Abstract
Background
The application of blood concentrates has gained popularity in dentistry in recent years. Platelet-rich fibrin (PRF) has been discussed frequently due to a high content of growth factors and the option of chair-side manufacturing in a simple centrifugation process. PRF is free from adjuvants and inexpensive to produce. The number of studies reporting beneficial effects of PRF in various clinical applications such as alveolar ridge preservation, sinus floor elevation, management and prevention of medical-related osteonecrosis of the jaw, third molar extractions, and guided bone regeneration in dentistry has increased recently. However, to date, neither clinical recommendations nor guidelines are available. The present narrative review aims to summarize the level of evidence on the clinical application of PRF within the field of oral surgery and implantology.
Summary
A literature search in Pubmed and Medline has identified 34 articles as a basis for this narrative review. The effectiveness of the clinical application of PRF has been analyzed for five indications within dentistry: medical-related osteonecrosis of the jaw, wisdom tooth extraction, guided bone regeneration, sinus floor elevation, and alveolar ridge preservation. The amount of data for third molar extractions, socket preservation, and guided bone regeneration is extensive. Less data were available for the use of PRF in combination with sinus floor elevations. There is a lack of studies with scientific evidence on PRF and medical-related osteonecrosis of the jaw; however, studies positively impact patient-related outcome measures. Most studies report on beneficial effects when PRF is additionally applied in intrabony defects. There is no evidence of the positive effects of PRF combined with bone graft materials during sinus floor elevation. However, some benefits are reported with PRF as a sole filling material.
Key Messages
Many recently published studies show the positive clinical impact of PRF. Yet, further research is needed to ensure the validity of the evidence.
Keywords: PRF, Oral surgery, Bone augmentation, Third molar extraction, MRONJ
Introduction
Blood concentrates have a lengthy history in dentistry and started with fibrin glues. The idea of this product is simple. Blood presence and its contents around the wound have always been the key to proper healing. Thus, establishing a blood clot at surgical sites appears to support natural healing processes [1, 2, 3].
The first described autologous platelet product used in dentistry was platelet-rich plasma (PRP) [4, 5]. It is produced in two centrifugation steps, depending on the applied protocol [6]. Platelets get activated by adding thrombin, usually of bovine descent, as well as calcium chloride or a combination of both. As a result, a liquid substance is fabricated. With certain technologies, its viscosity can be changed [6]. It contains approximately 5 times more platelets than noncentrifuged, natural blood and a variety of key growth factors that accelerate wound healing and tissue remodeling [7]. PRP stimulates certain cells' proliferation and differentiation, such as osteoblasts [4, 8]. It supports healing processes [9] and interacts with physiological processes, especially in the first days after surgeries, to prevent serious complications, like ankylosis [10]. PRP has been considered safe in clinical application with a low-risk of infections and allergies [11]. PRP further allows bleeding management during the treatment of patients with anticoagulant medications [12]. Despite all these positive effects, there are some limitations. In the literature, there are opposing results reported on the effects of PRP, especially on the promotion of bone healing [13, 14, 15]. In addition, PRP releases growth factors only once, right when it is applied [2]. Another disadvantage is its preparation, which requires time due to the two-step centrifugation process. As a result, PRP seems rather unlikely to become a routine clinical practice in the future [13].
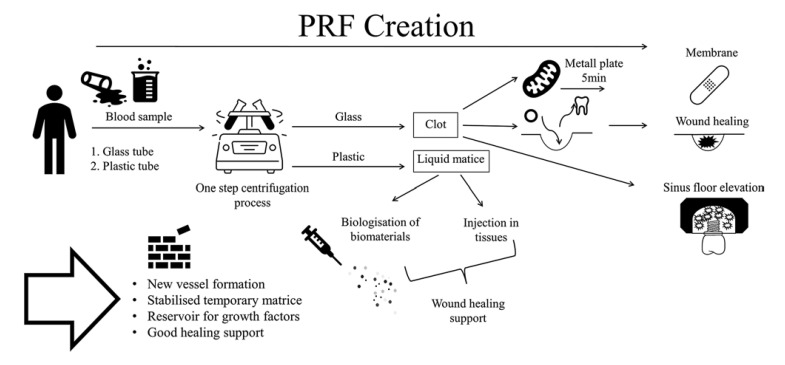
More than 10 years ago, Choukroun et al. [16] introduced platelet-rich fibrin (PRF). PRF is the first platelet concentrate easily generated in a single-stage centrifugation process free of anticoagulants [17] (see Fig. 1). PRP clots' size is rather small compared to PRF [13]. The matrix of PRF tends to be more penetrable than the one of PRP. This might ease the release of growth factors and other important substances of the wound healing cascade. Also, its permeability for cells simplifies the organization of the wound healing process [1, 3, 18]. There are two consistencies of PRF-solid [19] and liquid [20]. In oral surgery, the solid version of PRF is preferably often and specially used as a membrane, for example, to cover bone substitute in bone augmentation procedures. Membranes can be created by pressing PRF-clots with a plate [21]. With certain centrifugation protocols using glass tubes or silica-coated tubes, PRF forms as a solid clot. Being weighted with a panel PRF turns into a stable membrane used for guided bone regeneration (GBR) procedures in various trials [22, 23]. Further cylindric clots are frequently used for alveolar ridge preservation techniques or as filling material in cyst cavities [23]. Due to the centrifugation process, three fractions are visible: acellular plasma, fibrin, and erythrocytes on the bottom [21]. PRF consists of various blood cells, growth factors, and fibrinogen which play a crucial role in natural healing processes [21, 23]. The cell composition of PRF includes: platelets, lymphocytes, monocytes, neutrophils, and basophiles [24].
Fig. 1.
PRF creation.
The complex process of wound healing can be partitioned into several overlapping phases. After the immediate excess of blood to flush out the wound, the hemostasis and coagulation cascade are initiated. Surrounding vessels contract for a few min and platelets initiate the formation of a clot as a temporary matrix. This active matrix involving fibrin acts as a storage for invading cells. Vessels dilate, leukocytes enter the wound stimulated by activated platelets and their growth factor release. The inflammatory phase is initiated by release of IL-1 and IL-6 [25]. TGF-β1 regulates inflammatory processes and the synthesis of collagen type III [25, 26, 27], and VEGF leads to new blood vessel formation and stabilization. VEGF further supports the proliferation and division of fibroblasts and endothelial cells. New bone formation seems to be enhanced [28, 29]. Neutrophils perform wound cleansing during inflammatory phase by phagocytosis and secretion of proteinases. Monozytes differentiated to macrophages participate in the process of wound debridement and trigger the proliferative phase through the release of TGF-β, PDGF, and VEGF. Additionally, they act as antigen-presenters [25, 30] with the aim of granulation tissue formation; vessels grow in the proliferative phase. Collagen synthesis is aiming to close the wound in the first place and to decrease again as keratinocytes start re-epitheliazation. Finally, collagenases and elastases are released to dissolve intercellular bonds and restore the permeability of the tissue. Dense new formed vascular nets, granulocytes, macrophages, fibroblasts, and collagen bundles are the main components of the granulation tissue developed. During remodeling, collagen type III is exchanged to more robust collagen type I [25].
The PRF-clots head describes the part which is abutted in the red blood cells after the centrifugation process. Along the border, authors describe a high leukocyte content [28].
Complications stimulate the development of new methods. In recent years, the topic of PRF has reached interest in the field of clinical research. The number of published articles in dentistry has grown. Ghanaati et al. have summarized the first 15 years of PRF in a systematic review [31], The quantity of publications and reports on the application of PRF in dental surgery and tissue regeneration has significantly increased and deepened. The number of articles indexed in Pubmed related to the keyword combination “platelet-rich fibrin” AND “oral surgery” has expanded exponentially since 2006 from 8 publications to 125 publications in 2021. However, no clinical recommendations or guidelines for indications and clinical protocols can be extracted. Therefore, the present narrative review aims to give an overview of the current level of evidence on the application of PRF within the field of dentistry and the regeneration of oral tissues.
Main Text
Materials and Methods
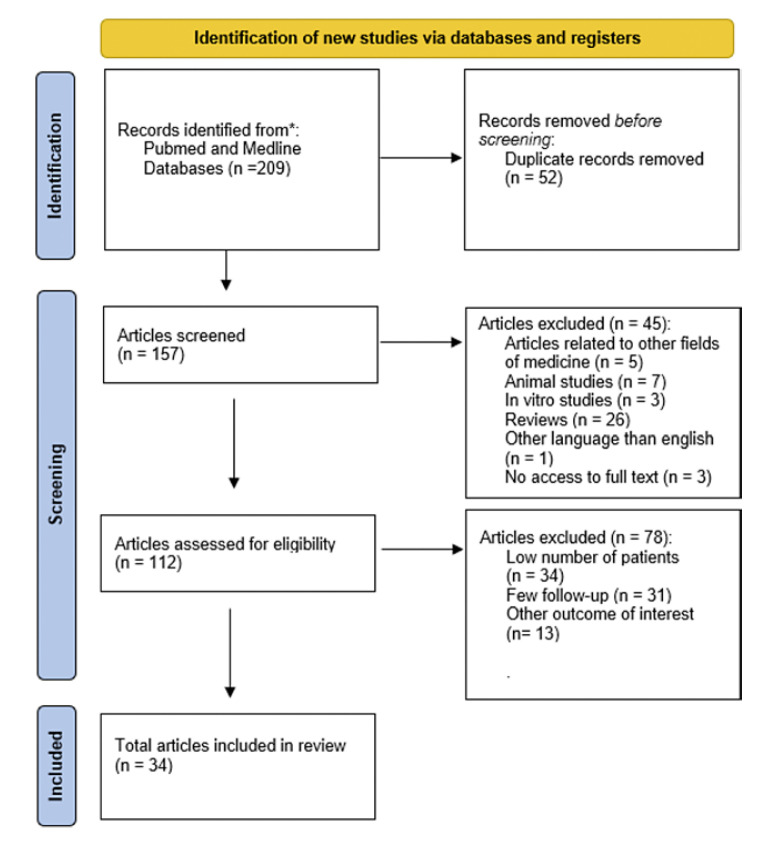
Literature search has been conducted via Pubmed and Medline. The following keywords have been used: “PRF” AND “Third molar” OR “SOCKET PRESERVATION” OR “SINUS FLOOR ELEVATION” OR “STICKY BONE” OR “GUIDED BONE REGENERATION” OR “ALVEOLAR RIDGE PRESERVATION” OR “MRONJ.”
PICO approach has been applied to verify the current knowledge effect of PRF in oral surgery.
Population
Adult human beings receiving third molar extractions, socket preservation, alveolar ridge preservation, sinus floor elevation, GBR, or a surgical intervention due to osteonecrosis of the jaw.
Intervention
Randomized clinical trial (RCTs)/case series/cohort studies/prospective studies or retrospective studies with additional application of PRF in oral surgery.
Comparison
RCTs/case series/cohort studies/prospective studies or retrospective studies without application of PRF in oral surgery.
Outcome
Healing of soft tissue, healing of hard tissue, the patient-centered outcome such as pain.
Inclusion Criteria
In vivo studies, RCTs, case series, cohort studies, prospective studies, retrospective studies, only studies published between 2018 and 2021. Ghanaati et al. summarized relevant clinical data about PRF in the field of dentistry up to 2017 [31].
Exclusion Criteria
Reviews, animal studies, in vitro studies, studies published in languages other than English (see Fig. 2).
Fig. 2.
Adapted after: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. doi: 10.1136/bmj.n71.
Scientific evidence, according to the US Agency for Healthcare Research and Quality, has been classified (see Table 1).
Table 1.
Level of scientific evidence adjusted according to the US Agency for Healthcare Research and Quality [32]
| Level of scientific evidence adjusted according to the US Agency for Healthcare Research and Quality | |
|---|---|
| Ia…. | Systematic reviews/Meta-analyses of RCTs |
| Ib…. | A minimum of one RCT |
| IIa…. | A minimum of one controlled study, well-designed, without randomization |
| IIb…. | A minimum of one other type of quasi-experimental study, well-designed |
| III…. | Descriptive studies such as comparative, correlation or case-control studies, nonexperimental, and well-designed |
| IV…. | Repot/opinions from expert committees |
Third Molar Extractions
The extraction of wisdom teeth is a frequently performed oral surgical intervention potentially causing distress in patients. This may be related to possible postoperative complications such as trismus, pain, and severe swelling, which are usually more frequent than infections, alveolar osteitis, and sensibility disorders [33]. In order to improve patients' postsurgical outcomes in regard to swelling, trismus, and pain, filling the defect with a PRF clot has been described in the literature [34].
Out of 22 articles published since 2018, only eight studies have been selected for this review. This selection is due to the number of patients, follow-up rate, and examination parameters. Studies with at least 20 patients were included. In total, a number of 315 patients were involved in the mentioned articles. Follow-up in the first week to report pain and swelling results up to 6 months to assess bone healing was included. Parameters investigated in the use of PRF for wisdom tooth removals are most likely pain assessment [29, 35, 36, 37, 38, 39, 40, 41], level of swelling [29, 35, 36, 37, 39, 41], effect on trismus [29, 41], the incidence of alveolar osteitis [38], soft tissue healing [29, 35, 39, 40, 41], and bone healing [29, 35, 36, 37, 39, 40, 41]. A follow-up to evaluate pain, swelling, trismus has been reported within the first postsurgical week. Incidence of alveolar osteitis and soft tissue healing has been examined in the first 3 months. Evaluation of bone healing has been performed generally after three to 6 months [29, 35, 36, 37, 38, 39, 40, 41]. All included studies are designed as split-mouth studies. Lower impacted third molars have been extracted, and in test groups, the alveolus has been filled with PRF, and control sides have been left for conventional healing. Gupta et al. [29] found significantly better results concerning pain, trismus, swelling, and soft tissue healing at day 3 in the PRF group (p < 0.05). Several other groups also observed reduced pain in the test group [29, 36, 37, 38, 39, 41]. Multiple research teams examined less swelling in the first postsurgical week [36, 37, 38, 39, 41]. Only one group, Ritto et al. [40] notified no statistically significant difference between test and control group related to pain and soft tissue healing. Additionally, Gupta et al. [29] showed that bone healing seemed to be influenced positively in the PRF group significantly (p < 0.05) measured radiographically. Five studies confirm these results with similar results [35, 37, 39, 40, 41]. In contrast, Sybil et al. [36] reported no statistically significant influence in bone height between the groups. One of the included studies [38] evaluated the presence or absence of alveolar osteitis showing more infectious events in the control groups.
Six studies with scientific evidence type Ib [29, 36, 37, 38, 40, 41] and two studies type IIa [35, 39]were taken in account. The extraction of wisdom teeth is a frequently performed intervention in oral surgery. Distinct advantages regarding patient-related outcome measures emerge from the studies listed above. In most of the studies, PRF appears to be a valuable supplement with clinical effect, especially in soft tissue healing [29, 36, 37, 38, 39, 41], There are some limitations. Publications with small case numbers predominate in this field. Studies about simultaneous upper and lower third molar extractions would be closer to practical relevance. Additional data reflecting these frequently occurring interventions are still missing.
Alveolar Ridge Preservation
Alveolar ridge preservation techniques are usually performed by filling the extraction socket with autogenous bone or substitute materials. An autogenous or exogenous barrier membrane can be applied to protect the bone graft particles from soft tissue ingrowth and dislocation [42]. Soft tissue closure is recommended in some cases [43]. To ensure the emergence profile around implants and to maintain a natural appearing gingival margin, the preservation of sufficient soft tissue is mandatory. In this field, PRF is used to support the preservation of soft tissues around extracted teeth. At the same time, these surgical interventions are also associated with postoperative discomforts such as pain, swelling, and the risk of inflammation. These could be reduced by the use of PRF [23]. The application in the included studies was performed either with PRF as a sole filling material [44, 45, 46, 47, 48, 49] or PRF in combination with bone graft materials and a collagen plug compared to spontaneous healing of the alveolus [49, 50, 51]. Eight out of 32 articles dealing with this topic were included in our analysis. Excluded articles had a low number of participants or little follow-up. A total of 294 patients were treated in the studies included. Follow-up is reported up to 6 months.
For this purpose, studied parameters were defined as dimensions of alveolar bone and its changes [44, 45, 46, 47, 48, 49, 50, 51], new bone formation [44, 46, 47, 51], pain [49, 50, 51], swelling [51], soft tissue healing [48, 49, 51, 52, 53]. Follow-up for soft tissue healing was documented in the first postsurgical week [44, 45, 47, 48] and for hard tissue healing between up to 6 months [44, 45, 46, 47, 48, 49, 50, 51]. Statistically, significant less reduction in bone dimension using PRF in combination with bone graft material was described in four articles [46, 47, 50, 51]. In addition, improved soft tissue healing due to PRF application could be detected in three included articles [45, 47, 48]. Ahmed et al. compared PRF as a sole filling material to natural healing as well as to PRF in combination with a collagen plug [45]. They found that PRF combined with a collagen plug was superior to socket preservation with PRF alone. These findings were reflected in the examination of bone dimensions [45]. Histomorphometric analyses were carried out by two groups and showed significantly higher percentages of new bone formation after 3 months using PRF as a sole graft material in clinical studies [46, 47]. In contrast, Aravena et al. [44] could not find significant differences in wound healing or bone formation and bone dimension [p = 0.78] between socket preservation using PRF as a sole graft material and natural healing. Pain in the first postsurgical week was slightly lower in the group treated with PRF, according to Kumar et al. [49]. Santhanakrishnan et al. and Yewale et al. [50, 51] reported no statistically significant differences in pain values between the groups. No statistically significant differences were observed in the study of Yewale et al. [51] evaluating patients' swelling.
Six studies at a level of evidence Ib [44, 45, 46, 49, 50, 51] and two studies at a level IIa were included in the analysis [47, 48]. Results on the application of PRF for the indication of alveolar ridge preservation are controversial. In the absence of evidence, no firm recommendation for the use of PRF can be made in this area.
Guided Bone Regeneration
PRF has been used for GBR to treat defects. With this type of intervention, success is particularly dependent on the healing readiness of the surrounding tissue. Inflammatory tissue is removed as thoroughly as possible and, if feasible, a restitutio ad integrum should be aimed for by creating ideal healing conditions. The application of PRF is intended to additionally support the formation of new tissues, both bone and soft tissue. Seven studies out of 31 have been analyzed in detail. Follow-up after 6 months is reported in each of the included studies. In total, 318 patients received treatment with PRF. Parameters examined in the seven studies were probing depth [52, 53, 54, 55], clinical attachment loss [52, 53, 54, 55], gingival recession [52, 55], bone fill [52, 53, 54, 56], bone height [53, 54, 57], percentage of vital bone [58], intrabony component [54], pain, bleeding [56], wound healing, and tooth mobility [53]. Intrabony defects have been treated with a variety of treatment options. Sun et al. [56] compared PRF and bone powder covered with PRF membranes and a flap curettage combined with GBR. Pain evaluation has been reported during 24 h postsurgical; however, bleeding was reported after 7 days. Both parameters were statistically significantly lower in the PRF group. After 60 days and 120 days, degrees of bone defects and bone density have been reexamined. The PRF group had significantly lower values in bone defect measurements (p < 0.05) after 60 days. The level of bone density was significantly higher after 60 days as well as after 120 days (p < 0.001). The study was carried out in patients with bone defects caused by peri-implantitis [56]. In the split-mouth, designed study of Bodhare et al. [52] bioactive glass has been either combined with PRF or applied as a sole filling material. Three-month follow-up and 6-month follow-up showed a significantly greater clinical attachment level, bone fill, and a reduction in probing depth in the PRF group [52]. Lei et al. [54] have compared three groups. Guided tissue regeneration used PRF, concentrated growth factors [CGF], and a control group treating periodontal intrabony defects. After 6 months, there was no difference in pocket depths, clinical attachment level gain, or bone height between PRF and CGF. However, both PRF and CGF achieved significantly better results concerning intrabony component depth and percentage of bone fill at the defect side than the control group [54]. A histological examination was performed by Hartlev et al. [58] six months after the surgical intervention. The group investigated the percentage of vital respectively nonvital bone and the number of blood vessels.
The test group was treated with autologous bone graft and PRF, the control group with bovine bone and a resorbable collagen membrane. In this article, no statistically significant difference of the examined parameters was found [58]. Similarly, Işik et al. like Hartlev et al. [57] conclude that both bovine bone graft alone and bovine bone graft combined with PRF deliver success used for GBR. Treatment of intrabony defects was compared by Pham et al. trying three different approaches. Open flap debridement combined with PRF, guided tissue regeneration, and open flap debridement alone. Periodontal parameters such as probing depth, clinical attachment level, tooth mobility, and bone defect fill were superior when defects were treated with open flap debridement and PRF, respectively, guided tissue regeneration [53]. Gingival recession defects were handled applying sticky bone, a mixture of bone allograft and liquid PRF, covered with collagen membranes coated with liquid PRF by Kapa et al. [55]. According to the case series, 6 months after the surgical intervention, positive effects in root coverage and increase of gingival thickness were noted [55].
The level of scientific evidence of the studies mentioned was Ib [52, 53, 55, 57, 58] and III [54, 55]. The evidence levels of the cited studies in this field appear to be high. The present studies included indicate potential benefits when using PRF during GBR procedures compared to control groups. However, also for this indication, no clear guideline can be extracted from the literature.
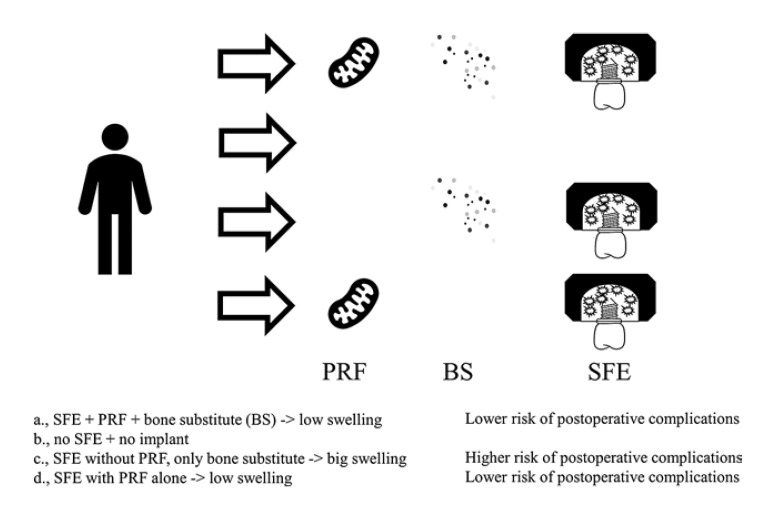
Sinus Floor Elevation
When jawbone loses its function after tooth removal, it recedes. However, a certain height and width of bone is a prerequisite for placing dental implants. Bone height can be reconstructed in the upper jaw using GBR techniques such as sinus floor elevation [59, 60, 61]. Sinus floor elevation is usually either performed through a lateral window or transcrestal approach [61, 62]. The cavity between the Schneiderian membrane and the bony sinus floor is usually filled with autogenous bone and or bone substitutes (see Fig. 3). When using a lateral window, the bone substitute is usually covered with a synthetic or xenogenic membrane [63]. In order to facilitate the integration of bone substitute in patients' maxilla, PRF has been used for a biologicalization of bone substitutes.
Fig. 3.
Sinus floor elevation with and without PRF.
This review includes only one study in which transcrestal sinus floor elevations were performed [64]. Three other studies with lateral sinus floor elevations were also analyzed [65, 66, 67]. One study examined lateral and transalveolar sinus floor elevations [68]. Studies included in this review consider 147 patients in total. Molemans et al. Cho et al. and Barbu et al. [64, 66, 68] used PRF as a sole filling material. Pichotano et al. and Irdem et al. [65, 67] combined PRF with deproteinized freeze-dried bovine bone mineral (DBBM) in the test group, whereas in control groups, SFE was performed with DBBM alone. Cho et al. [64] compared PRF as a sole filling material with saline as a control. The studies of Pichotano et al. Cho et al., and Irdem et al. [65, 67] were designed as split-mouth randomized controlled clinical trials. The case series of Barbu et al. [66] report about large sinus membrane perforation preserved with PRF as a sole filling material and simultaneous implant placement. Molemans et al. [68] did twenty-two transcrestal and six lateral SFE using a prospective, single-cohort study design. Most likely, newly formed bone was measured as a parameter. Histological analysis, radiographs, or Micro-CT were made after three to 12 months [65, 66, 67, 68]. Pichotano et al. observed implant survival, as did Cho et al. at a 1-year follow-up [64, 65] and Barbu et al. [66] at a 4-year follow-up. Two groups collected histological samples to detect the amount of vital bone [66, 67], fibrous tissue, and residue from bone graft [69]. PRF as a sole filling material in simultaneous SFE showed clinically integrated implants [68, 70]. A significantly higher intrasinusoidal bone level could be measured using PRF (2.6 ± 1.1 mm) as a sole filling material in comparison to a saline filling (1.7 ± 1.0 mm) (p < 0.5) [68]. Pichotano et al. reported statistically significant higher percentages of vital bone in the test group with PRF and DBBM (p = 0.0087). The amount of residual graft was higher in the control group with DBBM as a sole filling material (p = 0.0111) [71]. Using PRF as a filling material in surgeries with a large perforation of the Schneiderian membrane was tested by Barbu et al. [72]. The case series observed nine complicated sinus floor elevations resulting in successfully osseointegrated implants after 4 years of follow-up [72]. Surprisingly Irdem et al. found no statistically significant difference in none of the investigated parameters. Neither new bone formation was more, nor residual graft amount was lower, in the test group using PRF additionally to DBBM instead of DBBM alone in the control group [69]. The low number of cases may have had an influence on the result.
There are three studies about sinus floor elevation and PRF at a scientific evidence level Ib [68, 69, 71]. One study with scientific evidence level IIa [70] and one study at a level of III were included in this narrative review [72].
The results obtained from the involved studies report controversial data on the application of PRF during sinus floor elevation. However, potential clinical benefit and new pathways in bone augmentation might arise, that have to be confirmed in further investigations.
This applies to both transcrestal and lateral SFE. Especially, the use of PRF combined with bone substitutes and as sole filling material might to be a promising alternative to the use of bone substitute alone [64, 68].
Medication-Related Osteonecrosis of the Jaw
Medication-related osteonecrosis of the jaw is a big challenge in oral surgery. The necrosis usually occurs as a side effect in patients under i.v. or oral antiresorptive therapy to treat osteoporosis and osteoclastic metastasis. This group of patients requires particularly careful treatment. There are great difficulties in mucosal healing. Patients often suffer from dehiscence and exposed bone for weeks after extraction or surgical debridement of necrotic bone. Quality of life can be reduced dramatically, and treatment can be easily accompanied by complications [71]. Since oral surgery may sometimes be necessary during antiresorptive therapy, it is important to support fast and natural healing as much as possible in order to avoid dehiscence and wound healing disorders and necrosis. Covering wounds with PRF membranes might compensate healing disturbances.
To illuminate the effect of PRF used at surgical interventions for the treatment of osteonecrosis of the jaw, six of thirteen studies were reviewed in detail [73, 74, 75, 76, 77, 78]. Four studies compared surgical treatment with additional use of PRF and conventional treatment [73, 74, 75, 78]. Three studies observed management of preexisting MRONJ using PRF [74, 75, 77]. Overall, 443 patients were observed in the studies mentioned above. Giudice et al. [70] compared a test group adding PRF to the conventional surgical treatment of MRONJ to a control group. Guidice et al. [75] could show that the PRF group had a statistically significant better mucosal integrity, no infection and less pain after 1-month follow-up. However, after 6 months and 1 year, no differences were found. Zelinka et al. concluded a success rate of 85% after 12 months of treating patients with early stages of MRONJ. Early stages are defined as necrosis which could be removed completely [77]. Pain, exposed bone, mucosal closure, and signs of inflammation were defined as outcome parameters. Zelinka et al. [77] assumed additional PRF application in surgical therapy of early stages of MRONJ is effective. Comparing three different treatments, Tenore et al. investigated healing, a transition from higher to lower stage of MRONJ, and persistence of pain and bone exposure. In the test groups, surgery was performed, and antibiotics and PRF were applied. In a second control group, additional photobiomodulation was performed. As a consent of this article, therapy with PRF, surgery, antibiotics, and photobiomodulation via low-level laser therapy (LLLT) contribute to the management of MRONJ [74]. Nica et al. described a heterogenous patient cohort consisting of low-risk patients, patients suffering from MRONJ stage zero, and patients suffering MORNJ stage 1–3 for whom an extraction was necessary (see Table 2). Low-risk patients were treated with sutures and LLLT, stage 0 patients took antibiotics, sutures, and LLLT, stage 1–3 patients received a perioperative antibiosis and were treated with piezo-surgery and additional LLLT and PRF were applied at the wound. The study concluded a high rate of success in all groups and a total healing rate of 91.66% in the third group using PRF [73]. Two studies investigated the possible prevention of MRONJ followed by oral surgeries in patients at risk [76, 78]. The study participants received antiresorptive therapy for up to 6 months [73, 74, 78]. Pain [78], exposed bone, mucosal healing, symptoms indicating inflammation were the assessed outcomes [73, 74, 78]. Miranda et al. treated patients under antiresorptive or antiangiogenic agents requiring acute dental extraction. In the control group, extractions were performed carefully and minimally invasive to prevent postoperative complications such as MRONJ. Test group extractions sockets were filled with a PRF plug. In the control group, 19.23% of patients suffered from MRONJ, whereas none of the test group patients developed necrosis [78]. Şahin et al. analyzed 63 dental extractions treated with PRF in 44 patients with a risk of MRONJ. They reported uneventful healing in all cases and success preventing MRONJ after 6 months of follow-up [79].
Table 2.
Stages of MRONJ after [80]
| Stage 0 | Non-specific clinical signs such as difference in bone density, tooth mobility without periodontal disease, pain in mandibular, no exposed necrotic bone |
| Stage 1 | Necrotic bone exposed but asymptomatic, no infection |
| Stage 2 | Infected exposed necrotic bone accompanied by symptoms |
| Stage 3 | Infected exposed necrotic bone accompanied by symptoms additionally to severe signs of MROJ such as fracture, extra-oral fistula or extended osteolysis |
One study with scientific evidence level Ib was included [75]. One study was at level IIa [77] and four studies at level III [73, 74, 76, 78].
In conclusion, PRF could provide notable improvements for patients suffering from MORNJ. In the presented clinical studies, it seems that the prevention and management of MRONJ could benefit from a PRF application.
Discussion
PRF is created from whole blood samples without additional ingredients [75]. Thus, a low risk of side effects can be assumed. Another advantage is the simplicity of its preparation process [76] and by that the intervention it is not associated with high costs [77]. PRF is applicable as liquid as well as solid matrix [19, 78] and seems to encounter a range of reasonable clinical applications. A variety of different manufacturing protocols have been published in the literature; however, the lack of a standardized protocol creates confusion and difficulty in the comparison of data [31]. Various parameters are required for the definition of a protocol. In the literature, this was often not fully specified, which hindered the reproducibility of study protocols. The different devices and protocols used for PRF-creation seem to have an influence on the quality of the blood product [28]. In particular, the literature suggests that the reduction of centrifugal force has a positive effect on the release of growth factors in vitro [19, 20, 24]. According to the available data on the application of PRF within field of dentistry, the following conclusion seems to be plausible:
A considerable number of RCTs report beneficial outcomes when using PRF in third molar extractions. Focusing on patients' comfort and avoiding complications during postoperative care, supplemental use of PRF could be a valuable treatment option.
Results on the application of PRF for the indication of alveolar ridge preservation are controversial. There is a lack of evidence to make a clear statement for the benefit of PRF in this field of application.
In the prevention and therapeutic management of MRONJ, potential benefits might derive from an application of PRF. However, to date, the degree of scientific evidence is rather low. Larger RCTs are urgently needed to confirm or otherwise reject these preliminary observations since in this area any benefit for the patients seems to be most crucial.
Results on PRF application during sinus floor elevation and GBR are also heterogeneously. PRF might be a promising supplement to bone substitutes or even sole filling material during SFE justifying further clinical investigations. In addition, PRF obviously represents a possibility for managing perforated sinus membranes.
Conclusion
There is a tendency that the available data on PRF in dentistry may have a positive impact on patient-related postoperative outcomes. However, there is a huge heterogeneity of protocols and a lack of guidelines for clinical application for PRF. Further studies are urgently needed to justify the standardized use of PRF within oral surgery.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There were no funding sources.
Author Contributions
Katharina Zwittnig writing the manuscript; Khaled Mukaddam, Daniel Vegh, Valentin Herber, Michael Payer, Norbert Jakse, and Peter Schlenke editing and proofreading.
Acknowledgments
We thank Peter Zwittnig MD for proofreading the manuscript. The authors would like to thank Enago [www.enago.com] for the English language review. Parts of the article have been published in a Diploma-Thesis [Katharina Zwittnig].
Funding Statement
There were no funding sources.
References
- 1.Fan Y, Perez K, Dym H. Clinical uses of platelet-rich fibrin in oral and maxillofacial surgery. Dent Clin North America. 2020 Apr 1;64((2)):291–303. doi: 10.1016/j.cden.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Chou TM, Chang HP, Wang JC. Autologous platelet concentrates in maxillofacial regenerative therapy. Kaohsiung J Med Sci. 2020 May 1;36((5)):305–310. doi: 10.1002/kjm2.12192. [DOI] [PubMed] [Google Scholar]
- 3.Feigin K, Shope B. Use of platelet-rich plasma and platelet-rich fibrin in dentistry and oral surgery introduction and review of the literature. J Vet Dent. 2019 Jun 1;36((2)):109–123. doi: 10.1177/0898756419876057. [DOI] [PubMed] [Google Scholar]
- 4.Lang S, Loibl M, Herrmann M. Platelet-rich plasma in tissue engineering hype and hope. 2018 doi: 10.1159/000492415. [cited 2021 Dec 5]; Available from: www.karger.com/esrwww.karger.com/esr. [DOI] [PubMed] [Google Scholar]
- 5.Whitman DH, Berry RL, Green DM. Platelet gel an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofacial Surg. 1997 Nov 1;55((11)):1294–1299. doi: 10.1016/s0278-2391(97)90187-7. [DOI] [PubMed] [Google Scholar]
- 6.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates from pure platelet-rich plasma (P-PRP) to leucocyte-and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009 Mar;27((3)):158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Alsousou J, Thompson M, Hulley P, Noble A, Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery a review of the literature. J Bone Joint Surg Br. 2009 Aug;91-B((8)):987–996. doi: 10.1302/0301-620X.91B8.22546. [DOI] [PubMed] [Google Scholar]
- 8.Arpornmaeklong P, Kochel M, Depprich R, Kü Bler NR, Wü Rzler KK. Influence of platelet-rich plasma [PRP] on osteogenic differentiation of rat bone marrow stromal cells. An in vitro study. Int J Oral Maxillofac Surg. 2004;33((1)):60–70. doi: 10.1054/ijom.2003.0492. [DOI] [PubMed] [Google Scholar]
- 9.Naik B, Karunakar P, Jayadev M, Rahul Marshal V. Role of platelet rich fibrin in wound healing a critical review. J Conser Den. 2013 Jul;16((4)):284–293. doi: 10.4103/0972-0707.114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wikesjö UME, Sorensen RG, Kinoshita A, Jian Li X, Wozney JM. Periodontal repair in dogs effect of recombinant human bone morphogenetic protein-12 [rhBMP-12] on regeneration of alveolar bone and periodontal attachment: a pilot study. J Clin Periodontol. 2004 Aug;31((8)):662–670. doi: 10.1111/j.1600-051X.2004.00541.x. [DOI] [PubMed] [Google Scholar]
- 11.Anitua E, Sánchez M, Orive G. The importance of understanding what is platelet-rich growth factor [PRGF] and what is not. J Shoulder Elb Surg. 2011;1:4–23. doi: 10.1016/j.jse.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Della Valle A, Sammartino G, Marenzi G, Tia M, Lauro A, Ferrari F, et al. Prevention of postoperative bleeding in anticoagulated patients undergoing oral surgery use of platelet-rich plasma gel. J Oral Maxillofac Surg. 2003;61((11)):1275–1278. doi: 10.1016/s0278-2391(03)00727-4. [DOI] [PubMed] [Google Scholar]
- 13.Simonpieri A, del Corso M, Vervelle A, Jimbo R, Inchingolo F, Sammartino G, et al. Current knowledge and perspectives for the use of platelet-rich plasma [PRP] and platelet-rich fibrin [PRF] in oral and maxillofacial surgery Part 2 bone graft, implant and reconstructive surgery. Curr Pharm Biotechnol. 2012 Jun 12;13((7)):1231–1256. doi: 10.2174/138920112800624472. [DOI] [PubMed] [Google Scholar]
- 14.Grageda E. Platelet-rich plasma and bone graft materials A review and a standardized research protocol. Implant Dent. 2004 Dec;13((4)):301–309. doi: 10.1097/01.id.0000148555.91063.06. [cited 2021 Dec 19] Available from: https://journals.lww.com/implantdent/Fulltext/2004/13040/Platelet_Rich_Plasma_and_Bone_Graft_Materials__A.7.aspx. [DOI] [PubMed] [Google Scholar]
- 15.Jakse N, Tangl S, Gilli R, Berghold A, Lorenzoni M, Eskici A, et al. Influence of PRP on autogenous sinus grafts an experimental study on sheep. Clin Oral Implants Res. 2003 Oct;14((5)):578–583. doi: 10.1034/j.1600-0501.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 16.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin [PRF] a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006 Mar;101((3)):e37–e44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan JAJ, Mouhyi J, et al. Platelet-rich fibrin [PRF] a second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Med Oral Pathol Oral Radiol Endod. 2006 Mar;101((3)):e45–e50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Sun X, Yu J, Wang J, Zhai P, Chen S, et al. Platelet-rich fibrin as a bone graft material in oral and maxillofacial bone regeneration classification and summary for better application. Biomed Res Int. 2019;2019:3295756. doi: 10.1155/2019/3295756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.el Bagdadi K, Kubesch A, Yu X, Al-Maawi S, Orlowska A, Dias A, et al. Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin [PRF]-based matrices a proof of concept of LSCC [low speed centrifugation concept] Eur J Trauma Emerg Surg. 2019 Jun 1;45((3)):467–479. doi: 10.1007/s00068-017-0785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choukroun J, Ghanaati S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin [PRF] concentrates advances patients' own inflammatory cells platelets and growth factors the first introduction to the low speed centrifugation concept. Eur J Trauma Emerg Surg. 2018 Feb 1;44((1)):87–95. doi: 10.1007/s00068-017-0767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dohan Ehrenfest DM, del Corso M, Diss A, Mouhyi J, Charrier JB. Three-dimensional architecture and cell composition of a Choukroun's platelet-rich fibrin clot and membrane. J Periodontol. 2010 Apr;81((4)):546–555. doi: 10.1902/jop.2009.090531. [DOI] [PubMed] [Google Scholar]
- 22.Valladão CAA, Jr, Monteiro MF, Joly JC. Guided bone regeneration in staged vertical and horizontal bone augmentation using platelet-rich fibrin associated with bone grafts a retrospective clinical study. Int J of Implant Dent. 2020 Dec;6((1)):72. doi: 10.1186/s40729-020-00266-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miron RJ, Zucchelli G, Pikos MA, Salama M, Lee S, Guillemette V, et al. Use of platelet-rich fibrin in regenerative dentistry a systematic review. Clin Oral Invest. 2017;21((6)):1913–1927. doi: 10.1007/s00784-017-2133-z. [DOI] [PubMed] [Google Scholar]
- 24.Wend S, Kubesch A, Orlowska A, Al-Maawi S, Zender N, Dias A, et al. Reduction of the relative centrifugal force influences cell number and growth factor release within injectable PRF-based matrices. J Mater Sci Mater Med. 2017;28((12)):188. doi: 10.1007/s10856-017-5992-6. [DOI] [PubMed] [Google Scholar]
- 25.Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012 Aug;49((10)):35–43. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- 26.Niu Y, Li Q, Ding Y, Dong L, Wang C. Engineered delivery strategies for enhanced control of growth factor activities in wound healing. Adv Drug Deliv Rev. 2019 Jun;146:190–208. doi: 10.1016/j.addr.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Miron RJ, Fujioka-Kobayashi M, Bishara M, Zhang Y, Hernandez M, Choukroun J. Platelet-rich fibrin and soft tissue wound healing a systematic review. Tissue Eng Part B Rev. 2017 Feb 1;23((1)):83–99. doi: 10.1089/ten.TEB.2016.0233. [DOI] [PubMed] [Google Scholar]
- 28.Dohan Ehrenfest DM, Pinto NR, Pereda A, Jiménez P, Corso MD, Kang BS, et al. The impact of the centrifuge characteristics and centrifugation protocols on the cells growth factors and fibrin architecture of a leukocyte- and platelet-rich fibrin [L-PRF] clot and membrane. Platelets. 2018;29((2)):171–184. doi: 10.1080/09537104.2017.1293812. [cited 2021 Dec 5] Available from: https://doi-1org-10013b5kd0d18.han.medunigraz.at/101080/0953710420171293812. [DOI] [PubMed] [Google Scholar]
- 29.Gupta N, Agarwal S. Advanced PRF-clinical evaluation in impacted mandibular third molar sockets. J Stomatol Oral Maxillofacial Surg. 2021 Feb 1;122:43–49. doi: 10.1016/j.jormas.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Qing C. The molecular biology in wound healing & non-healing wound. Chin J Traumatol. 2017 Aug;20((4)):189–193. doi: 10.1016/j.cjtee.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghanaati S, Herrera-Vizcaino C, Al-Maawi S, Lorenz J, Miron RJ, Nelson K, et al. Fifteen years of platelet rich fibrin in dentistry and oromaxillofacial surgery How high is the level of scientific evidence? J Oral Implantol. 2018;44((6)):471–492. doi: 10.1563/aaid-joi-D-17-00179. [DOI] [PubMed] [Google Scholar]
- 32.Gonzallez-Garcia R. Scientific evidence in surgery for the treatment of temporomandibular joint internal derangement. Stomatological Dis Sci. 2019 May 29;:2019. [Google Scholar]
- 33.Bailey E, Kashbour W, Shah N, Worthington HV, Renton TF, Coulthard P. Surgical techniques for the removal of mandibular wisdom teeth. Cochrane Database Syst Rev. 2020 Jul;26;7((7)):CD004345. doi: 10.1002/14651858.CD004345.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang X, Shi P, Zhang P, Shen J, Kang J. Impact of platelet-rich fibrin on mandibular third molar surgery recovery a systematic review and meta-analysis. BMC Oral Health. 2019 Jul 25;19((1)):163. doi: 10.1186/s12903-019-0824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das D, Malhotra A, Kapur I, Sharma A, Gupta M, Kumar M. Comparative evaluation of bone regeneration with platelet-rich fibrin in mandibular third molar extraction socket a randomized split-mouth study. Natl J Maxillofac Surg. 2020;11((2)):241. doi: 10.4103/njms.NJMS_50_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sybil D, Sawai M, Faisal M, Singh S, Jain V. Platelet-rich fibrin for hard- and soft-tissue healing in mandibular third molar extraction socket. Ann Maxillofac Surg. 2020 Jan 1;10((1)):102–107. doi: 10.4103/ams.ams_228_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapse S, Surana S, Satish M, Hussain SE, Vyas S, Thakur D. Autologous platelet-rich fibrin can it secure a better healing? Oral Surg Oral Med Oral Pathol Oral Radiol. 2019 Jan 1;127((1)):8–18. doi: 10.1016/j.oooo.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Unsal H, Erbasar G. Evaluation of the effect of platelet-rich fibrin on the alveolar osteitis incidence and periodontal probing depth after extracting partially erupted mandibular third molars extraction. Niger J Clin Pract. 2018;21:201–206. doi: 10.4103/njcp.njcp_1_17. [DOI] [PubMed] [Google Scholar]
- 39.Dar MM, Shah AA, Najar A, Younis M, Kapoor M, Dar JI. Healing potential of platelet rich fibrin in impacted mandibular third molar extraction sockets. Ann Maxillofac Surg. 2018;8((2)):206. doi: 10.4103/ams.ams_181_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritto FG, Pimentel T, Canellas JVS, Junger B, Cruz M, Medeiros PJ. Randomized double-blind clinical trial evaluation of bone healing after third molar surgery with the use of leukocyte- and platelet-rich fibrin. Int J Oral Maxillofac Surg. 2019;48((8)):1088–1093. doi: 10.1016/j.ijom.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 41.Jeyaraj P, Chakranarayan A. Soft tissue healing and bony regeneration of impacted mandibular third molar extraction sockets following postoperative incorporation of platelet-rich fibrin. Ann Maxillofac Surg. 2018 Jan 1;8:10. doi: 10.4103/ams.ams_185_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buser D, Dula K, Hirt HP, Schenk RK. Lateral ridge augmentation using autografts and barrier membranes a clinical study with 40 partially edentulous patients. J Oral Maxillofac Surg. 1996;54((4)):420–432. doi: 10.1016/s0278-2391(96)90113-5. discussion 432-3. [DOI] [PubMed] [Google Scholar]
- 43.Tonetti MS, Jung RE, Avila-Ortiz G, Blanco J, Cosyn J, Fickl S, et al. Management of the extraction socket and timing of implant placement consensus report and clinical recommendations of group 3 of the XV European Workshop in Periodontology. J Clin Periodontol. 2019 Jun 1;46:183–194. doi: 10.1111/jcpe.13131. [DOI] [PubMed] [Google Scholar]
- 44.Christian Aravena P, Pilar Sandoval S, Eduardo Pizarro F, Isabel Simpson M, as Castro-Adams N, Eng M, et al. Leukocyte and platelet-rich fibrin have same effect as blood clot in the 3-Dimensional alveolar ridge preservation. A split-mouth randomized clinical trial. J Oral Maxillofac Surg. 2021 Mar;79((3)):575–584. doi: 10.1016/j.joms.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed N, Gopalakrishna V, Nagraj V, Imran M, Kumar P. Efficacy of PRF vs PRF + Biodegradable collagen plug in post-extraction preservation of socket. J Contemp Dent Pract. 2019;20((11)):1323–1328. [PubMed] [Google Scholar]
- 46.Canellas JVd S, da Costa RC, Breves RC, de Oliveira GP, Figueredo CMd S, Fischer RG, et al. Tomographic and histomorphometric evaluation of socket healing after tooth extraction using leukocyte- and platelet-rich fibrin a randomized, single-blind, controlled clinical trial. J Cranio-Maxillofac Surg. 2020 Jan 1;48((1)):24–32. doi: 10.1016/j.jcms.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Ruan Z, Shen M, Tan L, Huang W, Wang L, et al. Clinical effect of platelet-rich fibrin on the preservation of the alveolar ridge following tooth extraction. Exp Therap Med. 2018 Mar 1;15((3)):2277–2286. doi: 10.3892/etm.2018.5696. [cited 2021 Dec 12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srinivas B, Das P, Rana MM, Qureshi AQ, Vaidya KC, Ahmed Raziuddin S. Wound healing and bone regeneration in postextraction sockets with and without platelet-rich fibrin. Ann Maxillofac Surg. 2018 Jan 1;8:28. doi: 10.4103/ams.ams_153_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Girish Kumar N, Chaudhary R, Kumar I, Arora SS, Kumar N, Singh H. To assess the efficacy of socket plug technique using platelet rich fibrin with or without the use of bone substitute in alveolar ridge preservation a prospective randomised controlled study. Oral Maxillofac Surg. 2018 Jun 1;22((2)):135–142. doi: 10.1007/s10006-018-0680-3. [DOI] [PubMed] [Google Scholar]
- 50.Santhanakrishnan M, Ramesh N, Kamaleeshwari R, Subramanian V. Research Article Variations in Soft and Hard Tissues following Immediate Implant Placement versus Delayed Implant Placement following Socket Preservation in the Maxillary Esthetic Region A Randomized Controlled Clinical Trial. Biomed Res Int. 2021 Oct 4;2021:5641185. doi: 10.1155/2021/5641185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yewale M, Bhat S, Kamath A, Tamrakar A, Patil V, Algal AS. Advanced platelet-rich fibrin plus and osseous bone graft for socket preservation and ridge augmentation a randomized control clinical trial. J Oral Biol Craniofac Res. 2021;11((2)):225–233. doi: 10.1016/j.jobcr.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bodhare GH, Kolte AP, Kolte RA, Shirke PY. Clinical and radiographic evaluation and comparison of bioactive bone alloplast morsels when used alone and in combination with platelet-rich fibrin in the treatment of periodontal intrabony defects—a randomized controlled trial. J Periodontol. 2019;90((6)):584–594. doi: 10.1002/JPER.18-0416. [DOI] [PubMed] [Google Scholar]
- 53.Vu Pham TA, Pham AV. Intrabony defect treatment with platelet-rich fibrin. Guided tissue regeneration and open-flap debridement a randomized controlled trial. J Evid Based Dent Pract. 2021 Sep;21((3)):101545. doi: 10.1016/j.jebdp.2021.101545. [DOI] [PubMed] [Google Scholar]
- 54.Lei L, Yu Y, Han J, Shi D, Sun W, Zhang D, et al. Quantification of growth factors in advanced platelet-rich fibrin and concentrated growth factors and their clinical efficacy as adjunctive to the GTR procedure in periodontal intrabony defects. J Periodontol. 2020 Apr 1;91((4)):462–472. doi: 10.1002/JPER.19-0290. [DOI] [PubMed] [Google Scholar]
- 55.Kapa BP, Nk S, Gv G, Mehta DS. Coronally advanced flap combined with sticky bone and i PRF coated collagen membrane to treat single maxillary gingival recessions case series. Clin Adv Periodontics. 2021 May 15;12((3)):147–151. doi: 10.1002/cap.10164. [DOI] [PubMed] [Google Scholar]
- 56.Sun G, Cao L, Li H. Effects of platelet-rich fibrin combined with guided bone regeneration in the reconstruction of peri-implantitis bone defect. Am J Transl Res. 2021;13((7)):8397–8402. [PMC free article] [PubMed] [Google Scholar]
- 57.Işık G, Yüce Ö, Koçak-Topbaş N, Günbay T. Guided bone regeneration simultaneous with implant placement using bovine-derived xenograft with and without liquid platelet-rich fibrin a randomized controlled clinical trial. Clin Oral Investig. 2021 Sep;25((9)):5563–5575. doi: 10.1007/s00784-021-03987-5. [DOI] [PubMed] [Google Scholar]
- 58.Hartlev J, Erik Nørholt S, Spin-Neto R, Kraft D, Schou S, Isidor F. Histology of augmented autogenous bone covered by a platelet-rich fibrin membrane or deproteinized bovine bone mineral and a collagen membrane a pilot randomized controlled trial. Clin Oral Implants Res. 2020 Aug 1;31((8)):694–704. doi: 10.1111/clr.13605. [DOI] [PubMed] [Google Scholar]
- 59.Tatum H. Maxillary and sinus implant reconstructions. Dent Clin North Am. 1986 Apr;30((2)):207–229. [PubMed] [Google Scholar]
- 60.Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980;38((8)):613–616. [PubMed] [Google Scholar]
- 61.Riben C, Thor A. The maxillary sinus membrane elevation procedure augmentation of bone around dental implants without grafts-A review of a surgical technique. Int J Dentistry. 2012;2012:1–9. doi: 10.1155/2012/105483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pjetursson BE, Lang NP. Sinus floor elevation utilizing the transalveolar approach. Periodontol. 2000;66((1)):59–71. doi: 10.1111/prd.12043. [DOI] [PubMed] [Google Scholar]
- 63.Starch-Jensen T, Jensen JD. Maxillary sinus floor augmentation a review of selected treatment modalities. J Oral Maxillofac Res. 2017 Sep 30;8((3)):e3. doi: 10.5037/jomr.2017.8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cho YS, Hwang KG, Jun SH, Tallarico M, Kwon AM, Park CJ. Radiologic comparative analysis between saline and platelet-rich fibrin filling after hydraulic transcrestal sinus lifting without adjunctive bone graft A randomized controlled trial. Clin Oral Implant Res. 2020 Nov 1;31((11)):1087–1093. doi: 10.1111/clr.13655. [DOI] [PubMed] [Google Scholar]
- 65.Pichotano EC, Molon RS, Souza RV, Austin RS, Marcantonio E, Zandim-Barcelos DL. Evaluation of L-PRF combined with deproteinized bovine bone mineral for early implant placement after maxillary sinus augmentation a randomized clinical trial. Clin Implant Dent Relat Res. 2019 Apr 1;21((2)):253–262. doi: 10.1111/cid.12713. [DOI] [PubMed] [Google Scholar]
- 66.Barbu HM, Iancu SA, Hancu V, Referendaru D, Nissan J, Naishlos S. PRF-Solution in Large Sinus Membrane Perforation with Simultaneous Implant Placement-Micro CT and Histological Analysis. Membranes. 2021;11((6)):438. doi: 10.3390/membranes11060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Irdem H, Dolanmaz D, Esen A, Ünlükal N, Şimsek S. Evaluation of the effectiveness of liquid platelet-rich fibrin and deproteinized bovine bone mineral mixture on newly formed bone in maxillary sinus augmentation a split-mouth histomorphometric study. Niger J Clin Pract. 2021 Sep;24((9)):1366–1372. doi: 10.4103/njcp.njcp_692_20. [DOI] [PubMed] [Google Scholar]
- 68.Molemans B, Cortellini S, Jacobs R, Teughels W, Pinto N, Quirynen M. Simultaneous sinus floor elevation and implant placement using leukocyte- and platelet-rich fibrin as a sole graft material. Int J Oral Maxillofac Implants. 2019 Sep;34((5)):1195–1201. doi: 10.11607/jomi.7371. [DOI] [PubMed] [Google Scholar]
- 69.Tenore G, Zimbalatti A, Rocchetti F, Graniero F, Gaglioti D, Mohsen A, et al. Management of medication-related osteonecrosis of the jaw [MRONJ] using leukocyte- and platelet-rich fibrin [L-PRF] and photobiomodulation a retrospective study. J Clin Med. 2020 Nov 1;9((11)):3505. doi: 10.3390/jcm9113505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giudice A, Barone S, Giudice C, Bennardo F, Fortunato L. Can platelet-rich fibrin improve healing after surgical treatment of medication-related osteonecrosis of the jaw? A pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;126:390–403. doi: 10.1016/j.oooo.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 71.Beth-Tasdogan NH, Mayer B, Hussein H, Zolk O. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst Rev. 2017 Oct 6;10((10)):CD012432. doi: 10.1002/14651858.CD012432.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nica DF, Riviș M, Roi CI, Todea CD, Duma VF, Sinescu C. Complementarity of Photo-Biomodulation Surgical Treatment and Antibiotherapy for Medication-Related Osteonecrosis of the Jaws [MRONJ] Medicina. 2021 Feb 1;57((2)):1–14. doi: 10.3390/medicina57020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zelinka J, Blahak J, Perina V, Pacasova R, Treglerova J, Bulik O. The use of platelet-rich fibrin in the surgical treatment of medication-related osteonecrosis of the jaw 40 patients prospective study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2021;165((3)):3227. doi: 10.5507/bp.2020.023. [DOI] [PubMed] [Google Scholar]
- 74.Miranda M, Gianfreda F, Raffone C, Antonacci D, Pistilli V, Bollero P. The Role of Platelet-Rich Fibrin [PRF] in the Prevention of Medication-Related Osteonecrosis of the Jaw [MRONJ] BioMed Res Int. 2021;2021:4948139. doi: 10.1155/2021/4948139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miron RJ, Fujioka-Kobayashi M, Hernandez M, Kandalam U, Zhang Y, Ghanaati S, et al. Injectable platelet rich fibrin [i-PRF] opportunities in regenerative dentistry? Clin Oral Investig. 2017 Nov;21((8)):2619–2627. doi: 10.1007/s00784-017-2063-9. [DOI] [PubMed] [Google Scholar]
- 76.Zrnc TA, Metzler P, Zemann W, Ghanaati S. Dentistry a short synopsis about implementation and workflow [in German] Swiss Dent J. 2018;128:712–713. doi: 10.61872/sdj-2018-09-04. [DOI] [PubMed] [Google Scholar]
- 77.Karimi K, Rockwell H. The benefits of platelet-rich fibrin. Facial Plast Surg Clin North Am. 2019;27((3)):331–340. doi: 10.1016/j.fsc.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 78.Al-Maawi S, Herrera-Vizcaíno C, Orlowska A, Willershausen I, Sader R, Miron RJ, et al. Biologization of collagen-based biomaterials using liquid-platelet-rich fibrin New insights into clinically applicable tissue engineering. Materials. 2019 Dec 1;12((23)):3993. doi: 10.3390/ma12233993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Şahin O, Tatar B, Ekmekcioğlu C, Aliyev T, Odabaşi O. Prevention of medication related osteonecrosis of the jaw after dentoalveolar surgery an institution's experience. J Clin Exp Dent. 2020 Aug 1;12((8)):e771–e776. doi: 10.4317/jced.56837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Surgery M. Medication-related osteonecrosis of the Jaw-2014 update special committee on medication-related osteonecrosis of the Jaws. J Oral Maxillofac Surg. 2014 Oct;72((10)):1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]