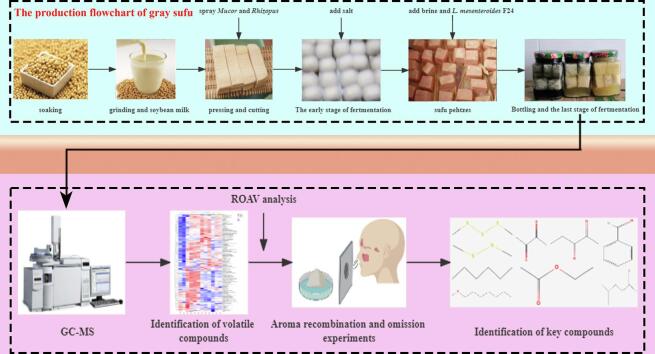
Graphical abstract
Keywords: Gray sufu, Leuconostoc mesenteroides subsp. mesenteroides, Auxiliary fermentation, Starter culture, Volatile profile, Key aroma compound
Highlights
-
•
Leuconostoc mesenteroides F24 was first used as an adjunct starter in gray sufu fermentation.
-
•
Inoculation of L. mesenteroides F24 changed the profiles of the volatile compounds.
-
•
Inoculation of L. mesenteroides F24 significantly improved the flavor quality of gray sufu.
-
•
Nine compounds were confirmed to be the key aroma-active compounds in gray sufu.
Abstract
Gray sufu is a traditional fermented bean product with strong flavor in China, but traditional fermentation methods often lead to its off-flavor. This study was performed to investigate the flavor quality characteristics of gray sufu fermented using L. mesenteroides F24. Results showed 220 volatile compounds in gray sufu, among which alcohols and esters were the main volatiles. Inoculation with L. mesenteroides F24 considerably affected the contents of flavor substances in gray sufu and substantially increased the main flavor compounds. In addition, 29 kinds of key volatile compounds were identified by analyzing the ROAVs. Four unique key flavor substances were found in gray sufu inoculated with L. mesenteroides F24. This study is the first report on the feasibility of L. mesenteroides F24 as a promising starter culture to improve the flavor quality of gray sufu. The results provide a theoretical basis for improving the processing and quality control of gray sufu.
1. Introduction
Gray sufu is a very distinct traditional fermented soybean product in China due to its unique flavor and creamy taste. This food is a popular side dish served with breakfast rice or steamed bread (Han, Rombouts, & Nout, 2001). Gray sufu is traditionally produced from salted pehtzes after their infusion with brine and tofu yellow serofluids, which are residues from making tofu (Hesseltine and Wang, 1986). These brines comprise sulfurous compounds, esters, alcohol, aldehydes, polyphenols, and aromatic compounds, which exhibit strong flavor properties (Sun et al., 2020). Moreover, given its diverse biological active substances (e.g., isoflavandiol, amino acids, and vitamin B12), the soybean and its fermentation process also provide gray sufu with various nutrients, which render it with oxidation resistance and capability to lower blood pressure and cholesterol and reduce bone loss (Gu et al., 2018).
Aroma is one of the most important quality attributes that contribute to the quality of gray sufu. The acceptance of gray sufu by the consumers is also dependent on the aroma. Hence, studying aroma characteristics is critical to improving the flavor and quality of this food (Wang et al., 2020). Its off flavor is the most important quality issues with gray sufu; it results from various factors, such as raw materials, fermentation time, fermentation temperature, and air quality, during fermentation. Therefore, investigating the favorable characteristics of gray sufu helps in improving its product quality, production processes, and food safety. Microbial regulation-based modern fermentation technology can control precisely the fermentation of gray sufu, and the results are critical to enhancing product quality and safety compared with traditional spontaneous fermentation. However, gray sufu is currently produced through an open fermentation process, which introduces numerous uncertainties, especially unpredictable microorganisms. The lack of high-quality starters is a significant bottleneck restricting the industrialization of gray sufu production. Thus, enterprises are actively exploring for a suitable starter to improve the flavor of gray sufu. Although previous studies have shown that microorganisms play a crucial role in the formation of the flavor of fermented soybean paste, rice wine and other fermented foods, their findings were based on unproven correlation analysis (An et al., 2020, Yang et al., 2021, Qian et al., 2023), and no verification experiment using a single strain as a starter has been performed.
Leuconostoc is found in various fermented foods, such as kimchi (Seo, Park, Kim, Lee, Na, & Son, 2018), pickled chili peppers (Ye et al., 2022), fermented coffee (Thayanna et al., 2022), and suancai (He et al., 2020). He et al. found significant positive correlation between Leuconostoc and several volatile compounds (VOCs), such as 1-hexanol, cis-hept-4-enol, 1-octanol, linalool, phenylethyl alcohol, 3-phenylpropanol, and 2,5-dimethyl-benzaldehyde. In our previous studies, Leuconostoc was the dominant bacterium during the fermentation of gray sufu (Sun et al., 2020). Therefore, we speculated that Leuconostoc may contribute significantly to its flavor. To confirm our hypothesis, we isolated a new strain of Leuconostoc from gray sufu and identified it as Leuconostoc mesenteroides subsp. mesenteroides F24 (L. mesenteroides F24). To our best knowledge, Leuconostoc has not been investigated as an adjunctive starter in the processing of gray sufu.
With the advancement of flavoromics, more interactions between flavors and VOCs of foods have been decoded. The most commonly used method for separating and quantifying VOCs from foodstuffs is gas chromatography-mass spectrometry (GC–MS) (Liao et al., 2020). Wang et al. (2020) detected 55 VOCs in unfermented stinky tofu brine using solid-phase microextraction (SPME-)-GC–MS. Meanwhile, An et al. identified the key flavor compounds in traditional fermented soybean paste of Northeast China using SPME-GC–MS. The results showed the feasibility of SPME-GC–MS to detect VOCs of gray sufu. Therefore, the main objectives of the current study were as follows: to identify the aroma compounds in gray sufu fermented by L. mesenteroides F24 using GC–MS; to assess the aroma contributions of these compounds to gray sufu fermented using L. mesenteroides F24 through quantification of aroma-active compounds and calculation of relative odor activity values (ROAVs); to determine the key aroma compounds in gray sufu fermented by L. mesenteroides F24 via aroma recombination and omission tests. The findings can potentially help elucidate the aroma-active compounds in gray sufu and provide a solid basis for L. mesenteroides F24 as an excellent adjunctive starter for gray sufu.
2. Materials and methods
2.1. Preparation and collection of gray sufu
The preparation of gray sufu was mainly divided into early and end fermentation. During the early fermentation, fresh soybean curds sprayed with a certain proportional mixed suspension of Mucor sp. and Rhizopus microsporus at the ratio of 1:1 (v/v) were placed on sterile wooden trays in a fermentation chamber with a controlled temperature of 28 °C and relative humidity (80%). After 7 days of fermentation, the curds were immersed in salt water (20%) to produce fresh sufu pehtzes. At the end of fermentation, the pehtzes were placed in a 350 mL glass jar containing a mixture of brine and tofu yellow serofluids, which are the soy whey residues obtained from making tofu. The adjunctive starter (L. mesenteroides F24) was added to the mixture of brine and tofu yellow serofluids at an initial cell density of approximately 106 CFU/mL. Two different batches were prepared using (1) spontaneous fermentation (group S) and (2) inoculation with L. mesenteroides F24 (group L). Then, the jars were sealed and kept at 28 °C for 28 days to complete the fermentation. Fresh pehtzes (0 days) and gray sufu were taken at random at days 7, 14, 21, 28, and 28 of fermentation. The mixture of brine and tofu yellow serofluids and pehtzes came from Changchun Zhu Laoliu Food Co., Ltd. Then, the samples at each time point were ground separately. All samples were stored at − 80 °C, and sample analyses were completed within two weeks.
2.2. Extraction of volatile compounds
VOCs from the samples were extracted using the method described by An et al. (2020), with several modifications. The frozen samples were ground, weighed, mixed with 10 μL of the internal standards (i.e., 2-octanol [40 μg/mL] and carbetamide [100 μg/mL]) per tube, and transferred to 10 mL gas-tight glass vessels. A carboxen/divinylbenzene/polydimethylsiloxane solid-phase microextraction (CAR/PDMS SPEM) tip (Agilent Technologies, USA) was inserted into each tube and incubated at 40 °C for 30 min. The fiber was then withdrawn and injected into the gas chromatograph.
2.3. GC × GC-TOF-MS analysis
An Agilent 7890A gas chromatograph equipped with a LECO Pegasus 4D mass spectrometer was used in this experiment. The VOCs were separated using a DB-WAX (30 m × 0.25 mm × 0.25 μm) and a DB-17MS (2 m × 100 μm × 0.10 μm) with an oven temperature programmer held at 40 °C for 3 min, ramped at 5 °C/min to 250 °C, and held for 5 min. The carrier gas was helium (>99.99%) with a constant flow rate of 1.0 mL/min. The mass spectrometer conditions were an electron ionization mode and an electronic energy of 70 eV. The interface temperature was set to 270 °C, the ion source temperature was 250 °C, and the mass scan range was 33–500 AMU.
2.4. Identification and quantification of aroma compounds
Each VOC was identified by comparing its mass spectrum from database libraries (NIST). Retention indices (RIs) and retention times were assessed with the data available in the published literature and online library. The VOCs were semi-quantitatively identified based on their peak areas relative to those of the internal standard. All samples were analyzed in triplicates.
2.5. Characterization of key aroma compounds
The ROAVs explain the contribution of each VOC to the aroma profile. Components with ROAV ≥ 1 were generally accepted to contribute to the overall flavor of the sample, whereas components with 0.1 ≤ ROAV < 1 had an important modifying contribution (Zhang et al., 2020). The ROAV was calculated according to Equation 1 and 2.
| (1) |
| (2) |
where OAV is the odor activity value (OAV) of an arbitrary VOC; OAVmax is the maximum OAV of all compounds in the sample; C and T are the detection and threshold concentrations of an aroma compound, respectively. Among them, the thresholds were taken from https://pubchem.ncbi.nlm.nih.gov/compound/.
2.6. Quantitative descriptive sensory analysis
Quantitative descriptive sensory analysis was conducted as described by Chen et al. (2021), with minor modifications. Ten panelists (five men and five women; average age: 27 years old) who have been familiar with the fermentation and flavor of gray sufu for a long time have been trained regularly to evaluate the sensory quality of gray sufu. Eight relevant flavor attributes, namely, fishy, fruity, gasoline, pungent, rancid, butter, sweet, and herbal, were selected after the panel discussion. Definitions and references for these terms were based on the ROAV analysis. The odor intensities were defined as 0 = no smell, 1 = very weak, 2 = weak, 3 = medium, 4 = strong, and 5 = very strong. The average of the final scores of the sensory group indicated the flavor profile of gray sufu. Each experiment was repeated thrice at room temperature.
2.7. Aroma recombination
Deodorized matrixes were prepared as described by Chen et al. (2021), with some modifications. VOCs with ROAVs ≥ 1 and the same amount of high-content VOCs were added to the deodorization matrix for recombinant models 1 and 2, respectively. The models were equilibrated at room temperature for 10 min. Then, the intensities of the eight different odor types produced by the chemicals were evaluated. Odor intensity was evaluated as described in Section 2.6. The recombinant samples were encoded and placed at room temperature, and the evaluators were instructed to sniff the samples for 90 s for evaluation. Each sample was assessed thrice at 10-min interval. The results were plotted in the form of a spider web diagram.
2.8. Omission experiments
A triangle test was used to select the VOCs that contributed greatly to the flavor of gray sufu (Tan et al., 2021). We omitted one or more VOCs from the recombinant model, including two complete recombinant models and one missing model. The omission model and complete recombination models were evaluated by ten panelists to select a model that differed considerably from the other two models.
2.9. Statistical analysis
All results from independent triplicates were expressed as mean ± standard deviation. Heatmaps were drawn using pheatmap (v3.3.2) in R. Data were analyzed using Graphpad Prism 8. For comparison between three or more samples, one-way analysis of variance followed by Tukey’s multiple comparison tests were used. In addition, P < 0.05, P < 0.01, and P < 0.001 were used to denote significance, high significance, and very high significance, respectively.
3. Results and discussion
3.1. GC × GC-TOF-MS analysis of VOCs in gray sufu fermented by L. Mesenteroides F24
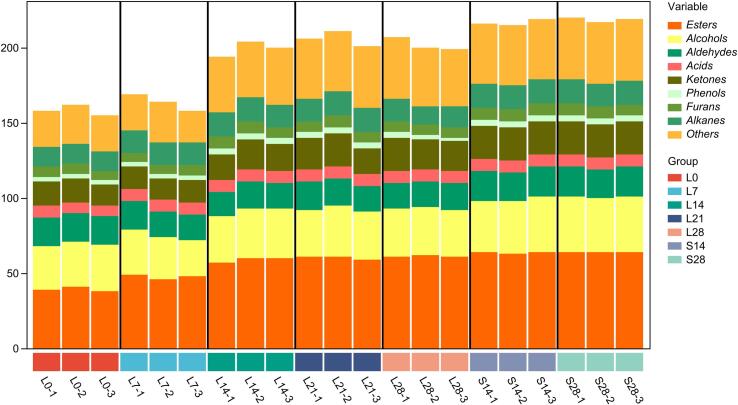
The concentrations of VOCs in gray sufu with spontaneous fermentation and inoculated with L. mesenteroides F24 were measured by headspace SPME (HS-SPME) and GC × GC-TOF-MS (Supplementary Table S1). A total of 220 VOCs were detected in gray sufu in spontaneous fermentation (group S) and inoculation with L. mesenteroides F24 (group L) at different fermentation periods. These VOCs can be divided into nine categories based on their chemical structures (Fig. 1). The structures and numbers of the chemicals detected in gray sufu on the 28th day of fermentation in group S were as follows: esters, 64; alcohols, 37; ketones, 22; aldehydes, 20; acids, 8; phenols, 4; furans, 8; alkanes, 16; and others, 41. Meanwhile, the following compounds were detected on the 28th day after the addition of L. mesenteroides F24: esters, 61; alcohols, 32; ketones, 20; aldehydes, 17; acids, 8; phenols, 3; furans, 7; alkanes, 14; and other compounds, 39. As shown in Fig. 1, ester and alcohol compounds were the dominant VOCs, and they accounted for 29.09% and 16.82% of the VOCs in group S and 30.34% and 15.92% of those in group L, respectively. Esters and alcohols are the most abundant volatiles in sufu products according to Chen et al. (2022).
Fig. 1.
Relative abundance of each volatile compound in gray sufu samples.
Among all flavor chemicals, esters account for>60% of the total mass of flavor substances. Given that most esters have low odor thresholds, they may contribute remarkably to odor (Xu et al., 2022). Esters are formed by lower saturated fatty acids and saturated fatty alcohols, which can mainly endow fruit, flower, sweet, and milk flavors to fermented food (Sidira et al., 2016, Xu et al., 2022). On the 28th day of fermentation, esters exhibited the highest types and contents (Fig. 1, Fig. 2). The contents of ester compounds (e.g., ethyl acetate [fruity], ethyl 4-methylpentanoate, methyl acetate [fruity], ethyl butyrate [fruity], methyl 4-methylpentanoate, and propyl butyrate [cream] [Table S1]) in group L after 28 days of fermentation were significantly higher than those in group S. These results indicate that inoculation with L. mesenteroides F24 enhanced ester synthesis and allowed the gradual accumulation of esters during fermentation, which provided gray sufu with a fruitier and creamier aroma (Zhong et al., 2021).
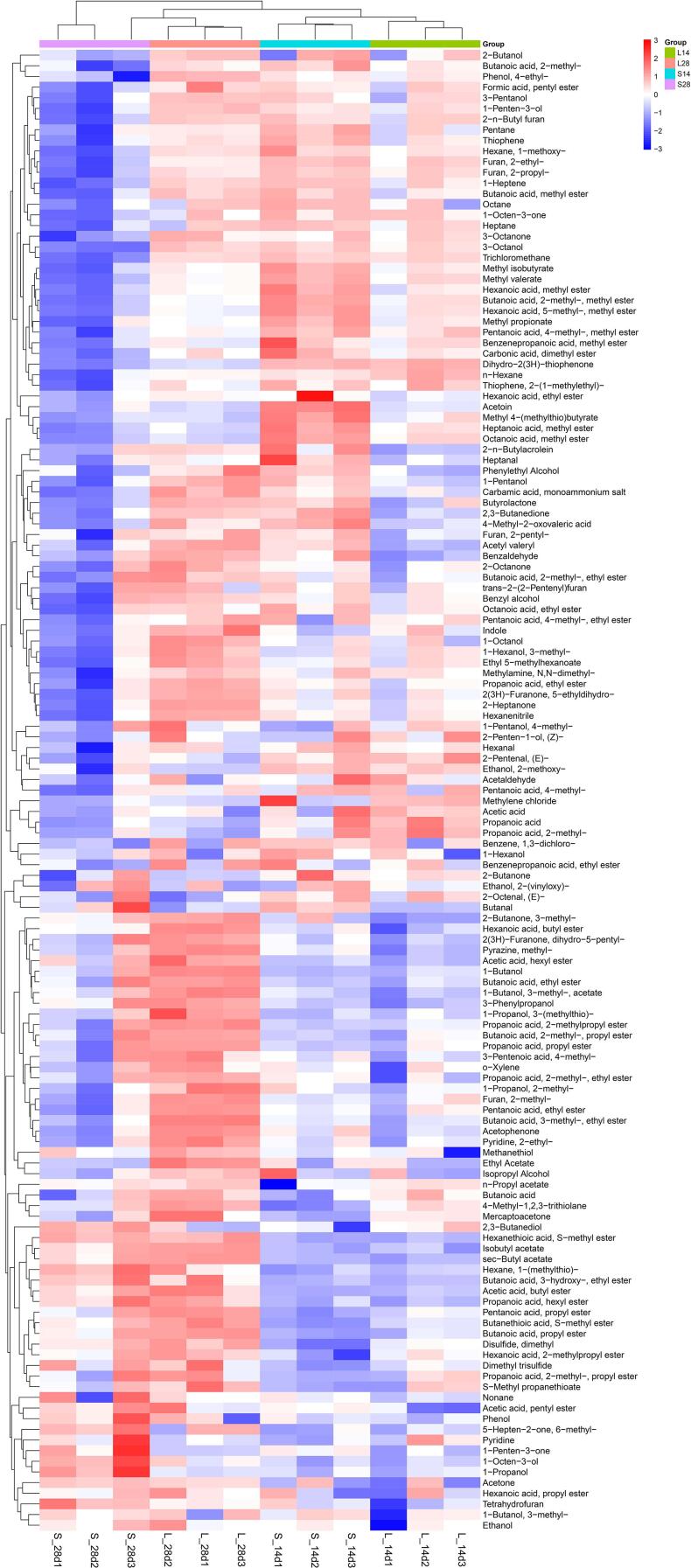
Fig. 2.
Heatmap and cluster analysis of volatile flavor compounds of gray sufu. Blue and red areas represent the minimum and maximum contents, respectively.
Alcohols, including ethanol, isopropyl alcohol, 1-propanol, 1-octanol, 1-heptanol, 1-hexanol, 1-pentanol, and 3-pentanol, were the second most widely detected compounds (Fig. 2). In this study, these alcohols showed higher contents during the fermentation of gray sufu. Yang et al. (2020) also observed that these alcohols are the main VOCs in raw soymilk. Ethanol was the most abundant alcohol compound produced during the fermentation of gray sufu. During fermentation, the change in volatiles and the accumulation of odor were mainly caused by three biochemical pathways, namely, ethanol fermentation metabolism, amino acid catabolism, and fatty acid catabolism. The biosynthesis of volatiles derived from ethanol fermentation was responsible for the “fermented” off-flavors (Xuan et al., 2022). By contrast, the introduction of L. mesenteroides F24 imparted a strong green and buttery aroma and significantly increased the contents of 1-octanol (green), 1-heptanol (herbal), 1-hexanol (fruity), and 1-penten-3-ol (fruity).
1-Octen-3-one (herbal), 2-octanone (cheese), 3-octanone (herbal), acetone (sweet), and acetophenone (floral) were the main ketone compounds detected during the fermentation of gray sufu. Ketones are mainly produced following the Maillard reaction and thermal modification of amino acids in food and oxidation of free fatty acids (Sevindik et al., 2022). Moreover, aldehyde contents, such as those of benzaldehyde (bitter almond), hexanal (leafy), nonanal (lime), and octanal (fruity), increased during the fermentation of group L. The formation of aldehydes may be related to the oxidative degradation of unsaturated fatty acids and the Strecker degradation of amino acids. In addition, microbial aminotransferase can convert free amino acids to α-ketoacids, which are further degraded to the corresponding aldehydes by various decarboxylases during fermentation (Ye et al., 2022). Therefore, such process may be the action mechanism of L. mesenteroides F24 that led to the accumulation of aldehyde compounds.
The volatile acids found in all samples were mainly acetic acid (sour), butanoic acid (penetrating and obnoxious odor), 2-methylbutanoic acid, propanoic acid (rancid odor), and isobutyric acid. Acids were the microbial metabolites produced during fermentation; they were responsible for the fruity, fatty, and sour aromas and contributed significantly to the sensory properties of gray sufu. During fermentation, the acid content gradually increased, possibly due to the oxidation of alcohols and hydrolysis of esters (Zhang et al., 2021). The contents of butanoic acid and 2-methylbutanoic acid in group L were higher than those in group S, possibly because branched-chain amino acids can be converted into organic acids through the first step of transamination after oxidation (Sevindik et al., 2022). Five phenolic substances, namely, phenol (medicinal), guaiacol (smoky), N-methyltyramine, 4-ethylphenol (woody), and 4-ethyl-2-methoxyphenyl (spicy), were also identified in this study. These compounds imparted a smoky, herbal, and spicy aroma to gray sufu (Sun et al., 2021).
Eight furan compounds were detected in gray sufu. Furans are mainly derived from the intermediate or final products of Maillard reactions between sugars and amino acids, primarily in fruity and smoky aroma (Sun et al., 2021). Hydrocarbons, which are possibly derived from the oxidation and degradation of fatty acids, are often aromatic and sweet, but higher thresholds, especially alkanes, have minimal contribution to the overall flavor (Ye et al., 2022). In addition, sulfur compounds, such as dimethyl disulfide, dimethyl sulfide, and dimethyl trisulfide, had been reported to act as aromatic components and precursors in reactions to produce more complex aromatic compounds. The contents of dimethyl disulfide, dimethyl sulfide, and dimethyl trisulfide were significantly higher in group L than in group S, and these compounds contributed to the production of more flavor substances during fermentation. Pyrazine is an important compound responsible for food flavor, and its flavor threshold is low. This compound contributed greatly to the formation of gray sufu flavor and mainly projected meaty, nutty, and roasted potato aromas. These compounds accumulated in the gray sufu in group L, which resulted in a more abundant flavor. Pyridine was generated later in fermentation. Pyridine forms when aldehydes and ketones are produced during lipid oxidation. Thus, the formation of pyridine is another consequence of the lipid oxidation pathway. Pyridine has a low threshold and various flavors. High concentrations of pyridine result in unpleasant pungent tastes, such as fishy, and its low concentrations lead to pleasant aroma characteristics, such as those of toast, corn, and cookies (Li et al., 2023).
3.2. Screening differential flavor metabolites in gray sufu fermented by F24
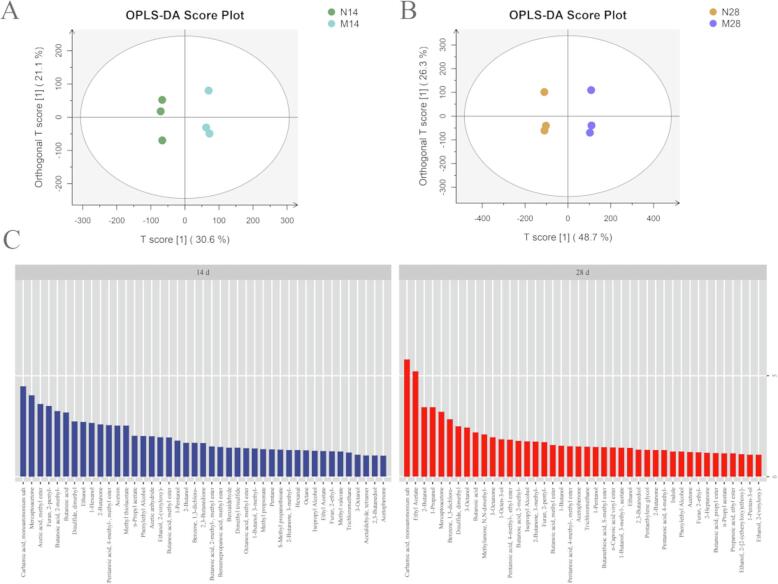
To further clarify the effect of adding L. mesenteroides F24 on the flavor of gray sufu, we analyzed the flavor compounds in groups S and L using the OPLS-DA model. As shown in Fig. 3A and 3B, group S was significantly distinct from group L when the fermentation was performed for 14 and 28 days. This result indicated that L. mesenteroides F24 had a significant effect on the flavor profile during spontaneous fermentation. In the current model, the values of R2X, R2Y, and Q2Y were 0.517, 0.998, and 0.721 for 14 days, respectively, and the corresponding values for 28 days were 0.75, 0.999, and 0.936. These findings showed that the model fitted well, and the predictability was acceptable (Fig. 3A, B). However, given that a large amount of data in OPLS-DA were not useful for classification, they greatly interfered with the analysis, thereby reducing the discrimination capability of the model (Kang et al., 2022). Therefore, the use of variable importance in the projection (VIP) is an effective method for selecting key flavor substances. Variables with VIP values > 1 are generally perceived to have significant differences between different categories and play an important role in classification. Thus, differential flavor metabolites were screened using VIP values > 1. As shown in Fig. 3C, the VIP values of 43 and 42 VOCs were > 1 on the 14th and 28th days of fermentation, respectively. On the 14th day, compared with those in group S, nine flavor compounds in group L were upregulated, and the other 34 were downregulated. The key VOCs of group L included mercaptoacetone, butanoic acid, dimethyl disulfide, methyl thiolacetate, n-propyl acetate, 2-butanol, dimethyl trisulfide, and 3-methyl-2-butanone (VIP > 1), which may be responsible for the fruity, penetrating, obnoxious, sulfurous, cooked-meat, wine, sweet, oily, apricot, and acetone-like odors. These aromas enhanced the characteristic flavor of gray sufu. Most of the nine compounds were sulfur-containing compounds, which were produced by amino acid degradation or lipid metabolism (Elena et al., 2022). This result indicated that L. mesenteroides F24 had significant effects on amino acid degradation and lipid metabolism. Although the addition of L. mesenteroides F24 reduced the content of some alcohols, esters, and aldehydes in the middle stage of fermentation, these compounds were significantly higher than those in group S on day 28 of fermentation, and they enhanced the fruity aroma of gray sufu. Thus, a correlation existed between L. mesenteroides F24 and the formation of volatile flavor substances, such as alcohols, aldehydes, and esters, was indicated. This finding was consistent with the report of He et al. (2020) and Lopez et al. (2022) studied the bread fermented by L. mesenteroides. The results showed that L. mesenteroides fermented significant branched-chain amino acids and metabolites, among which the BCAA metabolite is also related to flavor compounds (e.g., 3-methylbutyraldehyde) (Lopez et al., 2022). This phenomenon may also be the main reason why L. mesenteroides promoted the accumulation of flavor in gray sufu.
Fig. 3.
Orthogonal partial least square discriminant analysis of groups L (inoculation with L. mesenteroides F24) and S (spontaneous fermentation) on (A) day 14 and (B) day 28 of fermentation; (C) the VIP value of the differential metabolites.
3.3. Analysis of flavor compounds combined with flavoromics
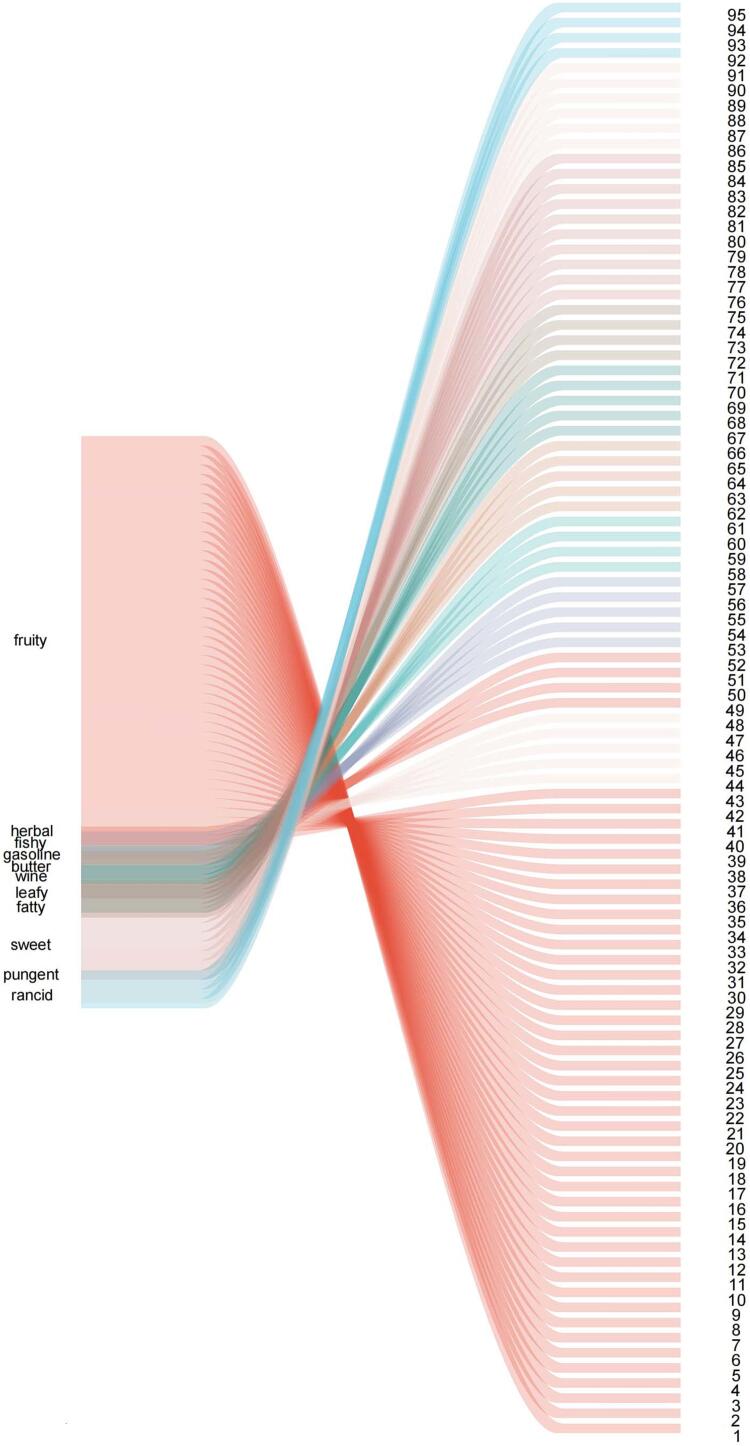
A total of 220 VOCs were detected in all samples (Fig. 1). These VOCs were further analyzed, and the aroma activities of 142 compounds were determined from PubChem database. Table 1 shows the aroma characteristics and odor of gray sufu. A total of 142 compounds formed the final flavor profile of gray sufu, with 95 compounds exhibiting important aroma attributes for all samples (Fig. 4): nos. 1–43, important fruity aroma; nos. 44–48, herbal aroma; nos. 49–52, fishy odor; nos. 53–57, gasoline odor; nos. 58–61, butter aroma; nos. 62–66, wine aroma; nos. 61–71, leafy aroma; nos. 72–75, fatty aroma; nos. 76–85, sweet aroma; nos. 86–91, pungent odor; and nos. 92–95, rancid odor. Moreover, the compounds with fruity aromas were mostly esters and alcohols. Esters are mostly short-chain fatty acid esters, such as ethyl acetate and ethyl butyrate. The esters accumulated in the fermentation group inoculated with L. mesenteroides F24, which indicates that the introduction of L. mesenteroides F24 improved the flavor quality of gray sufu. Short-chain fatty acid esters are a group of low-molecular-weight chemicals that are mainly produced by the esterification of alcohols with short-chain fatty acids. In fermented foods, these esters are mainly synthesized by enzymes from the carboxylic ester hydrolase superfamily (EC 3.1.1.-) in microorganisms, thus forming a unique flavor that affects the sensory quality and flavor characteristics (Xu et al., 2021). In addition, the unique flavor of gray sufu has been attributed to its unpleasant odor, such as gasoline, pungent, and rancid smell, due to the presence of nitrogen and sulfur-containing compounds. Nitrogen- and sulfur-containing compounds mainly include protein and amino acids in the substrate, which are converted into derivatives, such as ammonia, pyrazine, pyrrole, thiazole, trimethylamine, and amine compounds by microbial metabolism during fermentation (Lu, Liu, & Xu, 2022). Most of these flavor compounds also accumulated in large amounts on gray sufu during cofermentation, possibly due to the transformation of protein and amino acids in the substrate promoted by L. mesenteroides F24. These flavor compounds usually produce fishy and pungent odors. The above results indicated the overall flavor profile and change in the gray sufu inoculated with L. mesenteroides F24.
Table 1.
Aroma profile, odor characteristic, and the aromatic compounds in the gray sufu by HS–SPME–GC–MS.
| No | aCompound CID | Aroma compound | bOdor characteristic | cOdor Threshold(mg/kg) |
|---|---|---|---|---|
| 1 | 31,276 | 3-Methylbutyl Acetate | fruity, banana, sweet, fragrant | 0.017 |
| 2 | 8103 | 1-Hexanol | fruity, ethereal, green, flower, sweet | 0.2 |
| 3 | 6569 | 2-Butanone | fruity, ethereal, ether, camphor | 50 |
| 4 | 5,364,752 | Trans-2-Pentenal | pungent green, fruity | – |
| 5 | 7710 | 5-Pentyloxolan-2-One | a coconut-like | – |
| 6 | 19,707 | 2,3-Hexanedione | fruity, creamy, oily, sweet, fatty, caramel, butter | – |
| 7 | 70 | 4-Methyl-2-Oxovaleric Acid | fruity | – |
| 8 | 177 | Acetaldehyde | pungent, fruity, suffocating, fresh, green | 0.21 |
| 9 | 31,272 | Butyl Acetate | fruity | 0.31 |
| 10 | 8908 | Hexyl Acetate | sweet, fruity | – |
| 11 | 6584 | Methyl Acetate | fruity | 180 |
| 12 | 12,348 | Pentyl Acetate | banana-like | 0.26 |
| 13 | 7643 | Methyl 3-Phenylpropionate | fruity, wine, floral sweet | – |
| 14 | 11,552 | 3-Methylbutanal | apple-like | 0.06 |
| 15 | 24,020 | Ethyl 2-Methylbutyrate | fruity | – |
| 16 | 62,572 | Ethyl 3-Hydroxybutyrate | fruity, grape, green | – |
| 17 | 7945 | Ethyl 3-Methylbutanoate | fruity, vinous, apple-like | – |
| 18 | 7762 | Ethyl Butanoate | pineapple | – |
| 19 | 12,180 | Methyl Butyrate | apple-like | – |
| 20 | 7770 | Propyl Butyrate | pineapple, apricot | – |
| 21 | 8857 | Ethyl Acetate | fruity, ethereal, green, anise, weedy, balsam, sweet | 0.1 |
| 22 | 12,529 | Pentyl Formate | fruity | – |
| 23 | 19,602 | 2-Pentylfuran | fruity | – |
| 24 | 7826 | Methyl Heptanoate | fruity, orris | – |
| 25 | 7775 | Isobutyl Hexanoate | fruity, cocoa odor | – |
| 26 | 12,294 | Butyl Hexanoate | pineapple | – |
| 27 | 7824 | Methyl Hexanoate | pineapple, ethereal | – |
| 28 | 12,293 | Propyl Hexanoate | ethereal, pineapple-blackberry | – |
| 29 | 8038 | Isobutyl Acetate | fruity, floral | 0.037 |
| 30 | 6544 | Isophorone | fruity, cooling, green, leather, cedarwood, sweet | 5.4 |
| 31 | 65,425 | Methyl 4-(Methylthio)Butyrate | fruity, cabbage, pineapple, sulfury, cheese | – |
| 32 | 7909 | Methyl Isobutyl Ketone | fruity, ethereal | 0.88 |
| 33 | 11,160 | Methyl Isovalerate | fruity | 50 |
| 34 | 7997 | n-Propyl Acetate | mild, fruity odor | 0.18 |
| 35 | 16,106,703 | Pentanal | fruity, malt, bready, nutty, pungent | 0.07 |
| 36 | 527 | Propanal | suffocating, fruity | 1 |
| 37 | 7342 | Ethyl Isobutyrate | fruity odor | – |
| 38 | 12,571 | Propyl Isobutyrate | pineapple odour | – |
| 39 | 10,895 | Isobutyl Propionate | pineapple | – |
| 40 | 7749 | Ethyl Propionate | fruity, rum-like, ethereal odour | – |
| 41 | 12,217 | Pentyl Propionate | apple-like odor. | – |
| 42 | 7803 | Propyl Propionate | fruity (apple, banana, pineapple) odour | – |
| 43 | 7758 | sec-Butyl Acetate | fruity odor | – |
| 44 | 8129 | 1-Heptanol | herbal, violet, green, leafy, coconut, sweet | 0.2 |
| 45 | 61,346 | 1-Octen-3-one | herbal, earthy, metal, musty, mushroom, dirty | – |
| 46 | 8051 | 2-Heptanone | herbal, fruity, soap, coconut, sweet, spicy | 0.07 |
| 47 | 11,527 | 3-Octanol | herbal, mushroom, minty, nut, citrus, woody | – |
| 48 | 246,728 | 3-Octanone | herbal, lavender, mushroom, fresh, herb, sweet | 6 |
| 49 | 18,827 | 1-Octen-3-ol | raw, fishy, oily, earthy, fungal, chicken | – |
| 50 | 1146 | Trimethylamine | fishy, amine odor | 0.6 |
| 51 | 261 | Butanal | pungent, aldehyde | 0.009 |
| 52 | 19,310 | Dimethyl Trisulfide | cabbage, fish, sulfurous, cooked, sulfur, meaty | 0.003 |
| 53 | 8125 | 1-Octene | gasoline | 0.005 |
| 54 | 7500 | Ethylbenzene | sweet, gasoline-like odor | 0.09 |
| 55 | 8058 | n-Hexane | gasoline-like odor | 0.0064 |
| 56 | 8141 | Nonane | gasoline, alkane | 650 |
| 57 | 356 | Octane | gasoline-like | 150 |
| 58 | 12,020 | 1-Penten-3-ol | butter, pungent, tropical, horseradish, green | 3 |
| 59 | 6590 | Isobutyric Acid | sharp, butter | 1 |
| 60 | 650 | 2,3-Butanedione | butter, caramel, oily, creamy, sweet, pungent | 0.0086 |
| 61 | 179 | Acetoin | butter | – |
| 62 | 6560 | 2-Methyl-1-Propanol | solvent, ether, wine, bitter | 40 |
| 63 | 31,265 | Ethyl Caproate | wine-like odour | – |
| 64 | 7799 | Ethyl Octanoate | wine, brandy, fruity floral odour | – |
| 65 | 8091 | Methyl Octanoate | wine, fruity, orange | – |
| 66 | 6568 | 2-Butanol | wine, sweet, oily, apricot | 3.2 |
| 67 | 5,281,168 | 2-Hexenal | leafy, apple, cheesy, vegetable, fat, banana | – |
| 68 | 5,283,324 | Trans-2-Octenal | green-leafy odor | 0.061 |
| 69 | 6184 | Hexanal | leafy, fruity, sweaty, grass, fatty, aldehydic | – |
| 70 | 996 | Phenol | medicinal, acid,ink, creosote | 5.5 |
| 72 | 8093 | 2-Octanone | fatty, green, cheese, floral, bitter, fruity | 248 |
| 73 | 454 | Octanal | fat, green, aldehydic, lemon, citrus, fatty | – |
| 74 | 5,283,321 | 2,4-Heptadienal | fatty, green aroma | 0.03 |
| 75 | 9862 | 6-Methyl-5-Hepten-2-one | strong fatty, green citrus-like odour | – |
| 76 | 12,756 | Gamma-Caprolactone | herbaceous, sweet odour | – |
| 77 | 11,124 | Methyl Propionate | sweet, fruity, rum | 50 |
| 78 | 6344 | Methylene Chloride | sweet, pleasant odor, chloroform | 214 |
| 79 | 7237 | o-Xylene | sweet | 1.8 |
| 80 | 7654 | Phenethyl Acetate | sweet, rose, honey | – |
| 81 | 180 | Acetone | sweet, fruity,etherous | 20 |
| 82 | 241 | Benzene | bitter almond, fruit, vanilla | 4.68 |
| 83 | 13,357 | Methyl 2-Methylbutyrate | sweet, fruity, apple | – |
| 84 | 10,885 | Isobutyl Butyrate | a sweet, fruity, apple, pineapple | – |
| 85 | 7302 | Butyrolactone | a faint, sweet, caramel | 20 |
| 86 | 7918 | Acetic Anhydride | pungent, vinegar | 1.44 |
| 87 | 6561 | Isobutyraldehyde | pungent | 0.3 |
| 88 | 8030 | Thiophene | pungent | – |
| 89 | 7150 | Methyl Benzoate | a pungent, heavy, floral | – |
| 90 | 261 | Butyraldehyde | pungent, aldehyde | – |
| 91 | 8005 | 1-Chlorobutane | pungent | 1.64 |
| 92 | 62,444 | Methyl Thiobutyrate | putrid, rancid, sour, pungent cabbage odor | – |
| 93 | 1032 | Propanoic Acid | pungent disagreeable, rancid | 0.066 |
| 94 | 1049 | Pyridine | putrid, rancid, sickening, fishy, sour, amine | 0.95 |
| 95 | 8130 | Heptanal | citrus, wine-lee, rancid, ozone, fatty, herbal | – |
| 96 | 263 | 1-Butanol | oil, vanilla, fruit, fusel, sweet, balsam | 0.5 |
| 97 | 31,260 | Isoamyl Alcohol | oil, alcoholic, burnt, whiskey, malt, banana | 0.1 |
| 98 | 62,465 | 4-Ethyl-2-Methoxyphenol | sweet, spicy, medicinal | – |
| 99 | 957 | 1-Octanol | burnt, orange, rose, waxy, chemical, metal | 24 |
| 100 | 6276 | 1-Pentanol | balsamic, vanilla, balsam, sweet, fusel, oil | 0.4 |
| 101 | 31,234 | 3-Phenylpropanol | balsamic | – |
| 102 | 1031 | 1-Propanol | alcoholic, fermented, alcohol, musty, fusel, pungent | 7 |
| 103 | 522,834 | 2-Acetyl-1-Pyrroline | roast, nut, roasted, ham, sweet, nutty | – |
| 104 | 11,251 | 3-Methyl-2-Butanone | acetone | 2.5 |
| 105 | 5,364,920 | Trans-2-Penten-1-ol | green | – |
| 106 | 5,364,919 | Cis-2-Penten-1-ol | green | – |
| 107 | 5,284,503 | 3-Hexen-1-ol | grassy, green | – |
| 108 | 11,747 | 2,3-Pentanedione | creamy, nutty, pungent, cream, sweet, butter | 0.005 |
| 109 | 61,021 | Metaldehyde | menthol | – |
| 110 | 176 | Acetic Acid | sour, vinegar, pungent | 1.2 |
| 111 | 12,587 | 4-Methylpentanoic Acid | sour, penetrating | – |
| 112 | 7410 | Acetophenone | acacia, flower, must, pungent, hawthorn, almond | 65 |
| 113 | 6054 | Phenylethyl Alcohol | rose | 7.02 |
| 114 | 240 | Benzaldehyde | bitter almond, fruit, vanilla | 0.05 |
| 115 | 244 | Benzyl Alcohol | faint | 5.5 |
| 116 | 7284 | 2-Methylbutyraldehyde | unpleasant | 0.04 |
| 117 | 7523 | 2-Ethylpyridine | unpleasant | – |
| 118 | 1068 | Dimethyl Sulfide | wild radish, cabbage | 0.0098 |
| 119 | 517,232 | Carbamic Acid | ammonia | 46.8 |
| 120 | 12,021 | Dimethyl Carbonate | pleasant | – |
| 121 | 6212 | Trichloromethane | pleasant, etheric, nonirritating | 192 |
| 122 | 7865 | Methyl Formate | pleasant | 2000 |
| 123 | 8452 | Cyclopentanone | minty | – |
| 124 | 264 | Butanoic Acid | penetrating, obnoxious | 1 |
| 125 | 13,852 | Dihydro-2(3H)-Thiophenone | burnt, garlic | – |
| 126 | 12,232 | Dimethyl Disulfide | garlic, sulfurous, diffuse, intense onion | 0.03 |
| 127 | 78,925 | 2-(Methylthio)Ethanol | meat | – |
| 128 | 8019 | 2-Methoxyethanol | ether | 0.22 |
| 129 | 8028 | Tetrahydrofuran | ether | 31 |
| 130 | 14,505 | 2-Acetylfuran | coffee | – |
| 131 | 18,554 | 2-Ethylfuran | smoky, burnt | – |
| 132 | 10,797 | Methylfuran | spicy, smoky | – |
| 133 | 8900 | Heptane | ethereal, alkane, sweet | 230 |
| 134 | 8027 | Pyrrole | ethereal, nutty, warm, sweet | – |
| 135 | 798 | Indole | feces | 0.5 |
| 136 | 878 | Methanethiol | rotten cabbage | – |
| 137 | 31,289 | Nonanal | lime, grapefruit, fat, rose, green, fresh | 0.0035 |
| 138 | 31,242 | P-Ethylphenol | woody-phenolic, sweet | – |
| 139 | 11,529 | Butyl Propionate | earthy, sweet | – |
| 140 | 88,454 | Hexyl Propionate | earthy, acrid | – |
| 141 | 31,252 | 2,5-Dimethyl Pyrazine | earthy, potato | 1 |
| 142 | 521,869 | S-Methyl Propanethioate | fruity, milk | – |
| 143 | 7976 | Methylpyrazine | nutty, cocoa | 0.25 |
| 144 | 9256 | Thiazole | nutty, pyridine, meaty | – |
| 145 | 8200 | Tetraethylene Glycol | mild | – |
a,bAromatic compounds CID and aroma descriptor were derived from the web pages: (https://pubchem.ncbi.nlm.nih.gov/compound/).
cOdor thresholds were taken from the web pages: (https://pubchem.ncbi.nlm.nih.gov/compound/) and (Lu et al., 2022).
Fig. 4.
Tracing diagram of flavor compound origin. Note: the numbered compounds in the figure are shown in Table 1.
3.4. Identification of key flavor compounds in gray sufu fermented by L. Mesenteroides F24
Because the samples under study may contain dozens or even hundreds of volatile chemicals, absolute quantification is incredibly time-consuming and expensive. In the present study, our only goal is to screen a few compounds with the highest OAV. The relative odor activity value (ROAV) is a technique that allows for the analysis of the relative percent (C%) of these compounds (Cheng et al., 2023). This technique has also been widely used to identify the primary flavoring ingredients in food, such as fragrant rapeseed oil and camellia seed oil (Fang et al., 2022, Liang et al., 2023). A total of 43 main aroma components (ROAV > 0.1) were identified in groups L and S at different fermentation stages, and 29 key aroma components (ROAV > 1) were detected (Table S2). Although significant differences were found in the key aroma components among the samples, 18 key aroma components, such as dimethyl trisulfide, n-hexane, 2-methylbutyraldehyde, ethyl acetate, and pentanal, were identified in all samples, and they provided fishy, gasoline-like, unpleasant, fruity, amine-like, buttery, pungent, balsamic, creamy, bitter almond-like, garlic-like, feces-like, and lime-like odors. These aromas together constitute the main flavor characteristics of gray sufu. In addition, at the end of fermentation, compared with group S, group L contained four unique key flavor substances, namely, ethyl acetate, 2,3-pentanedione, isobutyl acetate, and 1-heptanol, which endowed gray sufu with fruity, creamy, floral, and a unique herbal aroma. Fermentation with L. mesenteroides F24 might have upregulated the key genes and proteins, such as alcohol dehydrogenase family, alcohol O-acetyltransferase, acetyl-CoA C-acetyltransferase, and acyl-CoA thioester hydrolase. However, this process might have also downregulated aldehyde dehydrogenase family to participate in the glycolysis/gluconeogenesis pathway, starch and sucrose metabolism pathway, amino sugar and nucleotide sugar metabolism pathway, tricarboxylic acid cycle, and pyruvic acid metabolism pathway, thus promoting the formation of ethyl acetate, alcohol and other esters (Liu, Qin, Hu, & Miao, 2023). In the future, through the complementary analysis of transcriptome and protein group, the formation mechanism of key flavors in gray sufu fermented by L. mesenteroides F24 will be explored.
3.5. Aroma recombination and omission tests
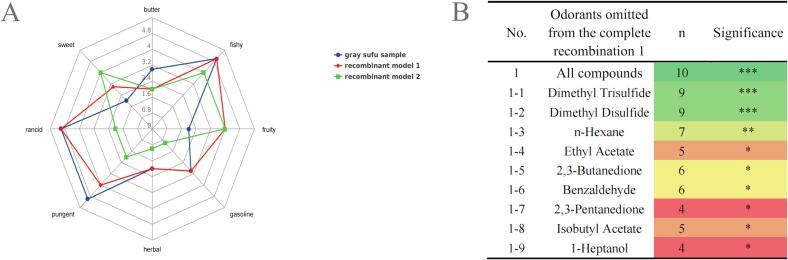
Based on the results of GC–MS detection, flavoromics, and ROVA analysis, the gray sufu flavor was more diversified in group L than in group S. Therefore, the gray sufu samples fermented for 28 days in group L were selected for the aroma omission and recombination experiments to verify the results of the ROAV analysis and GC–MS. Recombinant experimental models were prepared using an odorless matrix, aromatic compound standards with ROAV > 1 and the same amount of high-content VOCs, and approximately 60% ultrapure water (Chen et al., 2021). As shown in Fig. 5A, subtle differences were observed in the sweet, buttery, and pungent flavors between recombinant model 1 and the original sample, but without statistical difference (P > 0.05). This phenomenon may be related to masking, inhibition, or synergy between compounds with ROAV < 1 and ROAV ≥ 1 (Zhang, Gao, Xia, & Jiang, 2022). However, a significant difference (P < 0.05) was found in the flavor between the recombinant model 2 and the original samples, indicating that the results of GC–MS detection and ROAV analysis were reliable.
Fig. 5.
(A) Aroma profiles of the original sample and recombinant model. (B) Omission experiments using complete recombination 1; n: number of panelists who perceived correctly the differences in the triangle test; significance: *** (P < 0.001), very highly significant); ** (P < 0.01, highly significant); * (P < 0.05, significant).
Therefore, recombination model 1 was selected for the omission experiment. The experiment was omitted to study the effect of specific compounds or a group of compounds on the overall odor of gray sufu. As shown in Fig. 5B, 10 omission models were prepared, in which a single compound or all compound groups were missed. Three models showed extremely significant differences (P < 0.001), one missing model exhibited highly significant differences (P < 0.01), and six missing models indicated significant differences (P < 0.05). When all compounds were omitted, a very significant difference was observed (P < 0.001), indicating the successful establishment of an odorless matrix. Extremely significant differences (P < 0.001) were also detected when dimethyl trisulfide and dimethyl disulfide were removed separately. These compounds are associated with flavors such as fishy and radish (Table 1), which may be the main source of odor in gray sufu. In addition, the absence of n-hexane, ethyl acetate, 2,3-butanedione, benzaldehyde, 2,3-pentanedione, isobutyl acetate, and 1-heptanol contributed to the flavor to a certain extent. Thus, the key flavor substances of gray sufu were determined.
4. Conclusion
This study explored for the first time the feasibility and advantages of L. mesenteroides F24 for improving the flavor of gray sufu, a traditional fermented soybean product in China. The results revealed the important characteristics of VOCs in gray sufu, with alcohols and esters as the main VOCs. The results of multivariate statistical analysis showed that the content levels of VOCs in the gray sufu inoculated with L. mesenteroides F24 were significantly different from those in the naturally fermented one. The key odors in gray sufu were identified by the flavor database method and ROAV analysis. L. mesenteroides F24 significantly improved the aroma quality of gray sufu. The main flavor substances of gray sufu are dimethyl trisulfide, dimethyl disulfide, n-hexane, ethyl acetate, 2,3-butanedione, benzaldehyde, 2,3-pentanedione, isobutyl acetate and 1- heptanol. This study is the first comprehensive report on the use of L. mesenteroides F24 in assisting the fermentation of gray sufu. The findings may help in understanding the aroma quality of gray sufu and provide a theoretical basis for the processing and quality control of gray sufu products. Thus, L. mesenteroides F24 is a promising adjunct strain to promote the modern industrial production of traditional gray sufu.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Jilin Provincial Key Science and Technology Innovation Project (No. 20220508106RC) and the China Agriculture Research System of MOF and MARA (No. CARS-04).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100881.
Contributor Information
Dayong Ren, Email: rdy79@163.com.
Hansong Yu, Email: yuhs79@126.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article: Supplementary Table S1. Volatile compounds of gray sufu as identified by SPME-GC-MS
Supplementary Table S2. Screening of key aroma compounds based on ROAV≥1.
Data availability
No data was used for the research described in the article.
References
- An F.Y., Li M., Zhao Y., Zhang Y., Mu D.L., Hu X.Y. Metatranscriptome-based investigation of flavor-producing core microbiota in different fermentation stages of dajiang, a traditional fermented soybean paste of Northeast China. Food Chemistry. 2020;343 doi: 10.1016/j.foodchem.2020.128509. [DOI] [PubMed] [Google Scholar]
- Chen C., Liu Z., Hy A., Yu H.Y., Xu Z.Y., Tian H.X. Flavoromic determination of lactones in cheddar cheese by GC–MS–olfactometry, aroma extract dilution analysis, aroma recombination and omission analysis. Food Chemistry. 2021;368 doi: 10.1016/j.foodchem.2021.130736. [DOI] [PubMed] [Google Scholar]
- Chen Z.G., Zhang C.R., Du H., Chen C., Xue Q.L., Hu Y.J. Effect of starter cultures on dynamics succession of microbial communities, physicochemical parameters, enzyme activities, tastes and volatile flavor compounds during sufu fermentation. Food Chemistry Advances. 2022;1 doi: 10.1016/j.focha.2022.100057. [DOI] [Google Scholar]
- Cheng H., Mei J., Xie J. Analysis of key volatile compounds and quality properties of tilapia (Oreochromis mossambicus) fillets during cold storage: Based on thermal desorption coupled with gas chromatography-mass spectrometry (TD-GC-MS) LWT - Food Science and Technology. 2023;184 doi: 10.1016/J.LWT.2023.115051. [DOI] [Google Scholar]
- Elena B., Gregorio P., Andrea V., Shakil A.P., Ravi S., Stefania S. Investigation of sulfur-containing compounds in spears of green and white Asparagus officinalis through LC-MS and HS-GC–MS. Food Research International. 2022;162(PA) doi: 10.1016/j.foodres.2022.111992. [DOI] [PubMed] [Google Scholar]
- Fang Z.Y., Li G.Z., Gu Y., Wen C., Ye H., Ma J.L., Liang Z.Y. Flavour analysis of different varieties of camellia seed oil and the effect of the refining process on flavour substances. LWT - Food Science and Technology. 2022;170 doi: 10.1016/J.LWT.2022.114040. [DOI] [Google Scholar]
- Gu J.S., Liu T.J., Hou J., Pan L.L., Sadiq F.A., Yuan L. Analysis of bacterial diversity and biogenic amines content during the fermentation processing of stinky tofu. Food Research International. 2018;111:689–698. doi: 10.1016/j.foodres.2018.05.065. [DOI] [PubMed] [Google Scholar]
- Han B.Z., Rombouts F.M., Nout M.J.R. A Chinese fermented soybean food. International Journal of Food Microbiology. 2001;65(1):1–10. doi: 10.1016/S0168-1605(00)00523-7. [DOI] [PubMed] [Google Scholar]
- Hesseltine, C. W., Wang, H. L. (1986). Indigenous fermented food of non-Western origin. (Chapter 3).
- He Z., Chen H., Wang X., Lin X., Ji C., Li S. Effects of different temperatures on bacterial diversity and volatile flavor compounds during the fermentation of suancai, a traditional fermented vegetable food from northeastern China. LWT - Food Science and Technology. 2020;118(C) doi: 10.1016/j.lwt.2019.108773. [DOI] [Google Scholar]
- Kang C.D., Zhang Y.Y., Zhang M.Y., Qi J., Zhao W.T., Gu J. Screening of specific quantitative peptides of beef by LC–MS/MS coupled with OPLS-DA. Food Chemistry. 2022;387 doi: 10.1016/j.foodchem.2022.132932. [DOI] [PubMed] [Google Scholar]
- Liao X.L., Yan J.N., Wang B., Meng Q., Zhang L.Y., Tong H.R. Identification of key odorants responsible for cooked corn-like aroma of green teas made by tea cultivar ' Zhonghuang 1 ′. Food Research International. 2020;136 doi: 10.1016/j.foodres.2020.109355. [DOI] [PubMed] [Google Scholar]
- Li Y., Yuan L., Liu H., Liu H., Zhou Y., Li M. Analysis of the changes of volatile flavor compounds in a traditional Chinese shrimp paste during fermentation based on electronic nose, SPME-GC-MS and HS-GC-IMS. Food Science and Human Wellness. 2023;12(1):173–182. doi: 10.1016/j.fshw.2022.07.035. [DOI] [Google Scholar]
- Lopez L.M., Gabriele R., Alessandra F., Luigi L., Annalisa R. Metabolomics and gene-metabolite networks reveal the potential of Leuconostoc and Weissella strains as starter cultures in the manufacturing of bread without baker’s yeast. Food Research International. 2022;162(PA) doi: 10.1016/j.foodres.2022.112023. [DOI] [PubMed] [Google Scholar]
- Lu K., Liu L., Xu Z.Y., Xie W.C. The analysis of volatile compounds through flavoromics and machine learning to identify the origin of traditional Chinese fermented shrimp paste from different regions. LWT - Food Science and Technology. 2022;171 doi: 10.1016/j.lwt.2022.114096. [DOI] [Google Scholar]
- Liang Q., Xiong W., Zhou Q., Cui C., Xu X., Zhao L., Yao Y.Z. Glucosinolates or erucic acid, which one contributes more to volatile flavor of fragrant rapeseed oil? Food Chemistry. 2023;412(PP) doi: 10.1016/J.FOODCHEM.2023.135594. [DOI] [PubMed] [Google Scholar]
- Liu N., Qin L., Hu L., Miao S. Formation mechanisms of ethyl acetate and organic acids in Kluyveromyces marxianus L1–1 in Chinese acid rice soup. Food Science and Human Wellness. 2023;12(1):45–56. doi: 10.1016/j.fshw.2022.07.017. [DOI] [Google Scholar]
- Qian M., Ruan F.X., Zhao W.H., Dong H., Bai W.D., Li X.L. The dynamics of physicochemical properties, microbial community, and flavor metabolites during the fermentation of semi-dry Hakka rice wine and traditional sweet rice wine. Food Chemistry. 2023;416 doi: 10.1016/J.FOODCHEM.2023.135844. [DOI] [PubMed] [Google Scholar]
- Sun N., Zhang Y.T., Yu H.S., Zhu X.M., Zhu X.M., Ren D.Y. Microflora Structure and Flavor Components and Correlation between Them in Fermented Stinky Tofu (in chinese) Food Science. 2020;41(22):177–183. doi: 10.7506/spkx1002-6630-20190929-360. [DOI] [Google Scholar]
- Seo S.H., Park S.E., Kim E.J., Lee K.I., Na C.S., Son H.S. A GC-MS based metabolomics approach to determine the effect of salinity on Kimchi. Food Research International. 2018;105:492–498. doi: 10.1016/j.foodres.2017.11.069. [DOI] [PubMed] [Google Scholar]
- Sidira M., Kandylis P., Kanellaki M., Kourkoutas Y. Effect of curing salts and probiotic cultures on the evolution of flavor compounds in dry-fermented sausages during ripening. Food Chemistry. 2016;201:334–338. doi: 10.1016/j.foodchem.2016.01.084. [DOI] [PubMed] [Google Scholar]
- Sevindik O., Agirman B., Selli S., Kadiroglu P., Bordiga M., Capanoglu E., Guclu G. Impacts of selected lactic acid bacteria strains on the aroma and bioactive compositions of fermented gilaburu (Viburnum opulus) juices. Food Chemistry. 2022;378 doi: 10.1016/j.foodchem.2022.132079. [DOI] [PubMed] [Google Scholar]
- Sun X.Z., Du J.W., Xiong Y.Q., Cao Q.W., Wang Z., Li H.J. Characterization of the key aroma compounds in Chinese JingJiu by quantitative measurements, aroma recombination, and omission experiment. Food Chemistry. 2021;352 doi: 10.1016/j.foodchem.2021.129450. [DOI] [PubMed] [Google Scholar]
- Thayanna S.P., Nádia N.B., Lúcia P.S.P. Self-induced anaerobiosis coffee fermentation: Impact on microbial communities, chemical composition and sensory quality of coffee. Food Microbiology. 2022;103 doi: 10.1016/j.fm.2021.103962. [DOI] [PubMed] [Google Scholar]
- Tan F.L., Wang P., Zhan P., Tian H.L. Characterization of key aroma compounds in flat peach juice based on gas chromatography-mass spectrometry-olfactometry (GC-MS-O), odor activity value (OAV), aroma recombination, and omission experiments. Food Chemistry. 2021;366 doi: 10.1016/j.foodchem.2021.130604. [DOI] [PubMed] [Google Scholar]
- Wang Y., Gao Y., Liang W., Liu Y., Gao H. Identification and analysis of the flavor characteristics of unfermented stinky tofu brine during fermentation using SPME-GC–MS, e-nose, and sensory evaluation. Journal of Food Measurement and Characterization. 2020;14(1):597–612. doi: 10.1007/s11694-019-00351-w. [DOI] [Google Scholar]
- Xu Y.Q., Zhao J.R., Liu X., Zhang C.S., Zhao Z.G., Li X.T. Flavor mystery of Chinese traditional fermented baijiu: The great contribution of ester compounds. Food Chemistry. 2022;369 doi: 10.1016/j.foodchem.2021.130920. [DOI] [PubMed] [Google Scholar]
- Xuan X.T., Sun R.Y., Zhang X.Y., Cui Y., Lin X.D., Sun Y. Novel application of HS-GC-IMS with PCA for characteristic fingerprints and flavor compound variations in NFC Chinese bayberry (Myrica rubra) juice during storage. LWT - Food Science and Technology. 2022;167 doi: 10.1016/j.lwt.2022.113882. [DOI] [Google Scholar]
- Xu Y., Wang X., Liu X., Li X., Zhang C., Li W. Discovery and development of a novel short-chain fatty acid ester synthetic biocatalyst under aqueous phase from Monascus purpureus isolated from Baijiu. Food Chemistry. 2021;338 doi: 10.1016/j.foodchem.2020.128025. [DOI] [PubMed] [Google Scholar]
- Yang M.L., Huang J., Zhou R.Q., Qi Q., Peng C., Zhang L. Characterizing the microbial community of Pixian Doubanjiang and analysing the metabolic pathway of major flavour metabolites. LWT - Food Science and Technology. 2021;143 doi: 10.1016/j.lwt.2021.111170. [DOI] [Google Scholar]
- Yang Y., Wang B., Fu Y., Shi Y.G., Chen F.L., Guan H.N. HS-GC-IMS with PCA to analyze volatile flavor compounds across different production stages of fermented soybean whey tofu. Food Chemistry. 2020;346 doi: 10.1016/j.foodchem.2020.128880. [DOI] [PubMed] [Google Scholar]
- Ye Z., Shang Z.X., Zhang S.Y., Li M.Q., Zhang X.T., Ren H.B. Dynamic analysis of flavor properties and microbial communities in Chinese pickled chili pepper (Capsicum frutescens L.): A typical industrial-scale natural fermentation process. Food Research International. 2022;153 doi: 10.1016/j.foodres.2022.110952. [DOI] [PubMed] [Google Scholar]
- Zhang H., Huang D., Pu D., Zhang Y., Chen H., Sun B. Multivariate relationships among sensory attributes and volatile components in commercial dry porcini mushrooms (Boletus edulis) Food Research International. 2020;133 doi: 10.1016/j.foodres.2020.109112. [DOI] [PubMed] [Google Scholar]
- Zhong A.A., Chen W., Duan Y.F., Li K., Tang X.Y., Tian X. The potential correlation between microbial communities and flavors in traditional fermented sour meat. LWT - Food Science and Technology. 2021;149 doi: 10.1016/j.lwt.2021.111873. [DOI] [Google Scholar]
- Zhang X.J., Meng L.J., Lu Z.M., Chai L.J., Wang S.T., Shi J.S. Identification of age-markers based on profiling of Baijiu volatiles over a two-year maturation period: Case study of Lu-flavor Baijiu. LWT - Food Science and Technology. 2021;141 doi: 10.1016/j.lwt.2021.110913. [DOI] [Google Scholar]
- Zhang X.J., Gao P., Xia W.S., Jiang Q.X., Liu S.Q., Xu Y.S. Characterization of key aroma compounds in low-salt fermented sour fish by gas chromatography-mass spectrometry, odor activity values, aroma recombination and omission experiments. Food Chemistry. 2022;397 doi: 10.1016/j.foodchem.2022.133773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.