm, phase 2 trial. Int J Clin Oncol. 2021;26:1611–1618. doi: 10.1007/s10147-021-01959-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.