Abstract
The initial marketing of OxyContin in 1996 increased fatal drug overdoses over the course of the opioid epidemic. However, the long-term impacts of this marketing on complications of injection drug use (IDU), a key feature of the ongoing crisis, are undetermined. This study evaluated the effects of exposure to initial OxyContin marketing on the long-term trajectories of IDU-related outcomes in the U.S. We used a difference-in-differences analysis to compare outcomes in states with high vs. low exposure to initial marketing before and after the 2010 OxyContin reformulation, which spurred widespread substitution to illicit drug use. Exposure to initial OxyContin marketing statistically significantly increased rates of fatal synthetic opioid-related overdoses, acute hepatitis A, B, and C viral infections, and infective endocarditis-related deaths. The greatest burden of adverse long-term outcomes is in states that experienced the highest exposure to early OxyContin marketing. Our findings indicate that OxyContin marketing decisions from the mid-1990s increased viral and bacterial complications of IDU and illicit opioid-related overdose deaths 25 years later.
Introduction
The opioid crisis in the United States has caused over half a million deaths between 1999 and 2020.(1) This ongoing epidemic is frequently divided into a pharmaceutical period between the 1990s and 2010, a heroin period beginning in 2010, and a synthetic opioid period after 2013, mainly driven by the use of fentanyl.(2) In recent years, public health experts have also referred to the opioid epidemic as a “converging public health crisis,” as injection drug use (IDU) has driven increased transmission of infectious diseases, including HIV and the viral hepatitides.(3)
The introduction of OxyContin, a prescription opioid analgesic, was a key contributor to the opioid epidemic.(2, 4, 5) OxyContin, a Schedule II extended-release preparation of oxycodone, was developed by Purdue Pharma in anticipation of generic competition for the company’s MS Contin, a morphine-based drug used to treat pain in cancer patients.(6) Purdue sought to expand the market for OxyContin beyond cancer-related pain. To accomplish this, it promoted the drug as a treatment for people with moderate and chronic pain, a substantially larger patient pool than for cancer pain alone, even though physicians were historically reluctant to prescribe opioids in this setting due to the elevated risk of addiction.(5–8) Employing aggressive sales tactics and exploiting data on physician prescribing patterns, Purdue targeted the marketing of OxyContin to leading opioid prescribers, normalized its use as a treatment for non-cancer pain, and downplayed its potential for addiction.(5, 8, 9) These efforts were effective. Purdue launched OxyContin in 1996. By 2002, sales for OxyContin reached $1.5 billion, representing over 7 million prescriptions.(10) Eager to capitalize on Purdue’s success, other pharmaceutical companies followed suit, using similar tactics to introduce and market competing opioids.(7, 11)
Previous analyses have found that OxyContin marketing decisions at the time of the drug’s introduction led to long-term increases in opioid-related overdose deaths in the U.S.(7, 12) Using internal information on Purdue’s marketing strategies disclosed in court materials, these studies separately identified two novel sources of geographic variation in the introduction of OxyContin. First, Purdue was unwilling to promote OxyContin in five states with triplicate prescribing programs for Schedule II drugs.(12) Triplicate prescribing programs required prescriptions to be written on an official form, with the prescriber, pharmacy, and state each preserving a copy for documentation and governmental oversight.(12) This placed a regulatory burden on physicians and effectively decreased the potential market for OxyContin, thus shielding these states from Purdue’s promotional activities and preventing many overdose fatalities.(12) Second, Purdue originally promoted OxyContin to oncologists and primary care physicians treating cancer patients, and then strategically used primary care physicians as a conduit to reach non-cancer patients.(7) Areas with a greater cancer burden in the mid-1990s, and thus more exposure to OxyContin marketing, endured larger increases in overdose fatalities and adverse social and infant health outcomes compared to other areas.(7)
Despite this compelling evidence and the critical implications for contemporary public health, however, the effects of early OxyContin marketing on viral and bacterial complications of IDU have been unexplored to date. IDU with nonsterile syringes has long been recognized as an important risk factor for the transmission of HCV and HIV.(13, 14) Since the start of the heroin phase of the opioid crisis in 2010, IDU-related infectious diseases have dramatically increased in the U.S.(15) During this transition, states introduced policies like prescription drug monitoring programs to curb misuse, while the supply of heroin, which was inexpensive, potent, and strongly linked to IDU, dramatically expanded.(2, 4, 16–20) Notably, this transition has also been attributed to an August 2010 chemical reformulation of OxyContin, which made the drug “abuse deterrent”.(21, 22) Before the reformulation, the extended release pills could be crushed and the entire dose could be ingested through snorting or injecting. Afterwards, the pills were more difficult to crush and misuse, resulting in widespread substitution to heroin. A body of work has shown that this reformulation increased heroin- and fentanyl-related overdose deaths and the transmission of infectious diseases such as hepatitis B and C, yet the impact of marketing decisions over a decade earlier by Purdue on infectious sequelae of IDU are as yet undetermined.(21–25)
In this study, we evaluated the causal effects of initial OxyContin marketing on the long-term trajectories of IDU-related outcomes in the U.S. We used data from the CDC on all 50 states and the District of Columbia spanning the mid to late 1990s to 2020. Our analysis leveraged two separate events that affected the supply of OxyContin: geographic variation in marketing from its 1996 introduction and the supply shock resulting from its 2010 reformulation. We used a quasi-experimental, difference-in-differences framework to evaluate the extent to which states with high exposure to initial OxyContin marketing (the treatment group) experienced worse health outcomes compared to states with low exposure (the control group). We examined this difference before and after the 2010 reformulation.
Study Data and Methods
Exposure to initial OxyContin marketing
We employed a proxy for initial OxyContin marketing. This proxy combined two features of geographic variation in the introduction of OxyContin that was identified in previous studies: the targeting of physicians with cancer patients and the avoidance of states with triplicate prescribing programs.(7, 12) To construct this proxy, we first calculated each state’s cancer burden in 1993–1995, defined as cancer-related mortality rates per 100,000 averaged over 1993 to 1995 (similar to previous work).(7) We used data from CDC WONDER compressed mortality files and the SEER definition of all malignant cancers (ICD-9 codes 140–208 and 238.6).(26, 27) We did not age-adjust cancer-related mortality rates because we were interested in the total state cancer burden regardless of a state’s age distribution. We then categorized states into terciles of the 1993–1995 cancer burden.
Next, we assigned the states with triplicate prescribing programs in 1996 to the lowest tercile of the 1993–1995 cancer burden. With this step, we assumed that states with either triplicate prescribing programs or the lowest cancer burdens were exposed to less OxyContin marketing than all other states. Of the triplicate prescribing states, Illinois and New York were originally in the middle tercile, while California, Idaho, and Texas were already in the lowest tercile.(12)
Our proxy for initial OxyContin marketing thus categorized each state and the District of Columbia into a “high”, “middle”, or “low” exposure group at the time of its 1996 introduction (appendix figure S1).(28) We assessed our use of this proxy by examining the extent to which each exposure group experienced differential increases in shipments of oxycodone (which includes OxyContin), shipments of other opioids, and fatal prescription- and all opioid-related overdose rates over the next two decades. (See appendix A for additional details).(28) Consistent with the findings of previous analyses, appendix figure S2 generally shows a dose-response divergence.(7, 12, 28)
Outcomes
Our primary outcomes were rates of fatal heroin-related overdoses and synthetic opioid-related overdoses, which includes fentanyl; incidences of acute hepatitis A virus (HAV), acute hepatitis B virus (HBV), and acute hepatitis C virus (HCV); incidence of new diagnoses of HIV attributed to IDU; and rates of infective endocarditis-related deaths amongst people aged 15 to 54. IDU is an important risk factor for these outcomes.(29, 30) As secondary outcomes, we also examined overdose deaths related to stimulant overdoses, including cocaine and methamphetamines.
For mortality outcomes, we collected publicly available CDC WONDER multiple cause of death data from 1999 to 2020.(1) We identified drug overdose deaths and infective endocarditis-related deaths using ICD-10 codes. We restricted infective endocarditis deaths to people aged 15 to 54 to avoid the inclusion of deaths not associated with IDU (e.g. deaths due to cardiac device endocarditis).(31) We calculated mortality rates per 100,000 population using the associated state populations provided by CDC WONDER (compressed mortality files before 1999 and multiple cause of death data beginning in 1999).(1, 26) This population data was also used to weight our analyses. In addition, we used a data smoothing algorithm to estimate suppressed death counts and generated undercounting-adjusted overdose deaths for use in a robustness test. For specific ICD-10 codes and additional details, see appendix B.(28)
We obtained data on acute HAV, acute HBV, and acute HCV rates per 100,000 population by state from 1995 to 2020 from publicly available CDC Viral Hepatitis Surveillance reports.(32, 33) Data were not available for all states in all years, and surveillance systems and data quality may vary across states. For additional details, see appendix C.(28) The CDC has advised that hepatitis testing was disrupted by the COVID-19 pandemic, which may have substantially lowered the number of reported cases in 2020.(33)
Rates of new diagnoses of HIV attributed to IDU per 100,000 population from 2008 to 2019 were collected from AIDSVu, which compiles data from the CDC National HIV Surveillance System.(34) We calculated these rates as the rate of new diagnoses of HIV per 100,000 multiplied by the share of new diagnoses of HIV attributed to IDU.
Statistical analysis
We used a difference-in-differences framework to estimate the effect of high versus low exposure to initial OxyContin marketing. We examined outcomes before and after the 2010 OxyContin reformulation, which facilitated the use of illicit drugs and spread of infectious disease.(21–25) First, we compared long-term trends in the mean values of our outcomes by exposure group. Next, we restricted the sample to states in the high and low exposure groups and employed an event study model to estimate differences between the high and low exposure groups for each year pre- and post-reformulation, relative to the year before the reformulation, using linear regression. We also used linear regression to estimate average effects for the full post-reformulation period, and for three-year sub-periods to document dynamic effects (2010 to 2013, 2014 to 2017, and 2018 and later). We accounted for state characteristics that do not change over time and national shocks to all states using state and year fixed effects, respectively. All regressions were weighted by state population and standard errors were clustered by state. See appendix D for specifications.(28)
Our estimated coefficients represent the causal effects of exposure to initial OxyContin marketing under the identifying assumption that, in the absence of the 2010 OxyContin reformulation, outcome trends in the high exposure group would paralleled trends in the low exposure group. We examined this parallel trends assumption by assessing any statistically significant differences in trends between the high and low exposure groups prior to 2010.
As a robustness test, we incorporated controls for multiple state-level policy changes that may be associated with initial OxyContin marketing and outcomes. (See appendix E for additional information on these variables).(28) We also conducted a series of falsification tests to assess the validity of our results. First, we replaced our proxy for initial OxyContin marketing with early shipments of oxycodone and other opioids (such as hydrocodone, morphine, and codeine), separately, in our differences-in-differences strategy to investigate which would have a larger impact on outcomes. In addition, we similarly examined the extent to which other explanatory factors could drive our results, including high initial levels of poverty; high pre-existing use and misuse of drugs; high initial levels of chronic pain; high initial prevalence of chronic health conditions; and higher levels of IDU in states whose labor markets were the most adversely affected by the Great Recession (See appendix F for detailed information on our falsification tests).(28)
Limitations
Our study has several limitations. First, we rely on an aggregate proxy measure for OxyContin marketing, which categorizes states into three exposure levels, and use state-level surveillance data. Although the analyses would be strengthened by access to Purdue Pharma’s actual marketing data, as well as the use of more granular outcome data, this information is not publicly available. Second, due to differences in surveillance systems, data quality for our hepatitis outcomes may vary across states, though we do not believe this affects our findings. (This is discussed further in appendix C).(28) Next, our study design does not address all state-level policy changes that occurred in the post-reformulation period. Even though we use event studies to assess differences in trends between high and low exposure states before 2010, and conduct robustness tests to account for multiple important policies, subsequent events that we did not account for may differentially affect IDU-related morbidity and mortality. Finally, constraints on our study design and data do not permit a causal analysis of all relevant IDU-related outcomes. Although we include HIV attributed to IDU, this data is only available beginning in 2008, which limits our ability to assess parallel pre-trends and make causal claims. Furthermore, due to data limitations we are unable to examine additional outcomes of interest (e.g., skin and soft tissue infections and nonfatal overdoses). In addition, we have only included acute HCV infections in this study, as chronic HCV data likely reflects a substantial number of infections related to the transfusion of blood products prior to 1990 (14).
Results
Baseline state characteristics
Exhibit 1 presents mean state characteristics at baseline, before the 2010 OxyContin reformulation, by exposure group using data from the IPUMS USA American Community Survey 2005–2009 5-Year Sample.(35) Consistent with having a greater 1993–1995 cancer burden, the populations of states in the high exposure group were somewhat older than those in the low exposure group, and were disproportionately non-Hispanic, white, and native-born. High and low exposure groups were similar in terms of educational attainment, share of the population that is Black, share with income at or below the poverty line, and outcomes. Appendix table S1 presents means and variable information for all outcomes and years by exposure group.(28)
EXHIBIT 1:
Characteristics of U.S. states by exposure to initial OxyContin marketing before the 2010 reformulation
| Exposure group | 1 (Low)a |
2 (Middle)b |
3 (High)c |
|---|---|---|---|
|
| |||
| Number of states | 19 | 15 | 17 |
| Cancer-related mortality in 1993–95 (rate per 100,000) | 178.6 | 206.0 | 236.2 |
| Demographic characteristics in 2005–2009 (%) | |||
| Female | 50.4 | 50.9 | 51.2 |
| Aged 0 to 17 | 25.6 | 24.4 | 23.3 |
| Aged 18 to 44 | 38.5 | 36.8 | 36.1 |
| Aged 45 plus | 35.9 | 38.8 | 40.6 |
| Black (non-Hispanic) | 10.9 | 14.9 | 12.2 |
| Hispanic (any race) | 22.9 | 5.9 | 8.6 |
| White (non-Hispanic) | 56.8 | 74.4 | 74.6 |
| Foreign born | 17.7 | 7.0 | 9.8 |
| High school or less educational attainment | 63.0 | 64.0 | 65.2 |
| Some college or more educational attainment | 37.0 | 36.0 | 34.8 |
| Income at or below the poverty line | 15.8 | 15.9 | 15.9 |
| State population, averaged 2005–2009 (unweighted) | 7,786,712 | 3,975,627 | 5,506,866 |
| Outcomes, averaged 2005–2009 (rate per 100,000) | |||
| Fatal heroin-related overdoses | 0.8 | 1.1 | 0.8 |
| Fatal synthetic opioid-related overdoses | 0.7 | 0.9 | 0.9 |
| Acute HAVd | 1.2 | 0.8 | 0.9 |
| Acute HBVd | 1.4 | 1.3 | 1.7 |
| Acute HCVd | 0.2 | 0.4 | 0.3 |
| New diagnoses of HIV attributed to IDU per 100,000e | 1.6 | 1.5 | 1.7 |
| Infective endocarditis-related deathsf | 0.8 | 1.0 | 1.1 |
SOURCE Authors’ analysis of data on mortality and population counts from CDC WONDER; demographic characteristics from IPUMS USA American Community Survey 2005–2009 5-Year Sample; acute hepatitides, including hepatitis A virus (HAV), hepatitis B virus (HBV), and hepatitis C virus (HCV), from CDC Viral Hepatitis Surveillance reports; and new diagnoses of HIV attributed to IDU from AIDSVu. NOTES Cancer-related mortality and demographic characteristic means were weighted using the average of the 2005–2009 state populations. Outcome means were weighted using state populations.
Alaska, Arizona, California, Colorado, Georgia, Hawaii, Idaho, Illinois, Minnesota, Nevada, New Mexico, New York, South Carolina, Texas, Utah, Vermont, Virginia, Washington, and Wyoming.
Connecticut, Indiana, Kansas, Louisiana, Maryland, Michigan, Mississippi, Montana, Nebraska, New Hampshire, North Carolina, North Dakota, Oregon, South Dakota, and Wisconsin.
Alabama, Arkansas, Delaware, the District of Columbia, Florida, Iowa, Kentucky, Maine, Massachusetts, Missouri, New Jersey, Ohio, Oklahoma, Pennsylvania, Rhode Island, Tennessee, and West Virginia.
Outcome is missing data for some state-years.
Only 2008–2009 available.
For people aged 15 to 54.
Trends by exposure group
Before the 2010 reformulation of OxyContin, outcomes generally exhibited similar levels and trends by exposure group (appendix figures S3, S4, and S5).(28) After 2010, mean trends in the high exposure group diverged from the low exposure group for all outcomes, including fatal overdose rates (appendix figure S3), acute hepatitides rates (appendix figure S4), rates of new diagnoses of HIV attributed to IDU (appendix figure S5), and infective endocarditis-related mortality rates (appendix figure S5).(28) This divergence occurred immediately post-reformulation for fatal heroin-related overdoses, acute HBV, acute HCV, and infective endocarditis-related mortality, but was delayed for fatal synthetic opioid-related overdoses (2014, corresponding to reports of increases of fentanyl in the illicit drug supply(36)), HIV (2017), and acute HAV (2018). Consistent with a dose-response effect of initial OxyContin marketing, the middle exposure group similarly diverged from the low exposure group, but with a smaller magnitude, for all outcomes except HIV.
Event studies
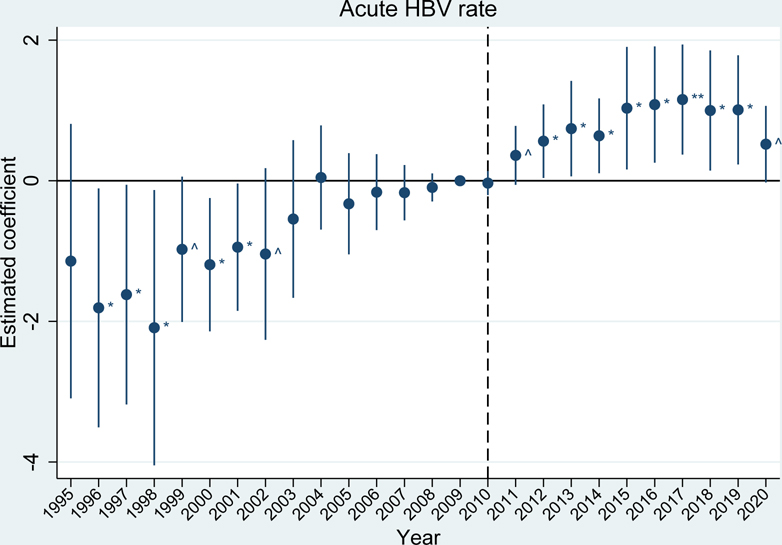
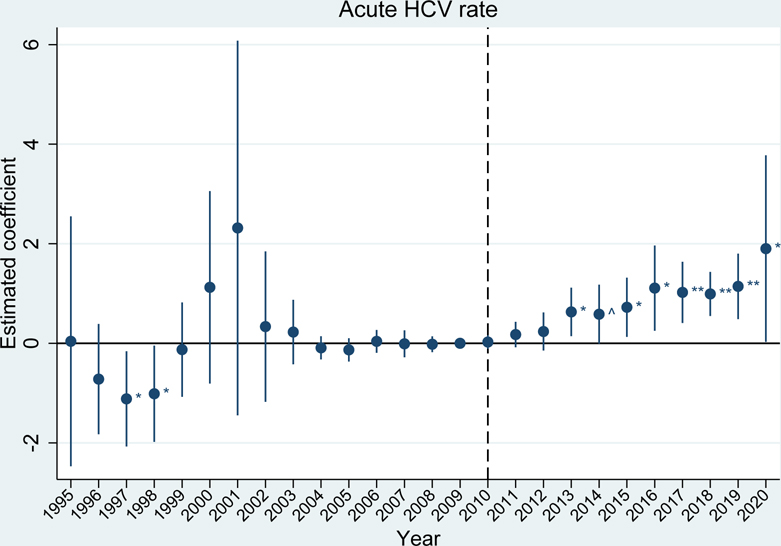
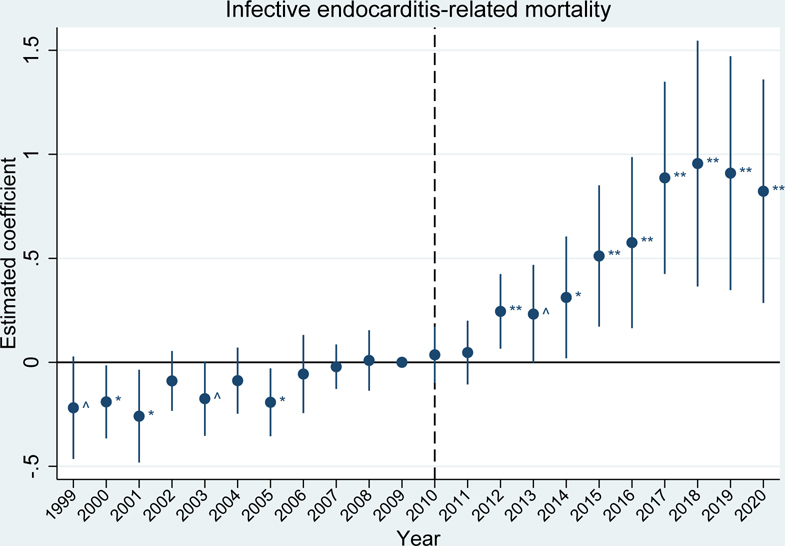
The event studies mirror these results. Prior to the 2010 OxyContin reformulation, outcome trends in the high exposure group were generally parallel to trends in the low exposure group for fatal overdose rates, acute hepatitides rates, and infective endocarditis-related mortality rates (exhibits 2, 3, and 4 and appendix figure S6).(28) For the acute hepatitides and infective endocarditis, trends were parallel in the immediate years leading up to 2010, but there were some statistically significant differences in earlier years. These differences in the hepatitides appear to be driven by elevated reports of hepatitis in a handful of states (e.g. acute HAV in Arizona in 1997 and acute HCV in Missouri and New Jersey in 2001). Due to this early volatility, our preferred estimates of average effects over the post-reformulation period use a sample that begins in 2004, once trends have stabilized. This better quantifies estimates relative to the immediate period before the reformulation (results for the full sample period are included in appendix table S2b).(28)
EXHIBIT 2.
Differences in incidence of acute HBV between U.S. states with high versus low exposure to initial OxyContin marketing
Source/Notes: SOURCE Authors’ analysis of the proxy for initial OxyContin marketing from CDC WONDER and rates of acute hepatitis B virus (HBV) per 100,000 from CDC Viral Hepatitis Surveillance reports. NOTES The vertical dotted line indicates the 2010 OxyContin reformulation. The estimated coefficient represents the estimated difference between the high and low exposure groups (95% confidence intervals are also shown). ^ p<0.10, * p<0.05, ** p<0.01. Differences are relative to the year before the OxyContin reformulation (2009). Event study models were estimated using linear regression, which included state and year fixed effects. All regressions were weighted by state population and standard errors were clustered by state.
EXHIBIT 3.
Differences in incidence of acute HCV between U.S. states with high versus low exposure to initial OxyContin marketing
Source/Notes: SOURCE Authors’ analysis of the proxy for initial OxyContin marketing from CDC WONDER and rates of acute hepatitis C virus (HCV) per 100,000 from CDC Viral Hepatitis Surveillance reports. NOTES The vertical dotted line indicates the 2010 OxyContin reformulation. The estimated coefficient represents the estimated difference between the high and low exposure groups (95% confidence intervals are also shown). ^ p<0.10, * p<0.05, ** p<0.01. Differences are relative to the year before the OxyContin reformulation (2009). Event study models were estimated using linear regression, which included state and year fixed effects. All regressions were weighted by state population and standard errors were clustered by state.
EXHIBIT 4.
Differences in infective endocarditis-related mortality rates between U.S. states with high versus low exposure to initial OxyContin marketing
Source/Notes: SOURCE Authors’ analysis of the proxy for initial OxyContin marketing and infective endocarditis-related mortality rates per 100,000 for people aged 15 to 54 from CDC WONDER. NOTES The vertical dotted line indicates the 2010 OxyContin reformulation. The estimated coefficient represents the estimated difference between the high and low exposure groups (95% confidence intervals are also shown). ^ p<0.10, * p<0.05, ** p<0.01. Differences are relative to the year before the OxyContin reformulation (2009). Event study models were estimated using linear regression, which included state and year fixed effects. All regressions were weighted by state population and standard errors were clustered by state.
After the reformulation, we see statistically significant differences between the high exposure group and the low exposure group for most outcomes. Notably, fatal synthetic opioid-related overdoses (beginning in 2014, appendix figure S6), rates of acute HBV (exhibit 2), rates of acute HCV (exhibit 3), and infective endocarditis-related mortality (exhibit 4), showed substantial, sustained divergences, while differences in fatal heroin-related overdoses and acute HAV are less extreme (appendix figure S6).(28) We are unable to investigate parallel pre-trends for HIV (appendix figure S6) due to limited data availability before 2010, and we do not see a statistically significant difference between the high and low exposure groups after 2010.(28)
Difference-in-differences
Our difference-in-differences estimates averaged over the full post-reformulation period, presented in appendix tables S2a and S2b, show that exposure to early OxyContin marketing statistically significantly increased fatal synthetic opioid-related overdoses by 5.3 deaths per 100,000; acute HAV rates by 2.1 cases per 100,000; acute HBV rates by 0.85 cases per 100,000; acute HCV rates by 0.83 cases per 100,000; and infective endocarditis-related mortality by 0.62 deaths per 100,000.(28)
Appendix tables S2a and S2b also present estimates averaged over three-year sub-periods to represent dynamic effects.(28) Although the estimated impact on acute HAV is statistically significant for the full post-reformulation period, our event study and dynamic effect findings suggest a delayed effect that emerges after 2018, likely driven by outbreaks in Kentucky, Tennessee, and West Virginia. This timing corresponds to initial reports of an outbreak of HAV in 2017–2018, representing a change in the recent epidemiology of HAV transmission in the U.S.(30) Before this outbreak, transmission generally occurred through food contamination, and afterwards, through person-to-person community spread via direct contact in populations who use drugs or are experiencing homelessness. As a result, we attribute this impact to exposure to initial OxyContin marketing. Impacts on fatal heroin-related overdoses and new diagnoses of HIV attributed to IDU were not statistically significant, with effects of 1.2 deaths per 100,000 (P=0.16) and −0.03 cases per 100,000 (P=0.92), respectively.
Secondary overdose mortality outcomes
In secondary outcomes, we found that fatal cocaine-related overdoses mirrored our findings for other outcomes, exhibiting mostly parallel pre-trends (except in 2008) between the high and low exposure groups and a dose-response divergence after 2010 (appendix figure S7).(28) Exposure to early OxyContin marketing yielded an average statistically significant increase of 1.9 deaths per 100,000 over the full post-reformulation period. While fatal psychostimulant-related overdoses (excluding cocaine), which include overdoses from methamphetamines, also exhibited parallel pre-trends, they did not exhibit a statistically significant divergence after 2010 (appendix figure S7).(28)
Robustness and falsification tests
Results from our robustness tests that include state-level policy indicators are similar to our main findings (appendix figures S8a and S8b and appendix table S3).(28) Furthermore, robustness tests that analyze underreporting-adjusted fatal heroin- and synthetic opioid-related overdoses are also similar to our main findings, but suggest we may be understating effects for these outcomes (appendix figure S9 and appendix table S3).(28)
Finally, our falsification tests demonstrate that the best explanation for these results is indeed exposure to initial OxyContin marketing. Replicating our analyses using early oxycodone shipments versus other opioids shipments shows oxycodone shipments most closely matches our findings (appendix figures S10a and S10b and appendix table S4).(28) Moreover, when examining the effects of alternative hypotheses, we find that the proxy for initial OxyContin marketing has the largest, and most statistically significant, positive impact (appendix figures S11a–S11e and appendix table S4).(28)
Discussion
This study provides evidence linking OxyContin marketing from 1996 to a host of complications of IDU decades later. We show that exposure to initial OxyContin marketing statistically significantly increased fatal synthetic opioid-related overdose rates, incidences of acute HAV, HBV, and HCV, and infective endocarditis-related mortality rates after the 2010 OxyContin reformulation. Our estimated effects for heroin-related overdose deaths and incidence of HIV attributed to IDU were not statistically significant, and we cannot causally interpret the HIV results. Nonetheless, the observed divergence in trends by exposure group for these outcomes provide suggestive evidence of impacts. The results for illicit opioid-related overdose deaths are consistent with evidence from previous work,(7, 12) although our findings on infectious complications of IDU are entirely novel.
Our findings are robust to the inclusion of multiple state policy variables. In addition, our falsification tests indicate that, first, early shipments of oxycodone, rather than early shipments of other opioids, best explain our results, and second, alternative hypotheses do a comparatively poor job of explaining these findings. Nonetheless, our analysis relies on a proxy measure for OxyContin marketing, uses state-level surveillance data, is unable to account for all post-2010 policy changes, and does not analyze all relevant outcomes. Future research should consider and address these constraints.
Despite these limitations, our results underscore the need for urgent policy actions to address the lingering impacts of OxyContin marketing, which have been magnified over time by the nature of addiction and its association with infectious diseases. Policymakers should provide aid to communities that continue to endure the consequences of OxyContin marketing through expanded access to treatments for opioid use disorder and increased availability of harm reduction services to prevent overdoses and the transmission of infectious diseases. Furthermore, policymakers should take action to proactively prevent future public health crises. This could be accomplished by implementing specific recommendations of the Stanford-Lancet Commission on the North American Opioid Crisis to limit the influence of the pharmaceutical industry on both opioid prescribers and regulators.(37)
A large literature has documented the effects of various policy and environmental exposures on long-term outcomes.(38, 39) This analysis contributes to an emerging literature that examines the long-term health, social, and economic consequences of another critical exposure: the targeted marketing of OxyContin to physicians treating cancer patients and states with fewer regulatory barriers.(7, 12)
Our findings are also consistent with the notion that the opioid epidemic is creating a “converging public health crisis” as it is “fueling a surge in infectious diseases”—particularly the viral hepatitides, infective endocarditis, and HIV.(3) Previous work has shown that these infections—often along with skin and soft-tissue infections and overdose—are associated with each other, offering further support to the observations about a converging health crisis among people who inject drugs.(31) In fact, this study provides additional, new evidence that these infections and health events (e.g. overdose) are clustering together across the U.S. with the greatest burden in states with the highest exposure to initial OxyContin marketing by Purdue.
Finally, many of the lawsuits against opioid manufacturers, including Purdue Pharma, have been concluded with settlements made or in process, though our findings could influence the enumeration of damages for any future or currently unresolved cases. At the very least, our study may inform allocation of funds awarded in settled cases. For example, funds could be used to address the infectious complications of IDU (e.g., supporting syringe exchange).
Conclusion
We found that exposure to Purdue Pharma’s OxyContin marketing in 1996 increased multiple complications of IDU after the 2010 OxyContin reformulation, including rates of fatal synthetic opioid-related overdoses, acute HAV, acute HBV, acute HCV, and infective-endocarditis related mortality. Our results suggest that the mortality and morbidity consequences of OxyContin marketing continue to be salient 25 years later. This study highlights a critical need for actions to address the spread of viral and bacterial infections and overdose associated with IDU, both in the states that were subject to Purdue’s promotional campaign and across the US more broadly.
Supplementary Material
Contributor Information
Julia M. Dennett, Department of Epidemiology of Microbial Diseases, Yale School of Public Health, Public Health Modeling Unit, 350 George Street, Ste 3rd Floor, New Haven, CT 06511, USA.
Gregg S. Gonsalves, Department of Epidemiology of Microbial Diseases, Yale School of Public Health, Public Health Modeling Unit, 350 George Street, Ste 3rd Floor, New Haven, CT 06511, USA.
References
- 1.Centers for Disease Control and Prevention, National Center for Health Statistics. Multiple cause of death 1999–2020 on CDC WONDER Online Database [Data file]. 2021. [cited 2022 Apr 15]. Available from: http://wonder.cdc.gov/mcd-icd10.html
- 2.Maclean JC, Mallatt J, Ruhm CJ, Simon K. Economic studies on the opioid crisis: a review. National Bureau of Economic Research Working Paper Series. 2020;No. 28067. [Google Scholar]
- 3.Schwetz TA, Calder T, Rosenthal E, Kattakuzhy S, Fauci AS. Opioids and infectious diseases: a converging public health crisis. J Infect Dis. 2019. Jul 2;220(3):346–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutler DM, Glaeser EL. When innovation goes wrong: technological regress and the opioid epidemic. J Econ Perspect. 2021;35(4):171–96. [Google Scholar]
- 5.Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan H, Girion L, Glover S. ‘You want a description of hell?’ OxyContin’s 12-hour problem. The Los Angeles Times; [Internet]. 2016. May 5 [cited 2022 May 4]. Available from: https://www.latimes.com/projects/oxycontin-part1 [Google Scholar]
- 7.Arteaga C, Barone V. A manufactured tragedy: the origins and deep ripples of the opioid epidemic. Unpublished working paper [Internet]. 2022. Mar 9 [cited 2022 Apr 14]. Available from: https://viquibarone.github.io/baronevictoria/Opioids_ArteagaBarone.pdf [Google Scholar]
- 8.Chakradhar S, Ross C. The history of OxyContin, told through unsealed Purdue documents. Stat [Internet]. 2019. Dec 3 [cited 2022 May 4]. Available from: https://www.statnews.com/2019/12/03/oxycontin-history-told-through-purdue-pharma-documents [Google Scholar]
- 9.Chow R. Purdue Pharma and OxyContin – a commercial success but public health disaster. Harvard Public Health Review. 2019;25. [Google Scholar]
- 10.United States General Accounting Office. Prescription drugs: OxyContin abuse and diversion and efforts to address the problem. Report to Congressional Requesters GAO-04–110. [Internet]. Washington D.C.: United States General Accounting Office; 2003. [cited 2022 May 25]. Available from: https://www.gao.gov/assets/gao-04-110.pdf [Google Scholar]
- 11.Horwitz S, Higham S, Bennett D, Kornfield M. Inside the opioid industry’s marketing machine. The Washington Post; [Internet]. 2019. Dec 6 [cited 2022 Jun 9]. Available from: https://www.washingtonpost.com/graphics/2019/investigations/opioid-marketing [Google Scholar]
- 12.Alpert A, Evans WN, Lieber EMJ, Powell D. Origins of the opioid crisis and its enduring impacts. The Quarterly Journal of Economics. 2022;137(2):1139–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Cock KM, Jaffe HW, Curran JW. Reflections on 30 years of AIDS. Emerg Infect Dis. 2011. Jun;17(6):1044–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiffman ML. The next wave of hepatitis C virus: The epidemic of intravenous drug use. Liver International. 2018;38(S1):34–9. [DOI] [PubMed] [Google Scholar]
- 15.Levitt A, Mermin J, Jones CM, See I, Butler JC. Infectious Diseases and Injection Drug Use: Public Health Burden and Response. The Journal of Infectious Diseases. 2020;222(Supplement_5):S213–S7. [DOI] [PubMed] [Google Scholar]
- 16.Mallatt J Policy-Induced Substitution to Illicit Drugs and Implications for Law Enforcement Activity. American Journal of Health Economics. 2022;8(1):30–64. [Google Scholar]
- 17.Quinones S. Dreamland: the true tale of America’s opiate epidemic. New York: Bloomsbury Press; 2015. [Google Scholar]
- 18.Bluthenthal RN, Chu D, Wenger LD, Bourgois P, Valente T, Kral AH. Differences in time to injection onset by drug in California: Implications for the emerging heroin epidemic. Drug Alcohol Depend. 2018. Apr 1;185:253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The Changing Face of Heroin Use in the United States: A Retrospective Analysis of the Past 50 Years. JAMA Psychiatry. 2014;71(7):821–6. [DOI] [PubMed] [Google Scholar]
- 20.Liebling EJ, Green TC, Hadland SE, Marshall BDL. Injection drug use and overdose among young adults who use prescription opioids non-medically. Addict Behav. 2018 2018/January/01/;76:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alpert A, Powell D, Pacula RL. Supply-side drug policy in the presence of substitutes: evidence from the introduction of abuse-deterrent opioids. American Economic Journal: Economic Policy. 2018;10(4):1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans WN, Lieber EMJ, Power P. How the reformulation of OxyContin ignited the heroin epidemic. The Review of Economics and Statistics. 2019;101(1):1–15. [Google Scholar]
- 23.Powell D, Pacula RL. The evolving consequences of OxyContin reformulation on drug overdoses. American Journal of Health Economics. 2021;7(1):41–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beheshti D Adverse health effects of abuse-deterrent opioids: evidence from the reformulation of OxyContin. Health Economics. 2019;28(12):1449–61. [DOI] [PubMed] [Google Scholar]
- 25.Powell D, Alpert A, Pacula RL. A transitioning epidemic: how the opioid crisis is driving the rise in Hepatitis C. Health Aff (Millwood). 2019;38(2):287–94. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention, National Center for Health Statistics. Compressed mortality file 1979–1998 on CDC WONDER Online Database [Data file]. [cited 2022 Apr 15]. Available from: http://wonder.cdc.gov/cmf-icd9.html
- 27.National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER cause of death recode 1969+ (03/01/2018) [Internet]. Bethesda (MD): National Cancer Institute; 2018. [cited 2022 Apr 21]. Available from: https://seer.cancer.gov/codrecode/1969_d03012018/index.html [Google Scholar]
- 28.To access the Appendix, click on the Details tab of the article online.
- 29.Marks LR, Nolan NS, Liang SY, Durkin MJ, Weimer MB. Infectious complications of injection drug use. Med Clin North Am. 2022. Jan;106(1):187–200. [DOI] [PubMed] [Google Scholar]
- 30.Foster M, Ramachandran S, Myatt K, Donovan D, Bohm S, Fiedler J, et al. Hepatitis A virus outbreaks associated with drug use and homelessness — California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:1208–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonsalves GS, David Paltiel A, Thornhill T, Iloglu S, DeMaria A Jr, Cranston K, et al. The dynamics of infectious diseases associated with injection drug use in Lawrence and Lowell, Massachusetts. Open Forum Infectious Diseases. 2021;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. Surveillance for acute viral hepatitis — United States, 2007. MMWR Surveillance Summaries 58 (No. SS-3). [Internet]. Atlanta (GA): Centers for Disease Control and Prevention; 2009. [cited 2022 May 3 and 2022 Aug 30]. Available from: https://www.cdc.gov/mmwr/PDF/ss/ss5803.pdf [Google Scholar]
- 33.Centers for Disease Control and Prevention. Viral hepatitis surveillance reports. [Internet]. Atlanta (GA): Centers for Disease Control and Prevention; 2012, 2017, 2019, and 2020 [cited 2022 May 3, 2022 Aug 30, and 2022 Sept 23]. Available from: https://www.cdc.gov/hepatitis/statistics/SurveillanceRpts.htm [Google Scholar]
- 34.Sullivan PS, Woodyatt C, Koski C, Pembleton E, McGuinness P, Taussig J, et al. A data visualization and dissemination resource to support HIV prevention and care at the local level: analysis and uses of the AIDSVu public data resource. J Med Internet Res. 2020;22(10):e23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruggles S, Flood S, Goeken R, Schouweiler M, Sobek M. IPUMS USA: Version 12.0 [Data file]. Minneapolis (MN): IPUMS; 2022. [cited 2022 Sept 13]. Available from: 10.18128/D010.V12.0 [DOI] [Google Scholar]
- 36.Department of Justice US, Drug Enforcement Administration, Diversion Control Division. NFLIS brief: fentanyl, 2001–2015 [Internet]. Springfield (VA): Drug Enforcement Administration, U.S. Department of Justice; 2017. [cited 2022 Oct 11]. Available from: https://www.nflis.deadiversion.usdoj.gov/publicationsRedesign.xhtml [Google Scholar]
- 37.Humphreys K, Shover CL, Andrews CM, Bohnert AS, Brandeau ML, Caulkins JP, et al. Responding to the opioid crisis in North America and beyond: recommendations of the Stanford–Lancet Commission. The Lancet. 2022;399(10324):555–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Currie J, Almond D. Human capital development before age five. In: Ashenfelter O, Card D, editors. Handbook of labor economics, volume 4, part B. Amsterdam; New York: North-Holland; New York City: Elsevier; 2011. p. 1315–486. [Google Scholar]
- 39.Almond D, Currie J, Duque V. Childhood circumstances and adult outcomes: act II. J Econ Lit. 2018;56(4):1360–446. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.