Abstract
Myocardial infarction (MI) leads to irreversible ischemic damage of the heart muscle and is the leading cause of heart failure. The ischemic cardiac injury triggers a potent local and systemic immune response. In the acute phase post-MI, neutrophils infiltrate the myocardium in large numbers and induce further cardiomyocyte death, expanding the infarcted area. The alarmin S100A8/A9 is a proinflammatory mediator primarily produced by myeloid cells, with an emerging role in MI. We previously demonstrated that short-term inhibition of S100A8/A9 during the inflammatory phase of the immune response to MI improves long-term cardiac function. In the present study, we investigated the effects of S100A8/A9 blockade on myocardial inflammation and post-ischemic myocardial injury in a mouse model of coronary artery ligation. Immunohistochemical (IHC) staining revealed that the presence of S100A9 is strongly correlated with neutrophil infiltration in the myocardium on days 1 and 3 post-MI. A 3-day treatment with the S100A8/A9 blocker ABR-238901 starting immediately after MI decreased the number of neutrophils and S100A9 presence in the myocardium and had a positive impact on cardiac damage, reducing infarction size. These findings promote S100A9 as an IHC biomarker of neutrophil infiltration and a promising immunomodulatory target to regulate neutrophil recruitment, reduce ischemic injury and promote long-term beneficial cardiac recovery after MI.
Keywords: myocardial infarction , inflammation , infarct size , S100A8/A9 blockade
Introduction
According to the Fourth Universal Definition of Myocardial Infarction (MI), the clinical definition of MI indicates the presence of acute myocardial injury, revealed by elevated cardiac biomarkers, in the setting of acute myocardial ischemia defined by symptoms, new ischemic electrocardiographic changes or imaging evidence of new regional wall motion abnormality in a pattern consistent with an ischemic etiology [1]. From a pathological standpoint, MI consists in myocardial cell death due to acute ischemia. Extensive cardiomyocyte death triggers a robust immune and inflammatory response that leads to myocardial healing and replacement of the damaged tissue with a collagen-based scar [2, 3]. During acute cardiac inflammation, high numbers of immune cells infiltrate the myocardium and initially exacerbate tissue damage, before conversion into a reparatory phenotype. Neutrophils are the first cells to invade the injured cardiac tissue, triggering the early inflammatory phase post-MI [4, 5]. Experimental and clinical studies have demonstrated both deleterious and beneficial roles of neutrophils in MI [6, 7, 8]. On one hand, neutrophils can induce the death of viable cardiomyocytes and further increase ischemic lesion [6, 9]. On the other hand, neutrophil depletion has been shown to lead to defective myocardial recovery and progressive decline in cardiac function, suggesting that neutrophil-driven mechanisms are also important to promote the post-acute reparatory phase [8].
S100A8/A9, a proinflammatory alarmin abundantly stored in neutrophils, increases rapidly in the circulation after the onset of MI [10, 11]. We have previously identified S100A8/A9 as a dual promoter of inflammation and repair after MI with important anti-inflammatory changes of left ventricle proteome [12]. Short-term S100A8/A9 blockade with the small molecule inhibitor ABR-238901 administered for the first three days post-MI improved cardiac function long-term, both after permanent coronary ischemia and after ischemia/reperfusion [13]. Conversely, long-term S100A9 blockade has a negative impact on cardiac recovery, counteracting the beneficial effects of short-term therapy due to inhibition of monocyte recruitment and their conversion into reparatory macrophages [14].
The inflammatory response is closely related to myocardial healing and fibrosis. Aggressive acute inflammation or insufficient healing may lead to cardiac rupture, followed by cardiac tamponade and death. Conversely, excessive cardiac fibrosis after MI leads to adverse cardiac remodeling, arrhythmias, and heart failure (HF) [15, 16]. Histopathological analysis allows classification of myocardial fibrosis into replacement fibrosis and reactive fibrosis [17]. Reparative fibrosis is a replacement fibrosis, as necrotic MI tissue is completely replaced by a collagen-based scar due to the insignificant regenerative ability of the adult mammalian heart post-MI [18]. Reactive fibrosis is an interstitial or perivascular fibrosis which is indirectly associated with cardiomyocyte death and may result from prolonged activation of fibrogenic stimuli [18, 19]. Post-MI cardiac fibrosis is a critical response to prevent ventricular rupture during the early stages of MI. Effective immunomodulatory therapies after MI should reduce the extent of the injury mediated by excessive acute inflammation, while leaving the repair mechanisms intact.
Mouse models of MI are essential in cardiovascular research, to assess the effects of potential treatments on cardiac function, immune cell infiltration and MI size [20]. We have previously shown that short-term S100A8/A9 blockade by ABR-238901 favorably tips the immune inflammation/repair balance in the infarcted heart and improves cardiac function long-term.
Aim
In the present work, we assessed the value of S100A9 as an immunohistological biomarker of neutrophil infiltration into the myocardium and we examined the effects of short-term S100A8/A9 blockade on MI size, as a potential explanation for the beneficial long-term effects of the treatment on cardiac function.
Materials and Methods
MI induction by permanent left coronary artery ligation
Wild-type (C57BL/6) female mice, 8–12 weeks of age, 20–25 g body weight, underwent permanent left anterior descending (LAD) coronary artery ligation as previously described [20, 21]. All animals were allowed to acclimatize for at least one week before being included in the study. Mice were housed in a controlled environment with a 12-hour light-dark cycle and free access to water and a regular mouse diet. All mice were purchased from the Cantacuzino National Research and Development Institute (Bucharest, Romania). All surgeries were performed at the Experimental Station of George Emil Palade University of Medicine, Pharmacy, Science, and Technology of Târgu Mureş (Romania), according to local protocols approved by the Scientific Research Ethics Commission of the University.
Briefly, mice were placed on a heated surgical pad to maintain their body temperature at around 37ºC. Subsequently, continuous inhalation anesthesia with 2–3% Isoflurane combined with 0.5 L/min oxygen was provided through a non-invasive delivery system (EZ-SA800 Single Animal System, Philadelphia, USA). The animal fur was removed with a standard trimmer for small animals, the skin was cleaned with Betadine and a small cut, approximately 1 cm in length, was made on the left side of the thorax. Next, the heart was rapidly popped-out through the fourth intercostal space and the LAD was ligated using a 6.0 silk suture at approximately 2–3 mm from the origin. The heart was placed back into the thorax immediately, the air was manually evacuated by gentle side pressure and the skin was sutured with 6.0 Prolene. The sham mice underwent the same surgical procedure except for the LAD ligation. MI was histologically confirmed by Hematoxylin–Eosin (HE) and Masson’s trichrome stainings.
Study design
The mice were included into three groups: sham-operated mice (sham group), MI-operated mice receiving phosphate-buffered saline (PBS) treatment (MI group) and MI-operated mice treated with 30 mg/kg ABR-238901 (a gift from Active Biotech AB, Lund, Sweden) diluted in PBS (MI+ABR group). ABR-238901 blocks the binding of both S100A9 and S100A8/A9 to their cognate receptors, Toll-like receptor (TLR)2, TLR4 and receptor for advanced glycation end-products (RAGE) [22]. Mice sacrificed on day 1 received one single intraperitoneal (i.p.) dose of PBS or ABR immediately after MI induction. All other mice included in the study received a total of three i.p. injections of either PBS or ABR administered immediately after MI and repeated after 24 and 48 hours. Mouse hearts were collected after 1 and 3 days post-MI and the histological analysis was performed as described below. The number of animals in each group is specified in the results section and in the figure legends.
Histology and immunostaining
Animal hearts were collected under general anesthesia induced by i.p. injection of a Ketamine–Xylazine (100/20 mg/kg body weight) solution. Heart tissue was fixed overnight in 10% neutral buffered formalin, embedded in paraffin, and sectioned into 4 μm-thick transverse sections for HE, Masson’s trichrome and immunohistochemical (IHC) staining. Serial sections at 300 μm intervals were collected, starting from the ventricular apex until the suture knot. Heart sections collected at 1 and 3 days post-MI were stained for HE, the mouse neutrophil marker lymphocyte antigen 6 family member G6D (Ly6G), S100A9 and Masson’s trichrome to evaluate histological changes and infarction size in the early acute inflammatory period after MI. We analyzed myocardial tissue sections collected from 5–6 different levels along the transversal axis of the heart, starting from the apex and up to the level of the coronary suture. All images were taken with an Axio Imager Z2 microscope with a color Axiocam 506 camera and processed using the ZenPro 3.2 software (all Zeiss, Germany) and QuPath version 0.3.0 (https://qupath.github.io).
We performed IHC staining for S100A9 and for Ly6G, a marker specifically expressed on the surface of mouse neutrophils [23]. Paraffin-embedded heart sections were rehydrated, and antigen retrieval was performed in retrieval solution (Leica Microsystems, Germany) using microwave (850 W) heating for 20 minutes, and endogenous peroxidases were quenched with 3% hydrogen peroxide (H2O2) for 10 minutes. Subsequently, the sections were rinsed in Tris-buffered saline (TBS) and unspecific binding sites were blocked with Protein Block Serum-Free solution (Dako, CA, USA) for 30 minutes at room temperature (RT). Purified monoclonal rat anti-mouse anti-Ly6G primary antibody (1:500 dilution; BD Biosciences, San Jose, CA, USA) or monoclonal rabbit anti-mouse anti-S100A9 primary antibody (1:800 dilution; Cell Signaling Technology, Danvers, MA, USA) were applied overnight at 4ºC. Next, sections were rinsed in TBS and incubated for one hour at RT with goat anti-rat immunoglobulin G (IgG) heavy and light (H&L) chains [Horseradish peroxidase (HRP)] secondary antibody (1:500 dilution; Abcam, Cambridge, UK) or with BrightVision poly-HRP goat-anti-rabbit Biotin-free (ready-to-use) secondary antibody (VWR, Amsterdam, The Netherlands), respectively. The signal was then detected using 3,3’-Diaminobenzidine (DAB), and finally the slides were counterstained with Hematoxylin according to the manufacturer’s instructions.
Statistical analysis
The GraphPad Prism 6.0 software (GraphPad, CA, USA) was used for statistical analysis. A p-value < 0.05 was considered to be statistically significant.
Results
S100A9 presence reflects neutrophil infiltration in the acute inflammatory phase post-MI
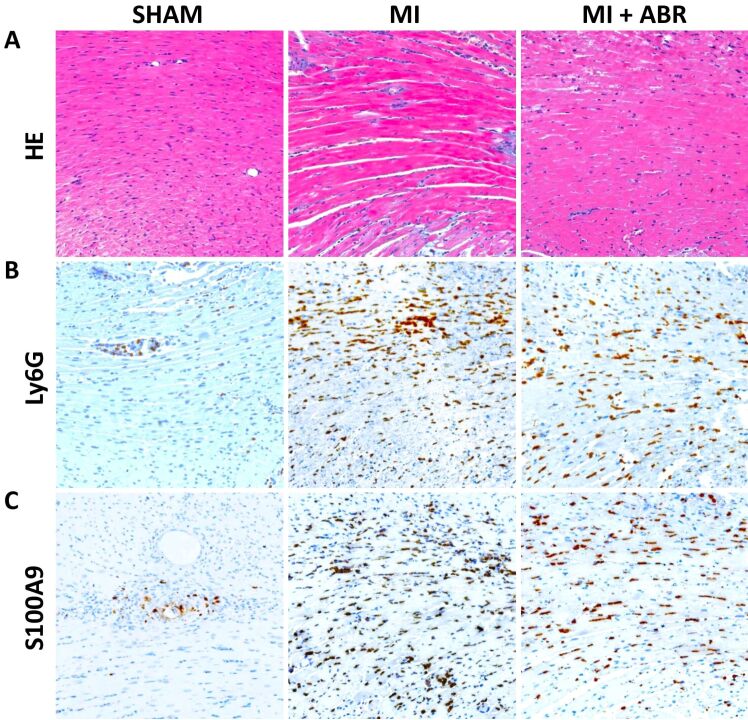
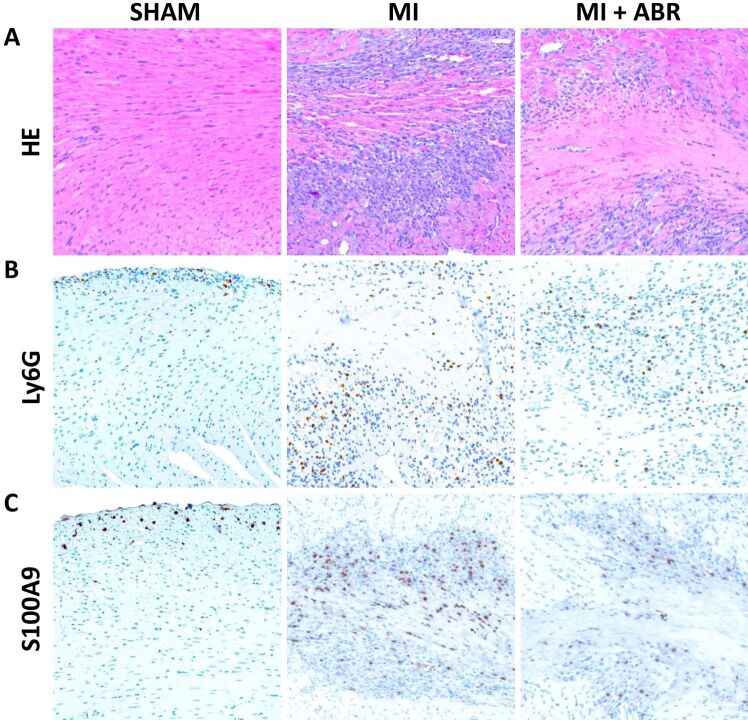
The first aim of our study was to examine the links between myocardial damage and the inflammatory infiltrate in the acute phase post-MI, and to validate S100A9 as a histological biomarker and treatment target to evaluate and control the infiltration of polymorphonuclear neutrophils into the infarcted myocardium. We focused on days 1 and 3 post-MI, at the beginning and the end of the acute inflammatory phase post-MI. We collected left ventricular sections from sham-operated mice and from mice with induced MI, which were treated for 1 or 3 days with PBS or ABR-238901 (ABR) i.p. On HE staining of the infarcted left ventricular areas, we can observe microscopic aspects of interstitial edema as blank spaces between the cells, nuclear pyknosis and loss of cardiomyocyte nuclei and striations, all features of cardiomyocyte necrosis (Figures 1A and 2A). The inflammatory infiltrate on day 1 mainly consists of neutrophils, as can be seen by comparing the areas of immune cells accumulation in the HE staining with the Ly6G staining in the MI and MI+ABR groups (Figure 1A and 1B). The myocardial destruction and the inflammatory infiltrate are much more pronounced on day 3, but the neutrophil presence is highly reduced compared to day 1 in both MI treatment groups [Figure 1A, 1B vs. Figure 2A, 2B]. In contrast, the sham-operated hearts only contain a few infiltrating neutrophils around the needle puncture (Figure 1B) and in the subendocardial zone (Figure 2B), probably infiltrating by contact from the surrounding areas because of pericardial rupture during surgery.
Figure 1.
Neutrophil infiltration and S100A9 presence in the myocardium on day 1 post-MI: (A) Representative images of HE staining of left ventricular sections from the sham and MI groups treated with PBS (MI) or ABR-238901 (MI+ABR), respectively, collected on day 1 after myocardial ischemia (400×); (B) Ly6G-positive staining identifying neutrophil infiltration (400×); (C) S100A9-positive immunohistochemical staining in the same areas as in (A) and (B) (400×). HE: Hematoxylin–Eosin; Ly6G: Lymphocyte antigen 6 family member G6D; MI: Myocardial infarction; PBS: Phosphate-buffered saline
Figure 2.
Neutrophil infiltration and S100A9 presence in the myocardium on day 3 post-MI: (A) Representative images of HE staining of left ventricular sections from the sham and the two MI groups receiving PBS (MI) or ABR-238901 (MI+ABR) treatment, collected on day 3 post-MI (400×); (B) Ly6G-positive neutrophil staining (400×); (C) S100A9 staining in the same areas as in (A) and (B) (400×
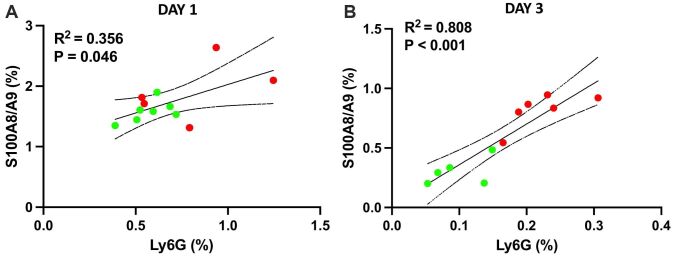
Visually, the location of the S100A9 staining (Figures 1C and 2C) coincides well with the infiltration of neutrophils into the myocardium, reflected by the Ly6G staining (Figures 1B and 2B). The ability of S100A9 to reflect the presence of neutrophils in the tissues is further confirmed by significant associations between the extent of the S100A9 and Ly6G staining, expressed as average percentage of left ventricular area, on both day 1 and day 3 post-MI and in both MI treatment groups (Figure 3A and 3B). These findings suggest that neutrophils are the major source of S100A9 in the infarcted myocardium and that the amount of S100A9 is a good histological biomarker of neutrophil infiltration.
Figure 3.
The S100A9 presence in the infarcted myocardium reflects neutrophil infiltration and the effect of the treatment. Correlations between neutrophil infiltration, expressed as Ly6G-positive staining, and S100A9 staining in myocardium collected on day 1 (A) and day 3 (B) post-MI. The stained area is reported as an average percentage of left ventricular area on five consecutive sections collected at 300 μm intervals
S100A8/A9 blockade inhibits inflammation after MI
Further, we studied the effects of S100A9 and S100A8/A9 blockade with ABR-238901 on the neutrophil infiltrate in the infarcted myocardium in the first three days post-MI, compared with PBS-treated controls. The treatment lowered the presence of inflammatory cells, as revealed by the HE staining. The effect was only discreet on day 1, after only one ABR-238901 dose (Figure 1A), but the difference between the groups became evident on day 3, at 24 hours after the third ABR-238901 dose (Figure 2A). We observed similar inhibitory effects of the treatment on the infiltration of Ly6G-positive neutrophils and on the S100A9 presence in the myocardium on days 1 and 3 [Figure 1B, 1C) vs. Figure 2B, 2C]. The difference between the treatment groups was further confirmed by quantification of Ly6G and S100A9-stained areas. On day 1, there was a tendency towards lower neutrophil and S100A9 staining in ABR-238901-treated mice, but there was still a considerable overlap between the two treatment groups (Figure 3A). On day 3, there was a better separation between the groups, both Ly6G and S100A9 being clearly higher in the myocardium of PBS-treated mice compared to animals receiving S100A8/A9 blockade (Figure 3B).
S100A8/A9 blockade reduces the extent of myocardial damage
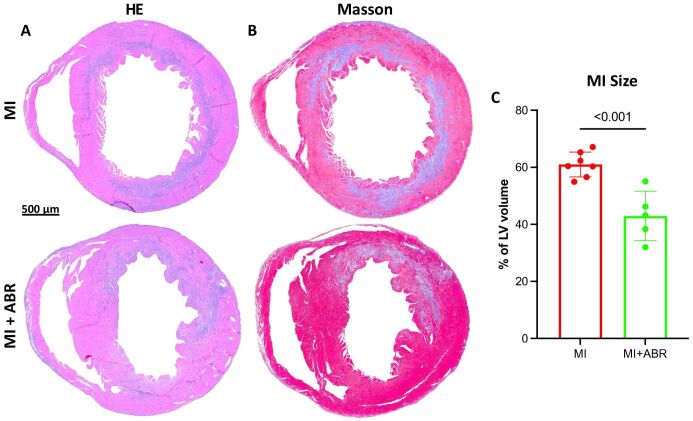
In addition, we assessed whether the reduction in neutrophil infiltration observed in mice receiving the S100A8/A9 blocker has a positive impact on myocardial damage. We compared the size of the MI at the end of the treatment, on day 3 post-MI, in mice treated with ABR-238901 or PBS. The location and extent of myocardial damage was identified on HE-stained sections below the coronary ligation, and further confirmed by Masson’s trichrome staining of the post-ischemic myocardial scar (Figure 4A and 4B). We found that the size of the MI, expressed as percentage of left ventricular volume below the coronary ligature, was significantly reduced by the 3-day inhibition of S100A8/A9 signaling (Figure 4C).
Figure 4.
Inhibition of S100A8/A9 signaling reduces the extent of MI on day 3 post-MI: (A) Representative HE staining of cardiac sections from the PBS- and ABR-238901-treated mice, three days after induced MI; (B) Masson’s trichrome staining of fibrous scar formation in the infarcted area; (C) Quantification of MI size, expressed as percentage of left ventricular (LV) volume
Discussions
Inflammation and fibrosis play crucial roles in the development of HF and cardiac remodeling after MI. An aggressive inflammatory response increases the extent of myocardial damage and excessive fibrosis leads to myocardial stiffening, diastolic dysfunction and increased intra-cavitary pressure, accelerating the progression towards clinically manifest HF [24, 25]. Clinical and experimental studies have consistently shown that a high degree of local and systemic inflammation is deleterious and is associated with increased mortality after MI [26, 27]. Timely reperfusion therapy by primary percutaneous coronary intervention is the “gold standard” treatment for reducing MI size [28], but there are no clinically available treatments able to efficiently modulate the aberrant inflammatory response and to improve cardiac healing after MI.
In this study, we assessed the neutrophil-mediated acute inflammatory response induced by ischemic myocardial damage in a mouse model of MI, and the ability of S100A8/A9 blockade to modulate neutrophil recruitment and reduce cardiac damage. We show that during the early inflammatory phase (the first three days after MI) the infarcted myocardium is massively infiltrated by inflammatory cells. Neutrophils dominate the inflammatory infiltrate on day 1 post-MI, but their numbers highly decline already on day 3, which is in line with previous literature [5]. We also show that the presence of S100A9 in the myocardium closely reflects the intensity of neutrophil infiltration. In a previous study, we found that soluble S100A8/A9 in plasma is closely associated with circulating neutrophil counts and with the risk for cardiovascular events in individuals from the general population [29]. Our current data further support the value of the protein as a biomarker of neutrophil presence in the infarcted tissue.
Secondly, we show that blockade of S100A8/A9 function with the small molecule blocker ABR-238901 significantly reduces neutrophil infiltration and S100A9 presence in the infarcted heart. The effect was not clearly defined on day 1, after one ABR-238901 dose, but became evident on day 3, at the end of the 3-dose treatment. This important finding suggests that S100A8/A9 has an autocrine effect on neutrophil recruitment and function, and that S100A8/A9 blockade not only inhibits its function but also reduces the presence of the protein in the myocardium. These results are in line with recent findings, showing that neutrophil secreted S100A8/A9 activates the NLR family pyrin domain containing 3 (NLRP3) inflammasome in neutrophils through an autocrine mechanism mediated by the TLR4 receptor [30]. The S100A8/A9-activated and NLRP3-primed neutrophils circulate back to the bone marrow (BM) and stimulate emergency hematopoiesis through increased interleukin-1beta (IL-1β) production [30, 31]. Our group has previously shown that systemic S100A8/A9 blockade inhibits neutrophil egression from the BM and spleen into the circulation and their recruitment into the cardiac muscle post-MI [13, 14]. Taken together, these data demonstrate that S100A8/A9 plays an important role in neutrophil production in the BM, myocardial recruitment, and effector function in the infarcted myocardium. The observed effects of S100A8/A9 blockade suggest that ABR-238901 can be used therapeutically to modulate the involvement of neutrophils in post-MI inflammation. Importantly, there are conflicting clinical and experimental results regarding the impact of neutrophils on post-MI cardiac damage and recovery. High levels of circulating neutrophils are associated with increased mortality and adverse prognosis in MI patients, as neutrophils significantly contribute to the sustained inflammatory response and overall severity of ischemic injury [32, 33]. Conversely, an important previous study has found that long-term neutrophil depletion in a mouse model of MI impaired the development of reparatory macrophages, leading to defective repair, excessive collagen deposition and progressive decline of cardiac function [8]. Here, we show that the short-term ABR-238901 treatment decreases but does not completely eliminate neutrophil infiltration into the infarcted myocardium. The contribution of neutrophils to cardiac repair is thereby maintained, and we have previously demonstrated that the 3-day treatment leads to improved cardiac function and has no negative impact on repair [13]. In contrast, extending the treatment long-term had a similar negative impact on cardiac repair and function as the long-term neutrophil depletion [14].
Besides its role as a proinflammatory mediator and potential treatment target, S100A8/A9 might also be valuable as a soluble biomarker reflecting local and systemic inflammation and residual cardiovascular risk in MI patients. Neutrophils are the main producers of S100A8/A9 in MI [34] and an earlier clinical study has shown that S100A8/A9, also called myeloid-related protein 8/14 (MRP8/14), is expressed in neutrophils and monocytes, but not platelets, in thrombi extracted from the site of coronary occlusion during percutaneous coronary interventions [35]. Importantly, the same study found that S100A8/A9 concentration in coronary blood is twice as high as in the systemic circulation during the acute coronary event, demonstrating that the activation of cells infiltrating the infarcted myocardium is the primary source of S100A8/A9 release. S100A8/A9 increased in the circulation faster than the myocardial damage markers myoglobin and troponin and was proposed as a potential marker for MI diagnosis [36]. The peak of plasma S100A8/A9 levels occurs at day 1 post-MI, coinciding with the peak of neutrophil recruitment [34]. Plasma levels slightly decrease thereafter but remain elevated in MI patients for at least the first four days post-MI [34]. A potent S100A8/A9 response during the acute phase appears to be detrimental in MI patients, as high circulating levels of S100A8/A9 have been associated with a poor long-term prognosis characterized by increased risk for major adverse cardiovascular events (MACE) [34, 36] and HF [13]. In this context, our current IHC data confirms the close relationship between neutrophil infiltration and S100A9 presence in the myocardium. We propose that plasma S100A8/A9 might be used as a clinical biomarker to evaluate the intensity of the local and systemic neutrophil response in MI and to follow the effect of the treatment.
Finally, we demonstrate that S100A8/A9 blockade with ABR-238901 reduces myocardial damage and the size of the myocardial scar on day 3 post-MI, at the end of the treatment. These loss-of-function data complement well previous gain-of-function findings, showing that treatment with recombinant S100A8/A9 exacerbated the ischemic injury and worsened HF in a mouse model of MI, due to enhanced RAGE signaling and activation of the proinflammatory NF-κB transcription factor [37]. The observed reduction in neutrophil infiltration and infarction size also provides further mechanistic explanation for our previously published study showing that the 3-day S100A8/A9 blockade tips the balance between inflammation and repair in the myocardium towards an anti-inflammatory and pro-reparatory phenotype, leading to long-term gain in cardiac function in both the permanent ischemia model used in the current work and in the clinically relevant model of ischemia/reperfusion.
Conclusions
In the current work, we demonstrate a close relationship between neutrophils and the proinflammatory mediator S100A8/A9 in the pathogenesis of MI, by using histological and IHC analysis of cardiac sections collected 1 and 3 days after coronary ischemia from a mouse model of coronary artery occlusion. We show that S100A9 presence closely reflects neutrophil infiltration into the myocardium, and that neutrophils dominate the inflammatory infiltrate on day 1 and thereafter rapidly decline. The neutrophil dynamics coincide with the presence of S100A9 in the myocardium and with the levels of circulating S100A8/A9 measured in MI patient plasma in a previous study. Further, we demonstrate that blockade of S100A8/A9 function with the small-molecule inhibitor ABR-238901 administered during the first three days post-MI lowers neutrophil infiltration and S100A9 presence in the myocardium and reduces the size of myocardial injury. Our results promote S100A8/A9 as a potential biomarker of neutrophil involvement in MI and as a promising treatment target to modulate neutrophil recruitment and function, reduce myocardial damage, promote healing, and prevent long-term HF.
Conflict of interests
The authors declare that they have no conflict of interests.
Source of funding
This work was supported by a grant of Ministry of Research and Innovation, CNCS‐UEFISCDI, project number PN‐III‐P4‐ID‐PCCF‐2016‐0172, within PNCDI III.
Acknowledgments
This article is part of a PhD Thesis from the Doctoral School of Medicine and Pharmacy within the George Emil Palade University of Medicine, Pharmacy, Science, and Technology of Târgu Mureş, titled “Angiogenic and Antifibrotic Effects of S100A8/A9 Blockade After Acute Myocardial Infarction”, which will be presented by Răzvan Gheorghiţă Mareş in 2023.
References
- 1.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 2.Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res. 2016;119(1):91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mares RG, Marinkovic G, Cotoi OS, Schiopu A. Innate immune mechanisms in myocardial infarction – an update. Rev Rom Med Lab. 2018;26:9–20. [Google Scholar]
- 4.Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K, Sano M. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013;62:24–35. doi: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Ma Y, Yabluchanskiy A, Iyer RP, Cannon PL, Flynn ER, Jung M, Henry J, Cates CA, Deleon-Pennell KY, Lindsey ML. Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res. 2016;110(1):51–61. doi: 10.1093/cvr/cvw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. 2004;61(3):481–497. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Carbone F, Nencioni A, Mach F, Vuilleumier N, Montecucco F. Pathophysiological role of neutrophils in acute myocardial infarction. Thromb Haemost. 2013;110(3):501–514. doi: 10.1160/TH13-03-0211. [DOI] [PubMed] [Google Scholar]
- 8.Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Weber C, Soehnlein O, Steffens S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2017;38(3):187–197. doi: 10.1093/eurheartj/ehw002. [DOI] [PubMed] [Google Scholar]
- 9.Puhl SL, Steffens S. Neutrophils in post-myocardial infarction inflammation: damage vs. resolution. Front Cardiovasc Med. 2019;6:25–25. doi: 10.3389/fcvm.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakuma M, Tanaka A, Kotooka N, Hikichi Y, Toyoda S, Abe S, Taguchi I, Node K, Simon DI, Inoue T. Myeloid-related protein-8/14 in acute coronary syndrome. Int J Cardiol. 2017;249:25–31. doi: 10.1016/j.ijcard.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Cai Z, Xie Q, Hu T, Yao Q, Zhao J, Wu Q, Tang Q. S100A8/A9 in myocardial infarction: a promising biomarker and therapeutic target. Front Cell Dev Biol. 2020;8:603902–603902. doi: 10.3389/fcell.2020.603902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boteanu RM, Suica VI, Uyy E, Ivan L, Cerveanu-Hogas A, Mares RG, Simionescu M, Schiopu A, Antohe F. Short-term blockade of pro-inflammatory alarmin S100A9 favorably modulates left ventricle proteome and related signaling pathways involved in post-myocardial infarction recovery. Int J Mol Sci. 2022;23(9):5289–5289. doi: 10.3390/ijms23095289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marinković G, Grauen Larsen, Yndigegn T, Szabo IA, Mares RG, de Camp, Weiland M, Tomas L, Goncalves I, Nilsson J, Jovinge S, Schiopu A. Inhibition of pro-inflammatory myeloid cell responses by short-term S100A9 blockade improves cardiac function after myocardial infarction. Eur Heart J. 2019;40(32):2713–2723. doi: 10.1093/eurheartj/ehz461. [DOI] [PubMed] [Google Scholar]
- 14.Marinković G, Koenis DS, de Camp, Jablonowski R, Graber N, de Waard, de Vries, Goncalves I, Nilsson J, Jovinge S, Schiopu A. S100A9 links inflammation and repair in myocardial infarction. Circ Res. 2020;127(5):664–676. doi: 10.1161/CIRCRESAHA.120.315865. [DOI] [PubMed] [Google Scholar]
- 15.Kain V, Prabhu SD, Halade GV. Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic Res Cardiol. 2014;109(6):444–444. doi: 10.1007/s00395-014-0444-7. [DOI] [PubMed] [Google Scholar]
- 16.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res. 2014;114(2):266–282. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schalla S, Bekkers SC, Dennert R, van Suylen, Waltenberger J, Leiner T, Wildberger J, Crijns HJ, Heymans S. Replacement and reactive myocardial fibrosis in idiopathic dilated cardiomyopathy: comparison of magnetic resonance imaging with right ventricular biopsy. Eur J Heart Fail. 2010;12(3):227–231. doi: 10.1093/eurjhf/hfq004. [DOI] [PubMed] [Google Scholar]
- 18.Frangogiannis NG. Cardiac fibrosis. Cardiovasc Res. 2021;117(6):1450–1488. doi: 10.1093/cvr/cvaa324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeda N, Manabe I. Cellular interplay between cardiomyocytes and nonmyocytes in cardiac remodeling. Int J Inflam. 2011;2011:535241–535241. doi: 10.4061/2011/535241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mares RG, Manu D, Szabo IA, Tomut ME, Pintican G, Cordos B, Jakobsson G, Dobreanu M, Cotoi OS, Schiopu A. Studying the innate immune response to myocardial infarction in a highly efficient experimental animal model. Rom J Cardiol. 2021;31(3):573–585. [Google Scholar]
- 21.Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 2010;107(12):1445–1453. doi: 10.1161/CIRCRESAHA.110.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Björk P, Björk A, Vogl T, Stenström M, Liberg D, Olsson A, Roth J, Ivars F, Leanderson T. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol. 2009;7(4):e97–e97. doi: 10.1371/journal.pbio.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee PY, Wang JX, Parisini E, Dascher CC, Nigrovic PA. Ly6 family proteins in neutrophil biology. J Leukoc Biol. 2013;94(4):585–594. doi: 10.1189/jlb.0113014. [DOI] [PubMed] [Google Scholar]
- 24.Stone GW, Selker HP, Thiele H, Patel MR, Udelson JE, Ohman EM, Maehara A, Eitel I, Granger CB, Jenkins PL, Nichols M, Ben-Yehuda O. Relationship between infarct size and outcomes following primary PCI: patient-level analysis from 10 randomized trials. J Am Coll Cardiol. 2016;67(14):1674–1683. doi: 10.1016/j.jacc.2016.01.069. [DOI] [PubMed] [Google Scholar]
- 25.wiątkiewicz I, Magielski P, Kubica J, Zadourian A, DeMaria AN, Taub PR. Enhanced inflammation is a marker for risk of post-infarct ventricular dysfunction and heart failure. Int J Mol Sci. 2020;21(3):807–807. doi: 10.3390/ijms21030807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seropian IM, Toldo S, Van Tassell, Abbate A. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol. 2014;63(16):1593–1603. doi: 10.1016/j.jacc.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Westman PC, Lipinski MJ, Luger D, Waksman R, Bonow RO, Wu E, Epstein SE. Inflammation as a driver of adverse left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol. 2016;67(17):2050–2060. doi: 10.1016/j.jacc.2016.01.073. [DOI] [PubMed] [Google Scholar]
- 28.Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. 2017;389(10065):197–210. doi: 10.1016/S0140-6736(16)30677-8. [DOI] [PubMed] [Google Scholar]
- 29.Cotoi OS, Dunér P, Ko N, Hedblad B, Nilsson J, Björkbacka H, Schiopu A. Plasma S100A8/A9 correlates with blood neutrophil counts, traditional risk factors, and cardiovascular disease in middle-aged healthy individuals. Arterioscler Thromb Vasc Biol. 2014;34(1):202–210. doi: 10.1161/ATVBAHA.113.302432. [DOI] [PubMed] [Google Scholar]
- 30.Sreejit G, Nooti SK, Jaggers RM, Athmanathan B, Ho Park, Al-Sharea A, Johnson J, Dahdah A, Lee MKS, Ma J, Murphy AJ, Nagareddy PR. Retention of the NLRP3 inflammasome-primed neutrophils in the bone marrow is essential for myocardial infarction-induced granulopoiesis. Circulation. 2022;145(1):31–44. doi: 10.1161/CIRCULATIONAHA.121.056019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sreejit G, Abdel-Latif A, Athmanathan B, Annabathula R, Dhyani A, Noothi SK, Quaife-Ryan GA, Al-Sharea A, Pernes G, Dragoljevic D, Lal H, Schroder K, Hanaoka BY, Raman C, Grant MB, Hudson JE, Smyth SS, Porrello ER, Murphy AJ, Nagareddy PR. Neutrophil-derived S100A8/A9 amplify granulopoiesis after myocardial infarction. Circulation. 2020;141(13):1080–1094. doi: 10.1161/CIRCULATIONAHA.119.043833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barron HV, Harr SD, Radford MJ, Wang Y, Krumholz HM. The association between white blood cell count and acute myocardial infarction mortality in patients > or =65 years of age: findings from the cooperative cardiovascular project. J Am Coll Cardiol. 2001;38(6):1654–1661. doi: 10.1016/s0735-1097(01)01613-8. [DOI] [PubMed] [Google Scholar]
- 33.Kaya MG, Akpek M, Lam YY, Yarlioglues M, Celik T, Gunebakmaz O, Duran M, Ulucan S, Keser A, Oguzhan A, Gibson MC. Prognostic value of neutrophil/lymphocyte ratio in patients with ST-elevated myocardial infarction undergoing primary coronary intervention: a prospective, multicenter study. Int J Cardiol. 2013;168(2):1154–1159. doi: 10.1016/j.ijcard.2012.11.074. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Chen B, Yang X, Zhang C, Jiao Y, Li P, Liu Y, Li Z, Qiao B, Bond Lau, Ma XL, Du J. S100a8/a9 signaling causes mitochondrial dysfunction and cardiomyocyte death in response to ischemic/reperfusion injury. Circulation. 2019;140(9):751–764. doi: 10.1161/CIRCULATIONAHA.118.039262. [DOI] [PubMed] [Google Scholar]
- 35.Altwegg LA, Neidhart M, Hersberger M, Müller S, Eberli FR, Corti R, Roffi M, Sütsch G, Gay S, von Eckardstein, Wischnewsky MB, Lüscher TF, Maier W. Myeloid-related protein 8/14 complex is released by monocytes and granulocytes at the site of coronary occlusion: a novel, early, and sensitive marker of acute coronary syndromes. Eur Heart J. 2007;28(8):941–948. doi: 10.1093/eurheartj/ehm078. [DOI] [PubMed] [Google Scholar]
- 36.Morrow DA, Wang Y, Croce K, Sakuma M, Sabatine MS, Gao H, Pradhan AD, Healy AM, Buros J, McCabe CH, Libby P, Cannon CP, Braunwald E, Simon DI. Myeloid-related protein 8/14 and the risk of cardiovascular death or myocardial infarction after an acute coronary syndrome in the Pravastatin or Atorvastatin Evaluation and Infection Therapy: Thrombolysis in Myocardial Infarction (PROVE IT-TIMI 22) trial. Am Heart J. 2008;155(1):49–55. doi: 10.1016/j.ahj.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volz HC, Laohachewin D, Seidel C, Lasitschka F, Keilbach K, Wienbrandt AR, Andrassy J, Bierhaus A, Kaya Z, Katus HA, Andrassy M. S100A8/A9 aggravates post-ischemic heart failure through activation of RAGE-dependent NF-κB signaling. Basic Res Cardiol. 2012;107(2):250–250. doi: 10.1007/s00395-012-0250-z. [DOI] [PubMed] [Google Scholar]