Highlights
-
•
Tuberculosis continues to be a major health concern around the world and kills an estimated 1.3 million people each year.
-
•
The aim of this study was to assess the survival status and risk factors of multidrug-resistant tuberculosis patients in Addis Ababa, Ethiopia, in 2022.
-
•
The obtained evidence in this study revealed that the death rate among research participants was high however, the successful treatment outcome was low.
Keywords: Multidrug-resistant tuberculosis, Survival probability, Treatment outcome
Abstract
Background
Tuberculosis continues to be a major health concern around the world. It kills an estimated 1.6 million people each year. The World Health Organization (WHO) removed Ethiopia from its list of thirty countries having a high prevalence of MDR/RR-TB in 2021. As a result, the aim of this study was to assess the current context of survival status and risk factors of multidrug-resistant tuberculosis patients in Addis Ababa, Ethiopia, in 2022.
Methods
An institutional-based retrospective cohort study with 245 patients was undertaken using multidrug-resistant tuberculosis patients who were recruited from January 1st, 2018 to December 30th, 2021, in St. Peter's specialized hospital. To find independent predictors of survival status, Cox regression analysis was used. An adjusted hazard ratio with a 95% confidence interval and a p-value of < 0.05 was used to establish association and statistical significance.
Results
The result of the study revealed that the incidence of mortality in this study was 13.1% (95% CI: 10.3–16.5). Moreover, being male (AOR = 3.7: 95% CI = 1.2, 11.4), old age (AOR = 14: 95% CI = 3.0, 60.4), site of TB (AOR = 0.2: 95% CI = 0.03, 0.6), and presence of comorbidity (AOR = 9.2: 95% CI = 2.4, 35.3), were independent predictors of time to death.
Conclusion
Generally, the death rate among research participants was high. Moreover, male gender, old age, site of tuberculosis, and presence of other comorbidity were predictors of mortality among MDR-TB patients.
1. Introduction
Tuberculosis (TB) is an infectious disease that is a leading cause of sickness, one of the top ten causes of mortality worldwide, and the leading cause of death from a single infectious agent (ranked above HIV/AIDS) [1]. It is the 9th leading cause of all deaths worldwide and the 2nd leading cause of death from all infectious diseases [2]. By 2021, there were an estimated 10.6 million new cases and 1.4 million among HIV-negative and 187,000 among HIV-positive a total of 1.6 million TB deaths worldwide [3]. TB is a treatable and preventable disease, but without treatment, the mortality rate is substantial (about 50%) [4].
Multidrug-resistant tuberculosis (MDR-TB) is a major global health risk, driving the ongoing TB epidemic and increasing morbidity and mortality worldwide [5]. Adherence and tolerability may be difficult to achieve once multidrug-resistant tuberculosis treatment is initiated [6]. Recent research reveals that MDR-TB is a significant contributor to post-TB lung disease (PTLD), which causes disability and frequently necessitates rehabilitation [7], [8]. MDR-TB treatment necessitates a course of second-line medications for at least nine months and up to twenty months, accompanied by counseling and monitoring for adverse outcomes [9].
According to anti-TB medication resistance surveillance statistics, 4.1% of new TB cases and 19% of previously treated TB cases worldwide have multidrug-resistant or rifampicin-resistant tuberculosis [10]. In Ethiopia, MDR-TB is an important health concern, affecting a productive segment of population, with an estimated prevalence of 1.03% among new and 6.52% among previously treated TB cases in 2019 [11].
MDR-TB patient death has been linked to a number of factors. According to various studies, the presence of any chronic disease such as HIV/AIDS, alcohol use, smoking, age, clinical complication, extra-pulmonary TB, therapeutic delay, body mass index (BMI), living in rural areas, poor drug adherence, hypokalemia, and a low CD4 count were risk factors for MDR-TB patient mortality [12], [13], [14], [15], [16], [17].
Ethiopia began MDR-TB treatment at St. Peter's Specialized Hospital in Addis Ababa in 2009 in response to the first 45 MDR-TB patients identified at St. Peter's TB Specialist Hospital [18], followed by Gondar University Hospital. In recent years, there has been a rapid scale-up at the national and regional state level, with over 67 MDR-TB Treatment Initiative Centers (TICs) that are facilities set up to care for MDR/RR TB patients [19]. The National TB and HIV Program in Ethiopia, together with partners, implemented a DR-TB mixed model of care, which was successful in increasing patient enrollment rates by a factor of twelve while also achieving treatment success rates of 75% and cure rates of 65% [20]. Moreover, Ethiopia was removed from the list of 30 countries with a high MDR/RR-TB burden by the World Health Organization (WHO) in 2021 [21]. Besides, identifying and managing risk factors in MDR-TB patients is the first step in developing effective therapeutic interventions and improving treatment outcomes. This study is therefore thought to provide current context of survival status and influencing risk factors for mortality in Multi Drug Resistant Tuberculosis patients. As a result, the purpose of this study was to assess the survival status and risk factors for mortality among multidrug-resistant tuberculosis patients in Addis Ababa, Ethiopia, in 2022.
2. Methods
2.1. Study design and setting
A retrospective follow-up study was conducted in Addis Ababa, the capital city of the Federal Democratic Republic of Ethiopia. It was carried out at Saint Peters specialized hospital, the country's largest TB referral centre, which started providing MDR-TB treatment at the end of 2009 to treat patients sent from all around the country. The hospital has a long history of tuberculosis management, and it was the first hospital in the country to manage TB cases since June 1961. The study sample consisted of MDR-TB patients who began MDR-TB medication between January 1, 2018, and December 30, 2021.
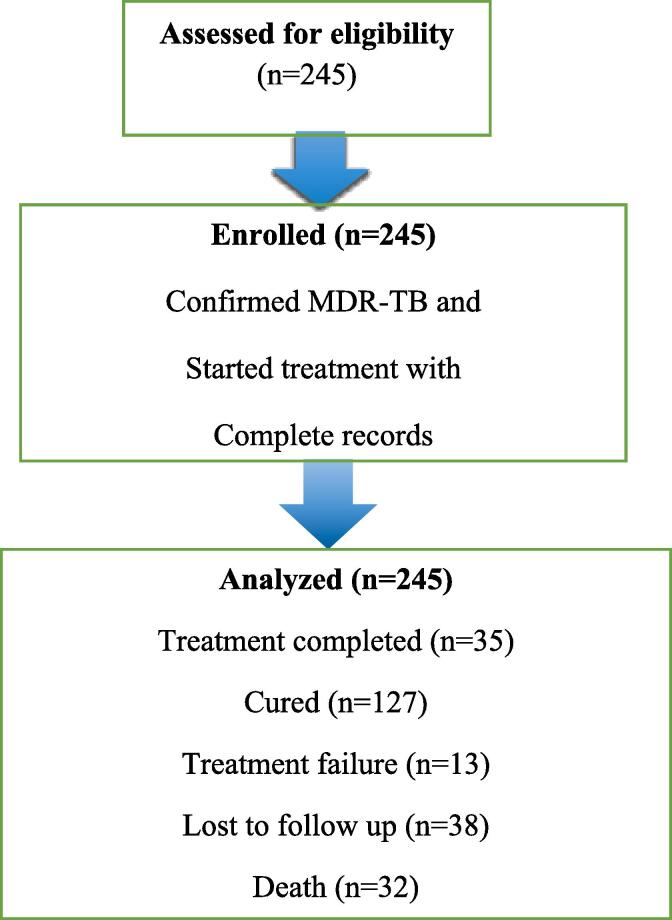
All MDR-TB patients who started therapy with second-line medicines at St. Peter's Specialized Hospitals between 2018 and 2021 were included in the study. The study includes the complete medical records of 245 MDR-TB patients who were enrolled at the St. Peter's Specialized Hospital during the index period (Fig. 1).
Fig. 1.
Sample recruitment chart of patients who received MDR-TB treatment in Addis Ababa, Ethiopia.
2.2. Variables and definition of the study
Survival status of MDR-TB patients was considered an outcome variable, as were socio-demographic factors such as age, gender, initial weight, initial height of the patients, smoking, alcohol use status, and clinical characteristics including tuberculosis treatment history, tuberculosis site (pulmonary or extrapulmonary), HIV co-infection, diabetes, hypertension, other co-morbidities, and the type of drug resistance at baseline, as well as the types of adverse drug reactions that occurred after treatment. These factors were evaluated as independent.
MDR-TB is caused by Mycobacterium tuberculosis strains that are resistant to at least isoniazid (INH) and rifampicin (RMP) [22];
Survival Status: The outcome of patients identified as censored or dead in their clinical data file from scheduled or unscheduled visits was designated as their survival status [23].
Time to death: It was determined as the interval between the date of a clear MDR-TB diagnosis and the date of death (in months) [24].
Ototoxicity is defined as ear damage, specifically damage to the cochlea or auditory nerve and, in some cases, the vestibular system. The loss of response at three consecutive frequencies was used to determine ototoxicity.
Serious adverse events (SAEs) are defined as any undesirable or harmful effect associated with the administration of drugs to treat MDR-TB that resulted in the suspension or withdrawal of one or more second-line anti-TB drugs. The term “suspends” considered to the temporary discontinuation of one or more drugs, whereas “withdrawal” referred to the permanent discontinuation of one or more drugs.
2.3. Data collection and analysis
Data were collected by reviewing patient charts. The data collection tool was adopted from different published articles with slight modifications for the local context. The check list was focused on clinical variables and sociodemographic characteristics.
Data were collected after one day of orientation for the data collectors. On-site supervision and feedback were provided on a daily basis for data collectors for consistency, completeness, and accuracy. Five percent of the total checklist was refilled, and ten percent of the data was double-entered to check for accuracy.
The raw data were entered into Epidata and transported to SPSS version 26 software for analysis. Descriptive statistical analysis was done using frequency, percentages, and tables. The bivariate cox-regression analysis was done to identify candidate variables using a p-value <2.5. Significant variables from the bivariate analysis with a p-value of 0.25 were included in the multivariable cox-regression analysis to identify independent predictors of survival status. An adjusted hazard ratio with 95% CI and a p-value < 0.05 was used to determine statistical significance.
3. Results
3.1. Socio-demographic characteristics
A total of 245 participants were included in this study. The majority of them, 140 (57.1%), were males, and 99 (40.4%) were below the age of 24 years, with a median age of 26. The majority of the patients in this study (62.4%) were city dwellers. In terms of substance use, 15 (6.1%) drank alcohol, and 7 (2.9%) were chat chewer (Table 1).
Table 1.
Sociodemographic characteristics of study participants in Addis Ababa, Ethiopia, 2022.
| Variables | Frequency | Percent (%) | |
|---|---|---|---|
| Sex | Male | 140 | 57.1 |
| Female | 105 | 42.9 | |
| Age | ≤24 | 99 | 40.4 |
| 25–30 | 38 | 15.5 | |
| 31–40 | 73 | 29.8 | |
| ≥41 | 35 | 14.3 | |
| Alcohols | Yes | 15 | 6.1 |
| No | 230 | 93.9 | |
| Chewing chat | Yes | 7 | 2.9 |
| No | 238 | 97.1 |
3.2. Clinical and medical conditions of patients
According to the findings of the study, the majority of 219 (89.4%) of the participants had pulmonary tuberculosis, and more than half (62.4%) of the MDR-TB patients had a history of TB treatment. One-third (37.6%) of MDR-TB patients were newly admitted, while 76 (31% were relapsing). The majority of cases (229, or 93.5%) were diagnosed using Gene Expert, with 26 (11.4%) using culture, 24 (9.8%) using line probe testing, and 16 (6.5%) using clinical diagnosis.
HIV co-infection was the most common comorbidity among MDR-TB cases (67.3%), while diabetes mellitus accounted for 6 (2.4%). In addition, almost 30 (12.2%) of MDR-TB patients had comorbidities other than HIV infection and diabetes mellitus, including anemia (4.5%), pneumonia (2.9%), and SAM (4.9%). Approximately 31 (13.1%) of MDR-TB patients experienced side effects during treatment. Hearing loss (6.1%) was the most common adverse effect, followed by ototoxicity (2.4%) (Table 2).
Table 2.
Clinical and medical characteristics of MDR-TB patients in Addis Ababa, Ethiopia, 2022.
| Variables | Frequency | Percent (%) | |
|---|---|---|---|
| Site of Tuberculosis | Pulmonary | 219 | 89.4 |
| Extra pulmonary | 26 | 10.6 | |
| Previous treatment for TB | Yes | 153 | 62.4 |
| No | 92 | 37.6 | |
| Registration group | New | 92 | 37.6 |
| Relapse | 76 | 31.0 | |
| After lost to follow up | 7 | 2.9 | |
| After Failure of first line anti TB | 37 | 15.1 | |
| After the failure of second line anti TB | 33 | 13.5 | |
| Diagnosis method | Gene-x pert | 229 | 93.5 |
| Line probe assay | 24 | 9.8 | |
| Solid culture | 16 | 6.5 | |
| Liquid culture | 12 | 4.9 | |
| Other (Clinical diagnosis) | 16 | 6.3 | |
| HIV-confection | Yes | 67 | 27.3 |
| No | 178 | 72.7 | |
| Diabetes mellitus | Yes | 6 | 2.4 |
| No | 239 | 97.6 | |
| Presence of other chronic diseases | Yes | 30 | 12.2 |
| No | 215 | 87.8 | |
| Other chronic diseases (n = 30) | Anemia | 11 | 4.5 |
| Pneumonia | 7 | 2.9 | |
| Severe acute malnutrition | 12 | 4.9 | |
| Sputum smears microscopy at baseline | Positive | 83 | 33.9 |
| Negative | 116 | 47.4 | |
| Unavailable | 30 | 12.2 | |
| Not applicable | 16 | 6.5 | |
| Culture results at baseline | Positive | 133 | 54.3 |
| Negative | 35 | 14.3 | |
| Unavailable | 61 | 24.9 | |
| Not applicable | 16 | 6.5 | |
| Serious adverse effect during MDR-TB treatment | Yes | 31 | 13.1 |
| No | 214 | 86.9 | |
| Serious adverse drug reaction type (n = 31) | Hearing loss | 15 | 6.1 |
| Psychosis | 7 | 2.9 | |
| Hypokalemia | 3 | 1.2 | |
| Ototoxicity | 6 | 2.4 |
3.3. Survival status and treatment outcome of MDR-TB patients
A total of 245 MDR-TB cases were followed for varying periods of treatment, ranging from 16 days to 694 days, with a median of 294 (IQR: 263–608) days, for a total of 86,218 person-days or 2,874 person-months of follow-up. During the follow-up period, 32 deaths were observed, resulting in a death rate of 13.4 per 100 person-years. At the end of treatment, the cumulative probability of survival for MDR-TB patients was 86.9%. The probability of surviving time varied significantly between groups, with a total mean survival time of 616.09 (95% CI = 590.8–641.4) days. Of all MDR-TB patients, 162 (66.1%) were effectively treated, meaning 127 (51.8%) were cured and 35 (14.3%) completed their treatment, whereas 32 (13.1%) died during treatment and 38 (15.5%) of patients were lost to follow-up (Table 3).
Table 3.
Treatment outcome of MDR-TB patients in Addis Ababa, Ethiopia, 2022.
| Treatment outcomes | Frequency | Percent (%) | 95% CI |
|---|---|---|---|
| Cured | 127 | 51.8 | 45.3, 58.4 |
| Treatment completed | 35 | 14.3 | 9.8, 18.8 |
| Treatment failure | 13 | 5.3 | 2.9, 8.6 |
| Lost to follow up | 38 | 15.5 | 11.4, 2 |
| Died | 32 | 13.1 | 9.0, 17.6 |
3.4. Factors associated with MDR-TB mortality
According to the results of the Cox multivariate analysis, sex, age, site of tuberculosis, and the presence of other chronic diseases were all substantially linked with death among MDR-TB patients. The findings showed that males were 3.7 times more likely than females to die (AHR: 3.7, 95% CI: 1.2, 11.4). Those aged 41 years or older were 14 times (AHR: 14, 95 %CI: 3.0, 60.4) more likely to die than patients aged 24 years. Patients with pulmonary tuberculosis had a 15% lower risk of death (AHR: 0.2, 95% CI: 0.03, 0.6) than patients with extrapulmonary tuberculosis. After adjusting for other covariates, patients with chronic conditions other than HIV and diabetes were 9.2 times more likely to die (AHR: 9.2, 95% CI: 2.4, 35.3) than patients without any additional chronic disease (Table 4).
Table 4.
Factors associated with MDR-TB mortality in Addis Ababa, Ethiopia, 2022.
| Variables | Category | Frequency | CHR (95% CI) | AHR (95% CI) | P-value |
|---|---|---|---|---|---|
| Sex | Male | 140 | 2.8 (1.2, 5.9) | 3.7 (1.2, 11.4) | 0.023* |
| Female | 105 | 1 | 1 | ||
| Age | ≤24 | 99 | 4.4 (1.4, 14.1) | 1 | |
| 25–30 | 38 | 1.3 (0.3, 5.1) | 2.6 (0.5, 12.2) | 0.32 | |
| 31–40 | 73 | 0.5 (0.2, 1.9) | 1.6 (0.29. 8.9) | 0.59 | |
| ≥41 | 35 | 1 | 14 (3, 60.4) | 0.0001** | |
| Baseline weight | <43 | 75 | 1.6 (0.7, 3.8) | 0.33 (0.8, 1.4) | 0.13 |
| ≥43 | 170 | 1 | 1 | ||
| Site of Tuberculosis | Pulmonary | 219 | 0.6 (0.2, 1.7) | 0.2 (0.03, 0.6) | 0.009** |
| Extra pul. | 26 | 1 | 1 | ||
| HIV-coinfection | Yes | 67 | 0.5 (0.2, 1.3) | 1.1 (0.3, 4.5) | 0.88 |
| No | 178 | 1 | 1 | ||
| Presence of other chronic diseases | Yes | 30 | 7.8 (3.4, 18.1) | 9.2 (2.4, 35.3) | 0.001** |
| No | 215 | 1 | 1 | ||
| SAE during MDR-TB treatment | Yes | 31 | 0.5 (0.2, 1.5) | 0.5 (0.1, 2.0) | 0.35 |
| No | 214 | 1 | 1 |
**Significant at P < 0.01, * Significant at P < 0.05.
4. Discussion
This study reviewed the survival status and risk factors of a cohort of multi-drug-resistant tuberculosis patients who began treatment at Saint Peter Specialized Hospital in Addis Ababa, Ethiopia, between 2018 and 2021. The incidence of mortality in this study was 13.4 (95% CI: 10.27–16.53) per 100 person-years of follow-up during treatment, and most patients died soon after starting treatment. This finding was consistent with a study conducted in Addis Ababa ten years ago with a result of 13.3% [25], and a study finding from Butajira, Arbaminch, and Shenengibe Hospitals in south and southwestern Ethiopia showed 11% [26]. The current finding, however, was higher than that of a previous study conducted in Yirgalem and Queen Eleni Hospital in southern Ethiopia: 8.44% [27], 8.2% in South-east Ethiopia [28], and 7.42% in central Ethiopia [29]. In contrast, this result was lower than a study conducted in eSwatini (21.3%) [30], western Ethiopia 21% [31], and a systematic review 38% [32]. The variation could be due to differences in service regions and the timing of care beginning, as well as a shift in time connected to greater patient awareness of their health and improvements in patient care.
The current study found 66.1% of successful treatment outcomes, which is consistent with the studies done in Northwest Ethiopia 63% [33] and Haiti, 66.4% [34]. However, it was lower than the national treatment success rate of 75.7% [35], 81.1% in Uganda [36], 76.9% in Addis Ababa [37] and 77.1% in Gonder compressive Specialized Hospital [38]. The difference in the current success rate could be attributed to progress over time, a patient condition during admission, program organization, setting, and a variety of other factors.
This study discovered that males were nearly four times more likely to die than females. Similar findings have been reported in different studies [39], [40], [41]. The possible explanation for this discovery could be that women are more likely to stick to treatment regimens than men, resulting in better health results. Males have a higher proclivity for alcohol and drug use, so stopping their prescription as a male has more economic consequences than females.
We discovered that patients aged 41 years or older were 14 times more likely to die than those aged<24 years. This finding is consistent with previous results indicating that older people are at a higher risk of dying during follow-up and having unsatisfactory MDR-TB treatment outcomes [42], [43]. In addition, older individuals might have a higher risk of mortality and respond poorly to anti-TB treatment because of factors such as physical decline, a higher number of comorbidities, malnutrition, and decreased immunity [44], [45].
This study also found that patients with pulmonary tuberculosis had a 15% lower risk of death than patients with extrapulmonary tuberculosis. This finding was similar to that of the Amhara region study [46], [47]. One possible explanation is the existence of many co-morbidities, which are substantial risk factors for the development of extrapulmonary tuberculosis. Moreover, people with chronic diseases other than HIV and diabetes were 9.2 times more likely to die than patients with no other chronic diseases. This conclusion is consistent with prior findings in Ethiopia [27] and India [41]. This could be linked to weakened immunity, and co-infection is frequently associated with poor response to TB treatments due to anti-tuberculosis drug nutrition absorption [48]. Besides, this type of TB is difficult to diagnose and results in a high death rate before and after treatment initiation [49].
4.1. Strengths and limitations of the study
The use of an adequate statistical model for a better estimate of risk factors was a main strength of this study. However, due to the nature of the data acquired during data collection, some critical characteristics such as the exact cause of death, patient compliance with therapy, and knowledge of the drug-resistance patterns of patient isolates were lacking. Moreover, end-of-treatment follow-up test results indicating that these patients were disease-free or not at treatment completion were difficult to obtain.
5. Conclusion
In general, the death rate among the study participants was high. Male sex, older age, site of tuberculosis, and presence of other chronic diseases were the major predictors of mortality among MDR-TB patients. Therefore, to obtain improved treatment outcomes, more attention should be placed on the management of drug-resistant TB patients with these features.
Consent for publication
Not applicable.
Funding
The authors received no specific funding for this work.
Ethical consideration
The researchers secured ethical approval from Yanet College's research and ethical review board with reference number ID YC/17/2022. A permission letter was obtained from Saint Peter's specialized hospital's higher officials. Patients' informed permission was ruled unnecessary because the data was purely secondary and there was no access to meet the study participants. Any specific personal identifiers, such as the patients' names, were not collected, and the confidentiality of any personal information was preserved throughout the study procedure.
CRediT authorship contribution statement
Genanew Kassie Getahun: Conceptualization, Data curation, Visualization, Investigation, Writing – original draft, Writing – review & editing. Elias Gezahegn: Conceptualization, Supervision, Data curation. Getabalew Endazenawe: Methodology, Supervision, Writing – review & editing. Tewodros Shitemaw: Supervision, Data curation. Zelalem Negash: Conceptualization, Writing – original draft, Writing – review & editing. Samuel Dessu: Conceptualization, Methodology, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We would like to acknowledge the study participants and data collectors for this study.
References
- 1.Global tuberculosis report 2022. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO.
- 2.Floyd K., Glaziou P., Zumla A., Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respir Med. 2018 Apr 1;6(4):299–314. doi: 10.1016/S2213-2600(18)30057-2. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. World Health Organization Global Tuberculosis Report 2021. URL: https://www. who. int/teams/global-tuberculosis-programme/tbreports/global-tuberculosis-report-2021. 2021.
- 4.Lönnroth K., Jaramillo E., Williams B., Dye C., Raviglione M. Tuberculosis: the role of risk factors and social determinants. Equity, social determinants and public health programmes. 2010;219:241. [Google Scholar]
- 5.Paul R. The threat of multidrug-resistant tuberculosis. J Glob Infect. 2018 Jul;10(3):119. doi: 10.4103/jgid.jgid_125_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Migliori G.B., Thong P.M., Alffenaar J.-W., Denholm J., Tadolini M., Alyaquobi F., et al. Gauging the impact of the COVID-19 pandemic on tuberculosis services: a global study. Eur Respir J. 2021;58(5):2101786. doi: 10.1183/13993003.01786-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menzies N.A., Quaife M., Allwood B.W., Byrne A.L., Coussens A.K., Harries A.D., et al. Lifetime burden of disease due to incident tuberculosis: a global reappraisal including post-tuberculosis sequelae. Lancet Glob Health. 2021;9(12):e1679–e1687. doi: 10.1016/S2214-109X(21)00367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akkerman O.W., Ter Beek L., Centis R., Maeurer M., Visca D., Muñoz-Torrico M., et al. Rehabilitation, optimized nutritional care, and boosting host internal milieu to improve long-term treatment outcomes in tuberculosis patients. Int J Infect Dis. 2020 Mar;1(92):S10–S14. doi: 10.1016/j.ijid.2020.01.029. [DOI] [PubMed] [Google Scholar]
- 9.Nahid P., Mase S.R., Migliori G.B., Sotgiu G., Bothamley G.H., Brozek J.L., et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med. 2019;200(10):e93–e142. doi: 10.1164/rccm.201909-1874ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girum T., Muktar E., Lentiro K., Wondiye H., Shewangizaw M. Epidemiology of multidrug-resistant tuberculosis (MDR-TB) in Ethiopia: a systematic review and meta-analysis of the prevalence, determinants and treatment outcome. Tropical Diseases, Travel Medicine and Vaccines. 2018 Dec;4(1):1–2. doi: 10.1186/s40794-018-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ethiopia, Federal Ministry of Health [FMOH] FMOH; Addis Ababa: 2021. Guidelines for Clinical and Programmatic Management of TB, TB/HIV, DR-TB and Leprosy in Ethiopia. [Google Scholar]
- 12.Asgedom S.W., Teweldemedhin M., Gebreyesus H. Prevalence of Multidrug-Resistant Tuberculosis and Associated Factors in Ethiopia: A Systematic Review. J Pathogens. 2018;2018:1–8. doi: 10.1155/2018/7104921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung-Delgado K., Guillen-Bravo S., Revilla-Montag A., Bernabe-Ortiz A., Caylà J.A. Mortality among MDR-TB cases: comparison with drug-susceptible tuberculosis and associated factors. PLoS One. 2015;10(3):e0119332. doi: 10.1371/journal.pone.0119332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Getahun GK, et al. Prevalence of Depression in Diabetes Mellitus Patients with Associated Co-Morbidities in Addis Ababa, Ethiopia, 2022. Diabetes Obes Int J 2022, 7(4): 000265.
- 15.Javaid A., Mehreen S., Khan M.A., Ashiq N., Ihtesham M., Khan A. Depression and its associated factors with multidrug-resistant tuberculosis at baseline. J Depress Anxiety. 2017;6(253):2167. 1044.1000253. [Google Scholar]
- 16.Gallo J., Pinhata J., Simonsen V., Galesi V., Ferrazoli L., Oliveira R. Prevalence, associated factors, outcomes and transmission of extensively drug-resistant tuberculosis among multidrug-resistant tuberculosis patients in São Paulo, Brazil: a cross-sectional study. Clin Microbiol Infect. 2018;24(8):889–895. doi: 10.1016/j.cmi.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Berhan A., Berhan Y., Yizengaw D. A meta-analysis of drug resistant tuberculosis in Sub-Saharan Africa: how strongly associated with previous treatment and HIV co-infection? Ethiop J Health Sci. 2013;23(3):271–282. doi: 10.4314/ejhs.v23i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Federal Ministry of Health. Guideline for program and clinical management of drug resistant tuberculosis. Addis Ababa, Ethiopia. 2013.
- 19.Tola H.H., Holakouie-Naieni K., Mansournia M.A., Yaseri M., Tesfaye E., Mahamed Z., et al. Intermittent treatment interruption and its effect on multidrug resistant tuberculosis treatment outcome in Ethiopia. Sci Rep. 2019;9(1):20030. doi: 10.1038/s41598-019-56553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molla Y., Jerene D., Jemal I., Nigussie G., Kebede T., Kassie Y., et al. The experience of scaling up a decentralized, ambulatory model of care for management of multidrug-resistant tuberculosis in two regions of Ethiopia. J Clin Tuberculosis Other Mycobacterial Diseases. 2017;7:28–33. doi: 10.1016/j.jctube.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Global Tuberculosis Report 2022. Geneva: World Health Organization. 27 October 2022.[Online] Accessed 27 October 2022.
- 22.Organization WH. Global diffusion of eHealth: making universal health coverage achievable: report of the third global survey on eHealth: World Health Organization; 2017.
- 23.Organization WH. Anti-tuberculosis drug resistance in the world. Fourth Global Report Geneva. The WHO/IUATLD Global Project on Anti-tuberculosis Drug Resistance Surveillance. Fourth Global Report. Geneva, WHO. WHO/HTM/TB/2008.394 Available at: http://whqlibdoc. who. int/hq/2008 …, 2013.
- 24.Pérez-Butragueño M., Ramos-Rincón J.-M., Tesfamariam A., Comeche B., Mohammed N., Tiziano G., et al. Impact of Xpert MTB/RIF in the Diagnosis of Childhood Tuberculosis in Rural Ethiopia. J Trop Pediatr. 2022;68(4) doi: 10.1093/tropej/fmac055. [DOI] [PubMed] [Google Scholar]
- 25.Getachew T., Bayray A., Weldearegay B. Survival and predictors of mortality among patients under multi-drug resistant tuberculosis treatment in Ethiopia: st. peter's specialized tuberculosis hospital, Ethiopia. J Int J Pharmaceut Sci Res. 2013;4(2):776. [Google Scholar]
- 26.Bade A.B., Mega T.A., Bajpai R.C. Survival status and its predictors among multi-drug resistance tuberculosis treated patients in Ethiopia: Multicenter observational study. PLoS One. 2020 Nov 9;15(11):e0241684. doi: 10.1371/journal.pone.0241684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Girum T., Tariku Y., Dessu S. Survival status and treatment outcome of multidrug resistant tuberculosis (MDR-TB) among patients treated in treatment initiation centers (TIC) in South Ethiopia: a retrospective cohort study. Ann Med Health Sci Res. 2017;7(5) [Google Scholar]
- 28.Mulugeta S.S., Tamiru S.D., Sultan H.H., Zerihun Z., Tadele G., Teklu T. Survival and predictors of mortality from multidrug resistant tuberculosis (MDR-TB) among patients treated at MDR-TB Referal Hospitals in Ethiopia: A retrospective cohort study. J Public Health Epidemiol. 2020;12(1):13–21. [Google Scholar]
- 29.Kassa G.M., Tadesse A., Gelaw Y.A., Alemayehu T.T., Tsegaye A.T., Tamirat K.S., et al. Predictors of mortality among multidrug-resistant tuberculosis patients in central Ethiopia: a retrospective follow-up study. Epidemiol Infect. 2020:148. doi: 10.1017/S0950268820002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verdecchia M., Keus K., Blankley S., Vambe D., Ssonko C., Piening T., et al. Model of care and risk factors for poor outcomes in patients on multi-drug resistant tuberculosis treatment at two facilities in eSwatini (formerly Swaziland), 2011–2013. PLoS One. 2018 Oct 17;13(10):e0205601. doi: 10.1371/journal.pone.0205601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ketema D.B., Muchie K.F., Andargie A.A. Time to poor treatment outcome and its predictors among drug-resistant tuberculosis patients on second-line anti-tuberculosis treatment in Amhara region, Ethiopia: retrospective cohort study. BMC Public Health. 2019 Dec;19(1):1. doi: 10.1186/s12889-019-7838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isaakidis P., Casas E.C., Das M., Tseretopoulou X., Ntzani E.E., Ford N. Treatment outcomes for HIV and MDR-TB co-infected adults and children: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2015 Aug 1;19(8):969–978. doi: 10.5588/ijtld.15.0123. [DOI] [PubMed] [Google Scholar]
- 33.Alene K.A., Viney K., McBryde E.S., Tsegaye A.T., Clements A.C. Treatment outcomes in patients with multidrug-resistant tuberculosis in north-west Ethiopia. J Trop Med Int Health. 2017;22(3):351–362. doi: 10.1111/tmi.12826. [DOI] [PubMed] [Google Scholar]
- 34.Bassili A., Fitzpatrick C., Qadeer E., Fatima R., Floyd K., Jaramillo E. A systematic review of the effectiveness of hospital-and ambulatory-based management of multidrug-resistant tuberculosis. J Am J Trop Med Hygiene. 2013;89(2):271. doi: 10.4269/ajtmh.13-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tola H, Holakouie-Naieni K, Mansournia MA, Yaseri M, Gamtesa DF, Tesfaye E, et al. National treatment outcome and predictors of death and treatment failure in multidrug-resistant tuberculosis in Ethiopia: a 10-year retrospective cohort study. 2021;11(8):e040862. [DOI] [PMC free article] [PubMed]
- 36.Izudi J., Tamwesigire I.K., Bajunirwe F. Treatment success and mortality among adults with tuberculosis in rural eastern Uganda: a retrospective cohort study. J BMC Public Health. 2020;20(1):1–10. doi: 10.1186/s12889-020-08646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aragaw N., Teferi M., Ali O., Tesema E., Ayele S., Jarso H., et al. Treatment outcomes and predictors of outcome in Multidrug Resistance Tuberculosis (MDR-TB) cohort of patients in Addis Ababa, Ethiopia. J Am J Heal Res. 2021;9(5):204. [Google Scholar]
- 38.Belachew T., Yaheya S., Tilahun N., Gebrie E., Seid R., Nega T., et al. Multidrug-Resistant Tuberculosis Treatment Outcome and Associated Factors at the University of Gondar Comprehensive Specialized Hospital: A Ten-Year Retrospective Study. J Infection Drug Resistance. 2022;15:2891–2899. doi: 10.2147/IDR.S365394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brust J.C., Gandhi N.R., Carrara H., Osburn G., Padayatchi N. High treatment failure and default rates for patients with multidrug-resistant tuberculosis in KwaZulu-Natal, South Africa, 2000–2003. Int J Tuberculosis Lung Disease. 2010;14(4):413–419. [PMC free article] [PubMed] [Google Scholar]
- 40.Hamusse S.D., Demissie M., Teshome D., Lindtjørn B. Fifteen-year trend in treatment outcomes among patients with pulmonary smear-positive tuberculosis and its determinants in Arsi Zone, Central Ethiopia. J Global Health Action. 2014;7(1):25382. doi: 10.3402/gha.v7.25382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parmar M.M., Sachdeva K.S., Dewan P.K., Rade K., Nair S.A., Pant R., et al. Unacceptable treatment outcomes and associated factors among India's initial cohorts of multidrug-resistant tuberculosis (MDR-TB) patients under the revised national TB control programme (2007–2011): evidence leading to policy enhancement. J PloS one. 2018;13(4):e0193903. doi: 10.1371/journal.pone.0193903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Javaid A., Shaheen Z., Shafqat M., Khan A.H., Ahmad N. Risk factors for high death and loss-to-follow-up rates among patients with multidrug-resistant tuberculosis at a programmatic management unit. J Am J Infect Control. 2017;45(2):190–193. doi: 10.1016/j.ajic.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 43.Murali S., Krishnamoorthy Y., Knudsen S., Roy G., Ellner J., Horsburgh C.R., et al. Comparison of profile and treatment outcomes between elderly and non-elderly tuberculosis patients in Puducherry and Tamil Nadu, South India. J PloS one. 2021;16(8):e0256773. doi: 10.1371/journal.pone.0256773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hochberg NS, Horsburgh Jr CRJCid. Prevention of tuberculosis in older adults in the United States: obstacles and opportunities. 2013;56(9):1240-7. [DOI] [PMC free article] [PubMed]
- 45.Negin J., Abimbola S., Marais B.J. Tuberculosis among older adults–time to take notice. Int J Infectious Diseases. 2015;32:135–137. doi: 10.1016/j.ijid.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 46.Limenih Y.A., Workie D.L. Survival analysis of time to cure on multi-drug resistance tuberculosis patients in Amhara region. Ethiopia J BMC Public Health. 2019;19(1):1–11. doi: 10.1186/s12889-019-6500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sreeramareddy C.T., Panduru K.V., Verma S.C., Joshi H.S., Bates M.N. Comparison of pulmonary and extrapulmonary tuberculosis in Nepal-a hospital-based retrospective study. J BMC Infectious Diseases. 2008;8(1):1–7. doi: 10.1186/1471-2334-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rabie H., Decloedt E.H., Garcia-Prats A.J., Cotton M.F., Frigati L., Lallemant M., et al. Antiretroviral treatment in HIV-infected children who require a rifamycin-containing regimen for tuberculosis. J Expert Opin Pharmacotherapy. 2017;18(6):589–598. doi: 10.1080/14656566.2017.1309023. [DOI] [PubMed] [Google Scholar]
- 49.Kourbatova EV, Leonard MK, Romero J, Kraft C, del Rio C, Blumberg HMJEJoE. Risk factors for mortality among patients with extrapulmonary tuberculosis at an academic inner-city hospital in the US. 2006;21(9):715-21. [DOI] [PubMed]