Abstract
Background & Aims
Frailty is prevalent in liver transplant (LT) candidates. It is considered an independent predictor of adverse outcomes pre- and post-transplant according to data obtained in the United States. We aimed to externally validate the liver frailty index (LFI) in a multicenter cohort of LT candidates.
Methods
Outpatients with cirrhosis were prospectively recruited from five Spanish centers (2018-2020). Patients were defined as “frail” by an optimal cut-off of LFI ≥4.5. Patients were followed for at least 6 months to study associations of pre-LT frailty with pre- and post-transplant mortality, length of hospital and intensive care unit (ICU) stays, risk of early (<30 days) and late (30-90 days) post-transplant complications, retransplantation and cardiovascular events.
Results
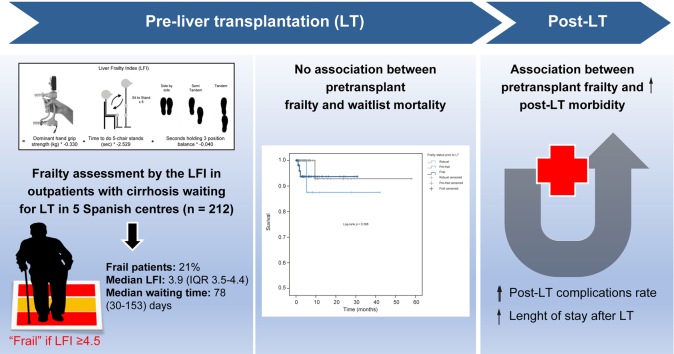
Of 212 patients included, 45 patients (21%) were frail pre-LT, and the median LFI was 3.9 (IQR 3.5–4.4). After a median waiting time of 78 days, 2% died or were delisted for clinical worsening. The LFI at baseline was not predictive of mortality/delisting in LT candidates in univariable or multivariable analyses after adjusting for age and MELD-Na score (hazard ratio 1.48; p = 0.586). In contrast, compared to non-frail patients, frail LT candidates had a significantly higher length of hospital stay (9 vs. 13 days; p = 0.001) and rate of early (<30 days) post-transplant complications (55% vs. 100%; p = 0.021).
Conclusions
In the context of a short LT waiting time, frailty does not impact pretransplant mortality and/or delisting. In contrast, LT frailty is predictive of higher post-transplant complication rates and length of hospital stay. Whether strategies aimed at pre- and/or re-habilitation are beneficial in settings with short waiting times needs to be confirmed in prospective studies.
Impact and implications
Literature is scarce on the actual impact of physical frailty on adverse outcomes in the liver transplant scenario outside North America. Evidence-based justification to extend the use of objective frailty tools in the decision-making processes in other liver transplant settings is needed. This study is the first to evaluate the predictive value of the liver frailty index in outpatients in the European liver transplant setting, showing that in a low MELD, high access system, frailty does not impact pretransplant mortality and/or delisting but is predictive of higher complication rates and longer post-transplant length of stay. In practical ways, physicians should consider physical frailty as a vital sign to be measured systematically and routinely during clinic visits; researchers are encouraged to initiate prospective studies to evaluate the benefit of applying strategies aimed at pre- and or re-habilitation in liver transplant settings with short waiting times.
Keywords: Frailty, cirrhosis, liver transplantation, outcomes, mortality, morbidity
Graphical abstract
Highlights
-
•
In a low MELD, high access system, frailty assessed in outpatients with cirrhosis waitlisted for liver transplantation:
-
•
The potential benefits of strategies aimed at pre- and/or rehabilitation should be assessed in prospective studies.
Introduction
Physical frailty is a prevalent condition in patients with cirrhosis and represents a clinical manifestation of muscle wasting, malnutrition and functional decline.1 In the liver transplant (LT) setting, physical frailty has emerged as a risk factor for poor outcomes both pre- and post-transplant mostly based on large prospective cohort studies from the United States and Canada.[2], [3], [4], [5], [6], [7] The American Societies of Transplantation and Liver Diseases have recently recommended incorporating frailty assessments in routine clinical practice in all outpatients with cirrhosis using one standardized frailty instrument to guide clinical decision making and to identify candidates for pre- and/or re-habilitation programs.8,9 Of the many frailty tools available, the liver frailty index is one with wider applicability in the ambulatory scenario given its low cost, ease of use, interrater reliability and repeatability.8,10 Results from the multicenter Functional Assessment in Liver Transplantation (FrAILT) Study, a large research network focused on physical frailty in patients with end-stage liver disease waiting for LT in the United States, highlighted the predictive validity of the liver frailty index both before and after LT. In the pretransplant setting, a strong association was found between the liver frailty index and waitlist mortality independently of standard markers of liver disease severity such as the model for end-stage liver disease (MELD) score (with or without the addition of serum sodium concentration – MELD-Na).5 Moreover, the incorporation of the liver frailty index into the MELD-Na score predicted waitlist mortality more accurately than MELD-Na alone, highlighting the complementarity of these two parameters in LT candidates.5 Recently, data in the post-transplant setting have been published showing that pretransplant liver frailty index predicts mortality and high health care utilization in LT recipients.7
Literature is scarce on the actual impact of physical frailty on adverse outcomes in the LT scenario outside North America. Particularly, in Europe, the liver frailty index has only been validated as a risk factor for pretransplant poor outcomes in a Slovakian single-center prospective cohort study based on an inpatient cohort of 168 adults with decompensated cirrhosis, not in an ambulatory population.11 In Spain, every year, 5–10% of patients included in the Spanish LT wait list, died or are delisted due to worsening of their clinical condition and frailty seems to be involved in these bad outcomes.12,13 However, there is a lack of reliable and objective frailty measurements to support the decision to list/delist a patient in the LT setting.
In this study we aimed to validate pretransplant liver frailty index as a predictor of adverse outcomes before and after LT in a Spanish multicenter cohort of patients with cirrhosis awaiting and subsequently undergoing LT. We hypothesized that as in North American studies, pretransplant frailty would be associated with poor outcomes both while on the LT waitlist and after transplant.
Patients and methods
Study design
We performed a prospective, longitudinal cohort study involving five LT centers in Spain. Adult patients with cirrhosis enrolled from November 7, 2018, to December 22, 2020, who had at least one frailty assessment by the liver frailty index while on the waiting list, were assessed.
Study outcomes
The primary outcome was waitlist mortality, which we defined as a combined outcome of death or delisting for being too sick for LT.
Secondary outcomes were: (1) post-transplant mortality, defined as death within 6 months after LT and (2) post-transplant morbidity which included the following metrics of health resources expenditure: (i) transplant hospitalization length of stay, defined as the number of days between LT date and the date of discharge; (ii) intensive care unit (ICU) length of stay, defined as the number of days in the ICU after LT surgery; (iii) early post-transplant complications, for adverse events occurring within 30 days after LT, defined and graded according to the Clavien-Dindo classification (mild: < grade IIIA; severe: ≥ Grade IIIA);14 (iv) late post-transplant complications, defined as adverse events requiring hospital readmission between 30 and 90 days after LT; (v) post-transplant cardiovascular events defined as acute coronary syndrome, ischemic/hemorrhagic stroke, heart failure, peripheral artery disease, arrhythmias (such as atrial fibrillation/flutter, except if appearing in the setting of sepsis, hemorrhage or during a surgery) occurring within 6 months after LT; and, (vi) need for retransplant in the 6 months after LT.
Study population and follow-up
Participants actively listed for LT and seen as outpatients at the following Spanish institutions were eligible: (i) La Fe University Hospital of Valencia, (ii) Clinic University Hospital of Barcelona, (iii) Reina Sofía University Hospital of Córdoba, (iv) Lozano Blesa University Hospital of Zaragoza and (v) Gregorio Marañón University General Hospital of Madrid. Patients were not enrolled if they had no underlying cirrhosis, were retransplant or combined LT candidates or had any acute decompensation of their liver disease at the time of the outpatient visit which would force their imminent hospitalization. We excluded patients whose last frailty assessment was performed more than 8 months prior to LT given that major changes in physical condition may occur in patients with end-stage liver disease in only a few months. After enrolment, patients’ outcomes were obtained prospectively. LT candidates were followed every 3-6 month while actively listed until death, delisting or transplant, whichever occurred first. After LT, each recipient was seen at 3 and 6 months concomitantly with regular clinic visits. Study duration for an individual patient was up to 32 months (a maximum of 26 months pretransplant and 6 months post-transplant).
The institutional review board from each center approved the study. Informed consent was obtained from each patient prior to participation in the study.
Frailty assessments
Our primary predictor was pretransplant frailty. All LT candidates underwent at least one objective measure of physical frailty while on the waiting list, using the liver frailty index, a continuous index specific for patients with cirrhosis calculated from the scores of three simple performance-based tests:
-
(i)
Dominant hand grip strength: the average of three measurements using a hand dynamometer, in kilograms.
-
(ii)
Chair stands: the number of seconds it takes a patient to do five chair stands with their arms folded across the chest.
-
(iii)
Balance testing: the number of seconds the patient can hold three positions (side, semi-tandem, and tandem) for a maximum of 10 s each.
These three tests were administered by trained study investigators. To calculate the liver frailty index, an online calculator was available at: http://liverfrailtyindex.ucsf.edu. Patients with a liver frailty index <3.2 were classified as “robust”, those with a liver frailty index between 3.2-4.4 were classified as “pre-frail” and those with a liver frailty index ≥4.5 were classified as “frail”, based on recommended cut-offs from a recently published American frailty experts opinion statement.8 Liver frailty index measurements were repeated every 6 months, coinciding with an outpatient clinic visit if the patient was still waiting for LT. For this study, the frailty assessment closest to the transplant date was used as the “pretransplant” frailty measurement for further analyses.
Additional data collection
Information regarding (i) demographics: age, sex, weight, height and BMI; (ii) baseline liver condition: etiology of liver disease (alcohol, non-alcoholic steatohepatitis [NASH], chronic HCV infection, cholestasic, chronic HBV infection, other), laboratory tests (creatinine, albumin, international normalized ratio, MELD-Na, MELD 3.0 and Child-Pugh score), history of ascites, hepatic encephalopathy and/or presence of hepatocellular carcinoma (HCC); (iii) cardiovascular comorbidities: history of hypertension, diabetes (type 1 or type 2) and/or cardiovascular disease, were extracted by study investigators from the clinic visit note closest to the liver frailty index assessment. Patients were considered to have a history of ascites or hepatic encephalopathy if it was reported in the electronic health record by the hepatologist who provided outpatient care to the patient. Particularly, ascites was categorized as “absent” if never reported, "mild-moderate" if controlled both with diuretic treatment or occasional paracentesis and "severe" if large-volume paracentesis were periodically required. Patients were classified as having diabetes or hypertension if this was listed in their past medical history or they were prescribed drug(s) to manage these diseases. History of cardiovascular disease was determined from medical chart and included coronary artery disease, heart failure, arrhythmias, peripheral artery disease and ischemic/hemorrhagic stroke.
Pre- and post-transplant outcomes were also obtained from the electronic health record. Per transplant protocol, all LT candidates and recipients requiring hospital admission are managed at the transplant center they belong to. Family and patients are required to notify the referenced transplant center of any hospitalization or death eventually, facilitating complete data capture regarding both waitlist mortality and post-transplant morbi-mortality.
Study size
An a priori power analysis was conducted for sample size estimation, based on data from the FrAI-LT study originally published in 2014.3 In that study, the prevalence of frail and robust patients among LT candidates was 17% and 83%, respectively. Waiting list mortality prevalence was 23% among frail patients and 11% among robust patients. With a significance criterion of α = 0.05 and power = 0.80, the sample size needed with this effect size is n = 306. Due to the COVID-19 pandemic, the obtained sample size (n = 212) was lower than initially estimated; and we performed a post hoc analysis. With the observed sample size, an 80% statistical power was also achieved for detecting waiting list mortality rates of 26% among frail patients and 11% among robust patients (similar effect size to initial calculations), thus confirming that the obtained sample size of n = 212 is more than adequate to test the study hypothesis.
Statistical analysis
We reported baseline characteristics of the entire cohort and by frailty categories using the liver frailty index to facilitate interpretation of characteristics that are generally associated with the frail phenotype. Continuous distributions were presented as medians (IQR) and discrete data were presented as frequencies (percentages). Differences in baseline characteristics by frailty categories were compared using chi-square or Mann-Whitney/Kruskal-Wallis tests for categorical and continuous variables, respectively.
Pre- and post-transplant outcomes were compared between frail and non-frail patients using chi-square, Fisher's exact and Mann-Whitney tests. The predicted probability of patient survival on the LT waitlist by pretransplant frailty categories (frail, pre-frail and robust) was determined with Kaplan-Meier curves. To precisely quantify the association between pretransplant liver frailty index (used as a continuous index) and both waitlist mortality and post-transplant outcomes, we used Cox and linear regression models, respectively. Then, to control for confounding factors, we adjusted for covariables associated with frailty in univariate analysis including MELD at transplant, recipient age, female sex, and cardiac comorbidities.
A cut-off p value <0.05 was used to determine statistical significance. SPSS 15.0, IL, USA, was used for all statistical analyses.
This manuscript adheres to the STROBE statement.15
Results
Participants
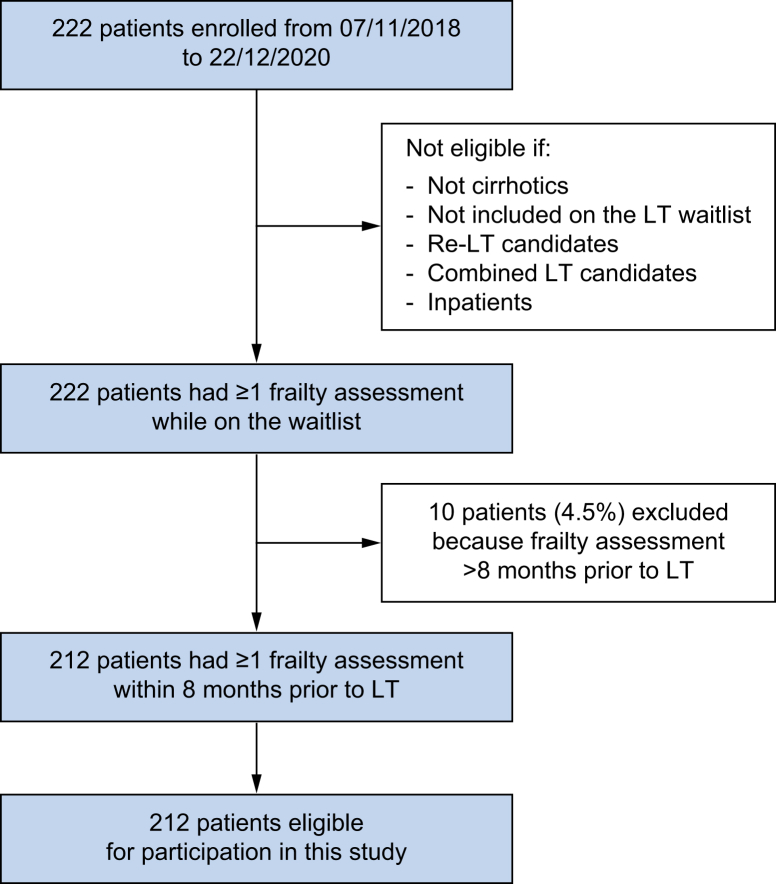
Of the 222 patients enrolled in the study during the study period, 10 patients (4.5%) were excluded because their last liver frailty index measurement was performed more than 8 months prior to LT. Finally, 212 were eligible for participation (Fig. 1).
Fig. 1.
Patient flow chart. LT, liver transplant.
Patient characteristics (Table 1)
Table 1.
Baseline characteristics of 212 patients with cirrhosis on the liver transplant waitlist and by frailty category†
| Pretransplant characteristics∗ | All (N = 212) | Robust (n = 26; 12%) | Pre-frail (n = 141; 67%) | Frail (n = 45; 21%) | p value∗∗ |
|---|---|---|---|---|---|
| Demographics | |||||
| Hospitals | |||||
| La Fe | 80 (38%) | 15 (58%) | 50 (35%) | 15 (33%) | 0.116 |
| Clínic | 83 (39%) | 9 (35%) | 55 (39%) | 19 (42%) | |
| Reina Sofia | 21 (10%) | 1 (4%) | 18 (13%) | 2 (4%) | |
| Lozano Blesa | 14 (7%) | 1 (4%) | 7 (5%) | 6 (13%) | |
| Gregorio Marañón | 14 (7%) | 0 | 11 (8%) | 3 (7%) | |
| Age, years | 60 (50-65) | 58 (55-63) | 59 (55-64) | 64 (59-68) | 0.006 |
| Female sex | 40 (19%) | 1 (4%) | 31 (22%) | 8 (18%) | 0.092 |
| Body mass index |
28 (25-31) |
29 (26-31) |
28 (25-30) |
27 (24-31) |
0.269 |
| Baseline liver condition | |||||
| Etiology of liver disease | |||||
| HCV | 79 (37%) | 16 (62%) | 54 (38%) | 9 (20%) | 0.041 |
| Alcohol | 74 (35%) | 7 (27%) | 46 (33%) | 21 (47%) | |
| NASH | 27 (13%) | 0 | 20 (14%) | 7 (16%) | |
| HBV | 14 (7%) | 3 (12%) | 8 (6%) | 3 (7%) | |
| Cholestasic | 2 (1%) | 0 | 2 (1%) | 0 | |
| Other | 16 (8%) | 0 | 11 (8%) | 5 (11%) | |
| HIV infection | 2 (8%) | 7 (5%) | 0 | 0.232 | |
| HCC | 121 (57%) | 24 (92%) | 83 (59%) | 14 (31%) | <0.001 |
| Laboratory tests | |||||
| MELD | 12 (9-18) | 8 (7-10) | 12 (9-17) | 16 (12-23) | <0.001 |
| MELD-Na | 12 (9-19) | 8 (7-10) | 12 (9-19) | 19 (12-27) | <0.001 |
| MELD 3.0 | 13 (9-19) | 7 (6-11) | 12 (9-18) | 18 (13-25) | <0.001 |
| Albumin, g/dl | 3.7 (3.2-4.2) | 4.5 (4.1-4.8) | 3.7 (3.2-4.2) | 3.2 (3.0-3.9) | <0.001 |
| Ascites | |||||
| Absent | 87 (41%) | 20 (77%) | 61 (43%) | 6 (13%) | <0.001 |
| Mild-moderate | 86 (41%) | 6 (23%) | 53 (38%) | 27 (60%) | |
| Severe | 39 (18%) | 0 | 27 (19%) | 12 (27%) | |
| Hepatic encephalopathy | 74 (35%) | 2 (8%) | 41 (29%) | 31 (69%) | <0.001 |
| Child-Pugh score |
7 (5-10) |
5 (5-6) |
7 (5-9) |
10 (7-11) |
<0.001 |
| Cardiovascular comorbidities | |||||
| Arterial hypertension | 72 (34%) | 6 (23%) | 49 (35%) | 17 (38%) | 0.426 |
| Dyslipidemia | 46 (22%) | 4 (15%) | 32 (23%) | 10 (22%) | 0.705 |
| Diabetes (type 1 or type 2) | 68 (32%) | 4 (15%) | 49 (35%) | 15 (33%) | 0.148 |
| History of cardiovascular disease |
22 (10%) |
2 (8%) |
13 (9%) |
7 (16%) |
0.427 |
| Pretransplant frailty | |||||
| Liver frailty index | 3.9 (3.5-4.4) | 2.9 (2.6-3.0) | 3.7 (3.5-3.9) | 4.8 (4.6-5.1) | <0.001 |
| Days between frailty assessment and transplant date | 41 (14-99) | 50 (20-129) | 46 (16-99) | 22 (6-65) | 0.199 |
Values in bold denote statistically significant results (p <0.05). HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease; MELD-Na, MELD-sodium; NASH, non-alcoholic steatohepatitis.
Defined by the liver frailty index as “Robust” if score <3.2, “Pre-frail” if score between 3.2-4.4 and “Frail” if score ≥4.5.
Median (interquartile range) or n (%).
Results of the Chi-square or Kruskal-Wallis tests.
Baseline characteristics of the 212 patients with cirrhosis included in this cohort are shown in Table 1. To summarize, 19% were women, median (IQR) age was 60 years (50-65) and median BMI was 28 kg/m2. The main etiologies of liver disease were chronic HCV in 37%, alcohol-related cirrhosis in 35% and NASH in 13%. Median MELD-Na score was 12 (IQR 9-19), MELD 3.0 was 13 (IQR 9-19) and Child-Pugh score was 7 (5-10). Personal history of hepatic encephalopathy was present in 35% of the cohort and 59% had ascites. Of LT candidates, 57% had a diagnosis of HCC prior to LT. Rates of hypertension, diabetes, dyslipidemia, and cardiovascular disease were 34%, 32%, 22% and 10%, respectively. Median follow-up time from enrolment was 8 months (IQR 6-10). Median LT waiting time was 78 (IQR 30-153) days and median (IQR) time from pretransplant liver frailty index measurement to transplant was 41 (14-99) days.
Pretransplant median liver frailty index score was 3.9 (IQR 3.5–4.4). Using cut-offs of <3.2, 3.2-4.4 and ≥4.5 for robust, pre-frail and frail patients, respectively, 26 (12%) LT candidates were robust, 141 (67%) were pre-frail and 45 (21%) were frail. Baseline patient characteristics by frailty categories are shown in Table 1. Compared to “pre-frail” and “robust” patients, patients classified as “frail” were significantly older, had higher Child-Pugh and MELD scores (in all its versions) and higher rates of personal history of ascites and hepatic encephalopathy (p <0.05). Frail patients were also more likely to have alcohol-related and NASH cirrhosis but less likely to have HCC than pre-frail or robust patients (p <0.05). The three groups were similar with respect to sex, body size, cardiovascular comorbidities and time between frailty assessment and LT (p >0.05).
Pretransplant outcomes
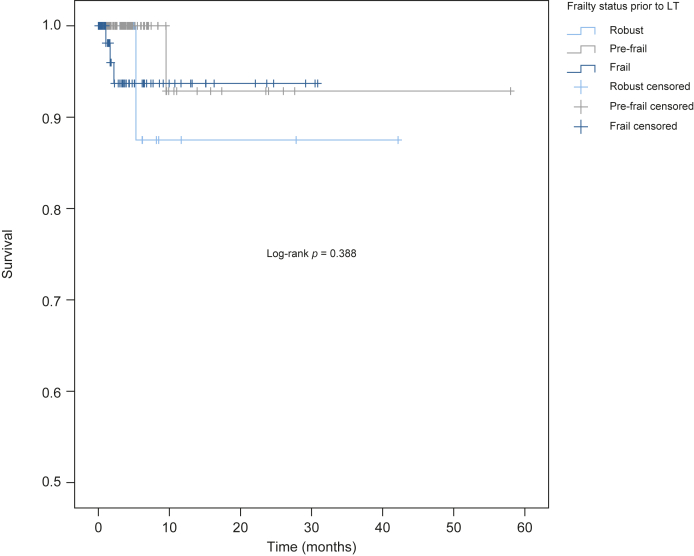
By the end of follow-up, only 5/212 patients (2%) had the primary outcome of death or delisting for being too sick for LT; 12/212 patients (6%) were removed from the waiting list for other reasons (improvement, tumor progression, social reasons and/or substance abuse), 180/212 (85%) underwent deceased donor LT, and 15/212 (7%) were actively waiting on the list at the end of the study period. There was a non-significant trend for higher mortality/waitlist removal in frail vs. non-frail patients (3/45 (7%) vs. 2/167 (2%), respectively, p = 0.056). No association was found between pretransplant frailty category (robust, pre-frail, frail) and patient probability of survival on the LT waitlist (log-rank p = 0.388 according to the Kaplan-Meier curve; Fig. 2). Univariate Cox regression models did not find an association between pretransplant liver frailty index (used as a continuous index) and waitlist mortality either (hazard ratio [HR] 1.66; p = 0.4819). Results did not change substantially after multivariable adjustment for age and MELD-Na score (HR 1.48; p = 0.586) (Table 2).
Fig. 2.
Kaplan-Meier survival curves of 212 liver transplant candidates by frailty category.
Frailty category defined by the pretransplant liver frailty index as “Robust” if score <3.2, “Pre-frail” if score between 3.2-4.4 and “Frail” if score ≥4.5). LT, liver transplant.
Table 2.
Pre- and post-transplant outcomes associated with pretransplant liver frailty index
| Pretransplant liver frailty index |
|||
|---|---|---|---|
| Variables | HR/β-coefficient/OR | 95% CI | p value |
| Pretransplant | |||
| Death/delisting for sickness | |||
| Univariable | HR = 1.66 | 0.49-5.68 | 0.419 |
| Multivariable∗ |
HR = 1.48 |
0.36-6.00 |
0.586 |
| Post-transplant | |||
| Length of intensive care unit stay | |||
| Univariable | β = 1.24 | 0.70-1.78 | <0.001 |
| Multivariable∗ | β = 0.85 | 0.26-1.44 | 0.005 |
| Length of transplant hospitalization | |||
| Univariable | β = 1.69 | 0.47-2.91 | 0.007 |
| Multivariable∗∗ | — | — | — |
| Early (<30 days) post-transplant complications | |||
| Univariable | OR = 2.05 | 1.29-3.26 | 0.002 |
| Multivariable∗ | — | — | — |
| Severe (≥ grade IIIA) early post-transplant complications14 | |||
| Univariable | OR = 1.38 | 0.80-2.38 | 0.251 |
| Multivariable∗ | OR = 1.31 | 0.75-2.31 | 0.345 |
| Late (30-90days) post-transplant complications | |||
| Univariable | OR = 1.85 | 1.13-3.03 | 0.014 |
| Multivariable∗ | OR = 2.60 | 1.47-4.59 | 0.001 |
| Retransplant within 6 months post-transplant | |||
| Univariable | OR = 1.96 | 0.89-4.31 | 0.095 |
| Multivariable∗∗ | — | — | — |
| Cardiovascular events within 6 months post-transplant | |||
| Univariable | OR = 1.69 | 0.70-4.10 | 0.243 |
| Multivariable∗∗ | — | — | |
| Death within 6 months post-transplant | |||
| Univariable | HR = 1.14 | 0.54-2.42 | 0.730 |
| Multivariable∗∗ | — | — | — |
Results from Cox regression (HR), linear regression (β coefficient) and logistic regression (OR), 95% CI and p value. Values in bold denote statistically significant results (p <0.05). HR, hazard ratio; OR, odds ratio.
Multivariable adjustment for covariables associated with frailty status in univariable analysis.
Results from univariable models concluded there were no significant variables to adjust for in multivariable analysis.
Post-transplant outcomes
For the entire cohort, median (IQR) length of stay for the LT hospitalization was 9[7], [8], [9], [10], [11], [12], [13], [14] days and median (IQR) time in the ICU was 3 (3-5) days. Compared to non-frail patients, frail LT candidates had significantly higher transplant length of stay (9 vs. 13 days; p = 0.001) and rates of early (<30 days) post-transplant complications (55% vs. 100%; p = 0.021). There was a trend toward greater time in the ICU during LT hospitalization among the frail vs. non-frail LT candidates (3 vs. 4 days; p = 0.082). No differences were found regarding the severity of early post-transplant complications and the rate of late complications (p >0.05). The two groups were similar with respect to the risk of retransplant, cardiovascular events, and death within 6 months following LT (p >0.05).
In univariable analysis, each 0.1 increase in the pretransplant liver frailty index (defining higher frailty) increased transplant hospitalization and ICU lengths of stay by 1.69 (p = 0.007) and 1.24 (p <0.001) days, respectively; and significantly increased the risk of early (odds ratio [OR] 2.05; p = 0.002) and late (OR 1.85; p = 0.014) post-transplant complications. No associations were found between pretransplant liver frailty index and the severity of early post-transplant complications (OR 1.38; p = 0.251) or with retransplant risk (OR 1.96; p = 0.095), cardiovascular events (OR 1.69; p = 0.243), or death (HR 1.14; p = 0.730) within 6 months after LT. The results remained similar after multivariable adjustment with covariables associated with frailty status in univariable analysis (Table 2).
Discussion
In the last decade, a new phenotype of the typical LT candidate has emerged consisting of a “frail” patient who is sicker, medically more complex and frequently presents with metabolic comorbidities, possibly related to the rising prevalence of obesity-related diseases and the greater proportion of elderly (between 60 and 69 and over 70 years old) candidates.8 Strong evidence from prospective, multicenter North American studies links frailty to poor pre- and post-transplant outcomes.2,4,5,7 However, well-designed studies validating the predictive utility of frailty in LT scenarios outside the United States are scarce.
Surprisingly, in our Spanish cohort of 212 outpatients with cirrhosis waiting for LT, the liver frailty index was not predictive of death/delisting in LT candidates both in univariate and multivariate analyses after adjusting for age and liver disease severity. In contrast, pretransplant frailty had an impact after transplantation, particularly in terms of health costs as it was associated with an increased rate of early and late post-transplant complications, as well as with prolonged transplant hospitalization and ICU stay.
It is likely that, in our setting, the lack of impact of frailty on pre-LT mortality is related to the short waiting time and low rate of pretransplant adverse events compared to studies performed in the United States, especially since 2014, coinciding with the introduction of the new direct-acting antivirals against HCV in our country.16 In fact, data from national transplant organizations show that both duration and waitlist outcomes are worse in the United States than in Spain. Particularly, in 2019, while the proportion of patients who died or were delisted for being too sick for LT in Spain was 8% and median (IQR) time on the waitlist was 42 (38-47) days;12 in the United States, the death/delisting rate was 18% and median time waiting for LT was 5.6 months (for patients with MELD scores between 15 and 34).17 Furthermore, in 2020, despite an 80% increase in median time on the LT waiting list in Spain, owing to the COVID-19 pandemic, waiting times in Spain continued to be significantly lower than elsewhere, e.g. in the United States (76 days vs. 4.2 months).18,19 In the present study, only 5/212 patients (2%) died or were delisted for being too sick for transplant after a median (IQR) LT waiting time of only 78 (30-153) days, which is consistent with data from the Spanish Transplant Organisation, and confirms better waitlist times and outcomes than in the American FrAILT cohort, where waitlist mortality was 17% (232/1405) and median (IQR) follow-up time was 245 (100-498) days.5 Therefore, in our Spanish cohort, the low number of pretransplant adverse events due to the short waiting time reduced the possibility of observing a statistically significant association between the liver frailty index and waitlist mortality (with HRs >1, therefore trending in the logical direction) and precluded the validation of the liver frailty index as a pretransplant prognostic tool complementary to the MELD score in our setting.
In contrast, we confirm that in the Spanish LT setting, the liver frailty index is associated with increased morbidity but not mortality within 6 months following LT. Particularly, the liver frailty index was useful to predict increased transplant hospitalization and ICU length of stay. A higher frailty was also associated with greater rates of early (<30 days) and late (30-90 days) post-transplant complications even though it did not predict greater severity of these post-surgical complications. Our findings are in line with those of the FrAILT study recently published by Lai JC et al. which included 1,166 adult LT recipients from eight centers in the United States with a pretransplant frailty assessment performed in the ambulatory setting using the liver frailty index. After a median follow-up of 36 months, the authors found that “frail” patients (defined by a liver frailty index ≥4.5) had prolonged transplant length of stay, ICU days, inpatient days within 3 months post-transplant and a higher rate of non-home discharge compared to non-frail patients.7 Thus, we both demonstrate a robust association between pretransplant frailty and greater health care utilization after LT. Moreover, both studies also agree that pretransplant frailty is not a reliable tool to predict short-term post-transplant mortality: in our Spanish cohort no association was found between pretransplant liver frailty index and death within 6 months after LT (HR 1.14; p = 0.730); in the FrAILT cohort, survival probability was significantly lower for “frail” than for “non-frail” LT recipients at 1, 3 and 5 years (log-rank p = 0.02) but the rate of death during LT hospitalization was similar in the two groups (3.2% vs. 1.8%; p = 0.16).
We would like to acknowledge the following limitations of our study. First, only outpatients were enrolled in this study with a median MELD-Na and MELD 3.0 of 12 and 13, respectively, lower than MELD-Na/MELD 3.0 scores on the LT list in many other studies or areas. The fact that only ambulatory patients were eligible for enrolment probably favored the selection of patients listed with HCC with low MELD scores and thus low risk of waitlist mortality. Indeed, in our cohort, 57% of patients had concomitant HCC at the time of LT. Therefore, as in the North American setting, the utility of pretransplant liver frailty index in hospitalized patients with cirrhosis and very high MELD scores is still unknown. Second, we only considered the liver frailty index measurement closest to the transplant date. While the extent of frailty may have changed between the liver frailty index measurement and the transplant date, the time between frailty assessment and LT was only 41 (14-99) days. Moreover, patients with a pretransplant frailty assessment performed more than 8 months prior to LT were excluded and we did not observe any significant difference in the median time between transplant date and frailty measurement by frailty category; overall, we do not believe that changes in liver frailty index while waitlisted could have substantially biased our results. Lastly, our study was conducted in five tertiary centers highly specialized in the care of patients with cirrhosis awaiting LT, potentially limiting the generalization of our results to the overall population of patients with end-stage liver disease.
Despite these limitations, our study is the first to evaluate the predictive value of the liver frailty index in outpatients in the European LT setting. Our research has picked up the lead from American Societies of Transplantation and Liver Disease by assessing physical frailty in Spanish outpatients with end-stage liver disease, with the aim of validating its utility in LT scenarios outside the United States. Data from our large, prospective, and multicenter study show that, in our setting, frailty does not impact pre- and post-transplant mortality and thus, should never be the only reason to deny a patient a LT. However, given that frailty does have an impact on post-transplant morbidity and health care utilization, our data should be read as an opportunity for programs to optimize the physical condition of frail patients.
To conclude, physical frailty should be considered as a vital sign and measured systematically and routinely during clinic visits. Our research provides the justification to initiate prospective studies to evaluate the benefit of applying strategies aimed at pre- and/or re-habilitation in settings where the waiting time for LT is not as long as that reported in previous studies from the United States.
Financial support
This research was funded by the Instituto de Salud Carlos III and co-funded by European Regional Development Fund “A way to make Europe” (grants number CM17/00006-LPR, PI19/01360-MB, and INT20/00061-MB), by the Generalitat Valenciana (grant AICO/2021/035-MB), and by the CIBER -Consorcio Centro de Investigación Biomédica en Red- [CB06/04/0065], Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea – European Regional Development Fund. No sponsor had a role in the study design, the data collection, the analysis and interpretation of data, the writing of the paper or the decision to submit the article for publication.
Authors’ contributions
Puchades: Study concept and design; acquisition of data; analysis and interpretation of data; study coordination and supervision; drafting of manuscript; critical revision of the manuscript for important intellectual content. Final approval of the version to be published; Agreement to be accountable for all aspects of the work. Herreras: Interpretation of data; critical revision of the manuscript for important intellectual content. Final approval of the version to be published; Agreement to be accountable for all aspects of the work. Ibañez: Analysis and interpretation of data; critical revision of the manuscript for important intellectual content. Final approval of the version to be published; Agreement to be accountable for all aspects of the work. Reyes: Acquisition of data and critical revision of the manuscript for important intellectual content. Final approval of the version to be published; Agreement to be accountable for all aspects of the work. Crespo: Acquisition of data; critical revision of the manuscript for important intellectual content. Final approval of the version to be published; Agreement to be accountable for all aspects of the work. Rodríguez-Perálvarez: Acquisition of data; critical revision of the manuscript for important intellectual content. Final approval of the version to be published; Agreement to be accountable for all aspects of the work. Cortés: Acquisition of data; critical revision of the manuscript for important intellectual content. Final approval of the version to be published; Agreement to be accountable for all aspects of the work. Serrano: Acquisition of data; critical revision of the manuscript for important intellectual content. Final approval of the version to be published; Agreement to be accountable for all aspects of the work. Fernández-Yunquera: Acquisition of data; critical revision of the manuscript for important intellectual content. Final approval of the version to be published; Agreement to be accountable for all aspects of the work. Montalvá: Interpretation of data, critical revision of the manuscript for important intellectual content. Final approval of the version to be published; Agreement to be accountable for all aspects of the work. Berenguer: Study concept and design; analyses and interpretation of data, study supervision, drafting of manuscript; critical revision of the manuscript for important intellectual content. Final approval of the version to be published; Agreement to be accountable for all aspects of the work.
Data availability statement
The research data is available on request to the corresponding author.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We would like to thank Juan Luis Gómez, bachelor’s degree in mathematics from the University of Valencia, for his statistical input.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100840.
Supplementary data
The following are the supplementary data to this article.
References
- 1.Puchades Renau L., Herreras López J., Cebrià I., Iranzo M.À., Cezón Serrano N., Di Maira T., et al. Frailty and sarcopenia in acute-on-chronic liver failure. Hepatol Commun. 2021 May 4;5(8):1333–1347. doi: 10.1002/hep4.1722. PMID: 34430779; PMCID: PMC8369934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carey E.J., Steidley D.E., Aqel B.A., Byrne T.J., Mekeel K.L., Rakela J., et al. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl. 2010 Dec;16(12):1373–1378. doi: 10.1002/lt.22167. PMID: 21117246. [DOI] [PubMed] [Google Scholar]
- 3.Lai J.C., Feng S., Terrault N.A., Lizaola B., Hayssen H., Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014 Aug;14(8):1870–1879. doi: 10.1111/ajt.12762. Epub 2014 Jun 16. PMID: 24935609; PMCID: PMC4107151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tandon P., Reddy K.R., O'Leary J.G., Garcia-Tsao G., Abraldes J.G., Wong F., et al. North American Consortium for the Study of End-Stage Liver Disease. A Karnofsky performance status-based score predicts death after hospital discharge in patients with cirrhosis. Hepatology. 2017 Jan;65(1):217–224. doi: 10.1002/hep.28900. Epub 2016 Nov 29. PMID: 27775842. [DOI] [PubMed] [Google Scholar]
- 5.Kardashian A., Ge J., McCulloch C.E., Kappus M.R., Dunn M.A., Duarte-Rojo A., et al. Identifying an optimal liver frailty index cutoff to predict waitlist mortality in liver transplant candidates. Hepatology. 2021 Mar;73(3):1132–1139. doi: 10.1002/hep.31406. Epub 2020 Oct 30. PMID: 32491208; PMCID: PMC7710552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai J.C., Segev D.L., McCulloch C.E., Covinsky K.E., Dodge J.L., Feng S. Physical frailty after liver transplantation. Am J Transpl. 2018 doi: 10.1111/ajt.14675. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai J.C., Shui A.M., Duarte-Rojo A., Ganger D.R., Rahimi R.S., Huang C.Y., et al. From the Multi-Center Functional Assessment in Liver Transplantation (FrAILT) Study Frailty, mortality, and health care utilization after liver transplantation: from the Multicenter Functional Assessment in Liver Transplantation (FrAILT) Study. Hepatology. 2022 Jun;75(6):1471–1479. doi: 10.1002/hep.32268. Epub 2021 Dec 29. PMID: 34862808; PMCID: PMC9117399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai J.C., Sonnenday C.J., Tapper E.B., Duarte-Rojo A., Dunn M.A., Bernal W., et al. Frailty in liver transplantation: an expert opinion statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Am J Transplant. 2019 Jul;19(7):1896–1906. doi: 10.1111/ajt.15392. Epub 2019 May 8. PMID: 30980701; PMCID: PMC6814290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai J.C., Tandon P., Bernal W., Tapper E.B., Ekong U., Dasarathy S., et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021 Sep;74(3):1611–1644. doi: 10.1002/hep.32049. Erratum in: Hepatology. 2021 Dec;74(6):3563. PMID: 34233031; PMCID: PMC9134787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C.W., Lebsack A., Chau S., Lai J.C. The range and reproducibility of the liver frailty index. Liver Transpl. 2019 Jun;25(6):841–847. doi: 10.1002/lt.25449. Epub 2019 Apr 29. PMID: 30884128; PMCID: PMC6542696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skladany L., Drotarova Z., Vnencakova J., Jancekova D., Molcan P., Koller T. Applicability and prognostic value of frailty assessment tools among hospitalized patients with advanced chronic liver disease. Croat Med J. 2021 Feb 28;62(1):8–16. doi: 10.3325/cmj.2021.62.8. PMID: 33660956; PMCID: PMC7976891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Organización Nacional de Trasplantes Sociedad Española de Trasplante Hepático. Actividad De Donación Y Trasplante Hepático España 2022. http://www.ont.es/wp-content/uploads/2023/06/DONACION-Y-TRASPLANTE-HEPATICO-2022.pdf Available at: Accessed.
- 13.Organización Nacional de Trasplantes Sociedad Española de Trasplante Hepático. Actividad De Donación Y Trasplante Hepático España 2022. http://www.ont.es/wp-content/uploads/2023/06/DONACION-Y-TRASPLANTE-HEPATICO-2022.pdf Available at: Accessed.
- 14.Clavien P.A., Barkun J., de Oliveira M.L., Vauthey J.N., Dindo D., Schulick R.D., et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009 Aug;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. PMID: 19638912. [DOI] [PubMed] [Google Scholar]
- 15.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P., STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014 Dec;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. Epub 2014 Jul 18. PMID: 25046131. [DOI] [PubMed] [Google Scholar]
- 16.Sáez-González E., Vinaixa C., San Juan F., Hontangas V., Benlloch S., Aguilera V., et al. Impact of hepatitis C virus (HCV) antiviral treatment on the need for liver transplantation (LT) Liver Int. 2018 Jun;38(6):1022–1027. doi: 10.1111/liv.13618. Epub 2017 Dec 2. PMID: 29105320. [DOI] [PubMed] [Google Scholar]
- 17.Kwong A.J., Kim W.R., Lake J.R., Smith J.M., Schladt D.P., Skeans M.A., et al. OPTN/SRTR 2019 annual data report: liver. Am J Transplant. 2021 Feb;21(Suppl 2):208–315. doi: 10.1111/ajt.16494. PMID: 33595192. [DOI] [PubMed] [Google Scholar]
- 18.Kwong A.J., Ebel N.H., Kim W.R., Lake J.R., Smith J.M., Schladt D.P., et al. OPTN/SRTR 2020 annual data report: liver. Am J Transplant. 2022 Mar;22(Suppl 2):204–309. doi: 10.1111/ajt.16978. PMID: 35266621. [DOI] [PubMed] [Google Scholar]
- 19.Organización Nacional de Trasplantes Sociedad Española de Trasplante Hepático. Actividad De Donación Y Trasplante Hepático España 2022. http://www.ont.es/wp-content/uploads/2023/06/DONACION-Y-TRASPLANTE-HEPATICO-2022.pdf Available at: Accessed.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The research data is available on request to the corresponding author.