Abstract
Background
Pancreatic cancer (PC) has a poor prognosis, with body weight loss commonly observed at diagnosis. However, the impact on PC prognosis of weight loss at the time of diagnosis on PC prognosis is unknown.
Methods
This retrospective, single-center study enrolled consecutively patients diagnosed with metastatic or locally advanced PC or resectable PC who were intolerant of or refused surgery. Patients who had lost more than 5% of their body weight or more than 2% and had a body mass index (BMI) of less than 20 kg/m2 at diagnosis were classified as experiencing body weight loss. Patients were subclassified into 2 groups: patients with and without weight loss. The study evaluated patient-related and PC-related factors affecting prognosis. Cox proportional hazards models were used to assess factors affecting prognosis. The primary endpoint was overall survival. Additionally, 1:1 propensity score matching was performed to reduce bias.
Results
In total, 220 patients were included in the study. The median age of the patients was 74 years, and 49.1% were male. Weight loss at diagnosis was observed in 43.2% of patients. There were no significant differences in clinical factors, except for anthropometric parameters, between the groups. The median survival time did not differ between the weight loss and no weight loss groups (149 and 173 days, respectively, P = .669). After matching, no significant differences in survival times were observed between the 2 groups.
Conclusions
This study found no association between weight loss at diagnosis and prognosis in patients with advanced PC treated with best supportive care or chemotherapy.
Keywords: body weight loss, pancreatic cancer, cancer cachexia, chemotherapy, best supportive care
Highlights
1. Patients with advanced pancreatic cancer often experience substantial and rapid weight loss, which affects their physical well-being, quality of life, and treatment tolerance.
2. Our study aims to improve our understanding of the relationship between weight loss at diagnosis, cachexia and prognosis in advanced pancreatic cancer.
3. Contrary to expectations, our study shows no significant association between weight loss at diagnosis and prognosis in patients with advanced pancreatic cancer.
Introduction
Cancer cachexia is a debilitating condition frequently observed in patients with various types of cancer, including pancreatic cancer (PC). It is characterized by weight loss, reduced physical well-being, poor quality of life, and decreased tolerance to cancer therapy. 1 According to the website of the National Cancer Institute in the United States, cachexia is estimated to occur in up to 80% of patients with advanced cancer, but varies depending on several factors. 2 Moreover, cachexia is recognized as a negative prognostic indicator due to its association with increased morbidity and mortality.3,4 In advanced PC, patients often experience rapid and significant weight loss, which not only affects their physical well-being and quality of life (QOL) but also limits their ability to tolerate cancer therapies.
Precisely diagnosing and evaluating cancer cachexia is challenging in clinical practice because of the difficulty in quantifying indicators used during the evaluation, such as muscle mass, strength, and function. According to the 2011 International Consensus Cancer Cachexia classification, 5 cancer cachexia is diagnosed when weight loss is greater than 5% or weight loss is greater than 2% in individuals already showing depletion as assessed by current BMI (<20 kg/m2) or skeletal muscle mass (sarcopenia). Cachexia is a complex syndrome characterized by the progressive loss of skeletal muscle mass, known as sarcopenia. 5 It should be distinguished from normal weight loss that can occur in cancer patients due to decreased appetite, malabsorption, or other causes. Unlike simple weight loss, cachexia cannot be fully reversed through nutritional intervention or weight gain, and the underlying mechanisms are not fully understood. This condition is believed to result from a combination of factors, including cytokine release, 6 oxidative stress, 7 and changes in the regulation of energy metabolism. 8
Body weight loss is a simple and easily measurable parameter frequently used to assess the presence of cachexia. Weight loss at diagnosis is a common and clinically relevant issue in advanced PC, as it may reflect the presence of cachexia and influence patient outcomes. Therefore, the main objective of this retrospective study was to evaluate the impact on prognosis of weight loss in the 6 months prior to diagnosis in patients with advanced PC who received best supportive care (BSC) or chemotherapy using the diagnostic criteria for cachexia proposed by Fearon et al. 5 Evaluating this association may contribute to our understanding of weight loss at diagnosis and cachexia in relation to the prognosis of advanced PC.
Materials and methods
This single-center retrospective study consecutively enrolled patients diagnosed and treated for metastatic, locally advanced, or resectable PC who were intolerant to or refused surgery at the Toyonaka Municipal Hospital between April 2013 and March 2022. Patients were diagnosed with adenocarcinoma using pathology samples obtained by endoscopic ultrasound-fine needle aspiration or endoscopic retrograde cholangiopancreatography. However, patients diagnosed by imaging evaluation and tumor marker elevation were also allowed to enroll in this study in cases that were not aggressively treated due to advanced age, poor activity of daily living (ADL), performance status (PS), or geriatric problems. All CT diagnoses were double-checked by a full-time radiologist at our hospital and certified by the Japan Radiological Society, and the attending physician made the final imaging diagnosis based on the report. Chemotherapy recommendations were based on the Pancreatic Cancer Clinical Practice Guidelines of the Japan Pancreas Society and included gemcitabine and nab-paclitaxel (GnP), modified FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan, and oxaliplatin), S1 alone, or gemcitabine (GEM) alone.
The study population was identified using the MegaOak online imaging system (NEC, Tokyo, Japan). Patients whose weights could not be evaluated prior to diagnosis were excluded. The reporting of this study conforms to STROBE guidelines. 9
Diagnostic Criteria for Significant Body Weight Loss
The diagnostic criteria for significant body weight loss were established using the diagnostic criteria for cachexia proposed by Fearon et al. 5 Body weight loss was considered significant if the patient had lost more than 5% of their body weight in the previous 6 months or more than 2% of their body weight and had a body mass index (BMI) of less than 20 kg/m2 at the time of PC diagnosis. All height and weight data were measured by medical staff on admission. We used data on body weight loss based on the patients' medical records, including documentation by healthcare professionals and any self-reported history of weight loss. Patients were classified into 2 groups: those with body weight loss and those without body weight loss based on these criteria.
Data Collection
Data were collected from electronic medical records at the time of PC diagnosis, and included patient-related factors, such as sex, age, BMI, waist circumference, subcutaneous fat area (SFA), visceral fat area (VFA), psoas muscle mass, psoas muscle index (PMI), Eastern Cooperative Oncology Group (ECOG)-PS, and geriatric assessment tools, including Geriatric (G)8, Vulnerable Elders Survey (VES)-13, age-adjusted Charlson Comorbidity Index (ACCI), neutrophil-to-lymphocyte ratio (NLR), and modified Glasgow Prognostic Score (mGPS). The factors related to PC were resectability, presence of distant metastasis, presence of biliary drainage, and tumor markers (serum carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9). The observation period was calculated from the time of PC diagnosis by imaging until all-cause death or last visit.
Assessment of Fat Area and Psoas Muscle Area
Computed tomography (CT) with Revolution GSI and Revolution EVO machines (GE Health care Japan, Tokyo, Japan) was used to evaluate the fat area, waist circumference, and psoas muscle to diagnose PC. We assessed the following body parameters using CT images: SFA, VFA at the umbilical level, and psoas muscle area at the level of the third lumbar vertebra (L3) using axial CT slices. These parameters were assessed using a SYNAPSE VINCENT image analysis system (Fujifilm, Tokyo, Japan). The fat area was set to a Hounsfield unit threshold ranging from −150 to −30. We measured the psoas muscle area using manual tracing. All images were measured by a single Dr. K.H., who has sufficient medical experience to minimize measurement error to reduce measurement discrepancies and increase the reliability of the results and followed uniform measurement methods. PMI was calculated by dividing the psoas muscle area by the square of the height. 10
Outcomes
The primary outcome measure was overall survival (OS), which was defined as the time from diagnosis to death from any cause. Secondary outcome measures included factors affecting prognosis, including patient-related factors, factors related to PC, and treatment modalities (chemotherapy or BSC).
Ethical Considerations
This study was conducted in accordance with the Declaration of Helsinki, and approval was obtained from the Institutional Review Board of Toyonaka Municipal Hospital (No. 2023-05-02). The requirement for informed consent was waived using the opt-out method on our hospital website.
Statistical Analysis
The statistical analysis was performed using JMP statistical software (ver. 16, SAS Institute Inc., Cary, NC, USA). To evaluate the influence of weight loss on survival, we used univariate and multivariate analyses with Cox proportional hazards models to assess whether the selected factors affected prognosis, and calculated the associated hazard ratios (HRs) with 95% confidence intervals (CIs). However, statistical sample size calculations were not performed due to a lack of evidence on which to base them. We set each assessment parameter to the following cutoff values according to previous reports. A VFA cutoff of 100 cm2 was used in accordance with guidelines set by the Japanese Visceral Fat Syndrome Study Committee of the Ministry of Health and Welfare of Japan. 11 The cutoff value for PMI in the Japanese population is 3.74 cm2/m2 for men and 2.29 cm2/m2 for women, which is based on the optimal PMI cutoff values for the diagnosis of skeletal muscle mass loss and sarcopenia in Japanese patients with chronic liver disease. 12 The cutoff values for VES-13 and NLR were 3 13 and 3, 14 respectively. We divided ECOG-PS into PS 0–1 and PS 2 or higher. The total G8 score ranged from 0 to 17, with a higher score indicating a better health status. 15 A score of G8 >14 was defined as normal, and a score of 14 or less was defined as abnormal according to the conventional classification. 15 We classified ACCI as low-risk (ACCI score 0–1), moderate-risk (ACCI score of 2–3), and high-risk (ACCI score 4 or higher) in the present study. The cutoff mGPS value was 1. 16 In the present study, we set the cutoff value of CA19-9 at 1000 IU/mL.
We also used the Kaplan‒Meier method to estimate overall survival and compared the survival curves between groups using the log-rank test. We then calculated propensity scores for prognosis with those significant factors to evaluate the impact of body weight loss and created a 1:1 matched study group with a .05 caliper width to minimize the impact of potential selection bias. We used a matrix imputation method to impute missing continuous variables. All calculated P values were two-sided, and differences for which P value <.05 was considered statistically significant.
Results
Patient Characteristics
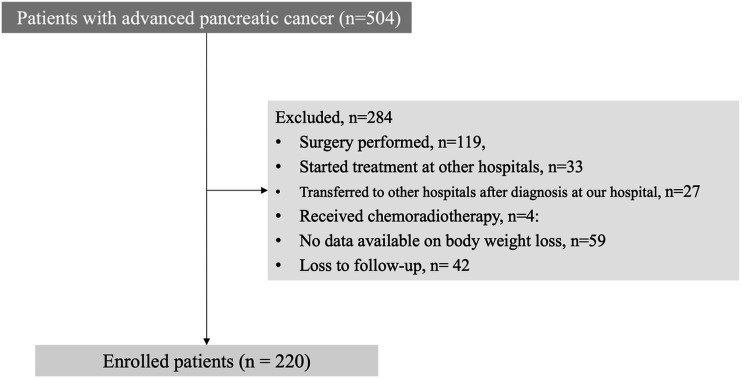
Between April 2013 and March 2022, 504 patients with an initial diagnosis of PC were admitted to our department. A total of 119 patients underwent surgery following evaluation; 33 patients began treatment in other hospitals and were subsequently transferred to our hospital; 27 patients were referred to other hospitals after the initial evaluation in our department; 4 patients received chemoradiotherapy; 59 patients were without available data on body weight information; and 42 patients were lost to follow-up. Therefore, 284 patients were excluded, and 220 patients were finally enrolled (Figure 1). Of these, 206 patients died during follow-up.
Figure 1.
Patient enrollment flowchart.
Table 1 summarizes the baseline characteristics of the enrolled patients. At the time of the initial evaluation for PC, the median age of the patients was 74 years (IQR: 69, 81), and 49.1% of the patients were men. Body weight loss at diagnosis was observed in 43.2% (95/220) of patients. The observation period from clinical diagnosis to the last visit ranged from 8 to 1611 days (median, 164 days; IQR, 67–281 days). The ECOG PS was 0 in 140 patients (63.6%), 1 in 44 (20.0%), and 2 or more in 36 patients (16.4%). 70 patients had diabetes, and 116 patients had chronic kidney disease. Regarding body parameters, the median BMI, waist circumference, SFA, VFA, and PMI (IQR) were 20.7 kg/m2 (18.4, 22.9), 79.6 cm (72,9, 85.4), 90.2 cm2 (41.5, 124.9), 50.4 cm2 (13.2, 78.9), 860.0 cm2 (635.6, 1216.6), and 3.48 cm2/m2 (2.77, 4.35), respectively. Regarding screening tools for health problems, the median (IQR) was 9.4 (9, 10) for the G8, 3.1 (1, 8) for the VES-13, 5 (4, 6) for the ACCI, and 3.8 (2.6, 6.3) for the NLR. The mGPS 0/1/2 was 120 (54.5%)/55 (25.0%)/45 (20.5%). Regarding cancer-related profiles, the most dominant tumor location was the pancreatic head in 90 patients (40.1%), followed by the pancreatic body in 62 patients (28.2%). Resectability was classified as resectable in 11 patients, unresectable locally advanced disease in 59, and unresectable metastatic disease in 150. Biliary drainage was required in 84 (38.2%) patients during the initial evaluation. The median levels of CEA and CA19-9 were 7.7 U/mL (3.8, 28.9) and 1750 U/mL (126, 19529), respectively.
Table 1.
Clinical Characteristics of Patients With Pancreatic Cancer.
| Characteristics, n (%) | |
|---|---|
| Patients | 220 |
| Patient-related factors | |
| Male sex, n (%) | 108 (49.1) |
| Age, median (years) | 74 |
| ECOG PS 0, 1, ≥2, n (%) | 140 (63.6), 44 (20.0), 36 (16.4) |
| Comorbidity | |
| Diabetes mellitus, yes, n (%) | 70 (31.8) |
| Chronic kidney disease, yes, n (%) | 116 (53.2) |
| Body parameters | |
| Body weight, median (kg) | 52.5 |
| Body weight loss, yes, n (%) | 95 (43.2) |
| BMI (kg/m2), median (IQR) | 20.7 (18.4, 22.9) |
| Waist circumference (cm), median (IQR) | 79.6 (72,9, 85.4) |
| Subcutaneous fat (cm2), median (IQR) | 90.2 (41.5, 124.9) |
| Visceral fat (cm2), median (IQR) | 50.4 (13.2, 78.9) |
| Psoas muscle mass (cm2), median (IQR) | 860.0 (635.6, 1216.6) |
| PMI (cm2/m2), median (IQR) | 3.48 (2.77, 4.35) |
| Screening tool for health problems | |
| G8, median (IQR) | 9.4 (9, 10) |
| VES-13, median (IQR) | 3.1 (1, 8) |
| ACCI, median (IQR) | 5 (4, 6) |
| NLR, median (IQR) | 3.8 (2.6, 6.3) |
| mGPS, 0/1/2 | 120/55/45 (54.5/25.0/20.5) |
| Cancer-related factors | |
| Main tumor location | |
| Head, head/body, body, body/tail, tail | 90 (40.1), 2 (.9), 62 (28.2), 2 (.9), 61 (27.7) |
| Resectability, R/UR-LA/UR-M | 11/59/150 (5.0/26.8/68.2) |
| Biliary drainage, yes, n (%) | 84 (38.2) |
| CEA, (U/mL) median (IQR) | 7.7 (3.8, 28.9) |
| CA19-9, (U/mL) median (IQR) | 1750 (126, 19529) |
| Treatment-related factors | |
| Best supportive care/chemotherapy, n (%) | 83/137 (37.7/62.2) |
| Chemotherapy regimen | |
| S1, n (%) | 7 (5.1) |
| GEM, n (%) | 37 (27.0) |
| GnP, n (%) | 82 (59.9) |
| mFOLFIRINOX, n (%) | 11 (8.0) |
Abbreviations: ECOG: Eastern Cooperative Oncology Group, PS: performance status, BMI: body mass index, PMI: psoas muscle index, R: resectable, UR: unresectable, LA: locally advanced, M: metastasis, G8: Geriatric 8, VES-13: Vulnerable Elders Survey, ACCI: age-adjusted Charlson Comorbidity Index, NLR: neutrophil-to-lymphocyte ratio, mGPS: modified Glasgow Prognostic Score, GEM: gemcitabine, GnP: gemcitabine and nab-paclitaxel, FOLFIRINOX:5-fluorouracil, leucovorin, irinotecan, and oxaliplatin.
Of the enrolled patients, 83 received BSC and 137 received chemotherapy. Of the 138 patients who received chemotherapy, 82 received GnP, 11 received mFOLFIRINOX, 7 received S1, and 37 received GEM as initial chemotherapy (Table 1).
Patients were subclassified into 2 groups according to the presence or absence of significant weight loss at diagnosis: the body weight loss group and the group without body weight loss. The body weight loss group included 95 patients, and the group without body weight loss included 125 patients. There were no significant differences in sex, age, ECOG-PS, presence of diabetes, or CKD; however, BMI was significantly lower in the body weight loss group, with a shorter waist circumference, smaller SFA, and VFA than in the group without body weight loss. The psoas muscle mass volume and PMI did not differ between groups. Regarding screening tools for health problems, the G8 was significantly lower, but all other parameters did not differ between the groups. Cancer-related factors, including resectability, need for biliary drainage, tumor markers, and treatment, did not differ between the groups (Table 2).
Table 2.
Comparison of Characteristics of Patients With and Without Body Weight Loss.
| Characteristics, n (%) | With Body Weight Loss | Without Body Weight Loss | |
|---|---|---|---|
| Patients, n | 95 | 125 | P Value |
| Patient-related factors | |||
| Male sex, n (%) | 48 (49.5) | 60 (52.0) | .7104 |
| Age, median (years) | 73 (69, 79) | 75 (68.5, 82.5) | .1936 |
| ECOG PS ≥2, n (%) | 11 (11.6) | 25 (20.0) | .0944 |
| Diabetes mellitus, yes, n (%) | 29 (30.5) | 41 (32.8) | .7199 |
| Chronic kidney disease, yes, n (%) | 50 (52.6) | 66 (53.7) | .8802 |
| Body parameters | |||
| Body weight, median (kg) | 50.8 (43.6, 57) | 54 (45.0, 63.7) | .0224 |
| BMI (kg/m2), median (IQR) | 19.7 (18.3, 21.9) | 21.8 (19.4, 23.3) | .0012 |
| Waist circumference (cm), median (IQR) | 79.3 (70.9, 82.1) | 80.5 (74.9, 86.7) | .0054 |
| Subcutaneous fat area (cm2), median (IQR) | 83.8 (29.9, 99.1) | 90.2 (54.1, 152.8) | .0027 |
| Visceral fat area (cm2), median (IQR) | 39.0 (8.3, 57.2) | 52.5 (17.8, 92.1) | .0211 |
| Psoas muscle mass (cm2), median (IQR) | 841.8 (617.9, 1221.4) | 886.4 (682.3, 1221.3) | .1518 |
| PMI (cm2/m2), median (IQR) | 3.22 (2.71, 4.14) | 3.68 (2.79, 4.40) | .0696 |
| Screening tool for health problems | |||
| G8, median (IQR) | 9.4 (8, 11) | 9.4 (9.4, 11) | .0222 |
| VES-13, median (IQR) | 3.1 (1,8) | 3.1 (1.6, 3.14) | .7383 |
| ACCI, median (IQR) | 4 (4,5) | 5 (4, 6) | .1320 |
| NLR, median (IQR) | 3.7 (2.5, 6.5) | 3.8 (2.7, 6.2) | .3013 |
| mGPS‡ 0/1/2 | 57/25/13 (60.0/26.3/13.7) | 63/30/32 (50.4/24.0/25.6) | .0919 |
| Cancer-related factors | |||
| R/UR-LA/UR-M | 5/28/62 (5.3/29.4/65.3) | 6/31/88 (4.8/24.8/70.4) | .7147 |
| Biliary drainage, yes, n (%) | 36 (37.9) | 48 (38.4) | .9391 |
| CEA, median (U/mL) | 7.8 (4, 27.2) | 7.6 (3.5, 31.4) | .4575 |
| CA19-9, median (U/mL) | 1484 (71, 15169) | 2455 (159.5, 22247.5) | .8058 |
| Best supportive care/chemotherapy, n (%) | 31/64 (32.6/67.4) | 51/74 (40.8/59.2) | .2146 |
| Chemotherapy regimen | |||
| S1/GEM/GnP/mFOLFIRINOX, n (%) | 4/23/31/6 (6.3/35.9/48.4/9.4) | 3/14/51/5 (4.1/19.2/69.9/6.9) | .0807 |
Abbreviations: ECOG: Eastern Cooperative Oncology Group, PS: performance status, BMI: body mass index, PMI: psoas muscle index, R: resectable, UR: unresectable, LA: locally advanced, M: metastasis, G8: Geriatric 8, VES-13: Vulnerable Elders Survey, ACCI: age-adjusted Charlson Comorbidity Index, NLR: neutrophil-to-lymphocyte ratio, mGPS: modified Glasgow Prognostic Score, GEM: gemcitabine, GnP: gemcitabine and nab-paclitaxel, FOLFIRINOX:5-fluorouracil, leucovorin, irinotecan, and oxaliplatin.
Overall Survival Curves of Patients With and Without Body Weight Loss
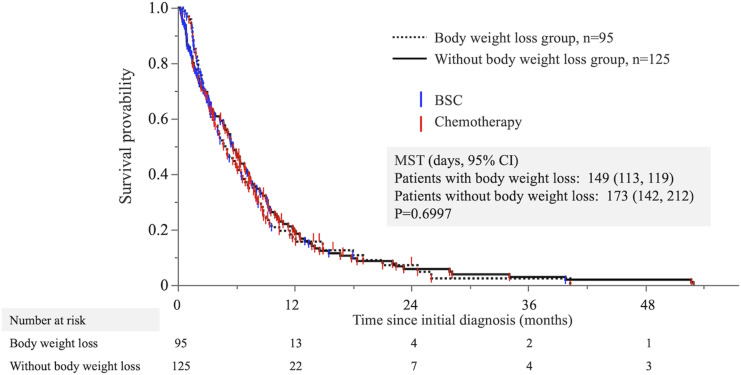
The overall survival (OS) times of patients with body weight loss and without body weight loss are plotted in Figure 2. The median OS time (MST) was 149 days in the body weight loss group and 173 days in the group without body weight loss. Participants that were provided BSC included 32.6% in the body weight loss group and 40.8% in the group without body weight loss, without any observed differences in the number of patients between the groups. The survival curves overlapped, and there was no significant difference between groups (log-rank P value: P = .6997).
Figure 2.
Overall survival times of patients with and without body weight loss. The Kaplan–Meier curve points do not indicate censoring but rather the observation period and treatment of each case.
Univariate and Multivariate Analyses With Cox Proportional Hazards Models for Predicting Prognosis in Patients With Advanced Pancreatic Cancer
We evaluated clinical factors for predicting PC prognosis. Univariate analysis showed that the variables PS >2, presence of CKD, BSC, metastatic lesions, NLR >3, mGPS 1/2, higher levels of CA19-9 and VES-13 > 3, and lower PMI were significantly associated with poor prognosis. However, body weight loss was not associated with poor prognosis (Table 3). The results of the multivariate analysis indicated that poor PS, body weight loss, BSC, metastatic lesions, NLR >3, higher CA19-9, and lower G8 were significantly associated with poor prognosis (Table 3).
Table 3.
Univariate and Multivariate Analyses With Cox Proportional Hazards Models for Clinical Factors Predicting Pancreatic Cancer Prognosis.
| Factors | Reference | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | ||
| Sex, female | Men | 1.14 | .86–1.50 | .3651 | .98 | .70–1.37 | .9066 |
| Age ≥75 years | <75 years | 1.24 | .94–1.64 | .1210 | .89 | .62–1.29 | .5614 |
| PS >2 | 0–1 | 2.79 | 1.93–4.04 | <.0001 | 1.80 | 1.04–2.73 | .0355 |
| Body weight loss, yes | No | 1.06 | .79–1.40 | .7004 | 1.50 | 1.11–2.03 | .0089 |
| Diabetes mellitus, yes | No | 1.02 | .76–1.37 | .8920 | 1.09 | .77–1.54 | .6244 |
| Chronic kidney disease*, yes | No | 1.34 | 1.00–1.77 | .0431 | 1.04 | .74–1.45 | .8374 |
| Biliary drainage, yes | No | 1.00 | .76–1.33 | .9775 | .91 | .67–1.26 | .5813 |
| Chemotherapy | BSC | .35 | .26–.47 | <.0001 | .36 | .24–.55 | <.0001 |
| UR-M†, yes | No | 2.00 | 1.44–2.78 | <.0001 | 2.01 | 1.43–2.84 | <.0001 |
| NLR ≥3 | <3 | 2.14 | 1.55–2.96 | <.0001 | 1.61 | 1.11–2.35 | .0130 |
| mGPS, 1–2 | 0 | 2.20 | 1.65–2.93 | <.0001 | 1.24 | .87–1.77 | .2415 |
| CA19-9, ≥1000 U/mL | <1000 U/mL | 1.60 | 1.21–2.13 | .0012 | 1.77 | 1.29–2.43 | .0004 |
| G8 <14 | >14 | .75 | .10–5.34 | .7741 | .09 | .01–.77 | .0275 |
| VES-13 >3 | <3 | 1.54 | 1.01–2.37 | .0464 | 1.36 | .85–2.18 | .2017 |
| ACCI, high risk | Low to medium risk | 1.14 | .79–1.65 | .4854 | 1.06 | .66–1.72 | .8033 |
| Visceral fat, >100 | <100 | 1.15 | .79–1.69 | .4651 | 1.16 | .73–1.45 | .5276 |
| PMI, low | Normal | 1.49 | 1.09–2.03 | .0110 | 1.32 | .93–1.89 | .1198 |
*Chronic kidney disease is defined as an eGFR of less than 60 mL/min.
†Among the three groups according to resectability, metastasis was a significantly poor prognostic factor, but there was no difference between locally advanced and resectable patients (resectable/locally advanced; HR 1.30, P = .4548). Therefore, we compared the 2 groups: patients with metastases and patients with resectable/locally advanced disease.
Abbreviations: UR: unresectable, M: metastasis, NLR: neutrophil-to-lymphocyte ratio, mGPS: modified Glasgow Prognostic Score, G8: Geriatric 8, VES-13: Vulnerable Elders Survey, ACCI: age-adjusted Charlson Comorbidity Index, PMI: psoas muscle index.
Propensity Score Matching Analysis
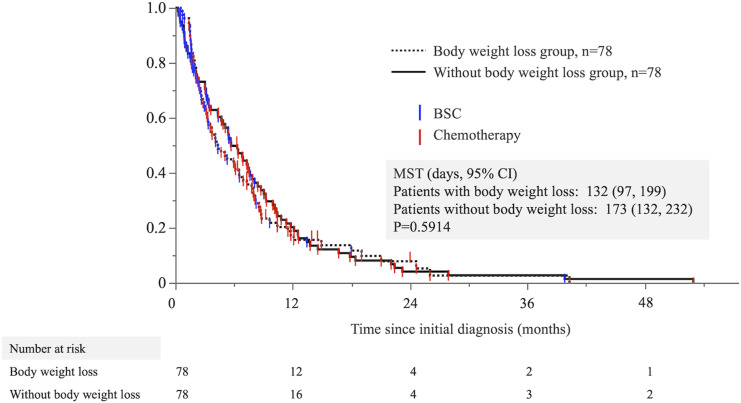
Multivariate logistic analysis showed that weight loss was an independent significant risk factor for predicting poor prognosis. We performed a 1:1 propensity-matching analysis to evaluate the impact of body weight loss on predicting poor prognosis using 6 significant factors by multivariate analysis, except for body weight loss, and including age and sex. We obtained 156 patients matched with and without body weight loss. Table 4 shows the results of the propensity score matching. After matching propensity scores, there were no significant differences between groups. OS was evaluated in propensity-matched patients in both groups. There was no significant difference in OS between the 2 groups, with a median OS of 132 and 173 days, respectively (log-rank P = .5914) (Figure 3).
Table 4.
Comparison of Characteristics of Patients With and Without Body Weight Loss After Propensity Score Matching.
| Propensity-Matched Cohort | |||
|---|---|---|---|
| Characteristics, n (%) | With Body Weight Loss | Without Body Weight Loss | P Value |
| Patients, n | 78 | 78 | |
| Male sex, n (%) | 39 (50.0) | 36 (46.2) | .7487 |
| Age | 73 (69, 79) | 76 (69, 82) | .1123 |
| PS ≥2, n (%) | 9 (11.5) | 9 (11.5) | 1.000 |
| Diabetes mellitus, yes | 24 (30.8) | 24 (30.8) | 1.000 |
| Chronic kidney disease *, yes | 42 (53.9) | 45 (58.4) | .6283 |
| Biliary drainage, yes | 29 (37.2) | 32 (41.0) | .7430 |
| Chemotherapy | 49 (62.8) | 51 (65.4) | .8675 |
| UR-M, yes | 57 (73.1) | 57 (73.1) | 1.000 |
| NLR ≥3 | 52 (65.8) | 57 (72.2) | .4917 |
| mGPS, 1–2 | 32 (41.0) | 37 (47.4) | .5192 |
| CA19-9, ≥1000 U/mL | 45 (57.7) | 45 (57.7) | 1.0000 |
| G8 ≤14 | 78 (100) | 78 (100) | 1.0000 |
| VES-13 ≥3 | 64 (82.1) | 69 (88.5) | .3667 |
| ACCI, high risk | 61 (78.2) | 66 (84.6) | .4107 |
| Visceral fat, ≥100 | 6 (7.7) | 12 (15.4) | .2094 |
| PMI, low | 25 (32.1) | 19 (24.4) | .3738 |
*Chronic kidney disease is defined as an eGFR of less than 60 mL/min.
Abbreviations: UR: unresectable, M: metastasis, NLR: neutrophil-to-lymphocyte ratio, mGPS: modified Glasgow Prognostic Score, G8: Geriatric 8, VES-13: Vulnerable Elders Survey, ACCI: age-adjusted Charlson Comorbidity Index, PMI: psoas muscle index.
Figure 3.
The overall survival times of patients with and without body weight loss after propensity score matching. The Kaplan–Meier curve points do not indicate censoring but rather the observation period and treatment of each case.
Discussion
This study aimed to investigate the association between weight loss at diagnosis and prognosis in patients with advanced PC. Our univariate analysis identified 9 factors that predicted poor prognosis in advanced PC: poor PS, CKD, selection of BSC, presence of metastatic lesion, higher NLR, higher mGPS, higher CA19-9, higher VES-13, and lower PMI. Similarly, the results of the multivariate analysis revealed 7 factors that were selected as poor prognostic indicators: poor PS, body weight loss at diagnosis, BSC selection, presence of metastatic lesion, higher NLR, higher CA19-9, and lower G-8. However, when we compared the prognostic value of weight loss at diagnosis between the 2 groups using propensity score matching, we found no significant differences in prognosis between patients with and without weight loss at diagnosis. One possible explanation for this discrepancy is that other factors adjusted for in the multivariate analysis may have stronger associations with prognosis than with weight loss at the time of diagnosis. In the propensity score analysis, weight loss at diagnosis was evaluated in relation to other factors, which may have weakened its association with prognosis. In addition, body weight loss may affect intolerance and the incidence of adverse events during chemotherapy. In this study, dose reductions of anticancer drugs were considered at the discretion of the treating physician, so these relationships were not fully examined. This could have influenced the present results and needs to be verified in future studies.
The prognostic value of weight loss at diagnosis in patients with PC remains controversial. Nemer et al. reported that weight loss >5% was not associated with worse survival in a multivariate analysis involving 123 patients with PC, but weight loss >8.4% was associated with worse survival in the univariate analysis. 17 In the current study, we used the definition of body weight loss as the loss of more than 5% of body weight or more than 2% of body weight with a BMI of less than 20 kg/m2, which was based on the International Consensus Cancer Cachexia classification. 5 It was suggested that the cutoff value of 5% weight loss, the criterion for cancer cachexia in this study, may have been too low to predict prognosis.
Davis et al. retrospectively reported that weight loss over 60 days during chemotherapy, but not at diagnosis, did not predict a poor prognosis in 93 patients with PC. 18 On the other hand, Yildirim et al. reported that a weight loss of 3.1% during chemoradiotherapy was an independent predictor of poor survival in 73 patients with locally advanced PC treated with chemoradiotherapy. 19 These conflicting results suggest that weight loss and survival may result from multiple factors. Almost all studies were retrospective, with heterogeneous designs, and the degree of weight loss and period over which weight loss was measured varied.
In the current study, we found that survival curves for both patients receiving BSC and chemotherapy showed that patients without body weight loss had better outcomes than those with body weight loss in the middle term of the observation period. However, survival curves in the early period just after diagnosis and after 1 year of observation appeared to overlap, leading to nonsignificant findings, even when the BSC and chemotherapy groups were evaluated separately (Supplementary Figure). Therefore, we speculate that this may account for why the log-rank analysis did not yield significant results. Weight loss at diagnosis may be associated with better outcomes at mid-term observation, except in patients with a short-term poor prognosis and in those observed over the long-term.
In this study, we focused on body weight loss at initial PC diagnosis. Salinas-Miranda et al. reported that greater skeletal muscle loss between prechemotherapy and first follow-up computed tomography may be associated with poorer prognosis in patients undergoing chemotherapy. 20 Nakano et al. reported that a rapid decline in visceral fat tissue over 1 month is closely associated with poor survival in patients with unresectable advanced PC 21 These results suggest that changes in body composition factors other than body weight, such as muscle mass and fat mass, from diagnosis to early treatment may impact prognosis. Further prospective studies to validate these findings, including the timing of evaluation, are needed.
The primary strength of this study was the large sample size (220 patients), which resulted in study population that is more representative of the real-world population of patients with advanced PC in Japan. The study included patients who were intolerant to or refused surgery, reflecting the patient population typical of clinical practice. The same trend was observed when the BSC and chemotherapy groups were examined separately, as shown in Supplementary Figure. Furthermore, this study used patient-related factors and geriatric assessment tools for a more accurate and comprehensive analysis.
This study had several limitations. First, this was a single-center retrospective study of Japanese subjects, which may limit the generalizability of the findings. Therefore, the study was subject to selection and recall bias of weight loss, which could have affected the accuracy of the results. However, we performed a propensity score matching analysis to reduce these biases. Second, the study did not include data on patient diet, physical activity, and body weight change during the clinical course after diagnosis owing to a lack of available data, which may have affected the results. However, the study aim was to examine the effects of weight loss at diagnosis but not during the clinical course. Third, we used the psoas muscle area at the L3 level, although the L3 level includes several muscles. However, PMI at the L3 level is well established and can provide valuable information about skeletal muscle mass. 22 Therefore, we used the psoas muscle as a surrogate marker because of its consistent appearance and ease of measurement on CT scans. In addition, since the measurements are made manually, the possibility of data variation cannot be completely eliminated. Fourth, although the chemotherapy regimens were based on Japanese clinical practice guidelines, variations in treatment between physicians could have affected the outcomes. Finally, we did not perform statistical sample size calculations because there is no evidence that initial body weight loss at diagnosis may affect the prognosis of patients with advanced pancreatic cancer. Therefore, it is possible that the number of cases was insufficient to derive the present results.
Conclusions
This study found that body weight loss at diagnosis was not associated with prognosis in patients with advanced PC treated with BSC or chemotherapy. The reason for this was not clear from this study but may be due to the poor prognosis related to advanced PC. Overall, this study provides valuable information regarding the relationship between weight loss and prognosis in patients with advanced PC. Further research on the prognostic impact of weight loss is needed. The prognosis of patients with PC is expected to improve in the future as treatment advances.
Supplemental Material
Supplemental Material for Impact of Initial Body Weight Loss on Prognosis in Advanced Pancreatic Cancer: Insights From a Single-Center Retrospective Study by Kana Hosokawa, Tsutomu Nishida, Daichi Hayashi, Miharu Kitazawa, Haruka Masuda, Katsuharu Tono, Yuhiko Katanosaka, Naohiro Sakamoto, Yoshifumi Fujii, Aya Sugimoto, Dai Nakamatsu, Kengo Matsumoto, Masashi Yamamoto, and Koji Fukui in Cancer Control
Authorship Contributions: All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Kana Hosokawaand Tsutomu Nishida. The first draft of the manuscript was written by Tsutomu Nishida and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
Ethical Statement
Ethics Approval
This study was conducted in accordance with the Declaration of Helsinki, and approval was obtained from the institutional review board of Toyonaka Municipal Hospital (No. 2023-05-02).
Patient Consent
The requirement for informed consent was waived using the opt-out method on our hospital website.
ORCID iDs
Tsutomu Nishida https://orcid.org/0000-0003-4037-9003
Masashi Yamamoto https://orcid.org/0000-0002-1107-6858
References
- 1.Mitsunaga S, Kasamatsu E, Machii K. Incidence and frequency of cancer cachexia during chemotherapy for advanced pancreatic ductal adenocarcinoma. Support Care Cancer. 2020;28(11):5271-5279. doi: 10.1007/s00520-020-05346-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Cancer Institute at the National Institutes of Health . Cancer Cachexia: After Years of No Advances, Progress Looks Possible. Updated August 23, 2022. https://www.cancer.gov/about-cancer/treatment/research/cachexia Accessed May 10, 2023. [Google Scholar]
- 3.Rich NE, Phen S, Desai N, et al. Cachexia is prevalent in patients with hepatocellular carcinoma and associated with worse prognosis. Clin Gastroenterol Hepatol. 2022;20(5):e1157-e1169. doi: 10.1016/j.cgh.2021.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go SI, Park MJ, Lee GW. Clinical significance of the cachexia index in patients with small cell lung cancer. BMC Cancer. 2021;21(1):563. doi: 10.1186/s12885-021-08300-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489. doi: 10.1016/s1470-2045(10)70218-7 [DOI] [PubMed] [Google Scholar]
- 6.AlSudais H, Rajgara R, Saleh A, Wiper-Bergeron N. C/EBPβ promotes the expression of atrophy-inducing factors by tumours and is a central regulator of cancer cachexia. J Cachexia Sarcopenia Muscle. 2022;13(1):743-757. doi: 10.1002/jcsm.12909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinch EC, Sullivan-Gunn MJ, Vaughan VC, McGlynn MA, Lewandowski PA. Disruption of pro-oxidant and antioxidant systems with elevated expression of the ubiquitin proteosome system in the cachectic heart muscle of nude mice. J Cachexia Sarcopenia Muscle. 2013;4(4):287. doi: 10.1007/s13539-013-0116-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalal S. Lipid metabolism in cancer cachexia. Ann Palliat Med. 2019;8(1):13-23. doi: 10.21037/apm.2018.10.01 [DOI] [PubMed] [Google Scholar]
- 9.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 10.Hamaguchi Y, Kaido T, Okumura S, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition. 2016;32(11-12):1200. doi: 10.1016/j.nut.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 11.Examination Committee of Criteria for 'Obesity Disease' in Japan, Japan Society for the Study of Obesity . New criteria for 'obesity disease' in Japan. Circ J. 2002;66(11):987. doi: 10.1253/circj.66.987 [DOI] [PubMed] [Google Scholar]
- 12.Ohara M, Suda G, Kimura M, et al. Analysis of the optimal psoas muscle mass index cut-off values, as measured by computed tomography, for the diagnosis of loss of skeletal muscle mass in Japanese people. Hepatol Res. 2020;50(6):715-725. doi: 10.1111/hepr.13499 [DOI] [PubMed] [Google Scholar]
- 13.Min L, Yoon W, Mariano J, et al. The vulnerable elders-13 survey predicts 5-year functional decline and mortality outcomes in older ambulatory care patients. J Am Geriatr Soc. 2009;57(11):2070. doi: 10.1111/j.1532-5415.2009.02497.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teo M, Mohd Sharial MS, McDonnell F, Conlon KC, Ridgway PF, McDermott RS. Prognostic role of neutrophil-to-lymphocyte ratio in advanced pancreatic ductal adenocarcinoma: impact of baseline fluctuation and changes during chemotherapy. Tumori. 2013;99(4):516. doi: 10.1177/030089161309900413 [DOI] [PubMed] [Google Scholar]
- 15.Takahashi M, Takahashi M, Komine K, et al. The G8 screening tool enhances prognostic value to ECOG performance status in elderly cancer patients: a retrospective, single institutional study. PLoS One. 2017;12(6):e0179694. doi: 10.1371/journal.pone.0179694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Ren D, Jin X, Wu H. The prognostic value of modified Glasgow Prognostic Score in pancreatic cancer: a meta-analysis. Cancer Cell Int. 2020;20:462. doi: 10.1186/s12935-020-01558-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nemer L, Krishna SG, Shah ZK, et al. Predictors of pancreatic cancer-associated weight loss and nutritional interventions. Pancreas. 2017;46(9):1152-1157. doi: 10.1097/MPA.0000000000000898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis M, Vanenkevort E, Varun S, et al. Is weight loss during chemotherapy for pancreatic cancer prognostic? Am J Hosp Palliat Care. 2022;40:585-591. doi: 10.1177/10499091221123049 [DOI] [PubMed] [Google Scholar]
- 19.Yildirim BA, Özdemir Y, Colakoglu T, Topkan E. Impact of presence and degree of pretreatment weight loss in locally-advanced pancreatic cancer patients treated with definitive concurrent chemoradiotherapy. Pancreatology. 2016;16(4):599-604. doi: 10.1016/j.pan.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 20.Salinas-Miranda E, Deniffel D, Dong X, et al. Prognostic value of early changes in CT-measured body composition in patients receiving chemotherapy for unresectable pancreatic cancer. Eur Radiol. 2021;31(11):8662-8670. doi: 10.1007/s00330-021-07899-6 [DOI] [PubMed] [Google Scholar]
- 21.Nakano O, Kawai H, Kobayashi T, et al. Rapid decline in visceral adipose tissue over 1 month is associated with poor prognosis in patients with unresectable pancreatic cancer. Cancer Med. 2021;10(13):4291-4301. doi: 10.1002/cam4.3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong M, Lin N, Wang L, et al. Age-specific reference values for low psoas muscle index at the L3 vertebra level in healthy populations: a multicenter study. Front Nutr. 2022;9:1033831. doi: 10.3389/fnut.2022.1033831 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Impact of Initial Body Weight Loss on Prognosis in Advanced Pancreatic Cancer: Insights From a Single-Center Retrospective Study by Kana Hosokawa, Tsutomu Nishida, Daichi Hayashi, Miharu Kitazawa, Haruka Masuda, Katsuharu Tono, Yuhiko Katanosaka, Naohiro Sakamoto, Yoshifumi Fujii, Aya Sugimoto, Dai Nakamatsu, Kengo Matsumoto, Masashi Yamamoto, and Koji Fukui in Cancer Control