Abstract
Diffuse dermal angiomatosis (DDA) is a rare, benign disease that can serve as the precursor to critical limb ischemia. Pruritic, erythematous plaques form from a proliferation of endothelial cells in response to dermal hypoxia. We present the case of a 63-year-old female patient with DDA of the left medial thigh, followed by ischemia of her distal extremities. Revascularization of her left leg resulted in resolution of the DDA and healing of her ulcers. DDA can be an important clue to identify significant peripheral vascular disease.
Keywords: Diffuse dermal angiomatosis, Lower limb ischemia, Peripheral arterial disease, Peripheral vascular disease, Revascularization
Diffuse dermal angiomatosis (DDA) is a form of reactive angioendotheliomatosis that is associated with severe atherosclerotic disease.1, 2, 3, 4, 5, 6 It is a benign disorder characterized by a diffuse proliferation of endothelial cells in the dermis due to vascular insufficiency.7,8 Clinically, patients present with nonhealing, painful, ulcerating plaques, commonly reported on the breast, but also found on the trunk, thigh, and lower extremities.5
We present the case of a 63-year-old woman with peripheral arterial disease (PAD) who presented with DDA on the medial aspect of her left thigh as the initial clinical sign of her critical limb ischemia. The patient provided written informed consent for the report of her case details and imaging studies.
Case report
A 63-year-old woman presented to her primary care physician with a pruritic, painful rash on her left medial thigh. She was treated with clindamycin/cephalexin for suspected cellulitis but returned with no improvement. After returning, she began complaining of left lower leg pain. A lower extremity venous duplex ultrasound examination showed the left femoral artery and popliteal artery with monophasic flow, consistent with more proximal stenosis or occlusion.
Because of the ultrasound findings, further workup was necessary to access her overall arterial status. The patient then presented to the vascular surgery clinic as a new patient with a 4-week history of painful pruritic plaques on her left inner thigh that had progressively enlarged over the preceding few days. Her medical history was notable for smoking, chronic obstructive pulmonary disease, obesity, and chronic kidney disease. Physical examination revealed a large indurated erythematous, painful, violaceous plaque with retiform areas of erythema at the periphery. Healing ulcers were also noted medially (Fig 1). No other areas of the skin or mucosa were affected. A pulse examination was notable for absent femoral and pedal pulses on the left. Her ankle brachial index at rest was 0.23 on the left and 0.72 on the right. The patient did not experience rest pain, had dry skin on her left foot, and noted her ambulation was limited by chronic obstructive pulmonary disease-induced shortness of breath. She was scheduled for an ankle brachial index test in 3 months.
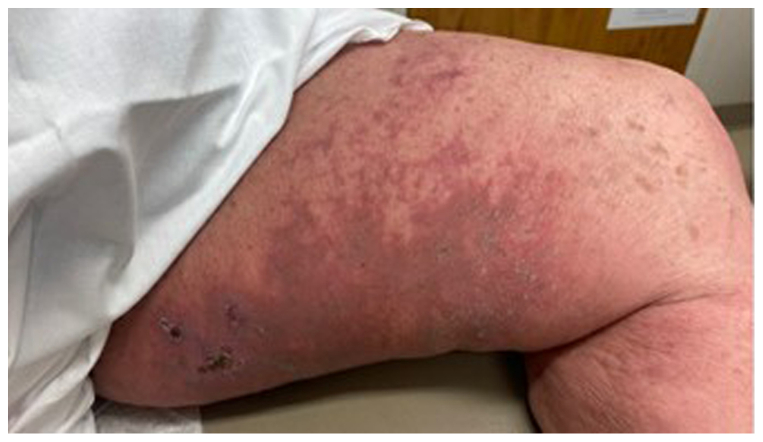
Fig 1.
Diffuse dermal angiomatosis (DDA) of left medial thigh. Photograph showing large indurated erythematous and violaceous plaque with retiform areas of erythema at the periphery and focal ulceration proximally involving the left medial thigh.
The patient's skin lesion remained persistent, and she was referred to dermatology, where a telescoping punch biopsy was performed. Pathologic examination showed a proliferation of vascular channels lined by small endothelial cells involving the superficial and deep dermis and the subcutis (Fig 2). The vascular structures contained red blood cells. No significant cytologic atypia was noted in the specimen, and mitoses were infrequent to absent. Immunohistochemistry for MIB-1 (Ki-67) revealed a low proliferative rate in the endothelial cells. Immunohistochemical markers for human herpesvirus 8 and C-myc were negative. A von Kossa stain failed to reveal significant calcification of the deeper vessels to suggest calciphylaxis. The histologic findings, along with the patient’s clinical presentation, were consistent with DDA. The recommended treatment was revascularization of the underlying left leg ischemia. She was also prescribed tramadol 50 mg for the pain and aspirin 81 mg to reduce the risk of cardiovascular events.
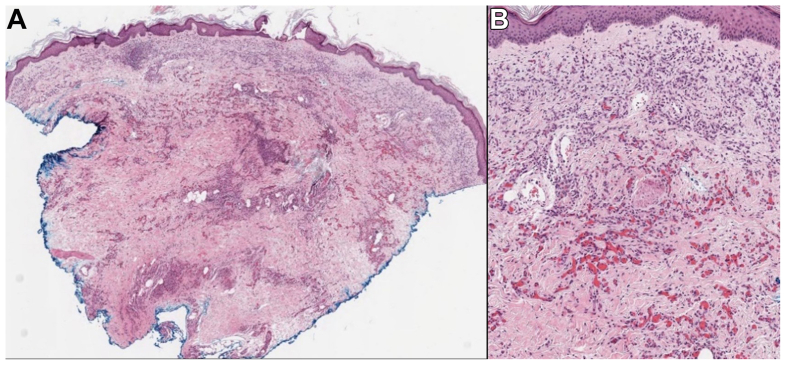
Fig 2.
Biopsy of erythematous plaque on the medial thigh. Small endothelial cells line a proliferation of vascular channels involving the superficial and deep dermis. (Hematoxylin and eosin stain, original magnification ×30 [A] and ×100 [B].)
Because of these findings, we made efforts to schedule earlier appointments with the patient but these were canceled. At follow-up 3 months later, the patient presented to the vascular surgery clinic with acute ischemic rest pain in her left foot and shortness of breath with limited ambulation. Discoloration of the toes was noted on the left foot that was concerning for critical lower limb ischemia due to severe aortoiliac occlusive disease. Computed tomography scans showed a stenotic infrarenal aorta, occlusion of the left common arteries, and occlusion of the left external iliac arteries (Fig 3).
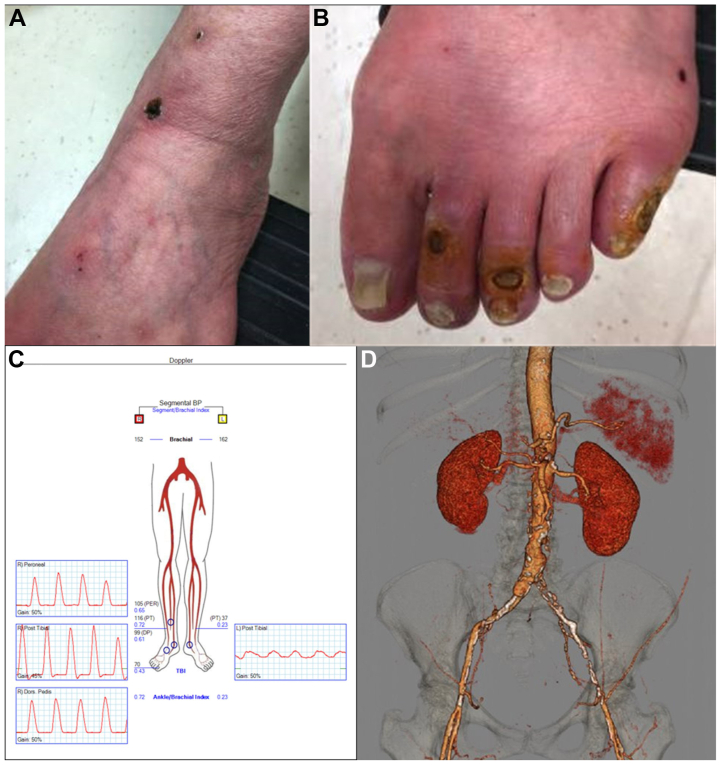
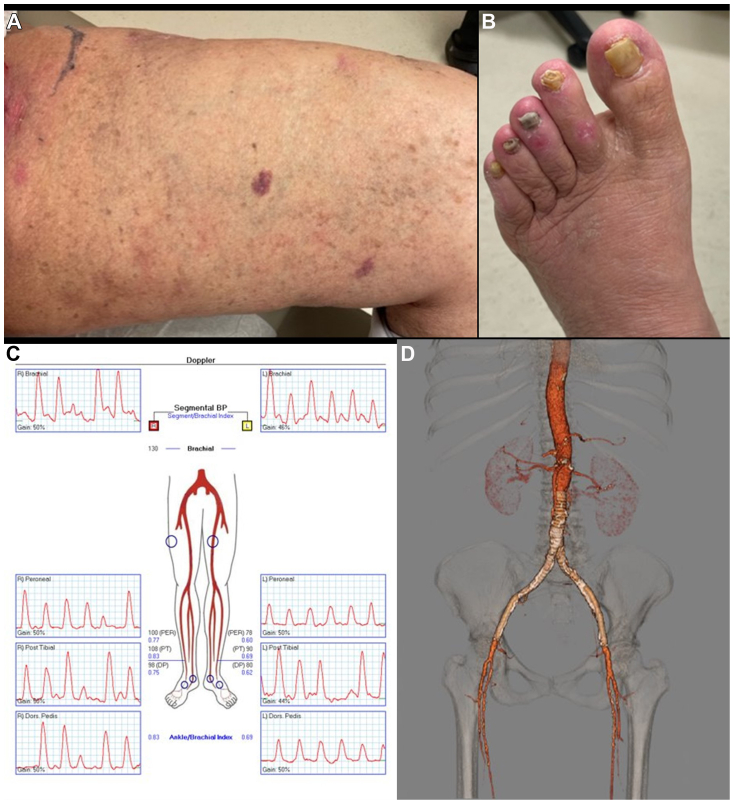
Fig 3.
Preoperative imaging evaluation before revascularization. Photographs showing critical limb ischemia at presentation 3 to 4 months after diagnosis of diffuse dermal angiomatosis (DDA) with ulcerations seen on the anterior aspect of the ankle (A) and second, third, and fifth toes (B). C, Lower extremity arterial Doppler study demonstrating moderate arterial occlusive disease in the right lower extremity and severe arterial occlusive disease in the left lower extremity. D, Three-dimensional computed tomography angiography reconstruction demonstrating severe aortoiliac occlusive disease.
Because of her clinical presentation and imaging results, revascularization was recommended. Owing to the extensive comorbidities, she underwent bilateral iliofemoral endarterectomy and deep femoral endarterectomy with a pericardial patch, stenting of the infrarenal aortic stenosis (11 mm × 79 mm and 11 mm × 30 mm VBX covered balloon expandable stents that were postdilated to 16 mm; W.L. Gore & Associates), stenting of bilateral common iliac arteries (two 8-mm × 79- mm VBX stents), stenting of the left external iliac artery (7-mm × 100-mm Viabahn self-expanding stent graft; W.L. Gore & Associates), and stenting of the right external iliac artery (8-mm × 100-mm Viabahn self-expanding stent graft; Fig 4). The patient tolerated the procedure well, with postoperative evidence of multiphasic dorsalis pedis and posterior tibial Doppler signals bilaterally. A follow-up computed tomography scan showed patent aorta bi-iliac stents. A dramatic improvement in her DDA and complete healing of all her ulcers were noted at follow-up 1 month later.
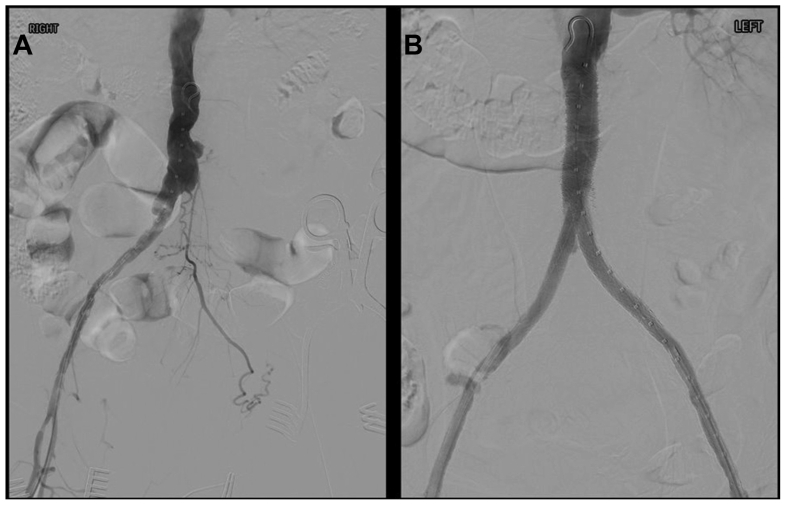
Fig 4.
Intraoperative imaging. A, Digital subtraction angiography before revascularization. B, Digital subtraction angiography at completion of bilateral femoral endarterectomy and aortoiliac stenting.
Discussion
DDA is a rare vascular finding caused by ischemia or inflammation of the tissue due to PAD, smoking, or other vascular abnormalities. DDA typically presents as a painful, purpuric plaque with ulceration, as seen in our patient (Fig 1). In a 2019 review of the literature, of 73 patients with DDA, only 9.6% had plaque on the thigh and 23.3% had vascular disease comorbidities such as hyperlipidemia and peripheral vascular disease (7 cases).9 Our case demonstrates a diagnosis of DDA as a precursor to severe PAD that progressed to ulceration of the left toes (Fig 2). Additionally, our surgical revascularization provided extensive repair of the patient's bilateral iliac and femoral artery occlusive disease with subsequent healing of the lesions.
Immunohistochemical expression of CD31 or ERG in the endothelial cells supports a vascular lesion; however, DDA can mimic vascular malignancies such as Kaposi sarcoma and angiosarcoma. Kaposi sarcoma, caused by a human herpesvirus, can be differentiated by immunohistochemical expression of human herpesvirus 8. Additionally, low Ki-67 levels through the MIB-1 stain, confirming a low proliferative index, support a diagnosis of DDA over angiosarcoma.
The standard treatment of DDA is the treatment of the underlying hypoxia and lifestyle changes such as weight loss and smoking cessation.10 Given the rarity of DDA, no consensus has been reached on its management and treatment. Some case reports noted the effective use of isotretinoin and corticosteroids to treat DDA in the breast owing to their anti-angiogenic properties.11 Revascularization provides a more curative solution to DDA. However, management of the underlying ischemia can be obtained with the use of steroids and anticoagulant therapy. With most DDA cases occurring in the breast, revascularization and stenting of the proximal left subclavian artery have also proved to be successful.12 In our patient with DDA on the thigh, our revascularization approach included bilateral iliofemoral endarterectomy, deep femoral endarterectomy with pericardial patch angioplasty, stenting of the infrarenal aortic stenosis, stenting of the bilateral common iliac arteries, and stenting of the bilateral external iliac arteries for left lower extremity critical limb ischemia. Bifemoral endarterectomy and aortoiliac stenting resulted in increased profusion to the distal extremities (Fig 4). At 3 months after her initial diagnosis of DDA, her PAD had progressed to ulcers on her second, third, and fifth toes, indicating the need for revascularization. Revascularization resulted in healing (Fig 5).
Fig 5.
Postoperative imaging evaluation after revascularization. Photographs showing evidence of healing 1 month after revascularization procedure of the medial thigh (A) and left foot (B). C, Lower extremity arterial Doppler study demonstrating improved arterial perfusion of bilateral lower extremities. D, Three-dimensional computed tomography angiography reconstruction demonstrating successful aortoiliac reconstruction.
Conclusions
DDA is a rare cutaneous disease that should warrant evaluation for progressing PAD. We describe a case of DDA in a patient whose PAD progressed to critical limb ischemia. Subsequent revascularization was successful and provided relief of her symptoms. Prompt diagnosis and treatment of DDA is essential to prevent ischemia and progression of PAD.
From the Eastern Vascular Society
Footnotes
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Author conflict of interest: none.
References
- 1.Krell J.M., Sanchez R.L., Solomon A.R. Diffuse dermal angiomatosis: a variant of reactive cutaneous angioendotheliomatosis. J Cutan Pathol. 1994;21:363–370. doi: 10.1111/j.1600-0560.1994.tb00713.x. [DOI] [PubMed] [Google Scholar]
- 2.Kimyai-Asadi A., Nousari H.C., Ketabchi N., Henneberry J.M., Costarangos C. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with atherosclerosis. J Am Acad Dermatol. 1999;40:257–259. doi: 10.1016/s0190-9622(99)70200-8. [DOI] [PubMed] [Google Scholar]
- 3.Kim S., Elenitsas R., James W.D. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with peripheral vascular atherosclerosis. Arch Dermatol. 2002;138:456–458. doi: 10.1001/archderm.138.4.456. [DOI] [PubMed] [Google Scholar]
- 4.Kirkland C.R., Hawayek L.H., Mutasim D.F. Atherosclerosis-induced diffuse dermal angiomatosis with fatal outcome. Arch Dermatol. 2010;146:684–685. doi: 10.1001/archdermatol.2010.111. [DOI] [PubMed] [Google Scholar]
- 5.Crickx E., Saussine A., Vignon-Pennamen M.D., et al. Diffuse dermal angiomatosis associated with severe atherosclerosis: two cases and review of the literature. Clin Exp Dermatol. 2015;40:521–524. doi: 10.1111/ced.12565. [DOI] [PubMed] [Google Scholar]
- 6.Sriphojanart T., Vachiramon V. Diffuse dermal angiomatosis: a clue to the diagnosis of atherosclerotic vascular disease. Case Rep Dermatol. 2015;7:100–106. doi: 10.1159/000430944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin S., Pitcher D., Tschen J., Wolf J.E. Reactive angioendotheliomatosis. J Am Acad Dermatol. 1980;2:117–123. doi: 10.1016/s0190-9622(80)80389-6. [DOI] [PubMed] [Google Scholar]
- 8.Rongioletti F., Rebora A. Cutaneous reactive angiomatoses: patterns and classification of reactive vascular proliferation. J Am Acad Dermatol. 2003;49:887–896. doi: 10.1016/s0190-9622(03)02100-5. [DOI] [PubMed] [Google Scholar]
- 9.Touloei K., Tongdee E., Smirnov B., Nousari C. Diffuse dermal angiomatosis. Cutis. 2019;103:181–184. [PubMed] [Google Scholar]
- 10.Azarfar A., Bég S. Diffuse dermal angiomatosis: case report of a distinct skin presentation. Rheumatology. 2020;59:e134–e135. doi: 10.1093/rheumatology/keaa311. [DOI] [PubMed] [Google Scholar]
- 11.Sheikh A.B., Javed N., Stoltze K. Diffuse dermal angiomatosis manifestation of underlying severe peripheral vascular disease. Eur J Case Rep Intern Med. 2020;7 doi: 10.12890/2020_001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tollefson M.M., McEvoy M.T., Torgerson R.R., Bridges A.G. Diffuse dermal angiomatosis of the breast: clinicopathologic study of 5 patients. J Am Acad Dermatol. 2014;71:1212–1217. doi: 10.1016/j.jaad.2014.08.015. [DOI] [PubMed] [Google Scholar]