Abstract
Otosclerosis is a bone condition affecting the stapes bone within the otic capsule, and its exact cause is still unknown. It is characterized by a lack of proper remodeling of newly formed vascular and woven bone, leading to the development of abnormal osteons and the formation of sclerotic bone. Bilateral otosclerosis is seen in 80% of patients and 60% of otosclerosis patients have a family history of the condition. The etiology of this disease is still unknown, there are lots of theories to explain it. The histopathological (HP) studies of otosclerosis showed that osteoblasts, osteoclasts, vascular proliferation, fibroblasts, and histiocytes were observed in the stapes footplate. The onset of the symptoms occurs by the early third decade of life, usually it doesn’t start later. In otosclerosis, the energy exerted by sound at the level of the tympanic membrane is reduced in the inner ear due to the fixation and rigidity of the ossicular chain, leading to hearing loss, especially for low frequencies. The primary clinical symptom of otosclerosis is conductive hearing loss but it is important to note that sensorineural hearing loss and mixed hearing loss can also occur as secondary symptoms of the condition. Another symptom present in patients with otosclerosis is tinnitus. The paper carried out a retrospective study of 70 patients diagnosed with otosclerosis in the Department of Otorhinolaryngology of Emergency City Hospital, Timişoara, Romania, between January 2021 to December 2022. Tissue fragments were processed at Service of Pathology by standard Hematoxylin–Eosin staining. The HP diagnosis was completed using Masson’s trichrome staining, Giemsa histochemical staining, and immunohistochemical (IHC) reactions with anti-cluster of differentiation (CD)20, anti-CD3, anti-CD4, anti-CD8, anti-CD34, and anti-CD31 antibodies. The microscopic examination showed a chronic diffuse inflammatory infiltrate that consisted predominantly of mature T-lymphocytes, immunohistochemically positive for CD3, CD4 and CD8. There were also present rare CD20-positive B-lymphocytes. Among the lymphocytes, relatively numerous mast cells were identified, highlighted histochemically by the Giemsa staining. They had numerous purple-violet intracytoplasmic granules. In the connective tissue support, a relatively rich vascular network was identified, consisting of hyperemic capillaries, highlighted immunohistochemically with anti-CD31 and anti-CD34 antibodies. Bone tissues trabeculae showed extensive areas of fibrosis. The collagen fibers were highlighted by Masson’s trichrome staining, being stained in green, blue, or bluish green.
Keywords: otosclerosis , immunohistochemistry , mast cells , T-lymphocytes
Introduction
Otosclerosis is a disorder that affects the stapes bone within the otic capsule. The cause of this condition is still unknown. The otosclerosis results as a failure in the appropriate remodeling of newly synthetized trabecular bone, being characterized by sclerosis of osseous extracellular matrix with perturbed osteons. The history of otosclerosis starts in 1704 when Valsalva reported for the first time that hearing loss is caused by stapes ankylosis caused by an inflammatory reaction in the middle ear [1]. Throughout history, Ádám Politzer discovered in his pathological studies that ankylosis is caused by a new bone that is overgrowing at the oval window and the stapes level. However, only in 1901 the term of otosclerosis is being introduced as a distinct pathology [1]. The etiology of this disease is still unknown, there are lots of theories to explain it. The histopathological (HP) studies of otosclerosis showed that osteoblasts, osteoclasts, vascular proliferation, fibroblasts, and macrophages were observed in the stapes footplate [2]. According to the published studies in terms of location, the otosclerotic plaque is most frequently at the level of the stapes footplate and anterior to the oval window, followed by the round window, pericochlear region and last but not least the anterior part of the internal auditory canal [3]. In the Ear Nose and Throat (ENT) treatise written by Anniko et al., it is underlined that there are roughly 15–20 cases of clinical otosclerosis per 100 000 people in Europe every year [2, 4]. In 80% of cases, otosclerosis is present bilateral. Sixty percent of patients with otosclerosis have a family history of this disease. According to Thys & Van Camp, 40% of the remaining cases of otosclerosis involve various factors, such as autosomal dominant inheritance with inconsistent penetrance in certain family members, phenocopies, new mutations, or rare cases transmitted through alternate modes of inheritance [1, 3, 5]. The disease doesn’t show clinical symptoms until after the age of 20, and in Central Europe, the fifth decade has the highest frequency. The illness runs in families, and females are affected twice more frequently than males [4]. Factors that influence the progression of the disease have been highlighted as follows: puberty, pregnancy, and menopause hormonal factors [3, 6]. Following the published literature, it was noticed that otosclerosis is a disease that predominates in the Caucasian population. This is rarely found in black, Indian, and oriental populations. The incidence is also low in the South American and Japanese population [3]. The onset of the symptoms occurs by the early third decade of life, usually it doesn’t start later [3, 6]. From the gender ratio point of view, it was remarked that there is a predominance for the female gender, the ratio of women to men being 2:1 [7]. In otosclerosis, the energy exerted by sound at the level of the tympanic membrane is reduced in the inner ear due to the fixation and rigidity of the ossicular chain, leading to hearing loss, especially for low frequencies [8]. Conductive hearing loss is the main clinical symptom of otosclerosis. In a few cases, the hearing loss could be sensorineural or mixed. These patients also accused tinnitus at presentation [3, 9]. The treatment methods used for otosclerosis are stapes surgery that is a minimally invasive procedure. Implantable hearing aids, such as middle ear or bone conduction implants, may be considered as options for managing otosclerosis. In more severe cases, cochlear implants can be an alternative solution. The effectiveness of different pharmaceutical treatments for otosclerosis remains uncertain and are not widely regarded as conventional treatment methods [7].
Aim
The paper highlighted the histological aspects found in osseous harvested specimens from patients with otosclerosis, with emphasis on immunohistochemical (IHC) phenotype of inflammatory cells presented around sclerotic bone and to characterize the vascularization of the surrounding connective tissue, for a better understanding of disease etiology.
Materials and Methods
Study patients’ selection
The paper carried out a retrospective study of all patients diagnosed with otosclerosis between January 2021 and December 2022 from the Hospital’s database at the Department of ENT, Emergency City Hospital, Timişoara, Romania. The Hospital is affiliated with the Victor Babeş University of Medicine and Pharmacy, Timişoara.
Patient demographic data, biological investigations, and medical history information were obtained from the patient observation sheet.
Inclusion criteria
To be included in the study, the patients should have presented: unilateral or bilateral mild, moderate, or severe mixed hearing loss or transmission hearing loss; type A tympanogram; absent Stapedius reflex; a surgery procedure (stapedotomy or stapedectomy) and fitting of a titanium (Ti) prosthesis at the same surgical time.
Exclusion criteria
As exclusion criteria were considered acute otic infections at the time of hospitalization and incomplete investigations like lack of audiogram.
During the afforded mentioned period, 73 patients were diagnosed with unilateral or bilateral otosclerosis, but three patients were excluded according with used selection criteria (a 39-year-old female patient because of the type of surgery applied, tympanoplasty not stapedotomy nor stapedectomy, a 38-year-old male patient because he refused the surgery, and a 51-year-old female patient was counted only once even if she had two hospital presentations during the period studied).
Laboratory method
Biopsy was performed in all cases. The harvested tissue fragments were sent to the Service of Pathology from Emergency City Hospital, Timişoara, and processed by standard histological procedure, after decalcification.
The harvested bone tissue fragments were processed in accordance with the international protocol, adapted to the requirements of the Department and recommendations of the Romanian Ministry of Health. All biopsies were fixed in buffered formaldehyde, then decalcified and stained using standard Hematoxylin–Eosin (HE) staining. The HP diagnosis was completed using Masson’s trichrome staining and Giemsa histochemical staining.
To phenotype the inflammatory cells and to characterize the blood vessel network, there were used IHC reactions for cluster of differentiation (CD)20, CD3, CD4, CD8, CD34, and CD31.
Table 1 consolidated all the data concerning the antibodies utilized for IHC reactions. All the reagents utilized for immunohistochemistry were supplied by Novocastra™ (Leica Biosystems). For all the antibodies, the inclusion time was 30 minutes.
Table 1.
Data related to the antibodies used for IHC reactions
|
Antibody |
Substrate |
Clone |
Dilution |
|
CD3 |
Monoclonal mouse |
LN10 |
1:500 |
|
CD4 |
Monoclonal mouse |
4B12 |
1:100 |
|
CD8 |
Monoclonal mouse |
4B11 |
1:500 |
|
CD20 |
Monoclonal mouse |
L26 |
1:150 |
|
CD34 |
Monoclonal mouse |
QBEnd/10 |
1:100 |
|
CD31 |
Monoclonal mouse |
JC70A |
1:100 |
CD: Cluster of differentiation; IHC: Immunohistochemical.
The slides were evaluated in conventional light microscopy, with the elaboration of an HP report that includes the patient’s personal data, the clinical diagnosis, the macroscopic examination of the specimens, as well as the HP diagnosis.
Statistical analysis
The statistics was run with Microsoft Excel software. For the ordinal variables, we calculated the median, minimum and maximum range values, frequencies, and percentages were used to describe the findings. For qualitative and dichotomous variables, there were calculated the frequency tables.
Ethical approvals
The Hospital Ethics Committee approved the presented study (No. I-28406/28.10.2022) and the patients signed the informed consent forms. Throughout the entirety of this study, the authors diligently adhered to all applicable guidelines, regulations, as well as ethics and safety protocols.
Results
Of the total of 70 patients, 69 were adults and one was a teenager, with the ages between 15 and 69.
The distribution of patients according to age highlighted that most subjects with otosclerosis were in between the ages 40 and 49 years old (39%), followed by the patients in the decade 50–59 years (19%) and 30–39 years (12%). In our study, the youngest patient was 15 years old and the oldest 69 years old. The average age of the studied group was 46 years, with a standard deviation of 9.47 years (Figure 1). Seventy-seven percent of subjects were females and 23% were males, with a 4:1 female to males’ ratio (Figure 1). Twenty-three percent come from a rural environment and 77% from an urban one (Figure 1).
Figure 1.
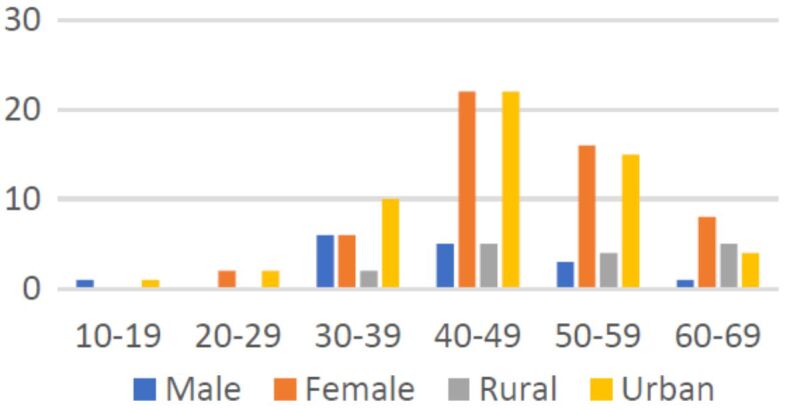
Distribution of patients according to age, gender, and place of living.
Twenty percent of patients had unilateral otosclerosis (14 out of 70), while 80% of them (56 out of 70) were diagnosed with bilateral disease (Figures 2 and 3). Besides hypoacusis, at admission, 54% of patients presented tinnitus, associated or not with vertigo (24%). Other symptoms were auricular fullness (four cases out of 70) and otalgia (three cases out of 70). The duration of symptoms was known for 50% of the patients included in the studied group. Symptoms with a duration of one to five years were described by 23 (33%) patients, between six and 10 years by 14% of patients and more than 20 years by only two patients (3%).
Figure 2.
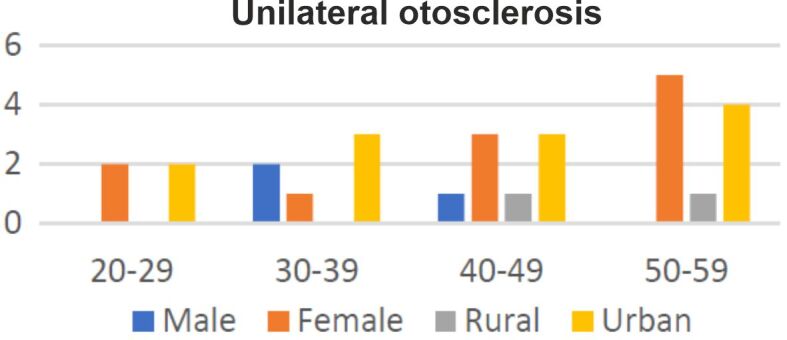
Distribution of patients diagnosed with unilateral otosclerosis according to gender and place of living
Figure 3.
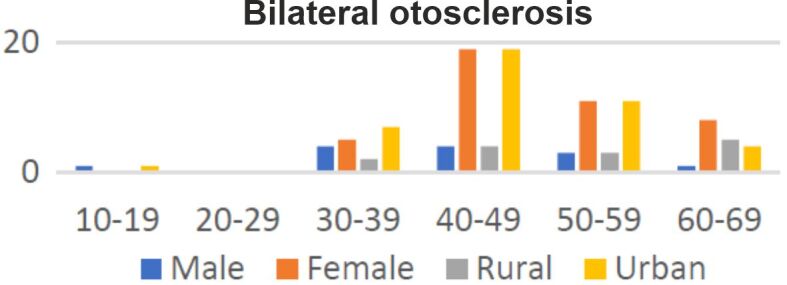
Distribution of patients diagnosed with bilateral otosclerosis according to gender and place of living.
Thirty percent of the patients (21 out of 70) have associated diseases like high blood pressure (seven cases), tumors (five cases), one benign and four malignant (thyroid, breast, Paget’s disease, ovarian cancer with costal metastasis), five cases of autoimmune thyroiditis, four cases of type 2 diabetes and three cases of hypercholesterolemia; other associated conditions, one case each, were coagulopathies, septal deviation, chronic hypertrophic rhinitis, type C hepatitis, herniated disc, chronic venous insufficiency, ovarian cyst, chronic uveitis, sinus tachycardia (Table 2).
Table 2.
The most frequent diseases associated with hearing loss
|
No. of cases |
Percentage |
|
|
Associated conditions |
21 |
30% |
|
High blood pressure |
7 |
10% |
|
Tumors |
5 |
7% |
|
Benign |
1 |
1% |
|
Malignant |
4 |
6% |
|
Autoimmune thyroiditis |
5 |
7% |
|
Type 2 diabetes |
4 |
6% |
|
Hypercholesterolemia |
3 |
4% |
Stapedotomy was performed on 67 out of 70 patients, while stapedectomy was used to correct the hypoacusis of a 27-year-old woman, and posterior stapedectomy was considered as a therapeutic method in two female cases (35-year-old and 42-year-old). In a 43-year-old woman, a reject of the Ti prosthesis was noted that had to be removed at three days post-implantation; in three cases, the surgical intervention consisted by reposition of Ti prosthesis (Table 3).
Table 3.
|
Patient |
Age [years] |
First surgery |
Actual surgery |
|
Male |
50 |
2017 |
2022 |
|
Female |
41 |
2020 |
2022 |
|
Female |
47 |
2020 |
2022 |
Sixty-one (87%) patients presented right ear hypoacusis, with 31 cases of conductive hearing loss, 27 mixt and three sensorineural. In nine cases, the right ear audiogram was normal (Figure 4). Sixty-five (93%) patients presented left ear hypoacusis, with 33 cases of conductive hearing loss, 26 mixt and six sensorineural. In five cases, the left ear audiogram was normal (Figure 5).
Figure 4.
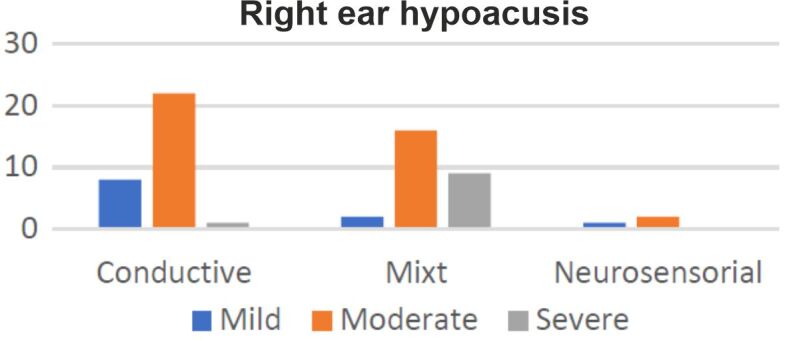
Distribution of right ear hypoacusis according to subtypes and intensity.
Figure 5.
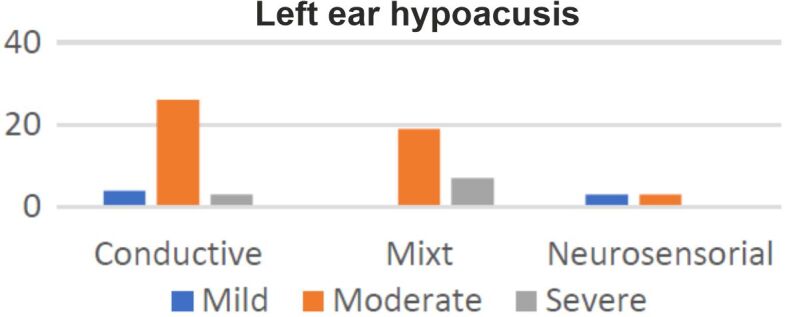
Distribution of left ear hypoacusis according to subtypes and intensity.
In one case of a 27-year-old female patient, diagnosed with moderate conductive hearing loss in the right ear, there were noted important family history events. At admission, the tympanogram was type As, with the absence of the stapedial reflex. The patient did not present symptoms such as vertigo or tinnitus. From the family history, she stated that her grandmother suffered from otosclerosis. The results punctuated a transmission of otosclerosis on the maternal line (Figure 6).
Figure 6.
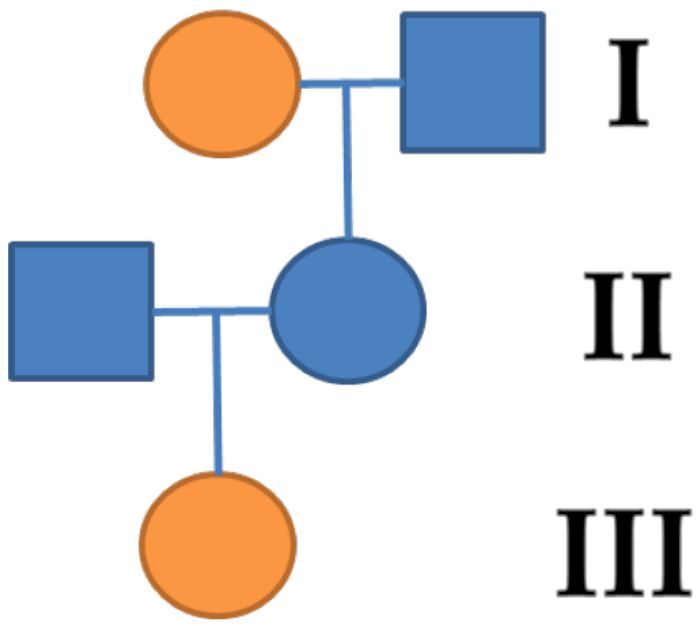
The genealogy of patient’s family. Orange symbols denote individuals with clinical features of otosclerosis.
The HP examination highlighted that on HE- and Masson’s trichrome-stained slides, in all cases, there was observed formation of osteoid, lined by osteoblasts and osteoprogenitor cells and surrounded by connective tissue, with areas of calcified osseous matrix that presented osteocytes disposed in lacunae between osseous lamella arranged in incomplete shaped osteons (Figures 7, 8, 9, 10, 11, 12, 13, 14).
Figure 7.
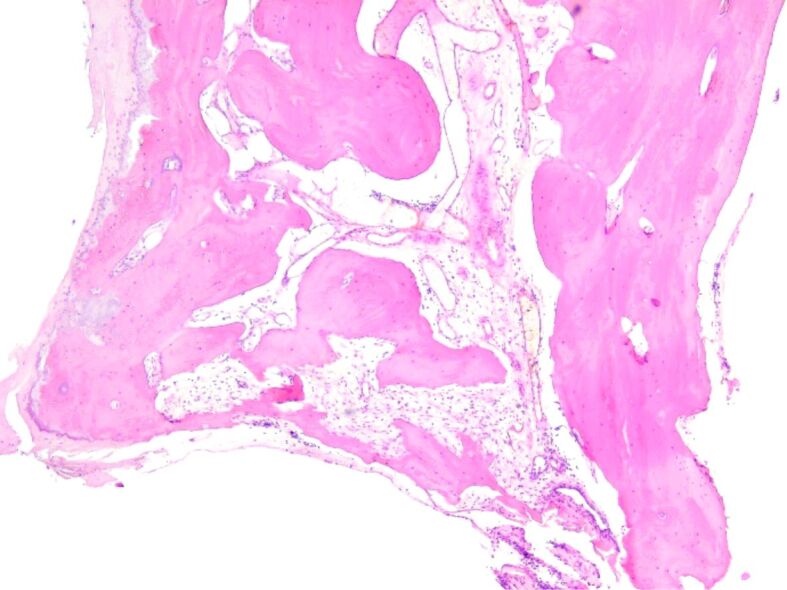
Incomplete shaped osteons, surrounded by connective tissue with interstitial edema and lymphocytes. HE staining, ×50. HE: Hematoxylin–Eosin.
Figure 8.
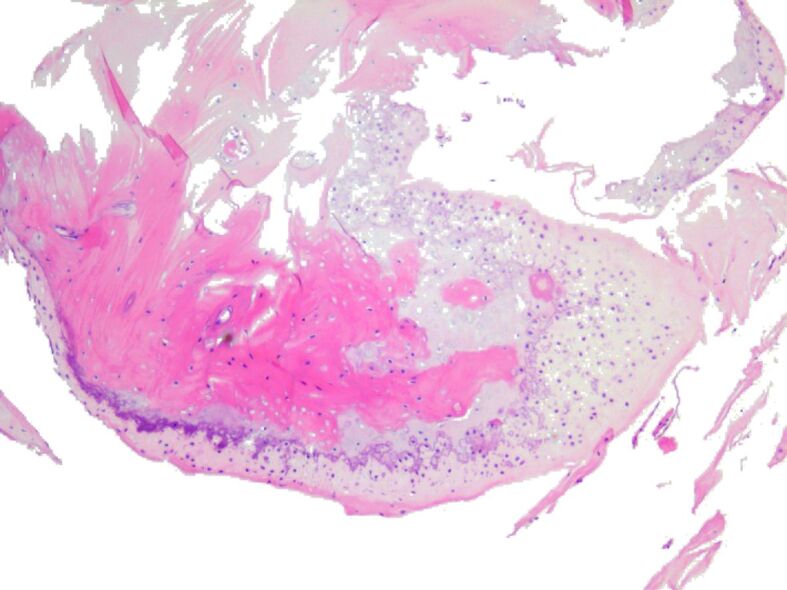
Newly-formed osteoid lined by osteoblasts and osteoprogenitor cells in a connective tissue with diffuse moderate lymphocytic infiltrate. HE staining, ×50.
Figure 9.
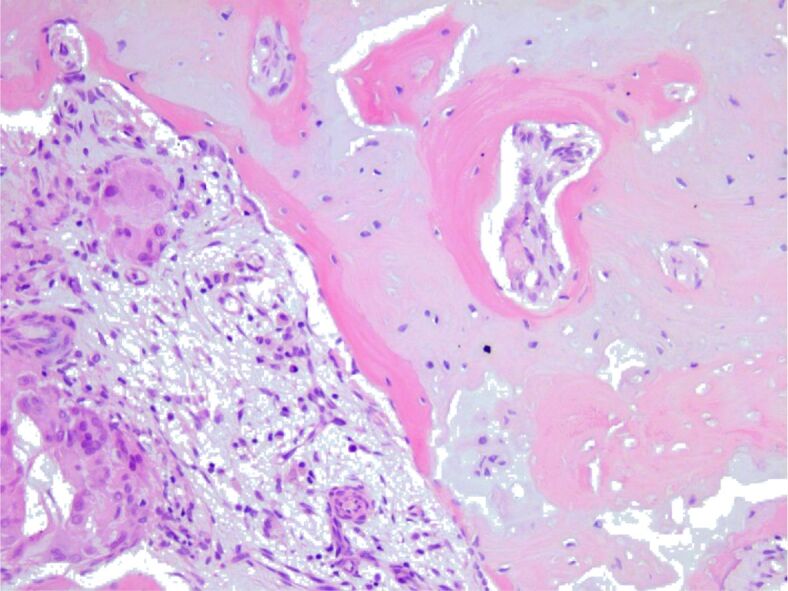
Newly-formed osteoid with osseous lamella arranged in osteons, and lacune with osteocytes, surrounded by connective tissue with lymphocytes, plasma cells, macrophages, and multinucleated giant cells. HE staining, ×200
Figure 10.
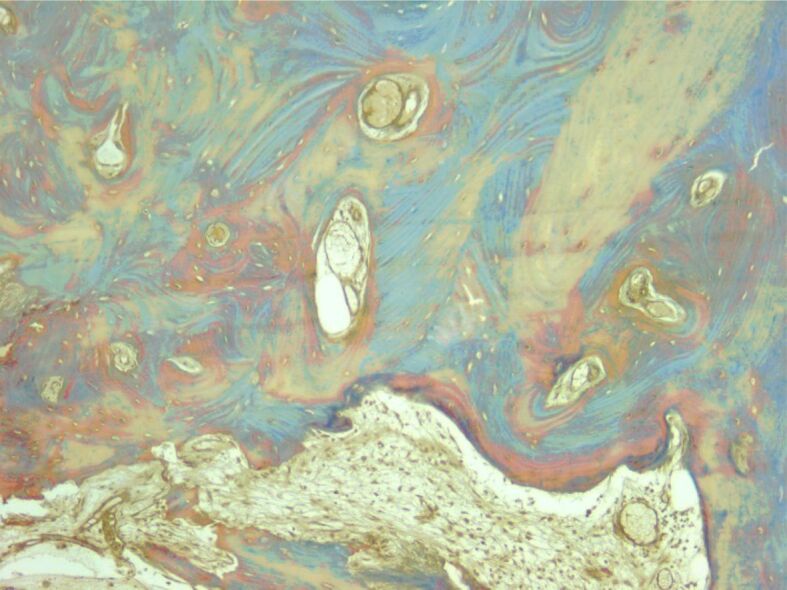
Osseous trabeculae formed intertrabecular spaces with bone marrow. Masson’s trichrome staining, ×100.
Figure 11.
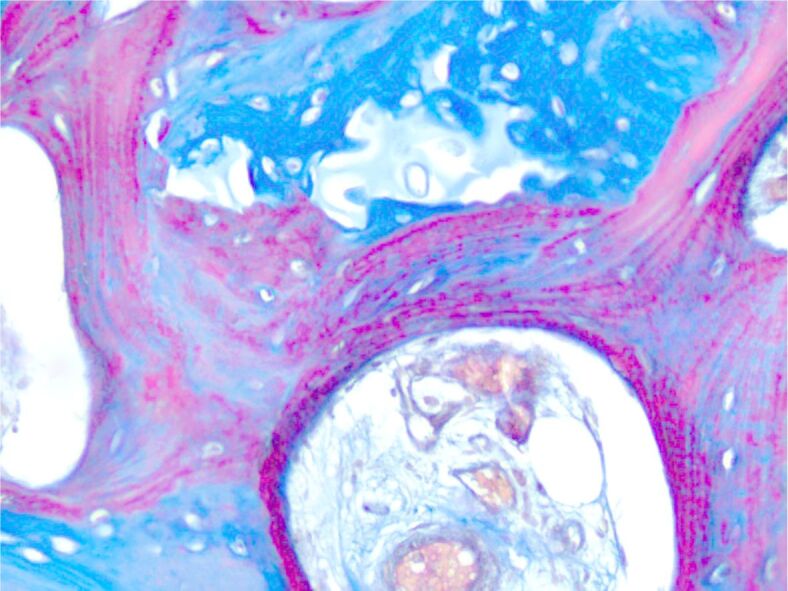
Newly-formed osteoid. Masson’s trichrome staining, ×200
Figure 12.
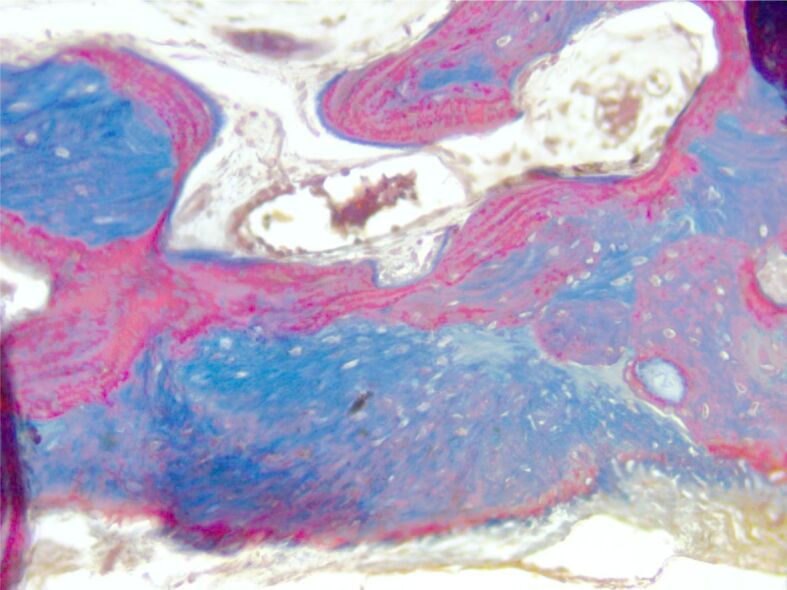
Dense sclerotic bone with prominent cement lines. Masson’s trichrome staining, ×200
Figure 13.
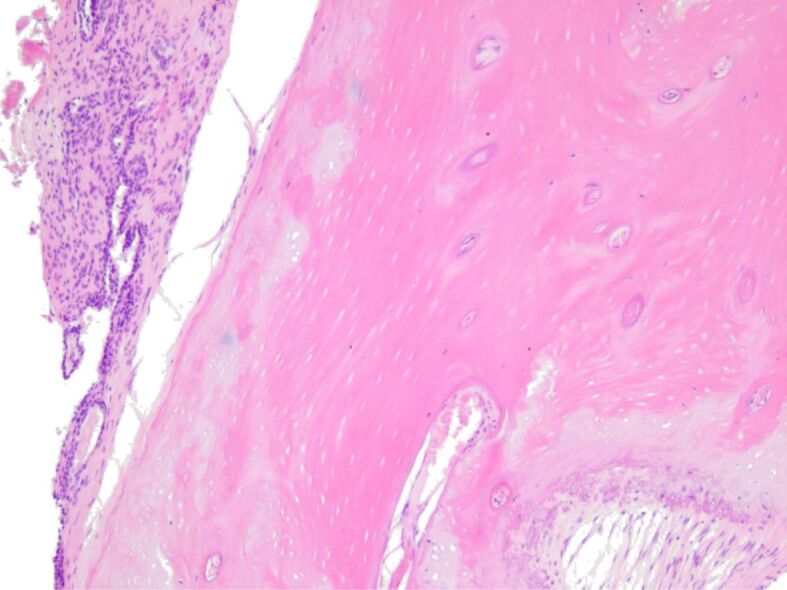
Diffuse mild lymphocytic infiltrate around the newly-formed osteoid. HE staining, ×100
Figure 14.
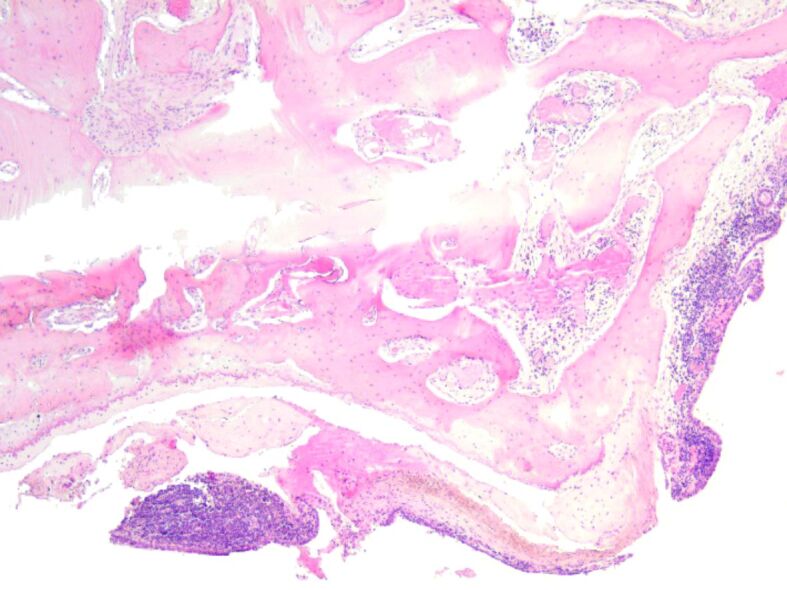
Heavy diffuse inflammatory infiltrate and with formation of small follicles around osteoid trabeculae. HE staining, ×50
The Giemsa staining revealed the presence of mast cells around small blood vessels (Figures 15 and 16).
Figure 15.
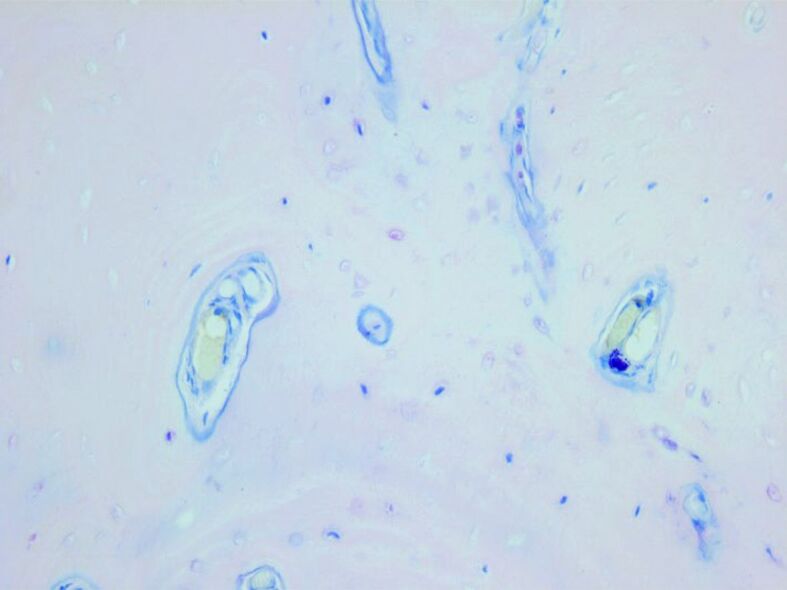
Small orthochromatic mast cells in perivascular space. Giemsa staining, ×100
Figure 16.
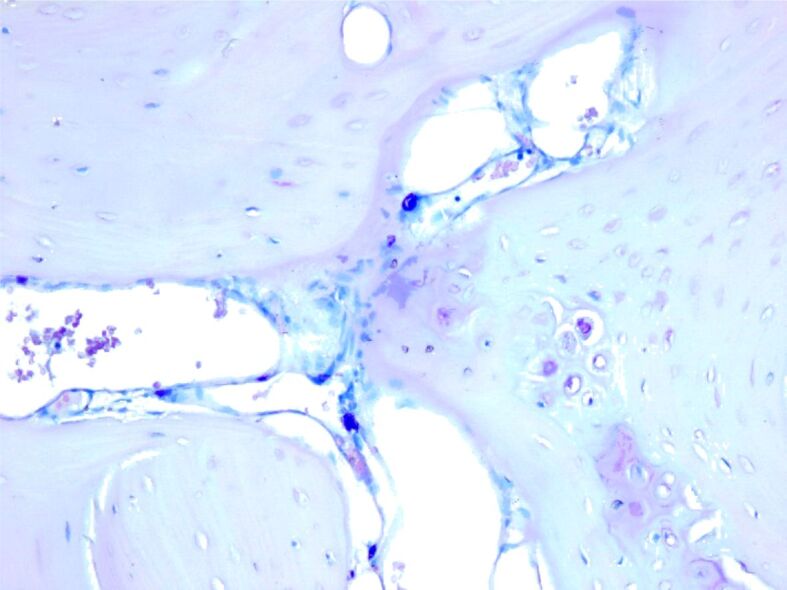
Mast cells filled with orthochromatic granules in the connective tissue around osseous trabeculae. Giemsa staining, ×50.
The inflammatory infiltrate consisted predominantly of mature T-lymphocytes, immunohistochemically positive for CD3. Most of the T-cells were CD4-positive, while only a few lymphocytes were positive for CD8 (Figures 17, 18, 19). A small number of lymphocytes were B-cells, CD20-positive (Figure 20).
Figure 17.
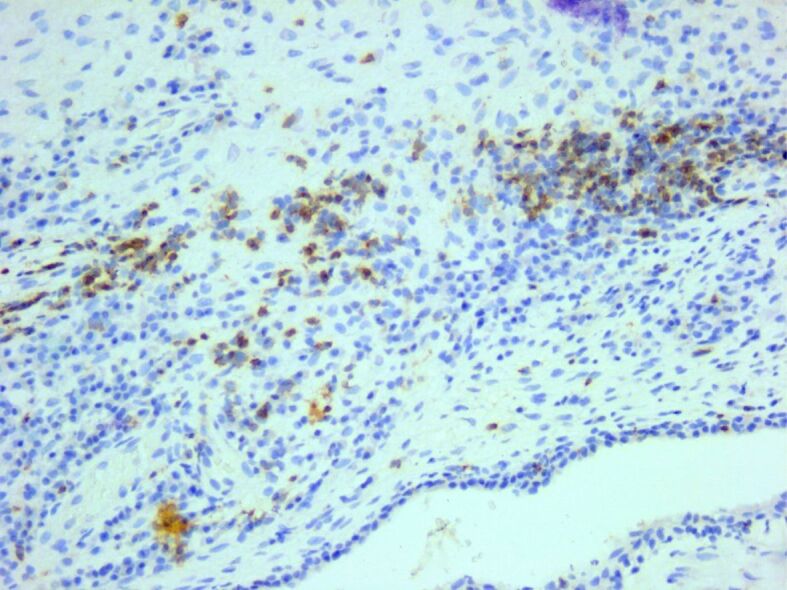
Diffuse inflammatory infiltrate composed of many CD3-positive T-cells. Anti-CD3 antibody immunomarking, ×200. CD: Cluster of differentiation
Figure 18.
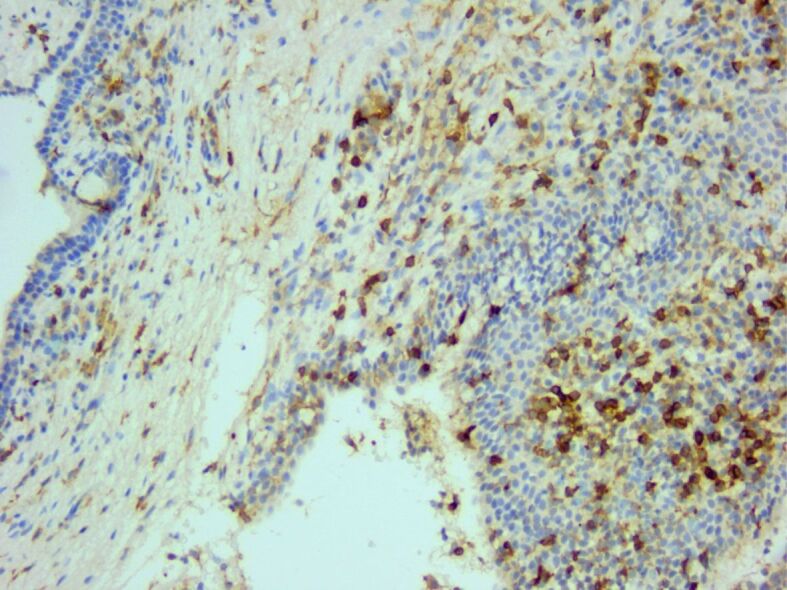
The inflammatory infiltrate consisted of many CD4-positive T-helper cells. Anti-CD4 antibody immunomarking, ×200
Figure 19.
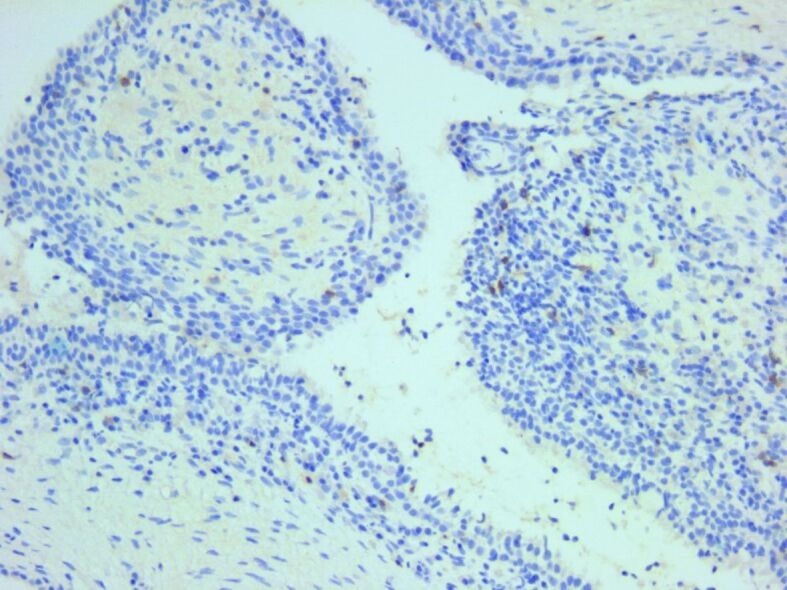
Only a few cytotoxic CD8-positive T-cells were identified in the inflammatory infiltrate. Anti-CD8 antibody immunomarking, ×200
Figure 20.
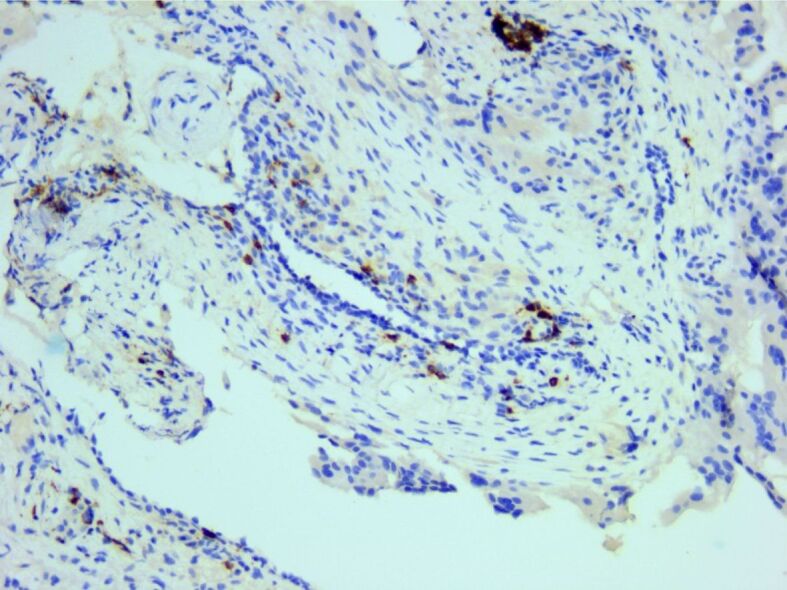
Inflammatory infiltrate also contained a small number of CD20-positive B-cells. Anti-CD20 antibody immunomarking, ×200
In the surrounding connective support, a relatively rich vascular network was identified, consisting of hyperemic capillary structures, highlighted immunohistochemically with anti-CD31 and anti-CD34 antibodies (Figures 21 and 22).
Figure 21.
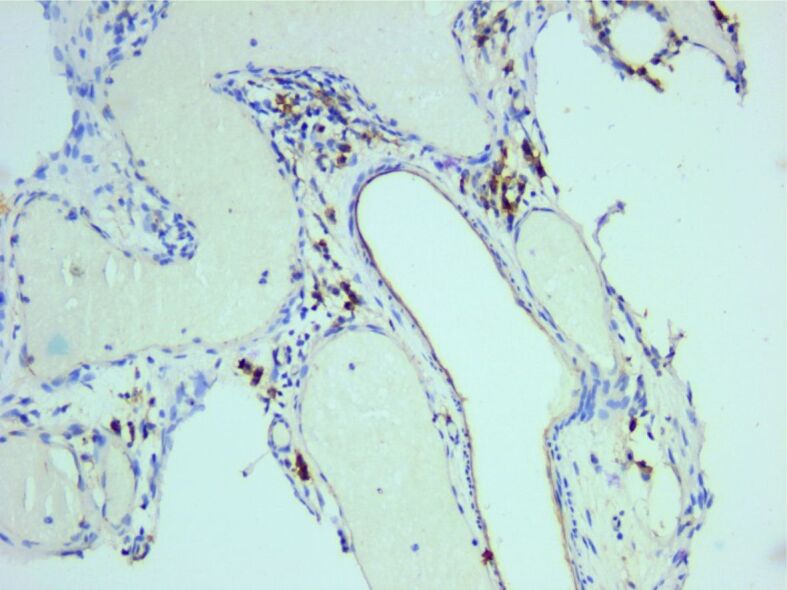
Rich vascular network highlighted by CD31 reaction. Anti-CD31 antibody immunomarking, ×200
Figure 22.
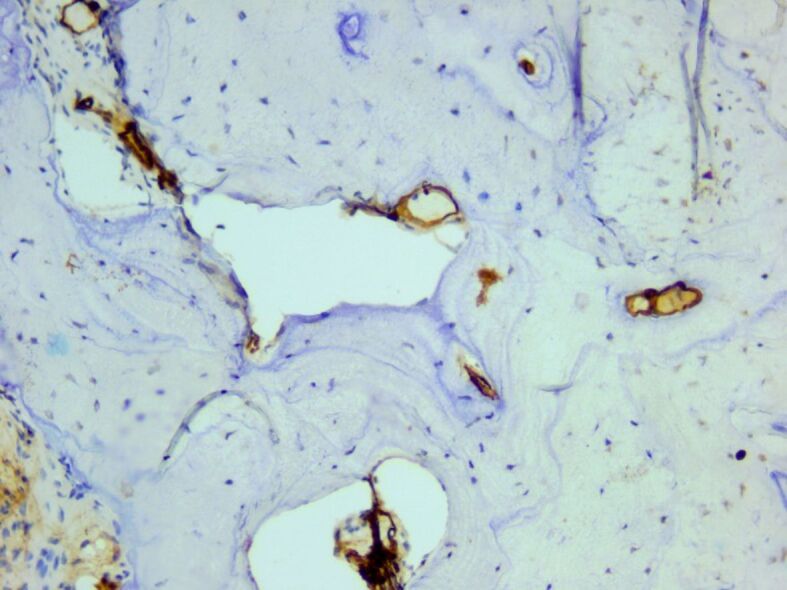
Newly-formed small blood vessels surrounding osseous trabeculae. Anti-CD34 antibody immunomarking, ×200.
Discussions
An intensive consultation and a complete audiological evaluation are the important keys in the correct diagnosis of otosclerosis, regardless of the patient’s age [10].
Otosclerosis is a disease that predominates in the Caucasian population. This is rarely found in black, Indian, and oriental populations. The incidence is also low in the South American and Japanese population [6, 11]. The studied group in the present article was composed only of Caucasians, but this could be also a demographic condition.
In otosclerosis, the energy exerted by sound at the level of the tympanic membrane is reduced in the inner ear due to the fixation and rigidity of the ossicular chain, leading to hearing loss, especially for low frequencies [9, 12]. The hearing loss is mainly conductive, but in few patients, a sensorineural or a mixed otosclerosis can occur [13, 14, 15]. In the present study, the most frequent mechanism of hearing loss was also conductive (44% for right ear and 47% for left ear), similar to the literature, but the number of cases with mixt hearing loss was also important (39% for right ear and 37% for left ear). Only a few cases presented a sensorineural hearing loss (4% for right ear and 9% for left ear).
Even if vertigo and tinnitus can be symptoms for many pathological conditions, are also present in patients with otosclerosis [6, 10, 16, 17, 18, 19]. If tinnitus was one of the most important accuse that referred 54% of the patients from this study to the hospital, vertigo was present only in 24% of cases.
According to the literature and similar to the present article, in 80% of cases, otosclerosis is diagnosed as a bilateral condition [20, 21].
Despite that in published literature, 60% of patients with otosclerosis have family members with this condition, in this study only one female patient described a similar condition presented by other family members [22, 23]. Other authors perceived that 40% of otosclerosis cases can be attributed to various factors, such as autosomal dominant inheritance with variable penetrance in family members, or rare cases transmitted through alternative modes of inheritance, but this fact was identified in only one patient of the present study [1, 6, 11].
The disease becomes clinical between the 30th year and 50th year of age, and the highest incidence is seen in the fifth decade in Central Europe. The diagnosis of otosclerosis is very rare under the age of 30 and over the age of 60 [24, 25]. In the present study, 39% of the patients were in their forties at the time of diagnosis, followed by 27% in their fifties. Only three patients out of 70 (4%) were at diagnosis less than 30-year-old.
Similar to those observed in different malignancies, also in otosclerosis there is a protection induced by sexual hormones [26]. Despite this protection, many authors found out that females are affected twice as often as males [2, 20, 27, 28]. In this study, women represented 77% of cases, with a 3.34:1 female to male ratio.
The most frequently associated diseases identified in the patients of the present study were high blood pressure, tumor conditions and autoimmune thyroiditis. Even if there are almost 100 years since few researchers suggested that could be an endocrine mechanism of otosclerosis, so far, no studies have been published in the English literature to demonstrate if there is a correlation between otosclerosis and autoimmune thyroiditis or different types of tumors [29, 30, 31]. These correlations should be well studied in the future, to be able to observe if exists an association between these pathologies and also an etiological pathway that could be interrupted by preventive therapies.
Some authors identified a correlation between Paget’s disease of bone and osteosclerosis [32, 33, 34, 35, 36, 37], condition that was also found in a 45-year-old female patient from present paper, but the association of these two diseases must be furthermore investigated.
Moreover, besides of the fact that high blood pressure was the most frequently observed associated conditions to otosclerosis during the patients of the present study, in the English literature there is no correlation cited between these diseases. A paper debates the possibility of a relation between high blood pressure and failure of the surgical procedure with necessity of reintervention [38, 39, 40]. In the studied group, three patients needed reintervention to reposition the prosthesis, but all of them had normal blood pressure.
It was demonstrated that bisphosphonates use can interfere with bone remodeling by acting on osteoclasts’ activity [41, 42]. This could be the reason why some authors proposed bisphosphonates as optimal candidates to prevent or to stop the evolution of osteosclerosis [32, 43, 44]. Besides this, the “gold standard” treatment method of otosclerosis remains surgical, through posterior stapedectomy, stapedectomy or stapedotomy and placement of an ossicular chain prosthesis [8].
HP studies of otosclerosis showed that osteoblasts, osteoclasts, vascular proliferation, fibroblasts, and macrophages were observed in the stapes footplate [4]. According to the published studies in terms of location, the otosclerotic plaque is most frequently at the level of the stapes footplate and anterior to the oval window, followed by the round window, pericochlear region and last but not least the anterior part of the internal auditory canal [7]. During the dynamic of otosclerotic disease, the bone can have three HP patterns: spongiotic bone, sclerotic bone with dense mineralized bone, and mixed bone [1, 10].
In the present study, the HP exams of pericochlear region, oval window, and stapes base highlighted the presence of osteoclasts and osteoblasts, around osseous trabeculae with foci of increased cellularity and areas of bone resorption and deposition. The collagen fibers of the newly formed bone showed fibrous thickening. The surrounding connective tissue presented mild to moderate inflammatory infiltrate and increased vascularization.
Even if the hearing loss due to chronic infections and formation of cholesteatomas and that from otosclerosis have different pathogenic pathways, the inflammatory infiltrate seems to be similar, consisting predominant from T-lymphocytes [45, 46, 47, 48].
Conclusions
The present study highlighted the epidemiological data of osteosclerosis and described its HP characteristics on morphological stained slides, HE and Masson’s trichrome. Moreover, Giemsa staining and IHC reactions helped to phenotype the inflammatory infiltrate and to demonstrate the presence of T-cells and mast cells around the newly formed trabeculae and also the aspects of small blood vessels. A better understanding of the pathophysiology of otosclerosis could offer a treatment option to cure this condition, that it is not realizable nowadays, with great impact on the patients’ quality of life.
Conflict of interests
The authors declare that they have no conflict of interests.
Author contribution
Claudia Raluca Bălaşa Vîrzob and Raluca Maria Cloşca equally contributed to the manuscript.
References
- 1.Rämö JT, Kiiskinen T, Seist R, Krebs K, Kanai M, Karjalainen J, Kurki M, Hämäläinen E, Häppölä P, Havulinna AS, Hautakangas H;, Palta P, Esko T, Metspalu A, Pirinen M, Karczewski KJ, Ripatti S, Milani L, Stankovic KM, Mäkitie A, Daly MJ, Palotie A. Genome-wide screen of otosclerosis in population biobanks: 27 loci and shared associations with skeletal structure. Nat Commun. 2023;14(1):157–157. doi: 10.1038/s41467-022-32936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anniko M , Bernal-Sprekelsen M , Bonkowsky V , Bradley PJ , Iurato S , et al., editors. Otorhinolaryngology, head and neck surgery . Berlin-Heidelberg : Springer-Verlag ; 2010 . [Google Scholar]
- 3.Foster MF, Backous DD. Clinical evaluation of the patient with otosclerosis. Otolaryngol Clin North Am. 2018;51(2):319–326. doi: 10.1016/j.otc.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Hederstierna C, Cureoglu S, Paparella MM. Undiagnosed severe cochlear otosclerosis as a cause of profound hearing loss. Otol Neurotol. 2013;34(3):e14–e15. doi: 10.1097/MAO.0b013e31826bf3bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thys M, Van Camp. Genetics of otosclerosis. Otol Neurotol. 2009;30(8):1021–1032. doi: 10.1097/MAO.0b013e3181a86509. [DOI] [PubMed] [Google Scholar]
- 6.Rudic M, Keogh I, Wagner R, Wilkinson E, Kiros N, Ferrary E, Sterkers O, Bozorg Grayeli, Zarkovic K, Zarkovic N. The pathophysiology of otosclerosis: review of current research. Hear Res. 2015;330(Pt A):51–56. doi: 10.1016/j.heares.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Michaels L, Soucek S. Origin and growth of otosclerosis. Acta Otolaryngol. 2011;131(5):460–468. doi: 10.3109/00016489.2010.532156. [DOI] [PubMed] [Google Scholar]
- 8.Batson L, Rizzolo D. Otosclerosis: an update on diagnosis and treatment. JAAPA. 2017;30(2):17–22. doi: 10.1097/01.JAA.0000511784.21936.1b. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho B, Hamerschmidt R, Telles JE, Richter N. Anatomopathology of the superstructure of the stapes in patients with otosclerosis. Int Arch Otorhinolaryngol. 2015;19(1):1–4. doi: 10.1055/s-0034-1382096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danesh AA, Shahnaz N, Hall JW. The audiology of otosclerosis. Otolaryngol Clin North Am. 2018;51(2):327–342. doi: 10.1016/j.otc.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Markou K, Stavrakas M, Karkos P, Psillas G. Juvenile otosclerosis: a case presentation and review of the literature. BMJ Case Rep. 2016;2016:bcr2015214232–bcr2015214232. doi: 10.1136/bcr-2015-214232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabbara B, Gauche C, Calmels MN, Lepage B, Escude B, Deguine O, Fraysse B, Marx M. Decisive criteria between stapedotomy and cochlear implantation in patients with far advanced otosclerosis. Otol Neurotol. 2015;36(3):e73–e78. doi: 10.1097/MAO.0000000000000692. [DOI] [PubMed] [Google Scholar]
- 13.Hill-Feltham PR, Johansson ML, Hodgetts WE, Ostevik AV, McKinnon BJ, Monksfield P, Sockalingam R, Wright T, Tysome JR. Hearing outcome measures for conductive and mixed hearing loss treatment in adults: a scoping review. Int J Audiol. 2021;60(4):239–245. doi: 10.1080/14992027.2020.1820087. [DOI] [PubMed] [Google Scholar]
- 14.Tomasoni M, Borsetto D, Deretti A, Arcuri M, Sordi A, Zorzi S, Redaelli de, Piazza C, Deganello A, Sorrentino T. Exploratory tympanotomy in conductive hearing loss with normal preoperative investigations. Acta Otorhinolaryngol Ital. 2022;42(6):569–581. doi: 10.14639/0392-100X-N1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SY, Han JJ, Oh SH, Lee JH, Suh MW, Kim MH, Park MK. Differentiating among conductive hearing loss conditions with wideband tympanometry. Auris Nasus Larynx. 2019;46(1):43–49. doi: 10.1016/j.anl.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Newman-Toker DE, Della Santina, Blitz AM. Vertigo and hearing loss. Handb Clin Neurol. 2016;136:905–921. doi: 10.1016/B978-0-444-53486-6.00046-6. [DOI] [PubMed] [Google Scholar]
- 17.Wright AE, McFarland J, Shoja MM. Archigenes and the syndrome of vertigo, tinnitus, hearing loss, and headache. Childs Nerv Syst. 2021;37(8):2417–2425. doi: 10.1007/s00381-019-04343-5. [DOI] [PubMed] [Google Scholar]
- 18.Jianu DC, Jianu SN, Dan TF, Motoc AGM, Poenaru M. Pulsatile tinnitus caused by a dilated left petrosquamosal sinus. Rom J Morphol Embryol. 2016;57(1):319–322. [PubMed] [Google Scholar]
- 19.Codreanu C, Tran Ba. Isolate vertigo crisis revealing an endolymphatic sac tumor. Rom J Morphol Embryol. 2010;51(2):387–389. [PubMed] [Google Scholar]
- 20.Crompton M, Cadge BA, Ziff JL, Mowat AJ, Nash R, Lavy JA, Powell HRF, Aldren CP, Saeed SR, Dawson SJ. The epidemiology of otosclerosis in a British cohort. Otol Neurotol. 2019;40(1):22–30. doi: 10.1097/MAO.0000000000002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quaranta N, Piccininni K, Romanello M, Lucidi D, Sergi B. The impact of intraoperative factors in otosclerosis outcomes: retrospective study in a tertiary centre. Acta Otorhinolaryngol Ital. 2019;39(3):197–204. doi: 10.14639/0392-100X-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMahon CM, Kifley A, Rochtchina E, Newall P, Mitchell P. The contribution of family history to hearing loss in an older population. Ear Hear. 2008;29(4):578–584. doi: 10.1097/AUD.0b013e31817349d6. [DOI] [PubMed] [Google Scholar]
- 23.Tavernier LJM, Vanpoucke T, Schrauwen I, Van Camp, Fransen E. Targeted resequencing of otosclerosis patients from different populations replicates results from a previous genome-wide association study. J Clin Med. 2022;11(23):6978–6978. doi: 10.3390/jcm11236978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marinelli JP, Totten DJ, Chauhan KK, Lohse CM, Grossardt BR, Vrabec JT, Carlson ML. The rise and fall of otosclerosis: a population-based study of disease incidence spanning 70 years. Otol Neurotol. 2020;41(9):e1082–e1090. doi: 10.1097/MAO.0000000000002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niedermeyer HP, Häusler R, Schwub D, Neuner NT, Busch R, Arnold W. Evidence of increased average age of patients with otosclerosis. Adv Otorhinolaryngol. 2007;65:17–24. doi: 10.1159/000098664. [DOI] [PubMed] [Google Scholar]
- 26.Dehelean CA, Soica C, Pinzaru I, Coricovac D, Danciu C, Pavel I, Borcan F, Spandidos DA, Tsatsakis AM, Baderca F. Sex differences and pathology status correlated to the toxicity of some common carcinogens in experimental skin carcinoma. Food Chem Toxicol. 2016;95:149–158. doi: 10.1016/j.fct.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Ricci G, Gambacorta V, Lapenna R, Della Volpe, La Mantia, Ralli M, Di Stadio. The effect of female hormone in otosclerosis. A comparative study and speculation about their effect on the ossicular chain based on the clinical results. Eur Arch Otorhinolaryngol. 2022;279(10):4831–4838. doi: 10.1007/s00405-022-07295-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian ZJ, Alyono JC. Effects of pregnancy on otosclerosis. Otolaryngol Head Neck Surg. 2020;162(4):544–547. doi: 10.1177/0194599820907093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drury DW. An endocrine factor in otosclerosis. Acta Oto-Laryngologica. 1927;10(2):90–105. [Google Scholar]
- 30.Wakwick HL, Stevenson HM. Is there an endocrine factor in otosclerosis. Acta Oto-Laryngologica. 1926;9(1):317–324. [Google Scholar]
- 31.orop VB, Borugă VM, Pînzaru IA, Barac IR, Utescu C, Maghiari AL, Baderca F, Bălan L, Şorop-Florea M, Dumitraşcu V, Anastasiu DM, Simu S, Radu D, Suciu O. Hormone treatment and UVB exposure influences on female mice regarding skin physiological parameters, biochemical parameters and organ histology. Rom J Morphol Embryol. 2020;61(3):879–887. doi: 10.47162/RJME.61.3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chole RA, McKenna M. Pathophysiology of otosclerosis. Otol Neurotol. 2001;22(2):249–257. doi: 10.1097/00129492-200103000-00023. [DOI] [PubMed] [Google Scholar]
- 33.Morrison AW, Bundey SE. The inheritance of otosclerosis. J Laryngol Otol. 1970;84(9):921–932. doi: 10.1017/s0022215100072698. [DOI] [PubMed] [Google Scholar]
- 34.Davis GL. Pathology of otosclerosis: a review. Am J Otolaryngol. 1987;8(5):273–281. doi: 10.1016/s0196-0709(87)80046-7. [DOI] [PubMed] [Google Scholar]
- 35.Kelemen G. Temporal bone showing otosclerosis, Paget’s disease and adenocarcinoma. Ann Otol Rhinol Laryngol. 1977;86(3 Pt 1):381–385. doi: 10.1177/000348947708600316. [DOI] [PubMed] [Google Scholar]
- 36.Petrescu PH, Izvernariu DA, Iancu C, Dinu GO, Berceanu-Văduva MM, Crişan D, Iacob M, Bucur VM, Răuţia IC, Prejbeanu IR, Dema S, Duţă CC. Pathological fracture of the femur in a patient with Paget’s disease of bone: a case report. Rom J Morphol Embryol. 2016;57(2):595–600. [PubMed] [Google Scholar]
- 37.Căruntu C, Zurac SA, Jugulete G, Boda D. Extramammary Paget’s disease in an HIV-positive patient. Rom J Morphol Embryol. 2017;58(3):1009–1015. [PubMed] [Google Scholar]
- 38.Normant S, Gendre A, Boucher S, Godey B, Bordure P, Michel G. Predictive factors of revision stapes surgery in otosclerosis. J Laryngol Otol. 2022;13:1–4. doi: 10.1017/S0022215122002572. [DOI] [PubMed] [Google Scholar]
- 39.Halily S, Abdulhakeem B, Oukessou Y, Rouadi S, Abada R, Roubal M, Mahtar M. CT scan findings impact on hearing thresholds in otosclerosis: a study of 108 patients. Ann Med Surg (Lond) 2022;77:103716–103716. doi: 10.1016/j.amsu.2022.103716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szyfter W, Gawęcki W, Bartochowska A, Balcerowiak A, Pietraszek M, Wierzbicka M. Conductive hearing loss after surgical treatment of otosclerosis - long-term observations. Otolaryngol Pol. 2020;75(1):1–6. doi: 10.5604/01.3001.0014.6216. [DOI] [PubMed] [Google Scholar]
- 41.Almăşan HA, Băciuţ M, Rotaru H, Bran S, Almăşan OC, Băciuţ G. Osteonecrosis of the jaws associated with the use of bisphosphonates. Discussion over 52 cases. Rom J Morphol Embryol. 2011;52(4):1233–1241. [PubMed] [Google Scholar]
- 42.Mânea HC, Urechescu HC, Balica NC, Pricop MO, Baderca F, Poenaru M, Horhat ID, Jifcu EM, Cloşca RM, Sarău CA. Bisphosphonates-induced osteonecrosis of the jaw - epidemiological, clinical and histopathological aspects. Rom J Morphol Embryol. 2018;59(3):825–831. [PubMed] [Google Scholar]
- 43.Brookler K. Medical treatment of otosclerosis: rationale for use of bisphosphonates. Int Tinnitus J. 2008;14(2):92–96. [PubMed] [Google Scholar]
- 44.Gogoulos PP, Sideris G, Nikolopoulos T, Sevastatou EK, Korres G, Delides A. Conservative otosclerosis treatment with sodium fluoride and other modern formulations: a systematic review. Cureus. 2023;15(2):e34850–e34850. doi: 10.7759/cureus.34850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Măru N, Pop F. Morphological considerations about middle ear cholesteatoma. Rom J Morphol Embryol. 2006;47(1):73–77. [PubMed] [Google Scholar]
- 46.Maniu A, Harabagiu O, Perde Schrepler, Cătană A, Fănuţă B, Mogoantă CA. Molecular biology of cholesteatoma. Rom J Morphol Embryol. 2014;55(1):7–13. [PubMed] [Google Scholar]
- 47.Harabagiu OE, Cosgarea M, Mogoantă CA, Leucuţa DC, Maniu AA. Keratinocyte growth factor and its receptor expression in chronic otitis media with and without cholesteatoma. Rom J Morphol Embryol. 2017;58(4):1333–1338. [PubMed] [Google Scholar]
- 48.Arnold W, Friedmann I. Immunohistochemistry of otosclerosis. Acta Otolaryngol Suppl. 1990;470:124–128. doi: 10.3109/00016488909138366. [DOI] [PubMed] [Google Scholar]


