Abstract
The formation, proliferation, and evolution of glioblastoma (GB) are significantly influenced by pathological angiogenesis. This is supported by several growth factor receptors, such as the vascular endothelial growth factor receptor (VEGFR). In this experiment, we examined how the Food and Drug Administration (FDA) approved VEGFR blockers Sorafenib and Axitinib affect the viability of GB cells in vitro. Cells were cultivated in 96-well culture plates for the experiments, afterwards Sorafenib and Axitinib were administered at doses ranging from 0.3 μM to 80 μM. 2,5-Diphenyl-2H-tetrazolium bromide (MTT) assay was used to assess the impact of VEGFR inhibition on high-grade glioma (HGG) cell lines. To observe the morphological changes in cell shape, we used a 10× magnification microscopy. Our results showed that both Axitinib and Sorafenib retarded GB1B culture proliferation in a dose- and time-dependent manner in comparison to control cohorts that had not received any treatment. The half maximal inhibitory concentration (IC50) value for Axitinib was 3.5839 μM after three days of drug administration and 2.2133 μM after seven days of drug administration. The IC50 value for Sorafenib was 3.5152 μM after three days of drug administration and 1.6846 μM after seven days of drug administration. After the treatment with Axitinib or Sorafenib, very few cells became rounded and detached from the support, others remained adherent to the culture substrate, but acquired a larger, flatter shape. Our results indicate that VEGFR might serve as a key target in the treatment of GB. Although it is known that in vitro some drugs block the VEGFR more potently, clinical evidence is required to show whether this actually translates to better clinical outcomes.
Keywords: Axitinib , Sorafenib , high-grade glioma , treatment , targeted therapy
Introduction
Because of their clinical significance, angiogenic biomarkers are potential therapeutic candidates for glioblastoma (GB), the most fatal malignant brain tumor with an average lifespan of fewer than 15 months. Angiogenesis is mediated by complex interactions between many pro- and anti-angiogenic molecules. Several receptor tyrosine kinases (RTKs) are known to be associated with tumor angiogenesis: vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor (PDGFR), epidermal growth factor receptor (EGFR), latrophilin and seven transmembrane domain-containing protein 1 receptor (ELTD1) and TEK receptor kinase (TIE2) [ 1, 2, 3, 4, 5]. Recently, a new angiogenic receptor, ELTD1, was reported by several research groups to be involved in both physiological and pathological angiogenesis [ 6, 7]. ELTD1 plays an important role in tumor angiogenesis. ELTD1 was found to be expressed in human glioma, its expression begins significantly higher in high-grade gliomas (HGGs) compared to low-grade gliomas (LGGs) [ 8]. Several classical and non-classical proangiogenic factors were described in recent years, their role in tumor angiogenesis progression is still under debate in the scientific community. Nine classical proangiogenic factors [i.e., VEGF, PDGF, FGF-2, platelet-derived endothelial cell growth factor/thymidine phosphorylase (PD-ECGF/TP), angiopoietins (Ang), hepatocyte growth factor (HGF), insulin-like growth factors (IGFs), tumor necrosis factor (TNF) and interleukin-6 (IL-6)] and three non-classical proangiogenic factors [i.e., stem cell factor (SCF), tryptase and chymase] were reported in the literature [ 9, 10, 11, 12]. The most frequent mechanisms that drive and maintain tumor angiogenesis are described to be concurrently: (i) increased VEGF, acidic and basic FGF, IL-8 and IL-6, hypoxia-inducible factor-1 alpha (HIF-1α) and Ang, together with (ii) downregulation of thrombospondins, angiostatin, endostatin and interferons [ 13, 14, 15, 16]. Proangiogenic growth factors (GFs) activate surface RTKs as well as other cell membrane receptors such as integrins. In tumor angiogenesis, VEGF is upregulated by hypoxia and a variety of other GFs [ 17]. The interaction between HIFs and RTKs has also been reported to be important for expansion of the new blood vessel formation [ 18, 19]. In this frame, VEGF (also called VEGF-A), as a crucial molecule controlling the development and microarchitecture of angiogenic vessel networks, being at the top of the list [ 20]. Several anti-angiogenic drugs work by binding directly to VEGF, blocking it from binding to the VEGFR. Over the last decade, five different VEGF/VEGFR targeted agents were approved by the Food and Drug Administration (FDA) for the treatment of cancer: Axitinib, Sorafenib, Sunitinib, Pazopanib and Bevacizumab. Many small-molecule RTK inhibitors (RTKIs) have also been used to block angiogenesis in malignant tumors. Sorafenib (Nexavar®, Bayer), a multi-targeted RTKI, has been FDA approved as a single agent in advanced renal cell carcinoma (RCC) and hepatocellular carcinoma (HCC) [ 21]. Another multi-targeted RTKI, Sunitinib (Sutent®, Pfizer), is also FDA approved for gastrointestinal stromal tumors and advanced RCC [ 22]. Axitinib is a more potent VEGFR inhibitor, selectively inhibiting VEGFRs 1, 2, and 3 tyrosine kinase activity. The drug has been approved for second line treatment of advanced RCC, showing antitumor activity both as a single-agent and in combination with other therapeutic approaches [ 23, 24]. VEGFR is strongly expressed in GB and progression from LGGs to HGGs is also mediated by this surface receptor [ 25]. Thus, VEGFR inactivation represents the most promising line of attack in GB. In May 2009, FDA approved Bevacizumab (Avastin®), a humanized anti-VEGF monoclonal antibody, for recurrent GB in United States. Although many clinical studies have shown the drug’s effectiveness as a single treatment or in combination with other therapeutic modalities in recurrent GB, the European Medicines Agency (EMA) denied this drug, due to a lack of evidence. The use of Bevacizumab drug in human GB is controversial, the effect of the drug on GB tumor size, recurrence, and vascularization is still unclear [ 26]. Several RTKIs have also been under investigation in the setting of recurrent HGG. Cediranib, Sunitinib, Pazopanib, Vandetanib, and Sorafenib are just some of the multi-kinase VEGFR inhibitors that were evaluated in GB [ 27, 28]. Yet, several phase III trials demonstrated relatively modest advantages when anti-angiogenic RTKIs were compared with conventional chemotherapy, despite the efficacy seen in clinical trials with several such RTKIs [ 29, 30]. These findings raise concerns about the impact of anti-angiogenesis medications on the tumor uptake of other chemotherapeutic drugs and the overall anti-tumor effect of combination therapy. Thus, the dominant role of VEGF system in the angiogenesis process, makes it the most attractive target in disrupting tumor angiogenesis and in this study, we investigated how VEGFR suppression affects GB cells viability in vitro. By utilizing the FDA-approved VEGFR inhibitors Sorafenib and Axitinib, we examined the impact of receptor inhibition on GB cells viability in vitro [ 31, 32, 33, 34].
Materials and Methods
Drugs and reagents
Sorafenib and Axitinib drugs were purchased from Redox Life Tech. Dulbecco’s Modified Eagle Medium (DMEM)/nutrient mixture F-12 Ham, with L-glutamine and sodium bicarbonate, without 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), sterile (MEM, D8062-500 mL, Sigma), Fetal Bovine Serum (FBS, F7524-500 mL, Sigma), Trypsin–Ethylenediaminetetraacetic acid (EDTA) solution 10X (T4174-100 mL, Sigma) and Cell Proliferation Kit I [2,5-Diphenyl-2H-tetrazolium bromide (MTT), RO11465007001, Roche Diagnostics] were supplied by Redox Life Tech.
Cell line establishment
The Bagdasar–Arseni Emergency Hospital in Bucharest, Romania provided us with surplus biological material from brain tumors that enabled our Laboratory to develop the GB cell line (GB1B), according to standard procedures [ 35, 36].
By signing the consent paperwork during their hospital stay, all patients agreed to donate their tissue for research purposes. In summary, fresh tumor tissues were diced in a Petri dish with an aseptic blade, and then combined with 0.5 mg/mL pronase, 0.25 mg/mL collagenase IV, and 0.4 mg/mL deoxyribonuclease (DNase). The specimens were placed in Hank’s buffered saline solution and kept in a shaking incubator for 30 minutes at 37ºC, and subsequently another 30 minutes at 4ºC. Moreover, the cell mixture was passed through a cell filter to create a suspension of just one cell. Phosphate-buffered saline (PBS) was used to clean the cells twice before they were seeded in six-well plates. Ultimately, a cell suspension was placed into tissue culture flasks and passaged 2–3 times. In this experiment, the same passage cells were used.
Cell culture
DMEM containing 10% FBS, 2 mM L-glutamine and antibiotics (100 IU/mL Penicillin, 100 IU/mL Streptomycin) were used to develop the cell line cultures. The cells were maintained in tissue-culture flasks and kept in a humidified incubator at 37ºC with a 95% air/5% carbon dioxide (CO2) environment. From the original biological material, the cell cultures were amplified 2–3 passages, and the third passage was preserved. When the confluence hit 30–50%, experiments were started. For our study, cells seeded in monolayers in 96-well culture plates (1–10×103 cells/well), under the same environmental conditions as during the amplification phase, were treated with varying concentrations of Axitinib or Sorafenib (0.3; 0.6; 1.25; 2.5; 5; 10; 20; 40; and 80 μM), for three days and seven days, respectively. We included adequate control groups that contained solely diluents and blanks.
Cell proliferation assay
Cellular proliferation was quantified using the MTT assay, which relies on the ability of cells with active metabolism to cleave yellow tetrazolium salt into purple formazan crystals. Cells at a confluence of 1×103 cells per well/200 μL medium were seeded in 96-well plates, incubated for eight hours, and then exposed to different doses of SU1498 (an inhibitor of VEGFR2) alone or in combination with radiation. The cells were incubated for three and seven days, after which 10 μL of the MTT labeling agent was added to each individual well. The cells were incubated for four hours, at 37°C, after which they were solubilized, and the optical density (OD) was measured at 595 nm. The percentage of cells in the control group used to measure cell viability. To observe the morphological changes in cell shape, we used a 10× magnification phase contrast microscopy.
IC50
The cells were exposed to increasing Axitinib or Sorafenib doses (0.3 μM; 0.6 μM; 1.25 μM; 2.5 μM; 5 μM; 10 μM; 20 μM; 40 μM; and 80 μM) for three and seven days and cell viability was determined by MTT. The free online Quest Graph™ IC50 Calculator offered by AAT Bioquest was used to solve the equation to estimate the inhibitory dosage that results in the death of 50% of cells (IC50). The Hill coefficient in the equation is negative for promotion activity and positive for the inhibition effect.
Statistical analysis
Using the Student’s t-test, mean values were statistically compared. Differences were deemed statistically significant if their p-value was less than 0.05.
Results
The cytotoxic effect of Axitinib on GB cells
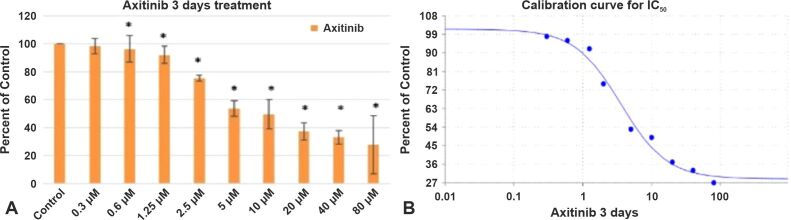
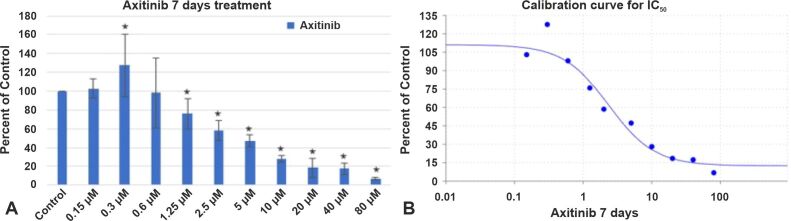
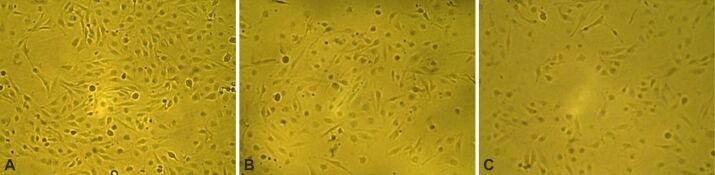
Axitinib (AG-013736), an oral tyrosine kinase inhibitor (TKI), exhibits promising anticancer efficacy in several other advanced malignancies, including GB [ 33, 37]. In this section, we have analyzed how VEGFR inhibition via Axitinib induced cell death in GB cells. For this reason, we used a GB cell line (GB1B) established in our Laboratory. The cells were cultivated in regular DMEM supplemented with 10% FBS, 2 mM of L-glutamine and a mix of two antibiotics (100 IU/mL Penicillin, 100 IU/mL Streptomycin). In the experimental setting, Axitinib was used to treat cells that were seeded in 96-well culture plates (0.5–1–3×103 cells/well, at doses ranging from 0.3 μM to 80 μM. The proliferation of the GB cells was measured after three and seven days. The GB1B cell line experienced the following notable effects after three days of exposure to increasing Axitinib concentrations: the treatment with 1.25 μM reduced GB1B cell survival by 8%, 2.5 μM Axitinib treatment produced 25% cell death in GB1B cells, 5 μM drug treatment reduced cell viability by 47%, 10 μM treatment decreased cell viability by 51%, 20 μM treatment produced 63% cell death, 40 μM drug treatment reduced cell survival by 66%, and proliferation was decreased by 73% with 80 μM treatment (Figure 1A). Higher cytotoxicity was seen in GB1B cells after extended exposure to a 7-day treatment with an increasing Axitinib concentration. The treatment with 1.25 μM drug impaired cell survival by 25%, 2.5 μM drug treatment reduced cell viability by 42%, 5 μM drug treatment reduced cell viability by 53%, 10 μM drug treatment decreased cell viability by 72%, 20 μM drug treatment reduced cell viability by 92%, 40 μM drug treatment produced a 93% reduction in cell viability, and 80 μM produced a 97% reduction in proliferation (Figure 2A). Axitinib’s IC50 value was 3.5839 μM after three days of treatment (Figure 1B) and 2.2133 μM after a 7-day course of treatment (Figure 2B). The cells that survived three and seven days of Axitinib treatment did not undergo a marked change in cell shape or size, as can be seen in Figure 3A, 3B, 3C. Assessment of phase contrast microscopy (10× magnification) on cells treated with Axitinib showed very few alterations in cell shape (rounded cell) and detachment from cell substrate (Figure 3A, 3B, 3C).
Figure 1.
The effect of Axitinib on GB1B proliferation (A) and the calibration curve for IC50 (B) at three days of treatment. Results are expressed as percentage of control. Data represents the mean and standard error of three separate experiments. Error bars are the mean ± SD for each drug concentration, representing the linear model fit to the data. *Represents significant difference from control (p<0.05). GB1B: Glioblastoma cell line; IC50: Half maximal inhibitory concentration; SD: Standard deviation.
Figure 2.
The effect of Axitinib on GB1B proliferation (A) and the calibration curve for IC50 (B) at seven days of treatment. Results are expressed as percentage of control. Data represents the mean and standard error of three separate experiments. Error bars are the mean ± SD for each drug concentration, representing the linear model fit to the data. *Represents significant difference from control (p<0.05)
Figure 3.
The effect of Axitinib on GB1B cells viability and morphology. The cells were exposed to 10 μM Axitinib for three and seven days. Microscopy pictures were taken at initial culture day (A), three days (B) and seven days (C) after the treatment with 10 μM Axitinib (10× magnification).
The cytotoxic effect of Sorafenib on GB cells
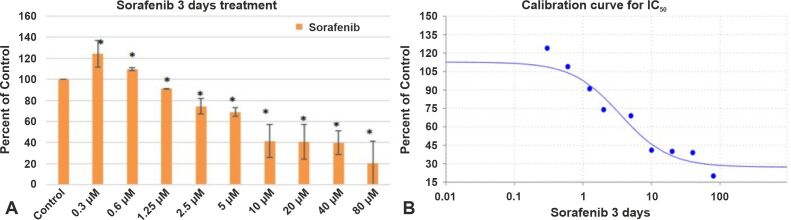
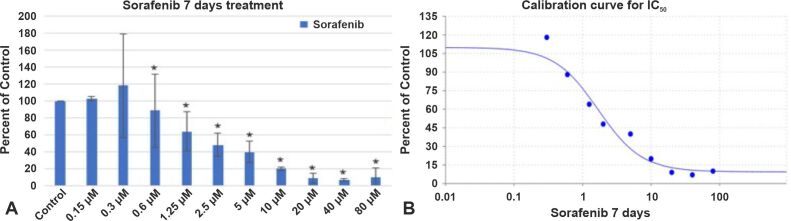
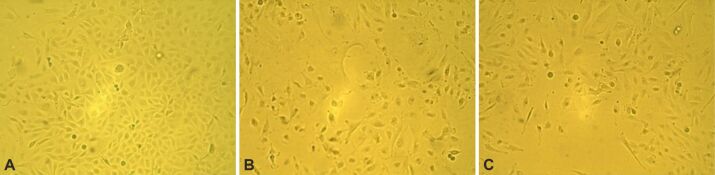
In a study by Jakubowicz-Gil et al., it has been reported that after Sorafenib treatment, autophagy was most frequently seen in T98G cells [ 38]. After three days of treatment with Sorafenib, the percentage of viable cells decreased as follows: 9% for a drug concentration of 1.25 μM, 25% for a drug concentration of 2.5 μM, 41% for a drug concentration of 5 μM, 59% for a drug concentration of 10 μM, 60% for a drug concentration of 20 μM, 61% for a drug concentration of 40 μM, and 80% for a drug concentration of 80 μM (Figure 4A). After a 7-day course of Sorafenib therapy, cell viability was significantly reduced, resulting in 46% cell death after a 1.25 μM treatment course, 52% cell death after a 2.5 μM treatment course, 60% cell death after a 5 μM treatment course, 80% cell death after a 10 μM treatment course, 91% cell death after a 20 μM treatment course, 93% cell death after a 40 μM treatment course, and 90% cell death after a 80 μM treatment course (Figure 5A). Sorafenib’s IC50 value was 3.5152 μM after a 3-day course of treatment (Figure 4B) and 1.6846 μM after a 7-day course of treatment (Figure 5B). Overall, our findings show that GB express VEGFR and might constitute a significant target in GB treatment. For most of these anchorage-dependent cells there were no changes in cell shape or size treated after Sorafenib treatment for three or seven days (Figure 6A, 6B, 6C).
Figure 4.
The effect of Sorafenib on GB1B proliferation (A) and the calibration curve for IC50 (B) at three days of treatment. Results are expressed as percentage of control. Data represents the mean and standard error of three separate experiments. Error bars are the mean ± SD for each drug concentration, representing the linear model fit to the data. *Represents significant difference from control (p<0.05).
Figure 5.
The effect of Sorafenib on GB1B proliferation (A) and the calibration curve for IC50 (B) at seven days of treatment. Results are expressed as percentage of control. Data represents the mean and standard error of three separate experiments. Error bars are the mean ± SD for each drug concentration, representing the linear model fit to the data. *Represents significant difference from control (p<0.05).
Figure 6.
The effect of Sorafenib on GB1B cells viability and morphology. The cells were exposed to 10 μM Sorafenib for three and seven days. Microscopy pictures were taken at initial culture day (A), three days (B) and seven days (C) after the treatment with 10 μM Sorafenib (10× magnification).
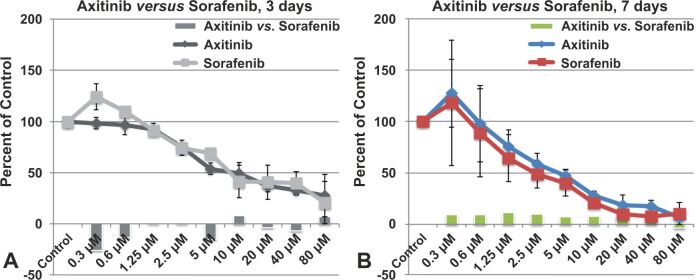
Comparison of cytotoxicity induced of Axitinib and Sorafenib by equimolar doses on GB cells
Axitinib was superior to Sorafenib (Nexavar®) and was approved for use in advanced RCC [ 39]. As seen in Figure 7A, Axitinib treatment provided to be cytotoxic for GB cells at very low concentrations, starting from 0.3 μM, while Sorafenib at concentrations of 0.3 μM and 0.6 μM induced cell proliferation in GB cells, becoming cytotoxic from a concentration of 1.25 μM. The cytotoxicity produced by Axitinib was approximately 26% higher than that produced by Sorafenib at concentrations of 0.3 μM and by 13% higher at concentrations of 0.6 μM. A very large difference in cytotoxicity was also recorded at the concentration of 5 μM, Axitinib producing 15% stronger cytotoxic effect than Sorafenib in GB1B cells. In general, the treatment with Axitinib was much more effective than the one with Sorafenib, at three days treatment. On the contrary, after seven days of treatment, Sorafenib treatment was superior to Axitinib treatment (Figure 7B). Except for the maximum concentration used in the study (80 μM), where the cytotoxic effect of the two drugs was comparable, at all other concentrations used, the cytotoxic effect of Sorafenib was 8–10% stronger than the cytotoxic effect produced by Axitinib (Figure 7B).
Figure 7.
Comparison of Axitinib and Sorafenib effect on GB1B proliferation, at three days (A) and seven days (B) of treatment. *Represents significant difference from control (p<0.05).
Discussions
In GB, VEGF is the most prevalent and significant mediator of angiogenesis. The use of VEGF system inhibition for the treatment of HGGs is becoming more and more popular. Several VEGFR inhibitors are being researched for HGGs. These drugs were investigated in a variety of clinical trials, with the results advancing our understanding of antiangiogenic therapy as a whole [ 5, 40, 41].
The recombinant humanized monoclonal antibody Bevacizumab (Avastin®) has also been investigated in both recurrent and newly diagnosed GB. Bevacizumab has shown significant activity in recurrent GBs, resulting in the FDA approved in 2009 [ 42, 43]. The combined treatment with Bevacizumab and Irinotecan for malignant gliomas treatment was also used in several clinical trials, and the results were promising [ 26]. Cancer immunotherapy has also raised the interest of the scientific community in recent years. In a meta-analysis study, it was found that dendritic cell (DC) therapy improves overall survival and progression-free survival (PFS) of HGG patients, both recurrent and newly diagnosed. Viral therapy also slightly improved overall survival but PFS was similar to the control arms [ 26, 44].
Axitinib demonstrated anti-tumor activity in preclinical and clinical studies, for different types of cancer, including thyroid cancer, epithelial ovarian cancer, and nasopharyngeal cancer [ 45, 46, 47]. Other angiogenic inhibitors including Trebananib, Aflibercept, Nintedanib, Cediranib, Imatinib, are also in ongoing clinical trials, but Axitinib effects are different from those of earlier treatments due to its more potent and active inhibition of VEGFR [ 48, 49].
In the treatment of brain tumors, Axitinib has been very little studied. One clinical study demonstrated that Axitinib induced direct cytotoxic effect against several patient-derived GB stem cells and also extended survival in preclinical orthotopic GB models, when administered as systemic single agent [ 32]. The results from a randomized phase II trial [ 37], showed that in recurrent GB patients, Axitinib treatment resulted in improved response rate. In the present study, we also found that Axitinib induced cytotoxicity in a patient-derived GB cell line; Axitinib’s IC50 value was 3.58 μM after three days of medical treatment and 2.21 μM after seven days of medical treatment. Axitinib treatment did not produce a marked change in cell shape or size. Assessment of phase contrast microscopy on cells treated with Axitinib showed very few alterations in cell shape (rounded cell) and detachment from cell substrate. Although the result from our and other research groups show that Axitinib is a potent anticancer drug in vitro, more clinical evidence is required to show whether this actually translates to better clinical outcomes.
Sorafenib, also known as Nexavar®, is a multikinase inhibitor that has been investigated in many solid tumors.
The medication has first been given clinical approval for the treatment of advanced RCC and HCC [ 50]. Nowadays, several clinical trials using Sorafenib are being conducted, including those for lung, thyroid, prostate, and breast cancer [ 51, 52, 53, 54]. In GB patients, Sorafenib as monotherapy or in combination with Temozolomide showed only limited benefit and considerable toxicity [ 55, 56]. When radiotherapy was added to Sorafenib and Temozolomide combination, same moderate outcome results were found, as compared to standard therapy alone, however the adverse effects were significantly increased [ 57]. Here, we found that Sorafenib was cytotoxic in GB1B cells, the IC50 value was 3.5152 μM after three days of medical treatment and 1.6846 μM after seven days of medical treatment. For most of these anchorage-dependent cells, there were no changes in cell shape or size after Sorafenib treatment for three or seven days.
In actuality, the FDA has authorized the use of four VEGFR TKIs: Sorafenib, Sunitinib, Pazopanib, and Axitinib; as well as one VEGF-direct antibody, Bevacizumab, for the management of metastatic RCC since 2005. A phase III trial was conducted evaluating the effectiveness of Axitinib with Sorafenib in individuals with metastatic RCC who have not had systemic treatment previously. According to this trial, Axitinib and Sorafenib showed a median PFS of 10.1 months and 6.5 months, respectively. Axitinib treatment resulted in a numerically longer PFS, compared to Sorafenib treatment, however this did not reach the required level of statistical significance, thus, the trial failure to increase PFS with Axitinib against Sorafenib for metastatic RCC was the final conclusion [ 58]. Our results showed that both Axitinib and Sorafenib retarded GB1B cell growth in terms of dose and duration in comparison with the untreated control groups. Axitinib treatment provided to be more cytotoxic on GB1B cells, at three days after the treatment, while Sorafenib treatment was superior to Axitinib treatment, at seven days after the treatment. For the majority of these GB1B anchorage-dependent cells, no significant change in shape or size could be observed after Axitinib or Sorafenib treatment. These results suggest that the treatment inhibits cell growth, rather than killing the cells. However, this observation is speculative, and the phenomenon must be studied in depth, in order to draw a conclusion.
Conclusions
GB treatment failure, as well as its progression and recurrence results from the fundamental nature of this disease, challenging the scientists to accelerate the study of the cellular mechanisms underlying tumor development, to provide better diagnostic methods and better therapeutic targets. Identifying biomarkers may increase the chance of GB patients’ survival, since they can facilitate timely diagnosis and aid in the selection of a targeted treatment. Overall, our results point to VEGFR as a potential therapeutic target for the treatment of GB. Although it is known that some drugs block the VEGFR more potently in vitro, clinical evidence is required to show whether this actually translates to better clinical outcomes.
Conflict of interests
The authors declare that they have no conflict of interests
Source of funding
This research was funded by Grant PN-III-P4-ID-PCE2020-1649, financed by UEFISCDI Authority, Romania.
Author contribution
Alexandru Opriţa and Mihaela Amelia Dobrescu equally contributed to this study
References
- 1.Wong MLH, Prawira A, Kaye AH, Hovens CM. Tumour angiogenesis: its mechanism and therapeutic implications in malignant gliomas. J Clin Neurosci. 2009;16(9):1119–1130. doi: 10.1016/j.jocn.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp Cell Res. 2006;312(5):630–641. doi: 10.1016/j.yexcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Alexandru O, Sevastre AS, Castro J, Artene SA, Tache DE, Purcaru OS, Sfredel V, Tataranu LG, Dricu A. Platelet-derived growth factor receptor and ionizing radiation in high grade glioma cell lines. Int J Mol Sci. 2019;20(19):4663–4663. doi: 10.3390/ijms20194663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carapancea M, Alexandru O, Fetea AS, Dragutescu L, Castro J, Georgescu A, Popa-Wagner A, Bäcklund ML, Lewensohn R, Dricu A. Growth factor receptors signaling in glioblastoma cells: therapeutic implications. J Neurooncol. 2009;92(2):137–147. doi: 10.1007/s11060-008-9753-8. [DOI] [PubMed] [Google Scholar]
- 5.Popescu AM, Alexandru O, Brindusa C, Purcaru SO, Tache DE, Tataranu LG, Taisescu C, Dricu A. Targeting the VEGF and PDGF signaling pathway in glioblastoma treatment. Int J Clin Exp Pathol. 2015;8(7):7825–7837. [PMC free article] [PubMed] [Google Scholar]
- 6.Masiero M, Simões FC, Han HD, Snell C, Peterkin T, Bridges E, Mangala LS, Wu SY, Pradeep S, Li D, Han C, Dalton H, Lopez-Berestein G, Tuynman JB, Mortensen N, Li JL, Patient R, Sood AK, Banham AH, Harris AL, Buffa FM. A core human primary tumor angiogenesis signature identifies the endothelial orphan receptor ELTD1 as a key regulator of angiogenesis. Cancer Cell. 2013;24(2):229–241. doi: 10.1016/j.ccr.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sevastre AS, Buzatu IM, Baloi C, Oprita A, Dragoi A, Tataranu LG, Alexandru O, Tudorache S, Dricu A. ELTD1 - an emerging silent actor in cancer drama play. Int J Mol Sci. 2021;22(10):5151–5151. doi: 10.3390/ijms22105151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Towner RA, Jensen RL, Colman H, Vaillant B, Smith N, Casteel R, Saunders D, Gillespie DL, Silasi-Mansat R, Lupu F, Giles CB, Wren JD. ELTD1, a potential new biomarker for gliomas. Neurosurgery. 2013;72(1):77–90; discussion 91. doi: 10.1227/NEU.0b013e318276b29d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 10.Montesano R, Vassalli JD, Baird A, Guillemin R, Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 1986;83(19):7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Rosa, Di Stasi, D’Andrea LD. Pro-angiogenic peptides in biomedicine. Arch Biochem Biophys. 2018;660:72–86. doi: 10.1016/j.abb.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Vafopoulou P, Kourti M. Anti-angiogenic drugs in cancer therapeutics: a review of the latest preclinical and clinical studies of anti-angiogenic agents with anticancer potential. J Cancer Metastasis Treat. 2022;8:18–18. [Google Scholar]
- 13.Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999;13(9):1055–1066. doi: 10.1101/gad.13.9.1055. [DOI] [PubMed] [Google Scholar]
- 14.Carmeliet P. Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet. 2003;4(9):710–720. doi: 10.1038/nrg1158. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5(4):434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 16.Delle Monache, Cortellini A, Parisi A, Pulcini F, Martellucci S, Mei C, Danubio ME, Mattei V, Angelucci A, Ficorella C. Expression of pro-angiogenic factors as potential biomarkers in experimental models of colon cancer. J Cancer Res Clin Oncol. 2020;146(6):1427–1440. doi: 10.1007/s00432-020-03186-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernández-Morales J, Hernández-Coronado CG, Guzmán A, Zamora-Gutiérrez D, Fierro F, Gutiérrez CG, Rosales-Torres AM. Hypoxia up-regulates VEGF ligand and downregulates VEGF soluble receptor mRNA expression in bovine granulosa cells in vitro. Theriogenology. 2021;165:76–83. doi: 10.1016/j.theriogenology.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 19.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 21.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM; Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 22.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 23.Tomita Y, Uemura H, Fujimoto H, Kanayama HO, Shinohara N, Nakazawa H, Imai K, Umeyama Y, Ozono S, Naito S, Akaza H; Key predictive factors of Axitinib (AG-013736)-induced proteinuria and efficacy: a phase II study in Japanese patients with cytokine-refractory metastatic renal cell carcinoma. Eur J Cancer. 2011;47(17):2592–2602. doi: 10.1016/j.ejca.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Kang YK, Yau T, Park JW, Lim HY, Lee TY, Obi S, Chan SL, Qin S, Kim RD, Casey M, Chen C, Bhattacharyya H, Williams JA, Valota O, Chakrabarti D, Kudo M. Randomized phase II study of Axitinib versus placebo plus best supportive care in second-line treatment of advanced hepatocellular carcinoma. Ann Oncol. 2015;26(12):2457–2463. doi: 10.1093/annonc/mdv388. [DOI] [PubMed] [Google Scholar]
- 25.Grau SJ, Trillsch F, Herms J, Thon N, Nelson PJ, Tonn JC, Goldbrunner R. Expression of VEGFR3 in glioma endothelium correlates with tumor grade. J Neurooncol. 2007;82(2):141–150. doi: 10.1007/s11060-006-9272-4. [DOI] [PubMed] [Google Scholar]
- 26.Artene SA, Turcu-Stiolica A, Ciurea ME, Folcuti C, Tataranu LG, Alexandru O, Purcaru OS, Tache DE, Boldeanu MV, Silosi C, Dricu A. Comparative effect of immunotherapy and standard therapy in patients with high grade glioma: a meta-analysis of published clinical trials. Sci Rep. 2018;8(1):11800–11800. doi: 10.1038/s41598-018-30296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batchelor TT, Sorensen AG, di Tomaso, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar R, Knick VB, Rudolph SK, Johnson JH, Crosby RM, Crouthamel MC, Hopper TM, Miller CG, Harrington LE, Onori JA, Mullin RJ, Gilmer TM, Truesdale AT, Epperly AH, Boloor A, Stafford JA, Luttrell DK, Cheung M. Pharmacokinetic-pharmacodynamic correlation from mouse to human with Pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007;6(7):2012–2021. doi: 10.1158/1535-7163.MCT-07-0193. [DOI] [PubMed] [Google Scholar]
- 29.Willett CG, Kozin SV, Duda DG, di Tomaso, Kozak KR, Boucher Y, Jain RK. Combined vascular endothelial growth factor-targeted therapy and radiotherapy for rectal cancer: theory and clinical practice. Semin Oncol. 2006;33(5 Suppl 10):S35–S40. doi: 10.1053/j.seminoncol.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vredenburgh JJ, Desjardins A, Herndon JE, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS. Bevacizumab plus Irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 31.Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, Gore M, Desai AA, Patnaik A, Xiong HQ, Rowinsky E, Abbruzzese JL, Xia C, Simantov R, Schwartz B, O’Dwyer PJ. Phase II placebo-controlled randomized discontinuation trial of Sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(16):2505–2512. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 32.Lu L, Saha D, Martuza RL, Rabkin SD, Wakimoto H. Single agent efficacy of the VEGFR kinase inhibitor Axitinib in preclinical models of glioblastoma. J Neurooncol. 2015;121(1):91–100. doi: 10.1007/s11060-014-1612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu-Lowe DD, Zou HY, Grazzini ML, Hallin ME, Wickman GR, Amundson K, Chen JH, Rewolinski DA, Yamazaki S, Wu EY, McTigue MA, Murray BW, Kania RS, O’Connor P, Shalinsky DR, Bender SL. Nonclinical antiangiogenesis and antitumor activities of Axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008;14(22):7272–7283. doi: 10.1158/1078-0432.CCR-08-0652. [DOI] [PubMed] [Google Scholar]
- 34.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME, Rusakov IG, Negrier S, Ou YC, Castellano D, Lim HY, Uemura H, Tarazi J, Cella D, Chen C, Rosbrook B, Kim S, Motzer RJ. Comparative effectiveness of Axitinib versus Sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 35.Alexandru O, Georgescu AM, Ene L, Purcaru SO, Serban F, Popescu A, Brindusa C, Tataranu LG, Ciubotaru V, Dricu A. The effect of curcumin on low-passage glioblastoma cells in vitro. J Cancer Res Ther. 2016;12(2):1025–1032. doi: 10.4103/0973-1482.167609. [DOI] [PubMed] [Google Scholar]
- 36.Tătăranu L, Georgescu AM, Buteică SA, Siloşi I, Mogoşanu GD, Purcaru ŞO, Alexandru O, Stovicek OP, Brînduşa C, Doşa M, Taisescu CI, Dricu A. Ligustrum vulgare hydroalcoholic extract induces apoptotic cell death in human primary brain tumour cells. Farmacia. 2017;65(5):766–771. [Google Scholar]
- 37.Duerinck J, Du Four, Bouttens F, Andre C, Verschaeve V, Van Fraeyenhove, Chaskis C, D’Haene N, Le Mercier, Rogiers A, Michotte A, Salmon I, Neyns B. Randomized phase II trial comparing Axitinib with the combination of Axitinib and Lomustine in patients with recurrent glioblastoma. J Neurooncol. 2018;136(1):115–125. doi: 10.1007/s11060-017-2629-z. [DOI] [PubMed] [Google Scholar]
- 38.Jakubowicz-Gil J, Langner E, Bądziul D, Wertel I, Rzeski W. Quercetin and Sorafenib as a novel and effective couple in programmed cell death induction in human gliomas. Neurotox Res. 2014;26(1):64–77. doi: 10.1007/s12640-013-9452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, Oudard S, Gore ME, Tarazi J, Hariharan S, Chen C, Rosbrook B, Kim S, Rini BI. Axitinib versus Sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14(6):552–562. doi: 10.1016/S1470-2045(13)70093-7. [DOI] [PubMed] [Google Scholar]
- 40.Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, Mason W, Mikkelsen T, Phuphanich S, Ashby LS, Degroot J, Gattamaneni R, Cher L, Rosenthal M, Payer F, Jürgensmeier JM, Jain RK, Sorensen AG, Xu J, Liu Q, van den. Phase III randomized trial comparing the efficacy of Cediranib as monotherapy, and in combination with Lomustine, versus Lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. doi: 10.1200/JCO.2012.47.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hutterer M, Nowosielski M, Haybaeck J, Embacher S, Stockhammer F, Gotwald T, Holzner B, Capper D, Preusser M, Marosi C, Oberndorfer S, Moik M, Buchroithner J, Seiz M, Tuettenberg J, Herrlinger U, Wick A, Vajkoczy P, Stockhammer G. A single-arm phase II Austrian/German multicenter trial on continuous daily Sunitinib in primary glioblastoma at first recurrence (SURGE 01-07) Neuro Oncol. 2014;16(1):92–102. doi: 10.1093/neuonc/not161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: Bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14(11):1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 43.Artene SA, Turcu-Stiolica A, Hartley R, Ciurea ME, Daianu O, Brindusa C, Alexandru O, Tataranu LG, Purcaru SO, Dricu A. Dendritic cell immunotherapy versus Bevacizumab plus Irinotecan in recurrent malignant glioma patients: a survival gain analysis. Onco Targets Ther. 2016;9:6669–6677. doi: 10.2147/OTT.S112842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vatu BI, Artene SA, Staicu AG, Turcu-Stiolica A, Folcuti C, Dragoi A, Cioc C, Baloi SC, Tataranu LG, Silosi C, Dricu A. Assessment of efficacy of dendritic cell therapy and viral therapy in high grade glioma clinical trials. A meta-analytic review. J Immunoassay Immunochem. 2019;40(1):70–80. doi: 10.1080/15321819.2018.1551804. [DOI] [PubMed] [Google Scholar]
- 45.Molina-Vega M, García-Alemán J, Sebastián-Ochoa A, Mancha-Doblas I, Trigo-Pérez JM, Tinahones-Madueño F. Tyrosine kinase inhibitors in iodine-refractory differentiated thyroid cancer: experience in clinical practice. Endocrine. 2018;59(2):395–401. doi: 10.1007/s12020-017-1499-7. [DOI] [PubMed] [Google Scholar]
- 46.Paik ES, Kim TH, Cho YJ, Ryu J, Choi JJ, Lee YY, Kim TJ, Choi CH, Kim WY, Sa JK, Lee JK, Kim BG, Bae DS, Han HD, Ahn HJ, Lee JW. Preclinical assessment of the VEGFR inhibitor Axitinib as a therapeutic agent for epithelial ovarian cancer. Sci Rep. 2020;10(1):4904–4904. doi: 10.1038/s41598-020-61871-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hui EP, Ma BBY, Loong HHF, Mo F, Li L, King AD, Wang K, Ahuja AT, Chan CML, Hui CWC, Wong CH, Chan ATC. Efficacy, safety, and pharmacokinetics of Axitinib in nasopharyngeal carcinoma: a preclinical and phase II correlative study. Clin Cancer Res. 2018;24(5):1030–1037. doi: 10.1158/1078-0432.CCR-17-1667. [DOI] [PubMed] [Google Scholar]
- 48.Aravantinos G, Pectasides D. Bevacizumab in combination with chemotherapy for the treatment of advanced ovarian cancer: a systematic review. J Ovarian Res. 2014;7:57–57. doi: 10.1186/1757-2215-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gross-Goupil M, François L, Quivy A, Ravaud A. Axitinib: a review of its safety and efficacy in the treatment of adults with advanced renal cell carcinoma. Clin Med Insights Oncol. 2013;7:269–277. doi: 10.4137/CMO.S10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 51.Blumenschein GR, Gatzemeier U, Fossella F, Stewart DJ, Cupit L, Cihon F, O’Leary J, Reck M. Phase II, multicenter, uncontrolled trial of single-agent Sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. J Clin Oncol. 2009;27(26):4274–4280. doi: 10.1200/JCO.2009.22.0541. [DOI] [PubMed] [Google Scholar]
- 52.Kim A, Widemann BC, Krailo M, Jayaprakash N, Fox E, Weigel B, Blaney SM. Phase 2 trial of Sorafenib in children and young adults with refractory solid tumors: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2015;62(9):1562–1566. doi: 10.1002/pbc.25548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steinbild S, Mross K, Frost A, Morant R, Gillessen S, Dittrich C, Strumberg D, Hochhaus A, Hanauske AR, Edler L, Burkholder I, Scheulen M. A clinical phase II study with Sorafenib in patients with progressive hormone-refractory prostate cancer: a study of the CESAR Central European Society for Anticancer Drug Research - EWIV. Br J Cancer. 2007;97(11):1480–1485. doi: 10.1038/sj.bjc.6604064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreno-Aspitia A, Morton RF, Hillman DW, Lingle WL, Rowland KM, Wiesenfeld M, Flynn PJ, Fitch TR, Perez EA. Phase II trial of Sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J Clin Oncol. 2009;27(1):11–15. doi: 10.1200/JCO.2007.15.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hassler MR, Ackerl M, Flechl B, Sax C, Wöhrer A, Widhalm G, Dieckmann K, Hainfellner J, Preusser M, Marosi C. Sorafenib for patients with pretreated recurrent or progressive high-grade glioma: a retrospective, single-institution study. Anticancer Drugs. 2014;25(6):723–728. doi: 10.1097/CAD.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 56.Zustovich F, Landi L, Lombardi G, Porta C, Galli L, Fontana A, Amoroso D, Galli C, Andreuccetti M, Falcone A, Zagonel V. Sorafenib plus daily low-dose Temozolomide for relapsed glioblastoma: a phase II study. Anticancer Res. 2013;33(8):3487–3494. [PubMed] [Google Scholar]
- 57.Hottinger AF, Ben Aissa, Espeli V, Squiban D, Dunkel N, Vargas MI, Hundsberger T, Mach N, Schaller K, Weber DC, Bodmer A, Dietrich PY. Phase I study of Sorafenib combined with radiation therapy and Temozolomide as first-line treatment of high-grade glioma. Br J Cancer. 2014;110(11):2655–2661. doi: 10.1038/bjc.2014.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hutson TE, Lesovoy V, Al-Shukri S, Stus VP, Lipatov ON, Bair AH, Rosbrook B, Chen C, Kim S, Vogelzang NJ. Axitinib versus Sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol. 2013;14(13):1287–1294. doi: 10.1016/S1470-2045(13)70465-0. [DOI] [PubMed] [Google Scholar]