Abstract
Serpentine cord formation in BACTEC 12B medium was evaluated as a rapid method for the presumptive identification of M. tuberculosis complex. Kinyoun acid-fast stained smears were prepared from 666 positive BACTEC 12B bottles and examined for the presence or absence of serpentine cording. Cord formation had a sensitivity, specificity, positive predictive value, and negative predictive value of 89.2, 99.2, 98.5, and 94.2%, respectively. The evaluation of the presence of cord formation in BACTEC 12B medium is reliable and permits the rapid presumptive reporting of M. tuberculosis.
The resurgence of tuberculosis in the United States has prompted recommendations from the Centers for Disease Control and Prevention (CDC) and others that emphasize the need to identify infectious patients rapidly (1, 9). Since the prompt availability of laboratory results is crucial to the proper management of tuberculosis patients, the recommendations have included the use of liquid medium for primary culture and the most rapid methods available for the identification of Mycobacterium tuberculosis. These rapid identification methods include the p-nitro-α-acetylamino-β-hydroxypropiophenone (NAP) test, DNA probes, or high-performance liquid chromatography (HPLC) analysis of mycolic acids. However, for many laboratories, economic pressures have precluded the use of these costly, commercially available identification procedures. Furthermore, although these rapid methods identify M. tuberculosis more quickly than conventional biochemicals (3, 4, 8), additional time is still required from the detection of a positive culture to the identification of an isolate (2–4).
A cost-effective method for the rapid presumptive identification of M. tuberculosis would be useful to all laboratories, regardless of the rapid identification method ultimately used for final organism identification. M. tuberculosis, when grown in liquid medium, often displays characteristic serpentine cording (6). Cord formation has been advocated as a guide for the cost-effective utilization of DNA probes for the identification of Mycobacterium species (5), but to date only two studies have evaluated the utility of cord formation for the presumptive identification of M. tuberculosis (7, 10). These studies yielded discordant data: the sensitivity of cord formation for the identification of M. tuberculosis was 22.9% in one study versus 88.3% in the other.
In view of the conflicting data published to date, the objective of the present study was to determine the reliability of serpentine cording in BACTEC 12B medium as a rapid method to report the presumptive identification of M. tuberculosis.
A total of 666 positive mycobacterial cultures from 344 patients were evaluated for the presence or absence of cording in BACTEC 12B vials from January 1993 through March 1997. Clinical specimens included 556 respiratory secretions (sputum, tracheal aspirate, and bronchoscopy samples), 48 stool samples, 16 bone marrow samples, 27 tissue samples, 4 wound samples, and 15 fluid samples. Blood specimens were not included because they are not routinely processed in BACTEC 12B bottles. Contaminated specimens were digested and decontaminated with N-acetyl-l-cysteine (Bristol Laboratories, Evansville, Ind.) and 2% NaOH (Ricca Chemical, Arlington, Tex.) for 15 min. The reaction was stopped by the addition of phosphate buffer, and the specimens were centrifuged at 3,000 × g for 15 min. Tissues and normally sterile body fluids were inoculated directly into the media. Each specimen was inoculated into one BACTEC 12B bottle (Becton Dickinson, Sparks, Md.) and one Middlebrook 7H11 slant (Becton Dickinson, Cockeysville, Md.). BACTEC bottles were incubated at 35°C and monitored for 6 weeks. Slants were incubated at 35°C in 5 to 10% CO2 and observed weekly for 8 weeks.
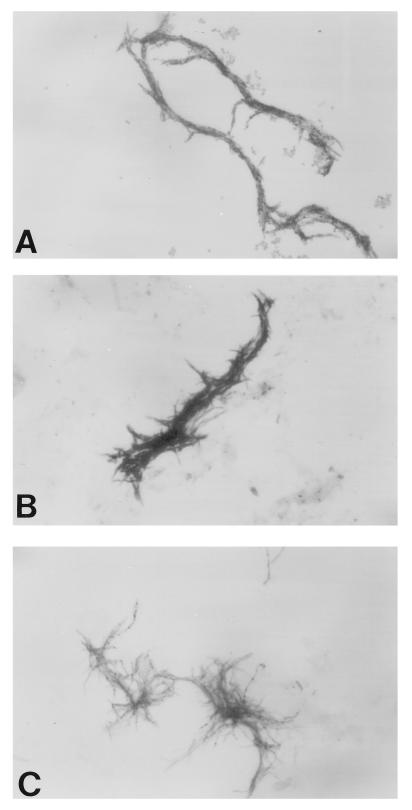
BACTEC 12B bottles with growth index values of ≥100 were vortexed briefly. Several drops of broth were smeared onto both rings of a two-ring glass slide, allowed to air dry completely, and stained with Kinyoun acid-fast stain. All smears were examined by a technologist and a supervisor for the presence or absence of acid-fast bacilli in serpentine cords. Serpentine cording was defined as tight, rope-like aggregates of acid-fast bacilli in which the long axes of the bacteria parallel the long axis of the cord (Fig. 1A). Microscopic morphology and organism orientation that did not meet the above criteria were considered negative for cording (Fig. 1B and C). Smears with only a few organisms on the slide were not evaluated for the presence of cording due to the insufficient quantity of organisms present. The presence or absence of cording was correlated with the final organism identification as determined by DNA probes or HPLC of mycolic acids by a reference laboratory.
FIG. 1.
Microscopic morphology of Mycobacterium species grown in BACTEC 12B broth and stained with Kinyoun acid-fast stain (magnification, ×900). (A) M. tuberculosis, exhibiting serpentine cording. (B) Mycobacterium species other than M. tuberculosis that exhibit loose aggregates, referred to as pseudocording. (C) Mycobacterium species other than M. tuberculosis that exhibit loosely massed organisms.
Kinyoun-stained smears from 666 positive BACTEC 12B bottles were examined for the presence of serpentine cording. A total of 50 (7.5%) smears were not evaluated for cording due to an insufficient number of organisms on the slide. These cultures had growth value indices that ranged from 102 to 999 and a median growth value index of 165. The remaining 616 positive cultures yielded 625 Mycobacterium isolates, including 223 (35.7%) isolates of M. tuberculosis and 402 (64.3%) isolates of Mycobacterium species other than M. tuberculosis (Table 1). A total of 202 smears demonstrated cording, and 414 did not (Table 1). A total of 24 of the 223 (10.8%) M. tuberculosis isolates did not exhibit cording. Of those 24 cultures, 13 (54.2%) were from patients from whom M. tuberculosis had been isolated previously, and the previous isolates had exhibited cording. In addition, 9 (37.5%) of the bottles achieved a growth index of ≥100 in ≤7 days. Thus, some specimens may not have incubated for a sufficient length of time to permit cord formation to develop. A total of 3 of the 202 (1.5%) bottles that were interpreted as cording positive were subsequently identified as either M. gordonae (2 cultures) or M. avium complex (1 culture). The medical records of these three patients were reviewed, and it was determined that, in these three instances, the false-positive result did not adversely impact patient care. In the two cases in which M. gordonae was subsequently identified, the patients were admitted with other respiratory diagnoses and three sputum specimens obtained for mycobacterial culture were smear negative. In both of these cases, the patients were discharged prior to culture positivity. For the third patient, for whom M. avium was eventually identified in a stool specimen, the three sputum and three stool specimens received for mycobacterial culture were smear negative. Clinical findings did not support a diagnosis of tuberculosis for this patient. The sensitivity, specificity, positive predictive value, and negative predictive value of cording for the presumptive identification of M. tuberculosis for all specimen types were 89.2, 99.2, 98.5, and 94.2%, respectively.
TABLE 1.
Mycobacteria isolated from positive BACTEC 12B cultures
| Species | No. (%) of cultures | Cord formation
|
|
|---|---|---|---|
| No. positive (%) | No. negative (%) | ||
| M. tuberculosis | 221 (35.9) | 198 (98.0) | 23 (5.6) |
| M. avium complex | 210 (34.1) | 1 (0.5) | 209 (50.5) |
| M. gordonae | 134 (21.8) | 2 (1.0) | 132 (31.9) |
| M. xenopi | 17 (2.8) | 0 | 17 (4.1) |
| M. kansasii | 10 (1.6) | 0 | 10 (2.4) |
| M. chelonae | 5 (0.8) | 0 | 5 (1.2) |
| M. fortuitum | 5 (0.8) | 0 | 5 (1.2) |
| M. marinum | 3 (0.5) | 0 | 3 (0.7) |
| M. scrofulaceum | 1 (0.2) | 0 | 1 (0.2) |
| M. genavense | 1 (0.2) | 0 | 1 (0.2) |
| M. avium complex and M. gordonae | 5 (0.8) | 0 | 5 (1.2) |
| M. tuberculosis and M. gordonae | 2 (0.3) | 1 (0.5) | 1 (0.2) |
| M. avium complex and M. chelonae | 2 (0.3) | 0 | 2 (0.5) |
| Total | 616 (100) | 202 (100) | 414 (100) |
The mycobacteriology laboratory plays a pivotal role in the control of tuberculosis through the rapid detection, isolation, and identification of Mycobacterium species. In an attempt to lower the incidence of tuberculosis transmission, the CDC has recommended the use of rapid identification methods (1). However, for many institutions, the implementation of these recommendations would require a significant expenditure of financial resources. The reporting of a presumptive identification of M. tuberculosis based on the presence of serpentine cording could potentially decrease laboratory turn-around time for result reporting without an increase in cost, since a smear must be made from positive BACTEC 12B bottles to determine if acid-fast organisms are present. In this study, the sensitivity of cording for the presumptive identification of M. tuberculosis was approximately 90%. This sensitivity is consistent with the results reported by Yagupsky (10) and considerably higher than the 22.9% sensitivity observed by Morris and Reller (7). In both the present study and the study by Morris and Reller, the BACTEC 12B bottles were vortexed prior to smear preparation. However, in the Morris and Reller study, the bottles were smeared when the growth index was ≥50. Thus, the bottles may have contained too few organisms to be evaluated for the presence of cord formation. In the present study, smears with a number of organisms insufficient for evaluation of the presence of cording were often from bottles with a low growth index (between 100 and 200). In this study, 10.8% of M. tuberculosis isolates did not exhibit cording. This is consistent with the findings of Yagupsky and colleagues (10). However, the positive and negative predictive values of cord formation remained high at 98.5 and 94.2%, respectively. In this study, the proportion of positive cultures with M. tuberculosis was high at 36.2%. Since some laboratories may not have such a large proportion of M. tuberculosis isolates, the positive and negative predictive values were recalculated by using 1, 5, 15, and 25% prevalences of M. tuberculosis among positive cultures. With these prevalences, the positive predictive values were 53.0, 85.4, 95.2, and 97.4%, respectively. The negative predictive values were 99.9, 99.4, 98.1, and 96.5%, respectively. In laboratories with a small proportion (1%) of positive M. tuberculosis cultures, the positive predictive value of cording for the identification of M. tuberculosis would be unacceptably low. However, the negative predictive value would remain high. Despite the occurrence of cording with two M. gordonae isolates and one M. avium complex isolate, the specificity of cording was 99.2%. Cording has previously been observed with M. kansasii, M. avium complex, M. szulgai, and M. chelonae (5, 10). In some instances, Mycobacterium species other than M. tuberculosis produce loose, incomplete “pseudocords” (Fig. 1B) that may be misinterpreted as true cording by the inexperienced microscopist. It should be noted that the recognition of serpentine cord formation in our laboratory improved during the course of the study. All three of the cording nontuberculosis mycobacteria were encountered early in the study. It is also important to recognize that smears with only a few organisms may not exhibit cord formation simply because of the paucity of organisms present. These smears should not be considered negative for cording. Reincubation of the bottle or concentration of the broth may aid in the demonstration of cording.
Based on the high positive and negative predictive values observed in this study, cord formation in BACTEC 12B medium is a reliable criterion for the presumptive identification of M. tuberculosis complex. In many laboratories, final identification procedures, such as DNA probes and HPLC, are batched to maximize cost effectiveness. In addition, the NAP test routinely requires a testing time of 5 days. Regardless of the method used to obtain a final identification of a Mycobacterium species, the examination of microscopic morphology in BACTEC 12B broth is a useful first step in the identification procedure. The evaluation of cording provides rapid preliminary information before the results of other identification methods are available. As a result of this study, our laboratory reports a presumptive identification of M. tuberculosis complex based on cord formation to physicians. The rapid and accurate detection of patients with tuberculosis has a significant impact on the care and treatment of the patient and on disease prevention. The evaluation of the presence of cord formation in BACTEC 12B medium is reliable and permits the rapid presumptive reporting of M. tuberculosis complex prior to the availability of results from other identification methods.
REFERENCES
- 1.Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities, 1994. Morbid Mortal Weekly Rep. 1994;43:24–25. , 64–65. [PubMed] [Google Scholar]
- 2.Chapin-Robertson K, Dahlberg S, Waycott S, Corrales J, Kontnick C, Edberg S C. Detection and identification of Mycobacterium directly from BACTEC bottles by using a DNA-rRNA probe. Diagn Microbiol Infect Dis. 1993;17:203–207. doi: 10.1016/0732-8893(93)90097-q. [DOI] [PubMed] [Google Scholar]
- 3.Christie J E, Callihan D R. The laboratory diagnosis of mycobacterial diseases: challenges and common sense. Clin Lab Med. 1995;15:279–306. [PubMed] [Google Scholar]
- 4.Jost K C, Jr, Dunbar D F, Barth S S, Headley V L, Elliott L B. Identification of Mycobacterium tuberculosis and M. avium complex directly from smear-positive sputum specimens and BACTEC 12B cultures by high-performance liquid chromatography with fluorescence detection and computer-driven pattern recognition models. J Clin Microbiol. 1995;33:1270–1277. doi: 10.1128/jcm.33.5.1270-1277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaminski D A, Hardy D J. Selective utilization of DNA probes for identification of Mycobacterium species on the basis of cord formation in primary BACTEC 12B cultures. J Clin Microbiol. 1995;33:1548–1550. doi: 10.1128/jcm.33.6.1548-1550.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Middlebrook G, Dubos R J, Pierce C. Virulence and morphological characteristics of mammalian tubercule bacilli. J Exp Med. 1947;86:175–184. doi: 10.1084/jem.86.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris A J, Reller L B. Reliability of cord formation in BACTEC media for presumptive identification of mycobacteria. J Clin Microbiol. 1993;31:2533–2534. doi: 10.1128/jcm.31.9.2533-2534.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfaller M A. Application of new technology to the detection, identification, and antimicrobial susceptibility testing of mycobacteria. Am J Clin Pathol. 1994;101:329–337. doi: 10.1093/ajcp/101.3.329. [DOI] [PubMed] [Google Scholar]
- 9.Tenover F C, Crawford J T, Huebner R E, Geiter L J, Horsburgh C R, Jr, Good R C. The resurgence of tuberculosis: is your laboratory ready? J Clin Microbiol. 1993;31:767–770. doi: 10.1128/jcm.31.4.767-770.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yagupsky P V, Kaminski D A, Palmer K M, Nolte F S. Cord formation in BACTEC 7H12 medium for rapid, presumptive identification of Mycobacterium tuberculosis complex. J Clin Microbiol. 1990;28:1451–1453. doi: 10.1128/jcm.28.6.1451-1453.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]