Abstract
We hereby make the first report of a case of mycosis caused by Purpureocillium lilacinum in CARD9 deficiency. A 40-year-old woman complained of lymph node swellings in the left cervical area. She also had chronic mucocutaneous candidiasis (CMC), and was found to have CARD9 deficiency. Lymphadenitis by P. lilacinum was confirmed. The diagnosis was difficult, as culturing the biopsy specimen at a cautiously selected temperature (25 °C) and genetic analysis were both required. Oral administration of voriconazole improved her lymphadenopathy.
Keywords: Purpureocillium lilacinum, Lymphadenitis, CARD9 deficiency
1. Introduction
CARD9 deficiency is an autosomal recessive disorder that leads to a patient's high susceptibility to fungal disease caused by a wide variety of pathogens. The patient suffers from repeated superficial candidiasis as well as some deep-seated mycoses, which are sometimes life-threatening [1]. In addition to Candida spp. and Aspergillus spp., which are major causative pathogens for fungal diseases, the causative fungi in CARD9 deficiency also include ‘non-major’ molds such as Exophiala dermatitidis and Phialophola verrucosa [2]. However, detailed information on the spectrum of the causative pathogens remains largely unavailable, and infection by Purpureocillium lilacinum has yet to be reported.
Culturing of the fungus and histopathological examinations often play a critical role in the diagnosis of fungal infection. However, in infections by P. lilacinum, which is listed as a ‘rare mold,’ the fungus that grew from clinical samples tends to be mistaken for a contaminant [3]. Another reason for the difficulty in its diagnosis lies in the histopathological findings. While filamentous fungi usually take on a hyphal form in aspergillosis and mucormycosis, P. lilacinum presents several forms including globose yeast-like structures, and as a result the fungus is sometimes mistaken for other molds [4].
In addition, the treatment of mycoses by P. lilacinum remains generally difficult because of their low sensitivity to a number of antifungal agents [4]. It is of primary importance therefore to identify the causative agent quickly and begin treatment in a timely manner.
In the present report, we describe a case of lymphadenitis caused by P. lilacinum in a patient with CARD9 deficiency. The hurdle facing the diagnosis of this infection was mostly the result of the particular requirement of the growth temperature and the misleading morphology on the histopathological examination of the fungus. Careful selection of the culturing condition and genetic analysis of the biopsied sample were required.
2. Case presentation
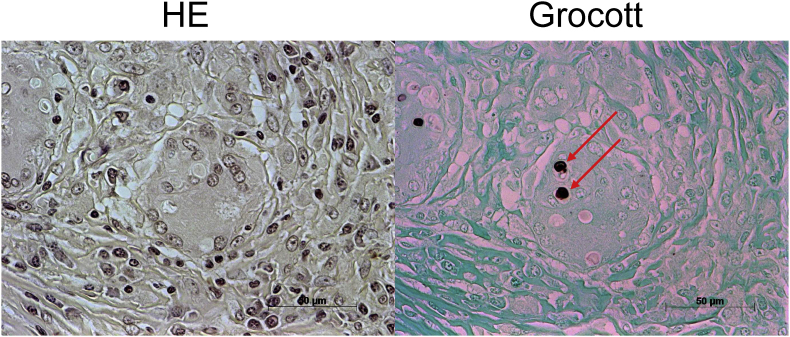
A 40-year-old woman attended our hospital on day 0, complaining of left cervical and supraclavicular lymphadenopathy. Her medical history was that, at the age of 18, she had enlarged nodes on her left neck, which then subsided spontaneously. At the age of 35, she had more enlarged nodes and visited a hospital. She had a lymph node in the cervical area biopsied on day −134, and histopathological examination showed no sign of malignant neoplasms but indicated granulomatous lymphadenitis with yeast-like structures (Fig. 1). There was no microbial or fungal growth.
Fig. 1.
Histopathological examination of resected lymph node shows granulomatous lymphadenitis with yeast-like pathogen (hematoxylin and eosin (HE) and Grocott methanamine silver stainings, 400 ×).
Although she had no significant family history, it was revealed that she had recurrent episodes of oral, nail and vaginal candidiasis from the age of six months (chronic mucocutaneous candidiasis (CMC)). Her candidiasis was cured by oral or transvaginal administration of azole for one or two weeks each time. Although no prophylaxis or follow-up had been carried out before, this time she was suspected of having some kind of innate immune deficiency. On day −21, genetic diagnosis revealed a homozygous frameshift mutation in the CARD9 region (c.820dup, p.Asp274fsTer61), which had been reported to be related to a higher susceptibility to fungi [5,6].
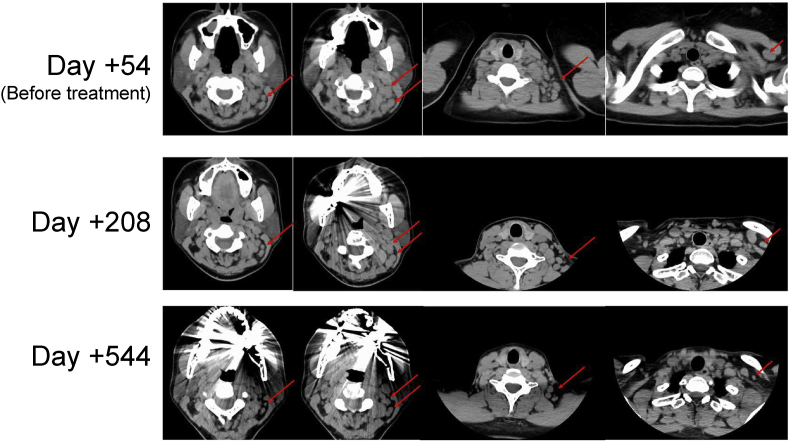
Because of the growing lymph nodes, she was then referred to our hospital for more detailed examinations of fungal lymphadenitis. The physical examination revealed cervical and supraclavicular lymph node swellings on the left side. Blood examination showed elevation of 1,3-β-D glucan (677.3pg/ml) and Aspergillus galactomannan antigen (4.7), while the values of neither soluble interleukin-2 receptor nor lysozyme in her serum were elevated. Interferon-gamma release assay (IGRA) was negative. Chest X-rays revealed swelling of left supraclavicular nodes, and CT scans showed enlarged lymph nodes in the upper-jugular, occipital, posterior-triangle, and supraclavicular areas (Fig. 2). Because CARD9-deficient patients are susceptible to fungi including Candida spp., Trichophyton spp., Phialophora spp. and Exophiala spp. [2], we suspected that she might also be suffering from fungal lymphadenitis. To identify the microbe causing her lymphadenitis, biopsy of the upper jugular lymph node was performed again on day +34.
Fig. 2.
Before the administration of voriconazole, lymph node swellings in left cervical and supraclavicular areas were detected by CT scanning. The size of the lymph nodes then gradually decreased following the initial treatment.
The resected lymph node was cultured in potato dextrose agar medium at 37 °C, but no fungi grew. We then cultured a sample at 25 °C, as some causative fungi of superficial mycoses are known to grow only at a lower temperature, and filamentous fungi were observed to grow. For its genetic identification, DNA was extracted by urea-phenol method and sequencing of the internal transcribed spacer region of the rRNA gene with the ITS1 and ITS4 primers [7], the data for which are available from DDBJ Sequenced Read Archive (accession number (LC774228)). Based on the BLAST search (https://www.ncbi.nlm.nih.gov/search/all/?term=BLAST) of the sequence in the ITS region, homology with the type strain of P. lilacinum NRRL 895 (GenBank accession: NR195946) was 99.8% (578/579 bp). Thus, the identification of P. lilacinum was made by phylogeny. Genetic analysis of the DNA, which was extracted from a pathological specimen with High Pure PCR Template Preparation Kit (Roche Diagnostics GmbH, Mannheim, Germany), also confirmed the presence of P. lilacinum. Based on these results, we arrived at a diagnosis of fungal lymphadenitis caused by P. lilacinum. The isolate was preserved as IFM 67776 at the Medical Mycology Research Center, Chiba University via the National Bio-Resource Project, Japan.
The antifungal susceptibility test was carried out based on the Clinical and Laboratory Standards Institute (CLSI) M38-Ed3 broth microdilution method, with slight modifications as described previously [8,9]. The 96-well plates were cultured at 30 °C instead of 35 °C because the pathogen failed to grow at 35 °C in potato dextrose agar medium. Since they grew slowly even at 30 °C, we evaluated the minimal effective concentrations (MECs) for echinocandins at 48hr and the minimal inhibitory concentration (MICs) for the other antifungal drugs at 72hr. The result showed that the MIC of voriconazole (VRCZ) was 2 μg/ml, which was lower when compared to other antifungals, although any breakpoints were not defined for P. lilacinum (Table 1).
Table 1.
Minimal effective concentrations (MECs) and minimal inhibitory concentrations (MICs) of Purpureocillium lilacinum isolated from the patient. MECs and MICs were evaluated at 48 hr and 72 hr, respectively.
| MCFG | CSFG | AMPH-B | 5-FC | FLCZ | ITCZ | VRCZ | |
|---|---|---|---|---|---|---|---|
| MEC/MIC (μg/ml) | 0.03 | 1 | >16 | >64 | 64 | >8 | 2 |
Abbreviations: MCFG, micafungin; CSFG, caspofungin; AMPH-B, amphotericin B; 5-FC, 5-fluorocytosine; FLCZ, fluconazole; ITCZ, itraconazole; VRCZ, voriconazole.
Based on these test results, antifungal treatment with VRCZ was begun on Day +61. Considering the side effects of the drug, we started voriconazole at 100mg/day and gradually increased its dose to 500mg/day while checking its blood trough concentration. Physical examination and CT scans then showed a gradual reduction in the size of lymph nodes both on the left side of the neck and the supraclavicular fossa (on Day +208, Fig. 2), and serum 1,3-β-D glucan and Aspergillus galactomannan antigen levels, which were thought to be elevated by the P. lilacinum infection, gradually decreased to 653.60pg/ml and 3.4, respectively (six months after initial treatment, on Day +229).
One year later (on day +544), continuing the same treatment, the patient was in stable condition, and CT scans showed greater reduction of the lymph nodes. The serum biomarkers continued to decrease (1,3-β-D glucan (323.60pg/ml) and Aspergillus galactomannan antigen levels (0.3)).
3. Discussion
To our knowledge, this is the first case report of hyalohyphomycosis by P. lilacinum in a CARD9-deficient patient. Careful selection of the culturing condition and genetic analysis of the biopsied sample enabled us to detect the causative pathogen.
CARD9 deficiency is an autosomal recessive disorder that leads to high susceptibility to certain fungi. Some patients with CARD9 deficiency have been reported to have characteristic CMC, followed by invasive fungal diseases [10]. The episodes of CMC in the present patient enabled us to consider CARD9 deficiency as an underlying disease and suspect fungus as a causative pathogen. Since the mortality rate of CARD9 deficient patients who have invasive fungal diseases is reportedly high (>20%) [1], it is important to diagnose the immune deficiency at an early stage, stay on high alert for fungal infections, and start appropriate treatment as soon as possible.
The species that cause fungal infection in this disorder are unique. Generally, in immunocompromised patients, such as in hematological malignancies and patients receiving strongly immunosuppressive agents, the causative fungi consist of Aspergillus spp., Candida spp., Cryptococcus spp., and Mucorales. In contrast, in CARD9 deficiency, the causative agents of the mycoses are not limited to these major pathogens, but also include other molds such as Tricophyton spp., Phialophora verrucosa [2], Exophilala spp. [1], and Trichosporon asahii [11].
Although no report has been presented on P. linacium infection in the literature, our case indicates that P. linacium should also be considered as a possible cause of this congenital disease. P. lilacinum, also previously known as Paecilomyces lilacinus, is an ascomycetous fungus. P. linacinum is ubiquitous in the environment and is widely found in soil and decaying material [4]. Its pathogenicity is not particularly high. It causes superficial fungal infections such as keratitis, but sometimes also causes deep-seated mycoses like endocarditis especially in immunocompromised patients [12]. This indicates that the fungus has the potential to invade human tissue subcutaneously and enter deeply into internal organs under certain conditions.
The relationship between the mutation type in the CARD9 region and the causative pathogens remains to be understood. In the present case, genetic diagnosis revealed that the patient carried c.820dup, p.Asp274fsTer61 in the CARD9 region, which mediates the production of signaling molecules such as NF-κB and MAPK in its downstream and is involved in regulating inflammatory responses [13]. Although reports on opportunistic infections based on this particular genetic mutation are limited, Exophiala dermatitidis [5] and Phialophora verrucosa [6] were reported to cause invasive fungal infections in patients with the same genetic mutation. Whether this mutation is responsible for particularly high susceptibility to certain fungal species including P. linacinum will need to be elucidated in the future.
Although microbiological and histopathological examinations are critical for the diagnosis of fungal infections, the histopathological findings in our case were very confusing. Most filamentous fungi take on a hyphal form in human tissues. However, in infections by P. lilacinum, which is also a filamentous fungus, histopathological examination often shows a yeast-like pathogen [4], which can be mistaken as Candida spp. or Cryptococcus spp. In our case, filamentous fungus grew at 25 °C after a yeast-like pathogen was found in the histopathological examination. This puzzling discrepancy made us suspect that the filamentous fungus might be a contaminant, leading us to conduct a genetic analysis of the biopsied specimen for the final diagnosis, which revealed that the filamentous fungus was not a contaminant but a true pathogen.
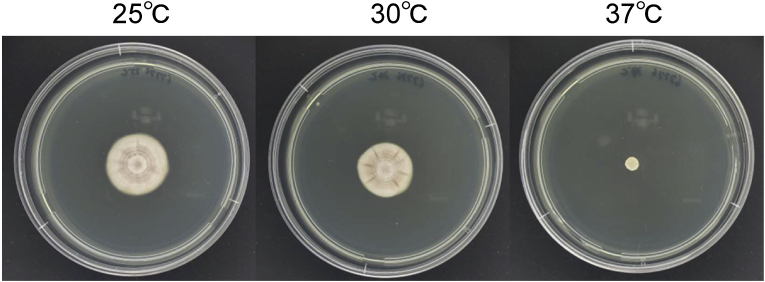
The “no growth” result for the microbiology was another problem in the diagnosis. Regular microbiological laboratories set the incubator temperature at 35–37 °C for the culturing of fungi, as this temperature is appropriate for the growth of Aspergillus spp. and Candida spp. However, P. linacinum is delicate in regard to the culturing temperature, as it usually grows at a range of 25–30 °C, and isolates of P. linacinum often fail to grow at 37 °C [14]. In fact, the isolate in our case finally started to grow when cultured at 25 °C (Fig. 3). These difficulties in detecting this pathogen suggest that the number of mycoses caused by P. lilacinum might have been underestimated.
Fig. 3.
At 7 days after inoculation with potato dextrose agar medium, the Purpureocillium lilacinum strain isolated from this patient was well cultured at 25 °C and 30 °C, but it failed to grow well at 37 °C.
Information concerning the treatment for P. lilacinum is limited. Sprute et al. reported that the combination of amphotericin B with an azole and the monotherapy of VRCZ were frequently used for its treatment [4]. In our case, oral administration of VRCZ effectively reduced the size of swollen lymph nodes and lowered the biomarker levels. The low MIC of this isolate to VRCZ was consistent with the data of several reports showing that P. lilacinum clinical isolates have low MICs to VRCZ and posaconazole [4,15]. Although the breakpoint has yet to be established [4,15], ECMM and ISHAM recommend VRCZ as a first-line therapy in Global Guideline for the Diagnosis and Management of Rare Mold Infections [3].
The lack of biomarkers also poses a challenge for the diagnosis and treatment of P. lilacinum infection. Our case suggests that 1,3-β-D glucan and Aspergillus galactomannan can be used as biomarkers of the infection. 1,3-β-D glucan is rich in cell walls of various fungal species, and it is widely used for the diagnosis of invasive fungal diseases [[16], [17], [18]]. Since the cell wall of P. linacinum is also considered to be rich in glucan, it seems reasonable that the beta glucan level is elevated in this fungal disease. Aspergillus galactomannan, which was also significantly elevated in our case, has been reported to have cross-reactivity with many fungal pathogens, such as Histoplasma spp [19]. and Fusarium spp [20]. In our case, there was no sign to suggest that invasive aspergillosis or other mycoses were present, and the elevation of galactomannan was apparently caused by the P. lilacinum infection. Furthermore, the levels of 1,3-β-D glucan and galactomannan were well correlated with the clinical course, which responded favorably to the treatment. Galactomannan, in particular, decreased quickly when VRCZ administration was begun, and this may have been caused by its rapid clearance from circulation [16].
In conclusion, we present the first report of a case of fungal infection with P. lilacinum in a CARD9-deficient patient. Rare molds such as P. lilacinum are sometimes difficult to recognize as causes, and they might be ignored both in culture and in histopathological examinations. Careful examinations should be carried out so as not to overlook the correct pathogen.
Declaration of competing interest
There were no conflicts of interest to declare.
Acknowledgements
The manuscript was technically edited by Mr. Arndt Gerz prior to submission.
Handling Editor: Dr Adilia Warris
References
- 1.Imanaka Y., Taniguchi M., Doi T., Tsumura M., Nagaoka R., Shimomura M., et al. Inherited CARD9 deficiency in a child with invasive disease due to Exophiala dermatitidis and two older but asymptomatic siblings. J. Clin. Immunol. 2021;41(5):975–986. doi: 10.1007/s10875-021-00988-7. [DOI] [PubMed] [Google Scholar]
- 2.Corvilain E., Casanova J.L., Puel A. Inherited CARD9 deficiency: invasive disease caused by Ascomycete fungi in previously healthy children and adults. J. Clin. Immunol. 2018;38(6):656–693. doi: 10.1007/s10875-018-0539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoenigl M., Salmanton-García J., Walsh T.J., Nucci M., Neoh C.F., Jenks J.D., et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European confederation of medical Mycology in cooperation with the International society for Human and animal Mycology and the American society for Microbiology. Lancet Infect. Dis. 2021;21(8):e246–e257. doi: 10.1016/S1473-3099(20)30784-2. [DOI] [PubMed] [Google Scholar]
- 4.Sprute R., Salmanton-García J., Sal E., Malaj X., Ráčil Z., Ruiz de Alegría Puig C., et al. Invasive infections with Purpureocillium lilacinum: clinical characteristics and outcome of 101 cases from FungiScope® and the literature. J. Antimicrob. Chemother. 2021;76(6):1593–1603. doi: 10.1093/jac/dkab039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling J., Pu Y., Gan J., Zhou W., Chen X., Zhang Q., et al. CARD9 deficiency in combination with invasive infection by Exophiala dermatitidis in a pediatric patient. Mycopathologia. 2022;187(2–3):299–303. doi: 10.1007/s11046-022-00628-7. [DOI] [PubMed] [Google Scholar]
- 6.Wang X., Zhang R., Wu W., Song Y., Wan Z., Han W., et al. Impaired specific antifungal immunity in CARD9-deficient patients with phaeohyphomycosis. J. Invest. Dermatol. 2018;138(3):607–617. doi: 10.1016/j.jid.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 7.White T.J.B.T., Lee S.B., Taylor J.W. In: PCR Protocols: A Guide to the Methods and Application. Innis M.A., Gelfan D.H., Sninsky J.J., White T.J., editors. Academic Press; San Diego: 1990. Amplification and direct sequencing of fungal ribosomal DNA for phylogenetics; pp. 315–322. [Google Scholar]
- 8.Arai T., Majima H., Watanabe A., Kamei K. A simple method to detect point mutations in Aspergillus fumigatus cyp51A gene using a Surveyor Nuclease assay. Antimicrob. Agents Chemother. 2020;64(4) doi: 10.1128/AAC.02271-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute . third ed. M38. CLSI; Wayne, PA, USA: 2017. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. [Google Scholar]
- 10.Glocker E.O., Hennigs A., Nabavi M., et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 2009;361(18):1727–1735. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Liu B., Zhang Y., et al. Disseminated trichosporosis in a young patient with CARD9 deficiency. Clin. Microbiol. Infect. 2022;28(5):681–683. doi: 10.1016/j.cmi.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Khalique Z., Hatipoğlu S., Rosendahl U., Mohiaddin R. Unusual complicated fungal endocarditis in a patient with vascular Ehlers-Danlos syndrome. Ann. Thorac. Surg. 2019;107(4):e269–e271. doi: 10.1016/j.athoracsur.2018.08.074. [DOI] [PubMed] [Google Scholar]
- 13.Liu X., Jiang B., Hao H., Liu Z. CARD9 signaling, inflammation, and diseases. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.880879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domsch WG K.H., Anderson Tarute-Heidi. second ed. IHW-Verlag Press; 2007. Compendium of Soil Fungi. [Google Scholar]
- 15.Corrêa-Moreira D., de Lima Neto R.G., da Costa G.L., de Moraes Borba C., Oliveira M.M.E. Purpureocillium lilacinum an emergent pathogen: antifungal susceptibility of environmental and clinical strains. Lett. Appl. Microbiol. 2022;75(1):45–50. doi: 10.1111/lam.13707. [DOI] [PubMed] [Google Scholar]
- 16.Malcolm D., Richardson D.W.W. fourth ed. Wiley-Black Well; 2012. Fungal Infection. [Google Scholar]
- 17.Donnelly J.P., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P.E., et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for Research and treatment of cancer and the mycoses study group education and Research consortium. Clin. Infect. Dis. 2020;71(6):1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theel E.S., Doern C.D. β-D-glucan testing is important for diagnosis of invasive fungal infections. J. Clin. Microbiol. 2013;51(11):3478–3483. doi: 10.1128/JCM.01737-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Min Z., Baddley J.W., Rodriguez J.M., Moser S.A., Patel M. Cross-reactivity of Aspergillus galactomannan in an HIV-infected patient with histoplasmosis. Med Mycol Case Rep. 2012;1(1):119–122. doi: 10.1016/j.mmcr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tortorano A.M., Esposto M.C., Prigitano A., Grancini A., Ossi C., Cavanna C., et al. Cross-reactivity of Fusarium spp. in the Aspergillus Galactomannan enzyme-linked immunosorbent assay. J. Clin. Microbiol. 2012;50(3):1051–1053. doi: 10.1128/JCM.05946-11. [DOI] [PMC free article] [PubMed] [Google Scholar]