Abstract
Introduction
The phase III IMbrave150 study established atezolizumab + bevacizumab as standard of care in patients with unresectable hepatocellular carcinoma (HCC). This exploratory analysis reports efficacy and safety results in patients with baseline Barcelona Clinic Liver Cancer (BCLC) stage B disease.
Methods
Patients with systemic treatment-naive unresectable HCC and Child-Pugh class A liver function were randomized 2:1 to receive 1,200 mg of atezolizumab plus 15 mg/kg of bevacizumab or 400 mg of sorafenib. Co-primary endpoints were overall survival (OS) and progression-free survival (PFS) per independent review facility (IRF)-assessed Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 in the BCLC stage B subgroup. Patients in this analysis had BCLC stage B disease at baseline per electronic case report form. Secondary efficacy endpoints included the objective response rate (ORR) and change in the sum of longest diameters (SLD) of target lesions from baseline per IRF RECIST 1.1 and modified RECIST (mRECIST) for HCC.
Results
Of 501 enrolled patients, 74 (15%) had BCLC stage B disease at baseline (atezolizumab + bevacizumab, n = 49; sorafenib, n = 24). For this group, median follow-up was 19.7 months. A trend toward improved OS and PFS per IRF RECIST 1.1 was observed with atezolizumab + bevacizumab versus sorafenib (OS: hazard ratio [HR]: 0.63; 95% confidence interval [CI]: 0.29, 1.34; PFS: HR: 0.64; 95% CI: 0.36, 1.12). ORRs per IRF RECIST 1.1 and HCC mRECIST were 43% and 50% with atezolizumab + bevacizumab and 26% and 30% with sorafenib, respectively. Percentage change in SLD of target lesions from baseline per IRF RECIST 1.1 and HCC mRECIST showed durable responses with atezolizumab + bevacizumab treatment. Safety data were consistent with known profiles of atezolizumab and bevacizumab, as seen in the overall study population.
Discussion/Conclusion
Efficacy benefits were observed with atezolizumab + bevacizumab in patients with baseline BCLC stage B disease, consistent with the intention-to-treat population.
Keywords: Barcelona Clinic Liver Cancer stage B, Atezolizumab, Bevacizumab, Unresectable hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) comprises 75–85% of primary liver cancers and represents a major global health problem [1]. Clinical decision-making and treatment allocation in HCC is typically performed using several established staging systems.
The Barcelona Clinic Liver Cancer (BCLC) staging system is one of the most widely used and is endorsed by both the European Association for Study of the Liver (EASL) and the American Association for the Study of Liver Disease (AASLD) for treatment allocation and prognostic classification of HCC [2]. The BCLC system classifies patients with HCC as very early (stage 0), early (stage A), intermediate (stage B), advanced (stage C), or terminal (stage D), based on tumor size and number, Eastern Cooperative Oncology Group (ECOG) performance status (PS), and Child-Pugh (CP) liver function [3].
The majority of patients included in clinical trials evaluating new treatments for unresectable HCC typically have intermediate (BCLC stage B) or advanced (stage C) HCC at baseline. Patients with intermediate BCLC stage B HCC have ECOG PS 0, preserved liver function (CP class A or B), multinodular and unresectable HCC without macrovascular invasion (MVI), or extrahepatic spread (EHS) [3]. Patients with advanced BCLC stage C disease also have preserved liver function (CP class A or B) and additionally present with either cancer-related symptoms (ECOG PS 1–2), MVI, or EHS [3].
BCLC stage B includes a heterogenous population of patients who have diverse liver function and can include patients with as few as 4 tumors to those with over 20 bilateral tumors, resulting in a wide range of survival outcomes [4]. Several strategies have been proposed to further categorize these heterogenous patients based on liver function and tumor burden, including the Bolondi system that proposed 4 substages (B1–B4) of BCLC stage B based on liver function (using CP scores) and tumor status (using the “beyond Milan” and “up-to-7” criteria) [5] and the Kinki criteria, a simplified modification of the Bolondi classification [6].
From 2007, until the approval of atezolizumab + bevacizumab in 2020, sorafenib was the standard of care and only first-line (1 L) therapeutic option for advanced unresectable HCC after demonstrating improved overall survival (OS) versus placebo [7]. Recent advances have resulted in the availability of multiple systematic treatment options for HCC, including 1 L lenvatinib and the combination of atezolizumab and bevacizumab (atezolizumab + bevacizumab), as well as second-line regorafenib, cabozantinib, ramucirumab, pembrolizumab, and nivolumab plus ipilimumab [8].
Of these treatments, the combination of atezolizumab, an anti-programmed death-ligand 1 monoclonal antibody, and bevacizumab, an anti-vascular endothelial growth factor (VEGF) monoclonal antibody, has emerged as the new standard of care for 1 L treatment of patients with unresectable HCC [8]. In the phase III IMbrave150 study (NCT03434379), atezolizumab + bevacizumab treatment resulted in statistically significant and clinically meaningful improvement in OS and progression-free survival (PFS) versus sorafenib after a median 8.6 months of follow-up [9].
These data supported the approval of atezolizumab + bevacizumab in over 85 countries for the treatment of patients with unresectable HCC who have not received prior systemic therapy [10, 11]. An updated analysis of IMbrave150 (median follow-up duration, 15.6 months) demonstrated maintenance of the clinically meaningful treatment benefit with atezolizumab + bevacizumab (median OS, 19.2 months) versus sorafenib (median OS, 13.4 months) [12]. At the time, this was the longest median OS reported in a phase III study of 1 L systemic therapy for unresectable HCC.
Recent EASL, AASLD, and Japan Society of Hepatology guidelines recommend atezolizumab + bevacizumab as the preferred treatment option in systemic treatment-naive patients with HCC [8, 13, 14]. The latest 2020 AASLD consensus guidelines recommend transarterial chemoembolization (TACE) and 1 L atezolizumab + bevacizumab in patients with BCLC stage B; atezolizumab + bevacizumab is recommended as the treatment of choice in patients unsuitable for TACE [14]. The Asia-Pacific Primary Liver Cancer Expert consensus statements and Japan Society of Hepatology guidelines also recommend upfront systemic therapy followed by locoregional therapy (LRT) in patients with BCLC stage B who are unsuitable for TACE [14, 15]. Here, we report exploratory efficacy and safety results in a subset of patients from IMbrave150 with BCLC stage B disease at baseline to understand the treatment efficacy of atezolizumab + bevacizumab in this population.
Methods
Study Design and Participants
This exploratory subgroup analysis evaluated efficacy and safety outcomes in patients with BCLC stage B who enrolled in the global, multicenter, open-label, randomized phase III IMbrave150 study (ClinicalTrials.gov, NCT03434379). Details of study design, eligibility criteria, and outcome measures were previously reported [9, 12].
Briefly, patients who were ≥18 years of age, had locally advanced, metastatic and/or unresectable HCC, an ECOG PS status of 0 or 1, CP class A liver function, and had not previously received systemic therapy for liver cancer were eligible for inclusion [9]. Patients with a history of autoimmune disease, who had coinfection with hepatitis B and C viruses, or who had untreated or incompletely treated esophageal or gastric varices with bleeding or high risk of bleeding were excluded from the study.
Patients in this analysis had BCLC stage B disease, defined as no EHS or MVI and ECOG PS 0, at baseline per electronic case report form. Patients with ≥1 metastatic site at enrollment were excluded.
Patients were randomized in a 2:1 ratio to receive either 1,200 mg of atezolizumab plus 15 mg/kg of bevacizumab intravenously every 3 weeks or 400 mg of sorafenib orally twice daily. Treatment was given until loss of clinical benefit as determined by the investigator after an integrated assessment of radiographic and biochemical data and clinical status (e.g., symptomatic deterioration such as pain secondary to disease) or unacceptable toxicity [9]. If patients transiently or permanently discontinued either atezolizumab or bevacizumab because of an adverse event (AE), single-agent therapy was allowed if the patient was experiencing clinical benefit. Dose modifications were permitted in the sorafenib arm but not in the atezolizumab + bevacizumab arm.
Tumors were assessed by computed tomography or magnetic resonance imaging at baseline and every 6 weeks until week 54, then every 9 weeks thereafter. Safety was continuously evaluated by recording vital signs and clinical laboratory test results and assessing the incidence and severity of AEs according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 [9].
Outcomes
The co-primary endpoints were OS (defined as time from randomization to death from any cause) and PFS (defined as time from randomization to disease progression per independent review facility [IRF]-assessed Response Evaluation Criteria in Solid Tumours [RECIST] version 1.1 or death from any cause, whichever occurred first) [9]. Secondary efficacy endpoints were PFS per investigator-assessed RECIST 1.1, objective response rate (ORR, defined as the percentage of patients with a confirmed complete [CR] or partial response [PR]) and change in the sum of longest diameters (SLD) of target lesions from baseline per IRF-RECIST 1.1 and per IRF-assessed modified RECIST (mRECIST) for HCC (HCC mRECIST) [16]. Results from the primary and 12-month updated analyses of these efficacy endpoints were reported previously [9, 12].
Statistical Analysis
Details of the statistical methods used in the IMbrave150 study were previously described [9, 12]. Efficacy was assessed in all patients in the intention-to-treat (ITT) population who had BCLC stage B disease per electronic case report form. All hazard ratios (HRs) were unstratified. Safety was assessed in the safety-evaluable population, defined as all patients who received any study treatment.
Results
Patients
IMbrave150 enrolled 501 patients, with 336 patients randomized into the atezolizumab + bevacizumab arm and 165 patients into the sorafenib arm of the ITT population (online suppl. Fig. S1; for all online suppl. material, see www.karger.com/doi/10.1159/000528272). Of these patients, 74 (15%) had BCLC stage B disease at baseline (atezolizumab + bevacizumab, 49; sorafenib, 24). Patients with BCLC stage B HCC disease had a median age of 66 years (range, 37–87 years) in the atezolizumab + bevacizumab arm and 71 years (range, 34–83 years) in the sorafenib arm. Twenty-five (51%) patients in the atezolizumab + bevacizumab arm and 15 (63%) patients in the sorafenib arm had some prior LRT (Table 1).
Table 1.
Patient demographics in patients with BCLC stage B disease at baseline
| Atezolizumab + bevacizumab (n = 49) | Sorafenib (n = 24) | |
|---|---|---|
| Age | ||
| Median (range), years | 66 (37–87) | 71 (34–83) |
| ≥65 years, n (%) | 27 (55) | 18 (75) |
| Male, n (%) | 43 (88) | 19 (79) |
| Race, n (%) | ||
| Asian | 26 (53) | 16 (67) |
| White | 19 (39) | 6 (25) |
| Unknown | 4 (8) | 2 (8) |
| Geographic region, n (%)a | ||
| Asia, excluding Japan | 11 (22) | 5 (21) |
| Rest of the world, including Japanb | 38 (78) | 19 (79) |
| Weight, median (range), kg | 70.4 (37.3–112.9) | 68.0 (53.7–115.0) |
| ECOG PS 0, n (%)a | 49 (100) | 24 (100) |
| HCC etiology, n (%)c | ||
| Hepatitis B virus | 14 (29) | 6 (25) |
| Hepatitis C virus | 13 (27) | 7 (29) |
| Nonviral | 22 (45) | 11 (46) |
| CP class, n (%) | ||
| A5 | 34 (71) | 18 (75) |
| A6 | 14 (29) | 6 (25) |
| Baseline SLD in target lesions, median (range), mmd | 61.0 (12.6–222.0) | 45.5 (11.5–162.0) |
| AJCC stage | ||
| IB | 1 (2) | 0 |
| II | 25 (51) | 13 (54) |
| IIIA | 21 (43) | 11 (46) |
| IIIB | 1 (2) | 0 |
| IVA | 1 (2) | 0 |
| AFP ≥400 ng/mL, n (%)a | 8 (16) | 3 (13) |
| Varices, n (%) | ||
| Present | 15 (31) | 10 (42) |
| Treated | 3 (20) | 6 (60) |
| Prior LRT for HCC, n (%) | 25 (51) | 15 (63) |
| Type of prior LRT, n (%) | ||
| TACE | 21 (43) | 13 (54) |
| Radiofrequency ablation | 12 (24) | 4 (17) |
| Percutaneous ethanol injection | 5 (10) | 0 |
| Transarterial embolization | 2 (4) | 1 (4) |
| Other | 2 (4) | 3 (13) |
AFP, alpha-fetoprotein; AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; HCC, hepatocellular carcinoma; SLD, sum of longest diameters.
Per electronic case report form, not interactive voice/web response system.
Rest of the world includes the USA, Australia, and Japan.
For patients whose cause of HCC was multifactorial as assessed by the investigator, the viral cause was prioritized over nonviral causes to define the primary etiology of the patient.
Per investigator assessment.
Baseline characteristics were generally well-balanced between arms in patients with BCLC stage B disease. Compared with patients in the sorafenib arm, a lower percentage of patients with BCLC stage B disease in the atezolizumab + bevacizumab arm were ≥65 years of age (75% vs. 55%), Asian (67% vs. 53%), had varices (42% vs. 31%), had treated varices (60% vs. 20%), and had prior LRT for HCC (63% vs. 51%).
Efficacy
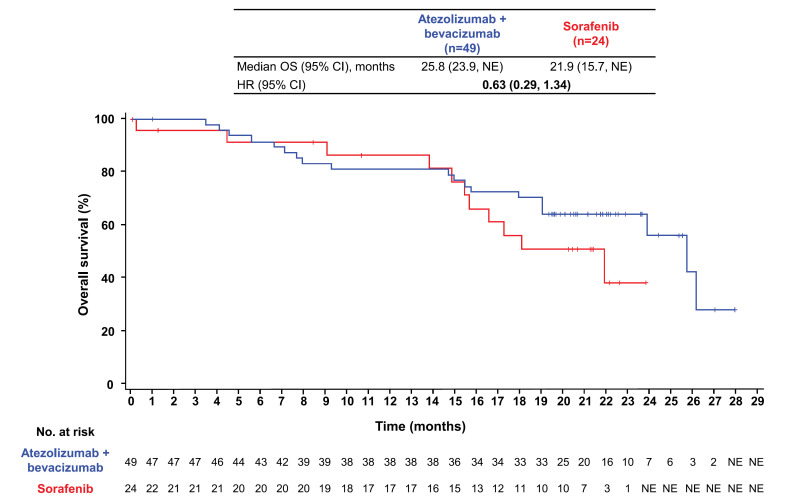
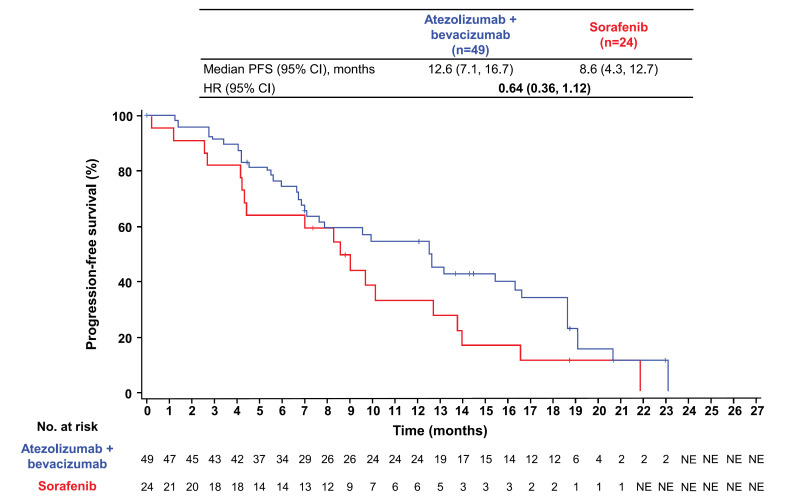
At the data cutoff of August 31, 2020, median follow-up duration in patients with BCLC stage B disease at baseline was 19.7 months (range, 0.0–28.0) overall, 20.1 months (range, 0.1–28.0) in the atezolizumab + bevacizumab arm, and 16.9 months (range, 0.0–23.9) in the sorafenib arm. Median OS in patients with BCLC stage B disease at baseline was 25.8 months (95% confidence interval [CI]: 23.9, not estimable) with atezolizumab + bevacizumab and 21.9 months (95% CI: 15.7, not estimable) with sorafenib (HR: 0.63; 95% CI: 0.29, 1.34; Figure 1). The atezolizumab + bevacizumab arm had a median PFS per IRF RECIST 1.1 of 12.6 months (95% CI: 7.1, 16.7) compared with 8.6 months (95% CI: 4.3, 12.7) in the sorafenib arm (HR: 0.64; 95% CI: 0.36, 1.12; Fig. 2). Median PFS per investigator-assessed RECIST 1.1 was 10.0 months (95% CI: 7.7, 14.3) in the atezolizumab + bevacizumab arm versus 4.2 months (95% CI: 2.8, 11.0) in the sorafenib arm (HR: 0.44; 95% CI: 0.25, 0.77; online suppl. Fig. S2).
Fig. 1.
Analysis of OS. Kaplan-Meier estimates of OS in the atezolizumab + bevacizumab and sorafenib arms in patients with BCLC stage B disease in the ITT population.
Fig. 2.
Analysis of PFS. Kaplan-Meier estimates of PFS per IRF-assessed RECIST 1.1 in the atezolizumab + bevacizumab and sorafenib arms in patients with BCLC stage B disease in the ITT population.
Confirmed ORR was 43% (n = 20) per IRF RECIST 1.1 and 50% (n = 23) per HCC mRECIST in patients receiving atezolizumab + bevacizumab (Table 2). CR was achieved in 5 patients (11%) per IRF RECIST 1.1 and 8 patients (17%) per HCC mRECIST. Progressive disease (PD) occurred in 2 patients (4%) per both IRF RECIST 1.1 and HCC mRECIST. Median time to first PR or CR was 4.1 months (95% CI: 1.3, 12.3) per IRF RECIST 1.1 and 2.8 months (95% CI: 1.2, 12.3) per HCC mRECIST.
Table 2.
Clinical response per IRF-assessed RECIST 1.1 or HCC mRECIST in patients with BCLC stage B disease
| RECIST 1.1 |
HCC mRECIST |
|||
|---|---|---|---|---|
| Atezolizumab + bevacizumab (n = 46) | Sorafenib (n = 23) | Atezolizumab + bevacizumab (n = 46) | Sorafenib (n = 23) | |
| Confirmed ORR, n (%) | 20 (43) | 6 (26) | 23 (50) | 7 (30) |
| (95% CI), %a | (28.9, 58.9) | (10.2, 48.4) | (34.9, 65.1) | (13.2, 52.3) |
| CR, n (%) | 5 (11) | 0 | 8 (17) | 1 (4) |
| PR, n (%) | 15 (33) | 6 (26) | 15 (33) | 6 (26) |
| SD, n (%) | 20 (43) | 11 (48) | 16 (35) | 11 (48) |
| DCR, n (%) | 40 (87) | 17 (74) | 39 (85) | 18 (78) |
| PD, n (%) | 2 (4) | 3 (13) | 2 (4) | 2 (9) |
| Not evaluable, n (%) | 1 (2) | 0 | 2 (4) | 0 |
| Missing, n (%) | 3 (7) | 3 (13) | 3 (7) | 3 (13) |
| Median DOR (95% CI),b months | 14.2 (10.9, 16.6) | 12.4 (4.7, NE) | 14.2 (11.8, 17.6) | 12.4 (6.1, NE) |
| Median time to first PR or CR (range),c months | 4.1 (1.3–12.3) | 4.2 (1.2–5.7) | 2.8 (1.2–12.3) | 3.0 (1.2–4.2) |
| Median time to first CR (range),c months | 6.9 (2.9–11.1) | NA | 5.0 (2.9–9.7) | 5.6 (5.6–5.6) |
BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; CR, complete response; DOR, duration of response; DCR, disease control rate; HCC, hepatocellular carcinoma; IRF, independent review facility; mRECIST, modified Response Evaluation Criteria in Solid Tumours; NA, not applicable; NE, not estimable; ORR, objective response rate; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumours; SD, stable disease.
Only patients with measurable disease at baseline were included in the analysis of ORR.
Only confirmed responders were included in the analysis of DOR.
Time to first PR or CR is defined as time from the date of randomization to the date of first PR/CR response.
Among patients receiving sorafenib, the confirmed ORR was 26% (n = 6) per IRF RECIST 1.1 and 30% (n = 7) per HCC mRECIST (Table 2). CR was achieved in 0 patients per IRF RECIST 1.1 and 1 patient (4%) per HCC mRECIST. PD occurred in 3 patients (13%) per IRF RECIST 1.1 and 2 patients (9%) per HCC mRECIST. Median time to first PR or CR was 4.2 months (95% CI: 1.2, 5.7) per IRF RECIST 1.1 and 3.0 months (95% CI: 1.2, 4.2) per HCC mRECIST.
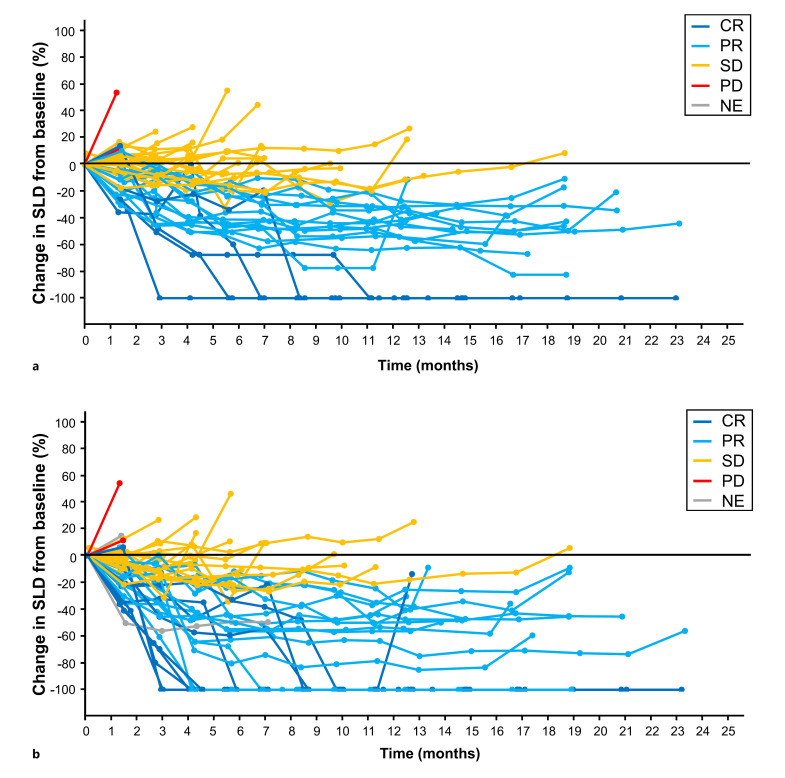
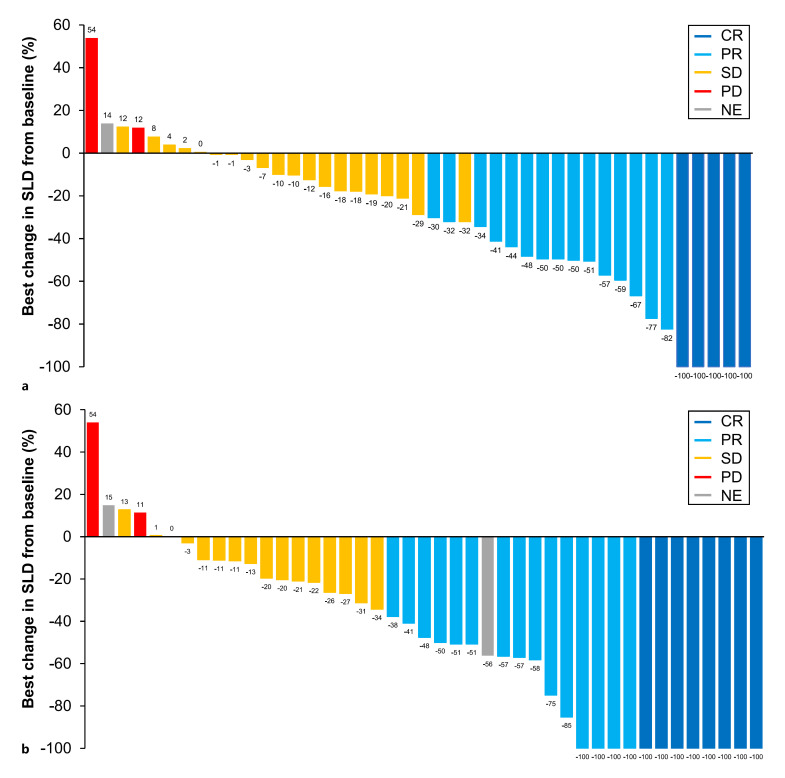
Percentage change in SLD of target lesions from baseline per IRF RECIST 1.1 and HCC mRECIST (Fig. 3) in the atezolizumab + bevacizumab arm shows a durable response (duration of response, 14.2 months; 95% CI: 10.9, 16.6; Table 2) in patients who achieved CR, PR, or stable disease. Waterfall plots of the best change in SLD of target lesions from baseline showed the depth of tumor shrinkage in patients receiving atezolizumab + bevacizumab per IRF RECIST 1.1 and HCC mRECIST (Fig. 4). In patients receiving atezolizumab + bevacizumab, 12 patients (28%) per IRF RECIST 1.1 and 21 (49%) per HCC mRECIST achieved >50% best decrease in SLD from baseline in target lesions (Fig. 4).
Fig. 3.
Change in SLD of target lesions from baseline. Spider plots of the change in SLD of target lesions from baseline by (a) IRF-assessed RECIST 1.1 and (b) HCC mRECIST in the atezolizumab + bevacizumab arm in patients with BCLC stage B with measurable disease at baseline (n = 46). Three of the 46 patients had missing SLD data and were not included in this analysis.
Fig. 4.
Best change in SLD of target lesions from baseline. Waterfall plots of the best change in SLD of target lesions from baseline by (a) IRF-assessed RECIST 1.1 and (b) HCC mRECIST in the atezolizumab + bevacizumab arm in patients with BCLC stage B with measurable disease at baseline (n = 46). Three of the 46 patients had missing SLD data and were not included in this analysis. Response categories indicated in the legend refer to the overall response and may not reflect the best response in the target lesion in some cases.
Safety
The safety-evaluable population included 48 patients in the atezolizumab + bevacizumab arm and 23 patients in the sorafenib arm who had BCLC stage B disease at baseline. The median duration of treatment was 12.5 months (range, 0–24) with atezolizumab and 11.4 months (range, 0–24 months) with bevacizumab (Table 3). The treatment duration of atezolizumab was <3 months in 17% of patients (n = 8) and ≥12 months in 54% of patients (n = 26). Bevacizumab treatment was administered for <3 months in 17% of patients (n = 8) and for ≥12 months in 48% of patients (n = 23). Patients were treated with sorafenib for a median of 4.8 months (range, 0–21), with 44% (n = 10) of patients receiving treatment for <3 months and 13% of patients (n = 3) receiving treatment for ≥12 months.
Table 3.
Safety summary
| Atezolizumab + bevacizumab (n = 48) | Sorafenib (n = 23)a | |
|---|---|---|
| Treatment duration | ||
| Median (range), months | Atezolizumab: 12.5 (0–24) | 4.8 (0–21) |
| Bevacizumab: 11.4 (0–24) | ||
| <3 months, n (%) | Atezolizumab: 8 (17) | 10 (44) |
| Bevacizumab: 8 (17) | ||
| 3 to <6 months, n (%) | Atezolizumab: 3 (6) | 3 (13) |
| Bevacizumab: 5 (10) | ||
| 6 to <9 months, n (%) | Atezolizumab: 8 (17) | 2 (9) |
| Bevacizumab: 9 (19) | ||
| 9 to <12 months, n (%) | Atezolizumab: 3 (6) | 5 (22) |
| Bevacizumab: 3 (6) | ||
| ≥12 months, n (%) | Atezolizumab: 26 (54) | 3 (13) |
| Bevacizumab: 23 (48) | ||
| All-grade AE, any cause, n (%) | 48 (100) | 22 (96) |
| Treatment-related all-grade AE | 43 (90) | 22 (96) |
| Grade 3/4 AE, n (%)b | 31 (65) | 16 (70) |
| Treatment-related grade 3/4 AEc | 22 (46) | 12 (52) |
| Serious AE, n (%) | 25 (52) | 8 (35) |
| Treatment-related serious AE | 10 (21) | 6 (26) |
| Grade 5 AE, n (%) | 4 (8) | 0 |
| Treatment-related grade 5 AE | 1 (2)c | 0 |
| AE leading to withdrawal from any component, n (%) | 12 (25) | 5 (22) |
| AE leading to dose interruption of any study treatment, n (%) | 31 (65) | 9 (39) |
| AE leading to dose modification of sorafenib, n (%)d | 0 | 12 (52) |
AE, adverse event.
Safety-evaluable population.
Highest grade experienced.
Patient experienced a treatment-related grade 5 AE of pneumonia.
No dose modification allowed for atezolizumab or bevacizumab.
In the atezolizumab + bevacizumab arm, the most common AEs were hypertension (20 patients [42%]), pruritus (18 [38%]), and proteinuria (17 [35%]) (online suppl. Table S1). Any-grade and grade 3/4 bleeding/hemorrhage AEs occurred in 25% and 10% of patients, respectively (online suppl. Table S2). No grade 5 bleeding/hemorrhage AEs were reported. Treatment-related AEs (TRAEs) occurred in 43 patients (90%); TRAEs were of grade 3/4 severity in 22 patients (46%) and grade 5 in 1 patient (2%) (Table 3).
In the sorafenib arm, the most common AEs were diarrhea (14 patients [61%]), palmar-plantar erythrodysesthesia syndrome (13 [57%]), and hypertension (9 [39%]). Any-grade and grade 3/4 bleeding/hemorrhage AEs occurred in 35% and 22% of patients, respectively. TRAEs occurred in 22 patients (96%) and were of grade 3/4 severity in 12 patients (52%). No grade 5 TRAEs occurred in patients receiving sorafenib.
Discussion
In this exploratory analysis of IMbrave150, OS and PFS benefits were observed with atezolizumab + bevacizumab versus sorafenib in patients with BCLC stage B HCC at baseline, consistent with the ITT analyses. A numerical increase in median OS was observed with atezolizumab + bevacizumab versus sorafenib. There was also a trend toward improved PFS with atezolizumab + bevacizumab treatment compared with sorafenib. In patients receiving atezolizumab + bevacizumab, durable responses were observed in complete and partial responders, and stable disease was prolonged. A high response rate was seen in the atezolizumab + bevacizumab arm, with a substantial proportion of patients achieving >50% reduction in tumor size. Together with the primary and updated analyses of IMbrave150 [9, 12], these analyses strengthen the evidence for atezolizumab + bevacizumab as standard of care for systemic treatment-naive patients with unresectable HCC, including those with BCLC stage B.
Treatment algorithms for patients with HCC are typically categorized using the BCLC staging system [2]. Patients with BCLC stage B disease are usually not candidates for tumor ablation or resection due to technical and prognostic reasons. Recent guidelines by EASL and AASLD recommend TACE and 1 L atezolizumab + bevacizumab systemic therapy as treatment options in patients with intermediate-stage HCC, with atezolizumab + bevacizumab being the treatment of choice in patients unsuitable for TACE or those who failed initial therapy [8, 13]. This exploratory analysis provides further clinical evidence to support these recommendations.
Similar to this analysis, several studies have evaluated the safety and efficacy of 1 L systemic therapy in patients with BCLC stage B HCC. Among patients with BCLC stage B disease in the SHARP trial, median OS with sorafenib was 14.5 months (HR vs. placebo, 0.72; 95% CI: 0.38, 1.38), and median PFS was 4.4 months (HR vs. placebo, 0.47; 95% CI: 0.23, 0.96) [17]. In the BCLC stage B subgroup of the REFLECT trial, median OS with lenvatinib was 18.5 months versus 17.3 months with sorafenib (HR: 0.91; 95% CI: 0.65, 1.28) and median PFS was 9.1 months and 5.5 months with lenvatinib and sorafenib, respectively (HR: 0.70; 95% CI: 0.50, 0.99) [18]. In the CheckMate 459 trial, 1 L nivolumab treatment did not significantly improve OS or PFS in the ITT population compared with sorafenib. Interestingly, in the BCLC stage B subgroup, there was a trend toward reduced OS with nivolumab versus sorafenib (HR: 1.35; 95% CI: 0.86, 2.11), albeit with a small sample size [19]. Overall, median OS and PFS observed with atezolizumab + bevacizumab in this analysis were longer than those reported for other 1 L systemic therapies in patients with BCLC stage B. These observations should be interpreted with caution as differences in study design and discrepancies in baseline populations preclude indirect comparisons between trials. The durable response (>12 months) and significant tumor shrinkage observed with atezolizumab + bevacizumab in this analysis may have contributed to the prolonged OS and PFS.
In this subgroup analysis, improved ORRs were observed in the atezolizumab + bevacizumab arm, with high CR rates compared with sorafenib. The percentage of patients who had PD with atezolizumab + bevacizumab in this analysis (4%) was lower than that seen in the IMbrave150 ITT population (19%) [12], in which the majority of patients (82%) had BCLC stage C disease at baseline. Furthermore, durable responses were observed in patients who achieved CR and PR with atezolizumab + bevacizumab. Almost half of the patients treated with atezolizumab + bevacizumab in this analysis achieved a >50% reduction in tumor size per HCC mRECIST. These data suggest that atezolizumab + bevacizumab may aid in preventing disease progression when administered in patients at an earlier stage of the disease.
The combination of programmed death-ligand 1 inhibition by atezolizumab and VEGF blockade by bevacizumab allows for simultaneous immune activation and inhibition of immunosuppression, reduced vascularity, and improved T-cell trafficking in the tumor microenvironment, contributing to the tumor response seen in this analysis [20, 21]. Atezolizumab enhances the recruitment and activation of dendritic cells and tumor infiltration of cytotoxic T lymphocytes, while bevacizumab suppresses the activity of T-regulatory cells and myeloid-derived suppressor cells, resulting in a synergistic immune response [20, 21, 22]. Unlike other VEGF-targeted tyrosine kinase inhibitor monotherapies that only contribute to a necrosis-like tumor response by decreasing blood flow in the tumor area [23], this analysis demonstrates the ability of atezolizumab + bevacizumab to achieve both necrosis-like tumor response and tumor shrinkage.
The combination of atezolizumab and bevacizumab is also able to maintain liver function for prolonged periods [24], possibly due to high specificity and affinity of monoclonal antibodies, resulting in low off-target effects. On the other hand, tyrosine kinase inhibitors such as sorafenib, regorafenib, cabozantinib, and lenvatinib are known to have off-target effects, inhibiting other receptors such as platelet-derived growth factor receptor, fibroblast growth factor receptor, and c-kit and possibly contributing to the increased occurrence of AEs [25, 26].
Data from the IMbrave150 study and this analysis support the continuation of atezolizumab + bevacizumab until PD or toxicity in patients who respond to treatment [9, 12]. As disease recurrence or progression is likely to occur when systemic therapy is discontinued (e.g., due to AEs) [27], patients who achieve a durable response with atezolizumab + bevacizumab treatment could require ongoing therapy. A potential option to improve prognosis in patients who achieved durable response with marked reductions in tumor size but are not cured is conversion to curative therapy. The durable responses seen in this study, with high tumor shrinkage rates, suggest that patients who achieve >50% reduction in tumor size with atezolizumab + bevacizumab therapy could be candidates for subsequent curative therapies such as liver resection or radiofrequency ablation. In a retrospective analysis of patients with intermediate-stage HCC who received 1 L atezolizumab + bevacizumab, curative conversion was achieved by following atezolizumab + bevacizumab with curative conversion therapy (termed ABC conversion) in 24–32% of patients [28, 29, 30]. ABC conversion employs a potentially curative treatment such as surgical resection or radiofrequency ablation after marked tumor shrinkage is achieved with atezolizumab + bevacizumab. However, further studies are required to identify patients who may benefit from conversion to LRT and to determine the optimal time to shift from systemic therapy to LRT or surgery.
The safety and tolerability profile of atezolizumab + bevacizumab in this analysis of patients with BCLC stage B HCC was consistent with the known safety profiles of each individual drug and with the underlying disease. No new or unexpected safety signals were identified for atezolizumab or bevacizumab in this study. Occurrence of TRAEs across all categories (all grade, grade 3/4, and serious AEs) was similar in the atezolizumab + bevacizumab and sorafenib arms. One grade 5 TRAE of pneumonia occurred in the atezolizumab + bevacizumab arm.
One limitation of this analysis is the lack of stratification in the BCLC stage B subgroup despite the minimal differences in baseline characteristics across arms. Second, this exploratory subgroup analysis had a small sample size and was not powered for statistical analyses. The limited sample size in this analysis prevented meaningful interpretation of results in additional patient subsets that were defined by other relevant characteristics such as etiology or prior LRT. Although this analysis provides valuable data on the efficacy of atezolizumab + bevacizumab in patients who had BCLC stage B disease at baseline, it is still unknown whether atezolizumab + bevacizumab is more efficacious than TACE, which currently remains the standard of care for intermediate-stage HCC.
As per the protocol of the global phase III IMbrave150 study, conversion surgery and LRT were not permitted after tumor shrinkage. Therefore, there are no available data on survival outcomes post 1 L atezolizumab + bevacizumab therapy and subsequent conversion surgery or LRT. The role of atezolizumab + bevacizumab in intermediate-stage patients who have not received prior LRT will be further explored in the ongoing phase IIIb ABC-HCC trial (NCT04803994) [31].
Conclusion
This exploratory subgroup analysis of IMbrave150 demonstrated an efficacy benefit with atezolizumab + bevacizumab treatment in patients with BCLC stage B HCC disease at baseline, consistent with the ITT population. These data strengthen the evidence for atezolizumab + bevacizumab as the standard of care for systemic treatment-naive patients with unresectable HCC, including patients with BCLC stage B who are not suitable for LRTs or whose disease is progressing on LRTs.
Statement of Ethics
IMbrave150 was carried out in accordance with the International Conference on Harmonisation guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. All patients gave written informed consent to participate in the study. Protocol approval was obtained from the Institutional Review Board (IRB) or Ethics Committee (EC) at each site. The first IRB approval for IMbrave150 was granted on December 19, 2017, from the City of Hope National Medical Center, Duarte, CA, USA (IRB No. 20172734; Western Institutional Review Board, Inc., Puyallup, WA, USA), in addition to multiple other EC/IRB approvals obtained across all participating sites in the different countries of enrollment. An independent data monitoring committee reviewed unmasked safety and trial conduct data approximately every 6 months until study unblinding. The study sponsor supplied the study drugs and collaborated with academic authors on the study design, data collection, data analysis, and data interpretation.
Conflict of Interest Statement
Masatoshi Kudo reports the following conflicts of interest: honoraria payment to self from Bayer, Chugai Pharmaceutical Co. Ltd., Eli Lilly, Eisai, Merck Sharp & Dohme, and Takeda; research funding to the institution from AbbVie, Chugai Pharmaceutical Co. Ltd., EA Pharma, Eisai, F. Hoffmann-La Roche Ltd., GE Healthcare, Gilead Sciences, Otsuka, Sumitomo Dainippon Pharma, Taiho, and Takeda; Editor-in-Chief of Liver Cancer. Richard S. Finn reports the following conflicts of interest: consulting fees to self from AstraZeneca, Bayer, CStone Pharmaceuticals, Eisai, Eli Lilly, Exelixis, F. Hoffmann-La Roche Ltd., Jiangsu Hengrui Pharmaceuticals, Merck, and Pfizer; research funding to the institution from Adaptimmune, Bristol Myers Squibb, Eisai, Eli Lilly, Merck, Pfizer, and F. Hoffmann-La Roche Ltd; Editorial Board Member of Liver Cancer. Peter R. Galle reports the following conflicts of interest: consulting fees to self from Adaptimmune, AstraZeneca, Bayer, Boston Scientific, Bristol Myers Squibb, Eisai, Eli Lilly, F. Hoffmann-La Roche Ltd., Guerbet, Ipsen, Merck Sharp & Dohme, and Sirtex Medical; honoraria payment to self from Adaptimmune, AstraZeneca, Bayer, Boston Scientific, Bristol Myers Squibb, Eisai, Eli Lilly, F. Hoffmann-La Roche Ltd., Guerbet, Ipsen, Merck Sharp & Dohme, and Sirtex Medical; advisory fees to self from Adaptimmune, AstraZeneca, Bayer, Boston Scientific, Bristol Myers Squibb, Eisai, Eli Lilly, F. Hoffmann-La Roche Ltd., Guerbet, Ipsen, Merck Sharp & Dohme, and Sirtex Medical; research funding to the institution from Bayer and F. Hoffmann-La Roche Ltd. Andrew X. Zhu reports the following conflicts of interest: consulting fees to self from Eisai, Eli Lilly, F. Hoffmann-La Roche Ltd., and Sanofi-Aventis; research funding to the institution from F. Hoffmann-La Roche Ltd. Michel Ducreux reports the following conflicts of interest: honoraria, consulting fees, or advisory fees to self from Amgen, AstraZeneca, Bayer, Eli Lilly, F. Hoffmann-La Roche Ltd., Ipsen, Merck Serono, Pierre Fabre, and Servier; speaker bureau participation for Amgen, Bayer, Eli Lilly, F. Hoffmann-La Roche Ltd., Ipsen, and Merck Serono; travel support from Bayer, Eli Lilly, F. Hoffmann-La Roche Ltd., Ipsen, Merck Sharp & Dohme, and Servier; research funding to the institution from Bayer and F. Hoffmann-La Roche Ltd. Ann-Lii Cheng reports the following conflicts of interest: research funding to the institution from F. Hoffmann-La Roche Ltd. Masafumi Ikeda reports the following conflicts of interest: honoraria to self from Bayer, Chugai Pharmaceutical Co. Ltd., Eisai, Eli Lilly, and Takeda; advisory/consulting fees to self from Chugai Pharmaceutical Co. Ltd., Eisai, Eli Lilly, Merck Sharp & Dohme, and Takeda; research funding to the institution from Bayer, Bristol Myers Squibb, Chugai Pharmaceutical Co. Ltd., Eisai, Eli Lilly, F. Hoffmann-La Roche Ltd., Merck Sharp & Dohme, and Takeda. Kaoru Tsuchiya reports the following conflicts of interest: advisory/consultancy fees to self from Chugai Pharmaceutical Co. Ltd., and Eisai; speaker bureau participation for Chugai Pharmaceutical Co. Ltd., Eisai, Eli Lilly, and Takeda; research funding to the institution from F. Hoffmann-La Roche Ltd. Ken-ichi Aoki reports the following conflicts of interest: employment by Chugai Pharmaceutical Co. Ltd. Jing Jia reports the following conflicts of interest: employment by F. Hoffmann-La Roche Ltd. Riccardo Lencioni reports the following conflicts of interest: advisory/consultancy fees to self from AstraZeneca, Bayer, Eisai, and F. Hoffmann-La Roche Ltd.; research funding to the institution from F. Hoffmann-La Roche Ltd.
Funding Sources
This study was sponsored by F. Hoffmann-La Roche Ltd. Third-party medical writing assistance was sponsored by Chugai Pharmaceutical Co. Ltd.
Author Contributions
Masatoshi Kudo contributed to conceptualization, methodology, investigation, resources, data curation, writing − review and editing, visualization, and supervision. Richard S. Finn, Peter R. Galle, and Andrew X. Zhu contributed to writing − review and editing and supervision. Michel Ducreux contributed to conceptualization, validation, investigation, and writing − review and editing. Ann-Lii Cheng contributed to conceptualization, validation, formal analysis, resources, data curation, and writing − review and editing. Masafumi Ikeda contributed to resources, writing − review and editing, and supervision. Kaoru Tsuchiya contributed to investigation, resources, and writing − review and editing. Ken-ichi Aoki contributed to conceptualization, methodology, resources, writing − review and editing, and visualization. Jing Jia contributed to formal analysis and writing − review and editing. Riccardo Lencioni contributed to conceptualization, methodology, and writing − review and editing.
Data Availability Statement
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing, the request platform is Vivli (https://vivli.org/ourmember/roche/). For up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient reidentification.
Supplementary Material
Supplementary data
Acknowledgments
The authors would like to thank the patients who participated in the trial, the patients' families, and the investigators and staff at all clinical study sites. Third-party medical writing assistance for this manuscript was provided by Akshaya Srinivasan, PhD, of Meditech Media Ltd.
Funding Statement
This study was sponsored by F. Hoffmann-La Roche Ltd. Third-party medical writing assistance was sponsored by Chugai Pharmaceutical Co. Ltd.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020 GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71((3)):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver Electronic address easloffice@easlofficeeu;European Association for the Study of the Liver EASL Clinical Practice Guidelines management of hepatocellular carcinoma. J Hepatol. 2018;69((1)):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma the BCLC staging classification. Semin Liver Dis. 1999;19((3)):329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 4.Kudo M. Heterogeneity and subclassification of Barcelona clinic liver cancer stage B Liver Cancer. 2016;5((2)):91–96. doi: 10.1159/000367768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, et al. Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32((4)):348–359. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]
- 6.Kudo M, Arizumi T, Ueshima K, Sakurai T, Kitano M, Nishida N. Subclassification of BCLC B stage hepatocellular carcinoma and treatment strategies proposal of modified Bolondi's subclassification (Kinki criteria) Dig Dis. 2015;33((6)):751–758. doi: 10.1159/000439290. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008. 24;359((4)):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic treatment of hepatocellular carcinoma an EASL position paper. J Hepatol. 2021;75((4)):960–974. doi: 10.1016/j.jhep.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382((20)):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 10.atezolizumab T (. Prescribing information Genentech Inc 2022. https://www.gene.com/download/pdf/tecentriq_prescribing.pdf .
- 11.Pharmaceuticals, Medical Devices Agency New drugs approved in FY 2020 (tecentriq and avastin) 2020. https://www.pmda.go.jp/files/000242574.pdf .
- 12.Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150 atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76((4)):862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 13.Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, et al. Trial design and endpoints in hepatocellular carcinoma AASLD Consensus Conference. Hepatology. 2021;73((Suppl 1)):158–191. doi: 10.1002/hep.31327. [DOI] [PubMed] [Google Scholar]
- 14.Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of hepatocellular carcinoma in Japan JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021;10((3)):181–223. doi: 10.1159/000514174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma asia-pacific primary liver cancer Expert consensus statements. Liver Cancer. 2020;9((3)):245–260. doi: 10.1159/000507370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30((1)):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 17.Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma subanalyses of a phase III trial. J Hepatol. 2012;57((4)):821–829. doi: 10.1016/j.jhep.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma a randomised phase 3 non-inferiority trial. Lancet. 2018;391((10126)):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 19.Yau T, Park J-W, Finn RS, Cheng A-L, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459) a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23((1)):77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 20.Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. 2016;7:12624. doi: 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castet F, Willoughby CE, Haber PK, Llovet JM. Atezolizumab plus bevacizumab a novel breakthrough in hepatocellular carcinoma. Clin Cancer Res. 2021;27((7)):1827–1829. doi: 10.1158/1078-0432.CCR-20-4706. [DOI] [PubMed] [Google Scholar]
- 22.Li SJ, Chen JX, Sun ZJ. Improving antitumor immunity using antiangiogenic agents mechanistic insights, current progress, and clinical challenges. Cancer Commun. 2021;41((9)):830–850. doi: 10.1002/cac2.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelley RK, Venook AP. Sorafenib in hepatocellular carcinoma separating the hype from the hope. J Clin Oncol. 2008;26((36)):5845–5848. doi: 10.1200/JCO.2008.19.7996. [DOI] [PubMed] [Google Scholar]
- 24.Kudo M, Finn RS, Cheng A-L, Zhu AX, Ducreux M, Galle P, et al. IMbrave150 albumin-bilirubin (ALBI) grade analyses in a phase III study of atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC) J Clin Oncol. 2021;39((3)) Virtual. Poster #932P. [Google Scholar]
- 25.Ivy SP, Wick JY, Kaufman BM. An overview of small-molecule inhibitors of VEGFR signaling. Nat Rev Clin Oncol. 2009;6((10)):569–579. doi: 10.1038/nrclinonc.2009.130. [DOI] [PubMed] [Google Scholar]
- 26.Gadaleta RM, Moschetta A. Dark and bright side of targeting fibroblast growth factor receptor 4 in the liver. J Hepatol. 2021;75((6)):1440–1451. doi: 10.1016/j.jhep.2021.07.029. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Zhang W, Jiang L, Chen Y. Recent advances in systemic therapy for hepatocellular carcinoma. Biomark Res. 2022;10((1)):3. doi: 10.1186/s40364-021-00350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudo M. A novel treatment strategy for patients with intermediate-stage HCC who are not suitable for TACE upfront systemic therapy followed by curative conversion. Liver Cancer. 2021;10((6)):539–544. doi: 10.1159/000519749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kudo M. Atezolizumab plus bevacizumab followed by curative conversion (ABC conversion) in patients with unresectable TACE-unsuitable intermediate-stage hepatocellular carcinoma. Liver Cancer. 2022;11((5)):399–406. doi: 10.1159/000526163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kudo M. New treatment paradigm with systemic therapy in intermediate-stage hepatocellular carcinoma. Int J Clin Oncol. 2022;27((7)):1110–1119. doi: 10.1007/s10147-022-02166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foerster F, Kloeckner R, Reig M, Chan SL, Chung JW, Merle P, et al. ABC-HCC a phase IIIb, randomized, multicenter, open-label trial of atezolizumab plus bevacizumab versus transarterial chemoembolization (TACE) in intermediate-stage hepatocellular carcinoma. J Clin Oncol. 2022;40((4_Suppl)):TPS498. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing, the request platform is Vivli (https://vivli.org/ourmember/roche/). For up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient reidentification.