Abstract
Ferroptosis, an iron-dependent form of regulated cell death, results in lipid peroxidation of polyunsaturated fatty acids in the cell membrane, which is catalyzed by iron ions and accumulated to lethal levels. It is mechanistically distinct from other forms of cell death, such as apoptosis, pyroptosis, and necroptosis, so it may address the problem of cancer resistance to apoptosis and provide new therapeutic strategies for cancer treatment, which has been intensively studied over the past few years. Notably, considerable advances have been made in the antitumor research of natural products due to their multitargets and few side effects. According to research, natural products can also induce ferroptosis in cancer therapies. In this review we summarize the molecular mechanisms of ferroptosis, introduce the key regulatory genes of ferroptosis, and discuss the progress of natural product research in the field of ferroptosis to provide theoretical guidance for research on natural product-induced ferroptosis in tumors.
Keywords: ferroptosis, natural product, antitumor, molecular mechanism
Introduction
Ferroptosis is a novel form of cell death proposed by Professor Stockwell in 2012. When ferrous ions promote lipid peroxidation in a manner similar to the Fenton reaction and accumulate to lethal levels, they rupture the membrane and cause cell death [1]. It is a dynamic process in that cells seek a balance between oxidative systems and ferroptosis defense systems during metabolism. Once the cellular oxidative activity intensifies or the antioxidant capacity is suppressed, ferroptosis occurs [ 2– 6] ( Figure 1). Tumor cells need hypermetabolism and high reactive oxygen level to work, but these are more likely to induce ferroptosis than normal cells. Ferroptosis activates tumor suppressors, stops cancer progression, and establishes a natural barrier for the body [ 7, 8]. However, oncogenic signal-mediated ferroptosis resistance contributes to tumor proliferation, metastasis, and treatment resistance [ 4, 7, 9]. Although tumor cells modulate ferroptosis-associated proteins to compensate for defense systems and survive temporarily, ferroptosis is a targetable vulnerability in cancer therapy. Thus, tumor cells face a dilemma: suppress metabolism and oxidative stress or promote ferroptosis defense mechanisms.
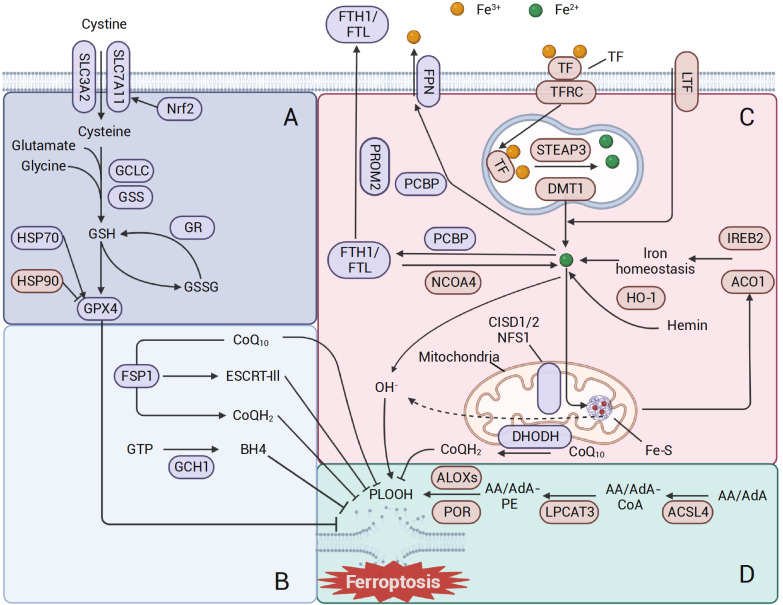
Figure 1 .
Molecular mechanisms of ferroptosis
(A) Canonical ferroptosis antioxidant systems. System xc– delivers cystine into the cell and then catalyzes the synthesis of GSH, thereby maintaining the activity of GPX4 to scavenge lipid peroxidation. (B) Other antioxidant systems. The GCH1-BH4 systems, ESCRT-III membrane repair and FSP1-CoQH2 can also suppress ferroptosis. (C) Iron metabolism. Many proteins regulate iron import, utilization, storage and export to balance iron level in cells. (D) Lipid peroxidation. PUFAs are synthesized in lipid peroxidation by the catalysis of ACSL4, LPCAT3, and ALOX.
Targeting ferroptosis may present new therapeutic opportunities for those who are drug resistant or insensitive to conventional therapies. Additionally, many studies have suggested that ferroptosis plays an important role in tumor suppression and immunity [ 9 , 10]. The proinflammatory factors produced by ferroptosis induce the tumor immune response and inhibit tumor growth, which solves the problem that apoptosis cannot induce a sufficient immune response because proinflammatory factors are removed rapidly [11]. Nevertheless, the regulatory mechanism of ferroptosis is still unknown in many aspects, as it involves amino acid, lipid metabolism, energy metabolism, redox, and iron homeostasis [10].
This review summarizes the latest views on the mechanisms of ferroptosis regulation, presents the key factors regulating ferroptosis, and discusses the progress in natural product research on ferroptosis.
Features of Ferroptosis
The morphological features of apoptosis are nuclear fragmentation, cell shrinkage, and apoptotic body formation [12]; necrosis is morphologically characterized by swelling of the cytoplasm and organelles [13]; and autophagy results in the formation of classical autophagic vesicles [13]. In contrast, ferroptosis is mainly characterized by alterations in mitochondria, including reduced volume, increased membrane density, reduced or absence of cristae, and ruptured outer membranes [1]. Biochemically, ferroptosis is characterized by glutathione (GSH) depletion, glutathione peroxidase 4 (GXP4) inactivation, aggregation of iron ions and lipid peroxide aggregation [1].
Regulatory Mechanisms of Ferroptosis
Iron metabolism
Ferrous ions play an important role in the ferroptosis regulatory network, mainly through the Fenton reaction and activation of iron-containing enzymes, which produce large amounts of lipid peroxides while supplying energy to cells [ 14, 15]. Thus, iron chelators can significantly inhibit ferroptosis [16]. Iron metabolism includes the uptake, export, storage, and utilization of iron. Cells maintain intracellular iron levels at an appropriate level by regulating iron metabolism. Dietary uptake and recycling of iron from senescent erythrocytes by macrophages are the main sources of iron. Fe 3+ binds to serum transferrin (TF) or lactotransferrin (LTF), which is then recognized by the transferrin receptor (TFRC) in the cell membrane [16]. Upon TFRC entering endosomes, Fe 3+ is reduced to Fe 2+ by six-transmembrane epithelial antigen of prostate 3 (STEAP3); it further passes through solute carrier family 11 member 2 (SLC11A2/DMT1) and is released into the cytoplasmic labile iron pool (LIP) [17]. Poly(C)-binding protein family proteins (PCBP) are ferrous ion chaperone proteins that bind with free Fe 2+ in the cytoplasm and deliver it to the corresponding proteins involved in iron utilization, storage, and export. First, Fe 2+ is transferred into mitochondria by mitochondrial transport proteins (SLC25A28 and SLC25A37) and then participates in various aspects of metabolic and biochemical processes, including energy metabolism, the synthesis of hemoglobin and iron-sulfur proteins, and storage in mitochondrial ferritin [18]. Normally, cancer cells utilize iron through the cysteine desulfurase (NFS1)-iron-sulfur cluster assembly enzyme (ISCU)-CDGSH iron-sulfur domain-containing protein 1 and 2 (CISD1/2) axis to inhibit mitochondrial lipid peroxidation and ferroptosis; when Fe 2+ in mitochondria is overloaded, it induces enzyme inactivation, impaired iron metabolism and ferroptosis [ 19– 21 ]. Second, PCBP transfers Fe 2+ to ferritin (FTH1/FTL) stores or exports excess Fe 2+ out of cells via the transporter SLC40A1 (FPN). In addition, prominin 2 (PROM2), a pentaspan transmembrane glycoprotein, exports ferritin and its storage iron through exosomes [22]. Theoretically, each step of iron metabolism provides the possibility for drugs to induce ferroptosis and combat tumors, and extensive evidence suggests that ferroptosis induced by iron overload inhibits tumor cell growth and proliferation [ 23, 24]. For instance, β-elemene and cetuximab upregulate heme oxygenase 1 (HO-1) and transferrin to induce ferroptosis in KRAS mutant colorectal cancer [25]. Temozolomide induces ferroptosis in glioblastoma cells via DMT1 [26]. Silencing of PCBP1 mediates ferroptosis in head and neck cancer [27].
Abnormalities in genes related to iron metabolism usually induce ferroptosis in tumor cells. Iron responsive element binding protein 2 (IREB2) is one of the major regulators of iron metabolism and regulates ferroptosis by affecting the expressions of TFRC, ISCU, FTH1, and FTL [ 1, 28]. Erastin induces ferroptosis in fibrosarcoma and breast cancer by regulating ferrous ion levels via IREB2 [ 1, 29]. Nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy recruits FTH to lysosomes for degradation, generating large amounts of ferrous ions in the labile iron pool [30]. Therefore, activation of NCOA4 and lysosomal activity elevates the level of ferrous ions and promotes ferroptosis [ 31, 32]. Additionally, inhibition of cytosolic glutamate oxaloacetate transaminase 1 (GOT1) enhances ferritinophagy and promotes ferroptosis [33]. In addition, a study showed that mitochondrial ferritin (FTMT) inhibits erastin-induced ferroptosis in neuroblastoma cells, suggesting that iron storage proteins play an important role in inhibiting ferroptosis [34]. Similarly, Nrf2 regulates iron metabolism through HO-1, and excessive activation of HO-1 catalyzes heme degradation to Fe 2+, which causes noncanonical ferroptosis. However, moderate upregulation of HO-1 may promote its cytoprotective effects by enhancing antioxidant activity [ 35, 36]. Therefore, targeting iron metabolism-related proteins is a potential strategy to induce ferroptosis in tumor cells.
Lipid peroxidation
Nonenzymatic lipid peroxidation
Nonenzymatic lipid peroxidation is a series of chain reactions driven by intracellular radicals, in which Fe 2+ and hydrogen peroxide (H 2O 2) produce hydroxyl radicals via the Fenton reaction. Hydroxyl radicals extract hydrogen from the PUFA at the phospholipid (PL) sn2 site to form a carbon-centered lipid radical (L –) and subsequently react with molecular oxygen (O 2) to generate a lipid peroxy radical (LOO –). LOO – can directly or indirectly form lipid peroxide (LOOH) and a new lipid radical by reacting with the adjacent PUFA or Fe 2+ to trigger another chain reaction. If PLOOH cannot be neutralized, the iron-catalyzed amplification reaction leads to an increase in membrane permeability and loss of membrane integrity, and then ferroptosis occurs in the cell. Artemisinin increases cellular free iron and lipid peroxidation and sensitizes cancer cells to ferroptosis [37]. Thus, iron chelators and lipophilic radical scavengers (RTAs) can be effective in preventing ferroptosis [ 10, 38 ].
Enzymatic lipid peroxidation
In enzymatic lipid peroxidation, acyl-CoA synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) are important driving factors. Long-chain PUFAs, mainly free arachidonic acid (AA) or adrenaline (AdA), are bound to CoA by ACSL4 to form the derivatives AA-CoA and AdA-CoA, which are then further processed by LPCAT3 to membrane phosphatidylethanolamine (AA-PE or AdA-PE). Previous studies have shown that the deletion of ACSL4 inhibits ferroptosis in many tumor cells [39]. The peroxidation of PLs, but not FAs, is more significant for ferroptosis; therefore, ACSL4 is thought to be central to ferroptosis [ 40, 41]. Arsenic trioxide induces antitumor ferroptosis by targeting ACSL4 [42]. In contrast, commonly used ALOX inhibitors have been reported to have RTA activity, which challenges the critical role of ALOX in ferroptosis [43]. Shah et al. [43] suggested that the importance of the enzymatic lipid peroxidation response for ferroptosis lies in bringing cells to the critical PLOOH threshold, rather than nonenzymatic lipid peroxidation being the actual driver of ferroptosis [44].
Antioxidant systems
Scavenging of reactive oxygen species (ROS) and lipid peroxidation via the system x c −-glutathione (GSH)-glutathione peroxidase 4 (GPX4) axis has long been considered a central component of the ferroptosis defense mechanism, in which many classical ferroptosis inducers and inhibitors have been identified ( Figure 1A and Table 1). An increasing number of pathways (mTOR, Nrf2, p53, etc.) with proteins (Hsp90 and Hsp70) have been reported for the regulation of system x c − and GPX4, enriching the regulatory network of this pathway in ferroptosis [ 7, 45– 47].
Table 1 Regulators of ferroptosis
|
Regulator |
Impact on ferroptosis |
Reagent |
Ref. |
|
Iron metabolism |
|
|
|
|
IREB |
Regulate the target genes that affect iron homeostasis to balance iron levels |
shRNA |
|
|
HO-1 |
Catalyze degradation of heme to iron |
Withaferin A BAY 11–7085 |
|
|
HSPB1 |
Decrease iron level to inhibit erastin-induced ferroptosis |
shRNA |
|
|
NCOA4 |
Cargo receptor for ferritinophagy, promote degradation of ferritin to iron |
shRNA |
|
|
FTH1 |
Store labile iron |
shRNA |
|
|
STEAP3 |
Regulated by FANCD2, convert iron from Fe 3+ to Fe 2+ |
|
|
|
NFS1 |
Synthesize iron-sulfur clusters using labile iron |
shRNA |
|
|
Lipid metabolism |
|
|
|
|
ACSL4 |
Catalyze PUFA to phospholipids to promote lipid peroxidation |
Thiazolidinedione |
|
|
LPCAT3 |
Insert acyl groups into lysophospholipids |
shRNA |
|
|
ALOX |
Catalyze the conversion of PUFA into lipid peroxidation |
AA861 PD146176 |
|
|
SQS |
Synthesize farnesyl pyrophosphate into squalene, resulting in reduced CoQ10 and failure to scavenge lipid peroxidation |
FIN56 |
|
|
HMGCR |
Synthesize mevalonate, the source of CoQ10 |
Statins |
|
|
Antioxidant system |
|
|
|
|
GPX4 |
Scavenge lipid peroxidation |
RSL-3 FIN56 ML162 FINO2 |
|
|
HO-1 |
Knockdown enhances erastin-induced ferroptosis |
shRNA |
|
|
Nrf2 |
Regulate the expression of the antioxidant gene including system x c – and FTH1 |
Trigonelline |
|
|
SLC7A11 |
Import cystine to synthesize GSH |
Erastin glutamate Sulfasalazine |
|
|
GCLC |
Synthesize GSH |
BSO |
|
|
FSP1 |
Reduce CoQ10 to CoQH2 to scavenge lipid peroxidation |
iFSP1 |
|
|
GCH1 |
Synthesize BH4 to scavenge lipid peroxidation |
Plasmids |
|
|
DHODH |
Synthesize CoQH2 to scavenge lipid peroxidation |
Brequinar |
Several mechanisms independent of GPX4, such as FSP1, CoQ10, BH4, and the ESCRT-III membrane repair system, have also been reported to prevent ferroptosis and lipid peroxidation ( Figure 1). Ferroptosis suppressor protein 1 (FSP1) uses NAD(P)H to reduce CoQ10 to CoQH2, thereby scavenging lipid peroxidation radicals [ 48, 49]. Unlike FSP1, although dihydrogen phosphate dehydrogenase (DHODH) also neutralizes lipid peroxidation by increasing CoQH2 synthesis, this process occurs mainly in mitochondria, so GPX4 and DHODH can collaborate to enhance the inhibition of mitochondrial lipid peroxidation, but cytoplasmic GPX4 and FSP1 cannot [ 50, 51]. Similarly, GTP cyclohydrolase 1 (GCH1) acts as a radical-trapping antioxidant by generating BH4 to inhibit ferroptosis [ 52– 54].
Regulators of Ferroptosis
GPX4
There is no doubt that GPX4 has been the focus of research on intracellular antioxidant mechanisms since the discovery of ferroptosis. Unlike most enzyme families, GPX4 is the only enzyme that can scavenge lipid peroxides in the GPX enzyme family [9]. Similarly, GPX4 is the main enzyme that catalyzes the reduction of PLOOH in cells [67]. It catalyzes phospholipid hydroperoxides (AA/AdA-PE-OOH) into the corresponding phosphatidyl alcohols (PLOH). In addition, GPX4 is a selenoprotein, and selenium increases GPX4 activity via a selenocysteine residue at U46 [ 15, 62, 68 , 69]. Deficiency of GPX4 or inhibition of GPX4 activity by binding to the active site leads to the accumulation of lipid peroxidation, which induces ferroptosis in cells or tissues, and lipophilic radical scavengers inhibit these processes [ 63, 70– 72]. Thus, GPX4 is considered an important inhibitor of ferroptosis, and ample compounds such as gallic acid, withaferin A, and oridonin were reported to induce ferroptosis in tumor cells through downregulation of GPX4 expression.
System x c –
Glutathione is a cofactor for many antioxidant enzymes and is also responsible for maintaining the activity of GPX4 [73]. System x c – is formed by the polymerization of two core proteins, SLC7A11 (xCT) and SLC3A2 (4F2hc), the former being responsible for the import of extracellular cystine, while the latter displaces intracellular glutamate [70]. Erastin induces ferroptosis by inactivating GPX4 indirectly through the depletion of GSH [1]. A number of compounds have been used to target the system x c –-GSH axis to promote ferroptosis or enhance sensitivity to ferroptosis and have received FDA approval, such as sulfasalazine and sorafenib [74]. Furthermore, erianin induces ferroptosis in a variety of tumor cells, including lung cancer, renal cell carcinoma, and bladder cancer cells, by inhibiting system xc – and depleting GSH [ 75– 77]. In conclusion, the system x c –-GSH pathway is one of the critical upstream mechanisms for the induction of ferroptosis.
FSP1
The ability of some tumor cells to proliferate without GPX4 has triggered the exploration of pathways other than GPX4 to prevent ferroptosis. Overexpression of FSP1 inhibits ferroptosis and is independent of ACSL4 and PUFA levels [48]. It has been shown that iFSP1, an inhibitor of FSP1, promotes ferroptosis in GPX4-deficient tumor cells [49]. In addition, FSP1 inhibits ferroptosis by activating ESCRT-III-dependent membrane repair [16].
ALOX
Free and esterified polyunsaturated fatty acids, mainly linoleic acid (LA) and arachidonic acid (AA), are catalyzed by lipoxygenase (ALOX) to produce various lipid hydroperoxides [78]. Studies have shown that inhibition or knockdown of lipoxygenase can inhibit ferroptosis in some cell types [ 79, 60 ]. Similarly, phosphatidylethanolamine binding protein 1 (PEBP1) promotes ferroptosis by directing ALOX15 to recognize polyunsaturated fatty acids on the cell membrane [80]. Although ALOX can sensitize cells to ferroptosis, it is not essential because of other enzymatic or nonenzymatic mechanisms of PL peroxidation. Previous studies suggested that ALOX plays a limited role in ferroptosis because the expression of ALOX is low in cell lines that are commonly studied for ferroptosis, and ALOX inhibitors usually have RTA activity [ 43, 81]. Furthermore, inhibition of ALOX does not prevent ferroptosis in cells lacking GPX4 [82]. Therefore, the regulation of ferroptosis by ALOX still needs further in-depth study.
ACSL4
ACSL4 is an enzyme involved in fatty acid metabolism that promotes ferroptosis by increasing the PUFA content in phospholipids and is therefore considered a specific biomarker and driver of ferroptosis [74]. It was found that protein kinase C betaII (PKCβII) is first activated and then phosphorylates ACSL4, promoting ACSL4-mediated PUFA-PL synthesis and ultimately leading to positive feedback amplification of ferroptosis [83]. Overexpression of ACSL4 may promote ferroptosis; conversely, deletion of ACSL4 moderates ferroptosis, suggesting that ACSL4 may be one of the main mechanisms of ferroptosis [ 2, 84 – 86].
Nrf2
Nrf2 is a major transcription factor of oxidative stress signaling that activates a large number of cytoprotective genes involved in multiple aspects of ferroptosis regulation, such as iron metabolism, oxidative defense, and redox systems. Nrf2 can reverse sorafenib-induced ferroptosis and has therefore been identified as an important defense mechanism for ferroptosis [57]. In recent years, a large number of Nrf2 target genes have been identified, covering iron metabolism (SLC40A1, FTH1, HO-1, etc.), GSH metabolism (SLC7A11, GPX4, GCLM, etc.), and ROS detoxification enzymes (TXNRD1, AKR1C family, NQO1, etc.) [74], further suggesting that Nrf2 is a valuable protein in the regulation of ferroptosis. Xiang et al. [76] found that inactivation of Nrf2 promoted erianin-induced ferroptosis in bladder cancer. Notably, Nrf2 may play a dual role in tumor progression, with both deficient and high expression of Nrf2 promoting tumor proliferation. Both nobiletin and tagitinin C elevates cellular ferrous ion levels via the Nrf2-HO-1 axis, which promotes ferroptosis in tumor cells [ 87 , 88].
Natural Products and Their Application for Ferroptosis
The role of natural products in cancer treatment can be summarized as follows: (1) natural products can be used for tumors that are sensitive to ferroptosis [9]; (2) natural products can induce ferroptosis to reverse drug resistance and enhance immunotherapy [ 11, 89]; (3) due to their multitarget characteristics and few side effects, they may induce ferroptosis in tumor cells while exerting protective effects or less toxicity in normal cells [ 90, 91].
Ferroptosis and natural products in drug resistance
Drug resistance has been a major challenge in the clinical treatment of cancers, and numerous studies have attempted to overcome it. Recent findings suggest that ferroptosis is associated with drug resistance. Using natural compounds to trigger ferroptosis offers great potential for drug-resistant cancers to enhance chemotherapeutic efficacy [89]. Mechanistically, mounting evidence suggests that SLC7A11 is overexpressed in many cancers; in particular, multiple factors reverse tumor suppression by stabilizing or upregulating SLC7A11 in sorafenib- and cisplatin-resistant cells [ 24, 89, 92, 93 ]. Similarly, Nrf2 has been found to be heavily upregulated in drug-resistant cells, since Nrf2 encodes a large number of antioxidant system proteins [ 24, 36 ]. In addition, suppression of GPX4 is an important strategy to overcome the resistance of cancer to chemotherapy [94]. Therefore, targeting ferroptosis has emerged as a potential therapy for drug resistance. In fact, many studies have reported that natural products induce ferroptosis to overcome drug resistance. Curcumin analog reverses temozolomide resistance in glioblastoma by downregulating GPX4 [95]. Compound 23 isolated from Jungermannia tetragona overcomes cisplatin resistance by targeting Prdx I/II and depleting GSH [96]. Furthermore, dihydroartemisinin increases cellular LIP and addresses cisplatin resistance in pancreatic ductal adenocarcinoma [97]. Additionally, artesunate induces ferroptosis in renal cell carcinoma by inhibiting GPX4, which reverses sunitinib resistance [98]. Additionally, natural products such as soyauxinium chloride, epunctanone, and ungeremine were reported to display cytotoxicity toward drug-resistant tumor cells via ferroptosis [ 99– 101].
Ferroptosis and natural products in immunotherapy
Tumor immunotherapy regulates the immune response and inhibits tumor growth by activating the body’s immune defense system and is considered an important therapy for tumors [ 102, 103 ]. However, poor immunogenicity, immune checkpoints, and immunosuppressive factors enable tumors to establish their unique immunosuppressive microenvironment, which limits the activity of effective T cells (Teffs) and resists the immune system from recognizing and attacking tumors [ 104 – 107]. Therefore, the indications for tumor immunotherapy are limited. First, the lack of tumor-specific antigens in cold (poorly immunogenic) tumors results in the inability of T cells to recognize tumor cells [ 108, 109]. Second, although Teffs bind to tumor cells, tumor cells can avoid attack via immune checkpoints (ICTs), such as PD-L1 and CTLA4 [ 110– 112]. In addition, tumor cells secrete specific factors to induce mast cells, which induce immunosuppression and promote tumor growth [ 113– 115]. Therefore, effective strategies to expand their indications and improve their efficiency have become the key element for cancer immunotherapy research [ 11, 112].
Natural products reshape the immunosuppressive tumor microenvironment and show great potential in enhancing the therapeutic efficacy of cancer immunotherapy. For example, capsaicin, ginsenoside Rg3, and resveratrol induce DAMP exposure to enhance the immunogenic cell death (ICD) effect [ 116– 118]. As adjuvants for vaccines, numerous reports have shown that saponins, polysaccharides and flavonoids from natural products can effectively enhance immunostimulatory effects and reverse immunosuppression [ 119– 123 ]. In addition, berberine, baicalin, and cordycepin downregulate PD-L1 expression in tumor cells [ 124– 126 ], and andrographolide, diosgenin, and geranium promote the efficiency of anti-PD-1/PD-L1 antibodies [ 127, 128 ].
As a novel mode of cell death mentioned in recent years, the relationship between ferroptosis and tumor immunity has attracted the attention of researchers. It was found that cancer cells undergoing ferroptosis could activate the tumor microenvironment through the release of DAMPs and create positive feedback of the immune response [ 129– 131 ]. Similarly, tumor-bearing mice treated with an anti-PD-L1 antibody show ferroptosis characteristics, such as an increase in lipid peroxidation [132]. The lipid peroxidation produced by ferroptosis could serve as a signal that promotes dendritic cell recognition of tumor antigens and improves tumor immunotherapy. After the combination of immunotherapy and ferroptosis inducers, infiltration of cytotoxic T lymphocytes was significantly increased [133]. In addition, immunotherapy may increase sensitivity to ferroptosis; thus, coadministration of ferroptosis inducers may generate a strong immune response and promote ferroptosis against tumors [131]. Lou et al. [134] showed that fascaplysin induced ferroptosis while upregulating PD-L1 expression, and increased sensitivity to anti-PD-L1 immunotherapy in non-small cell lung cancer. Although there are fewer reports that natural products activate tumor immune responses via ferroptosis, it is foreseeable that this will be a potential strategy for tumor suppression. Similarly, the cytotoxicity of ferroptosis on immunecells should be noted.
Advances in natural products in the field of ferroptosis
Artemisinin and its derivatives
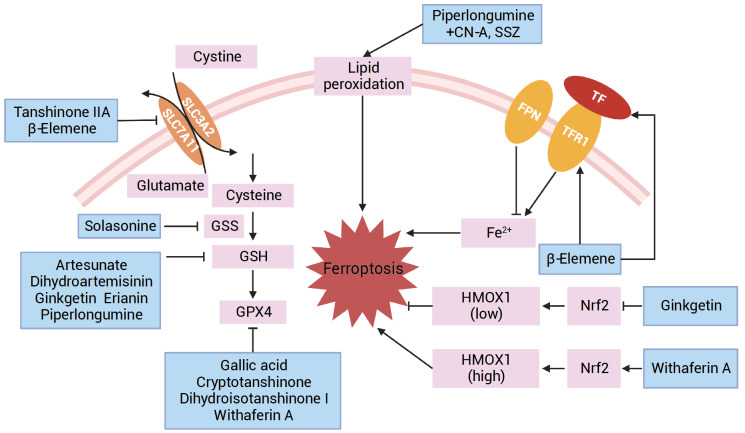
In addition to their antimalarial effects, the antitumor effects of artemisinin and its derivatives have been extensively studied. Artemisinin, dihydroartemisinin, and artesunate, on the one hand, increase ferrous ion level through lysosomal degradation of ferritin or upregulation of NCOA4 and DMT1 levels; on the other hand, they induce ferroptosis through downregulation of GSH and GPX4 levels [ 37, 135, 136] ( Figure 2). According to previous research, artemisinin and its derivatives regulate 20 genes related to iron metabolism, including SLC40A1, IREB, FTMT, and ISCU, to induce ROS production [137]. In addition, inhibition of the Nrf2-ARE pathway allows artesunate to reverse cisplatin resistance through ferroptosis, and coadministration of artesunate enhances the sensitivity of hepatocellular carcinoma cells to sorafenib [66].
Figure 2 .
Functions of natural products in inducing ferroptosis
Salvia officinalis extract
Tanshinone extracts include tanshinone IIA, dihydrotanshinone I, and cryptotanshinone. In normal cells, tanshinone IIA inhibits hepatocyte ferroptosis in an atherosclerosis model by upregulating the expressions of GPX4, SLC7A11, and FTH1 [138] ( Figure 2); in hippocampal cells, it inhibits ferroptosis by upregulating HO-1 expression to lower LPO and Fe 2+ levels [139]. In contrast, in gastric cancer cells, tanshinone IIA mediates the decrease in GSH and increase in ROS triggered by the downregulation of SLC7A11 level [91]. In addition, cryptotanshinone induces ferroptosis in lung cancer cells through HSPB1 and GPX4 upregulation and IREB2 downregulation [140]. Dihydroisotanshinone I reduces GPX activity or inhibits GPX4 protein expression, increasing malondialdehyde (MDA) and lipid ROS levels [ 141, 142].
Piperlongumine
Ferroptosis induced by piperlongumine mainly depends on the inactivation of antioxidant mechanisms and the accumulation of ROS in cells ( Figure 2). In a variety of cancer cell lines, including gastric, pancreatic, and colorectal cancers, piperlongumine inhibits GPX4 activity mainly by reducing GSH, leading to ROS accumulation [143]. It also significantly enhances the anticancer effect of ferroptosis by combining sulfasalazine, oxaliplatin, and APR-246 [144]. In contrast to cancer cells, activation of HO-1 by piperlongumine inhibits ferroptosis in normal cells; in vivo studies also showed that piperlongumine attenuates weight loss induced by oxaliplatin treatment [ 145 , 146].
Others
In addition to the natural products mentioned above, a large number of compounds have been reported in antitumor studies via ferroptosis mechanisms, encompassing the modulation of iron metabolism, lipid peroxidation, and antioxidant systems. Hence, the main mechanism by which other natural products induce ferroptosis is summarized in Figure 2 and Table 2.
Table 2 Ferroptosis-induced natural products
|
Compound |
Effect |
Cancer type |
Ref. |
|
Erianin |
Downregulate GPX4, GSH, and SLC7A11, and activation of CaM caused an increase in Fe 2+ |
Lung cancer |
|
|
Oridonin |
Decrease GGT1 activity to block GSH formation and reduce GPX4 activity |
Esophageal cancer |
|
|
Solasonine |
Suppress GPX4 and GSS to increase lipid ROS |
Hepatocellular carcinoma |
|
|
Pseudolaric acid B |
Upregulate p53 to inhibit SLC7A11 and GSH, upregulation of TFRC |
Glioma |
|
|
Withaferin A |
Increase Fe 2+ by tragting Keap1 to activate HO-1; inactivates glutathione peroxidase 4 |
Neuroblastoma |
|
|
Actinidia chinensis planch |
Suppress GPX4 and SLC7A11 to accumulate ROS |
Gastric cancer |
|
|
Gambogic acid |
Upregulate p53 to inhibit SLC7A11, GSH and GPX4; reduced SOD activity |
Melanoma |
|
|
Ginkgetin |
Decrease expression of SLC7A11 and GPX4, inactivation of Nrf2/HO-1 |
Non-small cell lung cancer |
|
|
Glycyrrhetinic acid |
Activate NADPH oxidases, and decrease GSH and GPX4 activity |
Triple-negative breast cancer |
|
|
Cucurbitacin B |
Accumulate iron ions, downregulate the expression of GPX4 |
Nasopharyngeal cancer |
|
|
6-Gingerol |
Upregulate LC3-II and NCOA4 to induce ferritinophagy and downregulation of GPX expression |
Lung cancer |
|
|
Polyphyllin II |
Activate NCOA4-induced ferritinophagy to increase iron ion |
Hepatocellular carcinoma; KRAS mutation harboring cancer |
|
|
β-Elemene |
Upregulate the expression of HO-1 and TF to elevate Fe 2+, and downregulation of GPX4, SLC7A11 and GS |
KRAS mutant colorectal cancer |
|
|
Gallic acid |
Promote TFRC, ATF4 and iron ion levels and inhibits GPX4 and SCL7A11 |
Colorectal cancer |
|
|
Oleanicacid |
Promote ACSL4 expression and iron ion to accumulate ROS and MDA |
Cervical cancer |
Conclusions and Perspectives
Targeting ferroptosis to antitumor effects will be a hot topic of scientific research, although the mechanism is not fully elucidated and needs further study. However, it is certainly the result of an imbalance between the intracellular redox protective system and iron metabolism. The sensitivity to ferroptosis varies among tumors, and therefore, an in-depth investigation of its different regulatory mechanisms would be instructive for the clinical application of targeting ferroptosis in tumors. Currently, the key regulator GPX4 has become an important therapeutic target for the development of anticancer drugs; as more research is conducted, more factors involved in ferroptosis will be identified, which will provide more targets and treatment options for clinical application.
At the same time, ferroptosis is a double-edged sword; on one hand, we use it to eliminate tumor cells, but on the other hand, we should also be concerned about the damage that ferroptosis may cause to normal cells. For example, GPX4 inhibits TMEM16A-mediated hepatic ischemia/reperfusion injury [159]. Additionally, DHODH inhibits ferroptosis in spinal cord injury [51]; similarly, ferroptosis exacerbates most cardiovascular diseases [160]. Notably, trastuzumab induces severe cardiotoxicity while treating breast cancer via ferroptotic cell death; however, SGLT2 inhibitors eliminate cardiotoxicity and show potent antitumor activity [161]. Targets such as SGLT2 deserve our attention because they can protect normal cells and kill tumor cells simultaneously.
Common clinical drugs have disadvantages such as poor selectivity and toxic side effects, which severely limit their efficacy. Natural products have multipathway and multitarget anticancer properties. How natural products regulate and interfere with ferroptosis for cancer treatment still needs further in-depth research. For example, as mentioned above, tanshinone IIA and piperlongumine can induce ferroptosis in tumor cells while also promoting antioxidation and inhibiting ferroptosis in normal cells [ 90, 145, 146]. Is there a threshold of intracellular Fe 2+ and lipid peroxidation levels that would allow tumor cells to undergo ferroptosis while leaving normal cells unaffected? Or whether there is a new target that induces ferroptosis in tumor cells only, once the mechanism is clarified, this will provide great diagnostic and therapeutic value to the clinic. In addition, the link between ferroptosis and other cell death modalities remains to be explored, and if the intrinsic link is clarified, this could provide a theoretical basis for the clinical combination of drugs. As the mechanism of natural products against tumors through ferroptosis will be further investigated, it may lead to new strategies for ferroptosis-based cancer therapy.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 82141203 and 81772798), the Shanghai Municipal Science and Technology Major Project (No. ZD2021CY001), the Three-year Action Plan for Shanghai TCM Development and Inheritance Program [No. ZY (2021-2023)-0401], and the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No. ZYYCXTDD-202004).
References
- 1.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. . 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu J, Minikes AM, Gao M, Bian H, Li Y, Stockwell BR, Chen ZN, et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. . 2019;572:402–406. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hangauer MJ, Viswanathan VS, Ryan MJ, Bole D, Eaton JK, Matov A, Galeas J, et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. . 2017;551:247–250. doi: 10.1038/nature24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. . 2017;547:453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou Y, Henry WS, Ricq EL, Graham ET, Phadnis VV, Maretich P, Paradkar S, et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature. . 2020;585:603–608. doi: 10.1038/s41586-020-2732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou Y, Palte MJ, Deik AA, Li H, Eaton JK, Wang W, Tseng YY, et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun. . 2019;10:1617. doi: 10.1038/s41467-019-09277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. . 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Shi J, Liu X, Feng L, Gong Z, Koppula P, Sirohi K, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol. . 2018;20:1181–1192. doi: 10.1038/s41556-018-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei G, Zhuang L, Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. . 2022;22:381–396. doi: 10.1038/s41568-022-00459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. . 2021;22:266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, Li M, Wang P, Bai Q, Cao X, Mao D. Recent organic photosensitizer designs for evoking proinflammatory regulated cell death in antitumor immunotherapy. Small Methods. . 2023;7:e2201614. doi: 10.1002/smtd.202201614. [DOI] [PubMed] [Google Scholar]
- 12.Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. . 2020;17:395–417. doi: 10.1038/s41571-020-0341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moujalled D, Strasser A, Liddell JR. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. . 2021;28:2029–2044. doi: 10.1038/s41418-021-00814-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. . 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA. . 2016;113:E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. . 2021;18:280–296. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 17.Fang X, Ardehali H, Min J, Wang F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol. . 2023;20:7–23. doi: 10.1038/s41569-022-00735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, Kang R, Kroemer G, Tang D. Organelle-specific regulation of ferroptosis. Cell Death Differ. . 2021;28:2843–2856. doi: 10.1038/s41418-021-00859-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci USA. . 2019;116:2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan H, Li X, Zhang X, Kang R, Tang D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun. . 2016;478:838–844. doi: 10.1016/j.bbrc.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 21.Kim EH, Shin D, Lee J, Jung AR, Roh JL. CISD2 inhibition overcomes resistance to sulfasalazine-induced ferroptotic cell death in head and neck cancer. Cancer Lett. . 2018;432:180–190. doi: 10.1016/j.canlet.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Brown CW, Amante JJ, Chhoy P, Elaimy AL, Liu H, Zhu LJ, Baer CE, et al. Prominin2 drives ferroptosis resistance by stimulating iron export. Dev Cell. . 2019;51:575–586. doi: 10.1016/j.devcel.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Yu C, Kang R, Tang D. Iron metabolism in ferroptosis. Front Cell Dev Biol. . 2020;8:590226. doi: 10.3389/fcell.2020.590226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajoolabady A, Tang D, Kroemer G, Ren J. Ferroptosis in hepatocellular carcinoma: mechanisms and targeted therapy. Br J Cancer. . 2023;128:190–205. doi: 10.1038/s41416-022-01998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen P, Li X, Zhang R, Liu S, Xiang Y, Zhang M, Chen X, et al. Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics. . 2020;10:5107–5119. doi: 10.7150/thno.44705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song Q, Peng S, Sun Z, Heng X, Zhu X. Temozolomide drives ferroptosis via a DMT1-dependent pathway in glioblastoma cells. Yonsei Med J. . 2021;62:843–849. doi: 10.3349/ymj.2021.62.9.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, You JH, Roh JL. Poly(rC)-binding protein 1 represses ferritinophagy-mediated ferroptosis in head and neck cancer. Redox Biol. . 2022;51:102276. doi: 10.1016/j.redox.2022.102276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gammella E, Recalcati S, Rybinska I, Buratti P, Cairo G. Iron-induced damage in cardiomyopathy: oxidative-dependent and independent mechanisms. Oxid Med Cell Longev. . 2015;2015:1–10. doi: 10.1155/2015/230182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozkan E, Bakar-Ates F. Etoposide in combination with erastin synergistically altered iron homeostasis and induced ferroptotic cell death through regulating IREB2/FPN1 expression in estrogen receptor positive-breast cancer cells. Life Sci. . 2023;312:121222. doi: 10.1016/j.lfs.2022.121222. [DOI] [PubMed] [Google Scholar]
- 30.Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. . 2016;26:1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torii S, Shintoku R, Kubota C, Yaegashi M, Torii R, Sasaki M, Suzuki T, et al. An essential role for functional lysosomes in ferroptosis of cancer cells. Biochem J. . 2016;473:769–777. doi: 10.1042/BJ20150658. [DOI] [PubMed] [Google Scholar]
- 32.Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh Iii HJ, Kang R, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. . 2016;12:1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kremer DM, Nelson BS, Lin L, Yarosz EL, Halbrook CJ, Kerk SA, Sajjakulnukit P, et al. GOT1 inhibition promotes pancreatic cancer cell death by ferroptosis. Nat Commun. . 2021;12:4860. doi: 10.1038/s41467-021-24859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang YQ, Chang SY, Wu Q, Gou YJ, Jia L, Cui YM, Yu P, et al. The protective role of mitochondrial ferritin on erastin-induced ferroptosis. Front Aging Neurosci. . 2016;8:308. doi: 10.3389/fnagi.2016.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassannia B, Wiernicki B, Ingold I, Qu F, Van Herck S, Tyurina YY, Bayır H, et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J Clin Invest. . 2018;128:3341–3355. doi: 10.1172/JCI99032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. . 2019;35:830–849. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Chen GQ, Benthani FA, Wu J, Liang D, Bian ZX, Jiang X. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ. . 2020;27:242–254. doi: 10.1038/s41418-019-0352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. . 2007;447:865–869. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. . 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M, Superti-Furga G, et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol. . 2015;10:1604–1609. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kagan VE, Mao G, Qu F, Angeli JPF, Doll S, Croix CS, Dar HH, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. . 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, Jin Z, Zhang S, Zhang X, Li P, Yang H, Ma Y. Arsenic trioxide elicits prophylactic and therapeutic immune responses against solid tumors by inducing necroptosis and ferroptosis. Cell Mol Immunol. . 2023;20:51–64. doi: 10.1038/s41423-022-00956-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah R, Shchepinov MS, Pratt DA. Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Cent Sci. . 2018;4:387–396. doi: 10.1021/acscentsci.7b00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng J, Conrad M. The metabolic underpinnings of ferroptosis. Cell Metab. . 2020;32:920–937. doi: 10.1016/j.cmet.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Swanda RV, Nie L, Liu X, Wang C, Lee H, Lei G, et al. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat Commun. . 2021;12:1589. doi: 10.1038/s41467-021-21841-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu S, Zhang Q, Sun X, Zeh Iii HJ, Lotze MT, Kang R, Tang D. HSPA5 regulates ferroptotic cell death in cancer cells. Cancer Res. . 2017;77:2064–2077. doi: 10.1158/0008-5472.CAN-16-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Z, Geng Y, Lu X, Shi Y, Wu G, Zhang M, Shan B, et al. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc Natl Acad Sci USA. . 2019;116:2996–3005. doi: 10.1073/pnas.1819728116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. . 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Goya Grocin A, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. . 2019;575:693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 50.Mao C, Liu XG, Zhang YL, et al. Dhodh-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021, 596: E13–E13 . [DOI] [PubMed]
- 51.Li D, Lu X, Xu G, Liu S, Gong Z, Lu F, Xia X, et al. Dihydroorotate dehydrogenase regulates ferroptosis in neurons after spinal cord injury via the P53-ALOX15 signaling pathway. CNS Neurosci Ther. . 2023;29:1923–1939. doi: 10.1111/cns.14150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soula M, Weber RA, Zilka O, Alwaseem H, La K, Yen F, Molina H, et al. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat Chem Biol. . 2020;16:1351–1360. doi: 10.1038/s41589-020-0613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thony B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochemical Journal. 2000, 347: 1–16 . [PMC free article] [PubMed]
- 54.Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci. . 2020;6:41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang LC, Chiang SK, Chen SE, Yu YL, Chou RH, Chang WC. Heme oxygenase-1 mediates BAY 11–7085 induced ferroptosis. Cancer Lett. . 2018;416:124–137. doi: 10.1016/j.canlet.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 56.Sun X, Ou Z, Xie M, Kang R, Fan Y, Niu X, Wang H, et al. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene. . 2015;34:5617–5625. doi: 10.1038/onc.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, Tang D. Activation of the p62‐Keap1‐NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. . 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song X, Xie Y, Kang R, Hou W, Sun X, Epperly MW, Greenberger JS, et al. FANCD2 protects against bone marrow injury from ferroptosis. Biochem Biophys Res Commun. . 2016;480:443–449. doi: 10.1016/j.bbrc.2016.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alvarez SW, Sviderskiy VO, Terzi EM, Papagiannakopoulos T, Moreira AL, Adams S, Sabatini DM, et al. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature. . 2017;551:639–643. doi: 10.1038/nature24637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seiler A, Schneider M, Förster H, Roth S, Wirth EK, Culmsee C, Plesnila N, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. . 2008;8:237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Shimada K, Skouta R, Kaplan A, Yang WS, Hayano M, Dixon SJ, Brown LM, et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. . 2016;12:497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. . 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaschler MM, Andia AA, Liu H, Csuka JM, Hurlocker B, Vaiana CA, Heindel DW, et al. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol. . 2018;14:507–515. doi: 10.1038/s41589-018-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garcia-Bermudez J, Baudrier L, Bayraktar EC, Shen Y, La K, Guarecuco R, Yucel B, et al. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature. . 2019;567:118–122. doi: 10.1038/s41586-019-0945-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adedoyin O, Boddu R, Traylor A, Lever JM, Bolisetty S, George JF, Agarwal A. Heme oxygenase-1 mitigates ferroptosis in renal proximal tubule cells. Am J Physiol Renal Physiol. . 2018;314:F702–F714. doi: 10.1152/ajprenal.00044.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roh JL, Kim EH, Jang H, Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. . 2017;11:254–262. doi: 10.1016/j.redox.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radical Biol Med. . 2019;133:144–152. doi: 10.1016/j.freeradbiomed.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 68.Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, Roveri A, et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell. . 2018;172:409–422. doi: 10.1016/j.cell.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 69.Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. . 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 70.Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. . 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weïwer M, Bittker JA, Lewis TA, Shimada K, Yang WS, MacPherson L, Dandapani S, et al. Development of small-molecule probes that selectively kill cells induced to express mutant RAS. Bioorg Med Chem Lett. . 2012;22:1822–1826. doi: 10.1016/j.bmcl.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Woo JH, Shimoni Y, Yang WS, Subramaniam P, Iyer A, Nicoletti P, Rodríguez Martínez M, et al. Elucidating compound mechanism of action by network perturbation analysis. Cell. . 2015;162:441–451. doi: 10.1016/j.cell.2015.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. . 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. . 2021;31:107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shen H, Geng Z, Nie X, Liu T. Erianin induces ferroptosis of renal cancer stem cells via promoting ALOX12/ P53 mRNA N6-methyladenosine modification . J Cancer. . 2023;14:367–378. doi: 10.7150/jca.81027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiang Y, Chen X, Wang W, Zhai L, Sun X, Feng J, Duan T, et al. Natural product erianin inhibits bladder cancer cell growth by inducing ferroptosis via NRF2 inactivation. Front Pharmacol. . 2021;12:775506. doi: 10.3389/fphar.2021.775506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen P, Wu Q, Feng J, Yan L, Sun Y, Liu S, Xiang Y, et al. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis . Sig Transduct Target Ther. . 2020;5:51. doi: 10.1038/s41392-020-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuhn H, Banthiya S, van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochim Biophys Acta. . 2015;1851:308–330. doi: 10.1016/j.bbalip.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. . 2016;26:165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wenzel SE, Tyurina YY, Zhao J, St. Croix CM, Dar HH, Mao G, Tyurin VA, et al. PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell. . 2017;171:628–641.e26. doi: 10.1016/j.cell.2017.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghandi M, Huang FW, Jané-Valbuena J, Kryukov GV, Lo CC, McDonald Iii ER, Barretina J, et al. Next-generation characterization of the cancer cell line encyclopedia. Nature. . 2019;569:503–508. doi: 10.1038/s41586-019-1186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jin G, Arai K, Murata Y, Wang S, Stins MF, Lo EH, van Leyen K. Protecting against cerebrovascular injury. Stroke. . 2008;39:2538–2543. doi: 10.1161/STROKEAHA.108.514927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang HL, Hu BX, Li ZL, et al. Pkcbetaii phosphorylates acsl4 to amplify. [DOI] [PubMed]
- 84.Brown CW, Amante JJ, Goel HL, Mercurio AM. The α6β4 integrin promotes resistance to ferroptosis. J Cell Biol. . 2017;216:4287–4297. doi: 10.1083/jcb.201701136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D, Liu D, et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. . 2019;26:2284–2299. doi: 10.1038/s41418-019-0299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH, Ajani JA, et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. . 2020;30:146–162. doi: 10.1038/s41422-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feng S, Zhou Y, Huang H, Lin Y, Zeng Y, Han S, Huang K, et al. Nobiletin induces ferroptosis in human skin melanoma cells through the GSK3β-mediated keap1/Nrf2/HO-1 signalling pathway. Front Genet. . 2022;13:865073. doi: 10.3389/fgene.2022.865073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wei R, Zhao Y, Wang J, Yang X, Li S, Wang Y, Yang X, et al. Tagitinin C induces ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal cancer cells. Int J Biol Sci. . 2021;17:2703–2717. doi: 10.7150/ijbs.59404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y, Wu X, Ren Z, Li Y, Zou W, Chen J, Wang H. Overcoming cancer chemotherapy resistance by the induction of ferroptosis. Drug Resist Updat. . 2023;66:100916. doi: 10.1016/j.drup.2022.100916. [DOI] [PubMed] [Google Scholar]
- 90.Xu L, Tang QQ. Research on the mechanism of tanshinone IIA inhibiting ferroptosis in HT22 hippocampus cells. Acta Universitatis Medicinalis Anhui. 2019, 54: 833–839
- 91.Guan Z, Chen J, Li X, Dong N. Tanshinone IIA induces ferroptosis in gastric cancer cells through p53-mediated SLC7A11 down-regulation. Biosci Rep. . 2020;40:BSR20201807. doi: 10.1042/BSR20201807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. . 2021;12:599–620. doi: 10.1007/s13238-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang W, Chen K, Lu Y, Zhang D, Cheng Y, Li L, Huang W, et al. ABCC5 facilitates the acquired resistance of sorafenib through the inhibition of SLC7A11-induced ferroptosis in hepatocellular carcinoma. Neoplasia. . 2021;23:1227–1239. doi: 10.1016/j.neo.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang C, Liu X, Jin S, Chen Y, Guo R. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer. . 2022;21:47. doi: 10.1186/s12943-022-01530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen TC, Chuang JY, Ko CY, Kao TJ, Yang PY, Yu CH, Liu MS, et al. AR ubiquitination induced by the curcumin analog suppresses growth of temozolomide-resistant glioblastoma through disrupting GPX4-mediated redox homeostasis. Redox Biol. . 2020;30:101413. doi: 10.1016/j.redox.2019.101413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun Y, Qiao Y, Liu Y, Zhou J, Wang X, Zheng H, Xu Z, et al. ent-Kaurane diterpenoids induce apoptosis and ferroptosis through targeting redox resetting to overcome cisplatin resistance. Redox Biol. . 2021;43:101977. doi: 10.1016/j.redox.2021.101977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Du J, Wang X, Li Y, Ren X, Zhou Y, Hu W, Zhou C, et al. DHA exhibits synergistic therapeutic efficacy with cisplatin to induce ferroptosis in pancreatic ductal adenocarcinoma via modulation of iron metabolism. Cell Death Dis. . 2021;12:705. doi: 10.1038/s41419-021-03996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Markowitsch SD, Schupp P, Lauckner J, Vakhrusheva O, Slade KS, Mager R, Efferth T, et al. Artesunate inhibits growth of sunitinib-resistant renal cell carcinoma cells through cell cycle arrest and induction of ferroptosis. Cancers. . 2020;12:3150. doi: 10.3390/cancers12113150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mbaveng AT, Bitchagno GTM, Kuete V, Tane P, Efferth T. Cytotoxicity of ungeremine towards multi-factorial drug resistant cancer cells and induction of apoptosis, ferroptosis, necroptosis and autophagy. Phytomedicine. . 2019;60:152832. doi: 10.1016/j.phymed.2019.152832. [DOI] [PubMed] [Google Scholar]
- 100.Mbaveng AT, Fotso GW, Ngnintedo D, Kuete V, Ngadjui BT, Keumedjio F, Andrae-Marobela K, et al. Cytotoxicity of epunctanone and four other phytochemicals isolated from the medicinal plants Garcinia epunctata and Ptycholobium contortum towards multi-factorial drug resistant cancer cells . Phytomedicine. . 2018;48:112–119. doi: 10.1016/j.phymed.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 101.Mbaveng AT, Noulala CGT, Samba ARM, Tankeo SB, Abdelfatah S, Fotso GW, Happi EN, et al. The alkaloid, soyauxinium chloride, displays remarkable cytotoxic effects towards a panel of cancer cells, inducing apoptosis, ferroptosis and necroptosis. Chem Biol Interact. . 2021;333:109334. doi: 10.1016/j.cbi.2020.109334. [DOI] [PubMed] [Google Scholar]
- 102.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. . 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Finck A, Gill SI, June CH. Cancer immunotherapy comes of age and looks for maturity. Nat Commun. . 2020;11:3325. doi: 10.1038/s41467-020-17140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. . 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.O′Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. . 2019;16:151–167. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 106.Hogg SJ, Beavis PA, Dawson MA, Johnstone RW. Targeting the epigenetic regulation of antitumour immunity. Nat Rev Drug Discov. . 2020;19:776–800. doi: 10.1038/s41573-020-0077-5. [DOI] [PubMed] [Google Scholar]
- 107.Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression—implications for anticancer therapy. Nat Rev Clin Oncol. . 2019;16:356–371. doi: 10.1038/s41571-019-0175-7. [DOI] [PubMed] [Google Scholar]
- 108.Branca MA. Rekindling cancer vaccines. Nat Biotechnol. . 2016;34:1019–1024. doi: 10.1038/nbt.3690. [DOI] [PubMed] [Google Scholar]
- 109.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. . 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. . 2005;54:307–314. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. . 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dong S, Guo X, Han F, He Z, Wang Y. Emerging role of natural products in cancer immunotherapy. Acta Pharmaceutica Sin B. . 2022;12:1163–1185. doi: 10.1016/j.apsb.2021.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Coleman JW. Nitric oxide: a regulator of mast cell activation and mast cell-mediated inflammation. Clin Exp Immunol. . 2002;129:4–10. doi: 10.1046/j.1365-2249.2002.01918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Frossi B, De Carli M, Daniel K, Rivera J, Pucillo C. Oxidative stress stimulates IL-4 and IL-6 production in mast cells by an APE/Ref-1-dependent pathway. Eur J Immunol. . 2003;33:2168–2177. doi: 10.1002/eji.200323995. [DOI] [PubMed] [Google Scholar]
- 115.Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. . 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 116.Granato M, Gilardini Montani MS, Filardi M, Faggioni A, Cirone M. Capsaicin triggers immunogenic PEL cell death, stimulates DCs and reverts PEL-induced immune suppression. Oncotarget. . 2015;6:29543–29554. doi: 10.18632/oncotarget.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Son K, Choi K, Lee SJ, Lee H. Immunogenic cell death induced by ginsenoside Rg3: significance in dendritic cell-based anti-tumor immunotherapy. Immune Netw. . 2016;16:75–84. doi: 10.4110/in.2016.16.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang J, Shen L, Li X, Song W, Liu Y, Huang L. Nanoformulated codelivery of quercetin and alantolactone promotes an antitumor response through synergistic immunogenic cell death for microsatellite-stable colorectal cancer. ACS Nano. . 2019;13:12511–12524. doi: 10.1021/acsnano.9b02875. [DOI] [PubMed] [Google Scholar]
- 119.Lacaille-Dubois MA, Wagner H. New perspectives for natural triterpene glycosides as potential adjuvants. Phytomedicine. . 2017;37:49–57. doi: 10.1016/j.phymed.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 120.Zhang XP, Li YD, Luo LL, Liu YQ, Li Y, Guo C, Li ZD, et al. Astragalus saponins and liposome constitute an efficacious adjuvant formulation for cancer vaccines . Cancer Biother Radiopharm. . 2018;33:25–31. doi: 10.1089/cbr.2017.2369. [DOI] [PubMed] [Google Scholar]
- 121.Fitzpatrick JM, Minogue E, Curham L, Tyrrell H, Gavigan P, Hind W, Downer EJ. MyD88-dependent and -independent signalling via TLR3 and TLR4 are differentially modulated by Δ9-tetrahydrocannabinol and cannabidiol in human macrophages. J Neuroimmunol. . 2020;343:577217. doi: 10.1016/j.jneuroim.2020.577217. [DOI] [PubMed] [Google Scholar]
- 122.Song YC, Huang HC, Chang CYY, Lee HJ, Liu CT, Lo HY, Ho TY, et al. A potential herbal adjuvant combined with a peptide-based vaccine acts against HPV-related tumors through enhancing effector and memory T-cell immune responses. Front Immunol. . 2020;11:62. doi: 10.3389/fimmu.2020.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang L, Zeng W, Wang L, Wang Z, Yin X, Qin Y, Zhang F, et al. Naringenin enhances the antitumor effect of therapeutic vaccines by promoting antigen cross-presentation. J Immunol. . 2020;204:622–631. doi: 10.4049/jimmunol.1900278. [DOI] [PubMed] [Google Scholar]
- 124.Zhang S, Zhou L, Zhang M, Wang Y, Wang M, Du J, Gu W, et al. Berberine maintains the neutrophil N1 phenotype to reverse cancer cell resistance to doxorubicin. Front Pharmacol. . 2020;10:1658. doi: 10.3389/fphar.2019.01658. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 125.Ke M, Zhang Z, Xu B, Zhao S, Ding Y, Wu X, Wu R, et al. Baicalein and baicalin promote antitumor immunity by suppressing PD-L1 expression in hepatocellular carcinoma cells. Int Immunopharmacol. . 2019;75:105824. doi: 10.1016/j.intimp.2019.105824. [DOI] [PubMed] [Google Scholar]
- 126.Hsu PY, Lin YH, Yeh EL, Lo HC, Hsu TH, Su CC. Cordycepin and a preparation from Cordyceps militaris inhibit malignant transformation and proliferation by decreasing EGFR and IL-17RA signaling in a murine oral cancer model . Oncotarget. . 2017;8:93712–93728. doi: 10.18632/oncotarget.21477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu W, Fan T, Li M, Zhang G, Guo W, Yang X, Jiang C, et al. Andrographolide potentiates PD-1 blockade immunotherapy by inhibiting COX2-mediated PGE2 release. Int Immunopharmacol. . 2020;81:106206. doi: 10.1016/j.intimp.2020.106206. [DOI] [PubMed] [Google Scholar]
- 128.Dong M, Meng Z, Kuerban K, Qi F, Liu J, Wei Y, Wang Q, et al. Diosgenin promotes antitumor immunity and PD-1 antibody efficacy against melanoma by regulating intestinal microbiota. Cell Death Dis. . 2018;9:1039. doi: 10.1038/s41419-018-1099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Linkermann A, Stockwell BR, Krautwald S, Anders HJ. Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat Rev Immunol. . 2014;14:759–767. doi: 10.1038/nri3743. [DOI] [PubMed] [Google Scholar]
- 130.Turubanova VD, Balalaeva IV, Mishchenko TA, Catanzaro E, Alzeibak R, Peskova NN, Efimova I, et al. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J Immunother Cancer. . 2019;7:350. doi: 10.1186/s40425-019-0826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao L, Zhou X, Xie F, Zhang L, Yan H, Huang J, Zhang C, et al. Ferroptosis in cancer and cancer immunotherapy. Cancer Commun. . 2022;42:88–116. doi: 10.1002/cac2.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu S, Min J, Wang F. Ferroptosis: an emerging player in immune cells. Sci Bull. . 2021;66:2257–2260. doi: 10.1016/j.scib.2021.02.026. [DOI] [PubMed] [Google Scholar]
- 133.Song R, Li T, Ye J, Sun F, Hou B, Saeed M, Gao J, et al. Acidity-activatable dynamic nanoparticles boosting ferroptotic cell death for immunotherapy of cancer. Adv Mater. . 2021;33:2101155. doi: 10.1002/adma.202101155. [DOI] [PubMed] [Google Scholar]
- 134.Luo L, Xu G. Fascaplysin induces apoptosis and ferroptosis, and enhances anti-PD-1 immunotherapy in non-small cell lung cancer (NSCLC) by promoting PD-L1 expression. Int J Mol Sci. . 2022;23:13774. doi: 10.3390/ijms232213774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin Cancer Biol. . 2017;46:65–83. doi: 10.1016/j.semcancer.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 136.Lin R, Zhang Z, Chen L, Zhou Y, Zou P, Feng C, Wang L, et al. Dihydroartemisinin (DHA) induces ferroptosis and causes cell cycle arrest in head and neck carcinoma cells. Cancer Lett. . 2016;381:165–175. doi: 10.1016/j.canlet.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 137.Ooko E, Saeed MEM, Kadioglu O, Sarvi S, Colak M, Elmasaoudi K, Janah R, et al. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine. . 2015;22:1045–1054. doi: 10.1016/j.phymed.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 138.He L, Liu YY, Wang K, Li C, Zhang W, Li ZZ, Huang XZ, et al. Tanshinone IIA protects human coronary artery endothelial cells from ferroptosis by activating the NRF2 pathway. Biochem Biophys Res Commun. . 2021;575:1–7. doi: 10.1016/j.bbrc.2021.08.067. [DOI] [PubMed] [Google Scholar]
- 139.Liang Z, Currais A, Soriano-Castell D, Schubert D, Maher P. Natural products targeting mitochondria: emerging therapeutics for age-associated neurological disorders. Pharmacol Ther. . 2021;221:107749. doi: 10.1016/j.pharmthera.2020.107749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tan GY. Effect of cryptotanshinone on ferroptosis-related gene expression in lung cancer cells. Chinese Pharmacological Bulletin. 2019, 1654–1658
- 141.Wu CY, Yang YH, Lin YS, Chang GH, Tsai MS, Hsu CM, Yeh RA, et al. Dihydroisotanshinone I induced ferroptosis and apoptosis of lung cancer cells. Biomed Pharmacother. . 2021;139:111585. doi: 10.1016/j.biopha.2021.111585. [DOI] [PubMed] [Google Scholar]
- 142.Lin YS, Shen YC, Wu CY, Tsai YY, Yang YH, Lin YY, Kuan FC, et al. Danshen improves survival of patients with breast cancer and dihydroisotanshinone I induces ferroptosis and apoptosis of breast cancer cells. Front Pharmacol. . 2019;10:1226. doi: 10.3389/fphar.2019.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu WH, Li CH, Jiang TL. Ferroptosis pathway and its intervention regulated by chinese materia medica. Zhongguo Zhong Yao Za Zhi. 2018, 43: 4019–4026 . [DOI] [PubMed]
- 144.Zeng YY WJ, LuoYB. Research advances on anti-tumor effect of natural compounds throngh regulating ferroptosis. China Cancer. 2021, 30: 867-874
- 145.Zhang P, Shi L, Zhang T, Hong L, He W, Cao P, Shen X, et al. Piperlongumine potentiates the antitumor efficacy of oxaliplatin through ROS induction in gastric cancer cells. Cell Oncol. . 2019;42:847–860. doi: 10.1007/s13402-019-00471-x. [DOI] [PubMed] [Google Scholar]
- 146.Tripathi SK, Biswal BK. Piperlongumine, a potent anticancer phytotherapeutic: Perspectives on contemporary status and future possibilities as an anticancer agent. Pharmacol Res. . 2020;156:104772. doi: 10.1016/j.phrs.2020.104772. [DOI] [PubMed] [Google Scholar]
- 147.Zhang J, Wang N, Zhou Y, Wang K, Sun Y, Yan H, Han W, et al. Oridonin induces ferroptosis by inhibiting gamma‐glutamyl cycle in TE1 cells. Phytother Res. . 2021;35:494–503. doi: 10.1002/ptr.6829. [DOI] [PubMed] [Google Scholar]
- 148.Jin M, Shi C, Li T, Wu Y, Hu C, Huang G. Solasonine promotes ferroptosis of hepatoma carcinoma cells via glutathione peroxidase 4-induced destruction of the glutathione redox system. Biomed Pharmacother. . 2020;129:110282. doi: 10.1016/j.biopha.2020.110282. [DOI] [PubMed] [Google Scholar]
- 149.Wang Z, Ding Y, Wang X, Lu S, Wang C, He C, Wang L, et al. Pseudolaric acid B triggers ferroptosis in glioma cells via activation of Nox4 and inhibition of xCT. Cancer Lett. . 2018;428:21–33. doi: 10.1016/j.canlet.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 150.Gao Z, Deng G, Li Y, Huang H, Sun X, Shi H, Yao X, et al. Actinidia chinensis Planch prevents proliferation and migration of gastric cancer associated with apoptosis, ferroptosis activation and mesenchymal phenotype suppression. Biomed Pharmacother. . 2020;126:110092. doi: 10.1016/j.biopha.2020.110092. [DOI] [PubMed] [Google Scholar]
- 151.Wang M, Li S, Wang Y, Cheng H, Su J, Li Q. Gambogenic acid induces ferroptosis in melanoma cells undergoing epithelial-to-mesenchymal transition. Toxicol Appl Pharmacol. . 2020;401:115110. doi: 10.1016/j.taap.2020.115110. [DOI] [PubMed] [Google Scholar]
- 152.Lou JS, Zhao LP, Huang ZH, Chen XY, Xu JT, Tai WCS, Tsim KWK, et al. Ginkgetin derived from Ginkgo biloba leaves enhances the therapeutic effect of cisplatin via ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR wild-type non-small-cell lung cancer . Phytomedicine. . 2021;80:153370. doi: 10.1016/j.phymed.2020.153370. [DOI] [PubMed] [Google Scholar]
- 153.Huang S, Cao B, Zhang J, Feng Y, Wang L, Chen X, Su H, et al. Induction of ferroptosis in human nasopharyngeal cancer cells by cucurbitacin B: molecular mechanism and therapeutic potential. Cell Death Dis. . 2021;12:237. doi: 10.1038/s41419-021-03516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tsai Y, Xia C, Sun Z. The inhibitory effect of 6-gingerol on ubiquitin-specific peptidase 14 enhances autophagy-dependent ferroptosis and anti-tumor in vivo and in vitro . Front Pharmacol. . 2020;11:598555. doi: 10.3389/fphar.2020.598555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lin PL, Tang HH, Wu SY, Shaw NS, Su CL. Saponin Formosanin C-induced ferritinophagy and ferroptosis in human hepatocellular carcinoma cells. Antioxidants. . 2020;9:682. doi: 10.3390/antiox9080682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. . 2008;15:234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hong Z, Tang P, Liu B, Ran C, Yuan C, Zhang Y, Lu Y, et al. Ferroptosis-related genes for overall survival prediction in patients with colorectal cancer can be inhibited by gallic acid. Int J Biol Sci. . 2021;17:942–956. doi: 10.7150/ijbs.57164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xiaofei J, Mingqing S, Miao S, Yizhen Y, Shuang Z, Qinhua X, Kai Z. Oleanolic acid inhibits cervical cancer Hela cell proliferation through modulation of the ACSL4 ferroptosis signaling pathway. Biochem Biophys Res Commun. . 2021;545:81–88. doi: 10.1016/j.bbrc.2021.01.028. [DOI] [PubMed] [Google Scholar]
- 159.Guo J, Song Z, Yu J, Li C, Jin C, Duan W, Liu X, et al. Hepatocyte-specific TMEM16A deficiency alleviates hepatic ischemia/reperfusion injury via suppressing GPX4-mediated ferroptosis. Cell Death Dis. . 2022;13:1072. doi: 10.1038/s41419-022-05518-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li C, Zhu X, Chen J, Xie X, Liang S, Liu X, Gong Q, et al. Multifaceted role of ferroptosis in cardiovascular disease. Acta Biochim Biophys Sin. 2023, 55: 183–193 . [DOI] [PMC free article] [PubMed]
- 161.Min J, Wu L, Liu Y, Song G, Deng Q, Jin W, Yu W, et al. Empagliflozin attenuates trastuzumab-induced cardiotoxicity through suppression of DNA damage and ferroptosis. Life Sci. . 2023;312:121207. doi: 10.1016/j.lfs.2022.121207. [DOI] [PubMed] [Google Scholar]