Summary
Background
Cervical cytology remains widely used as the initial tool in cervical cancer screening worldwide. WHO guidelines recommend replacing cytology with primary HPV testing to reach cervical cancer elimination goals. We assessed the performance of cytology and high-risk HPV testing to detect cervical precancer, cervical intraepithelial neoplasia (CIN) grade 3 or worse (CIN3+) among women aged 30–64 years participating in the ESTAMPA study.
Methods
Women were screened with cytology and HPV across ESTAMPA study centres in Latin America. Screen-positives were referred to colposcopy with biopsy collection and treatment as needed. Those with no evident precancer were recalled at 18-months for a second HPV test to complete disease ascertainment. Performance indicators for cytology and HPV to detect CIN3+ were estimated.
Findings
30,606 participants with available cytology and HPV results were included in the analysis. A total of 440 histologically confirmed CIN3s and 30 cancers were diagnosed. Cytology sensitivity for CIN3+ was 48.5% (95% CI: 44.0–53.0), whereas HPV testing had a sensitivity of 98.1% (95% CI: 96.3–96.7). Specificity was 96.5% (95% CI: 96.3–96.7) using cytology and 88.7% (95% CI: 88.3–89.0) with HPV. Performance estimates varied substantially by study centre for cytology (ranging from 32.1% to 87.5% for sensitivity and from 89.2% to 99.5% for specificity) while for HPV results were more consistent across sites (96.7%–100% and 83.6–90.8%, respectively).
Interpretation
The limited and highly variable sensitivity of cytology strongly supports transition to the more robust and reproducible HPV-based cervical screening to ensure progress towards global cervical cancer elimination targets in Latin America.
Funding
IARC/WHO, UNDP, HRP/WHO, NCI and local funders.
Keywords: Cervical cytology, HPV testing, Screening, ESTAMPA
Research in context.
Evidence before this study
The performance of cytology to detect cervical precancer and cancer has been studied largely among women from Europe and North America. There is ample evidence and agreement that HPV testing is a more sensitive and reproducible test for primary screening than cytology, which allows extension of screening intervals and can be self-collected. WHO guidelines recommend HPV testing with or without triage as the preferred alternative for cervical cancer screening. We conducted a PubMed search for systematic reviews and meta-analyses on June 1, 2022, using the search terms (“cytology” [Title] OR “Pap” [Title] OR “HPV testing” [Title]) AND ((“accuracy”) OR (“diagnosis”) OR (“performance”)) without language or date restrictions. Most of the evidence about the cytology performance evaluation came from high-income countries. Five studies from individual countries in Latin America reported evidence on the accuracy of cytology compared to HPV testing that was consistent with findings in other regions, but none included a large amount of data collected under a single protocol from multiple centres across the region.
Added value of this study
We present results of the largest to-date cervical cancer screening study in Latin America where we investigated the performance of cytology and HPV testing among more than 30,000 women from eleven centres in nine countries across the region. Women were screened with cytology and HPV testing and screen-positives were referred to a standardised colposcopy and treatment as needed with participation rates in follow-up above 90%. We detected more than 500 CIN3+ and assessed the performance of cytology and HPV testing using the combination of both as the gold standard. The sensitivity of cytology for CIN3+ was less than 50% compared to more than 95% for HPV testing. Furthermore, cytology varied widely between centres while HPV had comparable sensitivity in all the sites.
Implications of all the available evidence
Our results are consistent with previous evidence demonstrating that cytology does not perform accurately to detect precancer and cancer, whereas HPV testing is highly sensitive. The limited accuracy of cytology, which remains the main screening method in Latin American countries, together with limited coverage and inadequate follow-up of abnormalities likely explains the persistently high burden of disease in the region.
These findings provide regional evidence that can guide evidence-based decision by policy makers to accelerate the transition from cytology to HPV testing for primary cervical cancer screening instead of improving current cytology capacity. Our results may have an important impact to fill evidence gaps regarding screening with cytology and HPV testing in large populations not only from Latin American countries but may also contribute to other LMIC countries where cytology is still widely used.
Introduction
Cervical cancer is the fourth most common cancer in women worldwide and, although preventable, continues to be one of the leading causes of cancer death in low- and middle-income countries (LMIC). In Latin America and the Caribbean, cervical cancer is the third most common cancer with around 60,000 new cases and 31,000 deaths estimated for 2020, corresponding to age-standardised rates (ASR) of incidence and mortality of 14.9 and 7.6 per 100,000 women, respectively.1 Persistent high-risk HPV infection is a necessary condition for developing cervical cancer; HPV infections are present in more than 99% of invasive cervical cancer cases and in most high-grade cervical lesions.2
In 2018, the World Health Organization (WHO) made a global call for action towards the elimination of cervical cancer as a public health problem, setting elimination goal as an incidence rate of 4 or fewer cases of invasive cancers per 100,000 women. Achieving that goal rests on three key pillars with clear targets that each country should meet by 2030: 1) 90% of girls vaccinated against HPV by age of 15 years, 2) 70% of women screened using a high-performance test by age 35, and again by age of 45, and 3) 90% of detected precancerous lesions treated and 90% of cancers adequately managed.3
Conventional cytology has been the main cervical cancer screening method for decades and, combined with colposcopic examination of women with a positive cytology and histological assessment, has led to a substantial decrease in incidence and mortality in countries where it has been implemented systematically with high-coverage and frequent repetition of the test.4 However, screening programmes based on cytology have rarely been successful in reducing cervical cancer rates in LMIC, mainly due to difficulties to reach high coverage and to complete and maintain the complex traditional screening process starting from collecting the sample, performing cytology, informing screening results, doing colposcopy when necessary, and treating women with histologically confirmed lesions detected.5 In addition, cytology relies on subjective interpretation requiring constant training and supervision of cytologists to promote quality of screening.
HPV testing has shown to be highly effective at detecting precancerous lesions and preventing cervical cancer,6 which led to the 2021 WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention, to recommend HPV testing rather than cytology for cervical cancer screening.7 Nevertheless, cytology continues to be widely used, particularly in Latin America where evidence on the performance of cytology in large screening populations remains limited.8, 9, 10
Herein, we evaluated the performance in primary screening of cytology and HPV testing to detect CIN3+ in a large group of women aged 30–64 years participating in the ESTAMPA study.
Methods
Study design
ESTAMPA is a multicentre cross-sectional cervical cancer screening study in 12 study centres across nine countries in Latin America (Argentina, Bolivia, Colombia, Costa Rica, Honduras, México, Paraguay, Perú, and Uruguay) including more than 42,000 participants. The ESTAMPA protocol has been previously described.11 Briefly, women aged 30–64 years were screened with cytology and HPV testing and those with negative results for both tests exited the study, while those with abnormal cytology ASC-US or worse (ASC-US+) and/or with positive high-risk HPV test were referred to colposcopy with biopsy of observed lesions and histological assessment. Women with negative colposcopy or with no high-grade lesions (<CIN2) on histology were recalled to a follow-up visit 18 months after the initial screening for a second HPV test; those HPV-positive had a second colposcopy with biopsy collection as needed. Women with high-grade cervical lesions, either at enrolment or 18-month visit, were treated with large loop excision of the transformation zone (LLETZ) and exited the study.
Participants and specimens
Participants were women having initiated sexual activity, without history of cervical cancer or treatment for a precancerous lesion of the cervix in the 6 months prior to enrolment, and not planning to move out of the study area. At screening, exfoliated cervical cells were collected with a Cervex-Brush® (Rovers Medical Devices, Oss, The Netherlands) and prepared for conventional cytology by study clinicians. Residual cells remaining on the brush were rinsed into vials containing 20 mL of ThinPrep® PreservCyt® medium (Hologic Inc., Marlborough, MA, USA) for HPV testing and other biomarkers.
Cytology and HPV testing
Cytology smears were processed, stained, and reported by local laboratories according to the Bethesda System as negative for intraepithelial lesion or malignancy (NILM) or epithelial cells abnormalities (in either squamous and/or glandular cells). Squamous abnormalities include: atypical squamous cells of undetermined significance (ASC-US), low grade squamous intraepithelial lesion (LSIL), atypical squamous cells cannot exclude high-grade lesion (ASC-H), high-grade squamous intraepithelial lesion (HSIL) named here as cHSIL (HSIL for cytology), and squamous cells carcinoma (SCC); and glandular abnormalities include: atypical glandular cell-not otherwise specified (AGC-NOS), atypical glandular cells favour neoplastic (AGC-FN), adenocarcinoma in situ (AIS), and endocervical adenocarcinoma (ADC). The threshold for cytology positivity was ASC-US+. Cytology was interpreted by local accredited laboratories independently of HPV testing results. Specific training on cytologic interpretation was not provided by the study.
One study centre (Bogota, Colombia) processed and interpreted cytology only for HPV-positive women, as reflex test; participants from this study centre were excluded from this analysis. HPV testing was locally performed according to manufacturer's instructions using the Digene HC2© High-Risk HPV DNA Test (QIAGEN, Germantown USA) or COBAS® 4800 HPV Test (Roche Diagnostics, Mannheim, Germany). Local laboratories had regular participation in External Quality Assessment [either the College of Pathologists (CAP) scheme or the Quality Control for Molecular Diagnostics (QCMD)] and maintained good laboratory practices, including facilities and suitable laboratory environment, stock of reagents, equipment and staff competence as previously described in ESTAMPA.12
Histology
Local study pathologists interpreted H&E-stained slides and reported histology results under the Cervical Intraepithelial Neoplasia (CIN) nomenclature as follows: negative, CIN1, CIN2, CIN3, AIS or invasive cancer. Additionally, slides are being reviewed by an international panel of experts on cervical pathology without knowledge of screening results using the Lower Anogenital Squamous Terminology (LAST) nomenclature which uses p16 immunohistochemistry to improve diagnostic accuracy for histological high-grade squamous intraepithelial lesions (HSIL) named here as bHSIL (HSIL from biopsy).13
Study outcomes
The primary outcome (gold-standard) was histologically confirmed CIN3+ diagnosed by local pathologists from biopsy specimens collected during the colposcopic evaluation triggered by cytology and HPV results at enrolment or LLETZ triggered by a diagnosis of CIN2+. Additionally, diagnosis based on endocervical samples processed as histology were also considered part of the disease definition. Secondary outcomes were defined as follows: i) CIN2+ diagnosed at enrolment by local pathologists, ii) bHSIL diagnosed at enrolment and reported under the LAST nomenclature by external reviewers, and iii) CIN3+ diagnosed either at the enrolment or 18 months visit by local pathologists. Women without high-grade cervical disease (<CIN2 or < bHSIL) included those with CIN1 (or bLSIL), negative histology, or negative colposcopy at which cervical tissues were not collected. Women with negative screening results (i.e., NILM and HPV-negative) were also considered disease-free.
Statistical analysis
HPV positivity stratified by cytological results (NILM, ASC-US, LSIL, cHSIL+) and age (30–39, 40–49, 50–64 years old) was described regardless of disease status. Trends by age of HPV positivity within cytological grade were assessed using the score test for trends.
Sensitivity, specificity, positive predictive value (PPV) and complement of the negative predictive value (cNPV) with 95% exact binomial confidence intervals (95% CI) were estimated to assess performance of cytology and HPV testing for detection of primary and secondary outcomes. For specificity calculations, CIN2 cases were excluded because CIN2 is less likely to progress and has greater variability in diagnosis. Additionally, age-stratified (30–39, 40–49 or 50–64 years old) performance indicators with 95% CI for detection of CIN3+ and CIN2+ diagnosed at enrolment were also estimated using logistic regression, and comparisons by age groups were assessed using likelihood ratio tests. Differences in performance estimations between cytology and HPV testing were assessed using a McNemar test for paired proportions (for sensitivities and specificities) or a Chi-squared test for proportions (for predictive values) as adequate. In addition, performance estimates for detection of CIN3+ diagnosed at enrolment were calculated by study centre using mixed-effect logistic regression models under a meta-analysis approach to obtain overall pooled estimations, separately for sensitivity and for specificity, adjusting for study centre heterogeneity with corresponding I2 statistic. Forest plots were produced to depict the variation among study centres. All analyses were performed using R statistical software.
Ethical consideration
The ESTAMPA protocol was approved by the Ethics Committee of the International Agency for Research on Cancer of the World Health Organization (IARC/WHO) (IEC Project 12–27-A7), the Pan American Health Organization (PAHO) Ethical Committee, and Ethical Committees at study centres. The study is considered of minimal risk as procedures are standard clinical practice. All women were informed by trained providers of the procedures and signed informed consent. This study is registered with ClinicalTrials.gov (NCT01881659).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript, or in the decision to publish the results.
Results
Study population
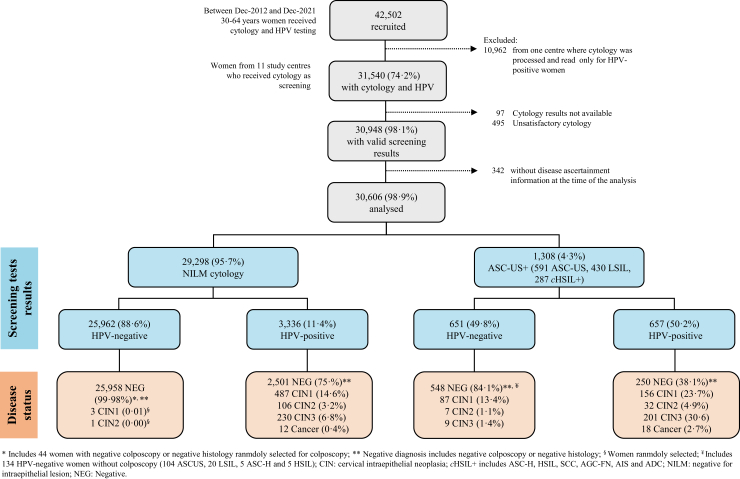
From December 2012 to December 2021, 42,502 women aged 30–64 years had valid screening tests results in the ESTAMPA study. 10,962 women from one study centre that used reflex cytology (smears were processed and read only for HPV-positive women) were excluded. In addition, participants without cytology (97), unsatisfactory cytology (495) or without disease ascertainment (342) were also excluded. The final analysis included 30,606 women; 25,962 (84.8%) were negative by both screening tests and 4644 (15.2%) had an abnormal cytology or a positive HPV test. Overall, 1308 (4.3%) participants had ASC-US+, whereas 3993 (13.0%) were positive for HPV. Cytology was NILM in 3336 HPV-positive women. Following initial colposcopy with biopsy, 616 CIN2+ cases were detected (146 CIN2, 440 CIN3 and 30 cancers) (Fig. 1).
Fig. 1.
Study population. ESTAMPA participants recruited between December 2012 and December 2021.
Supplementary Table S1 presents the characteristics of the study population. Mean age (±SD) of women was 44.4 (±9.3) years, 21,618 women (70.6%) were younger than 50 years. 15,286 (50.0%) reported having been screened with cytology every year, 10,847 (35.5%) within 2–5 years, 3808 (12.5%) >5 years, and 607 (2.0%) reported never having been screened.
The study population included 3312 (10.8%) and 320 (1.0%) women from two study centres in Argentina, 3155 (10.3%) from Bolivia, 1219 (4.0%) from Colombia, 8548 (27.9%) from Costa Rica, 4129 (13.5%) from Honduras, 804 (2.6%) from México, 6239 (20.4%) and 367 (1.2%) from two study centres in Paraguay, 658 (2.1%) from Perú, and 1855 (6.1%) from Uruguay.
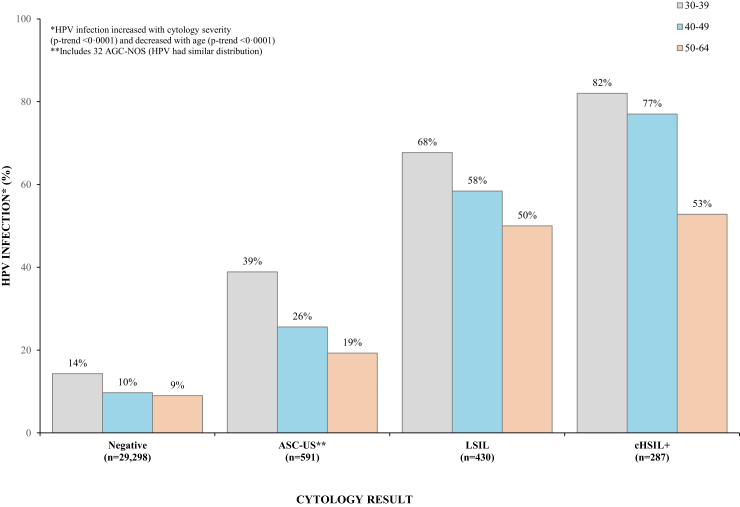
Fig. 2 depicts the association of cytology and HPV results by age, showing that HPV prevalence significantly increased with severity of cytology in all age groups ranging from 11.4% in women with NILM cytology to 73.2% in women with cHSIL+ (p-trend <0.0001) while significantly decreased with age regardless of cytologic results (p-trend <0.0001).
Fig. 2.
HPV prevalence by cytologic results and age.
Performance of cytology and HPV testing at enrolment
Table 1 presents cytology and HPV test results by disease status at enrolment. Cytology detected 39 (26.7%) of 146 histologically confirmed CIN2 cases, 210 (47.7%) of 440 CIN3 and 18 (60%) of 30 cancer cases, whereas HPV testing detected 138 (94.5%), 431 (98%) and 30 (100%), respectively. Overall, of the 470 CIN3+ cases, cytology detected 48.5% whereas HPV detected 98.1%.
Table 1.
Distribution of screening test results and disease status at enrolment.
| Screening results | Colposcopic-histologic diagnosis |
|||||
|---|---|---|---|---|---|---|
| Negative (%) | CIN1 (%) | CIN2 (%) | CIN3 (%) | Cancer (%) | Total (%) | |
| Cytology | ||||||
| NILM | 28,459 (97.3) | 490 (66.8) | 107 (73.3) | 230 (52.3) | 12 (40.0) | 29,298 (95.7) |
| ASC-US | 466 (1.6) | 79 (10.8) | 7 (4.8) | 37 (8.4) | 2 (6.7) | 591 (1.9) |
| LSIL | 237 (0.8) | 111 (15.1) | 20 (13.7) | 59 (13.4) | 3 (10.0) | 430 (1.4) |
| cHSIL+ | 95 (0.3) | 53 (7.2) | 12 (8.2) | 114 (25.9) | 13 (43.3) | 287 (0.9) |
| HPV testing | ||||||
| HPV- | 26,506 (90.6) | 90 (12.3) | 8 (5.5) | 9 (2.0) | 0 (0) | 26,613 (87.0) |
| HPV+ | 2751 (9.4) | 643 (87.7) | 138 (94.5) | 431 (98.0) | 30 (100) | 3993 (13.0) |
| Total | 29,257 (100) | 733 (100) | 146 (100) | 440 (100) | 30 (100) | 30,606 (100) |
The sensitivity, specificity and PPV of cytology for CIN3+ were 48.5% (95% CI: 43.9–53.1), 96.5 (95% CI: 96.3–96.7) and 17.4 (15.4–19.6), respectively. HPV testing had two-fold higher sensitivity than cytology (98.1%, 95% CI: 96.4–99.1, p < 0.0001) but lower specificity (88.7, 95% CI: 88.3–89.0, p < 0.0001) and lower PPV (11.5, 95% CI: 10.6–12.6, p < 0.0001) (Table 2). In addition, the risk of CIN3+ for women with negative cytology was 27 times higher than for HPV-negative women (cNPV 0.83% vs. 0.03%, respectively, p < 0.0001) (Table 2). There were no differences between the HC2© and the COBAS® 4800 tests in their performance for CIN3+ detection.
Table 2.
Performance of cytology and HPV testing to detect CIN3+ and CIN2+ diagnosed at enrolment by age.
| Cytology |
HPV |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All women | 30–39 years | 40–49 years | 50–64 years | p-value | All women | 30–39 years | 40–49 years | 50–64 years | p-value | |
| Test positivity | 1308 (4.3%) | 619 (5.3%) | 451 (4.6%) | 238 (2.6%) | 3993 (13.0%) | 1961 (16.7%) | 1124 (11.4%) | 908 (10.1%) | ||
| Performance characteristics | ||||||||||
| CIN3+ | ||||||||||
| Sensitivity | 48.5% (43.9–53.1) | 48.5% (42.5–54.5) | 50.6% (42.8–48.5) | 42.6% (30.0–55.9) | 0.60 | 98.1% (96.4–99.1) | 98.5% (96.5–99.5) | 97.4% (94.1–99.2) | 98.1% (92.1–99.9) | 0.80 |
| Specificity | 96.5% (96.3–96.7) | 95.9% (95.5–96.3) | 96.2% (95.8–96.6) | 97.6% (97.3–97.9) | <0.0001 | 88.7% (88.3–89.0) | 85.9% (85.2–86.5) | 90.3% (89.7–90.8) | 90.6% (89.9–91.1) | <0.0001 |
| PPV | 17.4% (15.4–19.6) | 20.2% (17.5–23.8) | 17.3% (14.0–21.0) | 9.7% (6.3–13.8) | <0.0001 | 11.5% (10.6–12.6) | 13.2% (11.7–14.7) | 13.3% (11.4–15.4) | 5.8% (4.4–7.5) | <0.0001 |
| cNPV | 0.8% (0.7–0.9) | 1.2% (1.0–1.4) | 0.81% (0.6–1.0) | 0.35% (0.2–0.5) | <0.0001 | 0.03% (0.02–0.06) | 0.04% (0.01–0.09) | 0.05% (0.01–0.11) | 0.01% (0–0.05) | 0.38 |
| TP | 228 | 127 | 78 | 23 | 461 | 258 | 150 | 53 | ||
| FP | 1041 | 466 | 364 | 211 | 3394 | 1611 | 940 | 843 | ||
| TN | 28,949 | 10,942 | 9296 | 8711 | 26,596 | 9797 | 8720 | 8079 | ||
| FN | 242 | 135 | 76 | 31 | 9 | 4 | 4 | 1 | ||
| CIN2+a | ||||||||||
| Sensitivity | 43.3% (39.4–47.4) | 42.9% (37.8–48.0) | 45.1% (38.2–52.1) | 40.9% (29.6–52.9) | 0.80 | 97.2% (95.6–98.4) | 98.0% (96.2–99.2) | 95.3% (91.8–97.7) | 98.5% (93.5–99.9) | 0.20 |
| Specificity | 96.5% (96.3–96.7) | 95.9% (95.5–96.3) | 96.2% (95.8–96.6) | 97.6% (97.3–97.9) | <0.0001 | 88.7% (88.3–89.0) | 85.9% (85.2–86.5) | 90.3% (89.7–90.8) | 90.6% (89.9–91.1) | <0.0001 |
| PPV | 20.4% (18.3–22.7) | 24.7% (21.4–28.2) | 19.3% (15.8–23.1) | 11.3% (7.7–15.8) | <0.0001 | 15.0% (13.9–16.1) | 17.8% (16.2–19.6) | 16.4% (14.3–18.6) | 7.2% (5.6–9.0) | <0.0001 |
| cNPV | 1.2% (1.1–1.3) | 1.8% (1.6–2.1) | 1.1% (0.9–1.4) | 0.5% (0.3–0.6) | <0.0001 | 0.06% (0.04–0.1) | 0.07% (0.03–0.14) | 0.1% (0.05–0.2) | 0.01% (0–0.05) | 0.03 |
| TP | 267 | 153 | 87 | 27 | 599 | 350 | 184 | 65 | ||
| FP | 1041 | 466 | 364 | 211 | 3394 | 1611 | 940 | 843 | ||
| TN | 28,949 | 10,942 | 9296 | 8711 | 26,596 | 9797 | 8720 | 8079 | ||
| FN | 349 | 204 | 106 | 39 | 17 | 7 | 9 | 1 | ||
Estimates with 95% confidence intervals shown. CIN2 cases excluded from specificity calculations.
p-values to assess difference in performance indicators between age groups by means of a logistic regression model.
Includes 1 CIN2 case detected in women randomly selected for colposcopy; TP: True positives; FP: False positives; TN: True negatives; FN: False negatives.
Similar results were found considering the reviewed histology (so far 70% completed), cytology had lower sensitivity than HPV for detection of histological bHSIL+ (45.4%, 95% CI: 40.4–50.5 vs. 97.9%, 95% CI: 96.1–99.1) and higher specificity (97.9%, 95% CI: 97.8–98.1 vs. 94.4%, 95% CI: 94.1–94.7).
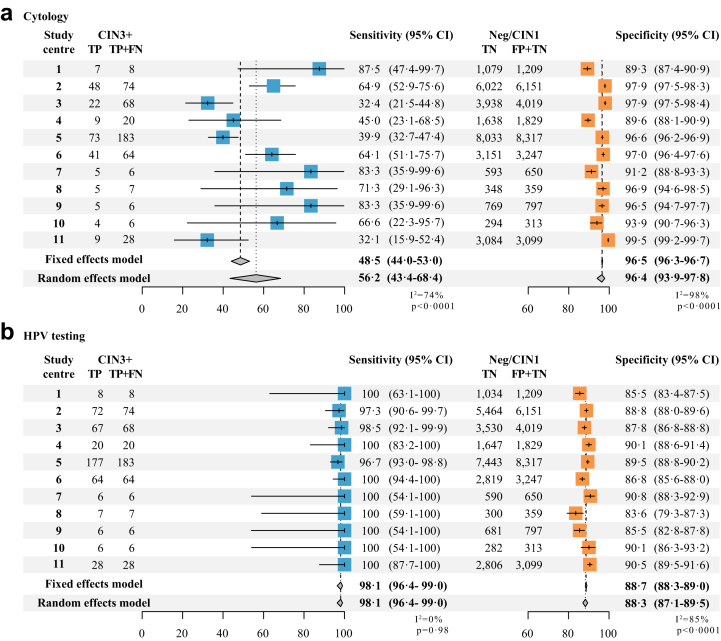
Moreover, there were no differences in sensitivity for CIN3+ detection by age for cytology (p = 0.60) nor for HPV (p = 0.80), whereas the specificity slightly increased with age (p < 0.0001) and the cNPV decreased for both tests (p < 0.0001). Similar results were found when assessing the performance for CIN2+ detection (Table 2). Additionally, sensitivity of cytology for CIN3+ detection varied significantly across study centres ranging from 32.1% to 87.5%, while HPV sensitivity was homogenous with no significant variation ranging from 96.7%–100%. Cytology specificity was slightly more variable than HPV specificity, however it was more consistent than sensitivity estimations. Pooled estimates adjusting for this heterogeneity were similar to unadjusted estimates but with wider confidence intervals (Fig. 3).
Fig. 3.
Performance Forest plots of cytology (a) and HPV testing (b) for CIN3+ detection for 11 study centres.
Performance of cytology and HPV testing to detect 18-months cumulative CIN3+
4068 screened-positive women with no high-grade disease (≤CIN1) detected at enrolment are being referred for a second follow-up visit at 18 months. 2940 (72.3%) have completed the follow-up at the time of this analysis and 1128 (27.7%) have either withdrew or were lost to follow-up (179, 15.9%) or are yet to complete the 18-month visit (949, 84.1%).
Among women who have attended the follow-up visit 63 CIN3+ (58 CIN3 and 5 cancers) and 26 CIN2 cases were additionally detected. Similar performance estimates for CIN3+ detection by each test were obtained when adding these cases. Cytology had lower sensitivity than HPV (45.6% vs. 97.9%), higher specificity (97.5% vs. 91.7%), higher PPV (24.5% vs. 17.0%) and higher cNPV (1.0% vs. 0.04%) (Supplementary Table S2).
Discussion
This is the first large-scale multicentre study evaluating the performance of cervical cytology and HPV in primary screening including more than 30,000 women across nine Latin American countries.8, 9, 10 There is overwhelming evidence showing that HPV testing is more sensitive compared to cytology for detecting precancerous lesions,14,15 and more effective at reducing cervical cancer.6,16,17
The 2021 published WHO guidelines for screening and treatment to prevent cervical cancer include results on modelling the harms and benefits, and cost-effectiveness of different screening approaches, using HPV testing followed by treatment of all positives or HPV testing followed by a triage test and treatment of triaged positives, as well as approaches using cytology. All HPV-based approaches showed to be more efficient than cytology given the reduction in screening costs associated with lower number of women who need cancer treatment, and economic gains in preventing unnecessary deaths. The guidelines therefore recommend the use of HPV testing self- or provider-collected samples as the preferred method for primary screening, rather than cytology. Moreover, HPV testing is considered one of WHO best buys (cost-effective intervention for the prevention and control of major noncommunicable diseases).7
The multicentre nature of the ESTAMPA study provided an opportunity to extensively explore the cytology performance in a region where cytology remains the main screening method, despite the global evidence and guidelines recommending primary HPV testing. We demonstrated that cytology had a limited sensitivity for detecting CIN3+ (48.5% and 43.4 for CIN2+) and was highly variable among study centres (ranging from 32.1% to 87.5%), whereas HPV testing showed a sensitivity for CIN3+ of 98.1% (97.2% for CIN2+) and was consistently very sensitive in all study centres. This main result is comparable with previous findings reporting that cytology has an overall sensitivity of 50% to detect high grade cervical lesions, with high variability (e.g., sensitivity range 26–87%).10,14,18
Cervical cancer screening programmes based on cytology have been successful in reducing the incidence of cervical cancer in high-income countries (HIC), but they have not been uniformly effective in LMIC.19 Several factors contribute to the variable impact of cytology-based screening. First, the low coverage of programmes and the complexity of the process that involves multiple visits of women with abnormal cytology to complete the diagnostic process and receive treatment.20 Second, cytology results are dependent on interpretation of a high-quality sample and its inherent subjectivity requires properly trained clinical staff and laboratories with rigorous quality assurance systems to ensure compliance with standards.21 Third, the low sensitivity of cytology demands repeating the test multiple times over the years to be effective. All these factors make good-quality cytology-based screening very expensive and not affordable in LMIC, as demonstrated by different efforts over time in Latin America.22, 23, 24
In the ESTAMPA study, management of women was rigorously implemented, with a large number of colposcopies performed with high participant compliance (>90% colposcopy attendance in all study centres) and CIN2+ cases treated.25 Our results show that even under ideal conditions (adequate access to treatment and rigorous follow-up of screened positives), the performance of cytology was still limited, missing 52% of CIN3+ cases (242/470) while HPV testing only missed 2% (9/470).
We remarkably observed that the baseline risk of CIN3+ was lower for HPV negative women (cNPV: 0.03%) than for women with negative cytology (cNPV: 0.83%), whose risk was comparable to that of the general population (∼1%),26 despite most women reporting being screened with cytology within the past 5 years. Additionally, 39.4% of CIN3+ cases (185/470) reported having been screened within the last year and 40.2% (189/470) within the previous 2–5 years. However, we noted that the risk of CIN3+ was 50% lower for those screened within the last year compared to under screened and/or unscreened women (data not shown), which indicates that, to be effective, cytology must be repeated regularly, whereas if HPV testing is used, the screening interval can be safely lengthened.6,27 Furthermore, in ESTAMPA the entire process (from the cervical sample collection to cytology reading and reporting) was performed following quality assurance guidelines, all clinicians received a standardised training to collect cervical samples before the study was launched, and although there was no specific training for cytology processing and interpretation, all professionals involved in cytology interpretation were very experienced, and cytology laboratories were fully accredited with at least 15 years of operation. Although cytology laboratories fulfilled principles of quality assurance, we still observed limited sensitivity in the majority, which is consistent with previous publications reporting that even under good-quality circumstances, the test has a limited performance.14,18
Primary HPV testing is gradually being introduced and it has been piloted in several countries including Brazil, Colombia, Costa Rica, Honduras, Paraguay, Peru and Uruguay, and other countries such as Argentina, El Salvador and Mexico have already introduced it in their cervical cancer screening programmes.28
An important limitation of the use of the HPV test for primary screening is its limited specificity for detecting the presence of precancer, with the possibility of overtreatment or the need to use additional tests for triage, with several promising alternatives in development and under evaluation in ESTAMPA. The existing capacity of cytology laboratories in Latin America could allow the use of cytology, possibly combined with HPV genotyping as triage of HPV-positive women while solid evidence of other triage tests is generated in the region. Cytology has been proposed to triage HPV-positive women and has been reported that the performance improve when HPV positivity is known by the cytology interpreters,29 we are currently evaluating this hypothesis within ESTAMPA. Furthermore, the switch to HPV as primary screening with cytology generates reductions in the overall cytology workload allowing centralisation of laboratories which could facilitate optimal quality assurance.
The use of co-testing with both cytology and HPV does not seem to be an option as it would perform nearly the same as HPV alone. We observed that cytology only detected 9 CIN3 cases (no cancers) among HPV screened-negative women; most of CIN3+ detection provided by co-testing were derived from the HPV testing component (100% CIN3+ detection for co-testing vs. 98% with HPV testing-only primary screening). Co-testing does not appear to be cost-effective since many cytology tests (at least 97.5% corresponding to HPV-negative women) would be underutilised at the expense of a high and unnecessary cost.
The strengths of this study include the large sample size, the design of ESTAMPA, and the high adherence to the study protocol and retention to colposcopy and treatment allowing adequate disease ascertainment. Additionally, HPV-positive women with no evident disease at enrolment were recalled at 18 months for a follow-up visit, which provided an opportunity to identify lesions that were not detected during the initial screening.
We noted two main limitations in our study. Firstly, the possibility of verification bias because biopsies were not collected in two groups of women: i) double screened negatives (<ASC-US and HPV-negative) who exited the study afterwards, and ii) screened positive women with negative colposcopy and no histology. Nevertheless, verification bias was mitigated by study design with the following: i) the use of two screening methods that allowed us to consider disease-free those double negative, a decision supported by the demonstrated low risk (0.08%) of CIN3+ after 5 years of negative results on cytology and HPV testing26; ii) the inclusion of a second HPV screen 18 months since initial screening for screened positives with no evident disease at enrolment that allowed us to maximise disease detection and safety of participants. In effect, at the time of analysis, among 406 screened-positive women with negative colposcopy at enrolment, we identified and treated 21 with CIN3+ (20 CIN3 and 1 cancer). Furthermore, estimates of performance were similar when considering as negative for disease those participants < CIN1 at enrolment who did not complete the second follow-up, suggesting absence of bias. Secondly, our results could have been strengthened by using reviewed histology done by an international panel of experts, which had not been completed at the time of analysis. Nonetheless, based on already 70% fully reviewed histology specimens, cytology had lower sensitivity than HPV for detection of histological bHSIL+ (45.4% vs. 97.9%) and higher specificity (97.9% vs. 94.4%).
The introduction of primary HPV testing brings different challenges, including the development and adoption of a new screening system, improvements of the health system capacities, training of primary care providers, educating women about HPV, resource constraints, widespread changes to laboratory, quality assurance and quality control and development of appropriate data systems, in addition to the necessity of dealing with stakeholder's beliefs, among others. Notably, the higher positivity of HPV testing compared to cytology demands use of simple screening algorithms, including screen-and-treat and screen, triage and treat to adequately manage the larger number of screened positives as recommended by WHO.7 Future studies on implementation research will be crucial to better understand these challenges and to provide actionable guidance for countries seeking to change from cytology to HPV testing and ensure sustainable scale-up of HPV-based screening programmes.30
Our findings confirm that cytology is not an effective method for primary cervical cancer screening in Latin America and strongly support the WHO recommendation to use HPV testing as the preferred test in primary cervical screening, a much better alternative to achieve the goal of eliminating cervical cancer in the region. Instead of directing efforts to improve current cytology capacity for primary screening, transition to HPV-based cervical screening should be prioritised and more efficient use of cervical screening resources in scenarios with several competing health needs should be encouraged.
Contributors
MA and RH conceived the ESTAMPA study and are the principal investigators responsible for its overall conduction. LF, ST, VV, GV, ACV, MV, GR, CT, MAP, AF, EK, LM, and AC were the local principal investigators responsible for recruitment, clinical management and data collection. YS and SM coordinated the training on cervical sample collection. Cytologies were read and reported by FDR, KR, RA, CC, OH, AM, DCR, HG, IR, DCH, JA, RB and LC. EG, DG and TD read and reported reviewed histological diagnosis. ATR wrote the manuscript with support from MA, RH, AB, JV, SL, NB and TD. JV and AB were responsible for data curation and had access and verified all data in the study. JV performed the statistical analysis that was verified by AB. All authors reviewed and edited the manuscript. All authors had full access to all the data in the study, have read and approved the final version of the manuscript, and accept responsibility for the decision to submit for publication.
Data sharing statement
The data used in this study selected several variables (age at recruitment, screening history, cytology and HPV testing results, colposcopy, histology, and results at the 18-month visit) from the EStudio multicéntrico de TAMizaje y triaje de cáncer de cuello uterino con pruebas del virus del PApiloma humano (ESTAMPA) study database, which is stored securely at the International Agency for Research on Cancer. Anonymised individual participant data or aggregated data would be available upon reasonable request to the corresponding author (ramirezt@iarc.who.int), after signing a contract, and with approval from the principal and local investigators.
Declaration of interests
All other authors declare no competing interests.
Acknowledgements
This project acknowledges the financial contribution from IARC (International Agency for Research on Cancer)/WHO; the UNDP (United Nations Development Programme)–UNFPA (United Nations Population Fund)–UNICEF–WHO–World Bank Special Program of Research, Development and Research Training in Human Reproduction (HRP/WHO), a co-sponsored program executed by the World Health Organization (WHO), the Pan American Health Organization (PAHO), the National Cancer Institute (NCI) UH2/3 CA202730; the NCI Center for Global Health, National Agency for the Promotion of Research, Technological Development and Innovation-PICT 0364–2016 (Ministry of Science, Technology and Innovation) and National Cancer Institute (Ministry of Health) of Argentina, National Cancer Institute of Colombia, Costa Rica Social Security Fund (CCSS), the National Council for Science and Technology (CONACYT) from Paraguay; and all local collaborative institutions. We would also like to thank the participants, all ESTAMPA team for their support, and the contributions of Dr Jose Alfredo Aleman from Mexico, who sadly passed away before publication.
ESTAMPA study group: From Early Detection, Prevention and Infections Branch, International Agency for Research on Cancer: Mary Luz Rol PhD, Eric Lucas MSc. From SMS-Oncology: María de la Luz Hernández PhD. From Grupo de Infección y Cáncer, Facultad de Medicina, Universidad de Antioquía: Gloria Inés Sánchez PhD. From Centro Javeriano de Oncología, Hospital Universitario San Ignacio: Raul Murillo MD. From Liga contra el Cáncer-Perú and US National Cancer Institute: Jose Jerónimo MD. From Advanced Center for Chronic Diseases, ACCDiS, Pontificia Universidad Católica de Chile: Catterina Ferreccio PhD. From Instituto de Investigaciones en Ciencias de la Salud, Universidad Nacional de Asunción: María Isabel Rodríguez PhD. From Instituto de Investigaciones en Microbiología, Universidad Nacional Autónoma de Honduras (UNAH): Yessy Cabrera BSc. From Secretaría de Salud in Tegucigalpa: Brenda Salgado MD. From Instituto Nacional de Enfermedades Infecciosas–ANLIS Malbrán: María Celeste Colucci BSc. From Hospital Nacional Profesor Alejandro Posadas: Maria Agustina Saino MD, Margarita Rodríguez de la Peña MD. From Facultad de Medicina, Universidad Mayor, Real y Pontificia de San Francisco Xavier de Chuquisaca: Daniel Llanos Fernández MD. From Laboratorio de Biología Molecular, Departamento de Patología Clínica, Centro Hospitalario Pereira Rossell: Laura García PhD. From Departamento de Anatomía Patológica y Citología, Hospital de la Mujer, Centro Hospitalario Pereira Rossell: Benedicta Caserta MD. From Patología Oncológica SAC: Franco Doimi MD. From Instituto de Salud Pública de México in Morelos: Eduardo Lazcano-Ponce PhD.
Footnotes
Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization. Some of the authors are present or former staff members of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100593.
Contributor Information
Arianis Tatiana Ramírez, Email: ramirezt@iarc.who.int.
ESTAMPA Study Group:
Mary Luz Rol, Eric Lucas, María de la Luz Hernández, Gloria Inés Sánchez, Raul Murillo, Jose Jerónimo, Catterina Ferreccio, María Isabel Rodríguez, Yessy Cabrera, Brenda Salgado, María Celeste Colucci, Maria Agustina Saino, Margarita Rodríguez de la Peña, Daniel Llanos Fernández, Laura García, Benedicta Caserta, Franco Doimi, and Eduardo Lazcano-Ponce
Appendix A. Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Walboomers J.M., Jacobs M.V., Manos M.M., et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Organization WH . 2020. Launch of the global strategy to accelerate the elimination of cervical cancer.https://www.who.int/news-room/events/detail/2020/11/17/default-calendar/launch-of-the-global-strategy-to-accelerate-the-elimination-of-cervical-cancer [Google Scholar]
- 4.Quinn M., Babb P., Jones J., Allen E. Effect of screening on incidence of and mortality from cancer of cervix in England: evaluation based on routinely collected statistics. BMJ. 1999;318(7188):904–908. doi: 10.1136/bmj.318.7188.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murillo R., Wiesner C., Cendales R., Piñeros M., Tovar S. Comprehensive evaluation of cervical cancer screening programs: the case of Colombia. Salud Publica Mex. 2011;53(6):469–477. [PubMed] [Google Scholar]
- 6.Ronco G., Dillner J., Elfström K.M., et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524–532. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- 7.second edition. 2021. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention.https://www.who.int/publications/i/item/9789240030824 [PubMed] [Google Scholar]
- 8.Terrazas S., Ibáñez C., Lagos M., et al. [Human papillomavirus testing in cervical cancer screening at a public health service of Santiago, Chile] Rev Med Chil. 2015;143(1):56–62. doi: 10.4067/S0034-98872015000100007. [DOI] [PubMed] [Google Scholar]
- 9.Salmerón J., Lazcano-Ponce E., Lorincz A., et al. Comparison of HPV-based assays with Papanicolaou smears for cervical cancer screening in Morelos State, Mexico. Cancer Causes Control. 2003;14(6):505–512. doi: 10.1023/a:1024806707399. [DOI] [PubMed] [Google Scholar]
- 10.Almonte M., Ferreccio C., Winkler J.L., et al. Cervical screening by visual inspection, HPV testing, liquid-based and conventional cytology in Amazonian Peru. Int J Cancer. 2007;121(4):796–802. doi: 10.1002/ijc.22757. [DOI] [PubMed] [Google Scholar]
- 11.Almonte M., Murillo R., Sanchez G.I., et al. Multicentric study of cervical cancer screening with human papillomavirus testing and assessment of triage methods in Latin America: the ESTAMPA screening study protocol. BMJ Open. 2020;10(5) doi: 10.1136/bmjopen-2019-035796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rol M.L., Picconi M.A., Ferrera A., et al. Implementing HPV testing in 9 Latin American countries: the laboratory perspective as observed in the ESTAMPA study. Front Med. 2022;9 doi: 10.3389/fmed.2022.1006038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darragh T.M., Colgan T.J., Cox J.T., et al. The lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136(10):1266–1297. doi: 10.5858/arpa.LGT200570. [DOI] [PubMed] [Google Scholar]
- 14.Cuzick J., Clavel C., Petry K.U., et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119(5):1095–1101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- 15.Koliopoulos G., Nyaga V.N., Santesso N., et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev. 2017;8 doi: 10.1002/14651858.CD008587.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rijkaart D.C., Berkhof J., Rozendaal L., et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13(1):78–88. doi: 10.1016/S1470-2045(11)70296-0. [DOI] [PubMed] [Google Scholar]
- 17.Kitchener H.C., Gilham C., Sargent A., et al. A comparison of HPV DNA testing and liquid based cytology over three rounds of primary cervical screening: extended follow up in the ARTISTIC trial. Eur J Cancer. 2011;47(6):864–871. doi: 10.1016/j.ejca.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Nanda K., McCrory D.C., Myers E.R., et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132(10):810–819. doi: 10.7326/0003-4819-132-10-200005160-00009. [DOI] [PubMed] [Google Scholar]
- 19.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 20.Murillo R., Almonte M., Pereira A., et al. Cervical cancer screening programs in Latin America and the Caribbean. Vaccine. 2008;26(Suppl 11):L37–L48. doi: 10.1016/j.vaccine.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Stoler M.H., Schiffman M. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285(11):1500–1505. doi: 10.1001/jama.285.11.1500. [DOI] [PubMed] [Google Scholar]
- 22.Flisser A., García-Malo F., Canepa MdeL., et al. Implementation and evaluation of a national external quality control program for cervical cytology in Mexico. Salud Publica Mex. 2002;44(5):431–436. doi: 10.1590/s0036-36342002000500007. [DOI] [PubMed] [Google Scholar]
- 23.Sepúlveda C., Prado R. Effective cervical cytology screening programmes in middle-income countries: the Chilean experience. Cancer Detect Prev. 2005;29(5):405–411. doi: 10.1016/j.cdp.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Longatto-Filho A., Maeda M.Y., Erzen M., et al. Conventional Pap smear and liquid-based cytology as screening tools in low-resource settings in Latin America: experience of the Latin American screening study. Acta Cytol. 2005;49(5):500–506. doi: 10.1159/000326195. [DOI] [PubMed] [Google Scholar]
- 25.Valls J., Baena A., Venegas G., et al. Performance of standardised colposcopy to detect cervical precancer and cancer for triage of women testing positive for human papillomavirus: results from the ESTAMPA multicentric screening study. Lancet Glob Health. 2023;11(3):e350–e360. doi: 10.1016/S2214-109X(22)00545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katki H.A., Schiffman M., Castle P.E., et al. Five-year risks of CIN 3+ and cervical cancer among women who test Pap-negative but are HPV-positive. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S56–S63. doi: 10.1097/LGT.0b013e318285437b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dijkstra M.G., van Zummeren M., Rozendaal L., et al. Safety of extending screening intervals beyond five years in cervical screening programmes with testing for high risk human papillomavirus: 14 year follow-up of population based randomised cohort in The Netherlands. BMJ. 2016;355:i4924. doi: 10.1136/bmj.i4924. [DOI] [PubMed] [Google Scholar]
- 28.Bruni L., Serrano B., Roura E., et al. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: a review and synthetic analysis. Lancet Glob Health. 2022;10(8):e1115–e1127. doi: 10.1016/S2214-109X(22)00241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright T.C., Stoler M.H., Aslam S., Behrens C.M. Knowledge of patients' human papillomavirus status at the time of cytologic review significantly affects the performance of cervical cytology in the ATHENA study. Am J Clin Pathol. 2016;146(3):391–398. doi: 10.1093/ajcp/aqw125. [DOI] [PubMed] [Google Scholar]
- 30.Broutet N., Jeronimo J., Kumar S., et al. Implementation research to accelerate scale-up of national screen and treat strategies towards the elimination of cervical cancer. Prev Med. 2022;155 doi: 10.1016/j.ypmed.2021.106906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.