Abstract
Objective:
To examine the association of multiple health behaviors to mental health functioning in male and female collegiate athletes.
Participants:
Prospective National Collegiate Athletic Association (NCAA) Division I athletes (n = 183) completed a health and wellness survey in the summer prior to joining the athletic program.
Methods:
Health behaviors (eating, sleeping, substance use, sexual, and aggressive behaviors) and mental health functioning (psychological distress and perceived stress) were assessed. Partial least squares (PLS) modeling was used as a multivariate approach to simultaneously examine the association of health behaviors to mental health functioning.
Results:
Aggressive behaviors, alcohol use, and fatigue were significantly associated with symptoms of psychological distress and stress in both males and females. Attention to nutrition, unhealthy dietary habits, and lower alcohol use was further related to psychological distress in female athletes only.
Conclusions:
Athletes’ eating, sleeping, substance use, and aggressive behaviors may provide insight into their mental health functioning.
Keywords: Alcohol, anxiety, depression, lifestyle, stress, student athletes
Decades of research on elite athletes have demonstrated that health behaviors, such as diet, sleep, and substance use critically impact competitive performance and risk of injury.1-3 In the general population, there is a growing consensus that these behaviors globally impact mental and physical health across the lifespan.4,5 Yet, the role of health behaviors on athlete mental health is understudied. This relationship is particularly important for collegiate athletes who often encounter elevated stress due to their status as both students and athletes,6,7 which may add to the general risk for psychological problems during this developmental period.8
Similar to nonathlete college students, there is a high prevalence of depression among collegiate athletes.9 However, heightened social stigma inherent within the culture of athletics9-11 increases the likelihood of underreporting mental health concerns and underutilization of counseling services in this population.11-13 It is therefore critical to establish novel approaches to identify potential psychological distress even when an athlete does not self-disclose. This study sought to examine whether the assessment of health behaviors (ie, eating, sleeping, substance use, sexual, aggressive behaviors) could provide insight into the mental health functioning of college athletes.
Prior studies have examined single health behaviors, including diet,14 sleep,15 aggressive behaviors,16 and substance use17 in collegiate athletes; few, however, have characterized how these behaviors interact to holistically affect physical health outcomes18 and none have assessed how these health behaviors interrelate to mental health outcomes. Understanding the association between mental health and eating, sleeping, substance use, sexual, and aggressive behaviors in athletes may help athletic trainers and other sports medicine personnel to identify collegiate athletes experiencing mental health problems earlier and even reduce the risk of later mental health problems. Thus, the aim of our study was to examine how co-occurring health behaviors related to mental health outcomes in a large sample of incoming collegiate athletes. The relationship between patterns of health behaviors and self-reported mental health symptoms (stress, anxiety, depression, and somatization) was assessed. We further examined whether the relationship between patterns of health behaviors and mental health outcomes differed between males and females.
Method
Participants
Prospective National Collegiate Athletic Association (NCAA) Division I athletes (n = 184) from a large Northeastern university completed a health behaviors survey at home prior to entry into the athletic program. One respondent was dropped from the sample due to missing data, leaving 183 athletes (mean age = 18.5, SD = 1.0) with complete surveys. Participants were asked to endorse male (54.6%), female (45.4%), or intersex (0%) in response to a single item on “gender”. All athletes were entering their first year at the university from public high school (80%), private high school (9%), religious-based schools (10%), charter schools (0.5%), or home school (0.5%). Most of the athletes reported never having to repeat a grade (93%); 5% reported receiving special education/intervention and 9% identified having a learning disability. Athletes identified their social class as lower (1%), middle (95%), or upper (4%); 17% reported being part of a free or reduced-fee lunch program in high school. Athletes participated in wide range of sports including football (17%), rowing (15%), track and field (14%), lacrosse (11%), soccer (8%), baseball (7%), basketball (5%), field hockey (4%), wrestling (4%), swimming/diving (3%), volleyball (3%), softball (3%), golf (3%), gymnastics (2%), tennis (0.5%), and cross-country (0.5%). The study was approved by the university’s institutional review board and each participant provided informed consent.
Measures
Collegiate athletes completed a 150-item health behavior survey that sought to characterize the basic health behaviors of incoming athletes. The survey was designed to broadly capture eating and hydration, fatigue and sleeping habits, aggressive behaviors, sexual behaviors, and substance use behaviors. Within these items, two psychometrically sound scales were embedded to measure the mental health outcomes of perceived stress and symptoms of anxiety and depression. Surveys were completed online using Qualtrics survey platform. A subset of items that specifically gauged five primary health behavior domains (eating, fatigue/sleep, aggression, sexuality, and substance use) were used in the present analyses.
Eating behaviors
Participants responded to 3 items regarding how often they have meals during the week with response options from 0 to 7 days (eg, “In a typical week, how many days did you eat breakfast?”), 8 items about the types of food they consume in a week (eg, “How often did you eat at a fast food restaurant?”) on a Likert scale from 1 (Not at all) to 5 (A lot), and 5 items regarding hydration (“How often did you drink plain water?”) rated on a similar scale. An exploratory factor analysis with principal axis factoring and orthogonal (varimax) rotation was performed to reduce the 11 dietary and 5 hydration items. The analysis identified two factors accounting for 33.3% of variance, corresponding to healthy and unhealthy dietary habits. The factor loadings are presented in Table 1. Items with loadings below .40 (ie, lunch, pre-made meals, and sports drinks) were considered weakly associated with the factor19 and excluded before computing factor scores. In addition, 5 items assessed attention to nutrition (eg, “In a typical week, how much attention did you pay to making sure you ate healthy proteins and fats?”) on a Likert scale from 1 (Not at all) to 5 (A lot). The nutrition attention items demonstrated strong internal consistency (Cronbach’s alpha .85) and thus were summed to produce a total attention to nutrition score, where higher values indicated greater attention. To assess criterion validity, we examined the association of attention to nutrition with eating habits. Total attention to nutrition was positively correlated with healthy factor scores (r = .57, p < .001), eating all meals (breakfast: r = .38, p < .001; lunch: r = .18, p = .015; dinner: r = .17, p = .02), fruits (r = .58, p < .001), pre-made/low calorie meals (r = .22, p = .003), vegetables (r = .46, p < .001), and salad (r = .40, p < .001), and negatively correlated with unhealthy factor scores (r = −.39, p < .001), eating fast food (r = −.23, p = .002), candy and cookies (r = −.27, p < .001), frozen food meals (r = −.24, p = .001), chips and salty snacks (r = −.35, p < .001), and drinking caffeinated soda (r = −.25, p = .001).
Table 1.
Factor loadings for the nutrition and hydration items.
| Item | Healthy | Unhealthy |
|---|---|---|
| Vegetables | 0.64 | −0.20 |
| Fruit | 0.61 | −0.24 |
| Breakfast | 0.50 | 0.00 |
| Salad | 0.50 | −0.08 |
| Water | 0.43 | −0.22 |
| Dinner | 0.42 | 0.07 |
| Other caffeinated beverages | 0.40 | −0.02 |
| Fast food | −0.19 | 0.61 |
| Candy/cookies | 0.02 | 0.58 |
| Chips or salty snacks | 0.03 | 0.56 |
| Caffeinated soda | −0.08 | 0.50 |
| Energy drinks | 0.09 | 0.44 |
| Frozen food meals | −0.17 | 0.42 |
| Lunch | 0.21 | 0.00 |
| Premade low calorie or diet | 0.17 | 0.16 |
| Sports drinks | −0.11 | 0.33 |
| Proportion of variance accounted for by each factor | 19.93 | 13.40 |
Bolded items loaded significantly onto the factor.
Energy and sleeping behaviors
Six items were adapted from the Profile of Mood States (POMS) Short Form to measure overall energy levels.20 Specifically, items from the vigor-activity and the fatigue-inertia subscales were used. These subscales have previously demonstrated good internal consistency.21 In this study, a factor analysis confirmed a two-factor structure that distinguished the fatigue and vigor items. Cronbach’s alpha was .65 for the fatigue items and .71 for the vigor items, possibly due to the low number of items in each subscale22 and the use of select items rather than the complete POMS. We selected specific items rather than the complete scale to limit the length of the overall survey, and due to the specific goals of the parent study from which our data were drawn. Each item was asked on a 5-point Likert scale ranging from 1 (Not at all) to 5 (Extremely). We reverse coded all items such that higher scores indicated more energy, and calculated a total energy score. Athletes also reported the typical number of hours of sleep they received each night over the past month.
Substance use behaviors
Alcohol and drug use items were adapted from the Youth Risk Behavior Survey (YRBS) by the Centers for Disease Control and Prevention (CDC).23 Two items were used to evaluate alcohol consumption and heavy drinking. Specifically, “In the past 30 days, how many days did you have at least one drink of alcohol?” to measure frequency of alcohol consumption, and “In the past 30 days, how many days did you have 5 (males)/4 (females) or more drinks of alcohol within a couple of hours?” to measure binge drinking.24 The 2 items assessing alcohol use were combined to create an alcohol composite score, where a higher score indicated heavier usage. The Rutgers Alcohol Problem Index (RAPI), a reliable and valid self-report survey,25,26 was used to measure alcohol problems. The 22-item questionnaire was modified to have a two-choice response selection to indicate the frequency of alcohol-related problems26 (1 = 0 times, 2 = 1 or more times) with Cronbach’s alpha of .74. The majority of collegiate athletes (81%) reported no problems with alcohol. Therefore, total RAPI score was dichotomized to distinguish those who reported (1) from those who did not report (0) alcohol-related problems.
A single item assessed whether participants had ever tried any of the following drugs: marijuana, cocaine, heroin, party drugs (eg, ecstasy or molly), diuretics, peptide/growth hormones, blood doping agents, anabolic steroids, or nutritional supplements with banned substances. As most collegiate athletes reported no drug use, a composite drug score was created where no lifetime drug usage was coded as 0, trying marijuana only was coded as 1, and trying other drugs or polydrug use including marijuana was coded as 2.
Aggressive behaviors
Aggressive behaviors were assessed using the 12-item Short-Form Buss–Perry Aggression Questionnaire (BPAQ), which consists of 4 subscales measuring hostility, anger, and physical and verbal aggression.27 Respondents rated items on a Likert scale from 1 (Extremely unlike me) to 6 (Extremely like me). Previous research has reported good reliability and validity of the BPAQ.28 This questionnaire had good internal consistency (Cronbach’s alpha = .89) and thus the total BPAQ score was used as an indicator of overall aggressive behaviors. Higher scores indicated more aggressive behaviors.
Sexual behaviors
Two items from the YRBS were included to assess how many times an individual regretted a sexual decision (A) while intoxicated and (B) not intoxicated.23 Response options ranged from 1 (Never) to 4 (More than 5 times). The majority of the sample (68%) reported no occasions of sexual regret with or without intoxication. Therefore, a dichotomous variable indicating whether a respondent had ever experienced sexual regret was created based on endorsing having ever experienced either.
Mental health outcome measures.
The Brief Symptom Inventory-18 (BSI-18) assessed severity of psychological distress, including depression, anxiety, and somatization, on a scale from 1 (Not at all) to 5 (Extremely).29 The BSI-18 has been reported to have good reliability and validity for collegiate athletes30 and showed good internal consistency in this sample (Cronbach’s alpha = .82). We calculated total scores such that higher scores indicated more overall symptoms of distress. The 4-item Perceived Stress Scale (PSS) assessed perceived stress, with responses from 1 (Never) to 5 (Very often).31 Two items were reverse coded per the scoring key. Although the Cronbach’s alpha in this sample was low (.55), a total score was nonetheless computed based on the strong psychometrics of the longer PSS from which the 4 items were drawn.31 Alternatively, the low alpha scores may be related to the specific sample and/or demand characteristics of this sample.
Data analysis
Differences between males and females in health behaviors and mental health outcome measures were compared with independent t-tests, chi-square tests, and Fisher’s exact test where appropriate. Analyses were performed using SPSS version 24.0. Partial least squares (PLS) was then used to assess the relationship between all health behaviors and mental health outcomes on the whole sample and split by males and females. PLS is a multivariate technique for relating two sets of variables to each other.32-35 Its data-driven approach requires no prior assumptions about the relationship between variables. As all comparisons are entered in one step, there is no need to correct for multiple comparisons.32 Singular value decomposition was performed on the covariance of the health behaviors and BSI/stress outcome variables to produce orthogonal dimensions or latent variables (LVs). These LVs represent the similarities and differences in covariance of the two sets of measures. The significance of LVs was computed by 1,500 permutation tests where the health behaviors were randomly reordered creating a sampling distribution to determine the probability of LVs occurring by chance.36 An LV was considered statistically significant when the probability of the singular value was less than .05.
Each independent variable is associated with a weight or “salience” on the LV (similar to a factor loading). To determine the stability of each variable’s contribution to the LV, salience-to-standard error, or bootstrap ratios (BSRs), were calculated with bootstrap estimation in which the observations of health behaviors and mental health outcome variables were resampled 500 times with replacement.37 BSRs are interpreted similarly to Z-scores such that when a BSR is larger than 2 it corresponds to a 95% confidence limit (p < .05) and is considered stable. PLS was performed with a PLS software package27 in MATLAB.
Results
Mean scores and proportions for all measures are reported in Tables 2 and 3. There were no significant differences in mental health outcomes (BSI-18 and PSS total scores) between males and females (ps > .05). Females had higher healthy factor scores (t(181) = 6.70, p < .001, Cohen’s d = 1.0) and total attention to nutrition scores (t(181) = 2.91, p = .004, Cohen’s d = .44) relative to males. Males reported more hours of sleep compared to females (t(181) = 3.03, p = .003, Cohen’s d = .45). Overall, the percentage of males and females who reported consuming alcohol was similar (86% and 77%, respectively; χ2(1) = 2.43, p = .12). However, males and females had a different pattern of alcohol use (χ2(3) = 9.80, p = .02; Table 3) with more males than females indicating that they have drank alcohol before but not recently. Due to low expected frequencies, we used Fisher’s exact test to compare drug composite scores. There was a significant difference in drug usage between males and females (p = .049), with males reporting more drug use than females. Levene’s test indicated a violation of homogeneity of variance for aggressive behavior scores only; corrected test statistics are reported. Males endorsed more aggressive behaviors (t(166.25) = 5.29, p < .001, Cohen’s d = .77) than females. There were no significant differences between males and females in unhealthy factor scores, energy levels, alcohol-related problems, or sexual regret.
Table 2.
Descriptive statistics of health behaviors for males and females.
| Male (n = 100) | Female (n = 83) | p-valuea | |
|---|---|---|---|
| Healthy factor scores | −0.35 (0.77) | 0.42 (0.77) | 0.000 |
| Unhealthy factor scores | 0.03 (0.88) | −0.04 (0.81) | 0.548 |
| Nutrition attention | 13.63 (5.05) | 15.70 (4.43) | 0.004 |
| Energy | 21.21 (2.86) | 21.19 (3.26) | 0.970 |
| Hours of sleep | 7.61 (1.27) | 7.06 (1.16) | 0.003 |
| BPAQ total | 21.53 (8.72) | 16.00 (5.26) | 0.000 |
| PSS total | 8.18 (2.38) | 8.41 (2.54) | 0.529 |
| BSI-18 total | 20.64 (3.98) | 21.13 (4.62) | 0.440 |
BPAQ: Buss–Perry Aggression Questionnaire; PSS: Perceived Stress Scale; BSI-18: Brief Symptom Inventory-18.
Mean scores with SD in parentheses.
Bolded values indicate p < .05 based on independent samples t-test
Table 3.
Frequencies of health behaviors in males and females.
| Male (n = 100) | Female (n = 83) | |
|---|---|---|
| Regret about Sexual Decisions | ||
| No | 62 (62.0%) | 62 (74.7%) |
| Yes | 38 (38.0%) | 21 (25.3%) |
| Alcohol | ||
| Don't drink | 14 (14.0%) | 19 (22.9%) |
| No recent drinking | 16 (16.0%) | 3 (3.6%) |
| Recent drinking, no recent binging | 26 (26.0%) | 28 (33.7%) |
| Recent binging | 44 (44.0%) | 33 (39.8%) |
| RAPI | ||
| No | 81 (81.0%) | 68 (81.9%) |
| Yes | 19 (19.0%) | 15 (18.1%) |
| Drugs | ||
| No drugs | 62 (62.0%) | 65 (78.3%) |
| Marijuana only | 31 (31.0%) | 16 (19.3%) |
| Marijuana & other drugs | 7 (7.0%) | 2 (2.4%) |
RAPI: Rutgers Alcohol Problem Index.
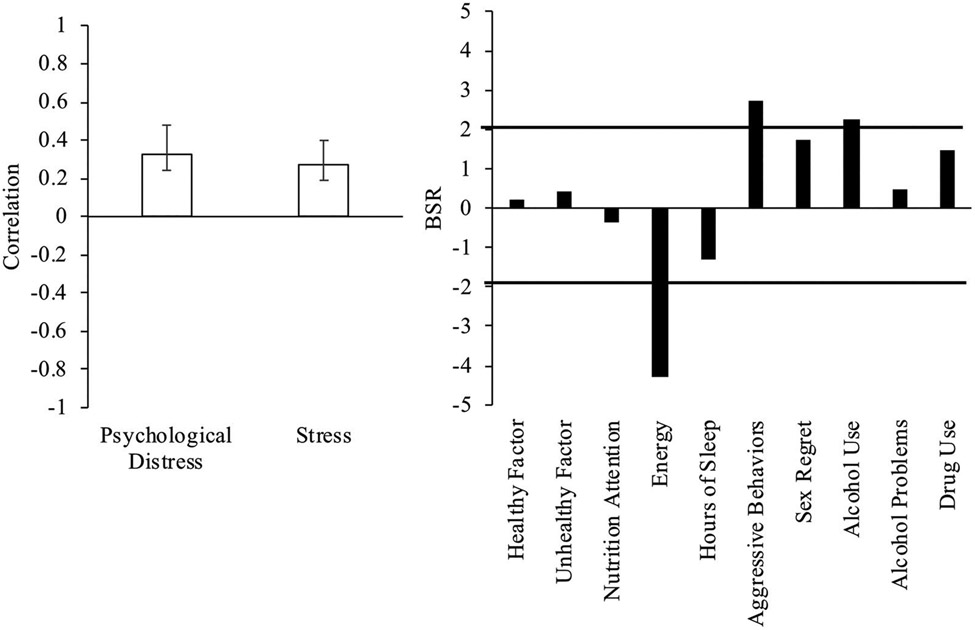
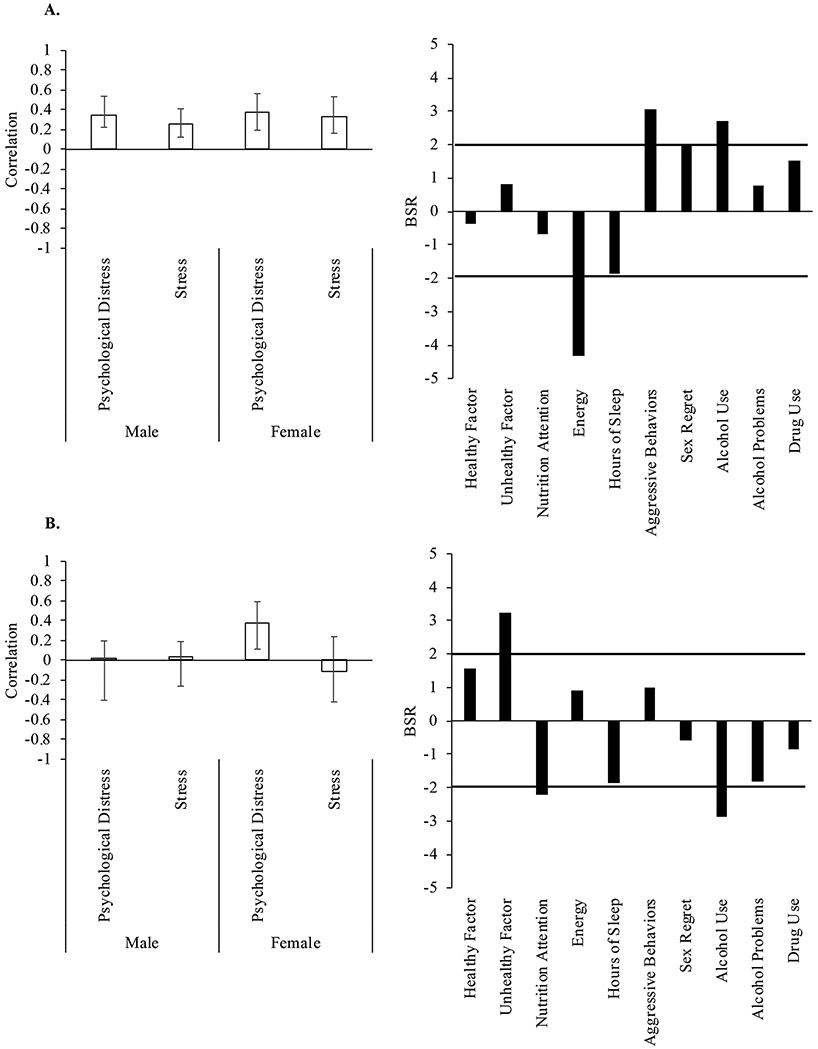
We assessed the relationship between health behaviors and mental health variables across the entire sample. One significant LV emerged (p < .001) accounting for 88.2% of the cross-block variance. Increased psychological distress and stress symptoms were associated with increased aggressive behaviors and alcohol use, and reduced energy across the whole sample of participants (Figure 1). We next examined the relationship between health behaviors and mental health variables in males and females separately. There were two significant LVs. The first LV (p < .001, accounting for 63.9% of cross-block variance) showed a similar pattern, where higher psychological distress and stress symptoms were associated with greater aggressive behaviors and more frequent alcohol use, and reduced energy for both males and females (Figure 2(A)). The second LV (p = .002, 24.3% of cross-block variance) demonstrated that higher psychological distress was associated with more unhealthy dietary habits, reduced attention to nutrition, and less frequent alcohol use in female athletes (Figure 2(B)). The effect was not significant for stress symptoms or in male athletes.
Figure 1.
Health behaviors and mental health outcomes in the overall sample. A significant latent variable (LV) from the PLS analysis of the whole sample indicated a significant relationship between energy, aggressive behaviors, and alcohol use and mental health outcomes. Psychological distress and stress were measured using the Brief Symptom Inventory (BSI) and Perceived Stress Scale (PSS), respectively. Left panel. BSI and PSS correlations. Error bars represent 95% confidence intervals. Right panel. Bootstrap ratios (BSR; analogous to a Z-score) for health behaviors; a BSR threshold of 2 corresponds to a p-value of p < .05, significance is represented by the solid line. Positive values indicate a positive correlation with psychological distress and stress scores; negative values indicate a negative correlation.
Figure 2.
Health behaviors and mental health outcomes in male and female athletes. (A) A significant latent variable (LV) from the PLS analysis separated by males and females indicated a significant relationship between energy, aggressive behaviors, and alcohol use and mental health symptoms for both males and females. (B) A second significant LV indicated a relationship between unhealthy dietary habits, nutrition attention, and alcohol health behaviors and psychological distress for females only. Left panels: Brief Symptom Inventory (BSI) and Perceived Stress Scale (PSS) correlations. Error bars represent 95% confidence intervals. Right panels: Bootstrap ratios (BSR) for health behaviors; a BSR threshold of 2 corresponds to a p-value of p < .05, significance is represented by the solid line. Positive values indicate a positive correlation with psychological distress and stress scores; negative values indicate a negative correlation.
Comment
Collegiate athletes, like their nonathlete peers, face a myriad of mental health problems9; however, athletes may be more hesitant to report these problems because of a perception that psychological issues signify weakness.10,11 Therefore, athletic trainers and other sports medicine personnel need new tools to identify mental health problems that do not rely on self-report of mental health symptoms. This study provides preliminary support that examining health behaviors may serve as such a tool. To our knowledge, this is the only study to examine the relationship of multiple health behaviors to mental health outcomes in a sample of incoming collegiate athletes.
Female athletes in our sample reported more healthy dietary habits, attention to nutrition, and recent drinking, whereas males reported more sleep, drug use, and aggressive behaviors. There were no differences between males and females in either mental health assessment nor in the link between health behaviors and mental health. In both male and female athletes, aggressive behaviors, alcohol use, and fatigue were associated with increased stress and psychological distress. In addition, a pattern of unhealthy dietary habits, reduced attention to nutrition, and less frequent alcohol use was related to psychological symptoms only in female athletes.
The positive association of aggressive behaviors, alcohol use, and fatigue with increased stress and psychological distress are supported by studies assessing these factors individually. Aggressive behaviors have been shown to predict depression and anxiety and be positively related to alcohol use in athletes.16,38 Increased alcohol use has been associated with experiencing more psychiatric symptoms.39 Fatigue has not previously been associated with mental health outcomes in collegiate athletes; however, a recent study found that collegiate athletes report substantial levels of daytime tiredness that was correlated with poor sleep quality.15 Further, poor sleep quality was associated with stress and depression among college students.40,41 The present study suggests that collegiate athletes with more impulsive behaviors like aggressive acts and greater alcohol consumption, combined with irregular sleep schedules and subsequent fatigue may be at a heightened risk for less effective emotional-regulation. Further characterization of whether prospective assessment of these health behaviors could predict mental health problems is needed.
The identification of a female-specific link between health behaviors and mental health is also in keeping with differences between males and females in lifestyle and risk for chronic diseases.42 Overall, less frequent alcohol use, more unhealthy dietary habits, and reduced attention to nutrition were associated with higher symptoms of psychological distress. The link between eating behaviors and mental health is consistent with the association of binge eating to negative feelings such as anger, depression, and feeling down in female collegiate athletes,43 and with female athletes tending to report greater body and body weight dissatisfaction and more body image related anxiety than males.14,43 It is also supported by evidence that female collegiate athletes receive more nutrition information44 and, in turn, have better nutrition knowledge than their male counterparts.45 It may be that healthy eating behaviors and attention to these behaviors could serve as indicators of positive self-perception, better overall health, and reduced symptoms of depression and anxiety in women. This observation warrants follow-up studies that specifically track whether eating behaviors could serve as an objective measure of mental health functioning in female athletes.
The observation that more frequent alcohol consumption in female athletes was associated with fewer psychological symptoms was unexpected. A tentative hypothesis is that social connectedness, or the sense of belonging to a social group, may mediate the association between alcohol use and psychological distress as collegiate athletes who report higher social connectedness tend to experience fewer symptoms of depression.40 Although not specifically tested in athletes, most studies on college students show that social motives for drinking are a significant predictor of alcohol consumption.46,47 Importantly, the results suggest better psychological functioning among female athletes who drink more often, but not who binge drink more often or experience alcohol-related problems. Thus, it may be that frequent, but not heavy drinking provides female athletes with a way to fit in with their peers, increasing feelings of belonging, and decreasing their psychological distress; however, a more nuanced approach to measuring alcohol use that considers both quantity and frequency, motivations and reasons for drinking, and expectancies from drinking is needed to clarify drinking behaviors and their relation to mental and physical health.
Taken together, these findings have implications for sports medicine personnel. Athletic trainers and other sports medicine personnel are responsible for athletes’ overall health and well-being, but they often lack training to identify mental health symptoms. However, their regular interactions with athletes put sports medicine personnel in the best position to recognize problematic behaviors. Health behaviors are easily observed and quantified, and perceived to be more socially acceptable to admit than symptoms of depression and anxiety. This study has identified patterns of health behaviors that may serve as proxies for mental health concerns. Future studies should track health behaviors over time to better understand their association with symptoms of psychological distress and stress.
Limitations
The current findings emphasize the importance of considering multiple health behaviors and using multivariate techniques to assess the interactive effect of these behaviors on mental health. The study, however, should be considered in light of its limitations. Participants were collegiate athletes from a single northeastern US university; different health behavior patterns may emerge in other university settings. Further, data were from a self-report survey that was completed prior to athletes’ official entry into the athletic program. Even though the athletes completed the survey at home, the veracity of their responses may have been affected by social desirability and concerns about potential consequences to endorsing certain behaviors. Moreover, due to time and resource restrictions, some questionnaires (eg, POMS) were not included in their entirety within the overall survey. Instead, specific items were selected that targeted health behaviors of interest. Finally, as this was a cross-sectional study, we are limited in being able to assess causal relationships. However, these findings provide a basis for future studies to follow behaviors over the course of a collegiate athlete’s career to examine whether they predict mental health outcomes.
Conclusions
This preliminary study sought to underscore the reciprocal connection between the body and mind. It suggests that measuring health behaviors, such as diet, sleep, exercise, and alcohol use, may circumvent the need to directly measure mental health symptoms, which many athletes seem hesitant to do.12 Collegiate athletes are a unique subpopulation in the college environment. The college experience of these young adults may be distinct from their nonathlete peers due to the extensive time commitments of sport participation. This may not only impact their social and academic schedules, but also their ability to access university health care centers. Collegiate athletes with mental health concerns often go unseen, undiagnosed, and untreated. The results of this study lay the ground work for developing novel early intervention approaches to identify potential mental health problems in this population by leveraging the skills and knowledge bases of sports medicine physicians, athletic training staff, and other sport-based professionals that routinely interact with collegiate athletes. If replicated, these findings support further study into the use of health behaviors as proxies for psychological wellness.
Funding
Funding for this study was provided by the New Jersey Commission on Brain Injury Research (CBIR13IRG028) and the National Institute on Alcohol Abuse and Alcoholism (K02AA025123).
Footnotes
Conflict of interest disclosure
The authors have no conflicts of interest to report. The authors confirm that the research presented in this article met the ethical guidelines, including adherence to the legal requirements, of the United States and received approval from the Institutional Review Board of Rutgers The State University of New Jersey.
References
- 1.Simpson NS, Gibbs EL, Matheson GO. Optimizing sleep to maximize performance: implications and recommendations for elite athletes. Scand J Med Sci Sports. 2017;27(3):266–274. doi: 10.1111/sms.12703. [DOI] [PubMed] [Google Scholar]
- 2.Beck K, Thomson JS, Swift RJ, et al. Role of nutrition in performance enhancement and postexercise recovery. Open Access J Sports Med. 2015;6:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vella LD, Cameron-Smith D. Alcohol, athletic performance and recovery. Nutrients. 2010;2(8):781–789. doi: 10.3390/nu2080781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halfon N, Verhoef PA, Kuo AA. Childhood antecedents to adult cardiovascular disease. Pediatr Rev. 2012;33(2):51–61. doi: 10.1542/pir.33-2-51. [DOI] [PubMed] [Google Scholar]
- 5.Dale H, Brassington L, King K. The impact of healthy lifestyle interventions on mental health and wellbeing: a systematic review. Mental Health Rev J. 2014;19(1):1–25. doi: 10.1108/MHRJ-05-2013-0016. [DOI] [Google Scholar]
- 6.Wilson G, Pritchard M. Comparing sources of stress in college student athletes and non-athletes. Athl Insight. 2005;7(1):1–8. [Google Scholar]
- 7.Martens MP, Dams-O’Connor K, Beck NC. A systematic review of college student-athlete drinking: prevalence rates, sport-related factors, and interventions. J Subst Abuse Treat. 2006;31(3):305–316. doi: 10.1016/j.jsat.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Hunt J, Eisenberg D. Mental health problems and help-seeking behavior among college students. J Adolesc Health. 2010;46(1):3–10. doi: 10.1016/j.jadohealth.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Wolanin A, Gross M, Hong E. Depression in athletes: prevalence and risk factors. Curr Sports Med Rep. 2015;14(1):56–60. doi: 10.1249/JSR.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 10.Gulliver A, Griffiths KM, Christensen H. Barriers and facilitators to mental health help-seeking for young elite athletes: a qualitative study. BMC Psychiatry. 2012;12(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown GT, Hainline B, Kroshus E, et al. Mind, Body and Sport: Understanding and Supporting Student-Athlete Mental Wellness. Indianapolis, IN: NCAA; 2014. doi: 10.1123/jcsp.2018-0082. [DOI] [Google Scholar]
- 12.Watson JC. College student-athletes’ attitudes toward help-seeking behavior and expectations of counseling services. J Coll Stud Dev. 2005;46(4):442–449. doi: 10.1353/csd.2005.0044. [DOI] [Google Scholar]
- 13.Moreland JJ, Coxe KA, Yang J. Collegiate athletes’ mental health services utilization: a systematic review of conceptualizations, operationalizations, facilitators, and barriers. J Sport Health Sci. 2018;7(1):58–69. doi: 10.1016/j.jshs.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pritchard ME, Milligan B, Elgin J, et al. Comparisons of risky health behaviors between male and female college athletes and non-athletes. Athl Insight. 2007;9(1):1–11. [Google Scholar]
- 15.Mah CD, Kezirian EJ, Marcello BM, et al. Poor sleep quality and insufficient sleep of a collegiate student-athlete population. Sleep Health. 2018;4(3):251–257. doi: 10.1016/j.sleh.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Nixon HL. Gender, sport, and aggressive behavior outside sport. J Sport Soc Iss. 1997;21(4):379–391. doi: 10.1177/019372397021004005. [DOI] [Google Scholar]
- 17.Yusko DA, Buckman JF, White HR, et al. Alcohol, tobacco, illicit drugs, and performance enhancers: a comparison of use by college student athletes and nonathletes. J Am Coll Health. 2008;57(3):281–290. doi: 10.3200/JACH.57.3.281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diehl KA, Thiel AB, Zipfel SC, et al. How healthy is the behavior of young athletes? A systematic literature review and meta-analyses. J Sports Sci Med. 2012;11(2):201–220. [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens JP. Applied Multivariate Statistics for the Social Sciences. 5th ed. New York, NY: Routledge; 2009. [Google Scholar]
- 20.Shacham S. A shortened version of the Profile of Mood States. J Person Assess. 1983;47(3):305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- 21.Curran SL, Andrykowski MA, Studts JL. Short form of the Profile of Mood States (POMS-SF): psychometric information. Psychol Assess. 1995;7(1):80–83. doi: 10.1037/1040-3590.7.1.80. [DOI] [Google Scholar]
- 22.Clark LA, Watson D. Constructing validity: basic issues in objective scale development. Psychol Assess. 1995;7(3):309–319. doi: 10.1037/1040-3590.7.3.309. [DOI] [Google Scholar]
- 23.Brener ND, Kann L, Kinchen SA. Methodology of the youth risk behavior surveillance system. MMWR Recomm Rep. 2004;53(RR-12):1–13. [PubMed] [Google Scholar]
- 24.National Institute of Alcohol Abuse and Alcoholism. NIAAA Council Approves Definition of Binge Drinking. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2004. NIAAA Newsletter - Vol. 3, p. 3. [Google Scholar]
- 25.White HR, Labouvie EW. Towards the assessment of adolescent problem drinking. J Stud Alcohol. 1989;50(1):30–37. doi: 10.15288/jsa.1989.50.30. [DOI] [PubMed] [Google Scholar]
- 26.Martens MP, Neighbors C, Dams-O’Connor K, et al. The factor structure of a dichotomously scored Rutgers Alcohol Problem Index. J Stud Alcohol Drugs. 2007;68(4):597–606. doi: 10.15288/jsad.2007.68.597. [DOI] [PubMed] [Google Scholar]
- 27.Bryant FB, Smith BD. Refining the architecture of aggression: a measurement model for the Buss–Perry Aggression Questionnaire. J Res Person. 2001;35(2):138–167. doi: 10.1006/jrpe.2000.2302. [DOI] [Google Scholar]
- 28.Kalmoe NP. Trait aggression in two representative U.S. surveys: testing the generalizability of college samples. Aggr Behav. 2015;41(2):171–188. doi: 10.1002/ab.21547. [DOI] [PubMed] [Google Scholar]
- 29.Derogatis L. Brief Symptom Inventory 18 (BSI-18): Administration, Scoring, and Procedures Manual. Vol. 1416. Bloomington, MN: Pearson; 2001. [Google Scholar]
- 30.Lancaster MA, McCrea MA, Nelson LD. Psychometric properties and normative data for the Brief Symptom Inventory-18 (BSI-18) in high school and collegiate athletes. Clin Neuropsychol. 2016;30(2):338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- 32.McIntosh AR, Lobaugh NJ. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 2004;23(Suppl. 1):S250–S263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 33.McKinnon MC, Palombo DJ, Nazarov A, et al. Threat of death and autobiographical memory: a study of passengers from Flight AT236. Clin Psychol Sci. 2015;3(4):487–502. doi: 10.1177/2167702614542280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esopenko C, Levine B. Autobiographical memory and structural brain changes in chronic phase TBI. Cortex. 2017;89:1–10. doi: 10.1016/j.cortex.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Palombo DJ, McKinnon MC, McIntosh AR, et al. The neural correlates of memory for a life-threatening event: an fMRI study of passengers from flight AT236. Clin Psychol Sci. 2016;4(2):312–319. doi: 10.1177/2167702615589308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovacevic N, Abdi H, Beaton D, et al. Revisiting PLS resampling: comparing significance versus reliability across range of simulations. In: Abdi H, Chin W, Esposito Vinzi V, et al. , eds. New Perspectives in Partial Least Squares and Related Methods. Springer Proceedings in Mathematics and Statistics. Vol. 56. New York, NY: Springer; 2013:159–170. [Google Scholar]
- 37.Sampson PD, Streissguth AP, Barr HM, et al. Neurobehavioral effects of prenatal alcohol: part II. Partial least squares analysis. Neurotoxicol Teratol. 1989;11(5):477–491. doi: 10.1016/0892-0362(89)90025-1. [DOI] [PubMed] [Google Scholar]
- 38.O’Brien KS, Kolt GS, Martens MP, et al. Alcohol-related aggression and antisocial behaviour in sportspeople/athletes. J Sci Med Sport. 2012;15(4):292–297. doi: 10.1016/j.jsams.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Miller BE, Miller MN, Verhegge R, et al. Alcohol misuse among college athletes: self-medication for psychiatric symptoms? J Drug Educ. 2002;32(1):41–52. doi: 10.2190/JDFM-AVAK-G9FV-0MYY. [DOI] [PubMed] [Google Scholar]
- 40.Armstrong S, Oomen-Early J. Social connectedness, self-esteem, and depression symptomatology among collegiate athletes versus nonathletes. J Am Coll Health. 2009;57(5):521–526. doi: 10.3200/JACH.57.5.521-526. [DOI] [PubMed] [Google Scholar]
- 41.Lund HG, Reider BD, Whiting AB, et al. Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Health. 2010;46(2):124–132. doi: 10.1016/j.jadohealth.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 42.Oksuzyan A, Gumà J, Doblhammer G. Sex differences in health and survival. In: Doblhammer G, Gumà J, eds. A Demographic Perspective on Gender, Family and Health in Europe. Cham: Springer; 2018:65–100. [Google Scholar]
- 43.Selby R, Weinstein HM, Bird TS. The health of university athletes: attitudes, behaviors, and stressors. J Am Coll Health. 1990;39(1):11–18. doi: 10.1080/07448481.1990.9936208. [DOI] [PubMed] [Google Scholar]
- 44.Jacobson BH, Sobonya C, Ransone J. Nutrition practices and knowledge of college varsity athletes: a follow-up. J Strength Cond Res. 2001;15(1):63–68. doi: 10.1519/00124278-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Heaney S, O’Connor H, Michael S, et al. Nutrition knowledge in athletes: a systematic review. Int J Sport Nutr Exerc Metab. 2011;21(3):248–261. doi: 10.1123/ijsnem.21.3.248. [DOI] [PubMed] [Google Scholar]
- 46.Ham LS, Hope DA. College students and problematic drinking: a review of the literature. Clin Psychol Rev. 2003;23(5):719–759. doi: 10.1016/S0272-7358(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 47.LaBrie JW, Hummer JF, Pedersen ER. Reasons for drinking in the college student context: the differential role and risk of the social motivator. J Stud Alcohol Drugs. 2007;68(3):393–398. doi: 10.15288/jsad.2007.68.393. [DOI] [PMC free article] [PubMed] [Google Scholar]