Abstract
Contact lenses are one of the most successful applications of biomaterials. The chemical structure of the polymers used in contact lenses plays an important role in determining the function of contact lenses. Different types of contact lenses have been developed based on the chemical structure of polymers. When designing contact lenses, materials scientists consider factors such as mechanical properties, processing properties, optical properties, histocompatibility, and antifouling properties, to ensure long-term wear with minimal discomfort. Advances in contact lens materials have addressed traditional issues such as oxygen permeability and biocompatibility, improving overall comfort, and duration of use. For example, silicone hydrogel contact lenses with high oxygen permeability were developed to extend the duration of use. In addition, controlling the surface properties of contact lenses in direct contact with the cornea tissue through surface polymer modification mimics the surface morphology of corneal tissue while maintaining the essential properties of the contact lens, a significant improvement for long-term use and reuse of contact lenses. This review presents the material science elements required for advanced contact lenses of the future and summarizes the chemical methods for achieving these goals.
Keywords: Silicone hydrogel, contact lens materials, surface modification, phosphorylcholine, antifouling, lubricity
Introduction
Because of the processing properties, mechanical strength, and optical properties of the polymer, some of the most successful examples of polymeric biomaterials are medical devices, such as contact lenses and intraocular lenses, which are used to restore vision. Typical contact lens materials are summarized in Table 1. The early contact lenses examined were glass. Poly(methyl methacrylate (MMA)) (PMMA) was later applied.1−3 These hard contact lenses have been used as a new vision correction device to replace eyeglasses since the late 1930s and contributed to the popularization of the convenience of using contact lenses. PMMA is a hydrophobic hard material that does not contain water and has an extremely low oxygen permeability, which is the limitation of this lens material. Subsequently, there were improvements in hard contact lens materials. In the later half of the 1970s, research and development of oxygen-permeable materials, classified as rigid gas permeable (RGP) contact lenses, progressed, and some methacryloyl group terminated-polydimethylsiloxane, siloxyl groups containing methacrylates, and fluoroalkyl methacrylates were combined with MMA, which is a monomer that has been put to practical use.4−6 Because these hard contact lenses have a hydrophobic nature, the surface of these contact lenses is easily soiled, and the proteins and lipids contained in tear fluid are strongly adsorbed.7,8 In addition, the poor wettability by water causes discomfort with the corneal and palpebral tissues upon use due to friction.9−12 To solve this problem, it is common to periodically soak contact lenses in a cleaning solution to remove dirt and use a lubricant to improve wearing comfort.13−15
Table 1. Characteristics of Contact Lens Materialsa.
| material | O2 permeability | stiffness | processing | |
|---|---|---|---|---|
| glass | none | rigid | – | cutting |
| PMMAa | none | rigid | + | cutting |
| PMMA-based gas permeableb | + + + | rigid | + | cutting, molding |
| PHEMAc | + | mediate | + | cutting |
| PHEMA-based | ++ | soft | + + | molding |
| silicone hydrogel | ++++ | soft to mediate | + + | molding |
Poly(methyl methacrylate).
Rigid gas permeable (RGP) material.
Poly(2-hydroxyethyl methacrylate).
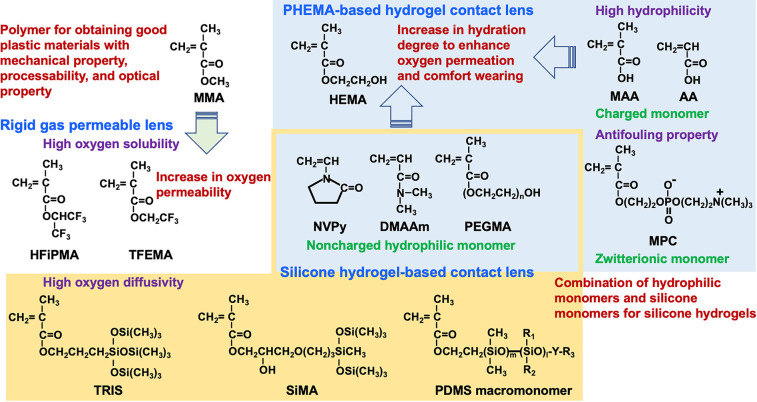
In the 1960s, a new, valuable, and distinctive monomer was created for contact lenses.16 Synthesized by Wichterie et al., 2-hydroxyethyl methacrylate (HEMA) is an important molecule used in the preparation of advanced contact lenses. It is a methacrylate monomer with a hydroxyl group in the side chain and has a good polymerization ability. The hydroxy group acts as a hydrophilic and reactive group for further functionalization. Poly(HEMA) (PHEMA) is insoluble in water, but the polymer chains hydrate and form hydrogels.17,18 Utilizing this feature, water-swollen soft contact lenses containing HEMA units as a single component have been developed. Soft contact lenses with flexible hydrogel structures have gained much support because they are more comfortable to wear and have improved oxygen permeability compared with hard contact lenses.19 The water content of the PHEMA-based hydrogels was ∼40 wt %.20 To increase the water content, N-vinylpyrrolidone (NVPy), acrylic acid (AA), and methacrylic acid (MAA) were added to HEMA to form a hydrogel.21−24 Poly(NVPy) and poly(AA) were water-soluble polymers. Currently, most soft hydrogel contact lens materials are prepared using combinations of HEMA and hydrophilic monomers with cross-linking agents.
Maintenance of tear fluid circulation and oxygen supply is essential to maintaining good condition of corneal tissue when wearing contact lenses. The oxygen permeability of soft contact lens materials is dependent on the total water content because oxygen permeates through the aqueous phase.25 Furthermore, considering the supply of oxygen and nutrients from tears to the corneal tissue, it is essential to maintain a high water content and to reduce the adsorption of tear components to the surface.26 However, because the mechanical properties of contact lenses are related to the properties of the lens material in its hydrated state, water content control is a crucial factor.27,28
The advent of soft contact lens materials has brought about a revolution in the contact lens market. In addition to resolving the issues of hard contact lenses that are discomfort and ocular stain, a new form of daily disposable use has been realized.29 The user was able to wear the new lens daily, increasing the efficiency of the care process for contact lenses. The key points for the development of reusable contact lenses are listed in Table 2. Herein, we summarize a method for chemically reproducing the structure and function of living organisms in an artificial system as a new contact lens material. Concerning biomimetic molecular design and material creation, we focused on methods to chemically control the bulk performance and surface properties of contact lenses.30
Table 2. Key Points for Reusable Contact Lenses.
| 1 | Wetting and lubricity under aqueous circumstances by tear, setting solution, etc. |
| 2 | Soft enough to be comparable to natural tissue |
| 3 | Enough mechanical strength for manufacturing and using |
| 4 | Sufficient optical properties as a lens |
| 5 | Antifouling property to avoid damage to both living tissue and lens performance |
| 6 | Superior oxygen permeability: oxygen solubility to the lens materials and diffusivity in the lens |
| 7 | Easy sterilization process |
Silicone Hydrogel as Contact Lens Material
Currently, commercial silicone hydrogel-based contact lenses account for 70% of the contact lens market and are more popular than PHEMA-based contact lenses (19%), RGP contact lenses (9%), and other contact lenses (2%).31 The global market of contact lenses is valued at 9.90 billion USD in 2022 and is projected to reach 10.35 billion USD by 2023 at a compound annual growth rate of ∼5.8%. Contact lenses are used to correct nearsightedness, farsightedness, astigmatism, or age-related visual impairment. According to the World Health Organization (WHO), myopia is prevalent in East Asian countries including Japan, South Korea, China, and Singapore. The future expansion of the contact lens market can be attributed to the increasing proportion of myopia symptoms and the promotion of contact lenses as a treatment for astigmatism, presbyopia, and other conditions. In addition, many people in developed countries prefer insurance coverage and tend to spend more on the products they need to take care of their eyes.32
Figure 1 shows the chemical structures of the monomers used for the development of contact lens materials. Due to the oxygen dissolution and solute diffusion properties of the silicone component, oxygen can be efficiently taken into the lens, even from an aqueous medium, and supplied to the corneal tissue.
Figure 1.
Chemical structure of representative monomers for the preparation of contact lenses. Monomers mainly used in PHEMA-based hydrogel contact lenses are shown with a blue background, and typical monomers used in silicone hydrogel-based contact lenses are shown with a yellow background. Hydrophilic monomers used in both contact lenses are shown in yellow blocks.
What kind of molecular design is required to increase the oxygen permeability of the siloxane bonds? This requires a new material-design concept. This is an application of polydimethylsiloxane (PDMS), which has many siloxane bonds, as a polymer. PDMS is a liquid at room temperature with a relatively free rotation of oxygen–silicon bonds in the siloxane bond (−Si(CH3)2–O−).33 Furthermore, silicone rubber obtained by cross-linking PDMS can maintain a rubber state with a low overall glass transition temperature and is used as an elastomer in the medical field. In addition, PDMS has been reported to have a high oxygen solubility coefficient.34−36 Although it is possible to increase the oxygen supply to the corneal tissue by incorporating these characteristics of PDMS as a contact lens material, cross-linking of the PDMS surface is covered with methyl groups, resulting in a low surface energy and low affinity for water or water wettability. Water wettability of the lens surface is a prerequisite for contact lens materials.37
Silicone compounds include 3-[tris(trimethylsiloxy)silyl]propyl methacrylate (TRIS), bis(trimethylsiloxy) methylsilylpropyl glycerol methacrylate (SiMA), a hydroxyl-group-terminated polydimethylsiloxane (H-PDMS), can prevent corneal hypoxia during lens wear, because of its high oxygen absorption.38 However, hypoxia complications, such as corneal swelling and blurred vision, limit the incorporation of silicone monomer units into contact lenses. These complications are associated with dry eye, a condition caused by increased hydrophobicity of the contact lens network.39 In addition, silicone-containing contact lenses have many limitations that cause ocular complications, such as poor surface wettability by water, the high elastic modulus of silicone, and low oil resistance based on hydrophobicity, leading to patient discomfort, dry eye, corneal erosion, giant papillary conjunctivitis, and other problems.40,41
Subsequently, a silicone hydrogel material was prepared by the copolymerization of hydrophilic monomers and TRIS, which was used in oxygen-permeable hard contact lenses. In the improvement of the hydrophilicity of TRIS, SiMA is developed, which is referred to as the “Tanaka monomer” for RGP contact lenses in the late 1970s.42 Because TRIS has poor compatibility with hydrophilic monomers such as HEMA and NVPy, the mechanical and optical properties of the resulting copolymers were not satisfactory. Therefore, practical applications took time. Contact lenses are formulated with wetting agents to reduce the hydrophobicity of silicone, improve the wettability of the surface and interior, and reduce the stiffness of the lens. HEMA, MAA, and NVPy are commonly used as hydrophilic components in conventional hydrogel lenses.43 Poly(ethylene glycol) (PEG) is well-known for its water solubility and resistance to protein adsorption.44 Ingredients such as HEMA, DMAAm, NVPy, MAA, PEG, and methacryloyl group-terminated PEG (PEGMA), are commonly used in the manufacture of commercial soft contact lenses. Generally, PEGMA can be used as a component to improve wettability.45 As the first generation of silicone hydrogel lenses, these hydrophilic monomers were commonly used with siloxane compounds, such as TRIS, SiMA, and H-PDMS, in the manufacture of lenses. For example, Tran et al. used TRIS, NVPy, MAA, and PEGMA (a polyether) to synthesize silicone hydrogels.43 According to the report, as the content of hydrophilic PEGMA in the copolymer gel increased, the hydration degree increased. However, a reduction in the elastic modulus and the oxygen permeation coefficient was observed. Therefore, the relationship between the degree of hydration and the oxygen permeability is an extension of conventional silicone hydrogel materials. Although a reduction in protein adsorption was observed, even when the content of PEGMA units was 20 wt %, the amount of albumin and lysozyme adsorbed was ∼20 μg/cm2, which is higher than that of common hydrogel lens materials. This may indicate that the PEG chains of PEGMA were not effectively oriented and concentrated on the water-contact surface of the lens material. This suggests that it is difficult to simultaneously achieve sufficient oxygen permeability and surface compatibility with water as a contact lens material by introducing siloxane groups alone.
Materials Requirements of Contact Lens for Compatibility to Corneal Tissue
Disposable soft contact lenses developed with PHEMA-based contact lenses are expected to reduce corneal infections; however, they do not decrease eye disorders.46,47 It been reported to affect the progression of myopia and predispose to dry eye.48,49 Silicone hydrogel contact lenses respond to the requirement of high oxygen permeability.33,42−44 Review articles have been published to explore how wearing silicone hydrogel lenses might affect the ocular surface and identify areas where research is required to improve biocompatibility.50 According to this study, silicone hydrogel lenses eliminate lens-induced hypoxia in most wearers and have less impact on corneal homeostasis than other lens types do. However, their mechanical interactions with ocular tissue and impact on tear structure and physiology are similar to those observed in typical soft lens wear. Although the ocular health benefits of silicone hydrogel lenses have increased the duration the lenses can be worn at night, the risk of infection is similar to that of other soft lens types. Regardless of the lens material, nighttime wear is less likely to occur during the day. This is a higher risk factor for infection than wear. Contact lens-associated bacterial keratitis is thought to be triggered by bacterial adhesion to corneal epithelial cells, which causes corneal infection. Cavanagh et al. clarified that bacteria adhere to corneal epithelial cells more easily when the contact lens has a lower oxygen permeability (Dk/t), where D is the diffusion coefficient, k is the partition coefficient, and t is the thickness.51 The permeability of the solute in a material is determined by the dissolution-diffusion model. This shows that the oxygen permeability (P: permeation coefficient) was independently governed by the solubility in the medium, represented by k, and the diffusion coefficient in the medium. In addition, when different media are present in the material, their volume fractions determine the permeation performance.
Figure 2 shows the relationship between the degree of hydration and oxygen permeability of the commercial contact lens. In conventional hydrogel soft contact lenses, oxygen permeability is related to the hydration degree of the contact lens materials.26 If a soft contact lens material with 100% water content is prepared, its oxygen permeation coefficient (P = Dk) is the Dk value of water (80 × 10–11 (cm2/s) (mL O2(mL × mmHg)). This is because the oxygen permeation of the hydrogel contact lenses was carried out through water. In contrast, silicone hydrogel contact lenses containing TRIS as silicone units have higher oxygen permeability.44 Silicone hydrogel lenses consist of a hydrophobic silicone component and a conventional hydrophilic monomer. Table 3 summarizes the representative properties of the commercially available silicone hydrogel contact lenses.52 It is known that the ratio of silicon atoms on the surface varies greatly, depending on the contact lens material, and this affects the water wettability and oxygen permeability of the surface.53
Figure 2.
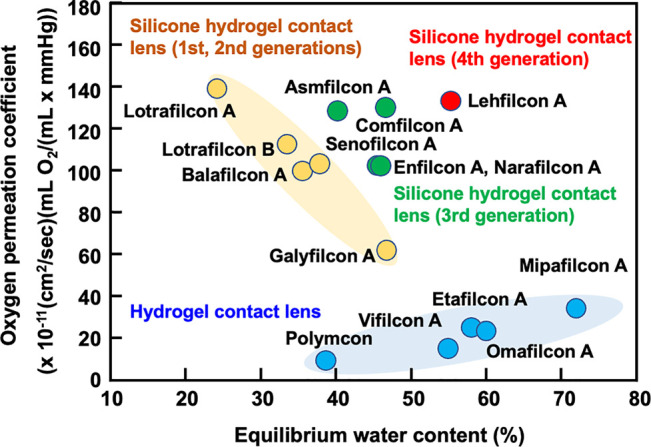
Relationship between equilibrium water content and oxygen permeation of contact lenses.
Table 3. Characteristics of Representative Silicone Hydrogel Contact Lens Materialsa.
| contact lens materials (USAN) | EWCb (%) | oxygen permeabilityc (Dk) | contact angle by water (degree) | modulus (MPa) | principle monomers |
|---|---|---|---|---|---|
| Lotrafilcon A | 24 | 140 | 1.4 | DMAAm, TRIS, siloxane macromonomer | |
| Lotrafilcon B | 33 | 110 | 44 | 1.0 | DMAAm, TRIS, siloxane macromonomer |
| Balafilcon A | 36 | 91 | 94 | 1.1 | NVPy, TPVC, NCVE, PBVC |
| Senofilcon A | 38 | 103 | 79 | 0.72 | DMAAm, HEMA, siloxane macromonomer, MPDMS |
| Asmofilcon A | 40 | 129 | 0.91 | ||
| Enfilcon A | 46 | 100 | 75 | 0.50 | |
| Narafilcon A | 46 | 100 | 95 | 0.65 | |
| Galyfilcon A | 47 | 60 | 66 | 0.43 | DMAAm, HEMA, siloxane macromonomer, MPDMS |
| Comfilcon A | 48 | 128 | 40 | 0.75 | |
| Lehfilcon A | 55 | 123 | 25 | 0.57 | |
| PHEMA-based hydrogel contact lens | |||||
| Polymacon | 38 | 8.4 | 30 | 0.81 | HEMA, cross-linker |
| Vifilcon A | 55 | 16 | HEMA, MAA, PVPy, cross-linker | ||
| Etafilcon A | 58 | 21 | 0.30 | HEMA, MAA, cross-linker | |
| Omafilcon A | 60 | 33 | 29 | 0.40 | HEMA, MPC, cross-linker |
| Mipafilcon A | 72 | 34 | HEMA, VAc, poly(MAA) |
Legend: HEMA, 2-hydroxyethyl methacrylate; MAA, methacrylic acid; NVPy, N-vinylpyrrolidone; VAc, vinyl acetate; MPC, 2-methacryloyloxyethyl phosphorylcholine; PVA, poly(vinyl alcohol); PVPy, poly(N-vinylpyrrolidone); DMAAm, N,N-dimethylacrylamide; IRIS, 3-{methacryloyloxy)propyltris(trimethylsiloxy)silane; TPVC, tris(trimethylsiloxysilyl)propyivinyl carbamate; NCVE, N-carboxyvinyl ester; PBVC poly(dimethylsiloxyl)-di(silylbutanol])- bis(vinyl carbamate); MPDMS monofunctional polydimethylsiloxyane
Equilibrium water content (w/w) × 100 (%).
Oxygen permeability × 10–11(cm2/s)(mL O2/mL × mmHg).
The required properties of advanced contact lens materials depend on the bulk and surface properties of the material.52−54 First, processing properties that allow the lens to conform to the eye’s shape are crucial. There was a time when hard contact lenses were made by cutting blocks of polymer to create a lens shape. During the transition to soft contact lenses, polymerization was achieved by mixing monomers and cross-linking agents in the mold. Furthermore, contact lenses are manufactured by photoinitiated polymerization of monomers in the mold.1,2,55 It is also necessary to design a monomer structure that is applicable under these polymerization conditions. In particular, the compatibility between the base monomer and functional monomer for imparting hydrophilicity or forming a cross-linking structure is an important parameter to ensure homogeneous optical properties and stable mechanical properties.
The factors that influence the comfort of wearing contact lenses are that the mechanical properties of the lens match the tissue of the eye, the surface of the lens is wetted with tear fluid and exhibits lubricity, and the surface adsorbs proteins and lipids as well as prevention of contamination, prevention of bacterial adhesion, and prevention of infection.56,57 Contact lens materials intended for long-term continuous wear must meet these requirements while achieving adequate oxygen permeation and circulation of the tear to the corneal tissue during wear.
Medical devices with PDMS as an element in a biological environment are expected to have the characteristics of an elastomer.58,59 Cross-linked PDMS has been used in vivo as a catheter and an implanted port. In contrast, it has been pointed out that the surface of cross-linked PDMS is hydrophobic, and its molecular diffusivity is high; therefore, it absorbs lipids, cholesterol, and other substances in body fluids, which changes the material properties. An improvement of this property is required for contact lens materials that can be worn continuously over a long period.
As described above, early silicone hydrogel contact lens materials were copolymers of hydrophilic monomers, silicone macromonomers, and small amounts of cross-linking monomers. Oxygen permeability was found to be greater than that of conventional soft hydrogel contact lenses, which justifies the material design concept. However, to enhance the hydration degree of the silicone hydrogel contact lens materials, the oxygen permeability was decreased, as shown in Figure 2. This was due to the phase separation of the silicone-rich and the water phases in the hydrogel materials, as shown in Figure 3.60−62 Oxygen can predominantly dissolve in the silicone phase and diffuse in the silicone phase when the silicone phase forms continuously. However, when the hydrophilic monomer unit composition was increased, the silicone phase was separated by an aqueous phase. Therefore, oxygen diffusion in the aqueous phase may be the rate-limiting factor for total oxygen permeation.
Figure 3.
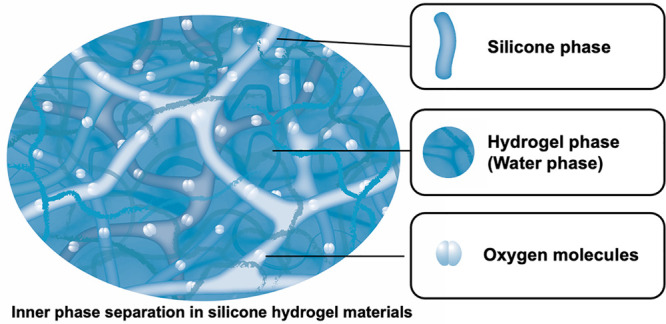
Schematic representation of phase separation in silicone hydrogel materials.
There have been some reports regarding corneal disorders that may be attributed to material properties.63−66 To resolve these issues, material design guidelines for silicone hydrogel lenses have been reconsidered. Some lens materials with improved characteristics have emerged. Research on material design methods to improve the surface and bulk properties of lens materials has also been conducted.
Importance of Surface Hydration on Contact Lens Materials
Various surface modification methods have been applied to impart functionality to the surface of medical devices.67−69 This depends on the environment in which the medical device is used and the desired function. Many research studies have focused on suppressing foreign body reactions when in contact with blood or biological tissue and mitigating the impact of the bulk properties of the materials needed to manufacture medical devices on biological tissues. Additionally, the ability to control the permeability of a substance with a thin surface treatment layer can be added, as in the case of drug-eluting coronary stents.
The contact lens surface properties are related to nearby water conditions. For convenience, this is the free energy of the surface of the material and is indicated by the contact angle of various droplets.28,45 However, if we consider this in more detail, we need an evaluation that focuses on the structure of the water. For example, PHEMA, which is used in soft contact lenses, is hydrophilic and contains hydroxyl groups. Nonionic NVPy and anionic AA are used as copolymers with HEMA to increase hydrophilicity, but the origin of this hydrophilicity is the hydration water of these hydrophilic monomer units in the polymer due to hydrogen bonding with water molecules and ionic hydration, respectively.70−73 In this case, the state of the water molecules around the polymer is affected by the functional groups.
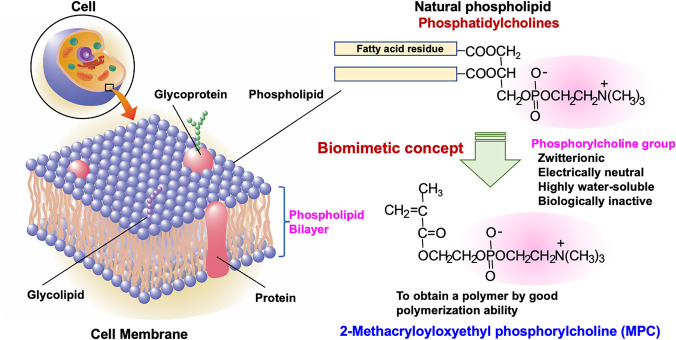
Since the 1990s, research on hydrophilic monomers has focused on the structure of phospholipid molecules in cell membranes (Figure 4). This is 2-methacryloyloxyethyl phosphorylcholine (MPC), designed using the biomimicking concept.74,75 The chemical structure of MPC is simple; a phosphorylcholine group, which is a typical phospholipid polar group, is attached to a polymerizable methacrylate group. Unlike conventional polymerizable phospholipids with diene or acetylene groups, MPC has good polymerization ability.76,77 Although MPC has charged groups, the molecule as a whole is neutral because the phosphate anion and trimethylammonium cation of the phosphorylcholine group form an inner salt, and these charges are neutralized.78 Therefore, it exhibits high hydrophilicity without being affected by changes in the ambient pH and ionic strength. MPC has been used for copolymerization with HEMA with a small amount of cross-linker in the development of a soft contact lens material, omafilcon A.79−81 Polymers containing MPC units can prevent protein adsorption because of their unique hydration characteristics. The water structure in the hydrogel of the MPC polymer was examined using thermal analysis.82,83 The free water-like water fractions were considerably higher than those of conventional hydrogels, such as PHEMA and polymers containing NVPy units. For the free-water-like water fraction, the number of proteins adsorbed on the surface of the hydrogels decreased dramatically to less than that of the monolayered adsorption. In addition, conformational changes were not observed in the attached protein at the interface of the MPC polymer hydrogel. Therefore, a weak interaction force is generated between the protein and the surface of the MPC polymer. The protein can detach reversibly and quickly after attachment to the surface.84
Figure 4.
Molecular design of 2-methacryloyloxyethyl phosphorylcholine inspired by cell membrane structure.
Regarding the water structure surrounding the polymer chains, Kitano et al. reported Raman spectroscopic observations of the effects of various hydrophilic monomer units on the structure of water.85,86 This can be understood as a parameter of the extent to which the monomer units in the water-soluble polymer break the hydrogen bonds of the surrounding water molecules, which is described as the N-value. This shows that a typical poly(NVPy) breaks approximately one hydrogen bond per monomer unit. PEG and poly(DMAAm), similar to nonionic water-soluble polymers, have N-values in the range of 1.0–2.3. In polyelectrolytes having charges, such as anions or cations, the N values are large and in the range of 3.6–5.9. This indicates that ionic hydration has a higher impact than hydrogen bonding hydration. In contrast, for zwitterionic poly(MPC), the N-value is −0.7, which is small and negative. Even in the case of MPC polymers with hydrophobic monomer units, poly(MPC-co-n-butyl methacrylate (BMA)), the N value was −0.4. This indicated a trend toward increased hydrogen bonding of water around the MPC unit in the polymer. These hydrated water molecules form bulk water-like cluster structures and are easily desorbed by molecular diffusion when proteins come into contact. Indeed, in the force curve measurement mode of atomic force microscopy, it has been observed that the adhesion force between the cantilever-immobilized protein and the PMPC surface is below the limit of measurement.87,88 In addition, the studies by Whitesides et al. discuss the molecular structure of functional groups in protein adsorption inhibition using self-assembling membranes and present a molecular science hypothesis that is ideal. They hypothesized that the molecular structure of a hydrophilic functional group should be hydrophilic, charge neutral, and have an intramolecular hydrogen bonding acceptor but no hydrogen bonding donor. From this, they report that the molecular structure of the phosphorylcholine group is optimal.89,90
Another unique hydration of the MPC polymer with a hydrophobic BMA moiety is temperature-dependent on the degree of hydration.74,78 The hydration degree of the conventional hydrogel containing PHEMA decreased with an increase in temperature between 10 and 60 °C. In contrast, the MPC polymer showed an opposite temperature dependence on the hydration degree; that is, the hydration of the MPC polymer increased with an increase in temperature. These phenomena indicate the difference in the hydration mechanisms considered for MPC polymer hydrogels. These unique hydration states are considered to have a considerable influence on the interfacial properties of the MPC polymers.
Furthermore, it has been recognized that the high lubricity on PMPC-grafted surfaces is due to the mobility of polymer chains upon hydration and the easy diffusion of water molecules in the hydrated layer.91
Surface Modification on Silicone Hydrogel Contact Lens with Hydrophilic Polymers
As described above, protein adsorption and lubricity on the surface of contact lens materials and the mechanical properties of the material are determined by the hydration state of the surface. Additionally, oxygen permeability, tear mobility, and refractive index are known to be affected by hydration within contact lens materials, which are important factors in material design. In general, silicone hydrogel contact lens materials tend to have a higher water wettability as the water content increases, making the material more flexible and more comfortable to wear. On the other hand, oxygen permeability is dominant in the water-containing portion of the silicone hydrogel contact lens material, so it decreases as it approaches diffusion in water. These are illustrated as shown in Figure 2.
The advantage of surface treatments is that they do not substantially affect bulk properties. To impart hydrophilicity and tissue compatibility to a silicone contact lens, this property should be expressed at the interface in contact with the cornea and eyelids.92,93 However, when hydrophilic functional groups or polymers are introduced in the preparation of silicone hydrogels, the oxygen permeability and mechanical strength are affected simultaneously. From this, the surface treatment for forming a hydrophilic polymer layer in the vicinity of the surface can assume characteristics in the manufacturing process of contact lenses and is effective in maintaining and managing them. In this review, studies on the formation of a hydrophilic graft polymer layer on the surface are introduced.39,94−99
The properties of the hydrophilic polymer layer formed on the surface of silicone hydrogel contact lenses must also remain unchanged in tear-fluid environments and be resistant to sterilization conditions. In particular, high ionic strength and stability in ocular environments in which proteins and lipids coexist are of paramount importance. Hydrophilic polymers interact strongly with water molecules during hydration. As mentioned above, this affects the formation of hydrogen bonds between the water molecules and the resulting cluster structure. Regarding polyelectrolytes, interactions with ions in the tear fluid must also be considered. Several silicone hydrogel contact lenses have been developed and conjugated with PEG, poly(NVPy), and other polyelectrolytes. These lenses maintained high oxygen permeability despite having a higher water content than earlier hydrophilic monomers and silicone macromonomer copolymers (Figure 2). Since PEG is the gold standard for obtaining protein-adsorption-resistant surfaces, various methods for the introduction of PEG on silicone hydrogel substrates have been examined.100,101 However, it should be noted that the surface density and chain length of PEG immobilized on the surface influence this property. In addition, PEG has only two reaction groups in one molecule at the terminal positions. Thus, the immobilization reaction has limitations.
Chen et al. reported that PDMS was surface-modified by passivating PEG with different molecular weights, monofunctional and bifunctional.102 Following the introduction of Si–H groups on the surfaces by acid-catalyzed equilibration in the presence of PDMS, PEG was linked via platinum-catalyzed hydrosilylation. The PEG-modified PDMS substrate noticeably reduced the level of protein adsorption because of the enhanced hydrophilic character of the surface. In addition, a high-density PEG-based polymer could be deposited on lotrafilcon A contact lenses via an allylamine plasma polymer interlayer. These coatings were stable upon autoclaving.39 Atomic force microscopy (AFM) experiments also revealed the structure of the coating and showed that the modulus of the contact lens surface was reduced in the presence of the PEG-based polymer. The clinical performance of PEG-based polymer-coated contact lenses was investigated in a 6 h controlled clinical study with lotrafilcon A control lenses in the contralateral eye. The clinical data demonstrated the high biocompatibility of the PEG-based polymer coatings, with all major clinical parameters showing results equivalent to those of commercial control lenses. The subsequent X-ray photoelectron spectroscopy (XPS) analysis of the worn PEO-coated lenses and lotrafilcon A control lenses also revealed reduced levels of in vivo biofouling, which were attributed to the presence of the high-density PEG-based polymer coating. However, small amounts of protein were still detected in the coatings. These results suggest that high-density PEG-based polymer coatings may improve the biocompatibility of contact lenses.103
Biomimetic Design of Silicone Hydrogel Contact Lenses
Biomimetic Morphologies on Silicone Hydrogel Contact Lenses
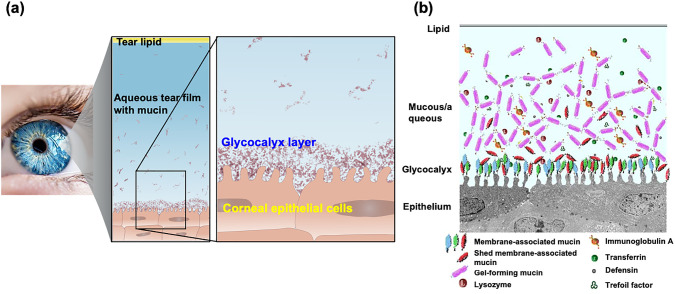
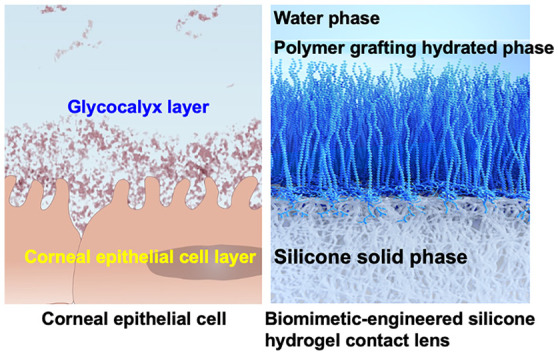
On the corneal surface, tear fluid is used as a lubricant to eliminate resistance during blinking. However, when a contact lens is inserted, the diffusion and movement of tear fluid on the surface are affected, increasing the frictional resistance and affecting the diffusion of oxygen contained in the tear fluid.104,105 These might affect ocular health. It is also assumed that the natural components in the tear fluid can affect the function and properties of contact lenses.106,107 Protein adsorption occurs on the surface of contact lenses within a short period after they are applied, resulting in a decrease in oxygen permeability due to the protein adsorption layer. There is also concern that lipids will be absorbed into the lens, affecting the bulk properties of the lens. Therefore, lenses are used and cleaned within a short period; alternatively, the period of use is limited, similar to that for disposable lenses. However, if the corneal surface structure could be mimicked on the contact lens surface, it would prevent significant interaction among the contact lens, the cornea, and the eyelid tissue. What is the structure of the corneal surface? Figures 5a and 5b show schematics of the surface structure of the corneal tissue. The cornea consists of a basement membrane composed primarily of collagen, where hydrophilic biomolecules, such as proteoglycans and mucins, bind, and the surface is in contact with the tear fluid.108 Dissolved mucins are also present in the tear fluid, and the outermost layer of the tear fluid phase is covered with lipids, which inhibits the evaporation of the tear fluid and ensures good lubrication. In addition, various proteins are present in the tear fluid and are responsible for antibacterial activity and immune response. For contact lenses that are exposed to such an environment for a long time, it is important to improve the surface properties in addition to the excellent bulk properties, such as optical and mechanical properties.109,110
Figure 5.
Structure of corneal epithelial surface. (a) Schematic diagram of glycocalyx on the surface of human corneal epithelial cells and (b) interface of corneal tissue.
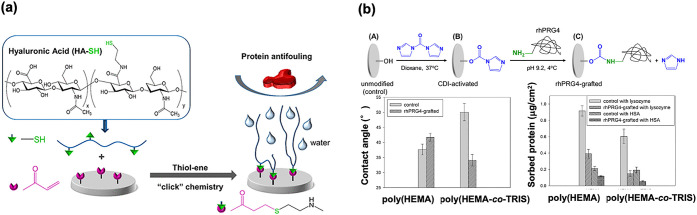
If a structure similar to that of the corneal surface can be constructed on the contact lens surface, lubrication and antifouling properties can be obtained along with improved water wettability.111−113 Research on surface treatment has been conducted based on the biomimetic molecular design concept. As shown in Figure 6a, the surface immobilization of hyaluronic acid on the model silicone hydrogel substrate was prepared by using poly(HEMA-co-TRIS). First, vinyl groups were introduced on the surface; then, the thiol group introduced hyaluronic acid by click chemistry with a thiol–ene selective addition reaction.114 The surface hydrophilicity increased after the modification, and protein adsorption and cell adhesion were examined. As a result, the surface modification process proceeded well and the surface was covered with hyaluronic acid. A reduction in the protein adsorption and cell adhesion was observed. Hyaluronic acid is used as an eye-drop component to prevent dry eye symptoms.115,116 Thus, it is concluded that this modification by the click reaction will be applicable for the surface modification of silicone hydrogel contact lenses. Another example is the immobilization of proteoglycans and lubricin to improve the lubricity of silicone hydrogel substrates.117,118 As shown in Figure 6b, on the surface of poly(HEMA-co-TRIS), proteoglycan 4, a mucinous glycoprotein molecule, reacted through active ester chemistry.119 The reaction was carried out under mild conditions in an aqueous medium. The hydrophilicity and protein adsorption resistance were significantly improved compared to those of the base substrate. Much advanced research on surface modification by natural molecules has been reported, including the coimmobilization of hyaluronic acid and recombinant human proteoglycan 4. It is a well-studied corneal tissue-mimicking system and is expected to have synergistic effects with both natural molecules.120
Figure 6.
Biomimetic surface preparation on the silicone hydrogel contact lens. (a) Immobilization of hyaluronic acid. [Reproduced with permission from ref (119). Copyright 2018, American Chemical Society, Washington, DC.] (b) Immobilization of proteoglycan and surface functions. [Reproduced with permission from ref (120). Copyright 2021, American Chemical Society, Washington, DC.]
Surface modification of silicone hydrogel substrates by plasma irradiation is also being performed; however, it is difficult to control the type and density of the polar groups generated. The direction of the polar group changes depending on the environment.121−123 It is impossible to maintain this state. Therefore, hydrophilic polymers with well-defined structures have been used. In addition, attempts have been made to improve the antifouling properties and prevent the loss of oxygen permeability during long-term continuous wear.
The use of phospholipid liposomes, constructed primarily of phosphatidylcholines, to improve the surface properties of silicone hydrogel contact lenses, has been investigated.124 In this method, an aqueous dispersion of liposomes composed of phosphatidylcholine is sprayed onto the contact lens surface. Phospholipid liposomes have been examined as cell surface-mimicking substrates in biological research and as cell-like vehicles for delivering drugs in the pharmaceutical field. This can be considered a type of biomimetic technology because a thin phospholipid layer exists on the surface of the tear fluid, which contributes to the prevention of tear evaporation and acquisition of lubricity. Since liposomes are molecular aggregates, they do not exhibit sufficient mechanical stability. The low molecular weight and lack of chemical bonds hinder the formation of a stable adsorption layer at the treatment interface. Polymerizable and reactive phospholipids have also been studied to stabilize liposomes.76,125,126 However, if only the characteristics of phospholipid molecules are considered, then the use of MPC polymers with phospholipid polar groups on the side chains is effective.
Phospholipid Polymer Modified Surface on Silicone Materials
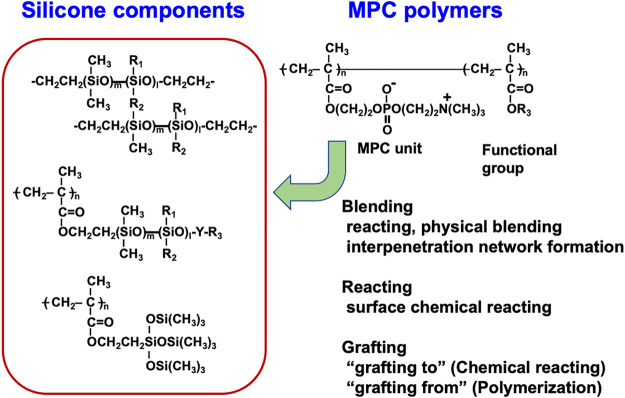
Composites of MPC polymers on the surface of silicone hydrogels are considered a promising biomimetic method for creating contact lens materials that can be worn continuously over long periods of long periods. Figure 7 shows the possible combination of the silicone hydrogel and MPC polymers. This is because the MPC polymer exhibited superior hydrophilic characteristics, oil repulsion properties, and protein adsorption resistance.127−129 Many processing methodologies for incorporating MPC polymers into PDMS have been reported.130−135 These include simple surface coating, the interpenetration of the MPC polymer chains by the swelling–deswelling process, and grafting by direct polymerization as “grafting from” or chemical reaction of reactive MPC polymers as “grafting to” processes.
Figure 7.
Method for compositing silicone-derived polymers with the MPC polymers.
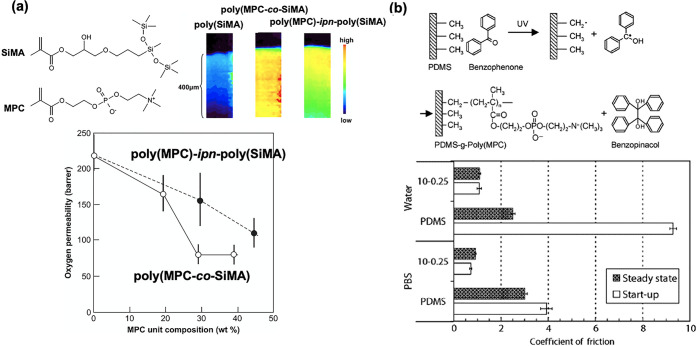
Shimizu et al. successfully formed an interpenetrating network structure on the surface by copolymerizing MPC with a small amount of cross-linking monomer in a cross-linked PDMS matrix (Figure 8a).136 The thickness of this layer was found to be ∼400 μm by microscopic infrared spectroscopy. The oxygen permeability was higher than that of conventional hydrogel contact lenses; however, it tended to decrease in dependence on the MPC polymer content, compared with that of untreated surfaces. This indicates that when such a thick hydrophilic polymer layer is formed on the surface, oxygen in the tear fluid must diffuse through this layer, and the characteristics of the silicone base material cannot be demonstrated efficiently. The swelling–deswelling process can be applied for the molecular fixation of amphiphilic block-type copolymers composed of poly(MPC) and poly(BMA) segments. This is also the preparation method for the interpenetrating network in the PDMS substrate. The polymer penetrated the substrate under swelling conditions in the cosolvent system and was fixed by molecular entanglement after evaporation of the solvent. The thickness of the modified layer can be controlled by controlling the concentration of the polymer and the swelling time. Seo et al. reported the successful fixation of block-type polymers using this process.131,137 The polymer segment was located at the water-contact surface and was stably fixed.
Figure 8.
Modification of silicone substrate by the MPC polymers. (a) Interpenetration network formation between PDMS and MPC polymers. The depth profile of the MPC polymer penetration is observed by infrared microscopy, and the relationship between MPC polymer content and oxygen permeability is indicated. [Reproduced with permission from ref (136). Copyright 2010, Elsevier.] (b) The chemical reaction of photoinduced graft polymerization of MPC on PDMS substrate and friction coefficient at the surface under an aqueous medium. [Reproduced with permission from ref (134). Copyright 2008, Elsevier.]
Studies have also reported the graft polymerization of MPC from the surface of the PDMS substrates. Goda et al. reported that a poly(MPC) layer could be efficiently formed on the surface of silicone by adsorbing benzophenone, which generates radicals through photoreaction, on the silicone surface and irradiating it with ultraviolet light in an MPC solution (Figure 8b).134,138 The polymerization proceeds via a normal radical polymerization mechanism. Therefore, the thickness of the poly(MPC) layer formed on the surface of the PDMS substrate is dependent on the monomer concentration. The density of the grafted polymer chains can be adjusted by adjusting the ultraviolet light irradiation time. This method renders the surface hydrophilic without compromising the characteristics of PDMS, resulting in a more lubricious and antifouling surface.
Surface-initiated living radical polymerization has been studied to clarify the molecular structure and to cover the surface with a polymer brush layer.127−129,139 In this method, an initiator for living radical polymerization is bound to the surface of a base material and a monomer is polymerized from this functional group. In living polymerization, the initiation reaction is a rate-determining process; the growth reaction rate is low, and there is no termination reaction. Thus, the polymerization reaction proceeds until there are no more monomers in the reaction system. As a result, polymer chains with the same degree of polymerization are formed from the surface-bonded initiating groups. Ideally, the density of the polymer chain is determined by the density of the initiator, and the degree of polymerization is determined by the ratio of the number of monomer molecules to the number of initiator molecules.
Characteristics of Biomimetic Silicone Hydrogel Contact Lenses
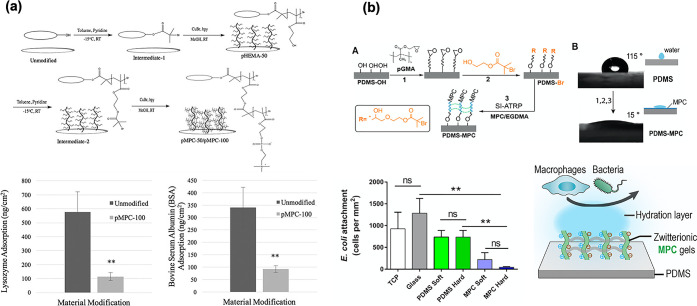
Spadafora et al. used the surface-initiated atom transfer radical polymerization (SI-ATRP) of MPC grafted onto a silicone hydrogel substrate to form a poly(MPC) brush layer (Figure 9a).140 The two different chain lengths of poly(MPC) with a controlled degree of polymerization significantly increased the surface wettability and equilibrium water content of the silicone hydrogel substrate compared to the untreated substrate. In other words, the surface water contact angle was as low as 16° for poly(MPC) with 50 degrees of polymerization. Complete wettability (∼0°) was observed for poly(MPC) with 100 degrees of polymerization. Protein adsorption was reduced by 83% for lysozyme and 73% for bovine serum albumin compared to the untreated silicone hydrogel substrate. However, no significant differences were observed between the different degrees of polymerization of poly(MPC).
Figure 9.
Surface modification of silicone substrate by surface-initiated atom transfer polymerization. (a) Immobilization of an initiator and grafting procedure on silicone hydrogel substrate. Protein adsorption resistance after grafting the poly(MPC) is indicated. [Reproduced with permission from ref (140). Copyright 2020, American Institute of Physics.] (b) Surface-initiated atom transfer polymerization of MPC on model PDMS substrate and bacteria adhesion inhibition performance is indicated. [Reproduced with permission from ref (132). Copyright 2019, American Chemical Society, Washington, DC.]
Qin et al. examined antifouling and antimicrobial silicones using covalent grafting with an MPC polymer layer (Figure 9b).132 The cells adhered to the original PDMS surface; however, they could not form stable adhesions on the MPC polymer gel surfaces. This is attributed to their superhydrophilicity and resistance to biofouling. Cytokine secretion assays confirmed that the MPC polymer gel was much less likely than the original PDMS to induce inflammatory macrophage activation. Finally, by surface-initiated atom transfer radical polymerization of MPC, the PDMS surface with a long-term stable poly(MPC) hydrogel layer reduced the contact angle from 110° to 20°. In addition, macrophage and bacterial adhesion were significantly reduced. These phenomena observed in MPC polymers are important for contact-lens applications. Improper wear and cleaning of contact lenses can increase the risk of eye infections. For infection prevention, the fact that bacteria do not easily adhere to contact lenses can be expected, in terms of safety.
To prepare the polymer brush-like structure, the so-called “grafting to” process is convenient and easy under aqueous conditions. As a feature of this methodology, it is possible to preliminarily synthesize and purify a polymer with a defined molecular weight and structure and use it in a highly safe state. In addition, it is possible to incorporate it into the manufacturing process of lenses to prevent the mixing of low-molecular-weight compounds during the reaction process and the introduction of reactive functional groups to the substrate surface. However, two steps are required: lens synthesis and surface treatment. In addition, it is necessary to select functional groups that enhance the efficiency of the surface treatment and determine the synthesis conditions that do not affect the properties of the contact lenses. A new contact lens material, lehfilcon A, was developed by chemically bonding MPC polymer chains, which have many amino groups near the terminal of the polymer chain, to the surface of the silicone hydrogel contact lenses.141−143
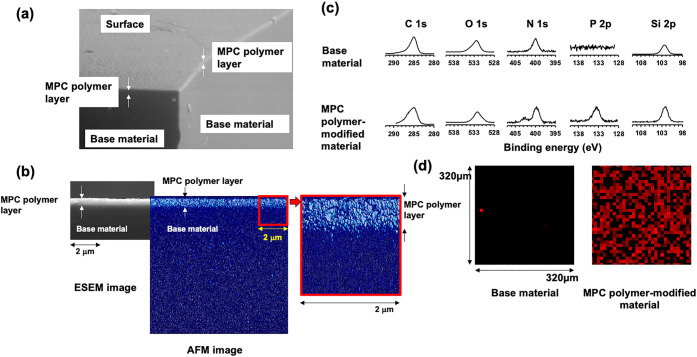
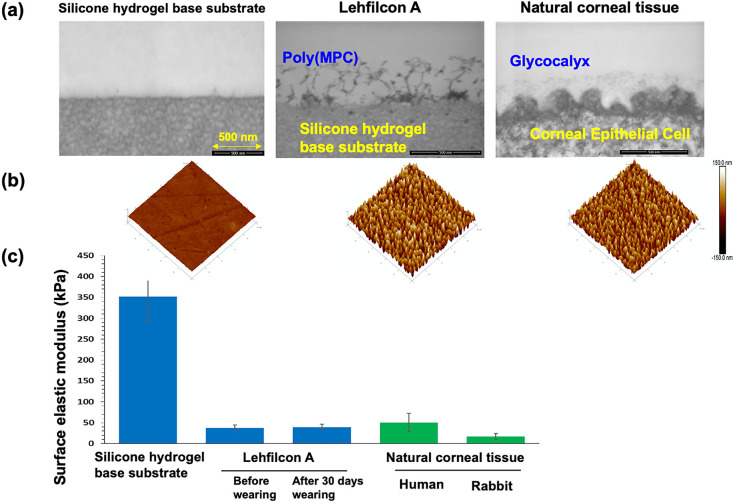
This lens material uses a silicone hydrogel as a substrate. Because the poly(MPC) chains are fixed and grafted at the ends, the MPC polymer chains are hydrated in an aqueous environment and further oriented toward the aqueous phase to form an interface.141 As shown in Figure 10a, an MPC polymer layer was observed on the surface of the silicone hydrogel using an environmental SEM (ESEM). In addition, AFM imaging of the fully hydrated lens further confirmed this submicrometer-thick MPC polymer layer (Figure 10b). As the surface is entirely covered with MPC polymer, it can be expected to improve the suppression of protein adsorption and lubricity, which are characteristics of MPC polymers. However, this thin hydrophilic polymer layer does not disturb oxygen permeation and is maintained at a high level, similar to the silicon hydrogel.
Figure 10.
Surface analysis of MPC polymer-grafted silicone hydrogel contact lens, lehfilcon A. Surface is observed by (a) ESEM under 100% humidity and (b) AFM under water. (c) Surface atom analysis is carried out by XPS and (d) the distribution of phosphate atoms at the surface is determined. [Reproduced with permission from ref (141). Copyright 2021, Elsevier.]
The MPC polymer chains present on the surface of lehfilcon A were also confirmed by XPS (Figure 10c). Each spectrum derived from C, O, N, and Si atoms is observed in a silicon hydrogel contact lens as a substrate. Contrastingly, after grafting the MPC polymer chain, in addition to these, a signal derived from the P atom is observed at 134 eV, and a new signal derived from N atoms is observed at 399 eV. These were attributed to the phosphate and trimethylammonium groups derived from the phosphorylcholine groups of the MPC polymer. It was found that a phosphorylcholine group was introduced onto the surface. A 2D mapping image of the distribution of P atoms on the surface is shown in Figure 10d.141 The P atoms were uniformly distributed on the MPC polymer-modified silicone hydrogel contact lenses. These results indicate that the MPC polymer chains are efficiently introduced to the surface via the “grafting to” method.
Furthermore, the results of observing the surface in the cross-sectional direction were reported.142 As shown in Figures 11a and 11b, a layer with fabric morphology can be observed on the surface of the silicone hydrogel contact lens grafted with MPC polymer chains. This resembled the shape of the glycocalyx present on the corneal surface (Figure 11a). This shape was also confirmed by AFM observations (Figure 11b). With the application of AFM technologies, the surface stiffness on the hydrated polymer layer can be evaluated as shown in Figure 11c.143,144 The elastic modulus of the surface of this contact lens was ∼350 kPa for the untreated silicone hydrogel contact lens and reduced to ∼30 kPa by the surface treatment.141,142 It was found that this is equivalent to the elastic modulus of human and rabbit corneas. In addition, it was confirmed that there was no change in the elastic modulus of the surface of the contact lens after wearing it for 30 days. This indicates that it is stable and that eyelid contact does not affect the surface properties. Thus, it was shown that the “grafting to” method can be applied without any substantial problems in attaching hydrated MPC polymer chains to the surface, and the resulting surface can provide physical properties comparable with those of natural cornea.
Figure 11.
(a) Transmission electron microscopy (TEM) images of a cross-section of the interface of silicone hydrogel substrate and that grafted with the MPC polymer, and natural corneal tissue. (b) AFM images at the surface are also indicated. (c) Surface electric modulus of various substrates and natural corneal tissue is indicated. [Reproduced with permission from ref (142). Copyright 2021, American Chemical Society, Washington, DC.]
The lubricity measurements on the silicone hydrogel contact lens and those modified with the MPC polymer revealed a considerable reduction in the dynamic friction coefficient by MPC polymer modification.141,145,146 The coefficient of friction of this surface is comparable to that of the corneal surface. These results indicate that surfaces grafted with MPC polymers can provide softness and lubricity equivalent to those of the corneal tissue.
Antifouling Properties on Biomimetic Silicone Hydrogel Contact Lenses
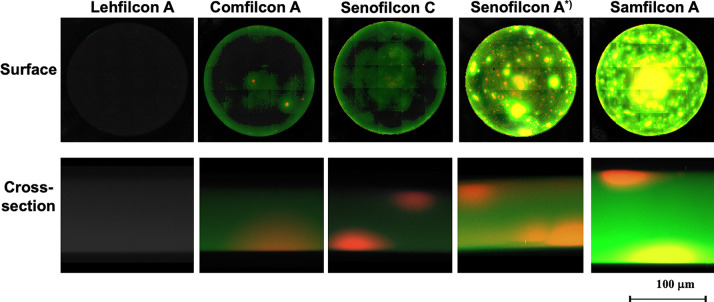
It has been mentioned that when contact lenses come into contact with tear fluid, proteins are adsorbed on the surface, causing staining and infections. In addition, when a protein adsorption layer is formed, it becomes resistant to oxygen permeation, and the oxygen supply to the cornea can be reduced. Previous studies have reported that hydrophilic polymer-grafted surfaces effectively reduce protein adsorption.129,147−150 Among the hydrophilic polymers that have been studied, the MPC polymers is recognized by many review papers to have a particularly excellent effect of inhibiting protein adsorption.75,81,82,112 Because the contact lens resides within the tear film, the aqueous layer that should be covered by the anterior lipid layer is much thinner. It also acts as a tear film, clearing it: the smooth surface of the eye along which the eyelid moves during blinking. Therefore, there is only a thin lipid layer on the outer surface of the soft hydrogel lens, and no lipid layer covers the hard lens. When little or no lipid layer is present, the tear film is easily destabilized and the lipids come into direct contact with the lens material. Hydrogel lenses are semipermeable in nature and can adsorb lipids at the surface and absorb them in the substrate, which presents another problem with varying degrees of lipid deposition.151 Although many experiments on protein adsorption on contact lens materials and their effects have been reported, relatively little information is available regarding the interactions between lipids and contact lens materials.152 The hydrophobic portion of the lipids was attached to the underlying contact lens material. The earliest observations of the interaction of lipids and hydrogel contact with their lenses were made by Hart et al.153,154 Various microscopic and histochemical staining analyses revealed that lipids were present and a major component of all deposits, with cholesteryl ester as the predominant lipid.155−157 As shown in Figure 12, the adsorption and absorption of lipids by the various silicone hydrogel contact lenses were determined by visualization with fluorescence staining. These silicone hydrogel contact lenses are immersed in an artificial tear lipid solution and then washed by a conventional procedure. This procedure was repeated for 14 days or 30 days.158 The fluorescence intensities differed for each silicone hydrogel contact lens. This result was assumed to depend on the chemical composition at the interface of the silicone hydrogel contact lens. Fluorescence was observed inside of a silicone hydrogel contact lens. These lipids can be absorbed in the silicone phase of the contact lens when they come into direct contact with tears. Therefore, the surface is sufficiently covered with a hydrophilic and oleophobic polymer layer, and lipid adsorption and absorption are considered to be blocked. It has also been reported that the contact angle of an oil droplet on a poly(MPC) graft surface measured in water is >170°.127 This indicates that the oil droplets did not adhere to the surface. On the surface of lehfilcon A, a similar polymer grafting layer is observed as shown in Figures 11a and 11b. Thus, lehfilcon A contact lens material can repel tear lipids.
Figure 12.
Lipid adsorption and absorption after the various silicone hydrogel contact lens immersed in the artificial tear lipid solution for 30 days or 14 days (*). Fluorescent staining of cholesterol ester lipids and triglyceride lipids was carried out and then observed by fluorescence microscopy. [Reproduced with permission from ref (158). Copyright 2022, The Association for Research in Vision and Ophthalmology.]
There are not many reports on the clinical evaluation of lehfilcon A lenses yet, but the results of evaluation of tear dynamics in patients with contact lens dry eye syndrome using lehfilcon A lenses have been investigated.159−161 As a result, after 30 days of wearing, the tear lipid film thickness tended to decrease, and the blink interval tended to increase. These results demonstrate that wearing lehfilcon A lenses improves the stability of tear fluid and reduces the subjective symptoms of the dry eye. As bacteria (S. marcescens) adhesion occurred, lehfilcon A contact lens reduces number of adherent bacteria and colonization of them at the surface compared with those of other silicone hydrogel materials and conventional hydrogel materials.162
Furthermore, when compared with conventional silicone hydrogel lenses, lens fit/mobility was optimal in 92.9% of eyes with lehfilcon A lenses and 89.2% of eyes with comfilcon A lenses at 3 months. The central focus of the lens was rated optimal in 98.7% of eyes for lehfilcon A lenses and 94.6% for comfilcon A lenses. Surface wetting of these contact lenses was acceptable in both lens groups across all of the participating study visits. Therefore, the lehfilcon A lenses demonstrated excellent visual acuity, optimal lens fitting characteristics, clean surfaces, high wettability, and low risk of adverse events after 3 months of lens wear. A more detailed evaluation is expected in the future.163
Conclusion and Future Perspective
In this review, we present the concept of biomimetic material design for silicone hydrogels, the latest contact lens material, and summarize the materials along this line. Bulk properties, such as mechanical properties, for stabilizing the shape and oxygen permeability for a sufficient supply of oxygen to the cornea, are important for reusable contact lenses that have been in contact with the cornea for a long time. Furthermore, a surface lubricity that achieves mechanical matching with the cornea and does not damage tissues is strongly desired. The prevention of fouling by tear components is essential to maintaining the initial properties of contact lenses for a long time. The design of material surfaces that mimic the structure and characteristics of the corneal surface has immense potential. The immobilization of a thin layer of water on the surface of the substrate based on a hydrophilic polymer layer, several hundred nanometers in thickness, can support the biomimetic concept of imparting new biocompatibility while taking advantage of the characteristics of the substrate.112,145 This provides a new material design concept that has had a significant impact on the history of material development for contact lenses.
Contact lenses have been developed as medical devices for vision correction. Previously, a material that greatly improved the comfort and safety of wearing was developed, realizing long-term continuous wearing goals. Currently, attention has focused on their role as interfaces for receiving information within the body and transmitting it to the outside of the body.164,165 For example, research is being conducted on sensing blood sugar levels from tears and diagnosing stress and cancer markers.166−168 In addition, silicone hydrogels with hydrophobic substrates can encapsulate sparingly soluble drugs and control their diffusion and permeation into the lysate, corresponding to their solubility. In particular, long-term wear can be expected to maintain sustained pharmacological activity. Research has also been conducted to slowly release a surface lubricant consisting of an amphiphilic polymer from silicone hydrogel contact lenses. This has the potential to be developed into a technology for administering bioactive biorelated molecules, such as proteins and nucleic acids, from contact lenses.169 We focus on the various potential applications of contact lenses. Molecular recognition and control of chemical reactions are similar to the biological reaction mechanisms that the living body performs on the surface of the cell membrane. It is believed that the biomimetic concept in this direction will show numerous developments.
Acknowledgments
We would like to thank Ms. Amanda Shows for her assistance in the schematic diagrams.
Author Contributions
Equally contributing authors. K.I., K.F., X.S., and J.Y.W. prepared the manuscript and figures. G.Y. and T.Y. made a deep discussion to polish up the manuscript. All authors read the manuscript before submission.
The authors declare no competing financial interest.
References
- Musgrave C. S. A.; Fang F. Contact lens materials: A materials science perspective. Materials (Basel) 2019, 12 (2), 261. 10.3390/ma12020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreddu R.; Vigolo D.; Yetisen A. K. Contact lens technology: From fundamentals to applications. Adv. Healthcare Mater. 2019, 8 (15), e1900368 10.1002/adhm.201900368. [DOI] [PubMed] [Google Scholar]
- Lloyd A. W.; Faragher R. G.; Denyer S. P. Ocular biomaterials and implants. Biomaterials 2001, 22 (8), 769–785. 10.1016/S0142-9612(00)00237-4. [DOI] [PubMed] [Google Scholar]
- Lembach R. G. Rigid gas permeable contact lenses. CLAO J. 1990, 16 (2), 129–134. [PubMed] [Google Scholar]
- Ferraz M. P. Biomaterials for ophthalmic applications. Appl. Sci. 2022, 12, 5886. 10.3390/app12125886. [DOI] [Google Scholar]
- Nicolson P. C. Continuous wear contact lens surface chemistry and wearability. Eye Contact Lens. 2003, 29 (1 Suppl), S30–S32. 10.1097/00140068-200301001-00009. [DOI] [PubMed] [Google Scholar]
- Luensmann D.; Jones L. Protein deposition on contact lenses: the past, the present, and the future. Contact Lens Anterior Eye. 2012, 35 (2), 53–64. 10.1016/j.clae.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Teichroeb J. H.; Forrest J. A.; Ngai V.; Martin J. W.; Jones L.; Medley J. Imaging protein deposits on contact lens materials. Optom. Vis. Sci. 2008, 85 (12), 1151–1164. 10.1097/OPX.0b013e31818e8ad6. [DOI] [PubMed] [Google Scholar]
- Vidal-Rohr M.; Wolffsohn J. S.; Davies L. N.; Cerviño A. Effect of contact lens surface properties on comfort, tear stability and ocular physiology. Contact Lens Anterior Eye. 2018, 41 (1), 117–121. 10.1016/j.clae.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Su C. Y.; Yeh L. K.; Fan T. W.; Lai C. C.; Fang H. W. Albumin acts as a lubricant on the surface of hydrogel and silicone hydrogel contact lenses. Polymers (Basel). 2021, 13 (13), 2051. 10.3390/polym13132051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievens C. W.; Rayborn E. Tribology and the ocular surface. Clin. Ophthalmol. 2022, 16, 973–980. 10.2147/OPTH.S360293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsom M.; Iwabuchi Y.; Sheardown H.; Schmidt T. A. Proteoglycan 4 and hyaluronan as boundary lubricants for model contact lens hydrogels. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106 (3), 1329–1338. 10.1002/jbm.b.33895. [DOI] [PubMed] [Google Scholar]
- Cheung S.; Lorentz H.; Drolle E.; Leonenko Z.; Jones L. W. Comparative study of lens solutions’ ability to remove tear constituents. Optom. Vis. Sci. 2014, 91 (9), 1045–61. 10.1097/OPX.0000000000000340. [DOI] [PubMed] [Google Scholar]
- Willcox M.; Keir N.; Maseedupally V.; Masoudi S.; McDermott A.; Mobeen R.; Purslow C.; Santodomingo-Rubido J.; Tavazzi S.; Zeri F.; Jones L. CLEAR - Contact lens wettability, cleaning, disinfection and interactions with tears. Contact Lens Anterior Eye. 2021, 44 (2), 157–191. 10.1016/j.clae.2021.02.004. [DOI] [PubMed] [Google Scholar]
- Guan A.; Li Z.; Phillips K. S. The effects of non-ionic polymeric surfactants on the cleaning of biofouled hydrogel materials. Biofouling 2015, 31 (9–10), 689–697. 10.1080/08927014.2015.1094690. [DOI] [PubMed] [Google Scholar]
- Wichterle O.; Lim D. Hydrophilic gels for biological use. Nature 1960, 185 (4706), 117–118. 10.1038/185117a0. [DOI] [Google Scholar]
- Maldonado-Codina C.; Efron N. Dynamic wettability of pHEMA-based hydrogel contact lenses. Ophthalmic Physiol. Opt. 2006, 26 (4), 408–418. 10.1111/j.1475-1313.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- Hoffman A. S. Hydrogels for biomedical applications. Adv. Drug Delivery Rev. 2002, 54 (1), 3–12. 10.1016/S0169-409X(01)00239-3. [DOI] [PubMed] [Google Scholar]
- Compañ V.; Guzmán J.; Riande E. A potentiostatic study of oxygen transmissibility and permeability through hydrogel membranes. Biomaterials. 1998, 19 (23), 2139–45. 10.1016/S0142-9612(98)00113-6. [DOI] [PubMed] [Google Scholar]
- Meakin J. R.; Hukins D. W.; Aspden R. M.; Imrie C. T. Rheological properties of poly(2-hydroxyethyl methacrylate) (pHEMA) as a function of water content and deformation frequency. J. Mater. Sci. Mater. Med. 2003, 14 (9), 783–787. 10.1023/A:1025088405674. [DOI] [PubMed] [Google Scholar]
- Lee D.; Lee N.; Kwon I. Efficient loading of ophthalmic drugs with poor load ability into contact lenses using functional comonomers. Biomater. Sci. 2018, 6 (10), 2639–2646. 10.1039/C8BM00586A. [DOI] [PubMed] [Google Scholar]
- Yañez F.; Concheiro A.; Alvarez-Lorenzo C. Macromolecule release and smoothness of semi-interpenetrating PVP-pHEMA networks for comfortable soft contact lenses. Eur. J. Pharm. Biopharm. 2008, 69 (3), 1094–103. 10.1016/j.ejpb.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Liu D. E.; Kotsmar C.; Nguyen F.; Sells T.; Taylor N. O.; Prausnitz J. M.; Radke C. J. Macromolecule sorption and diffusion in HEMA/MAA Hydrogels. Ind. Eng. Chem. Res. 2013, 52 (50), 18109–18120. 10.1021/ie402148u. [DOI] [Google Scholar]
- Liu D. E.; Dursch T. J.; Taylor N. O.; Chan S. Y.; Bregante D. T.; Radke C. J. Diffusion of water-soluble sorptive drugs in HEMA/MAA hydrogels. J. Controlled Release 2016, 239, 242–248. 10.1016/j.jconrel.2016.08.025. [DOI] [PubMed] [Google Scholar]
- Weissman B. A.; Schwartz S. D.; Gottschalk-Katsev N.; Lee D. A. Oxygen permeability of disposable soft contact lenses. Am. J. Ophthalmol. 1990, 110 (3), 269–273. 10.1016/S0002-9394(14)76343-3. [DOI] [PubMed] [Google Scholar]
- Efron N.; Morgan P. B.; Cameron I. D.; Brennan N. A.; Goodwin M. Oxygen permeability and water content of silicone hydrogel contact lens materials. Optom. Vis. Sci. 2007, 84 (4), 328–337. 10.1097/OPX.0b013e31804375ed. [DOI] [PubMed] [Google Scholar]
- Garrett Q.; Laycock B.; Garrett R. W. Hydrogel lens monomer constituents modulate protein sorption. Invest. Ophthalmol. Vis. Sci. 2000, 41 (7), 1687–1695. [PubMed] [Google Scholar]
- Baines M. G.; Cai F.; Backman H. A. Adsorption and removal of protein bound to hydrogel contact lenses. Optom. Vis. Sci. 1990, 67 (11), 807–810. 10.1097/00006324-199011000-00003. [DOI] [PubMed] [Google Scholar]
- Cho P.; Boost M. V. Daily disposable lenses: the better alternative. Contact Lens Anterior Eye. 2013, 36 (1), 4–12. 10.1016/j.clae.2012.10.073. [DOI] [PubMed] [Google Scholar]
- McGlinchey S. M.; McCoy C. P.; Gorman S. P.; Jones D. S. Key biological issues in contact lens development. Expert Rev. Med. Devices. 2008, 5 (5), 581–590. 10.1586/17434440.5.5.581. [DOI] [PubMed] [Google Scholar]
- Nichols J. J.; Fisher D. Contact lenses 2020. Contact Lens Spectr. 2021, 36, 24–29. [Google Scholar]
- Contact Lenses, Market Research Report, 2022; Global Industry Analysis: West Hartford, CT, USA. [Google Scholar]
- Tighe B. J. A decade of silicone hydrogel development: surface properties, mechanical properties, and ocular compatibility. Eye Contact Lens. 2013, 39 (1), 4–12. 10.1097/ICL.0b013e318275452b. [DOI] [PubMed] [Google Scholar]
- Wu S.; Zhang X.; Sun Y.; Yang H.; Lin B.; Han X.; Chen P. Study on the influence of crosslinking density and free polysiloxane chain length on oxygen permeability and hydrophilicity of multicomponent silicone hydrogels. Colloid Polym. Sci. 2021, 299, 1327–1335. 10.1007/s00396-021-04850-5. [DOI] [Google Scholar]
- Abada E. N.; Feinberg B. J.; Roy S. Evaluation of silicon membranes for extracorporeal membrane oxygenation (ECMO). Biomed Microdevices. 2018, 20 (4), 86. 10.1007/s10544-018-0335-z. [DOI] [PubMed] [Google Scholar]
- Nguyen H. T.; Massino M.; Keita C.; Salmon J. B. Microfluidic dialysis using photo-patterned hydrogel membranes in PDMS chips. Lab Chip 2020, 20 (13), 2383–2393. 10.1039/D0LC00279H. [DOI] [PubMed] [Google Scholar]
- Keir N.; Jones L. Wettability and silicone hydrogel lenses: a review. Eye Contact Lens. 2013, 39 (1), 100–108. 10.1097/ICL.0b013e31827d546e. [DOI] [PubMed] [Google Scholar]
- Lin C. H.; Yeh Y. H.; Lin W. C.; Yang M. C. Novel silicone hydrogel based on PDMS and PEGMA for contact lens application. Colloids Surf. B: Biointerfaces 2014, 123, 986–994. 10.1016/j.colsurfb.2014.10.053. [DOI] [PubMed] [Google Scholar]
- Begley C. G.; Caffery B.; Nichols K. K.; Chalmers R. Responses of contact lens wearers to a dry eye survey. Optom. Vis. Sci. 2000, 77 (1), 40–46. 10.1097/00006324-200001000-00012. [DOI] [PubMed] [Google Scholar]
- Jones L.; Senchyna M.; Glasier M. A.; Schickler J.; Forbes I.; Louie D.; May C. Lysozyme and lipid deposition on silicone hydrogel contact lens materials. Eye Contact Lens. 2003, 29, 75–79. 10.1097/00140068-200301001-00021. [DOI] [PubMed] [Google Scholar]
- Mann A.; Tighe B. Contact lens interactions with the tear film. Exp. Eye Res. 2013, 117, 88–98. 10.1016/j.exer.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Mutlu Z.; Shams Es-Haghi S.; Cakmak M. Recent trends in advanced contact lenses. Adv. Healthcare Mater. 2019, 8 (10), e1801390 10.1002/adhm.201801390. [DOI] [PubMed] [Google Scholar]
- Tran N. P. D.; Yang M. C. Synthesis and characterization of silicone contact lenses based on TRIS-DMA-NVP-HEMA hydrogels. Polymers 2019, 11, 944. 10.3390/polym11060944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thissen H.; Gengenbach T.; du Toit R.; Sweeney D. F.; Kingshott P.; Griesser H. J.; Meagher L. Clinical observations of biofouling on PEO coated silicone hydrogel contact lenses. Biomaterials 2010, 31, 5510–5519. 10.1016/j.biomaterials.2010.03.040. [DOI] [PubMed] [Google Scholar]
- Sun F. Q.; Li X. S.; Cao P. T.; Xu J. K. Enhancing hydrophilicity and protein resistance of silicone hydrogels by plasma induced grafting with hydrophilic polymers. Chin. J. Polym. Sci. 2010, 28, 547–554. 10.1007/s10118-010-9082-1. [DOI] [Google Scholar]
- Fleiszig S. M. J.; Kroken A. R.; Nieto V.; Grosser M. R.; Wan S. J.; Metruccio M. M. E.; Evans D. J. Contact lens-related corneal infection: Intrinsic resistance and its compromise. Prog. Retin. Eye Res. 2020, 76, 100804. 10.1016/j.preteyeres.2019.100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox M. D.; Holden B. A. Contact lens related corneal infections. Biosci. Rep. 2001, 21 (4), 445–461. 10.1023/A:1017991709846. [DOI] [PubMed] [Google Scholar]
- Cheng X.; Brennan N. A.; Toubouti Y.; Greenaway N. L. Safety of soft contact lenses in children: retrospective review of six randomized controlled trials of myopia control. Acta Ophthalmol. 2020, 98 (3), e346–e351. 10.1111/aos.14283. [DOI] [PubMed] [Google Scholar]
- Sorbara L.; Zimmerman A. B.; Mitchell G. L.; Richdale K.; Lam D. Y.; Kinoshita B. T.; Chalmers R. L.; Wagner H. Multicenter testing of a risk assessment survey for soft contact lens wearers with adverse events: A contact lens assessment in youth study. Eye Contact Lens. 2018, 44 (1), 21–28. 10.1097/ICL.0000000000000305. [DOI] [PubMed] [Google Scholar]
- Wang X.; Jacobs D. S. Contact Lenses for Ocular Surface Disease. Eye Contact Lens. 2022, 48 (3), 115–118. 10.1097/ICL.0000000000000879. [DOI] [PubMed] [Google Scholar]
- Ren D. H.; Petroll W. M.; Jester J. V.; Ho-Fan J.; Cavanagh H. D. The relationship between contact lens oxygen permeability and binding of Pseudomonas aeruginosa to human corneal epithelial cells after overnight and extended wear. CLAO J. 1999, 25 (2), 80–100. [PubMed] [Google Scholar]
- Jacob J. T. Biocompatibility in the development of silicone-hydrogel lenses. Eye Contact Lens. 2013, 39 (1), 13–19. 10.1097/ICL.0b013e31827dbb00. [DOI] [PubMed] [Google Scholar]
- Rex J.; Knowles T.; Zhao X.; Lemp J.; Maissa C.; Perry S. S. Elemental composition at silicone hydrogel contact lens surfaces. Eye Contact Lens. 2018, 44, S221–S226. 10.1097/ICL.0000000000000454. [DOI] [PubMed] [Google Scholar]
- Vidal-Rohr M.; Wolffsohn J. S.; Davies L. N.; Cerviño A. Effect of contact lens surface properties on comfort, tear stability and ocular physiology. Contact Lens Anterior Eye. 2018, 41 (1), 117–121. 10.1016/j.clae.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Ullah F.; Othman M. B.; Javed F.; Ahmad Z.; Akil H. Md. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 414–433. 10.1016/j.msec.2015.07.053. [DOI] [PubMed] [Google Scholar]
- Lu F.; Tao A.; Tao W.; Zhuang X.; Shen M. Thickness changes in the corneal epithelium and Bowman’s layer after overnight wear of silicone hydrogel contact lenses. BMC Ophthalmol. 2018, 18 (1), 286. 10.1186/s12886-018-0956-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L.; Muller S. J.; Radke C. J. Wettability of silicone-hydrogel contact lenses in the presence of tear-film components. Curr. Eye Res. 2004, 28 (2), 93–108. 10.1076/ceyr.28.2.93.26231. [DOI] [PubMed] [Google Scholar]
- Miranda I.; Souza A.; Sousa P.; Ribeiro J.; Castanheira E. M. S.; Lima R.; Minas G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct Biomater. 2022, 13 (1), 2. 10.3390/jfb13010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S. H.; Zhang S.; Hjort K.; Hilborn J.; Wu Z. PDMS-based elastomer tuned soft, stretchable, and sticky for epidermal electronics. Adv. Mater. 2016, 28 (28), 5830–5836. 10.1002/adma.201505372. [DOI] [PubMed] [Google Scholar]
- Zhao Z.; Xie H.; An S.; Jiang Y. The relationship between oxygen permeability and phase separation morphology of the multicomponent silicone hydrogels. J. Phys. Chem. B 2014, 118 (50), 14640–14647. 10.1021/jp507682k. [DOI] [PubMed] [Google Scholar]
- Zhao Z. B.; An S. S.; Xie H. J.; Han X. L.; Wang F. H.; Jiang Y. The Relationship between the hydrophilicity and surface chemical composition microphase separation structure of multicomponent silicone hydrogels. J. Phys. Chem. B 2015, 119 (30), 9780–9786. 10.1021/acs.jpcb.5b04202. [DOI] [PubMed] [Google Scholar]
- Wuchte L.; DiPasquale S.; Masterson A.; Vance A.; Goff J.; Arkles B.; Sulaiman S.; Byrne M. Characterization and analysis of extended-wear silicone hydrogel contact lenses utilizing novel silicone macromers. J. Biomed Mater. Res. A 2022, 110 (8), 1512–1523. 10.1002/jbm.a.37389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. M. The effects of silicone hydrogel lens wear on the corneal epithelium and risk for microbial keratitis. Eye Contact Lens. 2013, 39 (1), 67–72. 10.1097/ICL.0b013e31827c5b73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczotka-Flynn L.; Diaz M. Risk of corneal inflammatory events with silicone hydrogel and low dk hydrogel extended contact lens wear: a meta-analysis. Optom Vis Sci. 2007, 84 (4), 247–56. 10.1097/OPX.0b013e3180421c47. [DOI] [PubMed] [Google Scholar]
- Hamano H.; Maeda N.; Hamano T.; Mitsunaga S.; Kotani S. Corneal thickness change induced by dozing while wearing hydrogel and silicone hydrogel lenses. Eye Contact Lens. 2008, 34 (1), 56–60. 10.1097/ICL.0b013e318073cf5d. [DOI] [PubMed] [Google Scholar]
- Steffen R. B.; Schnider C. M. The impact of silicone hydrogel materials on overnight corneal swelling. Eye Contact Lens. 2007, 33 (3), 115–120. 10.1097/01.icl.0000242166.09513.aa. [DOI] [PubMed] [Google Scholar]
- Morra M.; Cassinelli C. Biomaterials surface characterization and modification. Int. J. Artif Organs. 2006, 29 (9), 824–833. 10.1177/039139880602900903. [DOI] [PubMed] [Google Scholar]
- Major R.; Gonsior M.; Sanak M.; Kot M.; Kustosz R.; Lackner J. M. Surface modification of metallic materials designed for a new generation of artificial heart valves. Int. J. Artif Organs. 2018, 41 (12), 854–866. 10.1177/0391398818794064. [DOI] [PubMed] [Google Scholar]
- Salehi-Nik N.; Amoabediny G.; Shokrgozar M. A.; Mottaghy K.; Klein-Nulend J.; Zandieh-Doulabi B. Surface modification of silicone tubes by functional carboxyl and amine, but not peroxide groups followed by collagen immobilization improves endothelial cell stability and functionality. Biomed Mater. 2015, 10 (1), 015024. 10.1088/1748-6041/10/1/015024. [DOI] [PubMed] [Google Scholar]
- Li I. T.; Walker G. C. Single polymer studies of hydrophobic hydration. Acc. Chem. Res. 2012, 45 (11), 2011–2021. 10.1021/ar200285h. [DOI] [PubMed] [Google Scholar]
- Chen Z. Surface hydration and antifouling activity of zwitterionic polymers. Langmuir. 2022, 38 (15), 4483–4489. 10.1021/acs.langmuir.2c00512. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Zheng J.; Lin C.; Yuan S. Molecular dynamics study on properties of hydration layers above polymer antifouling membranes. Molecules. 2022, 27 (10), 3074. 10.3390/molecules27103074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerch G. Polymer hydration and stiffness at biointerfaces and related cellular processes. Nanomedicine. 2018, 14 (1), 13–25. 10.1016/j.nano.2017.08.012. [DOI] [PubMed] [Google Scholar]
- Ishihara K.; Ueda T.; Nakabayashi N. Preparation of phospholipid polymers and their properties as polymer hydrogel membranes. Polym. J. 1990, 22 (5), 355–360. 10.1295/polymj.22.355. [DOI] [Google Scholar]
- Ishihara K. Revolutionary advances in 2-methacryloyloxyethyl phosphorylcholine polymers as biomaterials. J. Biomed Mater. Res. A 2019, 107 (5), 933–943. 10.1002/jbm.a.36635. [DOI] [PubMed] [Google Scholar]
- Okuno K.; Saeki D.; Matsuyama H. Phase separation behavior of binary mixture of photopolymerizable diacetylene and unsaturated phospholipids in liposomes. Biochim Biophys Acta Biomembr. 2020, 1862 (9), 183377. 10.1016/j.bbamem.2020.183377. [DOI] [PubMed] [Google Scholar]
- Pax H.; Blume A. Polymerizable phospholipids with lipoic acid as head group: synthesis and phase properties. Chem. Phys. Lipids. 1993, 66 (1–2), 63–74. 10.1016/0009-3084(93)90032-X. [DOI] [PubMed] [Google Scholar]
- Ishihara K.; Mu M.; Konno T.; Inoue Y.; Fukazawa K. The unique hydration state of poly(2-methacryloyloxyethyl phosphorylcholine). J. Biomater Sci. Polym. Ed. 2017, 28 (10–12), 884–899. 10.1080/09205063.2017.1298278. [DOI] [PubMed] [Google Scholar]
- Willis S. L; Court J. L; Redman R. P; Wang J.-H.; Leppard S. W; O’Byrne V. J; Small S. A; Lewis A. L; Jones S. A; Stratford P. W A novel phosphorylcholine-coated contact lens for extended wear use. Biomaterials. 2001, 22 (24), 3261–3272. 10.1016/S0142-9612(01)00164-8. [DOI] [PubMed] [Google Scholar]
- Lemp M. A.; Caffery B.; Lebow K.; Lembach R.; Park J.; Foulks G.; Hall B.; Bowers R.; McGarvey S.; Young G. Omafilcon A (Proclear) soft contact lenses in a dry eye population. CLAO J. 1999, 25 (1), 40–47. [PubMed] [Google Scholar]
- Goda T.; Ishihara K. Soft contact lens biomaterials from bioinspired phospholipid polymers. Expert Rev. Med. Devices. 2006, 3 (2), 167–174. 10.1586/17434440.3.2.167. [DOI] [PubMed] [Google Scholar]
- Ishihara K.; Nomura H.; Mihara T.; Kurita K.; Iwasaki Y.; Nakabayashi N. Why do phospholipid polymers reduce protein adsorption?. J. Biomed Mater. Res. 1998, 39 (2), 323–330. . [DOI] [PubMed] [Google Scholar]
- Ishihara K.; Iwasaki Y. Reduced protein adsorption on novel phospholipid polymers. J. Biomater Appl. 1998, 13 (2), 111–127. 10.1177/088532829801300203. [DOI] [PubMed] [Google Scholar]
- Ueda T.; Ishihara K.; Nakabayashi N. Adsorption-desorption of proteins on phospholipid polymer surfaces evaluated by dynamic contact angle measurement. J. Biomed Mater. Res. 1995, 29 (3), 381–387. 10.1002/jbm.820290313. [DOI] [PubMed] [Google Scholar]
- Kitano H.; Sudo K.; Ichikawa K.; Ide M.; Ishihara K. Raman spectroscopic study on the structure of water in aqueous polyelectrolyte solution. J. Phys. Chem. B 2000, 104 (47), 11425–11429. 10.1021/jp000429c. [DOI] [Google Scholar]
- Kitano H.; Imai M.; Mori T.; Gemmei-Ide M.; Yokoyama Y.; Ishihara K. Structure of water in the vicinity of phospholipid analogue copolymers as studied by vibrational spectroscopy. Langmuir. 2003, 19 (24), 10260–10266. 10.1021/la0349673. [DOI] [Google Scholar]
- Sakata S.; Inoue Y.; Ishihara K. Precise control of surface electrostatic forces on polymer brush layers with opposite charges for resistance to protein adsorption. Biomaterials. 2016, 105, 102–108. 10.1016/j.biomaterials.2016.07.043. [DOI] [PubMed] [Google Scholar]
- Sakata S.; Inoue Y.; Ishihara K. Quantitative evaluation of interaction force between functional groups in protein and polymer brush surfaces. Langmuir. 2014, 30, 2745–2751. 10.1021/la404981k. [DOI] [PubMed] [Google Scholar]
- Holmlin R. E.; Chen X.; Chapman R. G.; Takayama S.; Whitesides G. M. Zwitterionic SAMs that resist nonspecific adsorption of protein from aqueous buffer. Langmuir. 2001, 17, 2841–2850. 10.1021/la0015258. [DOI] [PubMed] [Google Scholar]
- Chapman R. G.; Ostuni E.; Takayama S.; Holmlin R. E.; Yan L.; Whitesides G. M. Surveying for surfaces that resist the adsorption of proteins. J. Am. Chem. Soc. 2000, 122, 8303–8304. 10.1021/ja000774f. [DOI] [Google Scholar]
- Kyomoto M.; Moro T.; Miyaji F.; Hashimoto M.; Kawaguchi H.; Takatori Y.; Nakamura K.; Ishihara K. Effects of mobility/immobility of surface modification by 2-methacryloyloxyethyl phosphorylcholine polymer on the durability of polyethylene for artificial joints. J. Biomed Mater. Res. A 2009, 90 (2), 362–371. 10.1002/jbm.a.32092. [DOI] [PubMed] [Google Scholar]
- Tighe B. J. A decade of silicone hydrogel development: surface properties, mechanical properties, and ocular compatibility. Eye Contact Lens. 2013, 39 (1), 4–12. 10.1097/ICL.0b013e318275452b. [DOI] [PubMed] [Google Scholar]
- Stapleton F.; Stretton S.; Papas E.; Skotnitsky C.; Sweeney D. F. Silicone hydrogel contact lenses and the ocular surface. Ocul Surf. 2006, 4 (1), 24–43. 10.1016/S1542-0124(12)70262-8. [DOI] [PubMed] [Google Scholar]
- Spadafora A.; Korogiannaki M.; Sheardown H. Antifouling silicone hydrogel contact lenses via densely grafted phosphorylcholine polymers. Biointerphases. 2020, 15 (4), 041013. 10.1116/6.0000366. [DOI] [PubMed] [Google Scholar]
- Wang J. J.; Liu F. Photoinduced graft polymerization of 2-methacryloyloxyethyl phosphorylcholine on silicone hydrogels for reducing protein adsorption. J. Mater. Sci. Mater. Med. 2011, 22 (12), 2651–2657. 10.1007/s10856-011-4452-y. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Hsu K. H.; Gharami S.; Butler J. E.; Hazra S.; Chauhan A. Interfacial polymerization of a thin film on contact lenses for improving lubricity. J. Colloid Interface Sci. 2020, 571, 356–367. 10.1016/j.jcis.2020.03.060. [DOI] [PubMed] [Google Scholar]
- Xu C.; He R.; Xie B.; Ismail M.; Yao C.; Luan J.; Li X. Silicone hydrogels grafted with natural amino acids for ophthalmological application. J. Biomater Sci. Polym. Ed. 2016, 27 (13), 1354–1368. 10.1080/09205063.2016.1201916. [DOI] [PubMed] [Google Scholar]
- Leigh B. L.; Cheng E.; Xu L.; Derk A.; Hansen M. R.; Guymon C. A. Antifouling photograftable zwitterionic coatings on PDMS substrates. Langmuir. 2019, 35 (5), 1100–1110. 10.1021/acs.langmuir.8b00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J.; Jin J.; Han Y.; Jiang W. Effect of end-grafted PEG conformation on the hemocompatibility of poly(styrene-b-(ethylene-co-butylene)-b-styrene). J. Biomater Sci. Polym. Ed. 2019, 30 (17), 1670–1685. 10.1080/09205063.2019.1657621. [DOI] [PubMed] [Google Scholar]
- Abreu-Rejón A. D.; Herrera-Kao W. A.; May-Pat A.; Ávila-Ortega A.; Rodríguez-Fuentes N.; Uribe-Calderón J. A.; Cervantes-Uc J. M. Influence of molecular weight and grafting density of PEG on the surface properties of polyurethanes and their effect on the viability and morphology of fibroblasts and osteoblasts. Polymers (Basel). 2022, 14 (22), 4912. 10.3390/polym14224912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T.; Tu C.; Zhou T.; Yu Z.; Wang Y.; Yu Q.; Yu K.; Jiang Z.; Gao C.; Yang G. Antifouling poly(PEGMA) grafting modified titanium surface reduces osseointegration through resisting adhesion of bone marrow mesenchymal stem cells. Acta Biomater. 2022, 153, 585–595. 10.1016/j.actbio.2022.09.058. [DOI] [PubMed] [Google Scholar]
- Chen H.; Zhang Z.; Chen Y.; Brook M. A.; Sheardown H. Protein repellant silicone surfaces by covalent immobilization of poly(ethylene oxide). Biomaterials. 2005, 26 (15), 2391–2399. 10.1016/j.biomaterials.2004.07.068. [DOI] [PubMed] [Google Scholar]
- Sun M.; Deng J.; Tang Z.; Wu J.; Li D.; Chen H.; Gao C. A correlation study of protein adsorption and cell behaviors on substrates with different densities of PEG chains. Colloids Surf. B Biointerfaces. 2014, 122, 134–142. 10.1016/j.colsurfb.2014.06.041. [DOI] [PubMed] [Google Scholar]
- Bai Y.; Ngo W.; Nichols J. J. Characterization of the thickness of the tear film lipid layer using high resolution microscopy. Ocul Surf. 2019, 17 (2), 356–359. 10.1016/j.jtos.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx S.; Eckstein J.; Sickenberger W. Objective analysis of pre-lens tear film stability of daily disposable contact lenses using ring mire projection. Clin Optom (Auckl). 2020, 12, 203–211. 10.2147/OPTO.S262353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. E.; Kim S. R.; Park M. Influence of tear protein deposition on the oxygen permeability of soft contact lenses. J. Ophthalmol. 2017, 2017, 5131764. 10.1155/2017/5131764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiah N. I.; Scales C. W.; Fuller G. G. The influence of protein deposition on contact lens tear film stability. Colloids Surf. B Biointerfaces. 2019, 180, 229–236. 10.1016/j.colsurfb.2019.04.051. [DOI] [PubMed] [Google Scholar]
- Gipson I. K.; Argüeso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int. Rev. Cytol. 2003, 231, 1–49. 10.1016/S0074-7696(03)31001-0. [DOI] [PubMed] [Google Scholar]
- Lum E.; Golebiowski B.; Gunn R.; Babhoota M.; Swarbrick H. Corneal sensitivity with contact lenses of different mechanical properties. Optom Vis Sci. 2013, 90 (9), 954–960. 10.1097/OPX.0000000000000016. [DOI] [PubMed] [Google Scholar]
- Bhamra T. S.; Tighe B. J. Mechanical properties of contact lenses: The contribution of measurement techniques and clinical feedback to 50 years of materials development. Cont Lens Anterior Eye. 2017, 40 (2), 70–81. 10.1016/j.clae.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Mao T.; Fang F. Biomimetic functional surfaces towards bactericidal soft contact lenses. Micromachines (Basel). 2020, 11 (9), 835. 10.3390/mi11090835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K. Biomimetic materials based on zwitterionic polymers toward human-friendly medical devices. Sci. Technol. Adv. Mater. 2022, 23 (1), 498–524. 10.1080/14686996.2022.2119883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L.; Zhao X.; Zhang Y.; Yang L.; Wang R.; Ma Z.; Liang Y. M.; Ma S.; Zhou F. Bioinspired polysaccharide derivative with efficient and stable lubrication for silicon-based devices. Biomacromolecules. 2022, 23 (9), 3766–3778. 10.1021/acs.biomac.2c00640. [DOI] [PubMed] [Google Scholar]
- Korogiannaki M.; Jones L.; Sheardown H. Impact of a hyaluronic acid-grafted layer on the surface properties of model silicone hydrogel contact lenses. Langmuir. 2019, 35 (4), 950–961. 10.1021/acs.langmuir.8b01693. [DOI] [PubMed] [Google Scholar]
- Weeks A.; Luensmann D.; Boone A.; Jones L.; Sheardown H. Hyaluronic acid as an internal wetting agent in model DMAA/TRIS contact lenses. J. Biomater Appl. 2012, 27 (4), 423–432. 10.1177/0885328211410999. [DOI] [PubMed] [Google Scholar]
- Yamasaki K.; Drolle E.; Nakagawa H.; Hisamura R.; Ngo W.; Jones L. Impact of a low molecular weight hyaluronic acid derivative on contact lens wettability. Contact Lens Anterior Eye. 2021, 44 (3), 101334. 10.1016/j.clae.2020.05.003. [DOI] [PubMed] [Google Scholar]
- Xu C.; He R.; Xie B.; Ismail M.; Yao C.; Luan J.; Li X. Silicone hydrogels grafted with natural amino acids for ophthalmological application. J. Biomater. Sci. Polym. Ed. 2016, 27 (13), 1354–1368. 10.1080/09205063.2016.1201916. [DOI] [PubMed] [Google Scholar]
- Dutta D.; Kamphuis B.; Ozcelik B.; Thissen H.; Pinarbasi R.; Kumar N.; Willcox M. D. P. Development of silicone hydrogel antimicrobial contact lenses with Mel4 peptide coating. Optom. Vis. Sci. 2018, 95 (10), 937–946. 10.1097/OPX.0000000000001282. [DOI] [PubMed] [Google Scholar]
- Korogiannaki M.; Samsom M.; Schmidt T. A.; Sheardown H. Surface-functionalized model contact lenses with a bioinspired proteoglycan 4 (PRG4)-grafted layer. ACS Appl. Mater. Interfaces. 2018, 10 (36), 30125–30136. 10.1021/acsami.8b09755. [DOI] [PubMed] [Google Scholar]
- Korogiannaki M.; Samsom M.; Matheson A.; Soliman K.; Schmidt T. A.; Sheardown H. Investigating the synergistic interactions of surface immobilized and free natural ocular lubricants for contact lens applications: A comparative study between hyaluronic acid and proteoglycan 4 (Lubricin). Langmuir. 2021, 37 (3), 1062–1072. 10.1021/acs.langmuir.0c02796. [DOI] [PubMed] [Google Scholar]
- De Smet N.; Rymarczyk-Machal M.; Schacht E. Plasma-induced surface modification of polydimethylsiloxane aimed at reducing salt and protein deposition. J. Biomater Sci. Polym. Ed. 2011, 22 (18), 2457–2473. 10.1163/092050610X540666. [DOI] [PubMed] [Google Scholar]
- Gill F. R.; Purslow C.; Murphy P. J. Atomic force microscopy analysis of the effect of plasma treatment on gas permeable contact lens surface topography. Cont Lens Anterior Eye. 2019, 42 (3), 265–272. 10.1016/j.clae.2019.02.010. [DOI] [PubMed] [Google Scholar]
- Yao K.; Huang X. D.; Huang X. J.; Xu Z. K. Improvement of the surface biocompatibility of silicone intraocular lens by the plasma-induced tethering of phospholipid moieties. J. Biomed Mater. Res. A 2006, 78 (4), 684–692. 10.1002/jbm.a.30741. [DOI] [PubMed] [Google Scholar]
- Wang M. T.; Ganesalingam K.; Loh C. S.; Alberquerque T.; Al-Kanani S.; Misra S. L.; Craig J. P. Compatibility of phospholipid liposomal spray with silicone hydrogel contact lens wear. Cont Lens Anterior Eye. 2017, 40 (1), 53–58. 10.1016/j.clae.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Hupfer B.; Ringsdorf H.; Schupp H. Liposomes from polymerizable phospholipids. Chem. Phys. Lipids. 1983, 33 (4), 355–374. 10.1016/0009-3084(83)90028-2. [DOI] [PubMed] [Google Scholar]
- Temprana C. F.; Duarte E. L.; Taira M. C.; Lamy M. T.; del Valle Alonso S. Structural characterization of photopolymerizable binary liposomes containing diacetylenic and saturated phospholipids. Langmuir. 2010, 26 (12), 10084–10092. 10.1021/la100214v. [DOI] [PubMed] [Google Scholar]
- Kobayashi M.; Terayama Y.; Yamaguchi H.; Terada M.; Murakami D.; Ishihara K.; Takahara A. Wettability and antifouling behavior on the surfaces of superhydrophilic polymer brushes. Langmuir. 2012, 28 (18), 7212–7222. 10.1021/la301033h. [DOI] [PubMed] [Google Scholar]
- Kobayashi M.; Terayama Y.; Hosaka N.; Kaido M.; Suzuki A.; Yamada N.; Torikai N.; Ishihara K.; Takahara A. Friction behavior of high-density poly(2-methacryloyloxyethyl phosphorylcholine) brush in aqueous media. Soft Matter. 2007, 3 (6), 740–746. 10.1039/b615780g. [DOI] [PubMed] [Google Scholar]
- Feng W.; Zhu S.; Ishihara K.; Brash J. L. Adsorption of fibrinogen and lysozyme on silicon grafted with poly(2-methacryloyloxyethyl phosphorylcholine) via surface-initiated atom transfer radical polymerization. Langmuir. 2005, 21 (13), 5980–5987. 10.1021/la050277i. [DOI] [PubMed] [Google Scholar]
- Fukazawa K.; Ishihara K. Simple surface treatment using amphiphilic phospholipid polymers to obtain wetting and lubricity on polydimethylsiloxane-based substrates. Colloids Surf. B Biointerfaces. 2012, 97, 70–76. 10.1016/j.colsurfb.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Seo J. H.; Ishihara K. Diffusion-induced hydrophilic conversion of polydimethylsiloxane/block-type phospholipid polymer hybrid substrate for temporal cell-adhesive surface. ACS Appl. Mater. Interfaces. 2016, 8 (33), 21839–21846. 10.1021/acsami.6b07414. [DOI] [PubMed] [Google Scholar]
- Qin X. H.; Senturk B.; Valentin J.; Malheiro V.; Fortunato G.; Ren Q.; Rottmar M.; Maniura-Weber K. Cell-membrane-inspired silicone interfaces that mitigate proinflammatory macrophage activation and bacterial adhesion. Langmuir. 2019, 35 (5), 1882–1894. 10.1021/acs.langmuir.8b02292. [DOI] [PubMed] [Google Scholar]
- Kang S.; Kim J.; Kim S.; Wufuer M.; Park S.; Kim Y.; Choi D.; Jin X.; Kim Y.; Huang Y.; Jeon B.; Choi T. H.; Park J. U.; Lee Y. Efficient reduction of fibrous capsule formation around silicone breast implants densely grafted with 2-methacryloyloxyethyl phosphorylcholine (MPC) polymers by heat-induced polymerization. Biomater Sci. 2020, 8 (6), 1580–1591. 10.1039/C9BM01802F. [DOI] [PubMed] [Google Scholar]
- Goda T.; Konno T.; Takai M.; Moro T.; Ishihara K. Biomimetic phosphorylcholine polymer grafting from polydimethylsiloxane surface using photo-induced polymerization. Biomaterials. 2006, 27 (30), 5151–5160. 10.1016/j.biomaterials.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Nakano H.; Kakinoki S.; Iwasaki Y. Long-lasting hydrophilic surface generated on poly(dimethyl siloxane) with photoreactive zwitterionic polymers. Colloids Surf. B Biointerfaces. 2021, 205, 111900. 10.1016/j.colsurfb.2021.111900. [DOI] [PubMed] [Google Scholar]
- Shimizu T.; Goda T.; Minoura N.; Takai M.; Ishihara K. Super-hydrophilic silicone hydrogels with interpenetrating poly(2-methacryloyloxyethyl phosphorylcholine) networks. Biomaterials. 2010, 31 (12), 3274–3280. 10.1016/j.biomaterials.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Seo J. H.; Matsuno R.; Takai M.; Ishihara K. Cell adhesion on phase-separated surface of block copolymer composed of poly(2-methacryloyloxyethyl phosphorylcholine) and poly(dimethylsiloxane). Biomaterials. 2009, 30 (29), 5330–5340. 10.1016/j.biomaterials.2009.06.031. [DOI] [PubMed] [Google Scholar]
- Goda T.; Matsuno R.; Konno T.; Takai M.; Ishihara K. Photografting of 2-methacryloyloxyethyl phosphorylcholine from polydimethylsiloxane: tunable protein repellency and lubrication property. Colloids Surf. B Biointerfaces. 2008, 63 (1), 64–72. 10.1016/j.colsurfb.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Ishihara K.; Fukazawa K. Cell-membrane-inspired polymers for constructing biointerfaces with efficient molecular recognition. J. Mater. Chem. B 2022, 10 (18), 3397–3419. 10.1039/D2TB00242F. [DOI] [PubMed] [Google Scholar]
- Spadafora A.; Korogiannaki M.; Sheardown H. Antifouling silicone hydrogel contact lenses via densely grafted phosphorylcholine polymers. Biointerphases. 2020, 15 (4), 041013. 10.1116/6.0000366. [DOI] [PubMed] [Google Scholar]
- Shi X.; Cantu-Crouch D.; Sharma V.; Pruitt J.; Yao G.; Fukazawa K.; Wu J. Y.; Ishihara K. Surface characterization of a silicone hydrogel contact lens having bioinspired 2-methacryloyloxyethyl phosphorylcholine polymer layer in hydrated state. Colloids Surf. B Biointerfaces. 2021, 199, 111539. 10.1016/j.colsurfb.2020.111539. [DOI] [PubMed] [Google Scholar]
- Shi X.; Sharma V.; Cantu-Crouch D.; Yao G.; Fukazawa K.; Ishihara K.; Wu J. Y. Nanoscaled morphology and mechanical properties of a biomimetic polymer surface on a silicone hydrogel contact lens. Langmuir. 2021, 37 (47), 13961–13967. 10.1021/acs.langmuir.1c02678. [DOI] [PubMed] [Google Scholar]
- Sharma V.; Shi X.; Yao G.; Pharr G. M.; Wu J. Y. Surface characterization of an ultra-soft contact lens material using an atomic force microscopy nanoindentation method. Sci. Rep. 2022, 12 (1), 20013. 10.1038/s41598-022-24701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M. M.; Johnson C. L.; Dunn A. C. Soft contact mechanics with gradient-stiffness surfaces. Langmuir. 2022, 38 (31), 9454–9465. 10.1021/acs.langmuir.2c00296. [DOI] [PubMed] [Google Scholar]
- Ishihara K. Highly lubricated polymer interfaces for advanced artificial hip joints through biomimetic design. Polym. J. 2015, 47, 585–597. 10.1038/pj.2015.45. [DOI] [Google Scholar]
- Moro T.; Takatori Y.; Ishihara K.; Konno T.; Takigawa Y.; Matsushita T.; Chung U. I.; Nakamura K.; Kawaguchi H. Surface grafting of artificial joints with a biocompatible polymer for preventing periprosthetic osteolysis. Nat. Mater. 2004, 3 (11), 829–836. 10.1038/nmat1233. [DOI] [PubMed] [Google Scholar]
- Inoue Y.; Ishihara K. Reduction of protein adsorption on well-characterized polymer brush layers with varying chemical structures. Colloids Surf. B Biointerfaces. 2010, 81 (1), 350–357. 10.1016/j.colsurfb.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Han Y.; Ma J.; Hu Y.; Jin J.; Jiang W. Effect of end-grafted polymer conformation on protein resistance. Langmuir. 2018, 34 (5), 2073–2080. 10.1021/acs.langmuir.7b03930. [DOI] [PubMed] [Google Scholar]
- Riedelová Z.; de Los Santos Pereira A.; Svoboda J.; Pop-Georgievski O.; Májek P.; Pečánková K.; Dyčka F.; Rodriguez-Emmenegger C.; Riedel T. The relation between protein adsorption and hemocompatibility of antifouling polymer brushes. Macromol. Biosci. 2022, 22 (11), e2200247 10.1002/mabi.202200247. [DOI] [PubMed] [Google Scholar]
- Emilsson G.; Schoch R. L.; Feuz L.; Höök F.; Lim R. Y.; Dahlin A. B. Strongly stretched protein resistant poly(ethylene glycol) brushes prepared by grafting-to. ACS Appl. Mater. Interfaces. 2015, 7 (14), 7505–7515. 10.1021/acsami.5b01590. [DOI] [PubMed] [Google Scholar]
- Lorentz H.; Heynen M.; Trieu D.; Hagedorn S. J.; Jones L. The impact of tear film components on in vitro lipid uptake. Optom Vis Sci. 2012, 89 (6), 856–867. 10.1097/OPX.0b013e318255ddc8. [DOI] [PubMed] [Google Scholar]
- Carney F. P.; Nash W. L.; Sentell K. B. The adsorption of major tear film lipids in vitro to various silicone hydrogels over time. Invest Ophthalmol Vis Sci. 2008, 49 (1), 120–124. 10.1167/iovs.07-0376. [DOI] [PubMed] [Google Scholar]
- Hart D. E.; Tidsale R. R.; Sack R. A. Origin and composition of lipid deposits on soft contact lenses. Ophthalmology. 1986, 93 (4), 495–503. 10.1016/S0161-6420(86)33709-6. [DOI] [PubMed] [Google Scholar]
- Hart D. E. Influence of contact lens material surface characteristics and replacement frequency on protein and lipid deposition. Optom Vis Sci. 1999, 76 (9), 616–617. 10.1097/00006324-199909000-00016. [DOI] [PubMed] [Google Scholar]
- Masoudi S.; Willcox M. A method for studying lipid adsorption to silicone hydrogel contact lenses. Biofouling. 2021, 37 (8), 862–878. 10.1080/08927014.2021.1978433. [DOI] [PubMed] [Google Scholar]
- Pitt W. G.; Perez K. X.; Tam N. K.; Handly E.; Chinn J. A.; Liu X. M.; Maziarz E. P. Quantitation of cholesterol and phospholipid sorption on silicone hydrogel contact lenses. J. Biomed Mater. Res. B Appl. Biomater. 2013, 101 (8), 1516–1523. 10.1002/jbm.b.32973. [DOI] [PubMed] [Google Scholar]
- Pucker A. D.; Nichols J. J. A method of imaging lipids on silicone hydrogel contact lenses. Optom Vis Sci. 2012, 89 (5), E777–E787. 10.1097/OPX.0b013e318253dea9. [DOI] [PubMed] [Google Scholar]
- Liang S.; Shows A.; Dunbar D.; Sharma V.; Shi X.; Wu J. Y. Antifouling studies of lehfilcon A silicone hydrogel contact lens. Invest. Ophthalmol. Vis. Sci. 2022, 63 (5332), A0230. [Google Scholar]
- Capote-Puente R.; Bautista-Llamas M. J.; Manzoni C.; Sánchez-González J. M. Pre-lens tear meniscus height, lipid layer pattern and non-invasive break-up time short-term changes with a water gradient silicone hydrogel contact lens. Life (Basel). 2022, 12 (11), 1710. 10.3390/life12111710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capote-Puente R.; Bautista-Llamas M. J.; Sánchez-González J. M. Tear film dynamics between low and high contact lens dry eye disease questionnaire (CLDEQ-8) score with a Lehfilcon A silicone hydrogel water gradient contact lens: A non-invasive methodology approach. Diagnostics (Basel). 2023, 13 (5), 939. 10.3390/diagnostics13050939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capote-Puente R.; Sánchez-González J. M.; Sánchez-González M. C.; Bautista-Llamas M. J. Evaluation of Celligent® biomimetic water gradient contact lens effects on ocular surface and subjective symptoms. Diagnostics (Basel). 2023, 13 (7), 1258. 10.3390/diagnostics13071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifer R.; Harris V.; Sanders D.; Crary M.; Shannon P. Evaluation of Serratia marcescens Adherence to Contact Lens Materials. Microorganisms. 2023, 11 (1), 217. 10.3390/microorganisms11010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley G.; Giedd B.; Hines B.; Bickle K.; Pearson C.; Lorentz H. Safety and efficacy of a new water gradient biomimetic monthly replacement spherical contact lens material (Lehfilcon A). Clin Ophthalmol. 2022, 16, 2873–2884. 10.2147/OPTH.S362926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Haick H.; Guo S.; Wang C.; Lee S.; Yokota T.; Someya T. Skin bioelectronics towards long-term, continuous health monitoring. Chem. Soc. Rev. 2022, 51 (9), 3759–3793. 10.1039/D2CS00207H. [DOI] [PubMed] [Google Scholar]
- Chien P. J.; Suzuki T.; Ye M.; Toma K.; Arakawa T.; Iwasaki Y.; Mitsubayashi K. Ultra-sensitive isopropanol biochemical gas sensor (Bio-Sniffer) for monitoring of human volatiles. Sensors (Basel). 2020, 20 (23), 6827. 10.3390/s20236827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Zhao Q.; Du X. Structurally coloured contact lens sensor for point-of-care ophthalmic health monitoring. J. Mater. Chem. B 2020, 8 (16), 3519–3526. 10.1039/C9TB02389E. [DOI] [PubMed] [Google Scholar]
- Inubushi S.; Kawaguchi H.; Mizumoto S.; Kunihisa T.; Baba M.; Kitayama Y.; Takeuchi T.; Hoffman R. M.; Sasaki R. Oncogenic miRNAs identified in tear exosomes from metastatic breast cancer patients. Anticancer Res. 2020, 40 (6), 3091–3096. 10.21873/anticanres.14290. [DOI] [PubMed] [Google Scholar]
- Badugu R.; Reece E. A.; Lakowicz J. R. Glucose-sensitive silicone hydrogel contact lens toward tear glucose monitoring. J. Biomed Opt. 2018, 23 (5), 1–9. 10.1117/1.JBO.23.5.057005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.; Dou J.; Wang Y.; Zhu L.; Yao G.; Kim Y. H.; Radke C. J.; Wu J. Y. Sustained release of a polymeric wetting agent from a silicone-hydrogel contact lens material. ACS Omega 2022, 7 (33), 29223–29230. 10.1021/acsomega.2c03310. [DOI] [PMC free article] [PubMed] [Google Scholar]