Summary
Laser irradiation is a powerful tool in inducing changes in lattice structures and properties of two-dimensional (2D) materials through processes such as heating, bleaching, catalysis, etc. However, the underlying mechanisms of such transformations vary dramatically in different 2D materials. Here, we report the structural transformation of layered titanium trisulfide (TiS3) to titanium disulfide (TiS2) after irradiation. We systematically characterized the dependence of the transformation on laser power, flake thickness, irradiation time, and vacuum conditions using microscopic and spectroscopic methods. The underlying mechanism is confirmed as the heat-induced materials decomposition, a process that also occurs in many other transition metal trichalcogenide materials. Furthermore, we demonstrate that this spatial-resolved method also enables the creation of in-plane TiS3-TiS2 heterostructures. Our study identifies a new family of 2D materials that undergo a structural transformation after laser irradiation and enriches the methods available for developing new prototypes of low-dimensional devices in the future.
Subject areas: Physics, Optics, Laser
Graphical abstract

Highlights
-
•
Local structural transformation from TiS3 to TiS2 using focused laser irradiations
-
•
Verification of heat-induced material decomposition mechanism of the transformation
-
•
Demonstration of in-plane TiS3-TiS2 heterostructures
Physics; Optics; Laser
Introduction
Structural transformation in van der Waals materials have been widely observed as the atomic thin layers can be influenced and modified through the interactions with energetic particles1,2,3,4,5 such as photons, electrons, and ions. The instability can be initiated via different mechanisms using these trigger methods. One useful way to modify the lattices with sub-micrometer spatial resolution in ultrafast timings is through optical irradiation. Typically, the laser spots with intense energy densities can raise the temperatures of the material at the focus, leading to a change of physical or chemical properties.1 This change can occur through processes such as oxidation, sublimation, lattice degradation,6,7,8,9 and phase transtions.10,11,12 While some studies have been conducted on the structural transformation of graphene and transition metal dichalcogenides (TMDs), how to identify these transitions in other van der Waals materials and the underlying mechanisms remains a question.
As a typical van der Waals family member, the group-IVB transition metal trichalcogenides (MX3, M: Ti, Zr, Hf; X: S, Se, Te) crystals are needle- or ribbon-like shapes, exhibiting unique physical properties such as metal-insulator transition,13 tunable charge density wave,14,15 anisotropic spectroscopic feature,16,17 large birefringence, and linear dichroism responses.18,19 Among them, the semiconducting titanium trisulfide (TiS3) exhibits a direct band gap size of 1 eV, similar to that of silicon,20 at its few-layer to monolayer limit,21,22 making it particularly attractive for research into the next generation electronic and optoelectronic devices,23,24 such as morphology-tailored transistors and broadband response photodetectors.25,26,27,28,29,30 Thus, the thermal stability of MX3 crystals is a critical issue for future applications. Previous studies have shown that they can be converted to transition metal dichalcogenides (MX2) through a pyrolysis effect in a vacuum at high temperatures.31,32 However, heating in ambient conditions results in a thermally activated oxidation process.33,34 Although the laser-induced damages and oxidations on MX3 flakes during spectroscopic characterizations35,36 have been noticed and detailed, a systematic investigation is still needed to understand the structural transformation of MX3 crystals under laser irradiations.
Here, we find that the monoclinic TiS3 can be converted to the trigonal titanium disulfide (TiS2) by irradiating it with a continuous-wave laser beam in a vacuum. The obtained 1T-TiS2 flakes have been characterized using microscopic, spectroscopic, and electrical methods, and we attribute the mechanism behind structural transformation to the local heating of TiS3 by the laser beam, causing the escape of one sulfur atom per unit cell. The transformation threshold power depends on the flake thickness, which is influenced by both the absorption in the layered materials and the heat dissipation through the flake and the substrate. The focused laser beam is scanned to convert part of the TiS3 flake into TiS2, creating an in-plane heterostructure with different the electrical properties. Moreover, we applied that this laser-irradiation process to ZrS3, ZrSe3, and NbSe3 which also underwent through a similar structural transformation. Our work thus has uncovered the transformation mechanism of the MX3 materials and expanded the application of the laser processing technology for building low-dimensional electronic and optoelectronic devices in the future.
Results and discussion
The TiS3 crystal, which belongs to the P21/m space group, has lattice constants of a = 4.973 Å, b = 3.433 Å, and c = 8.714 Å, respectively.27 As shown in the left part of Figure 1A, each titanium atom is surrounded by six sulfur atoms, forming bicapped tetrahedrons that extend along the crystallographic b-axis. The formation of such quasi-1D chains in the crystal results in the anisotropic geometries and properties of TiS3. Figure 1C shows an optical image of a mechanically exfoliated TiS3 flake, which has a ribbon-like shape with a large aspect ratio. The flake exhibits purple color under white light illumination, corresponding to a thickness of approximately 16 nm (17 layers), as confirmed by atomic force microscopy (AFM) characterization (inset of Figure 1C). The Raman spectrum of TiS3 is shown in Figure 1D as the red curve, which displays four peaks located at 172 cm−1, 295 cm−1, 363 cm−1, and 554 cm−1 within the collected spectral range. They are all identified as Ag modes of the monoclinic TiS3 crystal as reported.37,38 Since the intensities of these Raman modes are polarization dependent, we always set the laser polarization angle in parallel to the long edge of the ribbon during measurements to obtain the strongest Raman intensities.17,35
Figure 1.
Structural transformation in TiS3 induced by laser irradiation
(A) The top and side views of the crystal structures of monoclinic TiS3 and trigonal TiS2, respectively.
(B) Illustration of the laser irradiation on a TiS3 flake on a SiO2/Si substrate.
(C) An optical image of a ribbon-like TiS3 flake. The height profile characterized by AFM in the inset figure shows that the flake thickness is 16 nm. The laser-irradiated region is outlined by a dashed rectangle.
(D) Raman spectra collected from the unirradiated and irradiated regions of the corresponding TiS3 flake and a pristine TiS2 crystal, respectively.
(E) EDS spectra collected from the pristine and irradiated regions of a TiS3 flake. The S Kα and Ti Kα lines are indicated by the arrows, and the peak at 3.5 keV marked by the asterisk is the sum peak of Si Kα lines. The inserted table shows the extracted atomic stoichiometric ratio from two regions, as compared to the value from a TiS3 single crystal.
To investigate the effects of laser irradiations, a focused laser beam with a wavelength of 532 nm is applied to specific regions of the TiS3 flake, as shown in Figure 1B. The irradiation conditions can be precisely controlled by adjusting the laser power, step size, and dwelling time during the scanning process. For example, the flake presented in Figure 1C was locally irradiated in a 5 × 3 μm2 region using the raster scan mode with a step size of 100 nm and a dwell time of 0.1 s. The laser power was maintained at a constant 20 mW. The irradiation caused the flake color to change from blue to red in the irradiated region. The Raman spectrum collected from the irradiated region (blue curve in Figure 1D) shows the disappearance of all Ag modes of TiS3, with two new peaks, emerging at ∼ 220 cm−1 and 330 cm−1, respectively. These changes indicate the destruction of the TiS3 lattice and the formation of a new structure. The new structure could be correlated with trigonal TiS2, whose crystal structure is presented in the right part of Figure 1A. This Raman spectrum exhibits peak profiles similar to that acquired from the high-quality TiS2 crystal (green curve in Figure 1D, as we discover that it is difficult to exfoliate TiS2 thin flakes, thus we use TiS2 crystal instead, which would give similar results), with the two peaks identified as the Eg and A1g modes of 1T-TiS2, respectively.
We then use energy-dispersive X-ray spectroscopy (EDS) to measure changes in the element stoichiometric ratio induced by laser irradiation. Figure 1E shows a comparison of the spectra collected from the unirradiated and irradiated regions of a thin flake on a SiO2/Si substrate. In both spectra, several strong peaks can be observed in the analyzed spectral range, including the peaks of Ti Kα (4.51 keV) and S Kα (2.31 keV) lines. The additional peak located at 3.48 keV marked with an asterisk is attributed to the sum peak of the Si Kα line (1.74 keV) that originates from the underlying SiO2/Si substrate, which is an EDS spectral artifact.39 The Si Kα line is presented in Figure S1 as additional information. The intensity of the S Kα line significantly decreases while the intensity of the Ti Kα line maintains almost a constant, indicating the loss of sulfur element after laser irradiation. The quantitative analysis of the spectra gives a stoichiometric ratio of Ti:S = 28.07%:71.93% (1:2.56) for the pristine unirradiated flake, which deviates from the value of a TiS3 crystal (25.72%:74.28%), as listed in the table shown in the figure. Such deviation may be attributed to the large signal errors in thin flakes. On the contrary, the irradiated region gives a stoichiometric ratio of 36.36%: 63.64%, close to that of a TiS2 crystal. Therefore, by combining Raman and EDS analysis, we can deduce that laser irradiation results in a structural transformation from monocline TiS3 to trigonal TiS2.
We also discovered that the structural transformation process occurs uniformly in the laser-scanned regions. Figure 2A presents two Raman intensity maps collected from a 20 nm thick flake, with a laser-scanned region of 5 × 5 μm2. These two maps are formed using the Raman peaks at 367 cm−1 (top view) and 331 cm−1 (bottom view), which corresponds to the Ag mode of TiS3 and A1g mode of TiS2, respectively. The Raman modes of TiS3 disappear in the irradiated region while exhibiting uniform intensity distributions in the unirradiated region; Conversely, the Raman modes of TiS2 can only be observed in the irradiated region with uniform intensities. These Raman intensity distributions indicate that the original TiS3 is uniformly transformed to TiS2 during laser scanning, which is consistent with the uniform optical contrast in the converted region shown in Figure 1C. The treated flake in Figure 1C is characterized by the AFM height map as shown in Figure 2B, where the scanned region can also be clearly identified. The thickness histograms from both the irradiated and unirradiated regions show an increase in surface roughness from 0.6 nm to 1.3 nm, as indicated in Figure 2C. Meanwhile, the laser irradiation causes the reduction of flake thickness from 16 nm to approximately 8 nm, as presented by the height line profile across the irradiated region in Figure 2E. The laser-induced thinning effect is also observed in several TMD systems (MoS2, MoTe2, etc.).1,6,10 In addition, we investigated the distribution of surface potential on this flake using Kelvin probe force microscopy (KPFM), with the corresponding KPFM map shown in Figure 2D. The irradiated region different colors (green) compared to the pristine TiS3 (blue), indicating a higher surface potential and a lower work function caused by structural transformation. The potential difference is estimated to be ∼250 mV, as extracted from the corresponding potential line profile in Figure 2E. The difference is consistent with previous reports on the work function measurement for these two materials, where the defective TiS2-x exhibits a lower work function with a value difference of approximately 150 meV compared to TiS3.32,40
Figure 2.
Morphology characterizations of laser-irradiated area
(A) Raman intensity maps using the Ag mode of TiS3 centered at ∼ 367 cm−1 (top view) and the A1g mode of TiS2 centered at ∼ 331 cm−1 (bottom view), respectively.
(B) The AFM height image of the irradiated TiS3 flake shown in Figure 1B.
(C) Histograms of thickness distributions collected from the unirradiated (TiS3) and irradiated (TiS2) regions.
(D) The KPFM image of the region as shown in (B).
(E) Variation of flake thickness (red line, corresponding to the left axis) and surface potential (blue line, corresponding to the right axis) across the irradiated flake along the dashed line in (B).
To understand the mechanism underlying the structural transformation, we conducted a systematic study using Raman spectroscopy. We used single-spot irradiation instead of raster scanning since we did not observe significant differences. Figure 3A shows the Raman spectra collected from a 28 nm thick flake with increased laser powers. At a laser power of 7 mW, the Raman spectrum corresponds to pure TiS3 modes, indicating that the TiS3 maintains its original crystal structure. However, at 10 mW, additional modes located at 230 cm−1 and 330 cm−1 appear, indicating that the TiS3 is partially converted to TiS2 (black arrows indicated in the figure). The TiS3 modes no longer exist at the higher power of 15 mW, indicating that the TiS3 is fully converted to TiS2.
Figure 3.
Controllability of the structural transformation
(A) Raman spectra of a 28 nm thick TiS3 flake irradiated with different laser power in vacuum. The black arrows indicate the Raman modes of TiS2. All the spectra shown here were using single spot acquisition mode instead of raster scanning.
(B) Raman spectra of a 70 nm thick TiS3 flake with an excitation laser power of 12 mW and for durations ranging from 10 s to 120 s, and the spectrum excited at a higher power of 13 mW and for a duration of 10 s.
(C) Raman spectra of TiS3 flakes with different thickness excited at a laser power of 20 mW.
(D) Raman spectra of a 35 nm thick TiS3 flake irradiated with different laser powers in air. The purple line shows the spectrum of a transformed TiS2 from a BN covered TiS3 excited at a power of 20 mW. The additional green arrow indicates the appearance of TiO2 peak.
(E) The structure transformation diagram as a function of flake thickness and laser irradiation power.
Figure 3B shows the Raman spectra collected from a 70 nm thick flake at a constant power of 12 mW. The spectra that correspond to pure TiS3 are observed with increased irradiation times from 10 s to 120 s. However, when the laser power is slightly increased from 12 mW to 13 mW, the co-existence of TiS3 and TiS2 appear immediately after a short irradiation time of 10 s (orange curve in Figure 3B). This finding suggests the existence of a clear power threshold although it is thickness dependent. Since it has been reported that the thermal annealing to TiS3 in a vacuum at high temperatures can convert it to TiS2,31,32 while the focused laser irradiation can heat the flakes locally, we deduce that the transformation from TiS3 to TiS2 is also a heating-related process. This transformation could be described using the following thermal decomposition mechanism:
| (Equation 1) |
where extra sulfur atoms can escape above the TiS3 decomposition temperature approximately 460°C, which is estimated from the phase diagram of the Ti-S system due to the laser heating effect.41 The formation of TiS2 is accompanied with a direct vaporization of sulfur through a sublimation process. The higher temperature converts a larger ratio of TiS3 to TiS2, which is consistent with our observation in Figure 3A. Since the conversion occurs within the regions of the moving laser spot, the formation of polycrystalline structures is expected, which is consistent with the increase of surface roughness after irradiation. Meanwhile, compared to the thermal annealing process, laser irradiation can locally modify the lattice at a micrometer scale with through its diffraction-limited focused beam, while the conversion time can be largely reduced at typical annealing temperatures (e.g., 600°C in ref. 32), thus providing a higher flexibility and controllability to the structural transformation from TiS3 to TiS2.
Figure 3C shows the Raman spectra obtained from four TiS3 flakes with different thicknesses (7 nm, 28 nm, 70 nm, and 150 nm) after irradiation with a laser power of 20 mW. The TiS3 lattice is maintained in the 7 nm thin flake while the 28 nm and 70 nm flakes completely transform to TiS2. The 150 nm thick flake shows a hybrid composite of TiS3 and TiS2. The observed thickness-dependent structural evolution can be understood by considering the heat transport process within the flake. For thin flakes, such as the 7 nm flake in Figure 3C, the absorption is limited and the SiO2/Si substrate acts as a heat sink, thus preventing the raise of flake temperature to its decomposition temperature. However, for the thick flakes, the heat diffusion will generate a temperature gradient in the c-axis of the crystal. Only layers on the top part can be completely converted to TiS2 and the bottom layers are maintained as TiS3. Hence the hybrid structures of TiS3 and TiS2 are expected. This explanation is further supported by the use of substrates with different thermal conductivities, which strongly modulate the transformation temperatures. As demonstrated in Figures S2 and S3, the threshold laser power is as low as 4 mW to convert a TiS3 flake to TiS2 using a Polydimethylsiloxane (PDMS) substrate, whereas no transformation occurs at a power of 20 mW after transferring the TiS3 flake onto the Si substrate.
By investigating this transformation behavior on more flakes, we have established a diagram that correlate the structural transformation with the layer thickness and the excitation power. As shown in Figure 3E, the TiS3 lattices on SiO2/Si substrate are maintained at either thin layers (thickness <10 nm) or low laser powers (typically <10 mW), while pure TiS2 structures can be obtained with a proper thickness (between 10 and 150 nm) and high laser powers.
The ambient condition is also important for structural conversion. Figure 3D presents the Raman spectra of a 35 nm thick TiS3 flake in air during the laser irradiation. Instead of forming TiS3/TiS2 mixed structures, an additional peak located at 146 cm−1 is observed when the laser power is increased from 5 mW to 10 mW (indicated by the additional green arrow). The emerged peak can be correlated with the Eg mode of TiO2, indicating the oxidization of TiS3 during irradiation.42 This observation is consistent with previous reports on the instability of TiS3 in the atmosphere, where it is converted to TiO2 during heating treatment.33 Furthermore, even at the highest available laser power of 20 mW, the TiS3 is not fully converted to TiO2 and a combination of Raman peaks from TiS3, TiS2, and TiO2 are present as shown in Figure 3D. Hence, we deduce that the oxidation is a self-limited process that occurs near the surface, preventing further oxidation in the flake and covering the transformed TiS2 region. This oxidation effect can be minimized by encapsulating TiS3 with hexagonal-boron nitride (h-BN) layers. The corresponding Raman spectrum (the purple line in Figure 3E) only shows the modes of TiS2, with no signs of TiS3 or TiO2, indicating that the h-BN protected TiS3 can also be converted to TiS2.
The different electronic band structures of TiS3 and TiS2 show distinct electrical behaviors. Theoretical calculations report that the TiS3 thin layer is a semiconductor with a band gap of 0.96 eV, while the 1T-TiS2 tends to be a metal (density of states in Figures S4 in the supplemental information).21,43 We converted part of the TiS3 flake with laser-irradiation and formed three different regions, including the pristine TiS3, converted TiS2, and the TiS3/TiS2 heterojunction as shown in Figure 4A (inset), and then investigated the electrical properties of these regions respectively. Figure 4A presents the current-voltage (I-Vds) relation without the back-gate voltage (Vg) control. All I-Vds curves exhibit linear response, indicating Ohmic contacts between electrodes and the underlying channel materials. Meanwhile, larger resistance is observed in the TiS3/TiS2 channel compared with the values in both the TiS3 and TiS2 channels. The transconductance defined by also shows different responses between in these three channels. Figure 4B shows the back-gate voltage response for the three channels under constant drain-source voltage bias (Vds) of +1 V. The pristine TiS3 exhibits the n-type response with a low on-off ratio of ∼1.3 in the ±60 V region of the applied gate voltages. The low on-off ratio may be attributed to its sheet-like geometry with heavy doping, which is reported to exhibit a low on-off ratio with high electrical conductivity.27 Conversely, the converted TiS2 exhibits a weaker gate response, corresponding to a higher carrier density with a narrow band gap of TiS2, which might be attributed to the introduce of sulfur vacancies in the channel during the laser irradiation process. Moreover, the TiS3/TiS2 heterojunction shows a similar value of on-off ratio compared with TiS3 (∼2.2) while exhibits much lower drain-source current. Such lower drain-source current in the channel of heterojunction may be attributed to the band structure misalignment between TiS3 and TiS2, as shown in Figure 4C. Metallic TiS2 is calculated to have a larger work function (calculated to be ∼5.43 eV) than semiconducting TiS3 (∼4.84 eV), which is approximately 0.59 eV. Although this value may be reduced by additional doping in two materials that raise their Fermi levels, an additional potential barrier can still be established that reduces the junction current. Figure 4D shows the low-frequency noise spectra for these three regions. All of these spectra exhibit a typical 1/f slope (dashed line in the figure).44 The original TiS3 and its heterojunction with TiS2 have the same level of noise density despite their different drain-source current, while the value for the converted TiS2 is approximately two orders larger. Such difference might be attributed to the larger fluctuations of carrier numbers and the correlated mobility caused by the inducing of additional defects during laser irradiation. Although TiS3 has been reported to have ultrahigh photoresponse,28 all the three channels in our device show ignorable photoresponse (Figures S5 in the supplemental information). We also attribute it to the sheet-like geometry, which results poor photoresponse compared with TiS3 nanoribbons.27
Figure 4.
Electrical properties of the pristine TiS3, the transformed TiS2, and the TiS3/TiS2 junction
(A) Ids-Vds response for the channels of TiS3, TiS2, and TiS3/TiS2 junction, respectively. The applied back gate voltage bias (Vg) is 0 V. The inset figure is optical image of the devices, channels of which are indicated by the colored arrows; laser converted TiS2 regions are outlined by white dashed rectangles. The scale bar is 10 μm.
(B) Ids-Vg response of three channels under the constant bias voltage Vds = 1 V.
(C) Band alignment of TiS2-TiS2 heterostructure. The calculated values of work function for TiS2 and TiS3 are 5.43 eV and 4.84 eV, respectively. The calculated band gap for TiS3 is 0.96 eV.
(D) Low-frequency noise spectra for the three channels under the bias voltage Vds = 0.1 V and gate voltage Vg = 0 V. The ideal 1/f slope is presented in the figure as a reference.
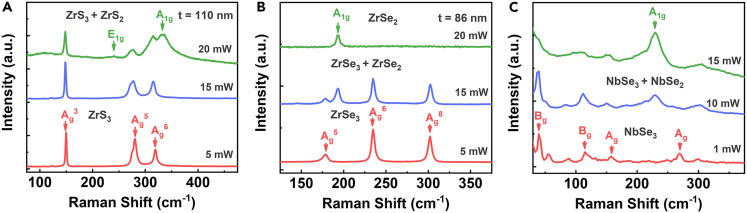
Besides TiS3, we discover that this laser irradiation induced structure transformation can occur in other MX3 materials. Figure 5A shows the Raman spectra of a 110 nm thick ZrS3 flake irradiated with different laser powers. At the highest power of 20 mW, additional Raman peaks corresponding to the E1g and A1g modes of ZrS2 shows up in addition to Raman modes of ZrS3, indicating a composition of ZrS3 and ZrS2. We also find the laser irradiation can completely covert an 86 nm thick ZrSe3 flake to ZrSe2 as shown in Figure 5B. Interestingly, this transformation is not limited in the group IV MX3 materials, as shown in Figure 5C, which displays the Raman spectra of NbSe3 under laser irradiation. The relatively low power of 10 mW can induce the hybrid structures of NbSe3 and NbSe2. This transformation process can be explained by the relatively lower crystallization temperatures of MX3 compared to the corresponding MX2 materials. Meanwhile, laser irradiations on transition-metal-tellurides, such as ZrTe3 and ZrTe5, form tellurium nanostructures instead of transition metal ditellurides (e.g., ZrTe2). Such results are also widely observed in other transition-metal-tellurides,45 possible due to the thermal instability of tellurides with higher sublimation temperatures of tellurium (Figure S6 in the supplemental information). Thus, the laser irradiation method provides a way to modulate the structures and properties of transition-metal-trichalcogenides with high controllability and spatial resolution.
Figure 5.
Structural transformation in other MX3 materials induced by laser-irradiation
(A) Raman spectra of ZrS3 irradiated with different laser powers in vacuum.
(B) Raman spectra of ZrSe3 irradiated with different laser powers in vacuum.
(C) Raman spectra of NbSe3 irradiated with different laser powers in vacuum.
Conclusions
In conclusion, we have systematically studied the structural transformation from the layered TiS3 to TiS2 induced by laser irradiations. A transformation diagram is established by investigating the relation between converted products and varied laser powers and flake thickness. The underlying mechanism is attributed to the pyrolysis of TiS3 caused by the irradiation-induced heating effect. The transformation can induce a change of the electrical transport properties and surface potential in the original van der Waals materials by controlling the amount of the converted TiS2 flakes. Moreover, such structural transformation occurs in many other trichalcogenide materials. Our work demonstrates the controllability of inducing structural transformations in trichalcogenides using laser irradiation, thus broadening their applications to low-dimensional electronic and optoelectronic devices in the future.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Other | ||
| Silicon Wafer (285 nm SiO 2/Si) | Yilan Microelectronics | http://www.yilanmicro.com/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Ya-Qing Bie (bieyq@mail.sysu.edu.cn) and Yangbo Zhou (yangbozhou@ncu.edu.cn).
Materials availability
All materials used and generated in this study will be made available on request from the lead contact with a completed Materials Transfer Agreement.
Experimental model and subject details
Sample preparation
High-quality TiS3 bulk crystals were synthesized using the conventional chemical vapor transport process.46 High purity Titanium (99.999%) and sulfur (99.9%) powders were mixed at a stoichiometric ratio of 1:3 and sealed in a quartz tube at high vacuum (<10− 4 Torr). The tube was placed in a two-zone furnace with the hot zone maintained at 550° C and the cold zone maintained at 450°C for seven days. Then the tube was cooled naturally to room temperature to obtain needle-like crystals. Other crystals used in the experiments (e.g., TiS2, ZrS3, and others) are either synthesized using the same method. The thin flakes were mechanically exfoliated and transferred onto SiO2/Si substrates using a PDMS-assisted stamp transfer method.47
Method details
Optical characterizations and laser irradiations
An optical microscope (Nikon Ci-Pol) was used to identify the geometry and location of the transferred TiS3 flakes on substrates. Their thickness was confirmed by AFM measurements. The Raman spectra were obtained using a commercial confocal Raman Microscope (Witec Alpha 300R). This system provides a 532 nm laser with diffraction-limited beam spot (<400 nm), and the delivered laser power can be precisely adjusted from 0.1 mW to 20 mW with 0.1 mW precision. The laser irradiation experiments were carried out on the same system, and the samples were placed in the vacuum chamber of an optical cryostat (CIA C04-002-007, pressure <10−2 Pa). The laser beam was focused on the flake using a glass thickness corrected objective lens (50 , N.A. = 0.7, spot size ∼460 nm). A different objective lens (100 , N.A. = 0.9, spot size ∼360 nm) was used for the laser scanning process in air as shown in Figure 3D.
AFM characterizations
The tapping mode in an AFM (Asylum Cipher ES) was applied to obtain the thickness information of the selected flakes. The kelvin probe force microscopy (KPFM) mode in the same instruments was also applied to investigate the surface potential variations after laser irradiations.
Device fabrication and electrical characterizations
TiS3 field-effect transistors with multiple electrical contacts were fabricated through the traditional microfabrication process. These contacts were first patterned by a standard electron beam lithography (EBL) process, followed by the deposition of a metal film (10 nm Ti and 50 nm Ni) with a lift-off process. The electrical characterizations to the fabricated devices were carried out by placing them in a vacuum probe station (Lakeshore CRX VF, vacuum pressure < mbar). All the electrical measurements were carried out using a semiconductor parameter analyzer (PDA FS-Pro) at room temperature without light illumination.
The EDS analysis
The EDS spectra from thin TiS3 flakes were collected using an energy-dispersive X-ray spectrometer (Oxford Instruments, UltimMax 65), which is installed on a Thermoscientific Scios2 dual beam scanning electron microscope. A 20 kV electron beam with current of 0.8 nA were used to collect the spectra.
Theoretical calculations
All the first-principles calculations were performed by density functional theory (DFT) based Vienna ab initio Simulation Package (VASP).48 A plane-wave basis set with a cutoff energy of 450 eV was employed.49 Perdew-Burke-Ernzerhof (PBE) within the generalized gradient approximation (GGA) is chosen for the exchange-correlation functional.50 The DFT+U method is carried out to deal with electron correlations for transition metal Ti atom with Ueff = 5.8 eV.51 The TiS3 and TiS2 were simulated by slab model with a 15 Å vacuum layer. The density of states and average electrostatic potential were computed using a 13 × 13×1 k-point mesh for the slab geometries. The convergence criterion was set to be 10−5 eV. The structures are fully relaxed until the force for all atoms is less than 10−3 eV/Å.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 11864022, 12164025, 61974167, 62264010, 12264026, 12004142, 12004154), the Natural Science Foundation of Jiangxi Province, China (Nos. 20192ACB21014, 20212BAB211023), and the Guangdong Program (No. 2019QN01X113).
Author contributions
Y.Z. and Y.Q.B. supervised the project. H.Z. and X.T. fabricated the samples, conducted the laser irradiation and the related measurements. Z.W. and S.W. conducted AFM measurements and analyzed the results. J.G. synthesized the crystals. L.F. conducted EDS measurements. X.L. and J.Y. did the DFT simulations. Y.Z., Y.Q.B., H.Z., and X.T. wrote the paper. All authors analyzed the results and approved the current version of the article. H.Z. and X.T. contributed equally to this work.
Declaration of interests
The authors declare no competing interests.
Published: September 11, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107895.
Contributor Information
Ya-Qing Bie, Email: bieyq@mail.sysu.edu.cn.
Yangbo Zhou, Email: yangbozhou@ncu.edu.cn.
Supplemental information
Data and code availability
-
•
Raman, AFM and electrical measurements data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original codes.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Kollipara P.S., Li J., Zheng Y. Optical Patterning of Two-Dimensional Materials. Research. 2020;2020 doi: 10.34133/2020/6581250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Z., Chen F. Ion beam modification of two-dimensional materials: Characterization, properties, and applications. Appl. Phys. Rev. 2017;4 doi: 10.1063/1.4977087. [DOI] [Google Scholar]
- 3.Fox D.S., Zhou Y., Maguire P., O’Neill A., Ó'Coileáin C., Gatensby R., Glushenkov A.M., Tao T., Duesberg G.S., Shvets I.V., et al. Nanopatterning and Electrical Tuning of MoS2 Layers with a Subnanometer Helium Ion Beam. Nano Lett. 2015;15:5307–5313. doi: 10.1021/acs.nanolett.5b01673. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Y., Jadwiszczak J., Keane D., Chen Y., Yu D., Zhang H. Programmable graphene doping via electron beam irradiation. Nanoscale. 2017;9:8657–8664. doi: 10.1039/C7NR03446F. [DOI] [PubMed] [Google Scholar]
- 5.Kim T.-Y., Cho K., Park W., Park J., Song Y., Hong S., Hong W.-K., Lee T. Irradiation Effects of High-Energy Proton Beams on MoS2 Field Effect Transistors. ACS Nano. 2014;8:2774–2781. doi: 10.1021/nn4064924. [DOI] [PubMed] [Google Scholar]
- 6.Castellanos-Gomez A., Barkelid M., Goossens A.M., Calado V.E., van der Zant H.S.J., Steele G.A. Laser-Thinning of MoS2: On Demand Generation of a Single-Layer Semiconductor. Nano Lett. 2012;12:3187–3192. doi: 10.1021/nl301164v. [DOI] [PubMed] [Google Scholar]
- 7.Hu L., Shan X., Wu Y., Zhao J., Lu X. Laser Thinning and Patterning of MoS2 with Layer-by-Layer Precision. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-15350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu J., Wu J., Carvalho A., Ziletti A., Liu H., Tan J., Chen Y., Castro Neto A.H., Özyilmaz B., Sow C.H. Bandgap Engineering of Phosphorene by Laser Oxidation toward Functional 2D Materials. ACS Nano. 2015;9:10411–10421. doi: 10.1021/acsnano.5b04623. [DOI] [PubMed] [Google Scholar]
- 9.Wang B., Peng R., Wang X., Yang Y., Wang E., Xin Z., Sun Y., Li C., Wu Y., Wei J., et al. Ultrafast, Kinetically Limited, Ambient Synthesis of Vanadium Dioxides through Laser Direct Writing on Ultrathin Chalcogenide Matrix. ACS Nano. 2021;15:10502–10513. doi: 10.1021/acsnano.1c03050. [DOI] [PubMed] [Google Scholar]
- 10.Cho S., Kim S., Kim J.H., Zhao J., Seok J., Keum D.H., Baik J., Choe D.-H., Chang K.J., Suenaga K., et al. Phase patterning for ohmic homojunction contact in MoTe2. Science. 2015;349:625–628. doi: 10.1126/science.aab3175. [DOI] [PubMed] [Google Scholar]
- 11.Mine H., Kobayashi A., Nakamura T., Inoue T., Pakdel S., Marian D., Gonzalez-Marin E., Maruyama S., Katsumoto S., Fortunelli A., et al. Laser-Beam-Patterned Topological Insulating States on Thin Semiconducting MoS2. Phys. Rev. Lett. 2019;123 doi: 10.1103/PhysRevLett.123.146803. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J., Han J., Peng G., Yang X., Yuan X., Li Y., Chen J., Xu W., Liu K., Zhu Z., et al. Light-induced irreversible structural phase transition in trilayer graphene. Light Sci. Appl. 2020;9:174. doi: 10.1038/s41377-020-00412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randle M., Lipatov A., Kumar A., Kwan C.-P., Nathawat J., Barut B., Yin S., He K., Arabchigavkani N., Dixit R., et al. Gate-Controlled Metal–Insulator Transition in TiS3 Nanowire Field-Effect Transistors. ACS Nano. 2019;13:803–811. doi: 10.1021/acsnano.8b08260. [DOI] [PubMed] [Google Scholar]
- 14.Huang C., Zhang E., Yuan X., Wang W., Liu Y., Zhang C., Ling J., Liu S., Xiu F. Tunable charge density wave in TiS3 nanoribbons. Chinese Phys. B. 2017;26 doi: 10.1088/1674-1056/26/6/067302. [DOI] [Google Scholar]
- 15.Papadopoulos N., Flores E., Watanabe K., Taniguchi T., Ares J.R., Sanchez C., Ferrer I.J., Castellanos-Gomez A., Steele G.A., van der Zant H.S.J. Multi-terminal electronic transport in boron nitride encapsulated TiS3 nanosheets. 2D Mater. 2019;7 doi: 10.1088/2053-1583/ab4ef3. [DOI] [Google Scholar]
- 16.Khatibi A., Godiksen R.H., Basuvalingam S.B., Pellegrino D., Bol A.A., Shokri B., Curto A.G. Anisotropic infrared light emission from quasi-1D layered TiS3. 2D Mater. 2019;7 doi: 10.1088/2053-1583/ab57ef. [DOI] [Google Scholar]
- 17.Kong W., Bacaksiz C., Chen B., Wu K., Blei M., Fan X., Shen Y., Sahin H., Wright D., Narang D.S., Tongay S. Angle resolved vibrational properties of anisotropic transition metal trichalcogenide nanosheets. Nanoscale. 2017;9:4175–4182. doi: 10.1039/C7NR00711F. [DOI] [PubMed] [Google Scholar]
- 18.Papadopoulos N., Frisenda R., Biele R., Flores E., Ares J.R., Sánchez C., van der Zant H.S.J., Ferrer I.J., D'Agosta R., Castellanos-Gomez A. Large birefringence and linear dichroism in TiS3 nanosheets. Nanoscale. 2018;10:12424–12429. doi: 10.1039/C8NR03616K. [DOI] [PubMed] [Google Scholar]
- 19.Hou S., Guo Z., Yang J., Liu Y.-Y., Shen W., Hu C., Liu S., Gu H., Wei Z. Birefringence and Dichroism in Quasi-1D Transition Metal Trichalcogenides: Direct Experimental Investigation. Small. 2021;17 doi: 10.1002/smll.202100457. [DOI] [PubMed] [Google Scholar]
- 20.Molina-Mendoza A.J., Barawi M., Biele R., Flores E., Ares J.R., Sánchez C., Rubio-Bollinger G., Agraït N., D'Agosta R., Ferrer I.J., Castellanos-Gomez A. Electronic Bandgap and Exciton Binding Energy of Layered Semiconductor TiS3. Adv. Electron. Mater. 2015;1 doi: 10.1002/aelm.201500126. [DOI] [Google Scholar]
- 21.Jin Y., Li X., Yang J. Single layer of MX3 (M = Ti, Zr; X = S, Se, Te): a new platform for nano-electronics and optics. Phys. Chem. Chem. Phys. 2015;17:18665–18669. doi: 10.1039/C5CP02813B. [DOI] [PubMed] [Google Scholar]
- 22.Dai J., Zeng X.C. Titanium Trisulfide Monolayer: Theoretical Prediction of a New Direct-Gap Semiconductor with High and Anisotropic Carrier Mobility. Angew. Chem. 2015;127:7682–7686. doi: 10.1002/ange.201502107. [DOI] [PubMed] [Google Scholar]
- 23.Island J.O., Molina-Mendoza A.J., Barawi M., Biele R., Flores E., Clamagirand J.M., Ares J.R., Sánchez C., van der Zant H.S.J., D’Agosta R., et al. Electronics and optoelectronics of quasi-1D layered transition metal trichalcogenides. 2D Mater. 2017;4 doi: 10.1088/2053-1583/aa6ca6. [DOI] [Google Scholar]
- 24.Tripathi N., Pavelyev V., Sharma P., Kumar S., Rymzhina A., Mishra P. Review of titanium trisulfide (TiS3): A novel material for next generation electronic and optical devices. Mater. Sci. Semicond. Process. 2021;127 doi: 10.1016/j.mssp.2021.105699. [DOI] [Google Scholar]
- 25.Niu Y., Frisenda R., Flores E., Ares J.R., Jiao W., Perez de Lara D., Sánchez C., Wang R., Ferrer I.J., Castellanos-Gomez A. Polarization-Sensitive and Broadband Photodetection Based on a Mixed-Dimensionality TiS3/Si p–n Junction. Adv. Opt. Mater. 2018;6 doi: 10.1002/adom.201800351. [DOI] [Google Scholar]
- 26.Liu S., Xiao W., Zhong M., Pan L., Wang X., Deng H.-X., Liu J., Li J., Wei Z. Highly polarization sensitive photodetectors based on quasi-1D titanium trisulfide (TiS3) Nanotechnology. 2018;29 doi: 10.1088/1361-6528/aaafa2. [DOI] [PubMed] [Google Scholar]
- 27.Island J.O., Barawi M., Biele R., Almazán A., Clamagirand J.M., Ares J.R., Sánchez C., van der Zant H.S.J., Álvarez J.V., D'Agosta R., et al. TiS3 Transistors with Tailored Morphology and Electrical Properties. Adv. Mater. 2015;27:2595–2601. doi: 10.1002/adma.201405632. [DOI] [PubMed] [Google Scholar]
- 28.Island J.O., Buscema M., Barawi M., Clamagirand J.M., Ares J.R., Sánchez C., Ferrer I.J., Steele G.A., van der Zant H.S.J., Castellanos-Gomez A. Ultrahigh Photoresponse of Few-Layer TiS3 Nanoribbon Transistors. Adv. Opt. Mater. 2014;2:641–645. doi: 10.1002/adom.201400043. [DOI] [Google Scholar]
- 29.Baraghani S., Abourahma J., Barani Z., Mohammadzadeh A., Sudhindra S., Lipatov A., Sinitskii A., Kargar F., Balandin A.A. Printed Electronic Devices with Inks of TiS3 Quasi-One-Dimensional van der Waals Material. ACS Appl. Mater. Interfaces. 2021;13:47033–47042. doi: 10.1021/acsami.1c12948. [DOI] [PubMed] [Google Scholar]
- 30.Lv T., Huang X., Zhang W., Deng C., Chen F., Wang Y., Long J., Gao H., Deng L., Ye L., Xiong W. High-Responsivity Multiband and Polarization-Sensitive Photodetector Based on the TiS3/MoS2 Heterojunction. ACS Appl. Mater. Interfaces. 2022;14:48812–48820. doi: 10.1021/acsami.2c12332. [DOI] [PubMed] [Google Scholar]
- 31.Nath M., Rao C.N.R. Nanotubes of Group 4 Metal Disulfides. Angew. Chem. Int. Ed. 2002;41:3451–3454. doi: 10.1002/1521-3773(20020916)41:18<3451::AID-ANIE3451>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Wu X.C., Tao Y.R., Gao Q.X. Preparation and field emission properties of titanium polysulfide nanobelt films. Nano Res. 2009;2:558–564. doi: 10.1007/s12274-009-9055-2. [DOI] [Google Scholar]
- 33.Ghasemi F., Frisenda R., Flores E., Papadopoulos N., Biele R., Perez de Lara D., van der Zant H.S.J., Watanabe K., Taniguchi T., D’Agosta R., et al. Tunable Photodetectors via In Situ Thermal Conversion of TiS3 to TiO2. Nanomaterials. 2020;10:711. doi: 10.3390/nano10040711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo J., Tao J., Zhang Z., Fei L., Li D., Jadwiszczak J., Wang X., Guo Y., Liao X., Zhou Y. Controllable Thermal Oxidation and Photoluminescence Enhancement in Quasi-1D van der Waals ZrS3 Flakes. ACS Appl. Electron. Mater. 2020;2:3756–3764. doi: 10.1021/acsaelm.0c00788. [DOI] [Google Scholar]
- 35.Osada K., Bae S., Tanaka M., Raebiger H., Shudo K., Suzuki T. Phonon Properties of Few-Layer Crystals of Quasi-One-Dimensional ZrS3 and ZrSe3. J. Phys. Chem. C. 2016;120:4653–4659. doi: 10.1021/acs.jpcc.5b12441. [DOI] [Google Scholar]
- 36.Pant A., Torun E., Chen B., Bhat S., Fan X., Wu K., Wright D.P., Peeters F.M., Soignard E., Sahin H., Tongay S. Strong dichroic emission in the pseudo one dimensional material ZrS3. Nanoscale. 2016;8:16259–16265. doi: 10.1039/C6NR05238J. [DOI] [PubMed] [Google Scholar]
- 37.Lipatov A., Loes M.J., Lu H., Dai J., Patoka P., Vorobeva N.S., Muratov D.S., Ulrich G., Kästner B., Hoehl A., et al. Quasi-1D TiS3 Nanoribbons: Mechanical Exfoliation and Thickness-Dependent Raman Spectroscopy. ACS Nano. 2018;12:12713–12720. doi: 10.1021/acsnano.8b07703. [DOI] [PubMed] [Google Scholar]
- 38.Pawbake A.S., Island J.O., Flores E., Ares J.R., Sanchez C., Ferrer I.J., Jadkar S.R., van der Zant H.S.J., Castellanos-Gomez A., Late D.J. Temperature-Dependent Raman Spectroscopy of Titanium Trisulfide (TiS3) Nanoribbons and Nanosheets. ACS Appl. Mater. Interfaces. 2015;7:24185–24190. doi: 10.1021/acsami.5b07492. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka R., Yuge K., Kawai J., Alawadhi H. Artificial peaks in energy dispersive X-ray spectra: sum peaks, escape peaks, and diffraction peaks. X Ray Spectrom. 2017;46:5–11. doi: 10.1002/xrs.2697. [DOI] [Google Scholar]
- 40.Hawkins C.G., Whittaker-Brooks L. Controlling Sulfur Vacancies in TiS2–x Cathode Insertion Hosts via the Conversion of TiS3 Nanobelts for Energy-Storage Applications. ACS Appl. Nano Mater. 2018;1:851–859. doi: 10.1021/acsanm.7b00266. [DOI] [Google Scholar]
- 41.Mikkelsen J.C. P-T-X phase diagram for Ti−S from (60÷75) atomic % sulfur. Nuov. Cim. B. 2007;38:378–386. doi: 10.1007/BF02723508. [DOI] [Google Scholar]
- 42.Ohsaka T., Izumi F., Fujiki Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978;7:321–324. doi: 10.1002/jrs.1250070606. [DOI] [Google Scholar]
- 43.Wang H., Qiu Z., Xia W., Ming C., Han Y., Cao L., Lu J., Zhang P., Zhang S., Xu H., Sun Y.-Y. Semimetal or Semiconductor: The Nature of High Intrinsic Electrical Conductivity in TiS2. J. Phys. Chem. Lett. 2019;10:6996–7001. doi: 10.1021/acs.jpclett.9b02710. [DOI] [PubMed] [Google Scholar]
- 44.Balandin A.A. Low-frequency 1/f noise in graphene devices. Nat Nano. 2013;8:549–555. doi: 10.1038/nnano.2013.144. [DOI] [PubMed] [Google Scholar]
- 45.Manjón F.J., Gallego-Parra S., Rodríguez-Hernández P., Muñoz A., Drasar C., Muñoz-Sanjosé V., Oeckler O. Anomalous Raman modes in tellurides. J. Mater. Chem. C. 2021;9:6277–6289. doi: 10.1039/D1TC00980J. [DOI] [Google Scholar]
- 46.Island J.O., Biele R., Barawi M., Clamagirand J.M., Ares J.R., Sánchez C., van der Zant H.S.J., Ferrer I.J., D’Agosta R., Castellanos-Gomez A. Titanium trisulfide (TiS3): a 2D semiconductor with quasi-1D optical and electronic properties. Sci. Rep. 2016;6 doi: 10.1038/srep22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castellanos-Gomez A., Buscema M., Molenaar R., Singh V., Janssen L., van der Zant H.S.J., Steele G.A. Deterministic Transfer of Two-dimensional Materials by All-dry Viscoelastic Stamping. 2D Mater. 2014;1 doi: 10.1088/2053-1583/1/1/011002. [DOI] [Google Scholar]
- 48.Kresse G., Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996;6:15–50. doi: 10.1016/0927-0256(96)00008-0. [DOI] [PubMed] [Google Scholar]
- 49.Kresse G., Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996;54:11169–11186. doi: 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- 50.Perdew J.P., Burke K., Ernzerhof M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 51.Dudarev S.L., Botton G.A., Savrasov S.Y., Humphreys C.J., Sutton A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B. 1998;57:1505–1509. doi: 10.1103/PhysRevB.57.1505. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Raman, AFM and electrical measurements data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original codes.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.